Abstract
In less than 6 months, COVID-19 spread rapidly around the world and became a global health concern. Hypertension is the most common chronic disease in COVID-19 patients, but its impact on these patients has not been well described. In this retrospective study, 82 patients diagnosed with COVID-19 were enrolled, and epidemiological, demographic, clinical, laboratory, radiological and therapy-related data were analyzed and compared between COVID-19 patients with (29 cases) or without (53 cases) hypertension. The median age of the included patients was 60.5 years, and the cohort included 49 women (59.8%) and 33 (40.2%) men. Hypertension (31 [28.2%]) was the most common chronic illness, followed by diabetes (16 [19.5%]) and cardiovascular disease (15 [18.3%]). The most common symptoms were fatigue (55 [67.1%]), dry cough (46 [56.1%]) and fever ≥ 37.3 °C (46 [56.1%]). The median time from illness onset to positive RT-PCR test was 13.0 days (range 3–25 days). There were 6 deaths (20.7%) in the hypertension group and 5 deaths (9.4%) in the nonhypertension group, and more hypertensive patients with COVID-19 (8 [27.6%]) than nonhypertensive patients (2 [3.8%]) (P = 0.002) had at least one comorbid disease. Compared with nonhypertensive patients, hypertensive patients exhibited higher neutrophil counts, serum amyloid A, C-reactive protein, and NT-proBNP and lower lymphocyte counts and eGFR. Dynamic observations indicated more severe disease and poorer outcomes after hospital admission in the hypertension group. COVID-19 patients with hypertension have increased risks of severe inflammatory reactions, serious internal organ injury, and disease progression and deterioration.
Subject terms: Viral infection, Epidemiology
Introduction
In 2020, COVID-19 spread rapidly around the globe, and the disease remains an international public health concern1. As a betacoronavirus in the 2β lineage, SARS-CoV-2 shares 79.5% sequence identity with SARS-CoV and 96% identity to a bat coronavirus at the whole-genome level2. By September 2020, the virus had spread to nearly 200 countries and regions, infecting more than 28 million people and killing nearly 1 million.
SARS-CoV-2 can infect multiple systems and organs through the binding of its spike protein to angiotensin converting enzyme II (ACE2); the SARS-CoV-2 spike has a higher affinity than the SARS-CoV spike for ACE2. As a functional receptor, the ACE2 protein is abundantly expressed in the epithelia of the human lung and small intestine3. Thus, the clinical spectrum of SARS-CoV-2 infection is broad, including fever, cough, dyspnea, decreased leukocyte counts or white blood cell counts, mild upper respiratory tract illness, and severe viral pneumonia4. System or organ malfunction, including shock, acute respiratory distress syndrome (ARDS), acute cardiac injury, and even death, can also occur in severe cases5. It is worth nothing that variations in the ACE system, including in ACE1 and ACE2, also contribute to the occurrence of hypertension6. However, the specific effects of SARS-CoV-2 infection on individuals with hypertension are unknown, and it is unclear whether hypertensive individuals with COVID-19 are at greater risk of serious outcomes4,7,8. In this study, we aimed to describe the epidemiology, clinical features, and pharmacotherapy response of COVID-19 inpatients and to further compare the available data between hypertensive and nonhypertensive patients admitted to Hubei No. 3 People’s Hospital of Jianghan University (the Third People’s Hospital of Hubei Province).
Methods
Study design and participants
This retrospective, single-center, observational study was conducted at Hubei No. 3 People’s Hospital of Jianghan University, Wuhan, China. Patients were hospitalized from Dec 31, 2019, to Feb 01, 2020, and the final date of follow-up was February 08, 2020. Hubei No. 3 People’s Hospital of Jianghan University is a designated COVID-19 hospital with more than 1500 beds. The diagnosis of COVID-19 was made based on the current New Coronavirus Pneumonia Prevention and Control Program (6th edition, in Chinese) released by the National Health Commission of China9 and was indicated by suspected symptoms, chest CT results and SARS-CoV-2 positivity on quantitative RT-PCR. Throat-swab specimens from all patients were collected at admission and kept in virus transport medium. Briefly, SARS-CoV-2 detection in respiratory specimens was conducted by the Chinese Center for Disease Control and Prevention, the Chinese Academy of Medical Science, the Academy of Military Medical Sciences, and the Wuhan Institute of Virology, the Chinese Academy of Sciences using real-time RT-PCR or next-generation sequencing technology. Other respiratory viruses, including influenza A virus (H1N1, H3N2, H7N9), influenza B virus, respiratory syncytial virus, parainfluenza virus, adenovirus, SARS coronavirus (SARS-CoV), and MERS coronavirus (MERS-CoV), were also detected by real-time RT-PCR. Sputum or endotracheal aspirates were also examined to identify potential pathogenic bacteria or fungi. Chest computed tomographic (CT) scans were carried out at least twice for each patient.
Patients hospitalized with COVID-19 are clinically classified as having mild, moderate, or severe disease, defined as follows: mild cases: mild clinical symptoms, no manifestations of pneumonia on imaging; moderate cases: symptoms such as fever and respiratory tract symptoms and imaging showing signs of pneumonia; severe cases: respiratory distress, respiratory rate ≥ 30 breaths/min, SpO2 ≤ 93% at rest, or PaO2/FIO2 ratio ≤ 300. In addition, cases with > 50% lesions progressing within 24 to 48 h on pulmonary imaging are considered severe.
Ethical approval
The research protocol was reviewed and approved by the Ethics Committee of Hubei No. 3 People’s Hospital of Jianghan University (202004). All procedures were carried out in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki. All patients had completed treatment at the beginning of the study, and the study did not interfere with diagnosis or treatment in any case. Therefore, the need for informed consent was waived by the Ethics Committee of Hubei No. 3 People’s Hospital of Jianghan University. In addition, all patient privacy and data were respected and protected.
Data collection
Eighty-two patients hospitalized with COVID-19 were included in this retrospective study, and 29 of these patients also had hypertension. Epidemiological, demographic, clinical, laboratory, X-ray and chest CT scan, treatment, and outcome data were extracted from electronic medical records with data collection forms. Eight researchers, including physicians and clinical pharmacists, reviewed the data collection forms and examined the data independently three times. COVID-19 patients were divided into two groups according to the presence or absence of hypertension. Hypertension was defined as clinic systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg without the use of antihypertensive medications. Subjects with a blood pressure < 140/90 mmHg but with a history of hypertension and who were taking antihypertensive medication at the time of admission were also included in the hypertension group10.
Statistical analysis
Categorical variables are reported as frequencies and percentages. Continuous variables are reported as mean (SD), median, and interquartile range (IQR) values. Comparisons of quantitative variables between groups were performed by the Wilcoxon rank sum test. Categorical variables are expressed as numbers (%) and were compared between groups using χ2 or Fisher’s exact tests. A two-sided p-value less than 0.05 was considered statistically significant. Statistical analysis was performed in SPSS (version 21.0).
Results
Baseline characteristics of hospitalized COVID-19 patients on admission
Figure 1 shows the flow chart for participant inclusion. Briefly, 295 cases with dates from Dec 31, 2019, to Feb 28, 2020, in the medical record system were initially screened, of which 213 were considered ineligible, including 73 cases admitted after 01 Feb, 23 cases without confirmed COVID-19 diagnosis, 22 duplicate records, 43 cases without available medical information, and 52 cases with missing core examination or therapy information. Ultimately, 82 patients were included in this study. The median age of the included patients was 60.5 years, and 49 (59.8%) were women (Table 1). The median time from first symptom to hospital admission was 7.0 days. Hypertension (29 [35.4%]) was the most common coexisting chronic illness, followed by diabetes (16 [19.5%]) and cardiovascular disease (15 [18.3%]). Fatigue (55 [67.1%]), dry cough (46 [56.1%]), and fever ≥ 37.3 °C (46 [56.1%]) were the most common symptoms. The median time from illness onset to positive RT-PCR test was 13.0 days (range 3–25 days). A significant difference in clinical types on admission was observed (P < 0.001) between the two groups. There was no significant difference in mortality rate between the hypertension group (6 [20.7%]) and the nonhypertension group (5 [9.4%]). Furthermore, no significant difference between the two groups was observed in the survival analysis (Fig. 2).
Figure 1.
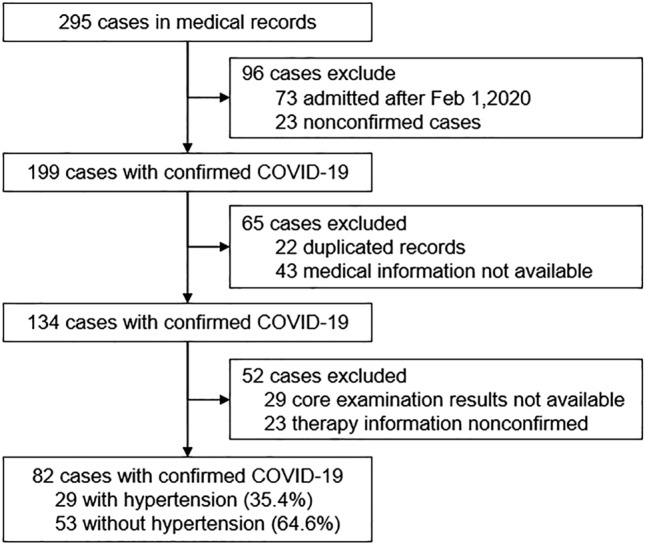
Flow chart for participant inclusion.
Table 1.
Demographics and clinical characteristics of patients with COVID-19 on admission.
| All patients (n = 82) | Hypertension group (n = 29) | Nonhypertension group (n = 53) | χ2/Z | P | |
|---|---|---|---|---|---|
| Age, years | 60.5 (46.8–69.0) | 66.0 (56.5–69.0) | 57.0 (40–68.5) | − 10.61 | < 0.001 |
| Female age | 60.0 (44.0–69.0) | 66.5 (57.8–71.5) | 63.5 (56.8–70.3) | − 14.798 | < 0.001 |
| male age | 61.0 (46.5–69) | 63.0 (53.0–69.0) | 57.5 (44.0–71.5) | − 0.967 | 0.334 |
| Sex | |||||
| Female | 49 (59.8) | 14 (48.3) | 19 (35.8) | 1.204 | 0.273 |
| Male | 33 (40.2) | 15 (51.7) | 34 (64.2) | ||
| Clinical type on admission | |||||
| Mild | 3 (3.7) | 0 (0.0) | 3 (5.7) | 43.931 | < 0.001 |
| Moderate | 68 (82.9) | 23 (79.3) | 45 (84.9) | ||
| Severe | 11 (13.4) | 6 (20.1) | 5 (9.4) | ||
| Illness onset to hospital admission, days | 7.0 (4.0–10.0) | 7 (5.5–10) | 7 (4–10) | − 1.129 | 0.259 |
| Chronic illnesses | |||||
| Hypertension | 29 (35.4) | 29 (100.0) | 0 (0) | – | – |
| Cardiovascular disease | 15 (18.3) | 9 (31.0) | 6 (11.3) | 4.814 | 0.028 |
| Diabetes | 16 (19.5) | 8 (27.6) | 8 (15.1) | 1.862 | 0.172 |
| COPD | 7 (8.5) | 4 (13.8) | 3 (5.7) | 1.568 | 0.210 |
| Malignancy | 1 (1.2) | 0 (0.0) | 1 (1.9) | 0.547 | 0.459 |
| Digestive system disease | 3 (3.7) | 2 (6.9) | 1 (1.9) | 1.318 | 0.251 |
| Cerebrovascular disease | 2 (2.4) | 2 (6.9) | 0 (0.0) | 3.747 | 0.054 |
| Nervous system disease | 3 (3.7) | 1 (3.4) | 2 (3.8) | 0.006 | 0.941 |
| Chronic liver disease | 3 (3.7) | 3 (10.3) | 0 (0.0) | 5.622 | 0.018 |
| More than one disease | 10 (12.2) | 8 (27.6) | 2 (3.8) | 9.926 | 0.002 |
| Signs and symptoms | |||||
| Fever (≥ 37.3 °C) | 46 (56.1) | 13 (44.8) | 33 (62.2) | 2.314 | 0.128 |
| ≥ 38 °C | 28 (34.1) | 6 (20.7) | 22 (41.5) | 3.361 | 0.057 |
| Fatigue | 55 (67.1) | 16 (55.2) | 39 (73.6) | 2.877 | 0.090 |
| Dry cough | 46 (56.1) | 13 (44.8) | 33 (62.3) | 2.314 | 0.128 |
| Shortness of breath | 23 (28.0) | 7 (24.1) | 16 (30.2) | 0.34 | 0.560 |
| Diarrhea | 15 (18.3) | 7 (24.1) | 8 (15.1) | 1.026 | 0.311 |
| Anorexia | 10 (12.2) | 1 (3.4) | 9 (17.0) | 3.167 | 0.075 |
| Myalgia | 9 (15.1) | 3 (10.3) | 6 (11.3) | 0.018 | 0.893 |
| Expectoration | 20 (24.3) | 10 (34.4) | 10 (18.9) | 2.478 | 0.115 |
| Pharyngalgia | 4 (4.9) | 2 (6.9) | 2 (3.8) | 0.389 | 0.533 |
| Nausea or vomiting | 7 (8.5) | 2 (6.9) | 5 (9.4) | 0.155 | 0.694 |
| Dyspnea | 5 (6.1) | 2 (6.9) | 3 (5.7) | 0.049 | 0.824 |
| More than three signs and symptoms | 40 (48.9) | 17 (58.6) | 23 (434) | 1.739 | 0.187 |
| Time from illness onset to positive RT-PCR test, days | 13 (6.0–19.0) | 13.0 (6.0–20.0) | 12.5 (6.0–15.5) | − 0.372 | 0.743 |
| Deaths | 11 (13.4) | 6 (20.7) | 5 (9.4) | 2.044 | 0.153 |
| Female | 5 (4.5) | 3 (10.3) | 2 (3.8) | 0.100 | 0.752 |
| Male | 6 (5.4) | 3 (10.3) | 3 (5.7) | ||
| Time from onset to hospital admission, days | 7.0 (5.0–10.0) | 7.0 (5.0–8.0) | 10 (5.5–20) | − 0.746 | 0.456 |
| Time from admission to death, days | 6.0 (4.0–12.0) | 7.5 (3.0–12.5) | 4.0 (2.5–9.5) | − 0.737 | 0.461 |
| Time from onset to death, days | 13.0 (10.5–19.5) | 13.5 (10.5–19.5) | 11.0 (10.5–28.5) | − 0.184 | 0.854 |
Data are presented as the median (IQR) or n/N (%), where N is the total number of patients with available data. p values comparing patients with or without hypertension cases are from χ2, Fisher’s exact test, or Wilcoxon rank sum test.
COVID-19 coronavirus disease 2019, IQR interquartile range, COPD Chronic Obstructive Pulmonary Disease.
Figure 2.
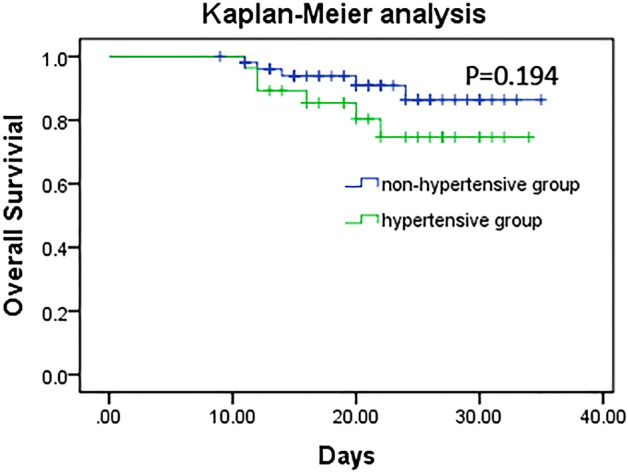
Survival analysis between the hypertension group and the nonhypertension group.
Laboratory markers in patients with or without hypertension
Major laboratory markers were recorded at hospital admission for all patients (Table 2). There were no significant differences in routine blood analysis parameters between the hypertension and nonhypertension groups. However, the hypertension group exhibited higher white blood cell counts (4.9 × 109/L), neutrophil counts (3.9 × 109/L), and neutrophil percentages (79.3%) and lower lymphocyte counts (0.72 × 109/L) and lymphocyte percentages (12.5%). Moreover, the median SAA in the hypertension group reached 630.1 mg/L, which was approximately double that (373.5 mg/L) in the nonhypertension group. Similarly, the median CRP level (79.2 mg/L) also exceeded that of the nonhypertension group (57.8 mg/L). In blood chemistry assays, no significant changes were found in AST (32.4 U/L vs 34.1 U/L), ALT (23.0 U/L vs 26.3 U/L), and GGT (29.0 U/L vs 30.3 U/L) between the two groups, and all medians were within the normal ranges. For renal injury, eGFR in hypertension group with COVID-19 was significantly decreased compared with that of nonhypertensive group (77.0 mL/min/1.73 m2 vs 113.0 mL/min/1.73 m2) (P = 0.017), while higher levels of urea nitrogen (4.7 mmol/L vs 4.2 mmol/L) and serum creatinine (70.5 μmol/L vs 57.0 μmol/L) were observed in the hypertension group. There were also elevated levels of NT-proBNP (166 ng/L vs 26 ng/L), lactate dehydrogenase (263.0 U/L vs 240.0 mU/L), and creatine kinase (143.0 U/L vs 64.0 U/L), with a significant increase in NT-proBNP in the hypertension group with COVID-19.
Table 2.
Laboratory findings of patients infected with COVID-19 on admission.
| Normal range | All patients (n = 82) | Hypertension group (n = 29) | Nonhypertension group (n = 53) | Z | P | |
|---|---|---|---|---|---|---|
| Blood routine tests | ||||||
| WBC, × 109/L | 3.5–9.5 | 4.7 (3.5–6.7) | 4.9 (3.7–7.4) | 4.5 (3.3–6.6) | − 1.093 | 0.274 |
| Neutrophil counts, × 109/L | 1.8–6.3 | 3.2 (2.2–5.2) | 3.9 (2.7–6.3) | 3.1 (2.2–4.9) | − 1.544 | 0.122 |
| N (%), | 40–75 | 76.0 (63.8–84.3) | 79.3 (69.2–85.7) | 71.6 (62.9–83.4) | − 1.852 | 0.064 |
| Lymphocyte counts, × 109/L | 1.1–3.2 | 0.8 (0.6–1.1) | 0.72 (0.59–0.10) | 0.81 (0.56–1.26) | − 0.652 | 0.515 |
| L (%) | 20–50 | 14.1 (9.1–19.2) | 12.5 (8.6–17.3) | 16.8 (12.2–21.3) | − 1.625 | 0.098 |
| Platelets, × 109/L | 125–350 | 192.0 (127.0–225.0) | 195 (165–293) | 188 (130–225) | − 0.492 | 0.623 |
| Hemoglobin, g/L | 130·0–175 | 127.0 (119.0–135.0) | 129.5 (114.8–137) | 126.5 (120–134.8) | − 0.067 | 0.947 |
| Infection-related biomarkers | ||||||
| PCT, ng/mL | 0.04–0.25 | 0.1 (0.0–0.2) | 0.07 (0.04–0.18) | 0.05(0.04–0.1) | − 1.679 | 0.093 |
| SAA, mg/L | 0.1–10 | 501.0 (111.3–102.0) | 630.1 (185.3–1010.5) | 373.5 (48.8–777.5) | − 1.509 | 0.131 |
| CRP, mg/L | 0–5 | 67.5 (27.2–102.0) | 79.2 (33.5–129.1) | 57.8 (20.4–83.1) | − 1.802 | 0.072 |
| ESR, mm/h | 0–20 | 47.8 (23.3–80.5) | 50.5 (24.9–88.0) | 47.8 (19–70) | − 0.723 | 0.470 |
| Blood biochemistry | ||||||
| AST, U/L | 8–40 | 33.6 (26.2–51.2) | 32.4 (24.2–49.8) | 34.1 (26.8–57.0) | − 0.982 | 0.326 |
| ALT, U/L | 5–35 | 26.1(17.0–40.0) | 23.0 (18.1–42.0) | 26.3 (14.7–39.4) | − 0.630 | 0.529 |
| GGT, U/L | 0–50 | 30.2 (18.0–50.6) | 29.0 (22.6–48.3) | 30.3 (16.2–55.4) | − 0.583 | 0.560 |
| Serum urea nitrogen, mmol/L | 3.5–7.2 | 4.4 (3.2–4.5) | 4.7 (3.4–5.9) | 4.2 (3.2–4.9) | − 1.324 | 0.185 |
| Serum creatinine, μmol/L | 44–120 | 60.0 (49.0–79.3) | 70.5 (51.8–80.8) | 57.0 (49.0–79) | − 1.473 | 0.142 |
| eGFR, mL/min/1.73 m2 | > 90 | 102.0 (84.0–119.3) | 77.0 (35.3–100.7) | 113.0 (91.9–125.9) | − 2.387 | 0.017 |
| Lactate dehydrogenase, U/L | 120–250 | 249.5 (200.8–353.8) | 263.0 (235–384.5) | 240.0 (193.0–318.5) | − 1.042 | 0.297 |
| Creatine kinase, U/L | 50–310 | 97.5 (55.7–179.8) | 143.0 (84.0–220.0) | 64.0 (50.0–154.0) | − 1.732 | 0.083 |
| NT-proBNP, ng/L | 25–500 | 115 (26–372) | 166 (70–1293) | 26 (20–120.5) | − 10.252 | < 0.001 |
| K, mmol/L | 3.5–5.5 | 3.7 (3.4–4.0) | 3.6 (3.5–4.1) | 3.7 (3.5–3.9) | − 0.635 | 0.525 |
| Ca, mmol/L | 2.0–2.6 | 2.1 (2.0–2.2) | 2.1 (2.0–2.1) | 2.1 (2.0–2.2) | − 0.112 | 0.911 |
| Albumin, g/L; | 40–55 | 39.0 (35.8–43.3) | 36.4 (34.6–39.9) | 40.0 (36.6–44.6) | − 2.218 | 0.026 |
| Glucose, mmol/L | 3.9–6.1 | 7.54 (6.26–9.23) | 7.8 (7.4–9.4) | 6.5 (5.5–10.0) | − 1.583 | 0.113 |
| Total bilirubin, μmol/L | 3.4–20.5 | 9.9 (8.4–13.5) | 9.8 (8.6–12.2) | 10.0 (8.0–13.8) | − 0.159 | 0.874 |
| Direct bilirubin, μmol/L | 0–6 | 3.7 (3.2–4.7) | 3.5 (3.2–4.7) | 3.9 (3.1–4.8) | − 0.817 | 0.413 |
| Total bile acid, μmol/L | 0–12 | 4.4 (2.5–6.9) | 3.8 (2.5–6.3) | 5.3 (2.5–9.1) | − 1.141 | 0.254 |
| Coagulation function | ||||||
| D-dimer, μg/mL | 0·0–1.5 | 0.5 (0.3–1.1) | 0.5 (0.3–1.0) | 0.5 (0.3–1.4) | − 0.157 | 0.875 |
| Prothrombin time, S | 9–14 | 10.4 (7.9–11.1) | 10.6 (9.7–11.1) | 10.8 (10.3–11.5) | − 1.109 | 0.267 |
| Activated partial thromboplastin time, S | 20–40 | 27.4 (11.7–31.9) | 29.7 (24.7–39.2) | 28.2 (26.9–32.0) | − 0.802 | 0.422 |
| Fibrin(-ogen) degradation products, mg/L | 0–5 | 3.7 (2.5–5.7) | 3.7 (3.0–5.5) | 3.7 (1.7–8.7) | − 0.217 | 0.828 |
Data are expressed as the median (IQR) or n/N (%), where N is the total number of patients with available data. p values comparing patients with or without hypertension are from χ2, Fisher’s exact, or Wilcoxon rank sum tests.
COVID-19 coronavirus disease 2019, IQR interquartile range, WBC white blood cell, PCT procalcitonin, SAA serum amyloid A, CRP C-reactive protein, ESR erythrocyte sedimentation rate, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT glutamine transpeptidase, eGFR estimated glomerular filtration rate, NT-proBNP N-terminal pro-brain natriuretic peptide.
Imaging findings
Of all 82 nonmedical COVID-19 patients on admission (Table 3), 14 (17.1%) patients showed unilateral pneumonia, and 64 (78.3%) developed bilateral pneumonia. Twenty-eight (34.2%) patients showed patchy shadows, while 22 (26.8%) patients showed multiple patchy shadows. Twenty-two (26.8%) patients also displayed ground glass opacities. In addition, hydrothorax occurred in 9 (11.0%) patients.
Table 3.
Chest X-ray and CT findings of COVID-19 patients on admission.
| All patients (n = 82) | Hypertension group (n = 29) | Nonhypertension group (n = 53) | χ2 | P | |
|---|---|---|---|---|---|
| Bilateral pneumonia | 64 (78.3) | 23 (79.3) | 41 (77.4) | 0.042 | 0.838 |
| Unilateral pneumonia | 14 (17.1) | 4 (13.8) | 10 (18.9) | 0.341 | 0.559 |
| Patchy shadows | 28 (34.2) | 9 (31.0) | 19 (35.8) | 0.249 | 0.618 |
| Multiple patchy shadows | 22 (26.8) | 11 (37.9) | 11 (20.7) | 2.871 | 0.093 |
| Ground glass opacity | 4 (4.9) | 1 (3.4) | 3 (5.7) | 0.198 | 0.657 |
| Hydrothorax | 9 (11.0) | 3 (10.3) | 6 (11.3) | 0.018 | 0.892 |
Data are expressed as the median (IQR) or n/N (%), where N is the total number of patients with available data. p values comparing patients with or without hypertension are from χ2 or Fisher’s exact tests.
COVID-19 Coronavirus Disease 2019, CT computed tomography.
Organ injuries and main treatments
On admission, common complications among 82 patients included ARDS (9 [11.0%]), sepsis (3 [3.7%]), acute renal injury (1 [1.2%]), and acute respiratory injury (1 [1.2%]) (Table 4). Although the differences between the two groups failed to reach statistical significance on hospital admission, the laboratory outcomes suggested that patients with hypertension had higher risks of multiple organ injuries in the kidney, heart, and lung. For COVID-19 treatment, 63 (76.8%) patients received antiviral therapy, including oseltamivir (56 [50.9%]), arbidol (46 [41.8%]), lopinavir/ritonavir (42 [38.2%]), and ganciclovir (28 [25.4%]). Many patients received glucocorticoid therapy (73 [89.0%]) and antibacterial therapy (55 [67.1]), including carbapenems (22 [20.0%]), quinolones (33 [30.0%]), and cephalosporins (14 [12.7%]). Fifty-five (67.1%) patients received both antiviral and antibacterial therapies. Oxygen therapy (24 [29.3%]) and immune globulin γ treatment (42 [51.3%]) were also applied.
Table 4.
Complications and treatments of COVID-19 patients.
| All patients (n = 82) | Hypertension group (n = 29) | Nonhypertension group (n = 53) | χ2 | P | |
|---|---|---|---|---|---|
| Complications | |||||
| ARDS | 9 (11.0) | 5 (17.2) | 4 (7.5) | 1.803 | 0.179 |
| Sepsis | 3 (3.7) | 2 (6.9) | 1 (1.9) | 1.402 | 0.236 |
| Acute renal injury | 1 (1.2) | 1 (3.4) | 0 | 1.893 | 0.169 |
| Acute respiratory injury | 1 (1.2) | 1 (3.4) | 0 | 1.893 | 0.169 |
| Treatment | |||||
| Antiviral therapy | 63 (76.8) | 22 (75.9) | 41 (77.4) | 0.024 | 0.878 |
| Two antiviral chemicals | 28 (34.1) | 11 (37.9) | 17 (32.1) | 0.286 | 0.593 |
| Three antiviral chemicals | 16 (19.5) | 6 (20.7) | 10 (18.9) | 0.040 | 0.842 |
| Glucocorticoid therapy | 73 (89.0) | 26 (89.7) | 47 (88.7) | 0.018 | 0.892 |
| Antibacterial therapy | 55 (67.1) | 18 (62.7) | 37 (69.8) | 0.509 | 0.476 |
| Anti-virus and antibacterial treatment | 34 (41.5) | 14 (48.3) | 20 (37.7) | 0.858 | 0.354 |
| Oxygen therapy | 24 (29.3) | 11 37.9) | 13 (24.5) | 1.626 | 0.202 |
| Immune globulin γ treatment | 42 (51.3) | 15 (51.7) | 27 (50.9) | 0.005 | 0.946 |
| Antihypertensive drugs | – | 25 (86.2) | – | – | – |
Data are expressed as n/N (%), where N is the total number of patients with available data. p values comparing patients with or without hypertension cases are from χ2 or Fisher’s exact test.
COVID-19 Coronavirus Disease 2019, ARDS Acute respiratory distress syndrome.
Dynamic characteristics of laboratory parameters in COVID-19 patients with or without hypertension
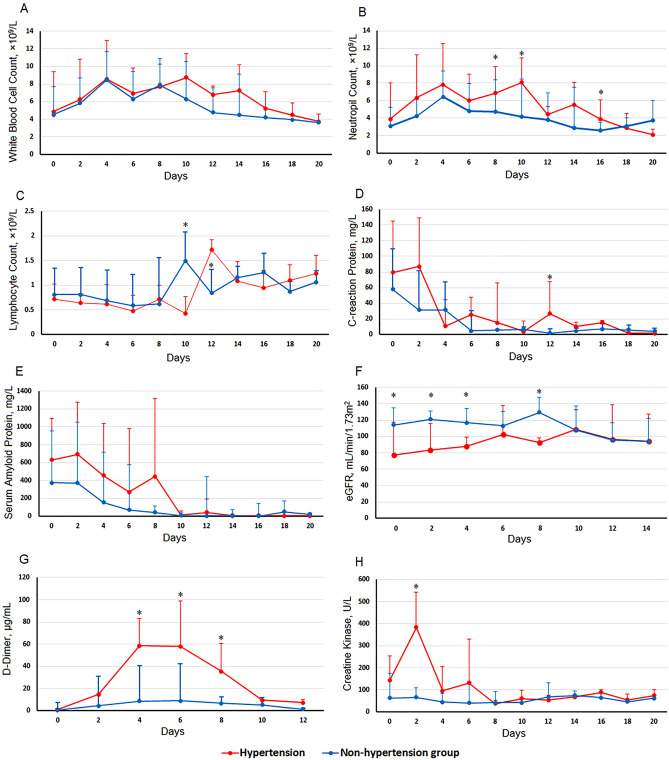
The dynamic laboratory features of COVID-19 patients, including 8 clinical parameters related to hematology, infection, coagulation function, and internal organ injury, were traced from hospital admission to 20 days in the hospital at a 2-day interval on the basis of no significant difference observed at hospital admission between the two groups (Table 1). As shown in Fig. 3A and B, white blood cell and neutrophil counts were higher in the hypertension group than in the nonhypertension group for almost the whole duration of hospitalization. Most patients had notable lymphopenia, with a 2-day delay in lymphocyte count increasing to the normal range compared to the nonhypertension group (Fig. 3C). Both CRP and SAA were higher in the hypertension group until 10 days after admission (Fig. 3D and E). The eGFR of patients with hypertension was markedly lower than that of nonhypertensive patients, maintaining an overall slow increase from the day of admission but a slight decrease from its peak on day 10; by contrast, there was a reduction of approximately 30 mL/min/1.73 m2 in the eGFR of the nonhypertension group from days 8 to 14 (Fig. 3F). The creatine kinase level in the hypertension group was higher on admission, increased to approximately 400 U/L on day 2, and then decreased (Fig. 3H). Similarly, the level of D-dimer in the hypertension group continued to increase until day 4 after admission or day 11 from onset and then declined after day 6 (Fig. 3G).
Figure 3.
Timelines of laboratory markers from hospital admission for COVID-19. Figure shows dynamic changes in white blood cell counts (A), neutrophil counts (B), lymphocyte counts (C), C-creation protein (D), serum amyloid A (E), eGFR (F), D-dimer (G), and creatine kinase (H). For eGFR and D-dimer, continuous data at 2-day intervals were available for 14 days and 12 days, respectively. *P < 0.05 for hypertension group vs nonhypertension group.
Discussion
In this investigation, we studied the epidemiology, clinical characteristics, and treatment of COVID-19, with a particular focus on potential differences in the disease course in the hypertensive population. Among the 82 included COVID-19 patients, there were 11 deaths (13.4%), which was consistent with the overall COVID-19 mortality rate at Jinyintan Hospital during the same period8. Nevertheless, national data showed that the case fatality had dropped to 2.3% by February 1111, suggesting that SARS-CoV-2 was more lethal at the early stage of the outbreak in January12.
Previously, hypertension has been identified as the most common chronic illness in more COVID-19 patients4,5,13,14. ACE2 is the receptor that mediates SARS-CoV-2 invasion in COVID-19, identical to SARS-CoV transmission, via the spike (S) glycoprotein–ACE2 binding pathway15–18. After infection, the ACE2 level was found to be reduced due to binding with the spike protein of SARS-CoV19, suggesting that SARS-CoV-2 may also reduce the level of ACE2 in infected cells, resulting in an imbalance between ACE1 and ACE2. Second, the renin-angiotensin II-aldosterone axis has been traditionally recognized as a key regulator of blood pressure in the development of hypertension, with AngII levels regulated by ACE. The balance between ACE1 and ACE2 is crucial for controlling the level of AngII. Thus, due to the imbalance of ACE1 and ACE2 induced by virus infection, the hypertensive population may tend to experience more serious organ injury.
In hypertensive COVID-19 patients, more severe clinical types or mortality were observed, suggesting that hypertension might be associated with the clinical outcomes of COVID-19. Our study also found that 31.0% of hypertensive patients also had other forms of cardiovascular disease, which has been associated with an increased risk of death in COVID-19 patients13,20. However, hypertension remains the most important risk factor leading to cardiovascular disease21. Accordingly, preexisting hypertension, rather than cardiovascular disease, might be the underlying cause of increased susceptibility to rapid disease progression and more severe COVID-19.
Laboratory results provided some evidence for this hypothesis. Hypertensive COVID-19 patients presented elevated levels of serum urea nitrogen, serum creatinine, lactate dehydrogenase, creatine kinase, and NT-proBNP and markedly reduced eGFR at admission. These altered parameters indicate that internal organs with high levels of ACE2 protein expression, such as the lung, kidney, and heart3,22, are more vulnerable to invasion and injury by SARS-COV-2. Simultaneously, SAA and CRP, which reflect systemic inflammation throughout the body, were also elevated. These results indicate that hypertensive patients tended to develop more severe COVID-19 not only through serious cytokine storms but also through reduced protection against organ injury due to imbalances in the ACE system23–25. Thus, the delay from illness onset to hospital admission could result in an increased risk of severe illness or death in hypertensive patients with COVID-19.
More dynamic changes were observed in hypertensive COVID-19 patients. White blood cell and neutrophil counts remained higher in the hypertension group than in the nonhypertension group for almost the entire period of hospitalization; SAA and CRP continued to increase until approximately 10 days after admission. In contrast with the nonhypertension group, in the hypertension group, lymphocyte counts continued to decline until 10 days after admission and returned to the normal range 2 days later. These findings suggested that more serious cytokine storms occurred in hypertensive COVID-19 patients.
Notably, in addition to ACE2, other receptors on the surface of human cells can facilitate the entry of SARS-COV-2, including TMPRSS226, sialic acid receptors27, and CD14728. Intriguingly, all of these factors are expressed by endothelial cells. Therefore, the endothelium, as one of the largest organs in the human body, is a key target organ in COVID-1929. Impairment of endothelium function can promote vasodilation, fibrinolysis, and antiaggregation30–32. Simultaneously, for hypertensive populations, endothelial dysfunction is also a key determinant of hypertension development and progression33,34. Therefore, hypertensive patients infected by SARS-COV-2 are vulnerable to more serious endothelial dysfunction and thus tend to develop a more severe COVID-19 phenotype, leading to a higher mortality rate.
In this study, 76.8% of patients were given antiviral agents based on previous experience in treating other coronavirus infections, such as SARS and MERS. Anti-influenza drugs, including oseltamivir and arbidol, were also used for empirical treatment during this unusual period. However, there are currently no agents proven to be an effective therapy for COVID-1935,36. Glucocorticoid therapy was also empirically used for severe complications in nearly 90% of patients. Nevertheless, the efficacy of glucocorticoids remains controversial because of their adverse effects37 and lack of consistently positive outcomes4,38. Recently, evidence from a randomized controlled trial showed that treatment with dexamethasone resulted in lowered mortality in COVID-19 patients39. Therefore, more reasonable glucocorticoid administration is still needed for COVID-19 treatment. Antibacterial therapy was also used in over 50% of patients to prevent or treat bacterial infections. Combined with the dynamic laboratory outcomes, parameters related to inflammation or organ function tended to improve 8–10 days after admission. However, these improvements could not be attributed to medication treatments alone.
This study had several limitations. First, only 82 patients with confirmed COVID-19 were included in the full analysis. A larger study population size would be helpful to more deeply understand the role of hypertension in the progression of COVID-19. Second, due to the retrospective study design, some information was unavailable, particularly the time from illness onset to the signs and symptoms in electronic medical records. Third, the positive rate of SARS-CoV-2 RNA detection in throat swabs is lower than that of other methods, and antibody assays were not available to assist in diagnosis by the end of January, resulting in the exclusion of suspected but unconfirmed cases from our analysis. Moreover, there are other potential risk factors, including age, chronic lung disease, cardiovascular disease, and even liver disease, that could lead to more severe disease and increased in-hospital death. However, this study initially identified hypertension as an important factor for the clinical outcomes of COVID-19. Further investigations of the mechanism in hypertensive populations are needed.
Acknowledgements
We thank all the patients involved in this study and their families, as well as the numerous doctors, nurses, pharmacists, and civilians working together to fight SARS-CoV-2. This work was supported by the Fundamental Research Funds for the Central Universities (2020kfyXGYJ038), the National Natural Science Foundation (81100128), and a Wuhan Science Grant for Distinguished Young Scholars (201751). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Author contributions
F.X. and J.Z. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. F.X., M.Z., and B.C. contributed equally and shared first authorship. Concept and design: F.X., M.Z., J.Z. Acquisition, analysis, or interpretation of data: F.X., B.C., W.A., M.C., P.Y., X.Z., Y.L., X.X., K.L., Q.Z., K.W., G.H., M.D., S.C. Drafting of the manuscript: F.X. Critical revision of the manuscript for important intellectual content: F.X., M.Z., B.C., X.Z., M.X. Statistical analysis: F.X., B.C., W.A., M.C. Administrative, technical, or material support: M.Z., S.L., Y.Z. Supervision: W.W., M.X., J.Z. All authors read, revised, and approved the final manuscript. All authors agree to this version of the manuscript and to the submission of this study to Scientific Reports and agree with the indicated contributions to this study and the order of attribution.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fei Xia, Mingwei Zhang, and Bo Cui.
Contributor Information
Fei Xia, Email: 53042940@qq.com.
Jianjun Zhang, Email: 2858147149@qq.com.
References
- 1.Hui DS, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health: The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou, P., Yang, X., Wang, X. & Hu, B. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv. (2020).
- 3.Hamming I, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Te RL, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015;116:960–975. doi: 10.1161/CIRCRESAHA.116.303587. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.New Coronavirus Pneumonia Prevention and Control Program (6th ed.) (In Chinese). (2020).
- 10.Lui LS, et al. 2018 Chinese guidelines for prevention and treatment of hypertension-a report of the revision committee of chinese guidelines for prevention and treatment of hypertension. J. Geriatr. Cardiol. 2019;16:182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Z. et al. Caution: The clinical characteristics of COVID-19 patients at admission are Changing. bioRixv. (2020).
- 13.Shi S, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JJ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 15.Xintian X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;1:1–4. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao, Y., Zhao, Z., Wang, Y. & Zhou, Y. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRixv. (2020).
- 17.Li W, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou P, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuba K, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in covid-19. N Engl J Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Jarraya F. Treatment of hypertension: Which goal for which patient? Adv. Exp. Med. Biol. 2017;956:117–127. doi: 10.1007/5584_2016_97. [DOI] [PubMed] [Google Scholar]
- 22.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yisireyili M, et al. Angiotensin receptor blocker irbesartan reduces stress-induced intestinal inflammation via AT1a signaling and ACE2-dependent mechanism in mice. Brain Behav. Immun. 2018;69:167–179. doi: 10.1016/j.bbi.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Simoes ESA, Silveira KD, Ferreira AJ, Teixeira MM. ACE2, angiotensin-(1–7) and mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 2013;169:477–492. doi: 10.1111/bph.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwenandar F, et al. Coronavirus disease 2019 and cardiovascular system: A narrative review. Int. J. Cardiol. Heart Vasc. 2020;29:100557. doi: 10.1016/j.ijcha.2020.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuyama S, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tortorici MA, et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, et al. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005;191:755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sardu C, et al. Hypertension, thrombosis, kidney failure, and diabetes: Is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 2020;9:1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godo S, Shimokawa H. Endothelial functions. Arterioscler. Thromb. Vasc. Biol. 2017;37:e108–e114. doi: 10.1161/ATVBAHA.117.309813. [DOI] [PubMed] [Google Scholar]
- 31.Kazmi RS, Boyce S, Lwaleed BA. Homeostasis of hemostasis: The role of endothelium. Semin. Thromb. Hemost. 2015;41:549–555. doi: 10.1055/s-0035-1556724. [DOI] [PubMed] [Google Scholar]
- 32.Santulli G. Endothelial cells: The heart attack of the clones. Sci. Transl. Med. 2018;10:1–10. doi: 10.1126/scitranslmed.aar7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv. Exp. Med. Biol. 2017;956:511–540. doi: 10.1007/5584_2016_90. [DOI] [PubMed] [Google Scholar]
- 34.Mordi I, Mordi N, Delles C, Tzemos N. Endothelial dysfunction in human essential hypertension. J. Hypertens. 2016;34:1464–1472. doi: 10.1097/HJH.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 35.de Wit E, van Doremalen N, Falzarano D, Munster V. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microb. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandhi RT, Lynch JB, Del RC. Mild or moderate covid-19. N. Engl. J. Med. 2020;1:10. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 37.Ho JC, et al. High-dose pulse versus nonpulse corticosteroid regimens in severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2003;168:1449–1456. doi: 10.1164/rccm.200306-766OC. [DOI] [PubMed] [Google Scholar]
- 38.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horby, P. et al. Dexamethasone in hospitalized patients with Covid-19: Preliminary report. N Engl J Med. (2020). [DOI] [PMC free article] [PubMed]