Abstract
The site-dependent load-deformation behavior of the human neurocranium and the load dissipation within the three-layered composite is not well understood. This study mechanically investigated 257 human frontal, temporal, parietal and occipital neurocranial bone samples at an age range of 2 to 94 years, using three-point bending tests. Samples were tested as full-thickness three-layered composites, as well as separated with both diploë attached and removed. Right temporal samples were the thinnest samples of all tested regions (median < 5 mm; p < 0.001) and withstood lowest failure loads (median < 762 N; p < 0.001). Outer tables were thicker and showed higher failure loads (median 2.4 mm; median 264 N) than inner tables (median 1.7 mm, p < 0.001; median 132 N, p = 0.003). The presence of diploë attached to outer and inner tables led to a significant reduction in bending strength (with diploë: median < 60 MPa; without diploë: median > 90 MPa, p < 0.001). Composites (r = 0.243, p = 0.011) and inner tables with attached diploë (r = 0.214, p = 0.032) revealed positive correlations between sample thickness and age. The three-layered composite is four times more load-resistant compared to the outer table and eight times more compared to the inner table.
Subject terms: Bone, Tissues, Anatomy
Introduction
A comprehensive understanding of the load-deformation behavior of the human neurocranium is paramount to reliably predict head impact scenarios or injury mechanisms using of computational head models1–3. The human neurocranium forms a three-layered composite consisting of two compact tables that enclose the cancellous diploë in a sandwich-like manner4. In contemporary finite element models, the diploë is either neglected or represented in an oversimplified manner due to the lacking or controversial material properties that are available in the scientific literature5. Previous research regarding the load-deformation behavior of the human neurocranium mainly focused on full-thickness composites6–17. Only few studies investigated the two tables7,18–23 or even the cancellous diploë layer individually13,18,24,25. However, an in-depth investigation that mechanically compares the inner and outer table with and without the adjacent diploë and their relation to the full-thickness three-layered neurocranial composite is missing to date, and, therefore, the contribution of the individual layers to the overall mechanical behavior remains unclear.
Flat bones of the cranial vault considerably vary in thickness, and even within the individual bones along defined axes17,26,27. Furthermore, it was shown that the thickness of the neurocranium steadily increases during the first two decades of life28,29, which potentially continues up to the age of 60 years30, and then decreases later in life31. However, the age-related change of the neurocranial thickness after the second decade of life was challenged by other studies26,32. A correlation between the load-deformation behavior of the cranial bone and its thickness was observed before6,17,33,34 with the latter being linked to the ratio between diploë to the two compact tables17. Moreover, the bone thickness was related to the dynamic impact response of the human neurocranium observed using finite element modeling33. The diploë of the occipital region was stated to be thicker compared to the other neurocranial bones, however, the occipital bone remained mechanically almost unexplored to date6,10.
This given study aimed at comparing the mechanical properties of the complete three-layered neurocranial composite with the individual behavior of the layers after being separated as a pioneering step to gain insight into the morpho-mechanicals of the human cranial vault. Investigating the large flat bones of the human neurocranium in an age range of almost one century will enable to observe the related load dissipation in relation to age, sex, layer thickness and time since death, and thus enhance the understanding of the trabecular impact on the complete bone biomechanics35.
Materials/methods
Retrieval and processing of human neurocranial samples
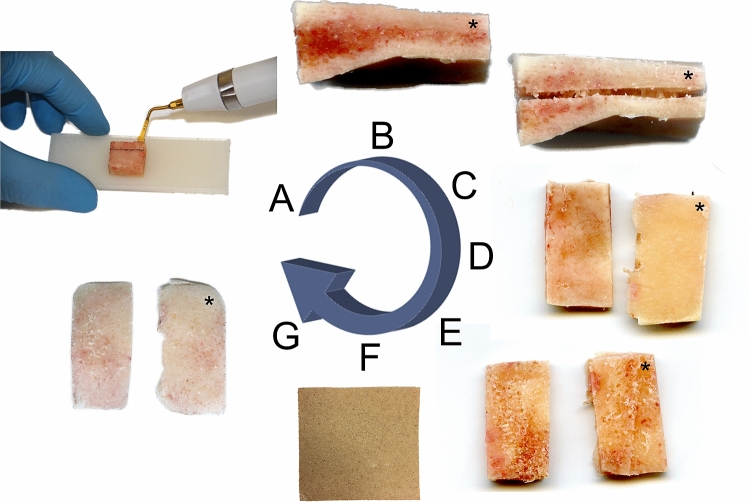
A total of 257 human neurocranial samples were retrieved from 73 cadavers (25 females, 48 males; age range 2–94 years) during forensic autopsies. Initially, samples of approximately 20 × 20 mm were retrieved from the frontal (n = 60), temporal left (n = 47), temporal right (n = 41), parietal (n = 53) and occipital (n = 56) region. More specifically, samples were retrieved according to the following rules: frontal bone: superior to the orbit at a level between the supraorbital margin and the coronal suture; temporal bone: squamous part; parietal bone: anterior–superior part between the sagittal and the squamous suture; occipital bone: in the middle of a line between the external occipital protuberance and the point where the sagittal suture connects with the lambdoid suture. The cadavers were stored at 4 °C prior to autopsy to prevent degradation of the tissues. Following the retrieval of the tissues at room temperature, the samples were precooled at 4 °C and then kept in a − 80 °C freezer in a chemically unfixed condition until further processing. The Ethics Committee of the University of Leipzig, Germany approved the retrieval of these tissues for the given purpose (protocol number 486/16-ek). All methods were carried out in accordance with relevant guidelines and regulations. When further processed, the samples were thawed and cut to a width of 10 mm with a bone cutter (PIEZOSURGERY® white, mectron s.p.a., Carasco, Italy; Fig. 1A) with a sawing blade of 0.5 mm thickness. The bone cutter automatically spills water on the blade during cutting to prevent burning of the sample while being cut. Thereafter, the samples were allocated into the following three groups: a “composite” group in which the mechanically-tested sample consisted of all three neurocranial bone layers (outer table, diploë and inner table; Fig. 1B), a “tables with diploë” group, in which the outer and inner table were separated in the middle of the diploë layer (Fig. 1C) and a “tables without diploë” group, in which the outer and inner tables were separated according to the former group, then followed by a complete removal of the diploë using sandpaper with a grit size of 60-grit to coarsely remove the diploë initially and using a 240-grit sandpaper to accurately remove the diploë close to the tables (grit sizes according to the Coated Abrasives Manufacturers’ Institute system; Fig. 1D–G). An attempt was made to separate the tables in the middle of the diploë leaving approximately 50% of the initial diploë on either table. According to the aforementioned separation procedure of the three-layered neurocranial bone the “tables with diploë” group resulted in two samples for mechanical testing (Touter + D, outer table + diploë; Tinner + D, inner table + diploë), which both still had approximately half of the diploë attached (Fig. 2). About 0.5 mm of the diploë was removed during the layer separation with the bone cutter (value equals the thickness of the sawing blade). The “tables without diploë” group also resulted in two samples for the mechanical tests (Touter, outer table; Tinner, inner table; Fig. 2). A summary of the number of samples per testing group, the retrieval site, age, post-mortem interval (PMI, time between death of the cadaver and the sample retrieval; range in this study 11–139 h) and sex ratio of the three groups is given in Table 1.
Figure 1.
The sample preparation for the mechanical testing is shown. (A) Sample cutting using an ultrasound bone cutter, (B) Three-layered full-thickness neurocranial composite (“full-thickness composite” group); (C) separated outer (*) and inner table (“tables with diploë” group); (D) view on surface of the outer (*) and inner table after separation; (E) view on diploë-facing side of the outer (*) and inner table after separation; (F) sandpaper (60-grit); (G) view on outer (*) and inner table according to (E) after diploë was removed with sandpaper (“tables without diploë” group).
Figure 2.
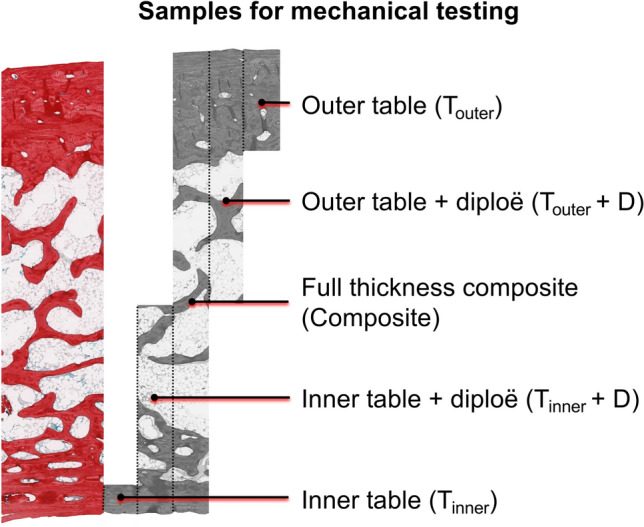
A picrosirius red-stained neurocranial bone sample is depicted to visualize the samples for mechanical testing on a histological level. Full-thickness composite samples formed the “composite” group, the outer and inner tables including the adjacent diploë the “tables with diploë” group and the outer and inner tables without the diploë the “tables without diploë” group.
Table 1.
The number of samples per donor for the mechanical testing groups and retrieval- and cadaver-related data are depicted.
| Group | Number of samples | F | TL | TR | P | O | Number of donors | Median age (IQR) (years) | Median PMI (IQR) (h) | Female:male ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Composite | 108 | 20 | 26 | 27 | 16 | 19 | 62 | 51 (39) | 71 (44) | 23:39 |
| Tables with diploë | 101 | 28 | 15 | 8 | 20 | 30 | 59 | 50 (39) | 71 (43) | 19:40 |
| Tables without diploë | 48 | 12 | 6 | 6 | 14 | 10 | 37 | 55 (40) | 63 (45) | 15:22 |
Composite, full-thickness composite group; F, frontal; O, occipital; P, parietal; PMI, post-mortem interval; IQR, interquartile range; F, frontal; O, occipital; P, parietal; TL/TR, temporal left and right.
Mechanical testing
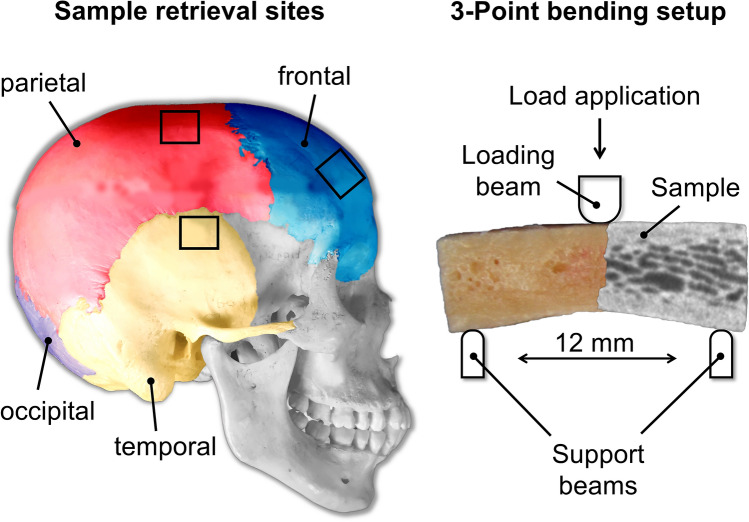
Prior to the mechanical testing, the thickness of each sample was determined with a digital caliper (Coolant Proof 200 mm, MeasumaX, Auckland, New Zealand; accuracy ± 0.001”). The samples were tested using a three-point bending setup on a universal testing machine (AllroundLine Table-top Z020; Zwick Roell, Ulm, Germany) equipped with an Xforce K load cell of 20 kN and testControl II measurement electronics (all Zwick Roell). The radii of the loading beam and the two support beams were 2 and 1 mm, respectively (Fig. 3). The samples were loaded until failure using a span length of 12 mm and a testing speed of 10 mm per minute. All tested samples were loaded from the scalp-facing surface to the brain-facing surface, corresponding to an in-vivo load application to the neurocranium from superficial to deep.
Figure 3.
The sample retrieval sites on the neurocranium and the three-point bending setup are depicted. The black squares exemplify the sample retrieval sites for all neurocranial regions except for the occipital one. A three-layered neurocranial sample of the composite group is shown as a fusion of a bare unprocessed sample and a micro-computed tomography image of the same to illustrate the sandwich-like structure more detailed.
Data processing and statistical analyses
Maximum force (Fmax), describing the maximum applicable force before failure of the tissue, was evaluated using the force readings from the machine. Bending strength (Bstrength) was calculated using Fmax, support span (12 mm) and measured width as well as thickness (both individual for each sample) under estimation of a bending beam with a rectangular cross-section as follows20:
Excel Version 16.16 (Microsoft Corporation, Redmond, WA) and GraphPad Prism software version 8 (GraphPad Software, La Jolla, CA, USA) were used for the statistical evaluation. The Shapiro–Wilk test was used to test Gaussian distribution of the samples. Parametric data of samples were then tested using an ordinary one-way ANOVA (parametric data) or a Kruskal–Wallis test (non-parametric data). For the overall comparison of mechanical parameters between the corresponding outer and inner tables (Touter + D vs. Tinner + D and Touter vs. Tinner) a Friedman test followed by an uncorrected Dunn’s test was applied. For a comparison of the outer and inner tables (Touter + D vs. Tinner + D and Touter vs. Tinner) for each sub-region (frontal, temporal left and right, parietal and occipital) a two-tailed paired t test was applied for parametric data and a two-tailed Wilcoxon test for non-parametric data. Bivariate correlations (Pearson’s r for parametric, Spearman’s ϱ for non-parametric data) were performed between the mechanical parameters and age of the deceased, PMI and thickness of the samples. Medians and interquartile ranges (IQRs) are given in text. p values of 0.05 or less were considered statistically significant.
Results
Three-layered “full-thickness composite” group showed regional differences in maximum force but not in bending strength
When comparing the complete bone composites among the five investigated regions, the left (886 N, IQR = 555 N) and right (763 N, IQR = 583 N) temporal bone samples showed a significantly lower Fmax compared to the parietal (1479 N, IQR = 757 N; both p = 0.002) and occipital (1781 N, IQR = 1099 N, left temporal: p = 0.003; right temporal: p = 0.004) samples (Fig. 4). There were no significant differences between frontal and temporal composites nor side-dependent differences for left and right temporal samples. Intact bones were similar and statistically non-different regarding their Bstrength. A summary of the mechanical values for these regions is given in Table 2.
Figure 4.
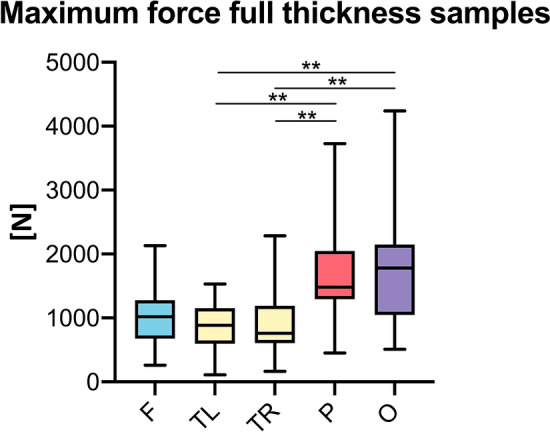
Temporal bone full-thickness composites revealed lower maximum forces compared to parietal and occipital samples. F, frontal; O, occipital; P, parietal; TL/TR, temporal left/right; **p < 0.01.
Table 2.
Summarized mechanical parameters.
| Pooled | Frontal | Temporal left | Temporal right | Parietal | Occipital | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fmax Composite (N) | 1054 (810) | 1019 (602) | 886 (555) | 763 (583) | 1479 (757) | 1781 (1099) | ||||||
|
Fmax Touter + D | Tinner + D (N) |
339 (275) | 206 (206) | 342 (309) | 241 (217) | 209 (144) | 145 (122) | 381 (185) | 167 (250) | 430 (361) | 284 (198) | 339 (318) | 151 (227) |
|
Fmax Touter | Tinner (N) |
264 (260) | 132 (143) | 259 (251) | 124 (181) | 196 (151) | 71 (47) | 147 (178) | 111 (117) | 377 (301) | 171 (137) | 360 (440) | 141 (200) |
| Bstrength Composite [MPa] | 67 (45) | 70 (71) | 72 (45) | 78 (45) | 59 (35) | 59 (39) | ||||||
| Bstrength Touter + D | Tinner + D [MPa] | 60 (42) | 59 (37) | 56 (50) | 63 (52) | 55 (36) | 59 (27) | 73 (34) | 59 (38) | 70 (76) | 56 (46) | 56 (36) | 58 (46) |
|
Bstrength Touter | Tinner [MPa] |
92 (52) | 90 (53) | 99 (64) | 91 (58) | 107 (73) | 111 (38) | 102 (130) | 119 (149) | 98 (40) | 78 (54) | 77 (43) | 68 (58) |
Fmax, maximum force; Touter + D, outer table + diploë; Tinner + D, inner table + diploë; Touter, outer table and Tinner, inner table; Median, interquartile ranges are given in parentheses.
The “tables without diploë” group revealed significantly different maximum forces between outer and inner tables as well as different sites of the neurocranium, but showed similar bending strengths
When all of the five regions of the neurocranium were pooled, Touter + D showed a significantly higher Fmax (median 339 N, IQR = 275 N) compared to Tinner + D (median 206 N, IQR = 206 N, p = 0.011), but both pooled sample cohorts were statistically non-different regarding their Bstrength. When each region was evaluated independently Fmax of Touter + D was also significantly higher compared to Tinner + D (frontal: p = 0.010; temporal left: p = 0.011; temporal right: p = 0.029; parietal: p < 0.001; occipital: p = 0.001). The Fmax comparison of Touter + D between regions revealed a significantly higher value for parietal samples (median 430 N, IQR = 361 N) compared to the left temporal samples (median 209 N, IQR = 144 N, p = 0.010). None of the remaining mechanical parameters differed between the regions on a statistically significant level. Moreover, Bstrength was similar and statistically non-different in each region in line with the pooled samples. A summary of the obtained mechanical values for this group is given in Table 2.
The “tables without diploë” group showed stronger outer compared to inner tables and weaker temporal regions compared to the remaining neurocranial bone locations, but similar bending strengths
When pooling the data of all regions, Touter revealed a significantly higher Fmax (median 264 N, IQR = 260 N) compared to Tinner (median 132 N, IQR = 143 N, p = 0.003), but was statistically non-different regarding its Bstrength. The Fmax of Touter was significantly higher compared to Tinner in the frontal (median Touter = 259 N, IQR = 251 N, median Tinner = 124 N, IQR = 181 N, p < 0.001), parietal (median Touter = 377 N, IQR = 301 N, median Tinner = 171 N, IQR = 137 N, p = 0.002) and occipital (median Touter = 360 N, IQR = 440 N, median Tinner = 141 N, IQR = 200 N, p = 0.013) regions, but non-different in both temporal regions. The Fmax comparison of Touter between regions revealed significantly lower values for both left (median 196 N, IQR = 151 N, p = 0.023) and right temporal (median 147 N, IQR = 178 N, p = 0.012) samples compared to parietal ones (median 377 N, IQR = 301 N). None of the remaining mechanical parameters differed between the regions on a statistically significant level. Bstrength was statistically non-different when comparing each region individually. A summary of the obtained mechanical values for this group is given in Table 2.
Comparison of mechanical parameters between groups
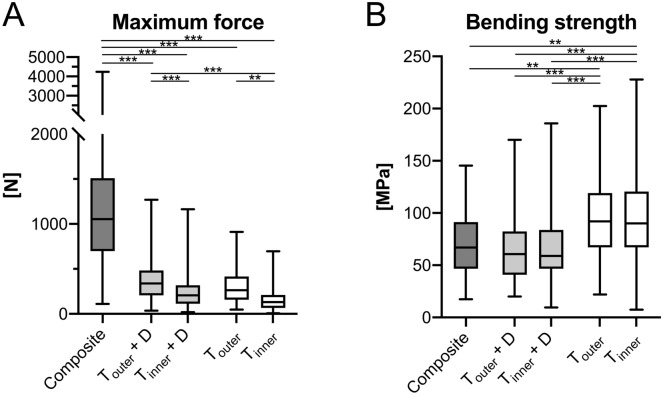
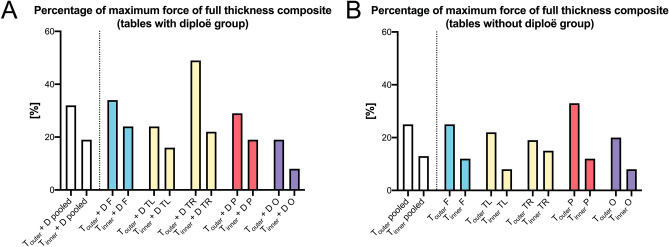
The Fmax values of the pooled group (median 1054 N, IQR = 810 N) were significantly higher compared to both the samples of the separated group (Touter + D: median 339 N, IQR = 275 N, p < 0.001; Tinner + D: median 206 N, IQR = 206 N, p < 0.001) as well as the samples of the separated and removed group (Touter: median 264 N, IQR = 260 N, p < 0.001; Tinner: median 132 N, IQR = 143 N, p < 0.001; Fig. 5A). Neither the Fmax values of Touter + D nor the one of Tinner + D were significantly different from the groups, in which the diploë was removed (Fig. 5A). While the Fmax values for the Touter + D group were statistically higher compared to Tinner (p < 0.001), the Tinner + D group was statistically non-different from the Touter group (Fig. 5A). The Bstrength of the composite group statistically differed from both the Bstrength of both layers of the separated and removed group (Touter, p = 0.003; Tinner, p = 0.004; Fig. 5B). The Bstrength of the Touter + D layer of the separated only group (median 60 MPa, IQR = 42 MPa) differed from both layers of the separated and removed group (median Touter = 92 MPa, IQR = 52 MPa, p < 0.001; median Tinner = 90 MPa, IQR = 53 MPa, p < 0.001; Fig. 5B). Equally, the Bstrength of the Tinner + D layer of the separated only group (median 59 MPa, IQR = 37 MPa) differed from both layers of the separated and removed group (Touter: p < 0.001; Tinner: p < 0.001) on a statistically significant level (Fig. 5B). When related to the Fmax, which the three-layered composite of the respective area withstood, Touter + D reached between 19% (occipital) and 49% (temporal right) of this force (Fig. 6A). Tinner + D only withstood between 8% (occipital) and 24% (frontal) of the Fmax of the three-layered composite (Fig. 6A). Similarly, when related to the Fmax, which the three-layered composite of the respective area withstood, Touter reached between 19% (temporal right) and 33% (parietal) of this force (Fig. 6B). Tinner only withstood between 8% (temporal left and occipital) and 15% (temporal right) of the Fmax of the three-layered composite (Fig. 6B).
Figure 5.
The maximum force (A) and bending strength (B) are depicted for the different groups. Composite, full-thickness group; Touter + D, outer table + diploë; Tinner + D, inner table + diploë; Touter, outer table; Tinner, inner table; **p < 0.01; ***p < 0.001.
Figure 6.
The maximum forces of (A) the “tables with diploë” group and the (B) “tables without diploë” group are given as a percentage of the “composite” group with the latter representing 100%. Touter + D, outer table + diploë; Tinner + D, inner table + diploë; Touter, outer table and Tinner, inner table; F, frontal; O, occipital; P, parietal; TL/TR, temporal left and right.
Age-, PMI-, sex-, and thickness correlations
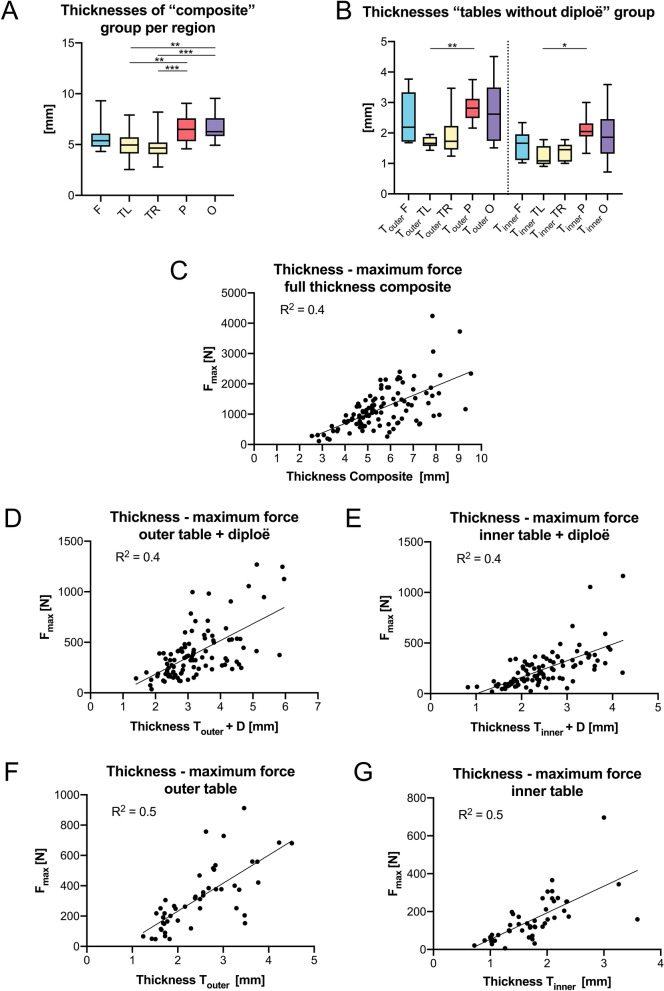
Both left and right temporal full-thickness composites were significantly thinner compared to parietal (temporal left: p = 0.004, temporal right: p < 0.001) and occipital (temporal left: p = 0.005, temporal right: p < 0.001) composites (Fig. 7A). Both Touter + D (p < 0.001) and Tinner + D (p < 0.001) were significantly thicker compared to the separated and diploë-removed group. For left temporal samples, both Touter and Tinner were significantly thinner compared to the parietal (Touter: p = 0.006; Tinner: p = 0.017) region (Fig. 7B). With the samples of all regions pooled, Touter was significantly thicker compared to Tinner (p < 0.001). However, when observing those regions individually, this difference was only observed for frontal (p = 0.001), parietal (p < 0.001) and occipital (p = 0.017) samples. The thickness values of the tested samples are depicted in Table 3.
Figure 7.
The mechanical parameters of neurocranial bone regions are depicted in relation to the thickness of the tested sample. (A) full-thickness “composite” group per region, (B) “tables without diploë” group, (C) correlation thickness—maximum force (Fmax) of “composite” group, (D) correlation thickness—Fmax outer table “tables with diploë” group (Touter + D), (E) correlation thickness—Fmax inner table “tables with diploë” group (Tinner + D), (F) correlation thickness—Fmax outer table (Touter) “tables without diploë” group, (G) correlation thickness—Fmax inner table (Tinner) “tables without diploë” group; F, frontal; O, occipital; P, parietal; TL/TR, temporal left and right; *p < 0.05; **p < 0.01; ***p < 0.001.
Table 3.
The thicknesses of the tested samples are depicted separated per region.
| Pooled | Frontal | Temporal left | Temporal right | Parietal | Occipital | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thickness Composite (mm) | 5.4 (1.8) | 5.4 (1.3) | 5.0 (1.6) | 4.7 (1.1) | 6.5 (2.3) | 6.3 (1.8) | ||||||
|
Thickness Touter + D | Tinner + D (mm) |
3.1 (1.2) | 2.4 (1.0) | 3.2 (1.7) | 2.4 (1.4) | 2.6 (0.3) | 2.0 (0.7) | 2.9 (1.0) | 2.4 (1.0) | 3.2 (1.3) | 2.6 (1.2) | 3.2 (1.4) | 2.5 (1.0) |
|
Thickness Touter | Tinner (mm) |
2.4 (1.3) | 1.7 (0.8) | 2.2 (1.6) | 1.7 (0.8) | 1.7 (0.3) | 1.1 (0.6) | 1.7 (0.8) | 1.5 (0.6) | 2.8 (0.6) | 2.1 (0.4) | 2.6 (1.8) | 1.9 (1.1) |
Touter + D, outer table + diploë; Tinner + D, inner table + diploë; Touter, outer table and Tinner, inner table; Interquartile ranges are given in parentheses.
Fmax of all groups showed a significant moderate to strong positive and linear correlation with the thickness of the samples (composite: r = 0.624, p < 0.001, Fig. 7C; Touter + D: r = 0.602, p < 0.001, Fig. 7D; Tinner + D: r = 0.705, p < 0.001, Fig. 7E; Touter: r = 0.769, p < 0.001, Fig. 7F; Tinner: r = 0.789, p < 0.001, Fig. 7G). The sample thickness of the composite group (r = 0.243, p = 0.011) and Tinner + D (r = 0.214, p = 0.032) samples of the separated only group showed a weak positive correlation with age. Bstrength of Tinner was the only mechanical parameter which significantly correlated with PMI (r = 0.302, p = 0.037). All mechanical parameters obtained in this study were independent of the sex of the cadaver irrespective of the tested subgroup. Apart from the weak negative correlation between Bstrength of the composite group (r = -0.285, p = 0.003), the mechanical parameters in this study were also independent of age.
Discussion
Mechanical properties of the human neurocranium have so far been obtained using three-point bending6,8,10,14–17, four-point bending36, compressive7,11,13,25, tensile7,13,16,19,22,24 and shear7 test protocols, as well as ultrasonic pulse transmission techniques21. The here presented study for the first time systematically investigated the contribution of the individual bone layers of the neurocranium to the mechanical behavior of the three-layered composite involving all major flat bones of the neurocranium in a large sample size over a broad age range. Overall, the thickness of the samples correlated with the applicable Fmax irrespective of the tested group in this given study. Temporal bone samples were significantly thinner and withstood lower loads compared to the parietal and occipital regions. Similarly, the Touter only revealed higher failure loads compared to Tinner when being thicker at the same time, which was true for the frontal, parietal and occipital samples, but not for the temporal samples of similar thickness. An exception to this ‘thicker bone—stronger bone’ relation was the Touter of the right temporal region, which showed a significantly lower Fmax value compared to the parietal region despite being of a similar thickness. This finding might be explained by the limited sample sizes in this study, with only six Touter samples for the subgroup at the right temporal region, which likely caused a statistical type I error. Lower sample sizes are prone to be biased by outliers that show, e.g., extremes, such as low mechanical resistance of a tested bone sample due to a decreased bone density10 or conditions that negatively affect the bone quality such as Paget’s disease37, referring to unknown conditions as pre-existing bone diseases were used as ultimate exclusion criteria during sampling. With regards to the diploë-table ratio of the neurocranium, two important observations were made in the given study. Firstly, the Tinner thickness was statistically non-different between all investigated regions. Secondly, the Touter thickness was statistically non-different between the different neurocranial regions investigated in this study apart from the left temporal Touter, which was thinner than the parietal site. Based on these observations and the assumption that the divergent value is biased, the here presented findings indicate that the thickness of the three-layered neurocranium is mainly determined by the thickness of the diploë rather than the outer or inner table. The covariation between diploë and cranial thickness is supported by a former radiographic study on 256 neurocranium samples measured on frontal, occipital and left and right euryon38. Temporal bones have a comparatively low amount of diploë4, which diminishes towards the inferior portion of the bone39. The results of this study showed that the intact temporal samples showed significantly lower loads compared to frontal, parietal and occipital samples, which supports the hypothesis that the diploë thickness is of high biomechanical importance when human neurocranium is simulated in computer models38.
The individual outer and inner tables only reach 25% and 13% of the maximum forces of the full-thickness composite
The individual layer tests in this study revealed that the mechanical characteristics of the human neurocranium are based on the arrangement of the three layers and their mutual connection rather than being a summative of the load resistance of the individual layers. When all samples were pooled Touter and Tinner reached only 25% and 13% of the Fmax value of the full-thickness composite. Cancellous bone has a lower compressive strength compared to compact bone in general13, and, therefore, the bare material properties of the diploë are insufficient to explain vastly higher load resistance of the intact neurocranial composite compared to the individual layers. The overall arrangement of the human neurocranium well corresponds to a special class of engineering materials—the sandwich-structured composite—with two thin but strong skin sheets and a lightweight but thick core connecting the strong skin sheets. This type of engineering composite with a core of a material with a lower strength provides an overall high bending stiffness and high bending strength with a much lower density compared to full thickness samples of the strong sheet material. In line with this, the “tables with diploë” group, in which approximately half of the diploë remained attached to the Touter and Tinner was mechanically indifferent from the group, in which the diploë was removed. Taking into account the complex trabecular orientation within the diploë without a direct connection of Touter and Tinner via trabeculae perpendicular to the surface of the tables, we hypothesize that the loads that are applied to the Touter from external in case of head impacts are dissipated via the diploic trabeculae to eventually act on larger areas on the Tinner compared to the area of impact on the Touter. Based on this load dissipation principle between the two tables, less load acts on the Tinner per area compared to the Touter, but the area this load is dissipated to via the trabeculae should be larger. Therefore, it is plausible that, in vivo, the Tinner is sufficiently load-resistant compared to the Touter even though being thinner, which provides as a biomechanical explanation for the thickness differences between the two layers. An alternative hypothesis of the observed thickness difference between the two tables is the exposure of the Touter to muscular loads, which are comparatively higher than intracerebral loads acting on the Tinner21, naturally omitting the necessity for a thicker Tinner. Despite containing significantly thicker samples, the “tables with diploë” group was statistically non-different compared to the “tables without diploë” group from a (bio)-mechanical perspective. These findings indicate that bone trabeculae require the respective second cortical table to effectively dissipate loads, likely to larger surface as described above or being able to store energy by being compressed between the two tables, while at the same time minimizing the weight of the bone composite.
The Bstrength in this study was similar and statistically non-different between the here investigated sites within one testing group from the various regions or when comparing the corresponding outer and inner tables within the groups with and without diploë, respectively. However, the outer and inner tables of the “tables with diploë” group revealed a significantly lower Bstrength compared with the tables, for which the cancellous bone was additionally removed. This is explained by the fact that the tables without attached diploë were significantly thinner compared to the ones with an attached diploë, but non-different in Fmax resistance at the same time. Significant thickness differences when comparing two materials are critically influencing the obtained Bstrength as the thickness is reflected in the Bstrength equation as a squared divisor20. Consequently, the compact Touter and Tinner show a higher Bstrength compared to the composite of the compact table with an attached similarly composed40 but more porous41 and weaker13 diploë layer that is adding significantly to the thickness of the sample, but not to its mechanical strength. The here reported Bstrength of 67 MPa is similar to the values of 85 MPa obtained from frontal and parietal regions of eight fresh-frozen cadavers using a testing velocity of 30,000 mm/min12 and the 64 to 86 MPa obtained from 114 unembalmed fronto-parietal samples using a testing speed of 0.06 mm/min10. A study involving Crosado-embalmed42 cadavers using an identical testing velocity as in the given study of 10 mm/min reported a lower Bstrength of 42 MPa and 53 MPa for the two investigated human neurocrania6, likely due to an embrittlement of the tissue following the chemical treatment or a statistical bias caused by the low sample size in the former work. The Bstrength of the composite group in this given study decreased with age, presumably caused by the concomitant age-related thickening of the samples without a concurrent increase of Fmax values. However, it should be noted that the found negative correlation between Bstrength and age revealed a limited “statistical strength” as the respective r value was low. The age-related thickening of the neurocranium is likely caused by a thickening of the diploë rather than the tables as thickening with age was seen in the separated group, but not in the separated and diploë-removed group in this study. The influence of age on the mechanical behavior of the neurocranium can be deemed vague rather than contradictory. Some authors report that mechanical parameters are independent of age17,19, whereas others detected age-related increases of elasticity14,18 and compressive strength18, but decreases of fracture loads43. Regarding the former, it must be considered that generally limited sample sizes and restricted age ranges might be insufficient to detect age-related mechanical changes caused by small effect sizes, which are simultaneously strongly affected by the other parameters such as sample thickness or the load application vector with respect to the anisotropic bone. The weak positive correlation between Bstrength and PMI of Tinner might have been caused by an increased collagen cross-linking post-mortem or by handling- and storage-related dehydration processes after death. As the load resistance of the here tested samples was not decreasing as a sign of tissue degradation in general, it is concluded that cadaveric bone retrieved during forensic autopsies can be used for the purposes, when cadavers or samples are kept cool constantly. Material properties of native bones are paramount to fabricate lifelike physical surrogates for surgical44 or forensic applications9,45. Moreover, material properties of the neurocranium are applied in computational simulations of the human head to simulate various head impact scenarios3. While this given study focused on the mechanical properties of human neurocranium only, it has to be noted that surrounding soft tissues such as the periosteum or the dura mater might be of importance to replicate the response of the human head to impact forces in a realistic manner9,46.
Limitations
Firstly, the given study is limited in sample size for each subgroup in spite of the large overall number of samples, which might have affected the here stated results via multiple group comparisons. However, robust post hoc tests were used for statistical analyses. Secondly, the bone samples are naturally convex towards the outer surface and, therefore, the bending stress assuming a straight beam with a rectangular cross-section represents an oversimplification, which unpreventably affected the results. Thirdly, the diploë removal might have been incomplete which could have influenced the here stated mechanical parameters. Fourthly, even though the specimens in this study were cut using a high-quality bone cutter that is certified to be used in clinical routine, the resulting dimensions minutely differed, which could have affected the given results. Fifthly, even though an attempt was made to separate the two tables in the middle of the diploë layer, this could not be achieved in every single sample due to the convex geometry of the neurocranial towards the outer surface. Sixthly, shear forces likely occurred due to the setup of this study and the structure of the tested tissue. This might have affected the measured Bstrength in this study. Seventhly, this given study did not determine the individual bone densities that are influenced by various conditions such as osteoporosis, which reflects on the bone’s mechanical strength47. Hence, the here reported statistical comparisons between the individual groups might have been biased by differences in bone densities. Lastly, all human neurocranial samples show a complex three-dimensionally curved geometry. As the sample curvature was not measured for each individual sample of this study, its potential influence on the here reported biomechanical parameters remains unknown.
Conclusion
The thicknesses of bones of the neurocranium critically influence their load-deformation properties. This study provides evidence that the neurocranial thickness is predominantly determined by the diploë, which thickens with age. The three-layered composite is up to four and eight times more load resistant than the individual outer and inner tables, respectively. Presuming storage of the cadaver at 4 °C at the earliest possible point after death, neurocranial samples retrieved during autopsy are suitable for mechanical testing purposes for at least five days post-mortem.
Acknowledgements
The authors acknowledge support from the German Research Foundation (DFG) and the University of Leipzig within the program of Open Access Publishing.
Author contributions
All authors were involved in the study design. J.Z., S.S. and B.O. performed the mechanical tests. N.H, KC.L., B.B., M.S. and NJ.W. gave technical advice on the conducted experiments. J.Z. drafted the first version of the manuscript. All authors reviewed, edited and approved the manuscript for submission.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Johann Zwirner, Email: medijo@gmx.de.
Niels Hammer, Email: niels.hammer@medunigraz.at.
References
- 1.Asgharpour Z, Baumgartner D, Willinger R, Graw M, Peldschus S. The validation and application of a finite element human head model for frontal skull fracture analysis. J. Mech. Behav. Biomed. Mater. 2014;33:16–23. doi: 10.1016/j.jmbbm.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Horgan TJ, Gilchrist MD. The creation of three-dimensional finite element models for simulating head impact biomechanics. Int. J. Crash. 2003;8:353–366. doi: 10.1533/cras.8.4.353.19278. [DOI] [Google Scholar]
- 3.Motherway J, Doorly MC, Curtis M, Gilchrist MD. Head impact biomechanics simulations: a forensic tool for reconstructing head injury? Leg. Med. (Tokyo) 2009;11(Suppl 1):S220–222. doi: 10.1016/j.legalmed.2009.01.072. [DOI] [PubMed] [Google Scholar]
- 4.Lillie EM, Urban JE, Weaver AA, Powers AK, Stitzel JD. Estimation of skull table thickness with clinical CT and validation with microCT. J. Anat. 2015;226:73–80. doi: 10.1111/joa.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamrad J, Marcian P, Borak L. On the level of computational model of a human skull: a comparative study. Appl. Comput. Mech. 2018;12:5–16. doi: 10.24132/acm.2018.385. [DOI] [Google Scholar]
- 6.Lee JHC, et al. An Investigation on the correlation between the mechanical properties of human skull bone, its geometry, microarchitectural properties, and water content. J. Healthc. Eng. 2019;2019:6515797. doi: 10.1155/2019/6515797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElhaney JH, et al. Mechanical properties on cranial bone. J. Biomech. 1970;3:495–511. doi: 10.1016/0021-9290(70)90059-x. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard RP. Flexure of layered cranial bone. J. Biomech. 1971;4:251–263. doi: 10.1016/0021-9290(71)90031-5. [DOI] [PubMed] [Google Scholar]
- 9.Ondruschka B, et al. A biomechanical comparison between human calvarial bone and a skull simulant considering the role of attached periosteum and dura mater. Int. J. Legal Med. 2019;133:1603–1610. doi: 10.1007/s00414-019-02102-4. [DOI] [PubMed] [Google Scholar]
- 10.Torimitsu S, et al. Statistical analysis of biomechanical properties of the adult skull and age-related structural changes by sex in a Japanese forensic sample. Forensic Sci. Int. 2014;234(185):e181–189. doi: 10.1016/j.forsciint.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Zhai X, Nauman EA, Moryl D, Lycke R, Chen WW. The effects of loading-direction and strain-rate on the mechanical behaviors of human frontal skull bone. J. Mech. Behav. Biomed. Mater. 2020;103:103597. doi: 10.1016/j.jmbbm.2019.103597. [DOI] [PubMed] [Google Scholar]
- 12.Motherway JA, Verschueren P, Van der Perre G, Vander Sloten J, Gilchrist MD. The mechanical properties of cranial bone: the effect of loading rate and cranial sampling position. J. Biomech. 2009;42:2129–2135. doi: 10.1016/j.jbiomech.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Evans FG, Lissner HR. Tensile and compressive strength of human parietal bone. J. Appl. Physiol. 1957;10:493–497. doi: 10.1152/jappl.1957.10.3.493. [DOI] [PubMed] [Google Scholar]
- 14.McPherson GK, Kriewall TJ. The elastic modulus of fetal cranial bone: a first step towards an understanding of the biomechanics of fetal head molding. J. Biomech. 1980;13:9–16. doi: 10.1016/0021-9290(80)90003-2. [DOI] [PubMed] [Google Scholar]
- 15.Delille R, Lesueur D, Potier P, Drazetic P, Markiewicz E. Experimental study of the bone behaviour of the human skull bone for the development of a physical head model. Int. J. Crashworth. 2007;12:101–108. doi: 10.1080/13588260701433081. [DOI] [Google Scholar]
- 16.Coats B, Margulies SS. Material properties of human infant skull and suture at high rates. J. Neurotrauma. 2006;23:1222–1232. doi: 10.1089/neu.2006.23.1222. [DOI] [PubMed] [Google Scholar]
- 17.Auperrin A, et al. Geometrical and material parameters to assess the macroscopic mechanical behaviour of fresh cranial bone samples. J. Biomech. 2014;47:1180–1185. doi: 10.1016/j.jbiomech.2013.10.060. [DOI] [PubMed] [Google Scholar]
- 18.Schroder WG, Harnisch S, Lippert H. Biomechanics of calvaria. IV. Resistance to compression of compact layers and diploe (author's transl) Unfallheilkunde. 1977;80:341–344. [PubMed] [Google Scholar]
- 19.Wood JL. Dynamic response of human cranial bone. J. Biomech. 1971;4:1–12. doi: 10.1016/0021-9290(71)90010-8. [DOI] [PubMed] [Google Scholar]
- 20.Robbins DH, Wood JL. Determination of mechanical properties of the bones of the skull. Exp. Mech. 1969;9:236–240. doi: 10.1007/BF02326542. [DOI] [Google Scholar]
- 21.Peterson J, Dechow PC. Material properties of the inner and outer cortical tables of the human parietal bone. Anat. Rec. 2002;268:7–15. doi: 10.1002/ar.10131. [DOI] [PubMed] [Google Scholar]
- 22.Boruah S, et al. Influence of bone microstructure on the mechanical properties of skull cortical bone—a combined experimental and computational approach. J. Mech. Behav. Biomed. Mater. 2017;65:688–704. doi: 10.1016/j.jmbbm.2016.09.041. [DOI] [PubMed] [Google Scholar]
- 23.Brooks T, Zwirner J, Hammer N, Ondruschka B, Jermy M. Preliminary observations of the sequence of damage in excised human juvenile cranial bone at speeds equivalent to falls from 1.6 m. Int J Legal Med. 2020 doi: 10.1007/s00414-020-02409-7. [DOI] [PubMed] [Google Scholar]
- 24.Schroder WG, Harnisch B, Lippert H. Biomechanics of calvaria. III. Resistance to tension of compact layers and diploe (author's transl) Unfallheilkunde. 1977;80:335–339. [PubMed] [Google Scholar]
- 25.Melvin JW, Robbins DH, Roberts VL. The mechanical behavior of the diploë layer of the human skull in compression. Dev. Mech. 1969;5:811–818. [Google Scholar]
- 26.Moreira-Gonzalez A, Papay FE, Zins JE. Calvarial thickness and its relation to cranial bone harvest. Plast. Reconstr. Surg. 2006;117:1964–1971. doi: 10.1097/01.prs.0000209933.78532.a7. [DOI] [PubMed] [Google Scholar]
- 27.Peterson J, Dechow PC. Material properties of the human cranial vault and zygoma. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003;274:785–797. doi: 10.1002/ar.a.10096. [DOI] [PubMed] [Google Scholar]
- 28.Roche AF. Increase in cranial thickness during growth. Hum. Biol. 1953;25:81–92. [PubMed] [Google Scholar]
- 29.Delye H, Clijmans T, Mommaerts MY, Sloten JV, Goffin J. Creating a normative database of age-specific 3D geometrical data, bone density, and bone thickness of the developing skull: a pilot study. J. Neurosurg. Pediatr. 2015;16:687–702. doi: 10.3171/2015.4.PEDS1493. [DOI] [PubMed] [Google Scholar]
- 30.Todd TW. Thickness of the male white cranium. Anat. Rec. 1924;27:245–256. doi: 10.1002/ar.1090270504. [DOI] [Google Scholar]
- 31.Sullivan WG, Smith AA. The split calvarial graft donor site in the elderly: a study in cadavers. Plast. Reconstr. Surg. 1989;84:29–31. doi: 10.1097/00006534-198907000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Lynnerup N. Cranial thickness in relation to age, sex and general body build in a Danish forensic sample. Forensic Sci. Int. 2001;117:45–51. doi: 10.1016/s0379-0738(00)00447-3. [DOI] [PubMed] [Google Scholar]
- 33.Ruan J, Prasad P. The effects of skull thickness variations on human head dynamic impact responses. Stapp Car Crash J. 2001;45:395–414. doi: 10.4271/2001-22-0018. [DOI] [PubMed] [Google Scholar]
- 34.Rahmoun J, Auperrin A, Delille R, Naceur H, Drazetic P. Characterization and micromechanical modeling of the human cranial bone elastic properties. Mech. Res. Commun. 2014;60:7–4. doi: 10.1016/j.mechrescom.2014.04.001. [DOI] [Google Scholar]
- 35.Keaveny TM, Morgan EF, Niebur GL, Yeh OC. Biomechanics of trabecular bone. Annu. Rev. Biomed. Eng. 2001;3:307–333. doi: 10.1146/annurev.bioeng.3.1.307. [DOI] [PubMed] [Google Scholar]
- 36.Davis MT, et al. The mechanical and morphological properties of 6 year-old cranial bone. J. Biomech. 2012;45:2493–2498. doi: 10.1016/j.jbiomech.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Gitto L, Arunkumar P, Maiese A, Bolino G. A simple depressed skull fracture in an old man with Paget disease: forensic implications in a rare case. Med. Sci. Law. 2015;55:44–49. doi: 10.1177/0025802414524384. [DOI] [PubMed] [Google Scholar]
- 38.Lynnerup N, Astrup JG, Sejrsen B. Thickness of the human cranial diploe in relation to age, sex and general body build. Head Face Med. 2005;1:13. doi: 10.1186/1746-160X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosma JF. Anatomy of the Infant Head. Baltimore: Johns Hopkins University Press Ltd; 1986. [Google Scholar]
- 40.Morgan EF, Unnikrisnan GU, Hussein AI. Bone mechanical properties in healthy and diseased states. Annu. Rev. Biomed. Eng. 2018;20:119–143. doi: 10.1146/annurev-bioeng-062117-121139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander SL, Rafaels K, Gunnarsson CA, Weerasooriya T. Structural analysis of the frontal and parietal bones of the human skull. J. Mech. Behav. Biomed. Mater. 2019;90:689–701. doi: 10.1016/j.jmbbm.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 42.Crosado B, et al. Phenoxyethanol-based embalming for anatomy teaching: an 18 years' experience with Crosado embalming at the University of Otago in New Zealand. Anat. Sci. Educ. 2020;13(6):778–793. doi: 10.1002/ase.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torimitsu S, et al. Effects of the freezing and thawing process on biomechanical properties of the human skull. Leg. Med. (Tokyo) 2014;16:102–105. doi: 10.1016/j.legalmed.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Hollensteiner M, et al. Novel bone surrogates for cranial surgery training. J. Mech. Behav. Biomed. Mater. 2017;72:49–51. doi: 10.1016/j.jmbbm.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 45.Falland-Cheung L, Waddell JN, Chun Li K, Tong D, Brunton P. Investigation of the elastic modulus, tensile and flexural strength of five skull simulant materials for impact testing of a forensic skin/skull/brain model. J. Mech. Behav. Biomed. Mater. 2017;68:303–307. doi: 10.1016/j.jmbbm.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 46.Zwirner J, Scholze M, Waddell JN, Ondruschka B, Hammer N. Mechanical properties of human Dura mater in tension—an analysis at an age range of 2 to 94 years. Sci. Rep. 2019 doi: 10.1038/s41598-019-52836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osterhoff G, et al. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47(Suppl 2):S11–20. doi: 10.1016/S0020-1383(16)47003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.