Abstract
Cancer-associated fibroblasts (CAFs) are a key component of the tumour microenvironment with evidence suggesting they represent a heterogeneous population. This study summarises the prognostic role of all proteins characterised in CAFs with immunohistochemistry in non-small cell lung cancer thus far. The functions of these proteins in cellular processes crucial to CAFs are also analysed. Five databases were searched to extract survival outcomes from published studies and statistical techniques, including a novel method, used to capture missing values from the literature. A total of 26 proteins were identified, 21 of which were combined into 7 common cellular processes key to CAFs. Quality assessments for sensitivity analyses were carried out for each study using the REMARK criteria whilst publication bias was assessed using funnel plots. Random effects models consistently identified the expression of podoplanin (Overall Survival (OS)/Disease-specific Survival (DSS), univariate analysis HR 2.25, 95% CIs 1.80–2.82) and α-SMA (OS/DSS, univariate analysis HR 2.11, 95% CIs 1.18–3.77) in CAFs as highly prognostic regardless of outcome measure or analysis method. Moreover, proteins involved in maintaining and generating the CAF phenotype (α-SMA, TGF-β and p-Smad2) proved highly significant after sensitivity analysis (HR 2.74, 95% CIs 1.74–4.33) supporting attempts at targeting this pathway for therapeutic benefit.
Subject terms: Non-small-cell lung cancer, Cancer microenvironment, Prognostic markers
Introduction
Non-small cell lung cancer (NSCLC) remains the leading cause of cancer death worldwide1–3. Despite more recent therapeutic advances, outcomes remain poor, with a 10-year survival rate of only 5%4. NSCLC shows a relatively low degree of tumour cell purity compared to other tumours, with high infiltration by immune and stromal cell populations5.
Fibroblasts are the most common stromal cell type in a range of solid tumours6–9, where they are referred to as cancer-associated fibroblasts (CAFs). CAFs are most commonly described as having an α-SMA-positive, “myofibroblastic” phenotype, analogous to that observed in wound healing10. These cells are associated with a number of the hallmarks of malignancy, including promotion of tumour invasion and metastasis, angiogenesis and immune evasion11–18. Unsurprisingly, this population correlates with poor prognosis in a range of malignancies9, 19–21. However, there is increasing evidence that CAFs are in fact a heterogeneous cell type, with a range of distinct phenotypes and functions22–24. For example, an inflammatory CAF sub-group has been described in a number of different tumours including pancreatic cancer25. Nevertheless, the relative contribution of specific populations is likely to vary by tumour type and has yet to be defined fully and for some cancer types, including NSCLC, the impact of CAFs on patient outcomes is less clear.
α-SMA is the most commonly used CAF marker26, but is also expressed by smooth muscle cells27 and pericytes28 and no single marker has been shown to reliably identify the entire CAF population. Indeed, FAP, another commonly-used CAF marker has been shown to identify both inflammatory and myofibroblastic CAFs in pancreatic ductal adenocarcinoma17 and breast cancer18. Other frequently used CAF markers include podoplanin and fibroblast-specific protein-1 (FSP-1)9, 29 with CAFs expressing the latter known to have immunomodulatory functions30–32. However, for others, the downstream functional pathways are yet to be characterised.
CAFs are an attractive therapeutic target, but despite promising data from pre-clinical models, the results of clinical trials targeting CAFs have been mixed33, 34. Characterisation of CAF phenotypes and their impact on outcomes has gained increasing interest in recent years, and there are now multiple studies profiling CAF heterogeneity at single-cell resolution22–24. To date, there have been many individual reports describing the prognostic effect of single CAF markers in NSCLC. The impact of CAFs seems to vary by marker and, in some cases, is contradictory (e.g. FAP35, 36). This may be explained, at least in part, by the known heterogeneity within this population, where common markers can be expressed by functionally distinct subgroups.
Although meta-analyses examining the relationship between protein marker expression and outcomes in NSCLC have been performed previously37, 38, these studies only focused on a small number of pre-determined markers and did not use methods to extract hazard ratios from studies which failed to quote them, leading to possible publication bias. Moreover, the number of studies published in the intervening period has increased significantly reflecting the increased interest in CAFs. Here, we perform a systematic review and meta-analysis of the literature assessing the prognostic effect of all CAF markers in NSCLC characterised thus far, as well as the cellular processes they are involved in. In addition, we also use several statistical methods, including a newly-published method39 which improves on the accuracy of extracted HRs when not quoted in the original studies. Assessing the prognostic significance of these markers is important in characterising the heterogeneity now widely accepted in CAFs whilst determining the most prognostic cellular pathways might help inform stromal targeting strategies in NSCLC.
Results
Study characteristics
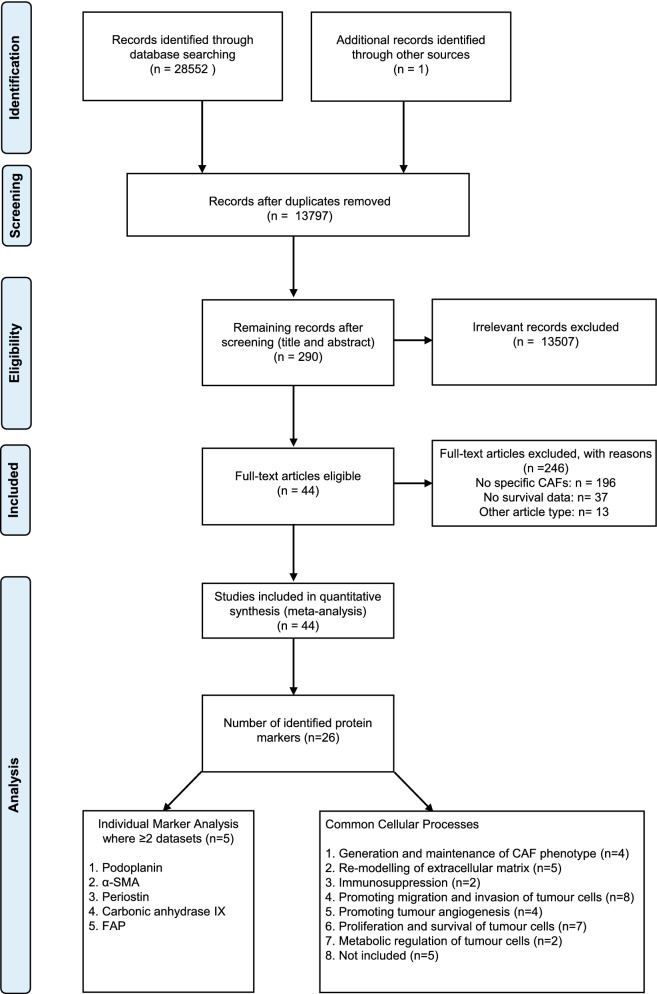
Of the 13,797 articles identified, 290 were eligible after screening titles and abstracts. Of these, 44 were included based on the full-text article, representing a total population of 7582 patients (Fig. 1). Cross-checking of previously published reviews on the roles of fibroblasts in lung cancer yielded one additional study that was not detected within the search strategy40. Of the 246 studies excluded, 196 described protein expression within the wider microenvironment, rather than specifically by fibroblasts which was the most common reason for study exclusion. A further 37 studies did not include survival statistics or a Kaplan–Meier (KM) plot from which these could be calculated. Twenty-six protein markers were identified from the 44 included articles. Five of these (podoplanin, carbonic anhydrase IX (CAIX), α-SMA, periostin and FAP) appeared in at least 2 separate cohorts, rendering them suitable for meta-analysis. Of all included markers, 21 (81%) were identified as a component of at least one common cellular process that defines, or is a hallmark, of cancer-associated fibroblasts41.
Figure 1.
Flow chart describing steps carried out in selecting articles.
The main characteristics of the included studies are shown in Supplementary Table S1. The earliest included study was published in 1998. However, the majority (30/44, 68%) were published within the last 5 years, likely reflecting the increased interest in CAFs. The median cohort size was 129 (range 52–729). Many studies reported cohorts focusing on lung adenocarcinoma (19/44, 43%) or a combination of adenocarcinoma and squamous cell carcinoma (17/44, 39%). In terms of treatment, 19 studies (43%) failed to report information on neo- or adjuvant therapy whilst 13 studies (30%) excluded patients who had received neo-adjuvant therapy. Almost half of studies (21/44, 48%) reported overall survival (OS) as the only survival outcome. The majority of studies reported a mix of univariate and multivariate hazard ratios (HR) (24/44, 55%) with 21/44 studies (48%) reporting a KM plot but no associated HR. To extract these missing HRs, we used a set of statistical techniques depending on the available information, including the Nlopt method39, a novel algorithm based on non-linear optimisation (see “Methods” section for more details). In these cases, the Nlopt method was used most frequently (14/21, 67%), with the Parmar42 (3/21, 14%) and Guyot43 method (4/21, 19%) also required in several instances. In total, the 44 included studies yielded 96 survival outcome measures.
Quality assessment
Assessment of study quality was determined by calculating a score based on the REMARK criteria44, 45 (summarised in Supplementary Figure S1; raw data given in Supplementary Table S2). The mean score was 14 (range 9.5–17) with most studies scoring moderately well against all domains of the REMARK criteria. The exception was the “data” domain in which all but one study46 registered low- to medium-quality scores. The data domain describes the flow of patients through the original study, as well as the relationship of the tumour marker to standard prognostic variables. In total, 3 studies had overall REMARK scores ≤ 50%, all of which were included in subsequent sensitivity analyses. Although the REMARK criteria were first published in 2005, in the studies included in this systematic review, there has not been a significant increase in scores over this time (R2 = − 0.017, P = 0.603; Supplementary Figure S2).
Individual marker results
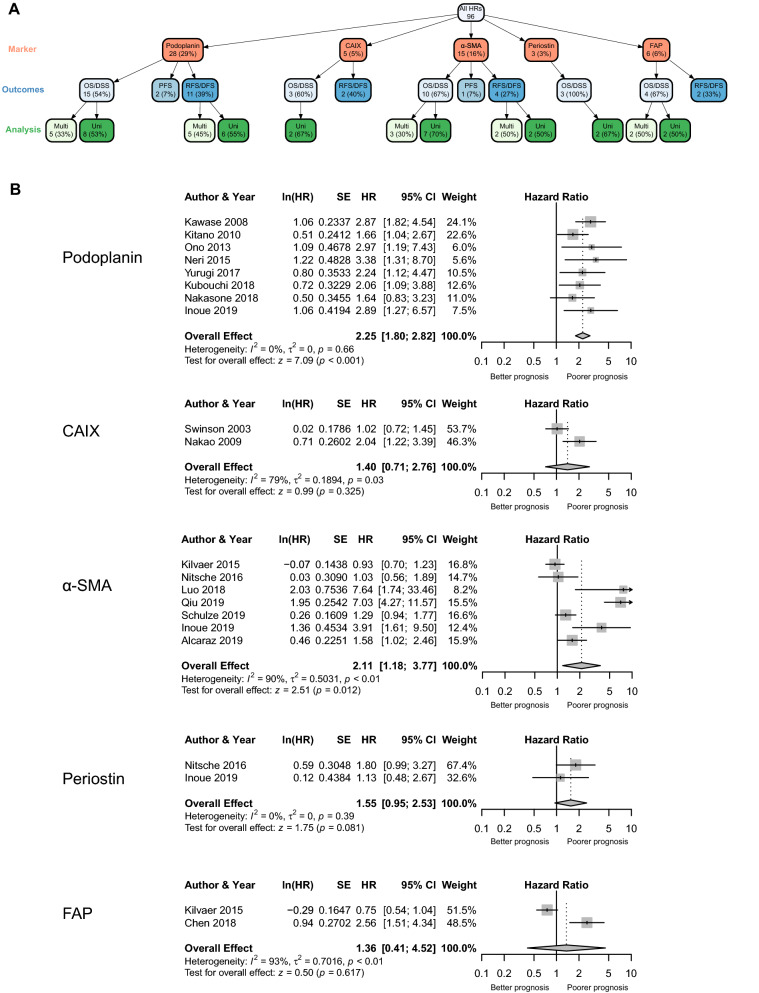
Podoplanin, α-SMA, FAP, periostin and CAIX all had at least two HRs calculated using similar outcome measures and assessed using either univariate or multivariate analysis deeming them eligible for meta-analysis. Calculating these separately was recommended in guidance published on carrying out meta-analyses on prognostic factors47. This approach resulted in twelve separate outcome measures of pooled HRs analysed using a random effects model as represented in the network tree (Fig. 2A). Example forest plots for univariate analysis of the OS/DSS outcome group for each marker are shown in Fig. 2B; full results are summarised in Table 1.
Figure 2.
Analysis of individual markers. (A) Tree network showing number of studies for each marker per outcome group and analysis method. Figure generated using the vtree package in R (version 3.5.2). (B) Random-effect forest plots of individual markers from the OS/DSS outcome group and univariate analysis method.
Table 1.
Summary of results from the random effects models for individual markers.
| Marker | Outcomes | Analysis | Studies | Random effects model | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| Overall effect (95% CIs) | P value | I2 (%) | τ2 | P value | ||||
| Podoplanin | OS/DSS | Univariate | 8 | 2.25 (1.80–2.82) | < 0.001 | 0 | 0.00 | 0.66 |
| Multivariate | 5 | 1.67 (1.03–2.73) | 0.040 | 67 | 0.19 | 0.02 | ||
| RFS/DFS | Univariate | 6 | 2.73 (2.11–3.54) | < 0.001 | 0 | 0.00 | 0.59 | |
| Multivariate | 5 | 2.19 (1.54–3.12) | < 0.001 | 0 | 0.00 | 0.41 | ||
| CAIX | OS/DSS | Univariate | 2 | 1.40 (0.71–2.76) | 0.320 | 79 | 0.19 | 0.03 |
| α-SMA | OS/DSS | Univariate | 7 | 2.11 (1.18–3.77) | 0.012 | 90 | 0.50 | < 0.01 |
| Multivariate | 3 | 3.21 (1.45–7.10) | 0.004 | 71 | 0.31 | 0.03 | ||
| RFS/DFS | Univariate | 2 | 8.12 (5.23–12.62) | < 0.001 | 0 | 0.00 | 0.93 | |
| Multivariate | 2 | 5.38 (3.34–8.67) | < 0.001 | 0 | 0.00 | 0.69 | ||
| Periostin | OS/DSS | Univariate | 2 | 1.55 (0.95–2.53) | 0.081 | 0 | 0.00 | 0.39 |
| FAP | OS/DSS | Univariate | 2 | 1.36 (0.41–4.52) | 0.620 | 93 | 0.70 | < 0.01 |
| Multivariate | 2 | 2.25 (1.39–3.63) | 0.001 | 0 | 0.00 | 0.68 | ||
Podoplanin and α-SMA were the most frequently reported of the five markers and were consistently associated with statistically significant poorer survival outcomes, regardless of outcome measure or analysis method (Table 1). However, significant test heterogeneity was found in a subset of these measures. As expected, HRs for RFS/DFS were always higher than OS/DSS although no other trends emerged when comparing survival outcomes from different groups. In contrast, CAIX (HR 1.40, 95% CI 0.71–2.76) and periostin (HR 1.55, 95% CI 0.95–2.53) did not show a significant correlation with survival. FAP expression was only associated with a statistically significant poor prognostic impact in the multivariate analysis from the OS/DSS outcome group (HR 2.25, 95% CI 1.39–3.63).
In all eight podoplanin and one α-SMA random effects models, there were a sufficient number of studies to carry out sub-group analysis based on histological subtype (Fig. 3A,B, Table 2). In the case of podoplanin, all were significantly associated with a poorer survival outcome, with the exception of multivariate analysis of the OS/DSS outcome group in adenocarcinoma and univariate analysis of the OS/DSS outcome group in squamous cell carcinoma. The univariate analysis of α-SMA in a cohort of patients with only adenocarcinoma was statistically significant (HR 5.91, 95% CI 3.49–10.00).
Figure 3.
Sub-group analysis of individual markers based on histological subtype. (A) Tree network showing number of studies for each marker per outcome group, analysis method and histological subtype. Figure generated using the vtree package in R (version 3.5.2). (B) Random-effect forest plots of individual markers from the OS/DSS outcome group and univariate analysis method with histological subtype indicated.
Table 2.
Subgroup analysis: summary of results from the random effects models for individual markers based on histology sub-type.
| Marker | Outcomes | Analysisa | Histologyb | Studies | Random Effects Model | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|
| Overall effect (95% CIs) | P value | I2 (%) | τ2 | P value | |||||
| Podoplanin | OS/DSS | U | A | 6 | 2.49 (1.90–3.26) | < 0.001 | 0 | 0.00 | 0.74 |
| S | 3 | 1.78 (0.91–3.48) | 0.093 | 58 | 0.20 | 0.093 | |||
| M | A | 3 | 1.28 (0.67–2.45) | 0.460 | 68 | 0.20 | 0.05 | ||
| S | 2 | 2.50 (1.58–3.95) | < 0.001 | 0 | 0.00 | 0.59 | |||
| RFS/DFS | U | A | 3 | 3.12 (2.17–4.49) | < 0.001 | 10 | 0.01 | 0.33 | |
| S | 3 | 2.30 (1.55–3.42) | < 0.001 | 0 | 0.00 | 0.89 | |||
| M | A | 3 | 2.59 (1.19–5.65) | 0.020 | 46 | 0.22 | 0.16 | ||
| S | 2 | 2.04 (1.30–3.18) | 0.002 | 0 | 0.00 | 0.94 | |||
| α-SMA | OS/DSS | U | A | 2 | 5.91 (3.49–10.00) | < 0.001 | 22 | 0.04 | 0.26 |
aU univariate, M multivariate.
bA adenocarcinoma, S squamous.
After sensitivity analysis excluding studies with low REMARK scores, two scores for podoplanin (OS/DSS > Univariate > All histology and OS/DSS > Univariate > adenocarcinoma only) remained unchanged (Supplementary Table S3).
CAF markers in cellular processes
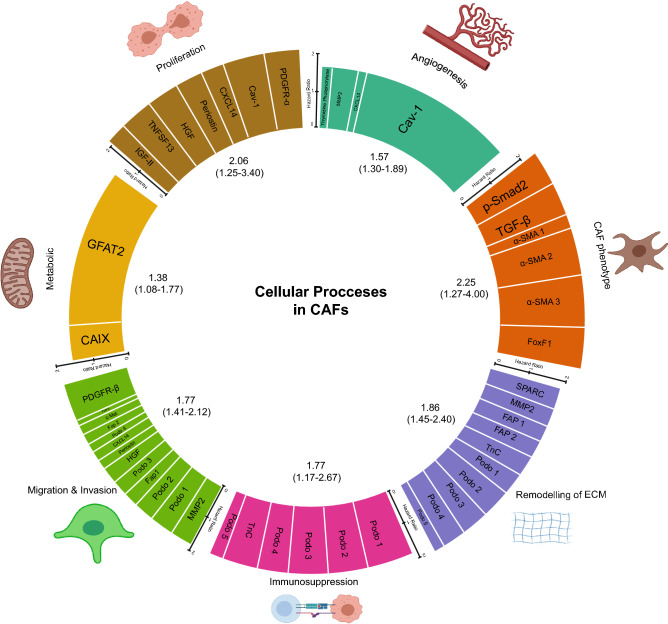
Our next aim was to assess the prognostic significance of the cellular processes known to be crucial in the function of CAFs. CAFs are known to have a variety of functions which influence cancer progression and which have been summarised in a number of recent reviews26, 41, 48. We established a consensus of functions from these reviews, creating a table of what are currently considered the most important functions or hallmarks of CAFs (Supplementary Table S4). Next, to determine the function of each of the identified markers, a separate literature search was performed focusing on studies which had identified a functional role of the marker specifically in CAFs. In several cases, this came from the functional studies published in the original paper, for example, c-Met49, GFAT250 and IGF-II51. Such studies generally included co-culture experiments in vitro or more complex mouse models in vivo or a combination of the two (Supplementary Table S5). Each of the markers was then assigned to one or more of the functions (Table 3). In two cases, CD34 and irisin have no clear role in CAFs currently so were excluded from this step of the analysis. In addition, three other markers, CD90, HSF-1, CD200 did not have outcome measures in the OS/DSS group so were also excluded from the analysis (see “Methods” section for more detail). In total, this resulted in 21/26 of the markers being attributed to at least one of 7 common processes. Analysis of the pooled HR for each process showed all were in fact associated with poorer survival (Table 4, Fig. 4) although generation and maintenance of the CAF phenotype (HR 2.25, 1.27–4.00 95% CIs) and enhancing the proliferation and survival of tumour cells (HR 2.06, 1.25–3.40 95% CIs) were the only processes with a HR above 2. A significant level of heterogeneity was again detected in 4 of the 7 cellular processes but in the case of the CAF phenotype, this was non-significant after sensitivity analysis excluding poor quality studies (Supplementary Table S3) and the pooled HR in fact increased to 2.74 (1.74–4.33 95% CIs).
Table 3.
Summary of the CAF functions and the role of each identified marker.
| CAF function | Identified markers |
|---|---|
| 1. Generation and maintenance of CAF phenotype | α-SMA, p-Smad2, TGF-β, FoxF1 |
| 2. Re-modelling of extracellular matrix | FAP, MMP2, SPARC, Tenascin-C, Podoplanin |
| 3. Immunosuppression | Podoplanin, Tenascin-C |
| 4. Promoting migration and invasion of tumour cells | c-Met, FAP, HGF, MMP2, PDGFR-β, Periostin, Podoplanin, CXCL14 |
| 5. Promoting tumour angiogenesis | Caveolin-1, CXCL14, MMP2, Thymidine Phosphorylase |
| 6. Proliferation and survival of tumour cells | Caveolin-1, CXCL14, HGF, IGF-II, PDGFR-α, Periostin, TNFSF13 |
| 7. Metabolic regulation of tumour cells | Carbonic anhydrase IX, GFAT2 |
| 8. Not included | |
| No known function in CAFs currently | CD34, Irisin |
| No outcome measure for OS/DSS | CD90, HSF-1, CD200 |
Table 4.
Summary of results from the random effects models for each cellular process. The list of proteins making up each cellular process can be found in Table 3.
| Cellular process | Studies | Random effects model | Heterogeneity | |||
|---|---|---|---|---|---|---|
| Overall effect (95% CIs) | P value | I2 (%) | τ2 | P value | ||
| Generation and maintenance of CAF phenotype | 6 | 2.25 (1.27–4.00) | 0.006 | 81 | 0.36 | < 0.01 |
| Re-modelling of extracellular matrix | 10 | 1.86 (1.45–2.40) | < 0.001 | 41 | 0.07 | 0.08 |
| Immunosuppression | 6 | 1.77 (1.17–2.67) | 0.007 | 63 | 0.16 | 0.02 |
| Promoting migration and invasion of tumour cells | 13 | 1.77 (1.41–2.12) | < 0.001 | 46 | 0.07 | 0.04 |
| Promoting tumour angiogenesis | 4 | 1.57 (1.30–1.89) | < 0.001 | 0 | 0.00 | 0.50 |
| Enhancing proliferation and survival of tumour cells | 7 | 2.06 (1.25–3.40) | 0.005 | 85 | 0.34 | < 0.01 |
| Metabolic regulation of tumour cells | 2 | 1.38 (1.08–1.77) | 0.010 | 20 | 0.01 | 0.26 |
Figure 4.
Ferris wheel plot summarising random-effect model HRs for each cellular process in CAFs. The height of each bar represents the HR for each process with the width of each bar indicating the % weight that each marker contributed to the random-effects model. The random-effect model HRs and 95% CIs are stated below each cellular process. Figure generated using Adobe Illustrator, 2020 (version 24.2). Icons representing each cellular process are from BioRender.com.
As with all meta-analyses, small-study effects should be examined to determine the extent of any publication bias. We produced funnel plots and tested asymmetry with linear regression in any meta-analysis with 7 or more studies (Supplementary Figure S3) due to the low power of these tests52. In the case of funnel plots for the univariate analysis of α-SMA in the OS/DSS outcome group and the invasion and proliferation meta-analysis (Supplementary Figure S3B, D, E), the plots were clearly asymmetrical but likely due in part to the heterogeneity that was also detected (Tables 1, 4). Only one of the plots was deemed to significantly deviate from a symmetrical distribution (cellular processes: invasion, P = 0.02) although this is in part likely due to the heterogeneity mentioned above. However, visual assessment of this funnel plot suggested some values missing where small studies with larger standard errors would be expected suggesting an element of publication bias in this particular random effects model.
Discussion
CAFs are a key component of the tumour microenvironment. Growing evidence suggests they are a heterogeneous population with respect to function53 and expression of both RNA and protein22, 54. We therefore performed a meta-analysis of all published protein markers of CAFs in NSCLC in an attempt to better characterise this heterogeneity by determining their prognostic significance. We implemented a search strategy focused on sensitivity, resulting in a number needed to read of 313. In addition, we calculated HRs from studies when these were not directly quoted using the most up-to-date extraction methods. In total, this yielded 26 different protein markers. These included well known markers of CAFs including α-SMA and FAP26, but also new potential markers identified from omic-type screens such as CD20055 and GFAT250. To ensure that this analysis was fibroblast-specific, we excluded studies which did not explicitly state if the protein marker was expressed by fibroblasts or just more generally within the stroma or tumour microenvironment.
Podoplanin is the best characterised of the identified markers, and in keeping with previous data37, was often associated with poor survival in this study. Podoplanin is a 44-kDa glycoprotein that was initially characterised as a platelet-aggregation factor on cancer cells from colorectal tumours56. It is also expressed by both lymphatic endothelium57 and inflammatory macrophages58. Functionally, podoplanin-positive fibroblasts in cancer have been shown to enhance the invasive properties of carcinoma cells59, play an important role in re-modelling of the ECM60–62, as well as promoting an immunosuppressive microenvironment63. Although podoplanin was consistently associated with poor survival in this study, a significant test heterogeneity was detected for the OS/DSS outcome group in multivariate analysis but when sub-grouping based on histological variant was carried out this became non-significant. The same analysis also revealed that squamous but not adenocarcinoma tumours were significantly associated with survival suggesting a possible difference in prognostic effect based on NSCLC histological subtype, explaining the heterogeneity originally identified. However, this trend was not observed in the RFS/DFS outcome measure suggesting further comparisons of podoplanin-positive fibroblasts in squamous and adenocarcinoma tumours are warranted.
α-SMA was also commonly reported and associated with a poorer survival. α-SMA is a member of a highly conserved group of proteins that regulate the cell cytoskeleton64. In fibroblasts, this protein is crucial in regulating the contractility of the cell which is itself required to both generate and maintain the CAF phenotype65. Although α-SMA was associated with poor survival, it tended to result in pooled HRs with larger variances. For example, in the case of analysing univariate HRs from OS/DSS outcomes, HRs ranged from 0.9 for Kilvaer et al.35 to over 7 for Qiu et al.66 whilst contributing similar weights to the random effects model. In comparing these two studies at either extreme, both featured cohorts of patients with stage I-IIIA NSCLC, excluding those treated with neo-adjuvant therapy and scored well on the REMARK criteria. Qiu et al.66 focused solely on adenocarcinoma cases with Kilvaer et al.35 considering both squamous cell carcinoma and adenocarcinoma. However, both studies used different immunohistochemistry scoring systems: Kilvaer calculated the dominant staining intensity in positive cells whereas the Qiu study used an index combining both staining intensity and extent. Such a difference in scoring might explain, at least in part, the variation seen in these two studies. Indeed, the use of different scoring criteria in biomarker research and subsequent difficulty in comparing studies is well known47, leading for calls to ensure scoring for markers is standardised67, and validates the need for a meta-analysis.
Three other markers were also suitable for meta-analysis in this study, CAIX, periostin and FAP. Unlike for α-SMA and podoplanin, fewer studies have been carried out on these markers so there was only one combination of outcome measures and forms of analysis for CAIX and periostin and two for FAP.
CAIX is a member of the carbonic anhydrase family, the expression of which is induced under hypoxic conditions by HIF-168. Whilst hypoxia is clearly an important aspect of tumourigenesis69 with the tumour microenvironment (including CAFs) playing an important role in its regulation70, CAIX expression in CAFs was not associated with reduced survival in this meta-analysis.
Periostin, an ECM protein produced by fibroblasts71, has previously been shown to enhance the proliferation and invasive potential of tumour cells72 but was also not associated with poor survival in NSCLC. In the case of FAP, meta-analysis produced conflicting results.
FAP is a type II integral membrane serine protease shown to be involved in ECM re-modelling and tumour cell migration73 and has been used as a marker of activated fibroblasts in a number of studies. In this meta-analysis, random effects models showed univariate analysis had no significant effect on prognosis whilst multivariate analysis did prove to be statistically significant. Notably, one of the univariate studies actually showed that increased CAF FAP expression was associated with improved survival in NSCLC46. Whilst not statistically significant in a mixed cohort, sub-group analysis of only squamous carcinoma cases was statistically significant, in contrast to findings of Chen et al.74 In what is emerging as a common theme, these two studies used different antibodies, grading systems and scored either whole slides or tissue micro-arrays; this may account for the discordant results in these studies. Such discrepancies are concerning though as FAP is currently regarded as a CAF target for molecular-based imaging75 and therapeutic targeting76. Without a clear understanding of its role in CAF biology, such trials might produce inconsistent results.
Some CAF markers were only analysed in a single study or did not share a common outcome group/form of analysis and were therefore excluded from the meta-analysis. These included several interesting studies; Chen et al.51 showed high expression of IGF-II in CAFs in a cohort of 80 patients resulted in a HR of 19.15 (95% CIs 6.32–58) for overall survival. In this study, CAFs from primary tumours were shown to promote stemness characteristics of lung cancer-stem cells (expression of Nanog and Oct3/4), an effect which was shown to be partly dependent on the expression of IGF-II. IGF-II in CAFs is known to accelerate tumour growth in cholangiocarcinoma xenograft models77 and promotes proliferation of anal squamous cell carcinoma cells78. Moreover, expression of IGF-II has been shown to promote differentiation of fibroblasts into myofibroblasts in idiopathic pulmonary fibrosis and scleroderma/systemic sclerosis-associated pulmonary fibrosis79. Together, this result suggests further examination of IGF-II expression in CAFs in NSCLC could lead to key biological pathways being elucidated or identification of additional sets of patients with poor survival.
Cav-1, a scaffold protein crucial to caveolae80 was also excluded from meta-analysis as the two studies which analysed its prognostic role only calculated either a univariate or multivariate hazard ratio. Along with FAP, Cav-1 was the only other marker that resulted in statistically significant outcome measures with opposite effects on survival. In the study by Shimizu et al.81, high expression of Cav-1 was associated with a decrease in overall survival (HR 2.78) whilst the study by Onion et al.82 showed high expression of Cav-1 was associated with improved survival (HR 0.64). The studies used different antibodies and scoring systems whilst the cohort used in the Shimizu study was larger and consisted of only patients with Stage I adenocarcinoma. The Onion study featured a cohort with Stage I–III NSCLC but did not state the histological classification of the tumours included. Given this, it is possible that the differences are due to histological subtype if the cohort in the Onion study was mainly composed of cases of squamous NSCLC. Loss of Cav-1 has previously been shown to correlate with poor survival in other cancers, for example prostate83 and breast84, in agreement with the Onion study but given the discrepancies identified in this analysis, further studies assessing the prognostic role of Cav-1 in CAFs in NSCLC would be warranted to clarify its prognostic role in lung cancer.
A variety of functions have been attributed to CAFs in recent years, leading to the question of whether all CAFs perform these functions, or whether there exist subsets of CAFs with different functions.
CAFs are increasingly recognised as a heterogeneous cell type. Recent studies have described transcriptomically-distinct CAF phenotypes in NSCLC, which may correspond to discrete functional subsets22, 23. Our aim was to therefore determine whether a set of protein markers, grouped together by function, would show prognostic differences. This in turn may suggest a subset of CAFs with specific functions leads to poorer survival outcomes. CAFs are crucial in depositing and re-modelling the ECM within a tumour41. Intrinsic to this is their ability to secrete growth factors and matrix proteases, promoting and enabling tumour cell migration and invasion85, 86. CAFs also promote angiogenesis87, the proliferation and survival of tumour cells88, and an immunosuppressive microenvironment by reducing T cell responses89.
Analysing the prognostic effect of these processes in CAFs showed all were in fact correlated with a poor survival outcome. However, proteins involved in the generation and maintenance of the CAF phenotype were most prognostic with a HR approaching 3 after sensitivity analysis. This suggests that although different functional subsets of CAFs might exist, conversion of a fibroblast into a CAF is a uniting feature, creating a population of cells which ultimately contribute to poor survival outcomes in NSCLC. Targeting of this process might thus prove an effective treatment strategy. Indeed, such an approach is currently a significant area of research with a recent study showing pharmacological inhibition of NOX4, a protein important in this conversion, reduced tumour growth in mouse xenograft models90. In addition, a number of clinical trials targeting proteins which are also important in CAF activation such as FGFR91 and TGF-β92, 93 are currently underway with their results awaited. Other attempts at targeting molecules, such as the vitamin D receptor, which aim to revert CAFs to a more normal state are also ongoing94. Thus, in the case of NSCLC, the results of this meta-analysis are in keeping with treatments targeting pathways important in generating and maintaining the CAF phenotype.
Although several significant survival associations were observed in this analysis, there are a number of limitations. Some issues common in research carried out on prognostic factors44 have already been mentioned, such as the use of different scoring methods and cut-off values for the same marker. In addition, a number of studies did not report whether patients received neoadjuvant or adjuvant therapy and those that did failed to report sub-group outcome analyses. Since adjuvant therapy is now commonplace in treating eligible patients with lung adenocarcinoma95, it is feasible that CAFs could exhibit both prognostic and predictive effects. Indeed, CAFs are known to mediate increased tissue tension, a factor known to affect drug delivery96. Thus, future studies should include outcome measures based on therapy where possible. Similarly, several studies examined cohorts with mixed histology, generally squamous and adenocarcinoma. In this meta-analysis, there was some evidence of outcome differences between histological subtypes suggesting that subtle trends may exist, which can only be identified with subtype analysis.
A further issue with the analysis of survival data was the adjustment factors used in calculating a multivariate HR. Such adjusted values are crucial in determining the independent effects of prognostic markers47 but whilst many studies described these factors and the model they used, there was a significant variation in the final adjustment factors. As suggested in guidance published on reporting prognostic studies47, analyses could include multivariate HRs with a core, agreed set of factors alongside other models facilitating more direct comparisons for studies such as this one. In general, scoring each study against the REMARK criteria captured elements of the limitations described above, further validating the approach in conducting sensitivity analyses. Interestingly, although the REMARK criteria have been in place since 2005, there has been no increase in these scores in the intervening years. This suggests that authors should still be encouraged to comply as fully as possible with these criteria, to ensure consistent publication of high-quality studies.
Another issue with reporting of survival outcomes was that many studies published a KM plot but no associated HR. Although we used well-established methods to extract these missing values, including a novel algorithm39 recently published which improves upon existing methods when the number at risk is not included below the KM plot, such techniques are still associated with varying degrees of error39, 97. However, if no attempt is made to obtain such values, a number of studies would have been excluded and in several cases resulted in non-significant values being ignored leading to publication bias, a significant concern in any meta-analysis47. We assessed this using funnel plots and an asymmetry was clear in three cases and significant in one (cellular process: invasion) but this was likely due to the associated heterogeneity identified in all cases, which is another well-known cause of funnel plot asymmetry52. Use of extraction methods would certainly reduce publication bias this but would only apply for univariate HRs as such methods require KM plots which are not generated in a multivariate analysis. On balance, although a degree of publication bias was present in one of our random effects model, this was not the case in the remaining models and so we do not believe publication bias was prevalent in this meta-analysis.
In conclusion, the aim of this study was to address the now widely accepted hypothesis that CAFs are a heterogeneous population41 which is therefore likely to mean distinct functional sub-groups of CAFs represented by different proteins/markers. The study was designed in such a way as to address both of these issues by: (1) summarising the prognostic significance of every protein so far examined in CAFs in NSCLC and (2) linking each of these proteins to a cellular process that is currently believed to be crucial in CAF function. This approach is based on the fact that proteins which are prognostically important might represent key proteins that are crucial to CAF biology as well as identifying functional sub-groups within CAFs generally. An additional approach as previously mentioned is the use of scRNA sequencing experiments to identify transcriptomically-different sub-populations of CAFs. Such experiments are already yielding exciting results22,23 and the combination of these analyses whilst also assessing the prognostic effect of any identified proteins, as in this study, has the potential to further our understanding of CAF biology and in particular, its heterogeneity.
Notwithstanding, the current results from this study show that, despite the limitations common in prognostic research and inherent to meta-analyses, CAF expression of podoplanin or α-SMA was consistently associated with poor survival in NSCLC. Moreover, the proteins and pathways required to generate and maintain the CAF phenotype might represent potential therapeutic targets in anti-cancer treatments in NSCLC.
Methods
This review was prospectively registered with PROSPERO (CRD42019130307), an International prospective register of systematic reviews (https://www.crd.york.ac.uk/prospero/). Guidelines for carrying out systematic reviews and meta-analysis of prognostic factor studies47 were followed where possible.
Search strategy
Literature was retrieved using Medline, Embase, Scopus, Web of Science, and Cochrane databases on the 29th January, 2020 with no date restriction. All results were then updated again with a search on 24th July, 2020. The full search strategy for each database is available in Supplementary Table S6.
Screening and selection of studies
All identified articles were exported into Rayyan98, a web-based application for carrying out systematic reviews. All titles, abstracts and full-text articles were independently screened by AI and SW with discrepancies resolved by consensus. The following P(atient) E(xposure) C(omparator) O(utcome), PECO was used to select articles: Patients: Individuals diagnosed with NSCLC (histological subtypes to include squamous, adenocarcinoma and large cell) who underwent surgical resection, treated with or without neoadjuvant or adjuvant therapy. Exposure: Tumour resections analysed for the presence of CAFs stained with antibodies against any protein marker using immunohistochemistry. For the definition of CAFs, an explicit statement in the methods or results section that fibroblasts, myofibroblasts, cells with a spindle-shaped morphology or similar were scored was required. Statements equivalent to positive staining within the tumour stroma or tumour microenvironment were not sufficient and such studies excluded. Comparator: Comparison of expression profiles (e.g. low/high, negative/positive) of the reported protein markers. Outcomes: The following survival outcomes were all considered for inclusion: overall survival (OS), disease-specific survival (DSS), progression-free survival (PFS), recurrence-free survival (RFS), and disease-free survival (DFS). Studies which failed to define the survival outcome were excluded.
Data extraction
Data extraction was carried out by AI and SW with the following information for every study collected: first author; year of publication; journal; protein marker; staging, histological subtype, size and treatment details for each cohort; scoring and cut-off criteria; survival outcome, HR including associated 95% confidence intervals (CI) and P value. If outcome measures were related to the absence and not presence of the identified marker, the HR and associated CIs were inverted. If different studies used the same or overlapping cohorts, the largest cohort was used for the random-effects models. In the case where a KM plot was included but no associated HR was quoted, three statistical methods were used to infer the HR value.
Methods to extract HRs from KM plots
Several methods exist to infer HRs from KM plots where they were not quoted within the article97. Here, we used the Parmar42 and Guyot43 methods as well as a new method, known as Nlopt39, based on the mathematical technique, non-linear optimisation. All three are associated with varying degrees of error97, but the Nlopt method is the most accurate when the number at risk (found at the bottom of a KM plot) are not included but a P value is; whereas the Guyot method is more accurate when the number at risk are included. The Parmar method was used when both the number at risk and P value were not included. All three methods rely on extracting a sufficient number of points from each KM plot. To carry this out, digitized KM plots were loaded into the Fiji distribution of Image J (version 1.52p; NIH, USA) and the axes calibrated using the Figure Calibration Plugin (Frederic V. Hessman, University of Gottingen). The specific guidance for extracting points for each method was then followed resulting in a number of X,Y points. In the case of the Parmar et al. method, we followed guidance from Tierney et al.99 to determine the minimum and maximum follow-up times for each study, as these values are crucial in extracting accurate HRs from KM plots when using this method. HRs and standard errors (SEs) for the Parmar et al. method were calculated in Excel, whilst we used the R script published with the Guyot et al. and Nlopt method to determine these HRs and SEs. The SE of the HRs were increased by 5 and 10% respectively for the Guyot/Nlopt method and Parmar method, reflecting the known error associated with each method39, 97.
Study quality assessment
To assess the quality of a study, a score from the REporting recommendations for tumour MARKer prognostic studies (REMARK) criteria was calculated for each included study by AI and SW. Discrepancies were resolved by consensus. Although checklists for assessing the quality of prognostic studies do exist (e.g. the QUIPS checklist100), the REMARK criteria are specific to tumour marker studies and have previously been used in meta-analyses of tumour markers101. The REMARK criteria is composed of twenty items split into several domains: introduction, patients, specimen characteristics, assay methods, study design, statistical analysis methods, data, and analysis, and discussion. Each article was scored 1 point per item, with a score of 0.5 for items where the study fulfilled some but not all of the criteria. Cut-offs for each domain were used to represent a low-, medium- and high-quality score. For assessment of overall quality, cut-offs for low, medium and high were ≤ 10, ≤ 15 and > 15 respectively. In the case of a random effects model including a low-quality study, sensitivity analysis was used to exclude these studies and the model re-analysed. The traffic light plot in Supplementary Figure S1 was produced using the robvis package102 in R. The relationship between year of publication and REMARK score was assessed using a linear model in R and plotted using the R package ggplot2103.
Defining the cellular processes key to CAF function
Cancer-associated fibroblasts have a wide range of functions which influence cancer progression and have been summarised in a number of recent reviews26, 41, 48, 104–106. These reviews were used as the basis to create a set of common cellular processes/functions crucial to CAF function (Supplementary Table S4).
Assigning individual markers to each cellular process
To determine the proposed function of an individual CAF marker, the literature was reviewed for functional studies which investigated the role of that particular protein in some aspect of cancer progression. The following strategy was used to search Medline as a way of identifying relevant articles:
(name of marker) AND (cancer OR tumour OR tumor) AND (fibroblast OR stroma)
Titles and abstracts were initially screened and the full-text reviewed if relevant. This strategy was used in preference to the alternative option of a bioinformatics approach using a database such as DAVID107 as the function recorded for each protein would not be specific for CAFs. Since there were only 26 identified markers, the decision to manually annotate the functional role of each marker was instead chosen as way of increasing the specificity of the highlighted functional process whilst accepting a potential loss of sensitivity. Functional studies that investigated the role of each marker in NSCLC were favoured but where these did not exist, other tumour types were used. Functional studies were occasionally determined in the same paper that also analysed the prognostic role of the particular protein in NSCLC. Functional studies generally included co-culture experiments with CAFs and tumour cells in tissue culture as well as mouse models whether these were injection studies or genetically-engineered strains. Each marker was then placed into the relevant cellular process as identified in the Methods described above. If no relevant functional process was identified, these proteins were excluded from the analysis.
Statistics
After extraction of all relevant data, we first combined similar survival outcomes resulting in three groups: OS/DSS, RFS/DFS and PFS. However, we considered HRs derived from either univariate or multivariate analysis separately, as recommended by Riley et al.47. In the case of an individual marker with at least 2 distinct cohorts based on the same outcome group and analysis method, a random effects model using the inverse variance method was used to create weighted HRs with 95% CIs and P value. A variable tree for the individual markers was generated using the vtree package108 in R. Heterogeneity was assessed by calculating I2 and Ʈ2 values with a P value generated to assess the statistical significance of the heterogeneity. The aggregate HRs for the cellular processes were calculated in the same manner but to ensure as many of the markers could be included in the analysis as possible we used HRs from the OS/DSS outcome group and combined multivariate and univariate HRs with the former used in preference to the latter where available. The ferris wheel plot was generated using ggplot2103 in R. The random effects model were carried out using the meta package109 in R. The icons representing the cellular processes in Fig. 4 are from BioRender.com. A P value of ≤ 0.05 was considered statistically significant for all tests carried out.
Software
Unless otherwise stated, all analysis and figures were generated in RStudio (Version 1.3.959) with version 3.5.2 of R. Panels of figures were assembled using Adobe Illustrator 2020 (Version 24.2).
Ethics statement
No animals or humans were used in generating data for this study.
Supplementary Information
Acknowledgements
AI was supported by the University of Southampton National Institute of Health Research (NIHR) Academic Foundation Programme and a University of Leeds NIHR Academic Clinical Fellowship. SW was supported by Cancer Research UK, the Medical Research Council Clinical Research Training Fellowships (MR/R001286/1) and a Pathological Society Trainees’ Small grant. This work was additionally funded by support from Cancer Research UK (grant nos. A20256, A27989) to GT. AI would like to thank Professor Tony Kendrick for help with designing the study protocol and his support throughout. We thank Paula Sands of the Health Sciences Library at the University of Southampton for invaluable help in generating the database search queries.
Author contributions
A.I. and G.T. developed the idea for the study. A.I. and B.S. developed the study protocol which this study is based on. A.I. and S.W. carried out the acquisition and analysis of data. E.G. helped design some of the figures. B.S. provided statistical help throughout. All authors were involved in writing the paper and had final approval of the submitted version.
Data availability
Any of the data generated in this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrew F. Irvine, Email: a.f.irvine@leeds.ac.uk
Gareth J. Thomas, Email: g.thomas@soton.ac.uk
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-81796-2.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl. Lung Cancer Res. 2016;5:288–300. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Research UK. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer. (2019).
- 5.Aran D, Sirota M, Butte AJ. Systematic pan-cancer analysis of tumour purity. Nat. Commun. 2015;6:8971. doi: 10.1038/ncomms9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 7.Rupp C, et al. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor–stroma interaction. Oncogene. 2014;34:815. doi: 10.1038/onc.2014.18. [DOI] [PubMed] [Google Scholar]
- 8.Servais C, Erez N. From sentinel cells to inflammatory culprits: cancer-associated fibroblasts in tumour-related inflammation. J. Pathol. 2013;229:198–207. doi: 10.1002/path.4103. [DOI] [PubMed] [Google Scholar]
- 9.Ishii G, Ochiai A, Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv. Drug Deliv. Rev. 2016;99(Part B):186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Roulis M, Flavell RA. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation. 2016;92:116–131. doi: 10.1016/j.diff.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Kraman M, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 12.Torres S, et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin. Cancer Res. 2013;19:6006–6019. doi: 10.1158/1078-0432.ccr-13-1130. [DOI] [PubMed] [Google Scholar]
- 13.Brentnall TA. Arousal of cancer-associated stromal fibroblasts: palladin-activated fibroblasts promote tumor invasion. Cell Adhes. Migr. 2012;6:488–494. doi: 10.4161/cam.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Mariathasan S, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018 doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford K, et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Res. 2020;80:1846–1860. doi: 10.1158/0008-5472.can-19-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieffer Y, et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-19-1384. [DOI] [PubMed] [Google Scholar]
- 18.Dominguez CX, et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ Myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 2020;10:232. doi: 10.1158/2159-8290.CD-19-0644. [DOI] [PubMed] [Google Scholar]
- 19.Tao L, Huang G, Song H, Chen Y, Chen L. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol. Lett. 2017;14:2611–2620. doi: 10.3892/ol.2017.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peña C, et al. STC1 expression by cancer-associated fibroblasts drives metastasis of colorectal cancer. Can. Res. 2013;73:1287. doi: 10.1158/0008-5472.CAN-12-1875. [DOI] [PubMed] [Google Scholar]
- 21.Quante M, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrechts D, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018;28:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 23.Hanley CJ, et al. Spatially discrete signalling niches regulate fibroblast heterogeneity in human lung cancer. bioRxiv. 2020 doi: 10.1101/2020.06.08.134270. [DOI] [Google Scholar]
- 24.Elyada E, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102–1123. doi: 10.1158/2159-8290.cd-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Öhlund D, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017;214:579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int. J. Cancer. 2020;146:895–905. doi: 10.1002/ijc.32193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latif N, Sarathchandra P, Chester AH, Yacoub MH. Expression of smooth muscle cell markers and co-activators in calcified aortic valves. Eur. Heart J. 2015;36:1335–1345. doi: 10.1093/eurheartj/eht547. [DOI] [PubMed] [Google Scholar]
- 28.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/s1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortez E, Roswall P, Pietras K. Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin. Cancer Biol. 2014;25:3–9. doi: 10.1016/j.semcancer.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Grum-Schwensen B, et al. S100A4-neutralizing antibody suppresses spontaneous tumor progression, pre-metastatic niche formation and alters T-cell polarization balance. BMC Cancer. 2015;15:44. doi: 10.1186/s12885-015-1034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Connell JT, et al. VEGF-A and Tenascin-C produced by S100A4(+) stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. U.S.A. 2011;108:16002–16007. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Chen L, Xiao M, Wang C, Qin Z. FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am. J. Pathol. 2011;178:382–390. doi: 10.1016/j.ajpath.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narra K, et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. J. Cancer Biol. Ther. 2007;6:1691–1699. doi: 10.4161/cbt.6.11.4874. [DOI] [PubMed] [Google Scholar]
- 34.Hofheinz RD, et al. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of Sibrotuzumab in patients with metastatic colorectal cancer. Oncol. Res. Treat. 2003;26:44–48. doi: 10.1159/000069863. [DOI] [PubMed] [Google Scholar]
- 35.Kilvaer TK, et al. Cancer associated fibroblasts in stage I-IIIA NSCLC: prognostic impact and their correlations with tumor molecular markers. PLoS ONE. 2015;10:e0134965. doi: 10.1371/journal.pone.0134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Y, Ni Y, He R, Liu W, Du J. Clinical implications of fibroblast activation protein-α in non-small cell lung cancer after curative resection: a new predictor for prognosis. J. Cancer Res. Clin. Oncol. 2013;139:1523–1528. doi: 10.1007/s00432-013-1471-8. [DOI] [PubMed] [Google Scholar]
- 37.Hu G, et al. Tumor-infiltrating Podoplanin+ Fibroblasts predict worse outcome in solid tumors. Cell Physiol. Biochem. 2018;51:1041–1050. doi: 10.1159/000495484. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Yao HH, Zhu ZQ, Ning ZL, Huang Q. Stromal myofibroblasts are associated with poor prognosis in solid cancers: a meta-analysis of published studies. PLoS ONE. 2016;11:e0159947. doi: 10.1371/journal.pone.0159947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Irvine AF, Waise S, Green EW, Stuart B. A non-linear optimisation method to extract summary statistics from Kaplan–Meier survival plots using the published P value. BMC Med. Res. Methodol. 2020;20:269. doi: 10.1186/s12874-020-01092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito RA, et al. Forkhead box F1 regulates tumor-promoting properties of cancer-associated fibroblasts in lung cancer. Cancer Res. 2010;70:2644–2654. doi: 10.1158/0008-5472.can-09-3644. [DOI] [PubMed] [Google Scholar]
- 41.Sahai E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998;17:2815–2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med. Res. Methodol. 2012;12:9. doi: 10.1186/1471-2288-1112-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McShane LM, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK) Br. J. Cancer. 2005;93:387–391. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG. Reporting recommendations for tumor marker prognostic studies (REMARK): an abridged explanation and elaboration. J. Natl. Cancer Inst. 2018;110:803–811. doi: 10.1093/jnci/djy1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kilvaer TK, et al. Tissue analyses reveal a potential immune-adjuvant function of FAP-1 positive fibroblasts in non-small cell lung cancer. PLoS ONE [Electronic Resource] 2018;13:e0192157. doi: 10.1371/journal.pone.0192157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley RD, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. doi: 10.1136/bmj.k4597. [DOI] [PubMed] [Google Scholar]
- 48.LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis. Models Mech. 2018 doi: 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tokunou M, et al. c-MET expression in myofibroblasts: role in autocrine activation and prognostic significance in lung adenocarcinoma. Am. J. Pathol. 2001;158:1451–1463. doi: 10.1016/S0002-9440(10)64096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W, et al. GFPT2-expressing cancer-associated fibroblasts mediate metabolic reprogramming in human lung adenocarcinoma. Can. Res. 2018;78:3445–3457. doi: 10.1158/0008-5472.CAN-17-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen WJ, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat. Commun. 2014;5:1–17. doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 52.Sterne JA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 53.Bartoschek M, et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018;9:5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puram SV, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck Cancer. Cell. 2017;171:1611–1624.e1624. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishibashi M, et al. CD200-positive cancer associated fibroblasts augment the sensitivity of Epidermal Growth Factor Receptor mutation-positive lung adenocarcinomas to EGFR Tyrosine kinase inhibitors. Sci. Rep. 2017;7:46662. doi: 10.1038/srep46662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato Y, et al. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J. Biol. Chem. 2003;278:51599–51605. doi: 10.1074/jbc.M309935200. [DOI] [PubMed] [Google Scholar]
- 57.Breiteneder-Geleff S, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 1999;154:385–394. doi: 10.1016/s0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerrigan AM, et al. Podoplanin-expressing inflammatory macrophages activate murine platelets via CLEC-2. J. Thromb. Haemost. 2012;10:484–486. doi: 10.1111/j.1538-7836.2011.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neri S, et al. Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int. J. Cancer. 2015;137:784–796. doi: 10.1002/ijc.29464. [DOI] [PubMed] [Google Scholar]
- 60.Wicki A, Christofori G. The potential role of podoplanin in tumour invasion. Br. J. Cancer. 2007;96:1–5. doi: 10.1038/sj.bjc.6603518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ito S, et al. Tumor promoting effect of podoplanin-positive fibroblasts is mediated by enhanced RhoA activity. Biochem. Biophys. Res. Commun. 2012;422:194–199. doi: 10.1016/j.bbrc.2012.04.158. [DOI] [PubMed] [Google Scholar]
- 62.Hoshino A, et al. Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: podoplanin in fibroblast functions for tumor progression. Cancer Res. 2011;71:4769–4779. doi: 10.1158/0008-5472.Can-10-3228. [DOI] [PubMed] [Google Scholar]
- 63.Sakai T, et al. Link between tumor-promoting fibrous microenvironment and an immunosuppressive microenvironment in stage I lung adenocarcinoma. Lung Cancer. 2018;126:64–71. doi: 10.1016/j.lungcan.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Zohar R, McCulloch CA. Multiple roles of alpha-smooth muscle actin in mechanotransduction. Exp. Cell Res. 2006;312:205–214. doi: 10.1016/j.yexcr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu X, et al. Relationship between stromal cells and tumor spread through air spaces in lung adenocarcinoma. Thoracic Cancer. 2019;10:256–267. doi: 10.1111/1759-7714.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McShane LM, et al. Reproducibility of p53 immunohistochemistry in bladder tumors. National Cancer Institute, Bladder Tumor Marker Network. Clin. Cancer Res. 2000;6:1854–1864. [PubMed] [Google Scholar]
- 68.Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004;64:6160–6165. doi: 10.1158/0008-5472.Can-03-2224. [DOI] [PubMed] [Google Scholar]
- 69.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.González-González L, Alonso J. Periostin: a matricellular protein with multiple functions in cancer development and progression. Front. Oncol. 2018;8:225. doi: 10.3389/fonc.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong L, et al. Expression of periostin in the serum of NSCLC and its function on proliferation and migration of human lung adenocarcinoma cell line (A549) in vitro. Mol. Biol. Rep. 2010;37:2285–2293. doi: 10.1007/s11033-009-9721-1. [DOI] [PubMed] [Google Scholar]
- 73.Lee HO, et al. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. doi: 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen L, et al. Clinical significance of FAP-alpha on microvessel and lymphatic vessel density in lung squamous cell carcinoma. J. Clin. Pathol. 2018;71:721–728. doi: 10.1136/jclinpath-2017-204872. [DOI] [PubMed] [Google Scholar]
- 75.Roy J, Hettiarachchi SU, Kaake M, Mukkamala R, Low PS. Design and validation of fibroblast activation protein alpha targeted imaging and therapeutic agents. Theranostics. 2020;10:5778–5789. doi: 10.7150/thno.41409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu R, Li H, Liu L, Yu J, Ren X. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol. Ther. 2012;13:123–129. doi: 10.4161/cbt.13.3.18696. [DOI] [PubMed] [Google Scholar]
- 77.Vaquero J, et al. The IGF2/IR/IGF1R pathway in tumor cells and myofibroblasts mediates resistance to EGFR inhibition in Cholangiocarcinoma. Clin. Cancer Res. 2018;24:4282–4296. doi: 10.1158/1078-0432.ccr-17-3725. [DOI] [PubMed] [Google Scholar]
- 78.Cacheux W, et al. Interaction between IGF2-PI3K axis and cancer-associated-fibroblasts promotes anal squamous carcinogenesis. Int. J. Cancer. 2019;145:1852–1859. doi: 10.1002/ijc.32178. [DOI] [PubMed] [Google Scholar]
- 79.Garrett SM, Hsu E, Thomas JM, Pilewski JM, Feghali-Bostwick C. Insulin-like growth factor (IGF)-II- mediated fibrosis in pathogenic lung conditions. PLoS ONE. 2019;14:e0225422. doi: 10.1371/journal.pone.0225422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann. Med. 2004;36:584–595. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 81.Shimizu K, et al. Clinicopathological significance of caveolin-1 expression by cancer-associated fibroblasts in lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 2017;143:321–328. doi: 10.1007/s00432-016-2285-2. [DOI] [PubMed] [Google Scholar]
- 82.Onion D, et al. Multicomponent analysis of the tumour microenvironment reveals low CD8 T cell number, low stromal caveolin-1 and high tenascin-C and their combination as significant prognostic markers in non-small cell lung cancer. Oncotarget. 2018;9:1760–1771. doi: 10.18632/oncotarget.18880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Vizio D, et al. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8:2420–2424. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X, Sun J, Hu S. Expression of caveolin-1 in breast cancer stroma as a potential prognostic biomarker of survival and progression: a meta-analysis. Wien. Klin. Wochenschr. 2017;129:558–563. doi: 10.1007/s00508-017-1173-3. [DOI] [PubMed] [Google Scholar]
- 85.Gaggioli C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 86.Calon A, et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O'Connell JT, et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16002–16007. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bremnes RM, et al. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011;6:209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. [DOI] [PubMed] [Google Scholar]
- 89.Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol. Res. 2014;2:187–193. doi: 10.1158/2326-6066.cir-14-0002. [DOI] [PubMed] [Google Scholar]
- 90.Hanley CJ, et al. Targeting the myofibroblastic cancer-associated fibroblast phenotype through inhibition of NOX4. J. Natl. Cancer Inst. 2018;110(1):4060751. doi: 10.1093/jnci/djx4060121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.https://ClinicalTrials.gov/show/NCT02699606.
- 92.https://ClinicalTrials.gov/show/NCT02688712.
- 93.https://ClinicalTrials.gov/show/NCT01373164.
- 94.https://ClinicalTrials.gov/show/NCT03520790.
- 95.Hirsch FR, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/s0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 96.Mohammadi H, Sahai E. Mechanisms and impact of altered tumour mechanics. Nat. Cell Biol. 2018;20:766–774. doi: 10.1038/s41556-018-0131-2. [DOI] [PubMed] [Google Scholar]
- 97.Saluja R, Cheng S, Delos Santos KA, Chan KKW. Estimating hazard ratios from published Kaplan–Meier survival curves: a methods validation study. Res. Synth. Methods. 2019;10:465–475. doi: 10.1002/jrsm.1362. [DOI] [PubMed] [Google Scholar]
- 98.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-1188-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann. Intern Med. 2013;158:280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 101.Creemers A, et al. A systematic review and meta-analysis of prognostic biomarkers in resectable esophageal adenocarcinomas. Sci. Rep. 2018;8:13281. doi: 10.1038/s41598-018-31548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McGuinness, L. robvis: An R package and web application for visualising risk-of-bias assessments. https://github.com/mcguinlu/robvis. (2019). [DOI] [PubMed]
- 103.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Berlin: Springer; 2016. [Google Scholar]
- 104.Kwa MQ, Herum KM, Brakebusch C. Cancer-associated fibroblasts: how do they contribute to metastasis? Clin. Exp. Metas. 2019;36:71–86. doi: 10.1007/s10585-019-09959-0. [DOI] [PubMed] [Google Scholar]
- 105.Bu L, et al. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene. 2019;38:4887–4901. doi: 10.1038/s41388-019-0765-y. [DOI] [PubMed] [Google Scholar]
- 106.Barbazán J, Matic Vignjevic D. Cancer associated fibroblasts: is the force the path to the dark side? Curr. Opin. Cell Biol. 2019;56:71–79. doi: 10.1016/j.ceb.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 107.Dennis G, Jr, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 108.Barrowman, N. vtree: Display Information About Nested Subsets of a Data Frame. https://cran.r-project.org/web/packages/vtree/index.html (2020).
- 109.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any of the data generated in this study are available from the corresponding author on reasonable request.