Abstract
To evaluate the prognostic factors in adult cancer patients with pneumococcal bacteremia, describe episode features and the phenotypic characteristics of the isolated strains. We evaluated the episodes in patients admitted to a cancer hospital between 2009 and 2015. The outcomes were defined as 48 h mortality and mortality within 10 days after the episode. The variables evaluated were: age, sex, ethnicity, ECOG, Karnofsky score, SOFA, cancer type, metastasis, chemotherapy, radiotherapy, neutropenia, previous antibiotic therapy, community or healthcare-acquired infection, comorbidities, smoking, pneumococcal vaccination, infection site, presence of fever, polymicrobial infection, antimicrobial susceptibility, serotype and treatment. 165 episodes were detected in 161 patients. The mean age was 61.3 years; solid tumors were the most prevalent (75%). 48 h and 10-day mortality were 21% (34/161) and 43% (70/161) respectively. The 48 h mortality- associated risk factors were SOFA and polymicrobial bacteremia; 10-day mortality-associated risk factors were fever, neutropenia, ECOG 3/4, SOFA and fluoroquinolones as a protective factor. Pneumococcal bacteremia presented high mortality in cancer patients, with prognosis related to intrinsic host factors and infection episodes features. Fluoroquinolone treatment, a protective factor in 10-day mortality, has potential use for IPDs and severe community-acquired pneumonia in cancer patients.
Subject terms: Cancer, Bacterial infection
Introduction
Streptococcus pneumoniae is an important causative agent of diseases around the world. Clinical manifestations range from mild presentations to invasive pneumococcal disease (IPD). IPDs are most prevalent in extremes of age—children under 2 years of age and adults over 65 years, as well as in patients with chronic pulmonary comorbidities, and immunocompromised hosts1. Cancer represents an important risk factor for IPD. In patients with lung cancer, the risk of IPDs is 13.41 times compared to the general population, and this risk is much higher for multiple myeloma2. IPDs cause high mortality in cancer patients compared to the general population, with reports of up to 40% within 7 days and 55% within 30 days after the first day of infection3–6. The need for further studies is shown by the high prevalence of pneumococcal bacteremia in the population with cancer, the high morbimortality related to them and the scarce world literature on epidemiology, etiology and bacteremia outcomes in these patients. Most of the available literature is restricted to those with hematological neoplasms, neutropenic and hematopoietic stem cell receptors. The objectives of this study were to evaluate prognostic factors in cancer patients in episodes of pneumococcal bacteremia and to describe the epidemiological, clinical and microbiological data, the phenotypic characteristics of the collected specimens (serotypes and antimicrobial resistance) and to correlate the S. pneumoniae serotypes with available vaccine coverage.
Methods
Study design and patients
A retrospective cohort study with inclusion of patients attended between January 2009 and July 2015 in the Institute of Cancer of the State of São Paulo (ICESP), Faculty of Medicine, University of São Paulo (FMUSP), with hematological malignancies and / or solid tumors, over 18 years of age, and who had at least one episode of S. pneumoniae bacteremia during the referred period. Bacteremia was defined as a clinical condition of infection with a blood culture positive for a microorganism. Each episode of bacteremia was considered as one case; other subsequent episodes in the same patient, during the period studied, were excluded from statistical analysis.
The Research Ethics Committee of the Faculty of Medicine of the University of São Paulo issued the opinion number 1,645,166 in which it states that the study in question is observational, retrospective and without intervention in the studied population and, therefore, without the possibility of ethical conflicts. Thus, this research was exempt from the application of terms of consent by our Ethics Committee. In this article, all methods have been carried out in accordance with the guidelines, relevant regulations and ethical principles for medical research guided by the Declaration of Helsinki.
Microbiological analysis
Streptococcus pneumoniae was identified using standard methodology7 and the serotypes were identified by Quellung reaction using antisera from the Statens Serum Institute (Copenhagen, Denmark). Antimicrobial susceptibility testing was assessed via the disk diffusion method to 1 µg oxacillin (OXA) screening for susceptibility to penicillin (PEN), chloramphenicol (CLO), erythromycin (ERY), clindamycin (CLI), vancomycin (VAN), levofloxacin (LEV) sulfamethoxazole-trimethoprim (TMP/SMX), and tetracycline (TET) as recommended by the Clinical Laboratory Standards Institute guidelines8. Strains with OXA resistance (halo ≤ 19 mm) were analyzed for minimum inhibitory concentration (MIC) to PEN and ceftriaxone (CRO). Susceptibility to antimicrobial agents (susceptible, intermediate and resistant) was based on 2016 CLSI breakpoints according to clinical diagnosis8. Strains were considered susceptible to PEN for treatment of pneumococcal meningitis when MIC was ≤ 0.06 mg/L. For treatment of other pneumococcal infections, we considered MIC ≤ 2 mg/L. Regarding sensitivity to CRO, we considered MIC ≤ 0.5 mg/L for pneumococcal meningitis and MIC ≤ 1 mg/L for other pneumococcal infections7,8.
Outcomes
The evaluated outcomes were 48-h mortality and 10-day mortality after the onset of episode of bacteremia.
Definitions and variables
Neutropenia was defined as absolute neutrophil count ≤ 500 cells/mm3. Severe neutropenia was defined as neutrophils ≤ 100 cells/mm3, and prolonged neutropenia, such as that lasting 7 days.
The following variables were collected from patients’ medical records: gender, age, ethnicity, cancer diagnosis, Karnofsky, ECOG (scale of performance status), SOFA score, presence of metastases, current neutropenia, severe neutropenia, prolonged neutropenia. Chemotherapy, monoclonal antibodies, radiotherapy, corticotherapy, antibiotic therapy (ABT) in the past month (one month before IPD starts), comorbidities, smoking, and previous pneumococcal vaccine.
In addition to the demographic data, the epidemiological characterization of the episode was performed: community infection or infection related to health care (HAI—defined as manifested, clinically or microbiologically, after 48 h of hospital admission or if it occurred in an outpatient, who had undergone any invasive procedure or chemotherapy within 30 days prior to infection), site of infection, antimicrobial treatment, and polymicrobial bacteremia (blood culture positive for S. pneumoniae and another agent concomitantly in the same sample).
Statistical analysis
All episodes were included for descriptive and statistical analysis of all variables. Mortality was described in 48 h and up to 10 days according to the characteristics evaluated and the association of characteristics with outcomes was verified using chi-square test, likelihood ratio test or Fisher's exact test and comparing the quantitative characteristics according to endpoints with use of Mann–Whitney test or Student's t-test. Odds Ratio (OR) of each variable was estimated with the respective 95% confidence intervals using bivariate logistic regression.
All variables with p < 0.10 in the bivariate tests were tested in the multiple logistic regression, and the variables of the final models were selected using the stepwise method with Backward selection with input and exit by 5%.
To perform analysis the software IBM-SPSS for Windows version 20.0 was used, and for tabulation of the data the software Microsoft Excel 2003 was used. The tests were performed with significance level of 5%.
Ethics approval and consent to participate
All data were analyzed anonymously and confidentially, with approval by the Research Ethics Committee of Clínicas Hospital of the University of Sao Paulo and received approval by CONEP (National Ethics Commission), Brazil. It was a retrospective cohort study; thus, it was not possible to apply consent to participate.
Results
In the study period, 165 episodes of S. pneumoniae bacteremia were identified in 161 patients. For the analysis, 4 patients who had two or more episodes (relapses) were excluded. Thus, 161 episodes in 161 patients were included in the statistical analysis.
The demographic and clinical characteristics of the individuals are described in Table 1. Data related to the episodes of infection are described in Table 2. Mortality in 48 h was 21% (34/161), and in 10 days 43% (70/161). Regarding onco-hematological tumors, there was 13% (5/40) mortality in 48 h and 28% (11/40) in 10 days. Among solid tumors, 24% (29/121) in 48 h and 49% (59/121) in 10 days.
Table 1.
Description of the demographic characteristics evaluated in 161 cancer patients with Streptococcus pneumoniae bacteremia in cancer patients.
| Demographic Characteristic | Description, n (%) |
|---|---|
| Sex | |
| Female | 60 (37.3) |
| Male | 101 (62.7) |
| Age (years) | |
| mean ± SD | 61.7 ± 11.9 |
| median (min; max.) | 62.1 (27.2; 91.1) |
| Ethnicity | |
| Asian | 2 (1.2) |
| White | 104 (64.6) |
| Black | 27 (16.8) |
| Mixed | 28 (17.4) |
| Neoplasm, n (%) | |
| Solid tumors | 121 (75.2%) |
| Head and necka | 24 (14.9) |
| GTI | 44 (27.3) |
| Respiratory tract | 23 (14.3) |
| Liver/bile ductes | 10 (6.2) |
| Breast | 6 (3.7) |
| Pancreas | 4 (2.5) |
| Female genitourinary | 4 (2.5) |
| Bones/cartilage/SST | 4 (2.5) |
| Male genitourinary | 1 (0.6) |
| Timo/parotid | 1 (0.6) |
| Hematological neoplasms | 40 (24.8%) |
| Multiple myeloma | 20 (12.4) |
| Non-Hodgkin's lymphoma | 14 (8.7) |
| Hodgkin's lymphoma | 4 (2.5) |
| Leukemias | 2 (1.2) |
| Comorbidities | |
| Systemic arterial hypertension | 75 (46.6) |
| Diabetes mellitus | 33 (20.5) |
| Congestive heart failure | 12 (7.5) |
| COPD | 78 (48.4) |
| Smoking | 88 (54.7) |
| Splenectomy | 1 (0.6) |
| AIDS/HIV | 8 (5) |
| Metastasis | 90 (55.9) |
| Karnofsky | |
| 90/100 | 62 (40.3) |
| 70/80 | 62 (40.3) |
| < 70 | 30 (19.5) |
| ECOG | |
| 0/1 | 86 (54.8) |
| 2/3/4 | 71 (45.2) |
GTI gastrointestinal tract (esophagus, stomach, small intestine, colon, anus and rectum), SST skin and soft tissue, COPD chronic obstructive pulmonary disease.
aHead and neck, means oral cavity, larynx and pharynx.
Table 2.
Description of the characteristics evaluated in 161 episodes of bacteremia by Streptococcus pneumoniae in cancer patients.
| Episode features | Description n/N (%) |
|---|---|
| Community-acquired infection | 53 (32.9) |
| Healthcare-acquired infections (HAIs) | 108 (67.1) |
| Primary site of infection, n (%) | |
| Lung | 128 (79.5) |
| Abdomen | 16 (9.9) |
| Bloodstream infection | 7 (4.3) |
| Central nervous system | 3 (1.9) |
| Oropharynx/sinuses | 2 (1.2) |
| Skin and soft tissues | 2 (1.2) |
| Biliary tract | 2 (1.2) |
| Urinary tract | 1 (0.6) |
| SOFA (points) | |
| Mean ± SD | 5.32 ± 3.55 |
| Median (min.; max.) | 5 (0; 17) |
| Fever in the 48 h preceding the episode | 97 (60.2) |
| Antibiotics past month | 50/160 (31.3) |
| Radiotherapy past month | 28 (17.4) |
| Chemotherapy past month | 94 (58.4)* |
| Monoclonal antibodies past month | 4 (2.5) |
| Corticotherapy past month | 50 (31.1) |
| Neutropenia | |
| Neutropenia past month | 43 (26.7) |
| Current neutropenia | 38 (23.6) |
| Current severe neutropenia | 34 (21.1) |
| Prolonged neutropenia past month | 10 (6.2) |
| Febrile neutropenia | 30 (18.6) |
| Strain susceptibility | |
| PEN susceptibility MIC ≤ 2 | 140/141 (99.29) |
| PEN susceptibility MIC ≤ 0.06 | 112/141 (79.43) |
| CRO susceptibility MIC ≤ 1 | 145/145 (100) |
| CHL susceptibility MIC ≤ 0.5 | 139/145 (95.86) |
| CLI susceptibility | 142/155 (91.61) |
| CLO susceptibility | 154/155 (99.35) |
| ERY susceptibility | 136/155 (87.74) |
| LEV susceptibility | 155/155 (100) |
| SXT susceptibility | 87 /154 (56.49) |
| TET susceptibility | 125/152 (82.23) |
| VAN susceptibility | 155/155 (100) |
| Vaccine serotypes PCV13a | 63/123 (51) |
| Vaccine serotypes PPV23b | 91/123 (74) |
| Pneumococcal vaccine prior to the episode | 4/9 (45) |
| Pneumococcal vaccine at any time | 9/161 (5.6) |
| Antimicrobial treatment | |
| Penicilinsc | 90/160 (56.3) |
| Glycopeptide/linezolid | 82/160 (51.3) |
| 3rd and 4th generation cephalosporin | 72/160 (45) |
| Fluoroquinolonesd | 29/160 (18.1) |
| CLI | 7/160 (4.4) |
| SXT | 1/160 (0.6) |
| Polymicrobial bacteremia | 24 (14.9) |
| Outcomes | |
| 48 h mortality | 33 (20.5) |
| 10-day mortality | 54 (33.5) |
*Data available in only 74 of 94 episodes that mentioned prior chemotherapy.
MIC minimum inhibitory concentration, PEN penicillin, CRO ceftriaxone, CHL chloramphenicol, ERY erythromycin, CLI clindamycin, VAN vancomycin, LEV levofloxacin, SXT trimethoprim-sulfamethoxazole, TET tetracycline.
aPCV vaccine 13, or pneumococcal conjugate vaccine 13.
bPPV 23 vaccine, or pneumococcal polysaccharide vaccine 23.
cOxacillin, amoxicillin, piperacillin-tazobactam.
dMoxifloxacin, levofloxacin.
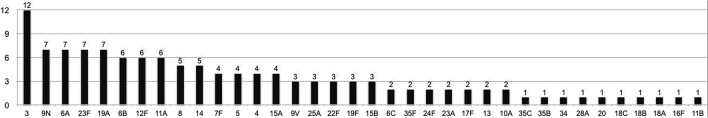
Serotype information was available for 123 strains. The most prevalent serotype was 3, composing 12 episodes (10%), followed by 9 N, 6A, 23F, 19A (7/123; 6% each), 11A, 12F, 6B (6/123; 5% each) and serotypes 8 and 14 (5/123; 4% each). The other serotypes found are summarized in Fig. 1. In 64 episodes (52%), the serotype belonged to the pneumococcal conjugate 13-valent vaccine (PCV13); additionally, other 35 episodes (28%) were due to serotypes belonged to the strains of the pneumococcal polysaccharide 23-valent vaccine 23 (PPV23). Twenty-five (20%) serotypes were non-vaccine types.
Figure 1.
Distribution of serotypes in 123 strains on episodes of Streptococcus pneumoniae bacteremia in cancer patients.
Information on pneumococcal vaccination was obtained for only 45 patients; of these, 9 had received the PPV23 and only 4 had received the vaccine prior to the bacteremia episode. In the patients with previous pneumococcal vaccination, the serotypes found was 16F (non-vaccine type—NVT), 9N, 11A and 6B (vaccine-type).
Regarding the specific treatment of pneumococcal bacteremia, 12 patients (7%) did not receive any antimicrobials. Among the remaining 149, we detected 18 different antimicrobial association schemes. Forty-eight patients (30%) received some penicillin (PEN) associated with a glycopeptide (GLYCO), 26 (16%) received some cephalosporin (CEPH) in monotherapy, 13 (8%) received CEPH + PEN + GLYCO, 10 (6%) received CEPH plus fluoroquinolone (FQ), 10 (6%) received only PEN in monotherapy, 8 (5%) received CEPH + GLYCO, 7 (4%) received CEPH + PEN, and 7 (4%) received only some FQ. Other schemes: CEPH + FQ + GLYCO (5; 3%); CEPH + Clindamycin (CLI) (3; 2%); CEPH + PEN + GLYCO + CLI; CEPH + FQ + GLYCO; PEN + FQ and FQ + GLYCO corresponded to 2 patients each (1%). CEPH + PEN + CLI; GLYCO; PEN + GLYCO + TMP/SMX and FQ + CLI corresponded to 1 patient each (1%). Therefore, 56% (90/161) of the patients received some penicillin or a penicillin derivative. Half of the patients (82/161; 51%) received GLYCO or linezolid, 45% (72/161) received 3rd and 4th generation CEPH (ceftriaxone, ceftazidime or cefepime), 18% (29/161) received antibiotics from the FQ group (levofloxacin or moxifloxacin), 4% (7/161) received CLI and one patient (1%) received TMP/SMX as treatment. Fifty patients (50/161, 31%) received prophylaxis or antimicrobial treatment in the 30 days prior to the episode of pneumococcal bacteremia. Only antimicrobial regimen introduced specifically to treat the pneumococcal bacteremia in question (empirical or directive use) were included in the descriptive and statistical analysis.
The results of the bivariate analysis and multiple models of variables related to mortality in 48 h and 10 days are displayed in Tables 3 and 4, respectively.
Table 3.
Factors potentially associated with 48 h mortality according to characteristics evaluated and results of bivariate and multivariate tests in 161 episodes of bacteremia caused by Streptococcus pneumoniae in cancer patients.
| Variable | Bivariate analysis/multivariate analysis | |
|---|---|---|
| OR (95% CI) | p value | |
| Sex | 0.183 | |
| Female | 1 | |
| Male | 1.77 (0.76–4.11) | |
| Age (years) | 0.98 (0.95–1.01) | 0.122** |
| Ethnicity | 0.061# | |
| Asian | & | |
| White | 0.71 (0.27–1.91) | |
| Black | 0.52 | |
| Mixed | 1 | |
| Comorbidities | ||
| Systemic arterial hypertension | 0.692 (0.317–1.51) | 0.353 |
| Diabetes mellitus | 0.094 (0.012–0.71) | 0.005 |
| Congestive heart failure | & | 0.128* |
| COPD | 0.737 (0.34–1.60) | 0.437 |
| Smoking | 0.628 (0.291–1.36) | 0.234 |
| Splenectomy | & | > 0.999* |
| AIDS/HIV | 1.312 (0.252–6.82) | 0.668* |
| Neoplasm | 0.870# | |
| Solid tumors | ||
| Head and necka | 1 | |
| GTI | 0.67 (0.22–1.98) | |
| Respiratory tract | 0.3 (0.07–1.32) | |
| Liver/biliary ducts | 0.5 (0.09–2.93) | |
| Breasts | 0.4 (0.04–4.02) | |
| Pancreas | 0.67 (0.06–7.48) | |
| Female genitourinary | 0.67 | |
| Bones/cartilage/SST | 0.67 (0.06–7.48) | |
| Male genitourinary | & | |
| Timo/parotid | & | |
| Hematologic neoplasms | ||
| Multiple myeloma | 0.22 (0.04–1.20) | |
| Non-Hodgkin's lymphoma | 0.33 (0.06–1.86) | |
| Hodgkin's lymphoma | 0.67 (0.06–7.48) | |
| Leukemias | & | |
| Karnofsky | 0.116 | |
| 90/100 | 1 | |
| < 90 | 1.96 (0.84–4.59) | |
| ECOG | 0.357 | |
| 0/1/2 | 1 | |
| 3/4 | 1.49 (0.64–3.50) | |
| Presence of metastasis | 1.496 (0.68–3.30) | 0.315 |
| Radiotherapy past month | 1.73 (0.68–4.37) | 0.244 |
| Chemotherapy past month | 1.56 (0.70–3.47) | 0.279 |
| Monoclonal antibodies past month | 1.3 (0.13–12.94) | > 0.999* |
| Corticotherapy past month | 1.14 (0.51–2.58) | 0.751 |
| Community-acquired infection | 0.86 (0.38–1.97) | 0.72 |
| Health-care infection | 1.16 (0.51–2.66) | 0.72 |
| Primary site of infection | 0.216# | |
| Lung | 1 | |
| Abdomen | 0.45 (0.1–2.08) | |
| Bloodstream infection | & | |
| Central nervous system | & | |
| Oropharynx/facial sinuses | & | |
| SST | & | |
| Biliary tract | & | |
| Urinary tract | & | |
| Fever in the 48 h preceding the episode | 0.64 (0.30–1.38) | 0.25 |
| Neutropenia | ||
| Neutropenia past month | 4.94 (2.19–11.14) | < 0.001 |
| Current neutropenia | 5.41 (2.37–12.39) | < 0.001 |
| Severe neutropenia past month | 4.54 (1.99–10.32) | < 0.001 |
| Current severe neutropenia | 4.78 (2.06–11.08) | < 0.001 |
| Prolonged neutropenia past month | 1.73 (0.42–7.08) | 0.429* |
| Febrile neutropenia | 2.87 (1.20–6.86) | 0.015 |
| Antibiotics last month | 0.83 (0.35–1.95) | 0.67 |
| SOFA | 1.4/1.37 (1.20–1.57)*** | < 0.001£/ < 0.001*** |
| Polymicrobial bacteremia | 5.52 (2.19–13.94)/3.78 (1.26–11.32) *** | < 0.001*/0.018 *** |
| Strain susceptibility | ||
| MIC PEN | 0.57 (0.18—1.86) | 0.771£ |
| MIC CRO | 0.87 (0.13–5.91) | 0.856£ |
| PEN susceptibility (MIC ≤ 2) | & | > 0.999* |
| CRO susceptibility (MIC ≤ 1) | & | & |
| CLI susceptibility | 1.36 (0.27–6.50) | > 0.999* |
| CHL susceptibility | & | 0.200* |
| ERY susceptibility | 2.3 (0.50–10.55) | 0.368* |
| LEV susceptibility | & | & |
| SXT susceptibility | 1.99 (0.84–4.68)) | 0.113 |
| TET susceptibility | 2.32 (0.65–8.27) | 0.184 |
| VAN susceptibility | & | & |
| Vaccination features | ||
| Vaccine serotype VCV 13b | 2.64 (1.00–6.99) | & |
| Vaccine serotype VPV 23c | 1.32 (0.45–3.9) | 0.046 |
| Pneumococcal vaccine before the episode | & | 0.618 |
| Pneumococcal vaccine in any time | & | > 0.999* |
| Antimicrobial treatment (ABT) | ||
| Instituted in the first 24 h | 0.47 (0.162–1.36) | 0.212* |
| 3rd and 4th generation cephalosporin | 0.38 (0.164–0.88) | 0.022 |
| Penicillind | 0.92 (0.424–1.98) | 0.825 |
| Fluoroquinolonese | 0.11 (0.014–0.85) | 0.012 |
| Glycopeptide/linezolid | 0.75 (0.346–1.61) | 0.455 |
| SMX/TMP | & | > 0.999* |
| Clindamycin | 1.57 (2.19–13.94) | 0.634* |
Chi-square test; *Fisher exact test; #Likelihood ratio test; **T-Student Test; £Mann–Whitney test; &Cannot estimate; ***Multiple logistic regression.
COPD chronic obstructive pulmonary disease, GTI gastrointestinal tract (esophagus, stomach, small intestine, colon, anus and rectum), SST skin and soft tissue, PEN peniciin, CRO ceftriaxone, CLO chloramphenicol, ERY erythromycin, CLI clindamycin, VAN vancomycin, LEV levofloxacin, SXT sulfamethoxazole- trimethoprim, TET tetracycline.
aHead and neck, oral cavity, larynx and pharynx.
bVaccine VCV 13. or pneumococcal conjugate vaccine 13.
cVaccine VPV 23. or pneumococcal polysaccharide vaccine 23.
dOxacillin, amoxicillin, piperacillin-tazobactam.
eMoxifloxacin, levofloxacin. MIC minimum inhibitory concentration.
Table 4.
Factors potentially associated with 10-day mortality according to characteristics evaluated and results of bivariate and multivariate tests in 161 episodes of bacteremia caused by Streptococcus pneumoniae in cancer patients.
| Variable | Bivariate analysis/multivariate analysis | |
|---|---|---|
| OR (95% CI) | p value | |
| Sex | 0.034 | |
| Female | 1 | |
| Male | 2.155 (1.05–4.42) | |
| Age (years) | 1.007 (0.98–1.04) | 0.643** |
| Ethnicity | 0.159# | |
| Asian | & | |
| White | 1.118 (0.46–2.72) | |
| Black | 0.739 (0.23–2.38) | |
| Mixed | 1 | |
| Comorbidities | ||
| Systemic arterial hypertension | 0.785 (0.41–1.52) | 0.471 |
| Diabetes mellitus | 0.37 (0.14–1.52) | 0.036 |
| Congestive heart failure | 0.373 (0.08–1.77) | 0.340* |
| COPD | 0.878 (0.46–1.69) | 0.698 |
| Smoking | 0.754 (0.39–1.45) | 0.399 |
| Splenectomy | & | > 0.999* |
| AIDS/HIV | 1.2 (0.28–5.22) | > 0.999* |
| Neoplasm | 0.056# | |
| Solid tumors | ||
| Head and necka | 1 | |
| GTI | 1.079 (0.4–2.93) | |
| Respiratory tract | 0.517 (0.16–1.71) | |
| Liver/bile ducts | 0.295 (0.05–1.69) | |
| Breasts | 0.591 (0.09–3.86) | |
| Pancreas | 1.182 (0.14–9.83) | |
| Female genitourinary | 0.394 (0.04 -4.35) | |
| Bones/cartilage/SST | 0.394 (0.04–4.35) | |
| Male genitourinary | & | |
| Timo/parotid | & | |
| Hematologic neoplasms | ||
| Multiple myeloma | 0.131 (0.03–0.70) | |
| Non-Hodgkin's lymphoma | 0.197 (0.04–1.08) | |
| Hodgkin's lymphoma | 0.394 (0.04–0.82) | |
| Leukemias | & | |
| Karnofsky | 0.053 | |
| 90/100 | 1 | |
| < 90 | 2.014 (0.98–4.12) | |
| ECOG | 0.032 | |
| 0/1/2 | 1 | 0.937 |
| 3/4 | 2.25 (1.06–4.77)/5.93 (2.04–17.22) *** | 0.937/0.001*** |
| Presence of metastases | 2.218 (1.11–4.41) | 0.022 |
| Radiotherapy past month | 1.354 (0.58–3.14) | 0.479 |
| Chemotherapy past month | 0.941 (0.49–1.83) | 0.858 |
| Monoclonal antibodies past month | 6.235 (0.63–61.43) | 0.110* |
| Corticotherapy past month | 1.331 (0.66–2.67) | 0.421 |
| Community-acquired infection | 1.029 (0.51–2.06) | 0.72 |
| Health-care infection | 0.972 (0.49–1.95) | 0.72 |
| Primary site infection | 0.018# | |
| Lung | 1 | |
| Abdomen | 0.372 (0.10–1.37) | |
| Bloodstream infection | & | |
| Central nervous system | & | |
| Oropharynx/facial sinuses | & | |
| SST | 1.612 (0.1–26.37) | |
| Biliary ducts | & | |
| Urinary tract | & | |
| Fever in the 48 h preceding the episode | 0.42 (0.21–0.82)/0.351 (0.14–0.88)*** | 0.01/(0.026)*** |
| Neutropenia | ||
| Neutropenia past month | 3.227 (1.56–6.67) | 0.001 |
| Current neutropenia | 3.91 (1.83–8.36)/4.006 (1.38–11.65)*** | < 0.001/0.011*** |
| Severe neutropenia past month | 3.369 (1.59–7.16) | 0.001 |
| Current severe neutropenia | 3.908 (1.78–8.59) | < 0.001 |
| Prolonged neutropenia past month | 1.347 (0.36–4.99) | 0.733* |
| Febrile neutropenia 48 h preceding the episode | 2.359 (1.05–5.29) | 0.034 |
| Antibiotics last month | 0.81 (0.40–1.67) | 0.571 |
| SOFA | 1.4 (1.24–1.60)/1.407 (1.2–1.66)*** | < 0.001£/ < 0.001*** |
| Strain susceptibility | & | 0.335* |
| MIC PEN | 1.16 (0.62–2.19) | 0.904£ |
| MIC CRO | 1.31 (0.27–6.23) | 0.369£ |
| PEN susceptibility (MIC ≤ 2) | & | 0.333* |
| CRO susceptibility (MIC ≤ 1) | & | & |
| CLI susceptibility | 1.089 (0.32–3.73) | > 0.999* |
| CHL susceptibility | & | 0.329* |
| ERY susceptibility | 1.98 (0.62–6.31) | 0.241 |
| LEV susceptibility | & | & |
| SXT susceptibility | 1.345 (0.68–2.68) | 0.398 |
| TET susceptibility | 2.017 (0.76–5.35) | 0.153 |
| VAN susceptibility | & | & |
| Vaccination | ||
| Vaccine serotype VCV 13b | 2.72 (1.21–6.14) | 0.014 |
| Vaccine serotype VPV 23c | 0.915 (0.38–2.2) | 0.842 |
| Pneumococcal vaccine before the episode | & | 0.539* |
| Pneumococcal vaccine in any time | & | & |
| Antimicrobial treatment | ||
| Instituted in the first 24 h | 0.359 (0.13–0.97) | 0.038 |
| 3rd and 4th generation cephalosporin | 0.331 (0.16–0.67) | 0.002 |
| Penicilinsd | 1.708 (0.87–63.36) | 0.119 |
| Fluoroquinolonese | 0.053 (0.01–0.40)/0.083 (0.01–0.71)*** | < 0.001/0.023*** |
| Glycopeptide/linezolid | 1.454 (0.75–2.81) | 0.266 |
| SMX/TMP | & | > 0.999* |
| Clindamycin | 0.777 (0.15–4.14) | > 0.999* |
| Polymicrobial bacteremia | 3.395 (1.39–8.28) | 0.005 |
Chi-square test; *Fisher exact test; #likelihood ratio test; **T-Student Test; £Mann–Whitney Test; &Cannot estimate; ***Multiple logistic regression.
COPD chronic obstructive pulmonary disease, GTI gastrointestinal tract (esophagus, stomach, small intestine, colon, anus and rectum), SST skin and soft tissue, PEN penicillin, CRO ceftriaxone, CLO chloramphenicol, ERY erythromycin, CLI clindamycin, VAN vancomycin, LEV levofloxacin, SXT trimethoprim-: sulfamethoxazole, TET tetracycline.
aHead and neck, oral cavity, larynx and pharynx.
bVaccine VCV 13, or pneumococcal conjugate vaccine 13.
cVaccine VPV 23. or pneumococcal polysaccharide vaccine 23.
dOxacillin, amoxicillin, piperacillin-tazobactam.
eMoxifloxacin, levofloxacin.MIC Minimum inhibitory concentration.
Table 3 shows that, together, only SOFA and polymicrobial bacteremia were statistically associated with mortality in 48 h (p < 0.05), the increase of one unit in SOFA resulted in a 37% increase in the chance of mortality in 48 h and the chance of mortality in 48 h in patients with polymicrobial bacteremia was 3.78 times the chance of in patients without polymicrobial bacteremia.
Table 4 shows that, together, patients who had fever within the 48 h prior to the data of positive blood culture had a 65% lower chance of mortality within 10 days. Patients with current neutropenia had a chance of mortality within 10 days 4.01 times higher than the patients without current neutropenia. Patients with ECOG 3/4 presented 5.93 times the chance of mortality in up to 10 days compared to patients with ECOG 0/1/2. The increase of one unit in SOFA resulted in an increase of 59% chance of mortality up to 10 days. The chance of mortality up to 10 days in patients who used quinolones was 92% less.
Discussion
Our sample consisted of 161 patients, 101 men (62.7%), with a mean age of 61.3 years. In fact, male gender and age over 65 years are risk factors reported for IPD, including in recurrent episodes9.
The most prevalent primary site of infection was the lung (128/161, 79.5%), followed by the abdomen (16/161, 9.9%). Pneumonia with lung as the primary site of infection was the most common clinical diagnosis among IPD in cancer patients, including pneumococcal bacteremia10,11.
In the present study, the four patients who presented two or more episodes had multiple myeloma (MM). The strong association of recurrent IPD and MM has been reported previously, and can be explained by intrinsic defects in humoral immunity and complement system, with opsonization dysfunction, an essential process in the granulocytic interaction with pneumococcus2.
The independent factors associated with mortality in 48 h in the multivariate analysis were the presence of polymicrobial bacteremia (PB) and SOFA. The 10-day mortality risk factors were current neutropenia, ECOG 3 or 4 and higher SOFA. The 10-day mortality protective factors were treatment with fluoroquinolones (FQ) and presence of fever.
Polymicrobial bacteremia has been associated with worse prognosis and unfavorable outcomes in several previous studies. In cancer patients, polymicrobial etiology significantly increases the risk of 30-day mortality and is associated with a higher propensity for severe sepsis and septic shock. This is an independent factor for worsening overall survival12,13.
The SOFA score is a condition that reflects organic failure in the context of sepsis14, being a consolidated predictor of prognosis in patients with cancer and independent factor for mortality. Each point earned in SOFA increased the chance of death by 17% during hospitalization in cancer patients in a previous study which 86% patients had solid tumors13. In the same study, ECOG was an independent predictor of in-hospital mortality when the value was 2, 3 or 4, which is consistent with other studies15,16 and with our finding in this present study, where ECOG 3 or 4 was a predictor of mortality in 10 days.
The prevalence of neutropenia in this study was 23.6%, consistent with other studies of pneumococcal bacteremia in oncology,18% to 26%17, and was a risk factor for 10-day mortality in the multivariate analysis. Numerous studies point to the presence of neutropenia as an independent and significant risk factor for mortality due to infection in cancer patients, mostly when dealing with pneumococcal bacteremia4,18.
Among the findings, treatment with FQ was a significant protective factor for 10-day mortality in the multivariate analysis, with a 92% lower chance of death. Previous studies have already shown consistent evidence about the effectiveness of the FQ in community-acquired pneumonia (CAP): a meta-analysis suggested that moxifloxacin alone had a higher pathogen eradication rate than a β-lactam (BL)-based combination therapy19, and also others studies showed that the use of FQ was associated with higher treatment success and better clinical outcomes compared to the established combination therapy of β-lactam antibiotic and a macrolide (ML) or without ML in general population20.
When bacteremic pneumonia in cancer patients arises, a population cohort showed that treatment with FQ or BL plus ML was a protective factor for 30-day mortality compared to BL monotherapy5. Similarly, in a retrospective chart review FQ (moxifloxacin) versus combination therapy in patients with severe CAP in intensive care unit (ICU) showed no difference in 30-day survival.
Thus, our findings are unprecedented and promising in favor of the use in monotherapy of FQ in IPD, which include pneumococcal bacteremia, bacteremic pneumonias and severe CAP.
The presence of fever in the 48 h before the onset of the episode was also a protective factor for 10-day mortality. Fever probably served as an alert, which led the patient to seek care earlier and, consequently, to receive antimicrobial treatment earlier, which is a decisive independent factor in sepsis-related mortality14.
Regarding antimicrobial susceptibility, our study presented a better susceptibility profile than other similar studies. Of all 141 strains tested, susceptibility to PEN was 79% (MIC ≤ 0.06 µg/mL) and 99% (MIC ≤ 2 µg/mL) which is higher than reported in the literature (74%, MIC ≤ 0.06 ug/mL21), including among cancer patients (78% MIC ≤ 0.1 ug/mL22 and 86% MIC ≤ 2 ug/mL23. In our study, no strains with high resistance to PEN were identified, and the MIC range was between 0.006 ug/mL and 4 ug/mL (median 0.016 ug/mL).
Of the 145 strains tested, the susceptibility to CRO was 100% (MIC ≤ 1 µg/mL, 4%? and MIC ≤ 0.5 µg/mL 96%). The median MIC was 0.016 µg/mL, with a range between 0.001 and 1 µg/mL. n other studies, the susceptibility (MIC ≤ 1 ug/mL) varied from 98%23 to 99%21.
Overall, pneumococcal susceptibility to other antimicrobials remained similar to those found in the literature, except small decrease of susceptibility to CLI and ERY21,23. It was not possible to statistically estimate the protective effect of pneumococcal vaccination due to the small number of patients known to be vaccinated (n = 9).
Among the 123 strains serotyped, 64 (52%) corresponded to those found in the PCV13 vaccine, and other 35 (28%) to the PPV23. 25 serotypes (20%) belonged to nonvaccine types, assuming that, hypothetically, 48 h and 10-day mortality would be potentially preventable through vaccination in 80% of the episodes of pneumococcal bacteremia.
The most prevalent serotypes found were serotype 3 (9.76%), followed by 19A, 23F, 6A, 9 N (5,69%) and 11A, 12F, 6B (4,88%). Of these, 3, 9 N and 6A are highly virulent, along with serotypes 19F and 6B. In particular, serotype 3 is described as being strongly encapsulated when compared to other serotypes24,25, which gives it a high virulence. In addition, types 19A and 23F have a high relative risk previously reported24.
In the present study, 48 h and 10-day mortality were, respectively, 20.5% and 33.5%. In similar studies, mortality in 48 h averaged 15% with a median of 13%18,26–28, and mortality at 30 days averaged 23.08%, with a median of 21.26%2,18,26,27,29,30. In previous multi-center studies21,31, there was a higher mortality rate from IPDs in general in Brazil and Latin America compared to other countries, which may explain, in part, the high mortality in this study. In addition, other factors possibly implicated would be infection with highly virulent serotypes23,24, especially serotype 3, the higher incidence of polymicrobial bacteremia, and the high incidence of neutropenia12.
The limitations of our study were its retrospective design, and the fact that it is a single center study with data obtained from medical records, which may have limited information available. There was no stratification of mortality by age and there was a shortage of vaccination history coverage information to estimate its protection role.
On the other hand, the study presents some aspects that make it unprecedented. Our case series was extensive (n = 161), and it is the only Brazilian study, so far, among the studies that evaluated prognostic factors in pneumococcal bacteremia in adult cancer patients. Due to the wide range of variables included, we could conclude several prognostic factors, confirm some previously known ones and include new hypotheses, such as the likely protective effect of FQ in the treatment of bacteremic pneumococcal pneumonia and severe CAP in cancer patients. This population is rarely targeted by clinical trials. Serotyping was performed in most cases (124/161), which provided important information about the prevalence, in addition to estimating the protective effect of the vaccine.
In conclusion, our study corroborated the high mortality associated with pneumococcal bacteremia in cancer patients. Factors associated with a worse prognosis were those intrinsically related to the host and to the episode itself. Despite the observation of high mortality rates in this study, the resistance rate to penicillin was lower than what previously was described in published series. The vast majority of isolated S. pneumoniae strains are included in the available vaccines, indicating the need for investment and optimization of vaccine focused prevention in cancer patients. FQ treatment as a protective factor in 10-day mortality shows its potential use for IPDs and severe CAP in cancer patients. Prospective studies should be conducted to confirm this finding in the future.
Abbreviations
- MIC
Minimal inhibitory concentration
- ECOG
Eastern Cooperative Oncology Group
- SOFA
Sequential Organ Failure Assessment score
Author contributions
N.S.F. assembled the data and drafted the manuscript. F.R., M.C.C.B. and S.C.G.A. assisted in MIC and serotype determination; F.M.T., P.R.B. and K.Y.I. assisted with the draft of manuscript; and E.A. designed, supervised, and assessed the study and drafted the manuscript. All authors have read, contributed, and approved the final manuscript.
Funding
Internal funding from the University of Sao Paulo, Brazil supported this study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in the spelling of the author Karim Yaqub Ibrahim which was incorrectly given as K. I. Ibrahim. As a result, the Author Contributions section was also adjusted.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/16/2021
A Correction to this paper has been published: 10.1038/s41598-021-96034-y
References
- 1.Anatoliotaki M, Valatas V, Mantadakis E, Apostolakou H, Mavroudis D, Georgoulias V, et al. Bloodstream infections in patients with solid tumors: Associated factors, microbial spectrum and outcome. Infection. 2004;32(2):65–71. doi: 10.1007/s15010-004-3049-5. [DOI] [PubMed] [Google Scholar]
- 2.Wong A, Marrie TJ, Garg S, Kellner JD, Tyrrell GJ, Group S. Increased risk of invasive pneumococcal disease in haematological and solid-organ malignancies. Epidemiol. Infect. 2010;138(12):1804–1810. doi: 10.1017/S0950268810000919. [DOI] [PubMed] [Google Scholar]
- 3.Richard V, Meunier F, Van der Auwera P, Dejace P, Daneau D, Klastersky J. Pneumococcal bacteremia in cancer patients. Eur. J. Epidemiol. 1988;4(2):242–245. doi: 10.1007/BF00144760. [DOI] [PubMed] [Google Scholar]
- 4.Carratala J, Roson B, Fernandez-Sevilla A, Alcaide F, Gudiol F. Bacteremic pneumonia in neutropenic patients with cancer: Causes, empirical antibiotic therapy, and outcome. Arch. Intern. Med. 1998;158(8):868–872. doi: 10.1001/archinte.158.8.868. [DOI] [PubMed] [Google Scholar]
- 5.Naucler P, Darenberg J, Morfeldt E, Ortqvist A, Henriques NB. Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax. 2013;68(6):571–579. doi: 10.1136/thoraxjnl-2012-203106. [DOI] [PubMed] [Google Scholar]
- 6.Shigayeva A, Rudnick W, Green K, Chen DK, Demczuk W, Gold WL, et al. Invasive pneumococcal disease among immunocompromised persons: Implications for vaccination programs. Clin. Infect. Dis. 2016;62(2):139–147. doi: 10.1093/cid/civ803. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Laboratory methods for diagnosis of meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae and Haemophilus influenzae (WHO Manual, WHO/IVB.11.09, Geneva, 2011). https://www.cdc.gov/meningitis/lab-manual/full-manual.pdf.
- 8.Perfomance standards for antimicrobial susceptibility testing, CLSI M100 (2016).
- 9.Mufson MA, Hao JB, Stanek RJ, Norton NB. Clinical features of patients with recurrent invasive Streptococcus pneumoniae disease. Am. J. Med. Sci. 2012;343(4):303–309. doi: 10.1097/MAJ.0b013e31822d9860. [DOI] [PubMed] [Google Scholar]
- 10.Marin M, Gudiol C, Garcia-Vidal C, Ardanuy C, Carratala J. Bloodstream infections in patients with solid tumors: Epidemiology, antibiotic therapy, and outcomes in 528 episodes in a single cancer center. Medicine (Baltimore). 2014;93(3):143–149. doi: 10.1097/MD.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backhaus E, Berg S, Andersson R, Ockborn G, Malmstrom P, Dahl M, et al. Epidemiology of invasive pneumococcal infections: Manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect. Dis. 2016;16:367. doi: 10.1186/s12879-016-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumbarello M, Spanu T, Caira M, Trecarichi EM, Laurenti L, Montuori E, et al. Factors associated with mortality in bacteremic patients with hematologic malignancies. Diagn. Microbiol. Infect. Dis. 2009;64(3):320–326. doi: 10.1016/j.diagmicrobio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Elting LS, Bodey GP, Fainstein V. Polymicrobial septicemia in the cancer patient. Medicine (Baltimore). 1986;65(4):218–225. doi: 10.1097/00005792-198607000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Vincent, J.L., Moreno, R., Takala, J., Willatts, S., Demendonca, A., Bruining, H., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 22(7), 707–710 (1996). [DOI] [PubMed]
- 15.Torres VB, Vassalo J, Silva UV, Caruso P, Torelly AP, Silva E, et al. Outcomes in critically ill patients with cancer-related complications. PLoS ONE. 2016;11(10):e0164537. doi: 10.1371/journal.pone.0164537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosolem MM, Rabello LS, Lisboa T, Caruso P, Costa RT, Leal JV, et al. Critically ill patients with cancer and sepsis: Clinical course and prognostic factors. J. Crit. Care. 2012;27(3):301–307. doi: 10.1016/j.jcrc.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Kumashi P, Girgawy E, Tarrand JJ, Rolston KV, Raad II, Safdar A. Streptococcus pneumoniae bacteremia in patients with cancer: Disease characteristics and outcomes in the era of escalating drug resistance (1998–2002) Medicine (Baltimore). 2005;84(5):303–312. doi: 10.1097/01.md.0000180045.26909.29. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Vidal C, Ardanuy C, Gudiol C, Cuervo G, Calatayud L, Bodro M, et al. Clinical and microbiological epidemiology of Streptococcus pneumoniae bacteremia in cancer patients. J. Infect. 2012;65(6):521–527. doi: 10.1016/j.jinf.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Yuan X, Liang BB, Wang R, Liu YN, Sun CG, Cai Y, et al. Treatment of community-acquired pneumonia with moxifloxacin: A meta-analysis of randomized controlled trials. J. Chemother. 2012;24(5):257–267. doi: 10.1179/1973947812Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 20.Vardakas KZ, Siempos II, Grammatikos A, Athanassa Z, Korbila IP, Falagas ME. Respiratory fluoroquinolones for the treatment of community-acquired pneumonia: A meta-analysis of randomized controlled trials. CMAJ. 2008;179(12):1269–1277. doi: 10.1503/cmaj.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahmel T, Asmussen S, Karlik J, Steinmann J, Adamzik M, Peters J. Moxifloxacin monotherapy versus combination therapy in patients with severe community-acquired pneumonia evoked ARDS. BMC Anesthesiol. 2017;17(1):78. doi: 10.1186/s12871-017-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castanheira M, Gales AC, Pignatari AC, Jones RN, Sader HS. Changing antimicrobial susceptibility patterns among Streptococcus pneumoniae and Haemophilus influenzae from Brazil: Report from the SENTRY Antimicrobial Surveillance Program (1998–2004) Microb. Drug Resist. 2006;12(2):91–98. doi: 10.1089/mdr.2006.12.91. [DOI] [PubMed] [Google Scholar]
- 23.Levin AS, Sessegolo JF, Teixeira LM, Barone AA. Factors associated with penicillin-nonsusceptible pneumococcal infections in Brazil. Braz. J. Med. Biol. Res. 2003;36(6):807–813. doi: 10.1590/S0100-879X2003000600017. [DOI] [PubMed] [Google Scholar]
- 24.Cardoso NT, Santos BA, Barbosa AV, Superti SV, Teixeira LM, Neves FP. Serotypes, antimicrobial resistance and genotypes of Streptococcus pneumoniae associated with infections in cancer patients in Brazil. Diagn. Microbiol. Infect. Dis. 2017;87(3):281–285. doi: 10.1016/j.diagmicrobio.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Ruckinger S, et al. Association of serotype with risk of death due to pneumococcal pneumonia: A meta-analysis. Clin. Infect. Dis. 2010;51(6):692–699. doi: 10.1086/655828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grabenstein JD, Musey LK. Differences in serious clinical outcomes of infection caused by specific pneumococcal serotypes among adults. Vaccine. 2014;32(21):2399–2405. doi: 10.1016/j.vaccine.2014.02.096. [DOI] [PubMed] [Google Scholar]
- 27.Zarco-Marquez S, Volkow-Fernandez P, Velazquez-Acosta C, Echaniz-Aviles G, Carnalla-Barajas MN, Soto-Nogueron A, et al. Invasive and complicated pneumococcal infection in patients with cancer. Rev. Invest. Clin. 2016;68(5):221–228. [PubMed] [Google Scholar]
- 28.Gudiol C, Royo-Cebrecos C, Laporte J, Ardanuy C, Garcia-Vidal C, Antonio M, et al. Clinical features, aetiology and outcome of bacteraemic pneumonia in neutropenic cancer patients. Respirology. 2016;21(8):1411–1418. doi: 10.1111/resp.12848. [DOI] [PubMed] [Google Scholar]
- 29.Imran MN, Leng PH, Yang S, Kurup A, Eng P. Early predictors of mortality in pneumococcal bacteraemia. Ann. Acad. Med. Singap. 2005;34(7):426–431. [PubMed] [Google Scholar]
- 30.Shelburne SA, 3rd, Tarrand J, Rolston KV. Review of streptococcal bloodstream infections at a comprehensive cancer care center, 2000–2011. J Infect. 2013;66(2):136–146. doi: 10.1016/j.jinf.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Chou MY, Brown AE, Blevins A, Armstrong D. Severe pneumococcal infection in patients with neoplastic disease. Cancer. 1983;51(8):1546–1550. doi: 10.1002/1097-0142(19830415)51:8<1546::AID-CNCR2820510832>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997–2002) Diagn. Microbiol. Infect. Dis. 2004;50(1):59–69. doi: 10.1016/j.diagmicrobio.2004.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.