Abstract
Background Oxidative stress is considered to be involved in the pathogenesis of coronary heart disease (CHD). Glutathione-S-transferase (GST) enzymes play important roles in antioxidant defenses and may influence CHD risk. The present meta-analysis was performed to investigate the link between glutathione S-transferase M1 (GSTM1) null genotype and CHD and to get a precise evaluation of interaction between GSTM1 null genotype and smoking by the case-only design.
Methods PubMed and EMBASE databases were searched through 15 December 2020 to retrieve articles. Odds ratios (ORs) were pooled using either fixed-effects or random-effects models.
Results Thirty-seven studies showed that GSTM1 null genotype was associated with risk of CHD in total population, Caucasians and Asians (for total population, OR = 1.38, 95% confidence interval (CI): 1.15, 1.65; for Caucasians, OR = 1.34, 95% CI: 1.04, 1.72; for Asians, OR = 1.40, 95% CI: 1.11, 1.77). After adjustment for heterogeneity, these relationships were still significant. After adjustment for heterogeneity, case-only analysis of 11 studies showed a positive multiplicative interaction between GSTM1 null genotype and smoking (ever smoking vs. never smoking) (OR = 1.27, 95% CI: 1.08, 1.50; I2 = 0%, P=0.553).
Conclusions The overall results indicated that GSTM1 null genotype was associated with a higher risk of CHD, and the association may be affected by smoking status. This is the first meta-analysis to prove a positive effect of the interaction between GSTM1 null genotype and smoking status on the risk of CHD. Well-designed studies are needed to investigate the possible gene–gene or gene–environment interactions.
Keywords: Coronary heart disease, Genetic polymorphism, GSTM1, Meta-analysis, Smoking
Introduction
Coronary heart disease (CHD) is the leading cause of mortality and a major cause of morbidity and disability all over the world [1,2]. CHD is an extremely multifactorial disease, which is influenced by both complex genetic and multiple environmental factors, as well as their interactions.
There is compelling evidence that cigarette smoking is one of the strong risk factors for CHD. Multiple chemicals in cigarette smoke can cause endothelial dysfunction, smooth muscle cell proliferation, generation of reactive oxygen species (ROS) and DNA damage, which can lead to atherosclerosis and, hence, CHD [3–6]. However, only a small number of smokers ultimately develop CHD. The differential susceptibility to CHD among smokers may be influenced by polymorphisms in genes encoding the metabolic enzymes, which play important roles in the detoxification of toxic chemicals generated by smoking.
The glutathione S-transferases (GSTs) are an important family of phase II isoenzymes which can detoxify electrophilic compounds generated by smoking, including toxins, DNA adducts, and carcinogens, mainly by changing them to harmless products through conjugation to glutathione [7,8]. In addition, GSTs can modulate the induction of other proteins and enzymes which are important for cellular functions, such as DNA repair [9].
Human cytosolic GST enzymes which comprise multiple isoenzymes are divided into eight separate classes: GSTM (mu), GSTP (pi), GSTT (theta), GSTA (alpha), GSTK (kappa), GSTO (omega), GSTS (sigma), and GSTZ (zeta) [10]. The Mu class of GSTs is encoded by the glutathione S-transferase M1 (GSTM1) gene, which is mapped to chromosome 1p13.3. Three alleles of the GSTM1 locus have been identified: GSTM1 null and two others (GSTM1a and GSTM1b) that differ by C→G substitution at base position 534. The C→G substitution leads to the substitution Lys→Asn at amino acid 172 [11]. Persons with homozygous deletions of the GSTM1 locus have been associated with no enzymatic functional activity and increased vulnerability to cytogenetic damage [12,13], and thus it was hypothesized to be linked with risk of CHD [14].
Our previous meta-analysis have proved that the null genotype of GSTT1 was associated with an increased risk of CHD [15]. Indeed, a great number of studies have investigated the association between GSTM1 genetic polymorphism and risk of CHD. However, results have been inconsistent [16–50], and the interaction between GSTM1 null genotype and smoking is unclear. To our knowledge, two previous meta-analyses [51,52] investigating the association between GSTM1 null genotype and CHD risk have yielded contradictory findings. One previous meta-analysis [51] reported that GSTM1 null genotype may be an independent risk factor for CHD and the other meta-analysis [52] indicated that a negative association exists between GSTM1 null genotype and CHD risk. To help clarify the inconsistent findings, we conducted a meta-analysis to investigate the association between polymorphism of GSTM1 and CHD risk. Furthermore, we performed a case-only design to get a more precise evaluation of interaction between GSTM1 null genotype and smoking on CHD risk.
Materials and methods
Search strategy and selection criteria
We searched electronic databases, including PubMed and Embase, for all articles published through 15 December 2020, which had investigated the association between GSTM1 genotype (null genotype vs. wildtype) and the risk of CHD. The terms used for searching included glutathione S-transferase, GST, GSTM1; gene, polymorphism; and coronary heart disease, CHD, myocardial infarction, MI, coronary artery disease, CAD, ischemic heart disease. References cited in retrieved articles and published review articles were also screened to identify additional publications. If there were several publications from the same study, we selected the most complete or most recent publication for meta-analyses. To minimize potential publication bias, studies without any special restriction were included.
The inclusion criteria were: (i) studies with case–control design examining the association between CHD risk and polymorphism of GSTM1; (ii) presenting original data for the calculation of odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs); (iii) clear definition of CHD. The exclusion criteria were: (i) case-only studies, animal studies, simply commentaries, case reports and review articles; (ii) studies with other genotypes of GST or other disease.
Data extraction and quality assessment
Characteristics abstracted from the articles included the name of the first author, year of publication, country, ethnicity, genotyping method, control source, number of cases, number of controls, cases null, controls null, Hardy–Weinberg equilibrium (i.e., the genotype distribution in the control population were in accordance with Hardy–Weinberg equilibrium: yes, no, not available), and adjustment covariates. When specific results were not reported, we used available tabular data to calculate them. When data were unavailable, we contacted the corresponding author by email for additional information. Different ethnicities were categorized as Caucasian, Asian, and Mixed. The bibliographic search, data extraction, and quality assessment were conducted independently by two authors, and any disagreements were resolved by consensus with a third investigator.
We assessed quality of included studies based on Newcastle–Ottawa Scale (NOS) [53]. The NOS is an 8-item instrument, and the detail of NOS grading standard is listed as follows: (i) selection, included adequate definition of patient cases, representativeness of patients cases, selection of controls, definition of controls, total score: 4; (ii) comparability, included Control for important factor or additional factor, total score: 2; (iii) exposure (case–control studies), included ascertainment of exposure (blinding), same method of ascertainment for participants, non-response rate, total score: 3. A star system of the NOS (range, 0–9 stars) has been developed for quality assessment (Supplementary Table S1). The mean value for all included studies was 7 stars.
Statistical analyses
Based on the genotype frequencies, crude ORs corresponding to 95% CI were calculated to measure the association between GSTM1 null genotype and risk of CHD. Cochran's χ2 based Q-statistic test and I2 test were performed to precisely assess possible heterogeneity, which quantified between-study heterogeneity irrespective of the number of studies [54]. If heterogeneity was considered significant at P<0.1 (Cochran's χ2 based Q-statistic test), a random-effects model (DerSimonian–Laird method) [55] was used to calculate the pooled ORs. Otherwise, the fixed-effect model [56] was conducted [57,58]. An I2 value less than 50% was considered to indicate low heterogeneity [59]. The meta-regression was performed to study the source of between-study heterogeneity [60]. The introduction of covariates for assessment of heterogeneity sources were publication year, ethnicity, sample size, and control source. If there was heterogeneity between studies, sources of heterogeneity were also investigated by stratified meta-analyses based on ethnicity (Asian, Caucasian); source of controls (population-based, hospital-based); sample size (number of cases <600 or >600). Sensitivity analysis, removing one study at a time, was also performed to evaluate the stability of the results. Besides, Galbraith plot was also conducted to spot the outlier as the possibly major source of between-study heterogeneity [61]. The outliers were considered as the possible major source of heterogeneity, and further meta-analysis after adjustment for heterogeneity was performed by excluding these studies. The potential publication bias was investigated by means of Begg's funnel plot and Egger's test [62].
To investigate the multiplicative interaction between GSTM1 null genotype and smoking (ever smoking vs. never smoking) on CHD risk, we also performed a case-only design in present meta-analyses [63,64]. All analyses were performed using Stata, version 11.0 (StataCorp, College Station, Texas). All tests were two-sided with a significance level of 0.05.
Results
Characteristics of the included studies
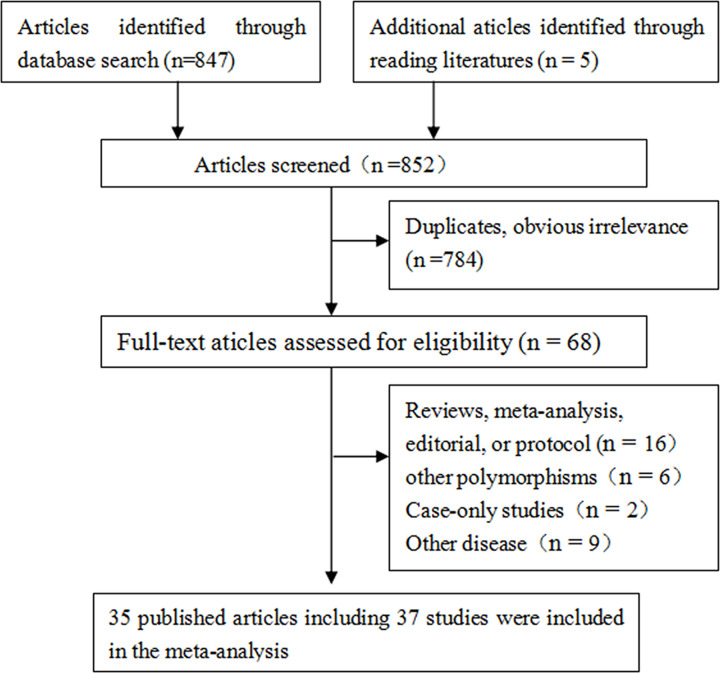
In total, 37 studies from 35 articles matching the search terms, comprising 16684 cases and 36510 controls, were retrieved from databases. A flow chart describing the exclusion/inclusion of individual articles has been presented as Figure 1. A total of 852 articles were found with our search criteria. One article contained three individual case–control studies [38] and one article was published in Chinese [36]. Table 1 showed characteristics of these 37 studies, 23 [16–23,26–29,33–35,37,38,41,44,45,48] were from Caucasian population, 13 [24,25,30,31,36,39,40,42,43,46,47,49,50] were from Asians, and 1 [32] was Mixed ethnicity. The number of cases varied from 29 to 2360, with a mean of 451, and the number of controls varied from 30 to 9099, with a mean of 988 (Table 1).
Figure 1. Flow chart depicting exclusion/inclusion of individual articles for meta-analysis.
Table 1. Characteristics of studies included in a meta-analysis of GSTM1 null genotype and CHD risk.
| First author | Year | Country | Ethnicity | Genotyping method | Control source | Number of cases | Number of controls | Cases null | Controls null | Hardy– Weinberg Equilibrium | Adjustment covariates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Evans [16] | 1996 | Saudi Arabia | Caucasian | PCR | PB | 90 | 884 | 57 | 504 | NA | NA |
| Wilson [17] | 2000 | U.K. | Caucasian | PCR | PB | 356 | 187 | 191 | 107 | NA | NA |
| Li [18] | 2000 | U.S.A. | Caucasian | PCR | PB | 400 | 890 | 178 | 354 | NA | Age, sex, race, LDL, HDL, hypertension and diabetes |
| Wang [20] | 2001 | U.S.A. | Caucasian | PCR | HB | 612 | 256 | 343 | 153 | NA | NA |
| Salama [19] | 2002 | U.S.A. | Caucasian | PCR | PB | 120 | 90 | 45 | 33 | NA | NA |
| Wilson [24] | 2003 | U.K. | Asian | PCR | PB | 170 | 203 | 70 | 107 | NA | NA |
| Palmer [23] | 2003 | U.K. | Caucasian | PCR | HB | 51 | 57 | 40 | 35 | NA | Age, smoking, duration of disease, sex, HDL, glucose, triglycerides, and blood pressure |
| Olshan [22] | 2003 | U.S.A. | Caucasian | PCR | PB | 526 | 868 | 252 | 352 | NA | Age, sex and race |
| Masetti [21] | 2003 | Italy | Caucasian | PCR | HB | 308 | 122 | 163 | 66 | NA | NA |
| Girisha [25] | 2004 | India | Asian | PCR | PB | 197 | 198 | 46 | 41 | Yes | NA |
| Tamer [26] | 2004 | Turkey | Caucasian | RT-PCR | PB | 148 | 247 | 67 | 103 | NA | NA |
| Hayek [28] | 2006 | U.K. | Caucasian | PCR | PB | 193 | 2399 | 88 | 1142 | NA | NA |
| Abu-Amero [27] | 2006 | Saudi Arabia | Caucasian | PCR | HB | 1054 | 762 | 655 | 117 | NA | Hypertension, cholesterol, obesity, smoking |
| Cornelis [29] | 2007 | Canada | Caucasian | PCR | PB | 2042 | 2042 | 980 | 531 | NA | Age, sex, area, smoking, waist-to-hip ratio, income, physical activity, history of diabetes and hypertension, intake of alcohol, and energy adjusted saturated fat and folate |
| Kim [30] | 2008 | Korea | Asian | PCR | HB | 356 | 336 | 198 | 191 | NA | Age, sex, hypertension, DM, BMI and lipid profile |
| Wang [31] | 2008 | China | Asian | PCR | HB | 277 | 277 | 89 | 59 | Yes | Diabetes, hypertension, smoking status |
| Martin [34] | 2009 | U.S.A. | Caucasian | PCR | PB | 67 | 63 | 41 | 19 | NA | NA |
| Manfredi [33] | 2009 | Italy | Caucasian | PCR | HB | 184 | 47 | 108 | 18 | NA | NA |
| Maciel [32] | 2009 | Brazil | Mixed | PCR | PB | 869 | 1573 | 557 | 789 | NA | NA |
| Ramprasath [39] | 2011 | India | Asian | PCR | HB | 290 | 492 | 128 | 150 | NA | NA |
| Bazo [35] | 2011 | Brazil | Caucasian | PCR | HB | 297 | 100 | 160 | 44 | NA | NA |
| Singh [40] | 2011 | India | Asian | PCR | PB | 230 | 300 | 56 | 65 | NA | Age, sex, BMI, smoking, alcohol, food habit, lipid profile and fasting glucose |
| Nomani [37] | 2011 | Iran | Caucasian | PCR | HB | 209 | 108 | 100 | 57 | NA | NA |
| Norskov CCHS [38] | 2011 | Denmark | Caucasian | RT-PCR | PB | 1769 | 8425 | 921 | 4414 | Yes | NA |
| Norskov CGPS [38] | 2011 | Denmark | Caucasian | RT-PCR | PB | 801 | 9099 | 411 | 4738 | Yes | NA |
| Norskov CIDHS [38] | 2011 | Denmark | Caucasian | RT-PCR | PB | 2360 | 4160 | 1203 | 2210 | NA | NA |
| Zhang [36] | 2011 | China | Asian | PCR | PB | 255 | 145 | 120 | 46 | NA | NA |
| Taspinar [44] | 2012 | Turkey | Caucasian | PCR | PB | 122 | 142 | 51 | 66 | NA | Age, gender, family history, smoking status, and diabetes |
| Kariz [41] | 2012 | Slovenia | Caucasian | PCR | HB | 206 | 257 | 64 | 91 | NA | Age, gender, diabetes, BMI, smoking, lipid parameters |
| Lakshmi [42] | 2012 | India | Asian | PCR | PB | 350 | 282 | 68 | 54 | Yes | Age, BMI, gender, diabetes, family history of CAD |
| Phulukdaree [43] | 2012 | South Africa | Asian | PCR | PB | 102 | 100 | 37 | 18 | Yes | NA |
| Cora [45] | 2013 | Turkey | Caucasian | PCR | PB | 324 | 296 | 182 | 143 | NA | Age, sex, smoking, diabetes, hypertension, family history, lipid profile |
| Yeh [46] | 2013 | Taiwan | Asian | PCR | HB | 458 | 209 | 253 | 121 | Yes | Age, sex, cigarette smoking, alcohol use, diabetes mellitus, and levels of serum total cholesterol and high-density lipoprotein cholesterol |
| Kadıoğlu [48] | 2016 | Turkey | Caucasian | PCR-RFLP | PB | 29 | 30 | 17 | 14 | Yes | Age, gender, hypertension and smoking habit |
| Bhat [47] | 2016 | India | Asian | PCR | PB | 200 | 200 | 62 | 36 | NA | Age, gender, body mass index, alcohol, total cholesterol, hypertension and family history of CAD |
| Mir [49] | 2017 | India | Asian | PCR | PB | 100 | 100 | 42 | 26 | Yes | NA |
| Bhatti [50] | 2018 | India | Asian | PCR | PB | 562 | 564 | 217 | 127 | NA | NA |
Abbreviations: AMI, acute myocardial infarction; AR, atherosclerosis; BMI, body mass index; CAD, coronary artery disease; HB, hospital-based; IHD, ischemic heart disease; MI, myocardial infarction; NA, not available; PB, population-based; PCR, polymerase chain reaction; RT-PCR, reverse transcription PCR.
GSTM1
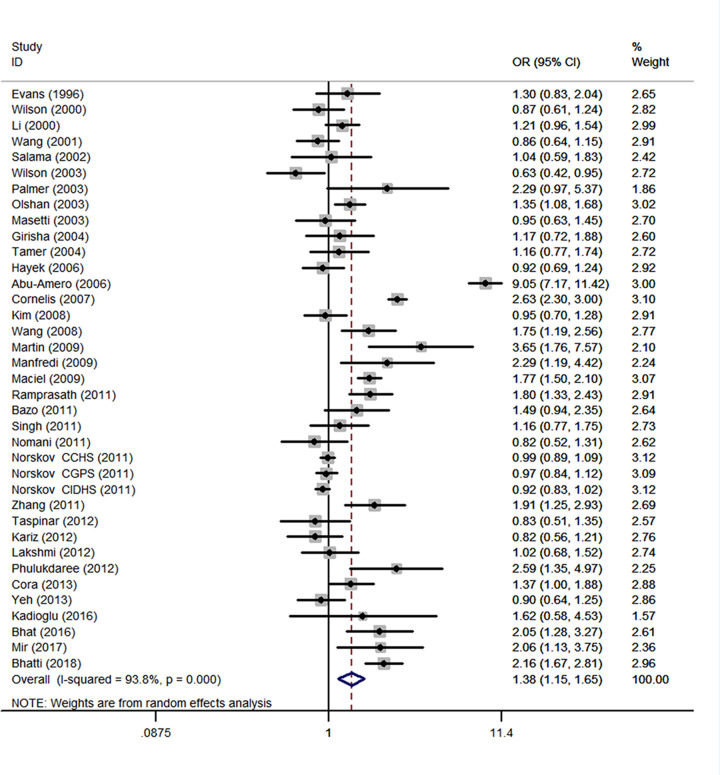
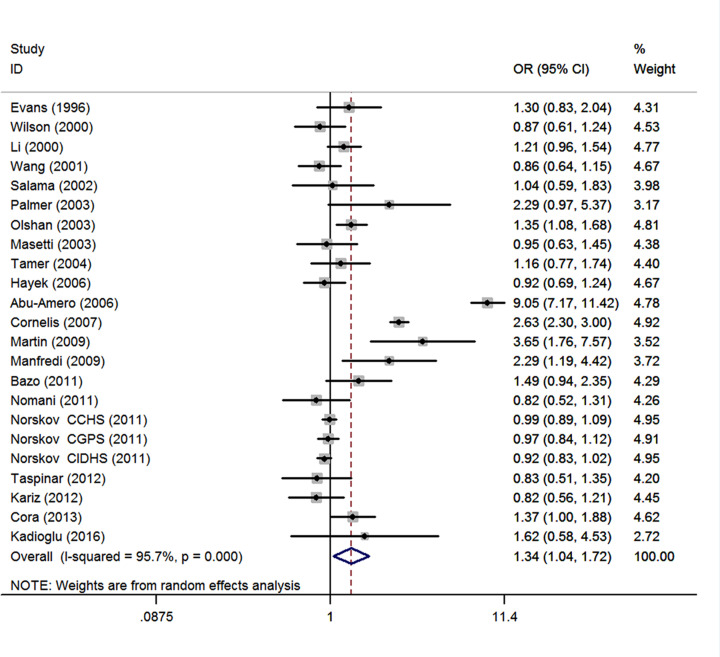
A total of 37 studies with 16684 cases and 36510 controls were retrieved based on the search criteria for CHD susceptibility related to the GSTM1 null polymorphism. Heterogeneity between studies was suggested (I2 = 93.8%; P<0.001), thus the random-effects model was used to pool data. The results indicated that the GSTM1 null genotype was significantly associated with CHD (OR = 1.38, 95% CI: 1.15, 1.65) (Figure 2). There was no evidence of publication bias (Begg's test, P=0.097; Egger's test, P=0.499 (Table 2). The meta-regression was conducted with the introduction of covariates including publication year, ethnicity, sample size, and control source. However, no covariate was identified as a potential source of between-study heterogeneity for any comparison. Sensitivity analyses indicated that the study by Abu-Amero et al. [27] was the main origin of heterogeneity in overall OR. After exclusion of the study [27], the heterogeneity was decreased (I2 = 88.6%). Besides, sensitivity analyses which yielded a range of ORs from 1.28 (95% CI: 1.12, 1.48) to 1.40 (1.17, 1.69) suggested that the results of this meta-analysis are stable. For meta-analysis of total studies, fifteen studies were spotted by Galbraith plot as possible major sources of heterogeneity [20,24,27,29,32–34,36,38,39,41,43,50]. There was no obvious between-study heterogeneity among remaining studies (I2 = 40.2%; P=0.027), and meta-analysis showed GSTM1 null genotype was also associated with increased risk of CHD (OR = 1.17, 95% CI: 1.05, 1.31) (Table 2). By stratifying the analysis by ethnicity, an OR of 1.34 (95% CI: 1.04, 1.72; I2 = 95.9%, P<0.001) (Figure 3) and 1.40 (95% CI: 1.11, 1.77; I2 = 78.6%, P<0.001) (Table 2) resulted in null genotype, among Caucasians and Asians, respectively. For meta-analysis of Caucasian studies, ten studies were spotted by Galbraith plot as possible major sources of heterogeneity [20,27–29,33,34,38,41]. After adjustment for heterogeneity by excluding these studies, the association was still significant in Caucasians (OR = 1.18, 95% CI: 1.07, 1.31; I2 = 18.1%, P=0.261). For meta-analysis of Asian studies, four studies were spotted by Galbraith plot as possible major sources of heterogeneity [24,30,46,50]. After adjustment for heterogeneity by excluding these studies, the association was still significant in Asians (OR = 1.60, 95% CI: 1.32, 1.95; I2 = 44.2%, P=0.073). Subgroup analysis by source of controls yield an OR of 1.47 (95% CI: 0.86, 2.51; I2 = 96.1%, P<0.001) and 1.33 (95% CI: 1.11, 1.58; I2 = 91.2%, P<0.001) resulted for null genotype, among hospital-based controls and healthy controls, respectively (Table 2). Stratified by sample size showed that the combined ORs were 1.32 (95% CI: 1.09, 1.61) for studies with the sample size < 600 and 1.40 (1.07, 1.84) for studies with the sample size > 600 (Table 2). Among smokers in 14 studies, people with the GSTM1 null genotype had an increased CHD risk with an OR of 1.64 (95% CI: 1.12, 2.40; I2 = 82.2%, P<0.001) (Table 2). Among non-smokers in 11 studies, people with the GSTM1 null genotype was not associated with CHD risk (OR = 1.26, 95% CI: 0.70, 2.27; I2 = 94.2%, P<0.001) (Table 2).
Figure 2. Meta-analysis of GSTM1 null genotype associated with CHD.
Each box represents the OR point estimate, and its area is proportional to the weight of the study. The diamond represents the overall summary estimate, with CI represented by its width.
Table 2. Subgroup analyses of studies included in a meta-analysis of GSTM1 null genotype and CHD risk.
| Null versus present | Studies | Cases/controls | OR (95% CI) | Heterogeneity | Model | P for Begg's test | P for Egger's test | |
|---|---|---|---|---|---|---|---|---|
| I2 | PH | |||||||
| Total studies | 37 | 16684/36510 | 1.38 (1.15, 1.69) | 93.8% | <0.001 | Random | 0.097 | 0.499 |
| Total studies (adjustment for heterogeneity1) | 22 | 5341/8322 | 1.17 (1.05, 1.31) | 40.2% | 0.027 | Random | 0.236 | 0.424 |
| Smoker | 14 | 2249/1300 | 1.64 (1.12, 2.40) | 82.2% | <0.001 | Random | 0.189 | 0.387 |
| Non-smoker | 11 | 1962/2195 | 1.26 (0.70, 2.27) | 94.2% | <0.001 | Random | 0.755 | 0.043 |
| Ethnicity | ||||||||
| Caucasians | 23 | 12268/31531 | 1.34 (1.04, 1.72) | 95.7% | <0.001 | Random | 0.045 | 0.605 |
| Caucasians (adjustment for heterogeneity2) | 13 | 2980/4021 | 1.18 (1.07, 1.31) | 18.1% | 0.261 | Fixed | 1.00 | 0.763 |
| Asians | 13 | 3547/3406 | 1.40 (1.11, 1.77) | 78.6% | <0.001 | Random | 0.583 | 0.903 |
| Asians (adjustment for heterogeneity3) | 9 | 2001/2094 | 1.60 (1.32, 1.95) | 44.2% | 0.073 | Random | 0.348 | 0.557 |
| Source of controls | ||||||||
| HB | 12 | 4302/3023 | 1.47 (0.86, 2.51) | 96.10% | <0.001 | Random | 0.244 | 0.238 |
| PB | 25 | 12382/33487 | 1.33 (1.11, 1.58) | 91.20% | <0.001 | Random | 0.199 | 0.418 |
| Sample size | ||||||||
| <600 | 20 | 3628/2973 | 1.32 (1.09, 1.61) | 68.5% | <0.001 | Random | 0.041 | 0.016 |
| >600 | 17 | 13056/33537 | 1.40 (1.07, 1.84) | 96.9% | <0.001 | Random | 0.650 | 0.472 |
Abbreviations: HB, hospital based; PB, population based.
PH: P-value based on Q test for between-study heterogeneity.
Adjustment for heterogeneity was performed by excluding 15 studies as the outliers and the possible major source of heterogeneity.
Adjustment for heterogeneity was performed by excluding 10 studies as the outliers and the possible major source of heterogeneity.
Adjustment for heterogeneity was performed by excluding 4 studies as the outliers and the possible major source of heterogeneity.
Figure 3. Meta-analysis of Caucasian studies.
Each box represents the OR point estimate, and its area is proportional to the weight of the study. The diamond represents the overall summary estimate, with CI represented by its width.
Smoking
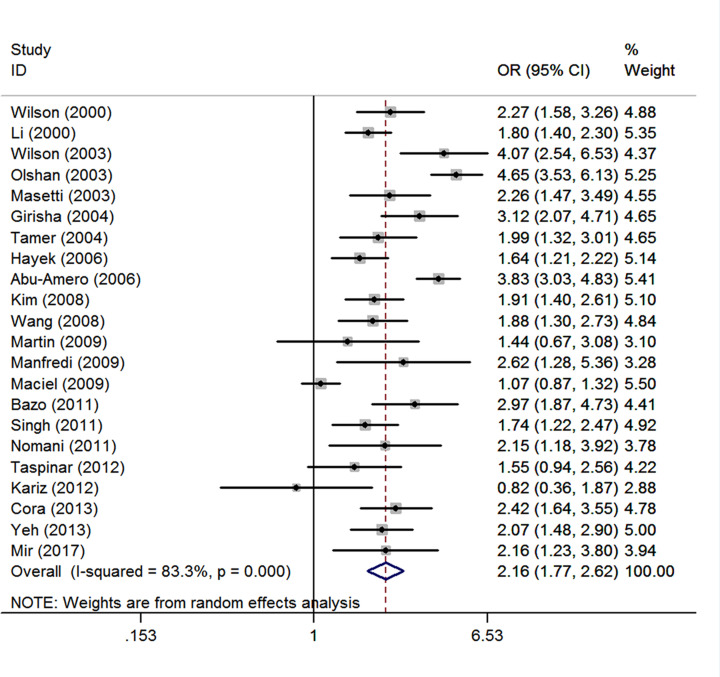
There are 22 studies [17,18,21,22,24–28,30–35,37,40,41,44–46,49] comprising 6816 CHD cases and 9822 controls. There was obvious between-study heterogeneity was detected among total 22 studies (I2 = 83.3%; P<0.001), and thus the random-effects model yielded an OR of 2.16 (1.77, 2.62) (Figure 4). The Begg's test (P=0.735) and Egger's test (P=0.808) showed no publication bias. After the exclusion of 4 studies [22,24,27,32] spotted by Galbraith plot as possible major sources of heterogeneity, there was no obvious between-study heterogeneity among those remained studies (I2 = 10.9%; P=0.324). Thus, the fixed-effects model was used to pool the ORs, and the result was not substantially changed (OR = 2.00, 95% CI: 1.82, 2.20). We performed a sensitivity analysis by omitting one study at a time, which yielded a range of ORs from 1.95 (95% CI: 1.77, 2.15) to 2.04 (1.84, 2.27).
Figure 4. Summary estimate (ORs and 95% CI) of CHD risk associated with smoking.
Each box represents the OR point estimate, and its area is proportional to the weight of the study. The diamond represents the overall summary estimate, with CI represented by its width.
GSTM1-smoking interplay
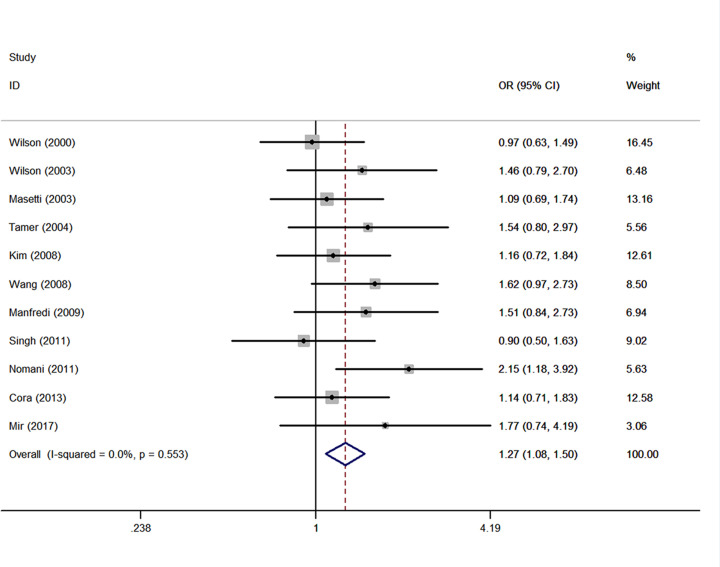
Twelve studies [17,21,24,26,27,30,31,33,37,40,45,49] included in the case-only analysis revealed a positive effect of the interaction between the GSTM1 null genotype and smoking status (ever smoking vs. never smoking) (OR = 1.49, 95% CI: 1.06, 2.08; I2= 80.9%; P<0.001). After omitting one study [27] which was spotted by Galbraith plot as the major sources of heterogeneity, the interaction between the GSTM1 null genotype and smoking on CHD risk was also statistically significant (OR = 1.27, 95% CI: 1.08, 1.50; I2 = 0%, P=0.553) (Figure 5).
Figure 5. Summary estimate (ORs and 95% CI) of the effect of interaction between the GSTM1 null genotype and smoking (ever vs. never) on CHD risk after adjustment for heterogeneity.
Each box represents the OR point estimate, and its area is proportional to the weight of the study. The diamond represents the overall summary estimate, with CI represented by its width.
Discussion
The current meta-analysis provided a comprehensive evaluation of the association between GSTM1 genetic polymorphism with risk of CHD. Moreover, to our knowledge, this is the first case-only designed analysis to prove a positive effect of the interaction between GSTM1 null genotype and smoking on CHD risk.
Two previous meta-analyses were performed to evaluated the association between GSTM1 genetic polymorphism with risk of CHD.The first one, performed in 2010 by Wang et al. [51], included 8020 cases and 11501 controls from 19 studies. They found a significant association between null polymorphism of GSTM1 and CHD risk. Afterwards, an updated meta-analysis conducted by Zhou et al. [52] showed that GSTM1 null genotype was not associated with increased risk of CHD in total population. In the present study, we identified 37 eligible studies, including 16684 CHD cases and 36510 controls, which could provide sufficient statistic power. Compared with previous meta-analyses, more than 11 relevant studies [36,37,39–42,45,47–50] were included in our analysis but not in theirs. Our meta-analysis showed that the GSTM1 null genotype was associated with a statistically elevated risk of CHD (OR = 1.38, 95% CI: 1.15, 1.65), which was consistent with the study by Wang et al. [51], but not the study by Zhou et al. [52]. After adjustment for heterogeneity by excluding these studies spotted by Galbraith plot, the results were still stable. By stratifying the analysis according to ethnicity, two previous meta-analyses [51,52] both found that GSTM1 null genotype was not associated with the risk of CHD for either Caucasians or Asians. However, our meta-analysis showed that the null genotype of GSTM1 may be associated with a higher risk of CHD in both Caucasians and Asians, which was inconsistent with two previous meta-analysis [51,52]. The results were still stable after adjustment for heterogeneity (Table 2). Two previous meta-analyses were relatively small and insufficient data were available for more exhaustive subgroup analysis. Among smokers in 14 studies, individuals with the null genotype of GSTM1 had a significantly increased CHD risk, which was consistent with two previous meta-analyses [51,52].
When interpreting the results of meta-analyses, heterogeneity assessment is necessary [65,66]. The I2 values surpassed the threshold of 50% in the present meta-analyses, indicating the presence of heterogeneity and insufficient power [59]. Meta-analyses might miss true effects when even modest between-study heterogeneity is present. Besides, low quality designed studies may result in incorrect conclusions [66]. In the present study, Galbraith plot was conducted to detect the outliers as the possible studies with low quality design and sensitivity analysis was further performed by omitting studies potted by Galbraith plot's method as the outliers. Fifteen studies were detected by Galbraith plot as possible major sources of heterogeneity in total studies, and ten studies were spotted by Galbraith plot as the possibly major sources of heterogeneity in Caucasian studies. When omitting those studies, the between-study heterogeneity decreased and there was no obvious heterogeneity among the remained studies (Table 2), which proved that those studies result in the heterogeneity. After adjustment for heterogeneity, meta-analyses showed that GSTM1 null genotype still increased risk of CHD in total population, Caucasians and Asians, respectively (Table 2). Errors and biases which led to heterogeneity were not known. Furthermore, there was limited knowledge on how much heterogeneity represented a true difference in genetic effects among different populations. Further studies need to focus on exploring the sources of heterogeneity.
Considering that CHD is a multifactorial trait and the impact of the GSTs on the progress of CHD may be modulated by age, gender and some other environmental and genetic influences, several subgroup meta-analyses were conducted in the present meta-analysis. In racial subgroups, meta-analysis showed GSTM1 null genotype increased risk of CHD both in Caucasians and in Asians. When stratifying by control source, significant association between null genotype of GSTM1 and CHD risk was observed population-based studies but not in hospital-based studies. By considering control source subgroups, Wang et al. [51] reported that GSTM1 null genotype was not associated with the risk of CHD in both population-based controls and hospital controls. The results may be biased by studies conducted by Abu-Amero et al. [27], Cornelis et al. [29], Nomani et al. [37], and Ramprasath et al. [39], because these studies included high-risk people with diabetes mellitus, hypertension, or obesity. Besides, what also needs to be pointed out is that the result should be interpreted with caution because of the relatively small sample size.
In present meta-analyses, the results suggest a positive multiplicative interaction (i.e., OR > 1) between smoking status and the GSTM1 null genotype on CHD risk. People with the GSTM1 null genotype were associated with CHD risk among smokers, but not among non-smokers in the present study. Cigarette smoking is a pro-inflammatory stimulus, and it is an important risk factor for CHD. Multiple chemicals in cigarette smoke can induce oxidative stress that results in smooth muscle cell proliferation, inflammation, vascular dysfunction DNA damage, and lipid peroxidation, which lead to atherosclerosis, and hence, CHD [3,4,6]. Animal experiments have proved that aromatic amines and polycyclic aromatic hydrocarbons (PAHs) in tobacco smoke can cause atherosclerotic lesions [67,68]. Moreover, DNA damage is present in cardiovascular disease patients [69]. Components in cigarette smoke can induce DNA adducts mitochondrial DNA damage in vascular cells [70] and DNA adducts in target tissues [5]. Oxidative stress and DNA damage play important roles in pathogenesis of atherosclerosis which is responsible for CHD. GSTs constitute the major defensive antioxidant system against oxidative stress by reducing ROS, which detoxify metabolites produced by oxidative stress and DNA damage within the cell and protect the cells against injury [71,72]. A homozygous deletion (0/0) or null genotype at the GSTM1 locus is related to enzyme function loss, which may be associated with susceptibility to CHD. Thus, there is biological evidence for the association between CHD risk and GSTM1 null genotype. The interaction between the GSTM1 null polymorphism and smoking status suggests that smoking is more detrimental to people who carry the GSTM1 null genotype. Although we pooled all published studies currently available on this topic, we thought our study was still far from conclusive, because many studies did not stratify the results according to smoking status. Besides, the sample sizes of these studies were small to modest, limiting their statistical power of the individual studies to detect interaction.
This meta-analysis had several limitations. First, the eligibility criteria for inclusion of controls were different. Some studies selected healthy individuals as controls, while the controls in other studies were selected from non-CHD individuals. Thus, selection bias might exist. Second, this meta-analyses were based on unadjusted estimates because many studies did not provide adjusted data. Third, some of these studies had relatively small sample sizes, which decreased their statistical power. Fourth, a possible publication bias may exist because only published studies were included, though there was no evidence of publication bias by visual examination of Begg's funnel plot or test results from Egger's test.
In conclusion, the present study showed that GSTM1 null genotype seems to be a risk factor for CHD. And the association may be affected by smoking status. The interaction between the GSTM1 null genotype and smoking status on CHD risk suggests that smoking is more detrimental to persons who carry the GSTM1 null genotype. Well-designed, population-based studies of adequate size are needed to investigate the possible gene–gene or gene–environmentinteractions in the association between gene polymorphisms and CHD risk.
Supplementary Material
Acknowledgements
We appreciate the contribution of all the members participating in the present study.
Abbreviations
- CHD
coronary heart disease
- CI
confidence interval
- GST
glutathione-S-transferase
- GSTM1
glutathione S-transferase M1
- NOS
Newcastle–Ottawa Scale
- OR
odds ratio
- ROS
reactive oxygen species
Contributor Information
Liegang Liu, Email: lgliu@mails.tjmu.edu.cn.
Jiansheng Liang, Email: niqc@whcdc.org.
Data Availability
The data used to support the findings of the present study are available from the corresponding author upon request.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author Contribution
Jiansheng Liang and Liegang Liu were the guarantors. They had full access to all of the data in the study and take responsibility for its integrity and the accuracy of the data analyses. They designed the study and also reviewed or revised the final manuscript. Yadong Song and Zhilei Shan led the conception; analyzed the data; drafted the article; provided critical review of the content. Yadong Song and Xiaoli Liu searched the databases according to the inclusion and exclusion criteria, reviewed search results by title and abstract, retrieved full-text articles to identify eligible trials, and extracted data. Xiaomin Chen, Cheng Luo, Liangkai Chen, Yimei Wang and Lin Gong evaluated methodological quality using criteria that were previously established, settled discrepancies by discussion in accordance with our selection criteria, and gave advice on meta-analysis methodology.
References
- 1.Fuster V.et al. (1992) The pathogenesis of coronary artery disease and the acute coronary syndromes (2). N. Engl. J. Med. 326, 310–318 10.1056/NEJM199201303260506 [DOI] [PubMed] [Google Scholar]
- 2.Pfisterer M.E., Zellweger M.J. and Gersh B.J. (2010) Management of stable coronary artery disease. Lancet 375, 763–772 10.1016/S0140-6736(10)60168-7 [DOI] [PubMed] [Google Scholar]
- 3.Irani K. (2000) Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res. 87, 179–183 10.1161/01.RES.87.3.179 [DOI] [PubMed] [Google Scholar]
- 4.Li R.et al. (2000) Glutathione S-transferase genotype as a susceptibility factor in smoking-related coronary heart disease. Atherosclerosis 149, 451–462 10.1016/S0021-9150(99)00483-9 [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi Y.et al. (2001) Oxidants in cigarette smoke extract modify low-density lipoprotein in the plasma and facilitate atherogenesis in the aorta of Watanabe heritable hyperlipidemic rabbits. Atherosclerosis 156, 109–117 10.1016/S0021-9150(00)00637-7 [DOI] [PubMed] [Google Scholar]
- 6.Martinet W.et al. (2002) Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 106, 927–932 10.1161/01.CIR.0000026393.47805.21 [DOI] [PubMed] [Google Scholar]
- 7.Guengerich F.P. (2000) Metabolism of chemical carcinogens. Carcinogenesis 21, 345–351 10.1093/carcin/21.3.345 [DOI] [PubMed] [Google Scholar]
- 8.Hayes J.D. and Strange R.C. (2000) Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 61, 154–166 10.1159/000028396 [DOI] [PubMed] [Google Scholar]
- 9.Hayes J.D., Flanagan J.U. and Jowsey I.R. (2005) Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 45, 51–88 10.1146/annurev.pharmtox.45.120403.095857 [DOI] [PubMed] [Google Scholar]
- 10.Lo H.W. and Ali-Osman F. (2007) Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Curr. Opin. Pharmacol. 7, 367–374 10.1016/j.coph.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 11.Rebbeck T.R. (1997) Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol. Biomarkers Prev. 6, 733–743 [PubMed] [Google Scholar]
- 12.Zhong S.et al. (1991) Glutathione S-transferase mu locus: use of genotyping and phenotyping assays to assess association with lung cancer susceptibility. Carcinogenesis 12, 1533–1537 10.1093/carcin/12.9.1533 [DOI] [PubMed] [Google Scholar]
- 13.Norppa H. (2004) Cytogenetic biomarkers and genetic polymorphisms. Toxicol. Lett. 149, 309–334 10.1016/j.toxlet.2003.12.042 [DOI] [PubMed] [Google Scholar]
- 14.(1990) International Commission for Protection Against Environmental Mutagens and Carcinogens. The possible involvement of somatic mutations in the development of atherosclerotic plaques. Report of ICPEMC Subcommittee 7/1. Conclusions and recommendations. Mutat. Res. 239, 143–148 [PubMed] [Google Scholar]
- 15.Song Y.et al. (2017) Glutathione S-Transferase T1 (GSTT1) null polymorphism, smoking, and their interaction in coronary heart disease: a comprehensive meta-analysis. Heart Lung Circ. 26, 362–370 10.1016/j.hlc.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 16.Evans D.A., Seidegard J. and Narayanan N. (1996) The GSTM1 genetic polymorphism in healthy Saudi Arabians and Filipinos, and Saudi Arabians with coronary atherosclerosis. Pharmacogenetics 6, 365–367 10.1097/00008571-199608000-00011 [DOI] [PubMed] [Google Scholar]
- 17.Wilson M.H.et al. (2000) Glutathione S-transferase M1 null genotype is associated with a decreased risk of myocardial infarction. FASEB J. 14, 791–796 10.1096/fasebj.14.5.791 [DOI] [PubMed] [Google Scholar]
- 18.Li R.et al. (2001) Interaction of the glutathione S-transferase genes and cigarette smoking on risk of lower extremity arterial disease: the Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis 154, 729–738 10.1016/S0021-9150(00)00582-7 [DOI] [PubMed] [Google Scholar]
- 19.Salama S.A.et al. (2002) Polymorphic metabolizing genes and susceptibility to atherosclerosis among cigarette smokers. Environ. Mol. Mutagen. 40, 153–160 10.1002/em.10106 [DOI] [PubMed] [Google Scholar]
- 20.Wang X.L.et al. (2002) Glutathione S-transferase mu1 deficiency, cigarette smoking and coronary artery disease. J. Cardiovasc. Risk 9, 25–31 10.1177/174182670200900104 [DOI] [PubMed] [Google Scholar]
- 21.Masetti S.et al. (2003) Interactive effect of the glutathione S-transferase genes and cigarette smoking on occurrence and severity of coronary artery risk. J. Mol. Med. (Berl.) 81, 488–494 10.1007/s00109-003-0448-5 [DOI] [PubMed] [Google Scholar]
- 22.Olshan A.F.et al. (2003) Risk of atherosclerosis: interaction of smoking and glutathione S-transferase genes. Epidemiology 14, 321–327 10.1097/01.EDE.0000059229.74889.CF [DOI] [PubMed] [Google Scholar]
- 23.Palmer C.N.et al. (2003) Association of common variation in glutathione S-transferase genes with premature development of cardiovascular disease in patients with systemic sclerosis. Arthritis Rheum. 48, 854–855 10.1002/art.10955 [DOI] [PubMed] [Google Scholar]
- 24.Wilson M.H.et al. (2003) Association between the risk of coronary artery disease in South Asians and a deletion polymorphism in glutathione S-transferase M1. Biomarkers 8, 43–50 10.1080/1354750021000042439 [DOI] [PubMed] [Google Scholar]
- 25.Girisha K.M.et al. (2004) T1 and M1 polymorphism in glutathione S-transferase gene and coronary artery disease in North Indian population. Indian J. Med. Sci. 58, 520–526 [PubMed] [Google Scholar]
- 26.Tamer L.et al. (2004) Glutathione S-transferase gene polymorphism as a susceptibility factor in smoking-related coronary artery disease. Basic Res. Cardiol. 99, 223–229 10.1007/s00395-004-0465-8 [DOI] [PubMed] [Google Scholar]
- 27.Abu-Amero K.K.et al. (2006) T null and M null genotypes of the glutathione S-transferase gene are risk factor for CAD independent of smoking. BMC Med. Genet. 7, 38 10.1186/1471-2350-7-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayek T.et al. (2006) A common variant in the glutathione S transferase gene is associated with elevated markers of inflammation and lipid peroxidation in subjects with diabetes mellitus. Atherosclerosis 184, 404–412 10.1016/j.atherosclerosis.2005.05.017 [DOI] [PubMed] [Google Scholar]
- 29.Cornelis M.C., El-Sohemy A. and Campos H. (2007) GSTT1 genotype modifies the association between cruciferous vegetable intake and the risk of myocardial infarction. Am. J. Clin. Nutr. 86, 752–758 10.1093/ajcn/86.3.752 [DOI] [PubMed] [Google Scholar]
- 30.Kim S.J.et al. (2008) Impact of glutathione S-transferase M1 and T1 gene polymorphisms on the smoking-related coronary artery disease. J. Korean Med. Sci. 23, 365–372 10.3346/jkms.2008.23.3.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L.S.et al. (2008) Association of GSTM1 and GSTT1 gene polymorphisms with coronary artery disease in relation to tobacco smoking. Clin. Chem. Lab. Med. 46, 1720–1725 10.1515/CCLM.2008.353 [DOI] [PubMed] [Google Scholar]
- 32.Maciel S.S.et al. (2009) Association between glutathione S-transferase polymorphisms and triglycerides and HDL-cholesterol. Atherosclerosis 206, 204–208 10.1016/j.atherosclerosis.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 33.Manfredi S.et al. (2009) Glutathione S-transferase T1- and M1-null genotypes and coronary artery disease risk in patients with Type 2 diabetes mellitus. Pharmacogenomics 10, 29–34 10.2217/14622416.10.1.29 [DOI] [PubMed] [Google Scholar]
- 34.Martin N.J.et al. (2009) Polymorphisms in the NQO1, GSTT and GSTM genes are associated with coronary heart disease and biomarkers of oxidative stress. Mutat. Res. 674, 93–100 10.1016/j.mrgentox.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 35.Bazo A.P.et al. (2011) DNA repair gene polymorphism is associated with the genetic basis of atherosclerotic coronary artery disease. Cardiovasc. Pathol. 20, e9–e15 10.1016/j.carpath.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 36.Zhang X.Y., Xu Y., Bao Y.Z.et al. (2011) Association of GSTT1 and GSTM1 gene polymorphisms with lipoprotein levels and coronary heart disease in the Chinese Han population. Chin. J. Crit. Care Med. 31, 502–506 [Google Scholar]
- 37.Nomani H.et al. (2011) The association between GSTT1, M1, and P1 polymorphisms with coronary artery disease in Western Iran. Mol. Cell. Biochem. 354, 181–187 10.1007/s11010-011-0817-2 [DOI] [PubMed] [Google Scholar]
- 38.Norskov M.S.et al. (2011) Copy number variation in glutathione S-transferases M1 and T1 and ischemic vascular disease: four studies and meta-analyses. Circ. Cardiovasc. Genet. 4, 418–428 10.1161/CIRCGENETICS.111.959809 [DOI] [PubMed] [Google Scholar]
- 39.Ramprasath T.et al. (2011) Potential risk modifications of GSTT1, GSTM1 and GSTP1 (glutathione-S-transferases) variants and their association to CAD in patients with type-2 diabetes. Biochem. Biophys. Res. Commun. 407, 49–53 10.1016/j.bbrc.2011.02.097 [DOI] [PubMed] [Google Scholar]
- 40.Singh N.et al. (2011) Glutathione S-transferase gene polymorphism as a susceptibility factor for acute myocardial infarction and smoking in the North Indian population. Cardiology 118, 16–21 10.1159/000324066 [DOI] [PubMed] [Google Scholar]
- 41.Kariz S., Nikolajevic Starcevic J. and Petrovic D. (2012) Association of manganese superoxide dismutase and glutathione S-transferases genotypes with myocardial infarction in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 98, 144–150 10.1016/j.diabres.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 42.Lakshmi S.V.et al. (2012) Role of CYP1A1 haplotypes in modulating susceptibility to coronary artery disease. Indian J. Biochem. Biophys. 49, 349–355 [PubMed] [Google Scholar]
- 43.Phulukdaree A.et al. (2012) GST polymorphisms and early-onset coronary artery disease in young South African Indians. S. Afr. Med. J. 102, 627–630 10.7196/SAMJ.5520 [DOI] [PubMed] [Google Scholar]
- 44.Taspinar M.et al. (2012) Impact of genetic variations of the CYP1A1, GSTT1, and GSTM1 genes on the risk of coronary artery disease. DNA Cell Biol. 31, 211–218 10.1089/dna.2011.1252 [DOI] [PubMed] [Google Scholar]
- 45.Cora T.et al. (2013) Glutathione S-transferase M1 and T1 genotypes and myocardial infarction. Mol. Biol. Rep. 40, 3263–3267 10.1007/s11033-012-2401-6 [DOI] [PubMed] [Google Scholar]
- 46.Yeh H.L.et al. (2013) GSTM1, GSTT1, GSTP1, and GSTA1 genetic variants are not associated with coronary artery disease in Taiwan. Gene 523, 64–69 10.1016/j.gene.2013.02.052 [DOI] [PubMed] [Google Scholar]
- 47.Bhat M.A. and Gandhi G. (2016) Association of GSTT1 and GSTM1 gene polymorphisms with coronary artery disease in North Indian Punjabi population: a case-control study. Postgrad. Med. J. 92, 701–706 10.1136/postgradmedj-2015-133836 [DOI] [PubMed] [Google Scholar]
- 48.Kadioglu E.et al. (2016) The role of oxidative DNA damage and GSTM1, GSTT1, and hOGG1 gene polymorphisms in coronary artery disease risk. Anatol. J. Cardiol. 16, 931–938 10.14744/AnatolJCardiol.2016.6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mir R.et al. (2016) Glutathione S-transferase M1 and T1 (rs4025935 and rs71748309) null genotypes are associated with increased susceptibility to coronary artery disease in Indian populations. Acta Cardiol. 71, 678–684 10.1080/AC.71.6.3178186 [DOI] [PubMed] [Google Scholar]
- 50.Bhatti J.S.et al. (2018) Genetic susceptibility of glutathione S-transferase genes (GSTM1/T1 and P1) to coronary artery disease in Asian Indians. Ann. Hum. Genet. 82, 448–456 [DOI] [PubMed] [Google Scholar]
- 51.Wang J.et al. (2010) Genetic polymorphisms of glutathione S-transferase genes GSTM1, GSTT1 and risk of coronary heart disease. Mutagenesis 25, 365–369 10.1093/mutage/geq014 [DOI] [PubMed] [Google Scholar]
- 52.Zhou D.et al. (2014) Glutathione S-transferase M1 polymorphism and coronary heart disease susceptibility: a meta-analysis involving 47,596 subjects. Heart Lung Circ. 23, 578–585 10.1016/j.hlc.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 53.Wells G.A., Shea B., O’Connell D.et al.. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm [Google Scholar]
- 54.Kavvoura F.K. and Ioannidis J.P. (2005) CTLA-4 gene polymorphisms and susceptibility to type 1 diabetes mellitus: a HuGE Review and meta-analysis. Am. J. Epidemiol. 162, 3–16 10.1093/aje/kwi165 [DOI] [PubMed] [Google Scholar]
- 55.DerSimonian R. and Laird N. (1986) Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 56.Mantel N. and Haenszel W. (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 22, 719–748 [PubMed] [Google Scholar]
- 57.He J.et al. (2015) No association between MTR rs1805087 A > G polymorphism and non-Hodgkin lymphoma susceptibility: evidence from 11 486 subjects. Leuk. Lymphoma 56, 763–767 10.3109/10428194.2014.935370 [DOI] [PubMed] [Google Scholar]
- 58.Fu W.et al. (2017) NFKB1 -94insertion/deletion ATTG polymorphism and cancer risk: evidence from 50 case-control studies. Oncotarget 8, 9806–9822 10.18632/oncotarget.14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moonesinghe R.et al. (2008) Required sample size and nonreplicability thresholds for heterogeneous genetic associations. Proc. Natl. Acad Sci. U.S.A. 105, 617–622 10.1073/pnas.0705554105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson S.G. and Higgins J.P. (2002) How should meta-regression analyses be undertaken and interpreted? Stat. Med. 21, 1559–1573 10.1002/sim.1187 [DOI] [PubMed] [Google Scholar]
- 61.Galbraith R.F. (1988) A note on graphical presentation of estimated odds ratios from several clinical trials. Stat. Med. 7, 889–894 10.1002/sim.4780070807 [DOI] [PubMed] [Google Scholar]
- 62.Begg C.B. and Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 63.Piegorsch W.W., Weinberg C.R. and Taylor J.A. (1994) Non-hierarchical logistic models and case-only designs for assessing susceptibility in population-based case-control studies. Stat. Med. 13, 153–162 10.1002/sim.4780130206 [DOI] [PubMed] [Google Scholar]
- 64.Khoury M.J. and Flanders W.D. (1996) Nontraditional epidemiologic approaches in the analysis of gene-environment interaction: case-control studies with no controls!. Am. J. Epidemiol. 144, 207–213 10.1093/oxfordjournals.aje.a008915 [DOI] [PubMed] [Google Scholar]
- 65.Zintzaras E. and Ioannidis J.P. (2005) Heterogeneity testing in meta-analysis of genome searches. Genet. Epidemiol. 28, 123–137 10.1002/gepi.20048 [DOI] [PubMed] [Google Scholar]
- 66.Ioannidis J.P., Patsopoulos N.A. and Evangelou E. (2007) Uncertainty in heterogeneity estimates in meta-analyses. BMJ 335, 914–916 10.1136/bmj.39343.408449.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albert R.E.et al. (1977) Effect of carcinogens on chicken atherosclerosis. Cancer Res. 37, 2232–2235 [PubMed] [Google Scholar]
- 68.Penn A. and Snyder C. (1988) Arteriosclerotic plaque development is ‘promoted’ by polynuclear aromatic hydrocarbons. Carcinogenesis 9, 2185–2189 10.1093/carcin/9.12.2185 [DOI] [PubMed] [Google Scholar]
- 69.Murgia E.et al. (2007) Micronuclei, genetic polymorphisms and cardiovascular disease mortality in a nested case-control study in Italy. Mutat. Res. 621, 113–118 10.1016/j.mrfmmm.2007.02.015 [DOI] [PubMed] [Google Scholar]
- 70.Yang Z.et al. (2007) The role of tobacco smoke induced mitochondrial damage in vascular dysfunction and atherosclerosis. Mutat. Res. 621, 61–74 10.1016/j.mrfmmm.2007.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dusinska M.et al. (2001) Glutathione S-transferase polymorphisms influence the level of oxidative DNA damage and antioxidant protection in humans. Mutat. Res. 482, 47–55 10.1016/S0027-5107(01)00209-3 [DOI] [PubMed] [Google Scholar]
- 72.Turkanoglu A.et al. (2010) Association analysis of GSTT1, GSTM1 genotype polymorphisms and serum total GST activity with ischemic stroke risk. Neurol. Sci. 31, 727–734 10.1007/s10072-010-0330-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of the present study are available from the corresponding author upon request.