Abstract
Ovarian cancer is the most lethal gynecological cancer. Numerous subtypes exist, each with distinct risk factors and prognosis. What underlies these subtypes and their progression is not clear, although inflammation through NFκB may play a key role. We performed a study on a series of well-characterized in vitro ovarian cancer models including TOV21G, TOV112D and OV90 originally derived from clear cell, endometrioid and high grade serous carcinoma respectively. Cells were treated with 0–100 ng/ml TNFα over 6–72 h. The NFκB pathway was inhibited by a series of NFκB pathway inhibitors, 100μM PDTC, 1μM PS-1145 and 200nM TPCA and the influence on cellular viability and inflammation was measured via an MTS assay and qPCR respectively. TNFα stimulation of NFκB was confirmed via Western blot. We found TNFα facilitated continued growth of TOV21G and TOV112D cells in an NFκB independent method. In contrast, TNFα inhibited OV90 cell growth in an NFκB dependent manner. TNFα stimulated production of IL-6, IL-8, MCP-1 and RANTES on all three cells lines, but only IL-6 and IL-8 were via NFκB mediated mechanisms. These results indicate TNFα may have diverse effects mediated through both NFκB and non-NFκB pathways on ovarian cancer cells. Understanding the role for TNFα in each subtype may have significant implications for charting disease progression and designing personalized treatments.
Keywords: Serous, Clear cell, Endometrioid, Ovarian cancer, Inflammation, TNFα, Epithelial
Serous, Clear cell, Endometrioid, Ovarian cancer, Inflammation, TNFα, Epithelial
1. Introduction
Ovarian cancer is the most lethal gynaecological carcinoma, and the fifth most common cause of cancer deaths [1]. Due to ineffective screening and no early clinical symptoms 75% of ovarian tumours are diagnosed at an advanced stage with fewer than 20% of women surviving long term [2]. A better understanding of the factors that lead to early pathogenesis could significantly improve outcomes. Epidemiological evidence strongly links ovarian cancer to inflammation. Risk factors, such as pelvic inflammatory disease [3] and incessant ovulation [4] support a role for inflammation in ovarian cancer pathology. Conditions that suppress ovulation and the inflammatory reaction that accompanies it [5] are associated with a reduced risk.
Ovarian cancer is a collection of molecularly and aetiologically distinct diseases subtypes [6] with diverse pathogenic mechanisms. High-grade serous carcinoma (HGSC) may originate from the fallopian tubes [7, 8] assisted by inflammation [9] and with an immune-reactive signature [10]. In contrast, endometrioid (EC) and clear cell (CCC), but not HGSC are associated with a malignant transformation of endometriosis lesions [11], a benign condition that produces an inflammatory microenvironment [12, 13]. Mucinous carcinoma (MC), while rare and their origin as yet unclear, may arise in association with teratomas [14, 15].
Tumor necrosis factor (TNF) α is an inflammatory cytokine, found at high concentrations in the ovarian cancer microenvironment [16]. It stimulates the transcription of other inflammatory mediators including interleukin 6 (IL-6), IL-8, monocyte chemotactic protein 1 (MCP-1) and Regulated on Activation normal T cell Expressed and Secreted (RANTES) [17]. Transcription is mediated through the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) transcription factor complex [17]. The role of TNFα and the NFκB complex in the progression of various ovarian cancer subtypes is not yet known.
We performed an in vitro study with a series of commercially available cell lines derived from distinct ovarian cancer subtypes to examine the effect of TNFα and the NFκB pathway on cellular proliferation and inflammation and their potential to contribute to ovarian cancer progression.
2. Materials and methods
2.1. Cell culture and treatment conditions
Cells derived from epithelial ovarian cancer: TOV-21G (ATCC® CRL-11730™)- clear cell carcinoma, TOV-112D (ATCC® CRL-11731™)- endometrioid carcinoma and OV-90 (ATCC® CRL-11732™)- serous adenocarcinoma were acquired from American Tissue Cell Culture (ATCC) with accompanying short tandem repeat (STR) profiling to ensure cell identity and experiments performed within passages 1–3 from receipt. Cells were cultured in 45% MCDB Media 105 (Lucerna Chem) and 45% Media 199 (Gibco, Life Technologies), with 10% fetal calf serum (FCS) and penicillin and streptomycin (Invitrogen) at 37 °C in a humidified atmosphere containing 5% CO2. For experimental treatments cells were grown to approximately 80% confluence and incubated with 0.5% FCS media overnight to synchronize the cell cycle.
To determine TNFα (R&D Systems, Minnesota, USA) influence on cell viability 72 h treatments were performed with TNFα at a maximum of 100 ng/ml and 1:9 serial dilutions. Analysis of NFκB activation and cytokine production was performed in the presence of 0, 10 or 100 ng/ml TNFα for 6 h. NFκB pathway inhibition on TNFα-stimulated cells was determined with 100μM PDTC to target NFκB and with both 1μM PS-1145 and 200nM TPCA for the upstream IKKβ inhibition with concentrations selected from previously published material or above their known IC50 values determined in similar cell types [18, 19, 20]. Subsequent 1:3 serial dilutions were performed with each compound. An initial one-hour pre-incubation was performed with the inhibitor alone, followed by a 1-hour incubation with both 100 ng/ml TNFα and the inhibitor.
2.2. MTS assay
Cells were seeded at approximately 1 × 104 cells into each well of a 96 well plates (Nunclon™ Delta Surface, Thermo Fisher Scientific) and incubated in media with 10% FCS. At 80% confluence, they were serum-starved with 0.5% FCS and treatments performed as described above. After 72 h cells were incubated in CellTiter 96 AQueous One Solution Reagent (Promega, Wisconsin, USA) diluted in normal media (1:5). Absorbance at 490 nm was measured after 2–3 h. Proliferation rate for each treatment is compared against its corresponding control and is expressed as the percentage of viable cells after treatment compared to the viable cells in the control treatment [21].
2.3. Reverse transcription and quantitative real-time PCR
Cells were collected in QIAzol Lysis Reagent (Qiagen, Venlo, Netherlands) and RNA was isolated on RNeasy Mini spin columns (Qiagen). RNA quality was determined using a NanoDrop Spectrophotometer 1000 (Witec, Luzern, Switzerland) and one μg of RNA transcribed into cDNA using Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega, Wisconsin, USA) using 1ug of starting RNA following the manufacturer's protocol.
The expression of four selected reference genes (ACTB, GAPDH, RPL13A and YWHAZ) and four genes of interest (IL-6, IL-8, MCP-1 and RANTES) were determined. Experiments were performed using TaqMan® Fast Advanced Master Mix and gene expression assay (Applied Biosystems, Foster City, CA, USA). Samples were run in duplicates on Rotor-Gene 2000 Real Time Cycler (Corbett Research, Qiagen). PCR amplification efficiency was determined by LinRegPCR software [22] and the relative mRNA expression values calculated by Biogazelle qbasePLUS (Bio-Rad, Hercules, CA, USA) [23].
2.4. Western blotting
After treatment whole cell lysate was collected in Protein lysis buffer (RIPA Buffer, 1% EGTA, 1% EDTA, 1% Triton-x and 1% protease inhibitor) and centrifuged at 12′000 x g for 20 min and the supernatant retained. 26μl of cell lysate was separated on a NuPage Bolt 4–12% Bis-Tris Plus gels (Life Technologies) and transferred to nitrocellulose membranes (iBlot® 2 NC Mini Stacks, Life Technologies) using the iBlot 2 dry transfer (Life Technologies). Immunodetection was performed on the SNAP i.d.™ Protein Detection System (Millipore USA). Non-specific binding was blocked with 0.05% bovine serum albumin (BSA) in Tris-buffered saline and antibody incubation performed for 30min with a primary rabbit anti-phosphorylated p65 (Ser536) monoclonal antibody (Cell Signaling, Danvers, Massachusetts, USA, 3033, 1:500), and a mouse anti-Actinβ monoclonal antibody (Abcam, UK, ab8226, 1:500). Membranes were incubated for 15 min with the secondary IRDye® 800CW Donkey anti-Rabbit antibodies (LI-COR, Lincoln, NE, USA, 926–32213, 1:3000) or IRDye® 680RD Donkey anti-Mouse antibodies (LI-COR, 926–68072, 1:4000).
Quantification of protein expression was performed with the Odyssey Imager (LI-COR) and accompanying LI-COR software. All values were normalized to the average value for each treatment on individual membranes.
2.5. Statistical analyses
TNFα influence on cell viability, mRNA expression, involvement of the NFκB pathway inhibitors and phos-p65 protein expression was performed with a non-parametric Kruskal-Wallis one-way analysis of variance (ANOVA) test with a Dunn's multiple comparison post-hoc analysis. Analysis of cell viability after NFκB inhibition in either the presence or absence of TNFα was determined with a two-way ANOVA with a post-hoc Sidak multiple comparison test. All analyses were performed with GraphPad Prism, version 5.00 (GraphPad Prism Software, La Jolla, Ca, USA). P < 0.05 were considered statistically significant.
3. Results
3.1. TNFα influence on cell viability and inflammation
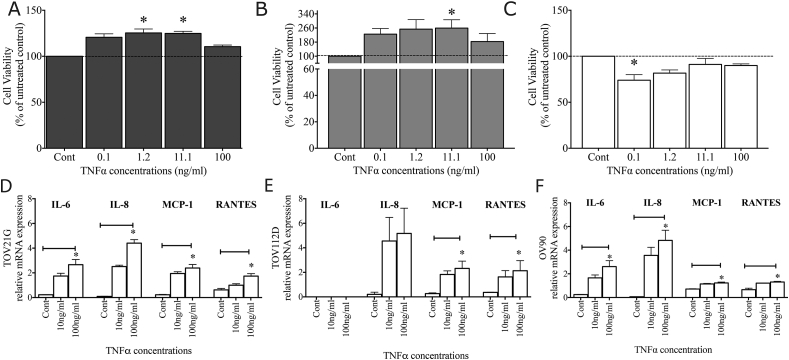
A one-way ANOVA identified a significant increase in TOV21G viability after TNFα treatment (p = 0.0021) (Figure 1A) with a significant difference after 1.2 ng/ml and 11.1 ng/ml TNFα treatment. TOV112D cell viability also increased after TNFα treatment (p = 0.0488) (Figure 1B) with the largest difference after 11.1 ng/ml of TNFα. In contrast, OV90 cell viability was significantly decreased by TNFα treatment (p = 0.0073) (Figure 1C) with a significant decrease after 0.1 ng/ml of TNFα.
Figure 1.
TNFα influence on ovarian epithelial cell number and inflammatory response. TNFα treatment of (A) TOV21G increased cell numbers above control (100%) at 1.2 ng/ml (125.3 ± 4.3%, p < 0.05) and 11.1 ng/ml (124.8 ± 2.3%, p < 0.05). (B) TNFα treatment of TOV112D increased cell numbers above control at 11.1 ng/ml (259.3 ± 47.49%, p < 0.05). (C) TNFα treatment of OV90 cells significantly reduced the number of viable cells at 0.1 ng/ml (73.95 ± 6.0%, p < 0.05). (D) TNFα treatment (100 ng/ml) significantly increased TOV21G production of IL-6 (control; 0.23 ± 0.02 v 100 ng/ml; 2.66 ± 0.42), IL-8 (control; 0.10 ± 0.02 v 100 ng/ml; 4.4 ± 0.49), MCP-1 (control = 0.23 ± 0.03 v 100 ng/ml = 2.4 ± 0.3) and RANTES (control 0.62 ± 0.12 v 100 ng/ml = 1.74 ± 0.20). (E) TOV112D showed no production of IL-6 both with and without a 100 ng/ml TNFα treatment. It did however produce a non-significant increase in IL-8 mRNA expression (control = 0.21 ± 0.18 v 100 ng/ml = 5.17 ± 2.06) and a significant increase in MCP-1 (control = 0.27 ± 0.06 v 100 ng/ml = 2.32 ± 0.60), and RANTES (control = 0.36 ± 0.02 v 100 ng/ml = 2.13 ± 0.82) mRNA expression. (F) A 100 ng/ml TNFα treatment of OV90 cell significantly increased the production of IL-6 (control = 0.25 ± 0.02 v 100 ng/ml = 2.61 ± 0.50), IL-8 (control = 0.06 ± 0.01 v 100 ng/ml = 4.83 ± 0.86), MCP-1 (control = 0.71 ± 0.03 v 100 ng/ml = 1.23 ± 0.07) and RANTES mRNA (control = 0.66 ± 0.13 v 100 ng/ml = 1.30 ± 0.05). ∗ <0.05. For all experiments n = 3.
An inflammatory response was induced after treatment with 100 ng/ml TNFα in TOV21G cells. There was a significant increase in IL-6 (p = 0.023), IL-8 (p = 0.015), MCP-1 (p = 0.023) and RANTES (p = 0.027) mRNA expression in these cells (Figure 1D). TOV112D cells showed no expression of IL-6 in either control, or after TNFα treatment and there was no statistically significant increase in IL-8 mRNA (p = 0.0714) after 100 ng/ml of TNFα. There was however a significant increase in MCP-1 (p = 0.034) and RANTES mRNA (p = 0.034) after 100 ng/ml TNFα (Figure 1E). In OV90 there was also a significant increase in IL-6 (p = 0.023), IL-8 (p = 0.034), MCP-1 (p = 0.041) and RANTES mRNA expression (p = 0.023) after 100 ng/ml TNFα treatment (Figure 1F).
3.2. TNFα stimulates p65 NFκB activity in ovarian cancer epithelial cells
Western blots identified specific 65kDa and 42kDa bands for phos-p65 and actinβ respectively (Figure 2) (Supplementary Figure 1). An ANOVA test showed a significant difference in phos-p65 in TOV21G (p = 0.0499) (Figure 2A) (Supplementary Figure 1A) and TOV112D (p = 0.025) (Figure 2B) (Supplementary Figure 1B) after TNFα treatment. A post-hoc, individual comparison test confirmed a significant increase after treatment with 10 ng/ml TNFα for the TOV112D cells. In the OV90 cells there was also an increase in phos-p65 after TNFα treatment (p = 0.0036) with a significant increase compared to control after treatment with 100 ng/ml TNFα (p < 0.05) (Figure 2C) (Supplementary Figure 1C).
Figure 2.
TNFα stimulated NFκB activity TNFα treatment stimulated phosphorylation of p65 NFκB in (A) TOV21G, (B) TOV112D and (C) OV90 ovarian cancer cells. A significant increase was observed in the TOV112D cells with 10 ng/ml TNFα (control = 0.77 ± 0.12 v 100 ng/ml = 1.04 ± 0.05) and in the OV90 cells with 100 ng/ml TNFα (control = 0.70 ± 0.02 v 100 ng/ml = 1.26 ± 0.05). ∗ <0.05. For all experiments n = 3.
3.3. NFκB inhibition and the inflammatory response
In TNFα-stimulated TOV21G cells IL-6 mRNA production was significantly (p = 0.0067) reduced from control by PDTC treatment. PDTC had no significant influence on IL-8, MCP-1 or RANTES (Figure 3A). In TNFα-stimulated TOV112D no IL-6 expression was observed, nor was there a significant reduction from control for IL-8, MCP-1 or RANTES by inclusion of any inhibitor (Figure 3B). In TNFα-stimulated OV90 cells both IL-6 and IL-8 mRNA were significantly reduced by inhibition of the NFκB pathway with the most significant effect occurring with PDTC on IL-6 (p = 0.028) and IL-8 (p = 0.038) (Figure 3C). No significant influence on either MCP-1, or RANTES mRNA expression was observed with PDTC. PS1145 and TPCA had no significant influence on any cytokine or chemokine concentration in any of the three cell lines.
Figure 3.
NFκB suppression of TNFα-stimulated inflammation. (A) TNFα stimulated IL-6 production was inhibited by PDTC in TOV21G (Control = 1.00 ± 0.07 v PDTC = 0.39 ± 0.10). (B) No IL-6 expression was observed in the TOV112D cells and all three inhibitors had no influence on IL-8. (C) In the OV90 cells PDTC inhibited both TNFα stimulated IL-6 (control = 1.01 ± 0.09 v PDTC = 0.37 ± 0.16) and IL-8 (control = 1.03 ± 0.41 v PDTC = 0.41 ± 0.04). No influence on either MCP-1 or RANTES was observed in any of the three cell lines. ∗ <0.05, ∗∗ <0.01. For all experiments n = 3.
3.4. NFκB inhibition of TNFα mediated cell viability
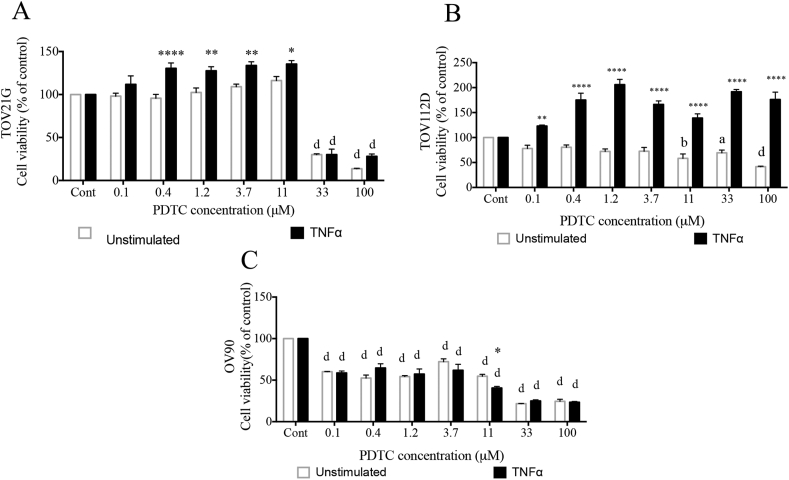
In the TOV21G cells PDTC did not significantly influence cell viability below concentrations of 11μM. The addition of TNFα increased the number of viable cells even in the presence of PDTC between 0.4 - 11μM (p < 0.0001). At 33μM and above PDTC cell viability was significantly repressed both with (p < 0.0001) and without TNFα (p < 0.0001) (Figure 4A).
Figure 4.
TNFα mediated cell viability after NFκB inhibition (A) TOV21G cells treated with PDTC retained the TNFα stimulated cell proliferation at concentrations of PDTC up to 33μM. After 33μM PDTC cell proliferation remained suppressed from control (100%) either with (30.9 ± 6.4%) or without 100 ng/ml of TNFα (30.1 ± 1.0%). (B) TOV112D cells treated with PDTC had a significant decrease in cell viability from PDTC concentrations of 11μM (58.39 ± 8.69%), although the stimulatory effect of 100 ng/ml of TNFα were not inhibited. (C) The viability of OV90 cells treated with PDTC decreased at all concentrations of PDTC with a significant reduction from concentrations above 0.1μM PDTC (60.1 ± 0.4%). TNFα did not stimulate cell viability under any conditions. Stars represent differences between TNFα (100 ng/ml) and unstimulated, ∗ <0.05, ∗∗ <0.01, ∗∗∗ <0.001, ∗∗∗∗ <0.0001. Letters represent difference from control, a <0.05, b < 0.01, c < 0.001, d < 0.0001. For all experiments n = 3.
In TOV112D cells PDTC consistently decreased cell viability from the lowest concentrations, reaching significance at concentration of 11μM (p < 0.01) and above. The addition of TNFα significantly increased cell viability irrespective of PDTC concentrations (p < 0.0001) (Figure 4B). In the OV90 cells PDTC mediated a significant reduction in cell viability from 0.1μM PDTC (p < 0.0001). Addition of TNFα did not stimulate cell viability irrespective of PDTC concentrations (Figure 4C). There was no difference with and without TNFα, although a further reduction with TNFα treatment was observed at the single concentration of 11μM PDTC.
4. Discussion
To examine the role of TNFα stimulated NFκB activity in ovarian cancer we used established ovarian cancer cell lines derived from three different subtypes. We chose these three cell lines as the source of the tumor was documented, they were commercially available and their identity validated via STR profiling. We found a significant variation in TNFα response between the cell lines with TOV21G and TOV112D showing a dose-response increase in cell viability and OV90 showing a decrease in cell viability. The decrease in OV90 was not mediated through an NFκB pathway. Although TNFα stimulated mRNA transcription of cytokines and chemokines from all three cell lines only IL6 and IL8 were stimulated predominantly via an NFκB mediated mechanism.
Previous studies have confirmed the utility of these cell lines as models for CCC (TOV21G), EC (TOV112D) and HGSC (OV90) [24,25]. One study screened 32 different ovarian cancer cell lines and found TOV21G was the prototypical model for CCC and that TOV112D is applicable to EC carcinoma [24]. Another study characterized 25 ovarian tumor cell lines and both TOV112D and OV90 were considered standard ovarian cancer cell lines with clear histological subtype related to their tumour of origin [25].
In this study inhibition of NFκB significantly reduced TOV112D and OV90 cell viability at all PDTC concentrations, whereas TOV21G viability was inhibited only at high concentrations. The inclusion of 100ng/ml TNFα stimulated TOV21G and TOV112D cell proliferation even the presence of an NFκB inhibitor, suggesting TNFα stimulated growth in these cells can occur in an NFκB independent mechanism. In contrast, TNFα did not rescue OV90 cell viability after inhibition with PDTC, suggesting NFκB is vital to the mechanism in this cell line. The results generated from our study with these three cell models suggest TNFα may play a role in facilitating CCC and EC, but not HGSC progression through non-NFκB mediated mechanisms.
Both CCC and EC ovarian cancer subtypes are closely associated with chronic inflammatory diseases, such as endometriosis [26]. These diseases while benign are accompanied by an increase in peritoneal fluid TNFα concentrations [27]. Peritoneal fluid TNFα concentrations range from <10 pg/ml [27] to 200 pg/ml [28]. In addition a 1,000 fold increase in local production can also occur in cancer cells [29]. In this study we used TNFα concentrations ranging from 100 pg/ml to 100 ng/ml. Concentrations that were sufficient to inhibit OV90 cell growth and increase both TOV21G and TOV112D cell proliferation. Continued exposure to inflammation has been associated with the acquisition of mutations that could contribute to a malignant transformation [30, 31].
Malignant cell lines constitutively secrete cytokines as a consequence of oncogenic mutations and dis-regulated signaling pathways [32]. We found TNFα increased the mRNA expression of all the cytokines consistently in all three cell lines, thus it is unlikely the differences in cell viability were mediated by generalized inflammatory changes of the microenvironment. Interestingly, there was an absence of IL-6 production by TOV112D cells. It is possible IL-6 expression is lost due to changes in culturing cells however, it has also been observed that the EC subtype has a suppressed IL-6 expression compared to HGSC and MC subtypes [33].
As with all studies the limitations of this study should be acknowledged. We have performed an in vitro analysis based on cell lines derived from human tumours. While providing the opportunity to model disease in the laboratory and reducing the burden on both patients, as well animals, in vitro studies are removed from the in vivo environment. It is always difficult to know how well they recapitulate the tumours they are intended to model and how well the results translate to the clinical environment. The results of these studies therefore need to be validated in more powerful models. However while the results are limited they do provide valid preliminary data to design larger studies and apply precious resources, such as laboratory animal, that should only be used if warranted after careful consideration.
In summary, ovarian cancer consists of a variety of subtypes with the distinct risk factors and prognoses. We found in vitro models of different ovarian cancer subtypes had significantly different response to TNFα and the pathway through which it is mediated that could have implications for disease progression. Increasing the number of cell lines, or employing relevant animal models would complement this study, however, many current tumor cell lines are of uncertain origin and their source is unclear and animal models should only be employed if there is sufficient justification to warrant their sacrifice. More work is needed on subtype specific initiation and progression in ovarian cancer to better characterize this deadly disease.
Declarations
Author contribution statement
Brett McKinnon: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Vida Kocbek: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Sara Imboden, Kostantinos Nirgianakis: Analyzed and interpreted the data.
Michael Mueller: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Swiss National Science Foundation (320030_140774).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Jemal A., Siegel R., Xu J., Ward E. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Malkasian G.D., Jr., Melton L.J., 3rd, O'Brien P.C., Greene M.H. Prognostic significance of histologic classification and grading of epithelial malignancies of the ovary. Am. J. Obstet. Gynecol. 1984;149(3):274–284. doi: 10.1016/0002-9378(84)90227-8. [DOI] [PubMed] [Google Scholar]
- 3.Shu X.O., Brinton L.A., Gao Y.T., Yuan J.M. Population-based case-control study of ovarian cancer in Shanghai. Canc. Res. 1989;49(13):3670–3674. [PubMed] [Google Scholar]
- 4.Ness R.B., Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J. Natl. Cancer Inst. 1999;91(17):1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 5.Risch H.A., Marrett L.D., Howe G.R. Parity, contraception, infertility, and the risk of epithelial ovarian cancer. Am. J. Epidemiol. 1994;140(7):585–597. doi: 10.1093/oxfordjournals.aje.a117296. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan S., Coward J.I., Bast R.C., Jr., Berchuck A., Berek J.S., Brenton J.D. Rethinking ovarian cancer: recommendations for improving outcomes. Nat. Rev. Canc. 2011;11(10):719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kindelberger D.W., Lee Y., Miron A., Hirsch M.S., Feltmate C., Medeiros F. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am. J. Surg. Pathol. 2007;31(2):161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 8.Vang R., Shih Ie M., Kurman R.J. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology. 2013;62(1):44–58. doi: 10.1111/his.12046. [DOI] [PubMed] [Google Scholar]
- 9.Ose J., Schock H., Tjønneland A., Hansen L., Overvad K., Dossus L. Inflammatory markers and risk of epithelial ovarian cancer by tumor subtypes: the EPIC cohort. Canc. Epidemiol. Biomarkers Prev. 2015;24(6):951–961. doi: 10.1158/1055-9965.EPI-14-1279-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearce C.L., Templeman C., Rossing M.A., Lee A., Near A.M., Webb P.M. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13(4):385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nothnick W., Alali Z. Recent advances in the understanding of endometriosis: the role of inflammatory mediators in disease pathogenesis and treatment. F1000Research. 2016;5:F1000. doi: 10.12688/f1000research.7504.1. Faculty Rev-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bersinger N.A., Dechaud H., McKinnon B., Mueller M.D. Analysis of cytokines in the peritoneal fluid of endometriosis patients as a function of the menstrual cycle stage using the Bio-Plex® platform. Arch. Physiol. Biochem. 2012;118(4):210–218. doi: 10.3109/13813455.2012.687003. [DOI] [PubMed] [Google Scholar]
- 14.Ronnett B.M., Seidman J.D. Mucinous tumors arising in ovarian mature cystic teratomas: relationship to the clinical syndrome of pseudomyxoma peritonei. Am. J. Surg. Pathol. 2003;27(5):650–657. doi: 10.1097/00000478-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Snir O.L., Buza N., Hui P. Mucinous epithelial tumours arising from ovarian mature teratomas: a tissue genotyping study. Histopathology. 2016;69(3):383–392. doi: 10.1111/his.12959. [DOI] [PubMed] [Google Scholar]
- 16.Grivennikov S.I., Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011;70(Suppl 1):i104–i108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 17.Ahn K.S., Aggarwal B.B. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 18.Redfern R., Pei Y., McDermott A. Cytokine stimulated expression of human beta-defensin-2 by corneal epithelial cells is mediated via activation of NFkB. Invest. Ophthalmol. Vis. Sci. 2002;43(13):1639. [Google Scholar]
- 19.Bain J., Plater L., Elliott M., Shpiro N., Hastie C.J., McLauchlan H. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid M.A., Lowman X.H., Pan M., Tran T.Q., Warmoes M.O., Ishak Gabra M.B. IKKβ promotes metabolic adaptation to glutamine deprivation via phosphorylation and inhibition of PFKFB3. Genes Dev. 2016;30(16):1837–1851. doi: 10.1101/gad.287235.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocbek V., Grandi G., Blank F., Wotzkow C., Bersinger N.A., Mueller M.D. TNFα-induced IKKβ complex activation influences epithelial, but not stromal cell survival in endometriosis. Mol. Hum. Reprod. 2016;22(11):768–777. doi: 10.1093/molehr/gaw054. [DOI] [PubMed] [Google Scholar]
- 22.Ruijter J.M., Ramakers C., Hoogaars W.M.H., Karlen Y., Bakker O., van den Hoff M.J.B. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37(6):e45–e. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinnon B.D., Evers J., Bersinger N.A., Mueller M.D. Induction of the neurokinin 1 receptor by TNFα in endometriotic tissue provides the potential for neurogenic control over endometriotic lesion growth. J. Clin. Endocrinol. Metabol. 2013;98(6):2469–2477. doi: 10.1210/jc.2013-1019. [DOI] [PubMed] [Google Scholar]
- 24.Anglesio M.S., Wiegand K.C., Melnyk N., Chow C., Salamanca C., Prentice L.M. Type-specific cell line models for type-specific ovarian cancer research. PloS One. 2013;8(9) doi: 10.1371/journal.pone.0072162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ince T.A., Sousa A.D., Jones M.A., Harrell J.C., Agoston E.S., Krohn M. Characterization of twenty-five ovarian tumour cell lines that phenocopy primary tumours. Nat. Commun. 2015;6(1):7419. doi: 10.1038/ncomms8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinton L.A., Gridley G., Persson I., Baron J., Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am. J. Obstet. Gynecol. 1997;176(3):572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 27.Overton C., Fernandez-Shaw S., Hicks B., Barlow D., Starkey P. Peritoneal fluid cytokines and the relationship with endometriosis and pain. Hum. Reprod. 1996;11(2):380–386. doi: 10.1093/humrep/11.2.380. [DOI] [PubMed] [Google Scholar]
- 28.Cheong Y.C., Shelton J.B., Laird S.M., Richmond M., Kudesia G., Li T.C. IL-1, IL-6 and TNF-alpha concentrations in the peritoneal fluid of women with pelvic adhesions. Hum. Reprod. 2002;17(1):69–75. doi: 10.1093/humrep/17.1.69. [DOI] [PubMed] [Google Scholar]
- 29.Szlosarek P.W., Grimshaw M.J., Kulbe H., Wilson J.L., Wilbanks G.D., Burke F. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol. Canc. Therapeut. 2006;5(2):382–390. doi: 10.1158/1535-7163.MCT-05-0303. [DOI] [PubMed] [Google Scholar]
- 30.Yan B., Wang H., Rabbani Z.N., Zhao Y., Li W., Yuan Y. Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Canc. Res. 2006;66(24):11565–11570. doi: 10.1158/0008-5472.CAN-06-2540. [DOI] [PubMed] [Google Scholar]
- 31.Babbar N., Casero R.A., Jr. Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Canc. Res. 2006;66(23):11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 33.Kryczek I., Gryboś M., Karabon L., Klimczak A., Lange A. IL-6 production in ovarian carcinoma is associated with histiotype and biological characteristics of the tumour and influences local immunity. Br. J. Canc. 2000;82(3):621–628. doi: 10.1054/bjoc.1999.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.