Summary
Sepsis is a leading cause of morbidity and mortality associated with significant impairment in memory T cells. These changes include the upregulation of co-inhibitory markers, a decrease in functionality, and an increase in apoptosis. Due to recent studies describing IL-27 regulation of TIGIT and PD-1, we assessed whether IL-27 impacts these co-inhibitory molecules in sepsis. Based on these data, we hypothesized that IL-27 was responsible for T cell dysfunction during sepsis. Using the cecal ligation and puncture (CLP) sepsis model, we found that IL-27Rα was associated with the upregulation of TIGIT on memory CD4+ T cells following CLP. However, IL-27 was not associated with sepsis mortality.
Subject Areas: Molecular Biology, Immunology
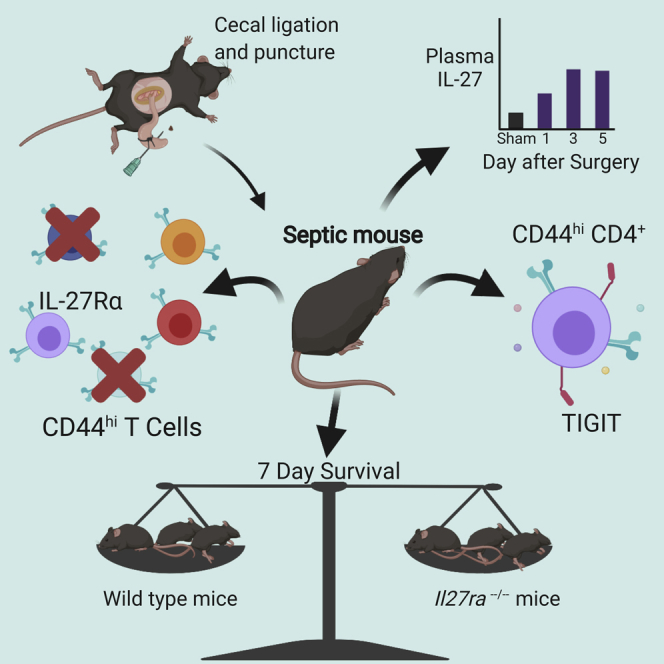
Graphical Abstract
Highlights
-
•
Numbers of IL-27Rα+ memory T cells are decreased following cecal ligation and puncture
-
•
TIGIT is expressed on more IL-27Rα+ versus IL-27Rα− memory CD4+ T cells during sepsis
-
•
Il27ra−/− and WT T cells exhibit similar effector function and apoptosis during sepsis
-
•
IL-27 signaling does not impact sepsis mortality
Molecular Biology; Immunology
Introduction
Sepsis is defined as a life-threatening organ dysfunction resulting from the body's dysregulated response to infection (Singer et al., 2016). It is a significant source of morbidity and mortality worldwide—in 2017 alone, an estimated 11 million people died from sepsis, accounting for 20% of all deaths that year (Rudd et al., 2020). Rapid recognition of sepsis and initiation of supportive care is effective at reducing mortality in the early stages of sepsis (Ferrer et al., 2009; Seymour et al., 2017), but a substantial proportion of sepsis patients die between 31 days and 2 years (Prescott et al., 2016). Mortality is associated with long-term immune system dysfunction that begins shortly after sepsis onset (Boomer et al., 2011).
Sepsis-induced immune dysfunction is multi-faceted, characterized by changes in both innate and adaptive cell responses. Current evidence suggests that alterations in the T cell repertoire lead to adaptive immune system dysfunction. We previously published data describing alterations in the T cell compartment following sepsis. We found significant reductions in the number of total CD4+ and CD8+ T cells using the cecal ligation and puncture (CLP) model of sepsis (Ramonell et al., 2017; Chen et al., 2017). Naive and memory CD4+ T cell numbers are significantly reduced following CLP. Although the number of naive CD8+ T cells is similar between CLP and sham-surgery mice at all time points, the number of memory CD8+ T cells is reduced following sepsis (Serbanescu et al., 2016; Xie et al., 2019a). One day after CLP surgery, the number of memory CD8+ T cells is reduced by 44% and remains reduced for three days after surgery (Serbanescu et al., 2016). Co-inhibitory markers such as TIGIT and PD-1 are also highly upregulated on T cells and linked to their apoptosis during sepsis (Boomer et al., 2012; Chen et al., 2017; Sjaastad et al., 2018; Ammer-Herrmenau et al., 2019; Xie et al., 2019a, 2019b).
Recently, the immunosuppressive cytokine IL-27 was associated with TIGIT and PD-1 expression on T cells during cancer (Chihara et al., 2018) and toxoplasmosis (Delong et al., 2019). Furthermore, IL-27 inhibited memory T cell responses during secondary malaria infection (Gwyer Findlay et al., 2014). We therefore hypothesized that IL-27 signaling is responsible for the upregulation of TIGIT and PD-1 on memory T cells during sepsis and ultimately for sepsis mortality. Previous studies also observed that the level of serum IL-27 increases following sepsis in mouse models and human septic patients (Wirtz et al., 2006; Nelson et al., 2010; Wong et al., 2012, 2013, 2014; Bosmann et al., 2014b; Cao et al., 2014; Hanna et al., 2015; Gao et al., 2016; Yan et al., 2016). Produced by antigen-presenting cells (Pflanz et al., 2002), IL-27 primarily regulates the effector response of dendritic cells and T cells during infections (Villarino et al., 2003; Yoshimura et al., 2006; Guzzo et al., 2012; Clement et al., 2016; Patin et al., 2016; Sowrirajan et al., 2017; Wehrens et al., 2018). Memory T cells express particularly high levels of the IL-27 receptor (IL-27Rα) (Villarino et al., 2005).
To determine whether IL-27 signaling is responsible for co-inhibitory molecule expression on memory T cells during sepsis, we analyzed the phenotype and function of IL-27Rα+ on memory T cells following cecal ligation and puncture (CLP). IL-27 receptor expression was associated with TIGIT, but not PD-1, expression on memory CD4+ T cells, and impaired production of IFNγ. However, IL-27 signaling was not associated with sepsis mortality. These results suggest that IL-27Rα signaling upregulates TIGIT on memory CD4+ T cells during sepsis but does not affect sepsis mortality.
Results
Sepsis increases plasma IL-27 but is associated with a reduction in the frequency and number of IL-27Rα+ CD4+ and CD8+ T cells
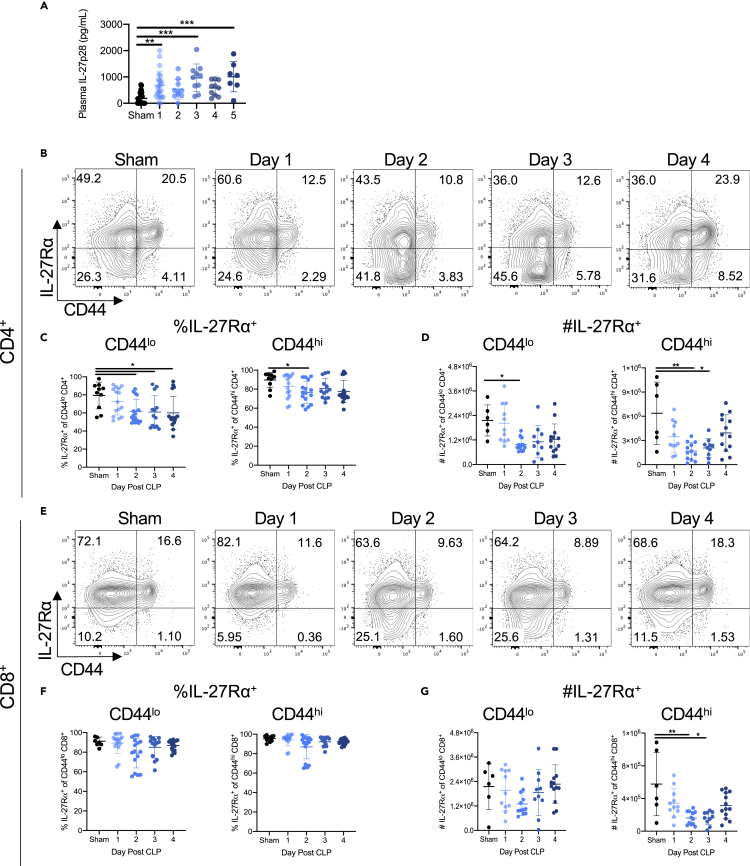
Although plasma IL-27 has been previously shown to increase at early time points during sepsis, it is not known how long it remains elevated (Wirtz et al., 2006; Nelson et al., 2010; Wong et al., 2012, 2013, 2014; Bosmann et al., 2014b; Cao et al., 2014; Hanna et al., 2015; Gao et al., 2016; Yan et al., 2016). To address this, mice underwent cecal ligation and puncture (CLP) or sham surgery to induce sepsis (see Transparent Methods). Plasma was collected on days 1 through 5 days following surgery. Septic animals had increased plasma IL-27p28 on days 1, 3, and 5 compared with mice that underwent sham surgery (Figure 1A). Although systemic IL-27p28 increased following CLP, it was unclear what proportion of T cells expressed the IL-27 receptor (IL-27Rα). Multi-color flow cytometry was used to determine the frequency and number of IL-27Rα expressing T cells in the spleens of CLP and sham mice. A high frequency of CD4+ and CD8+ T cells expressed IL-27Rα in unmanipulated mice (Figure S1). After CLP surgery, the frequency of IL-27Rα+ cells among CD44lo naive CD4+ T cells was reduced compared with sham controls on days 2 and 4 (Figures 1B and 1C). In contrast, the frequency of IL-27Rα+ CD44hi memory CD4+ T cells decreased on day 2 following CLP but increased back to sham levels on days 3 and 4 (Figures 1B and 1C). Because sepsis causes lymphopenia, we also assessed the absolute cell numbers. We found that absolute numbers of IL-27Rα+ CD44lo and CD44hi CD4+ T cells were significantly reduced after CLP, with a greater reduction in the CD44hi population (Figure 1D). The frequency of IL-27Rα+ CD44lo or CD44hi CD8+ T cells was not altered after CLP (Figures 1E and 1F). However, the absolute number of IL-27Rα+ CD44hi (but not CD44lo) CD8+ T cells was reduced on days 2 and 3 after CLP (Figure 1G). Moreover, neither the number of IL-27Rα− CD44hi nor IL-27Rα− CD44lo T cells was not decreased following CLP (Figure S2).
Figure 1.
Sepsis results in a reduction in the frequency and number of IL-27Rα+ T cells
Following cecal ligation and puncture (CLP) or sham surgery (sham), animals were euthanized on the indicated days. Plasma was collected for IL-27p28 ELISAs on days 1 through 5 and spleens harvested for analysis by flow cytometry on days 1 through 4.
(A) Concentration of IL-27p28 in the plasma of sham and CLP mice on days 1 through 5 following surgery.
(B) Representative flow cytometric plots showing the frequency of IL-27Rα expressing CD4+ T cells in sham and CLP mice on days 1–4 after surgery.
(C) The frequency of CD4+ CD44lo naive (left) and CD4+ CD44hi memory (right) T cells expressing the IL-27Rα in sham and CLP mice on days 1–4 after surgery.
(D) The absolute number of CD4+ CD44lo naive (left) and CD4+ CD44hi memory (right) T cells expressing the IL-27Rα in sham and CLP mice on days 1–4 after surgery.
(E) Representative flow cytometric plots showing the frequency of IL-27Rα expressing CD8+ T cells in sham and CLP mice on days 1–4 after surgery.
(F) The frequency of CD8+ CD44lo naive (left) and CD8+ CD44hi memory (right) T cells expressing the IL-27Rα in sham and CLP mice on days 1–4 after surgery.
(G) The absolute number of CD8+ CD44lo naive (left) and CD8+ CD44hi memory (right) T cells expressing the IL-27Rα in sham and CLP mice on days 1–4 after surgery. All summary data were pooled from three independent experiments, with n = 7–18 mice per group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Error bars represent the mean ± the standard deviation.
IL-27 signaling is associated with the upregulation of TIGIT on memory CD4+ T cells in septic mice
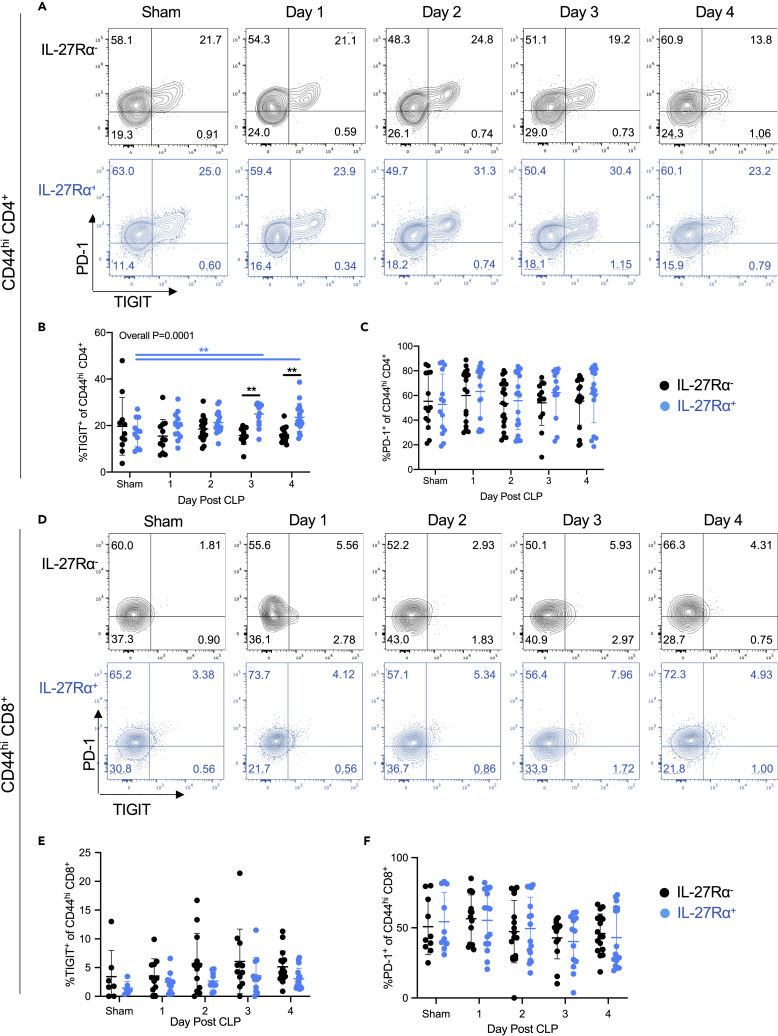
Based on the literature showing that IL-27 signaling regulates the expression of TIGIT and PD-1 (Delong et al., 2019; Chihara et al., 2018), we sought to determine whether IL-27Rα expression associates with the frequency of TIGIT and PD-1 expression on memory T cells during sepsis. To do this, we assessed the frequency of the co-inhibitory molecules TIGIT and PD-1 (gating strategy shown in Figure S2) in IL-27Rα+ and IL-27Rα– CD44hi CD4+ T cells on days 1–4 after CLP. Beginning on day 3 and continuing to day 4 following CLP, the frequency of TIGIT+ cells in the IL-27Rα+ CD4+ memory population increased relative to the IL-27Rα− CD4+ memory population (Figures 2A and 2B). When comparing sham mice with CLP mice on days 1, 2, 3, and 4 following surgery, TIGIT expression was increased on IL-27Rα+ memory CD4 T cells (p = 0.01), with p = 0.004 when comparing sham versus day 3 post-CLP and p = 0.04 when comparing sham versus day 4 post-CLP (Figures 2A and 2B). When making the same comparisons for IL-27Rα− memory CD4+ T cells, the overall difference was not significant (p = 0.37) and p > 0.99 on days 3 and 4 after CLP. This suggests that sepsis increases the expression of TIGIT selectively on the IL-27Rα+ cell population (Figures 2A and 2B). However, we found no difference in the frequency of PD-1+ cells between IL-27Rα+ versus IL-27Rα– memory CD4+ T cell populations (Figures 2A and 2C). There were no significant differences in the frequencies of TIGIT+ (Figures 2D and 2E) or PD-1+ (Figures 2D and 2F) T cells at any point following CLP. In contrast to the memory CD4+ T cell compartment, memory CD8+ T cells had an indistinct population of TIGIT+ and PD-1+ cells (Figure 2E). These results demonstrate that IL-27 receptor expression is associated with TIGIT expression on CD44hi CD4+ T cells.
Figure 2.
IL-27Rα expression is associated with an increased frequency of TIGIT expression on memory CD4+ T cells during sepsis
The gating strategy for PD-1 and TIGIT is shown inFigure S2.
(A) Representative flow cytometric plots showing PD-1 and TIGIT expression on CD44hi memory CD4+ T cells lacking (black) or expressing (blue) IL-27Rα in wild-type mice on days 1–4 after sham surgery (“sham”) or CLP.
(B) The frequency of TIGIT+ expressing IL-27Rα− (black) and IL-27Rα+ (blue) CD44hi memory CD4+ T cells in sham and CLP wild-type mice on days 1–4 after surgery.
(C) The frequency of PD-1+ expressing IL-27Rα− (black) and IL-27Rα+ (blue) CD44hi memory CD4+ T cells in sham and CLP wild-type mice on days 1–4 after surgery.
(D) Representative flow cytometric plots showing TIGIT and PD-1 expression on CD44hi memory CD8+ T cells lacking (black) or expressing (blue) IL-27Rα in sham mice and CLP wild-type mice on days 1–4 after surgery.
(E) The frequency of TIGIT+ expressing IL-27Rα− (black) and IL-27Rα+ (blue) CD44hi memory CD8+ T cells in sham and CLP wild-type mice on days 1–4 after surgery.
(F) The frequency of PD-1+ expressing IL-27Rα− (black) and IL-27Rα+ (blue) CD44hi memory CD8+ T cells in sham and CLP wild-type mice on days 1–4 after surgery. Data were pooled from three independent experiments, with n = 7–17 mice per group. ∗∗p < 0.01. Error bars represent the mean ± the standard deviation.
IL-27 signaling is associated with reduced proliferation, but not apoptosis, of CD44hi CD8+ T cells in septic mice
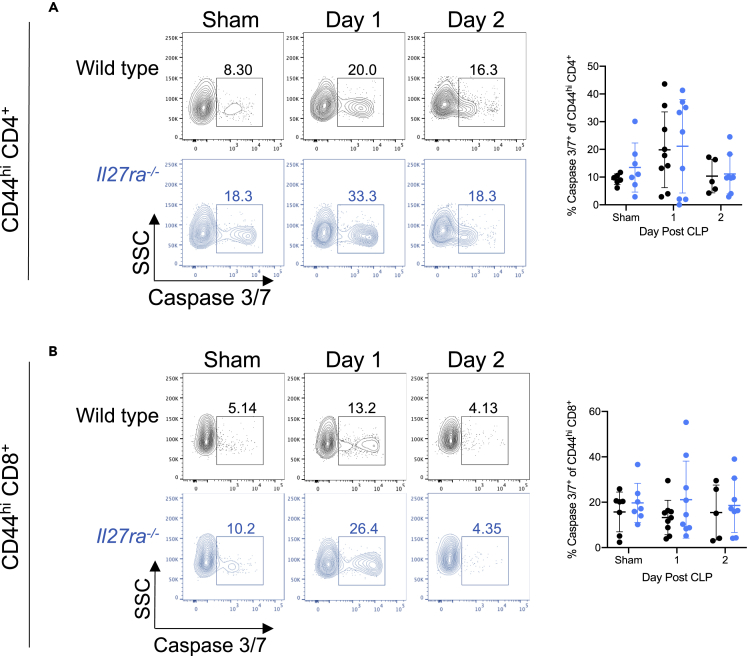
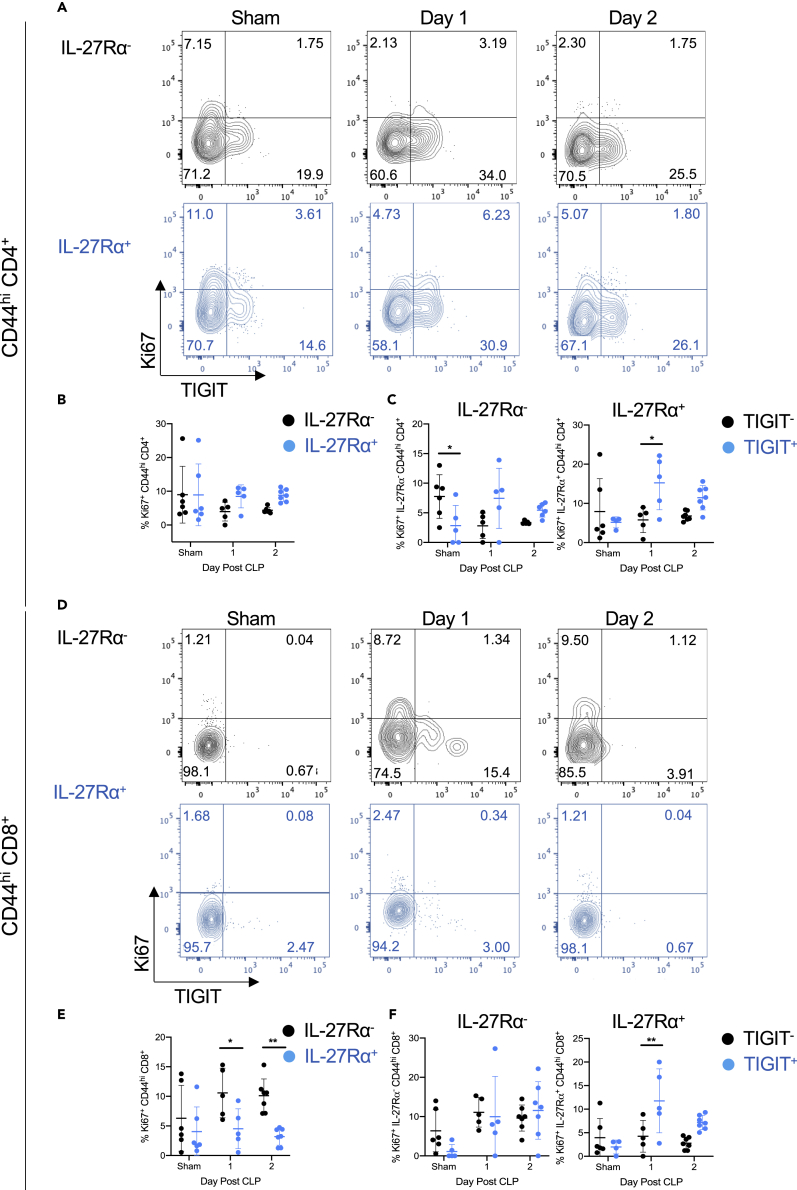
To determine whether IL-27 modulates the apoptosis or proliferation of T cells during sepsis, we next assessed whether the IL-27 signaling was associated with activity of the apoptotic proteases Caspase 3 and Caspase 7 or expression of the proliferation marker Ki67. We measured active Caspase 3/7 on memory T cells using flow cytometry and found that CD44hi CD4+ T cells exhibit similar frequencies of active caspase 3/7+ cells in both wild-type and Il27ra−/− mice (Figure 3A). Also, the frequency of caspase 3/7+ apoptotic cells is similar between the CD44hi CD8+ T cells in wild-type and Il27ra−/− septic mice (Figure 3B). The frequency of proliferating (Ki67+) cells among CD4+ CD44hi memory T cells was similar in IL-27Rα− versus IL-27Rα+ populations (Figures 4A and 4B). We next looked at the association between TIGIT expression and proliferation in the IL-27Rα− and IL-27Rα+ populations. Among IL-27Rα− memory CD4+ T cells, TIGIT+ cells exhibited reduced frequencies of proliferating cells compared with TIGIT− cells in non-septic animals but similar frequencies following CLP (Figure 4C). In contrast, TIGIT+ cells proliferated more than TIGIT− IL-27Rα+ memory CD4+ T cells one day following CLP (Figure 4C). In the CD44hi memory CD8+ T cell compartment, IL-27Rα expression associated with reduced proliferation 1 and 2 days after CLP (Figures 4D and 4E). Among IL-27Rα− cells, there was no difference in proliferation based on TIGIT expression at any time point analyzed. In contrast, among IL-27Rα+ T cells, TIGIT+ cells proliferated more than TIGIT− cells one day after CLP surgery (Figure 4F). These results indicate that the numerical reduction in IL-27Rα+ memory CD4+ T cells following CLP is unrelated to a deficit in proliferation but that reduced proliferation may be responsible for the reduced numbers in the CD8+ T cell compartment. In addition, TIGIT expression is associated with higher proliferation in IL-27Rα+, but not IL-27Rα−, memory T cells after CLP.
Figure 3.
IL-27 signaling is not associated with CD44hi T cell apoptosis in septic mice
(A) Representative flow cytometric plots showing Caspase 3/7 (x axis) by SSC (y axis) on CD44hi memory CD4+ T cells in wild-type and Il27ra−/− mice on days 1 and 2 after sham surgery (“sham”) or CLP surgery (left). The frequency of apoptotic (Caspase 3/7+) CD44hi memory CD4+ T cells is summarized on the right.
(B) Representative flow cytometric plots showing Caspase 3/7 (x axis) by SSC (y axis) on CD44hi CD8+ T cells after sham or CLP surgery in wild-type and Il27ra−/− mice on days 1 and 2 after surgery (left). The frequency of apoptotic (Caspase 3/7+) CD44hi memory CD8+ T cells is summarized on the right. Data are representative of two experiments with n = 5–9 mice per group. Error bars represent the mean ± the standard deviation.
Figure 4.
IL-27 signaling is associated with reduced proliferation of CD44hi CD8+ T cells in septic mice
Wild-type mice underwent sham (“sham”) or CLP surgery, and splenocytes were harvested 1 to 2 days later for flow cytometric analysis.
(A) Representative flow cytometric plots showing TIGIT (x axis) versus Ki67 (y axis) expression in IL-27Rα− (black) and IL-27Rα+ (blue) CD44hi CD4+ T cells.
(B) Summary graph showing the frequency of Ki67+ CD44hi CD4+ T cells between the IL-27Rα− (black) and IL-27Rα+ (blue) populations.
(C) Summary graphs showing the frequency of Ki67+ CD44hi CD4+ T cells within TIGIT− (black) and TIGIT+ (blue) cells of the IL-27Rα− (left) and IL-27Rα+ (right) populations.
(D) Representative flow cytometric plots showing TIGIT (x axis) versus Ki67 (y axis) expression in IL-27Rα− (black) and IL-27Rα+ (blue) CD44hi CD8+ T cells.
(E) Summary graph showing the frequency of Ki67+ CD44hi CD8+ T cells between the IL-27Rα− (black) and IL-27Rα+ (blue) populations.
(F) Summary graphs showing the frequency of Ki67+ CD44hi CD8+ T cells within TIGIT− (black) and TIGIT+ (blue) cells of the IL-27Rα− (left) and IL-27Rα+ (right) populations. Data were pooled from two independent experiments with n = 5–7 per group. ∗p < 0.05, ∗∗p < 0.01. Error bars represent the mean ± the standard deviation.
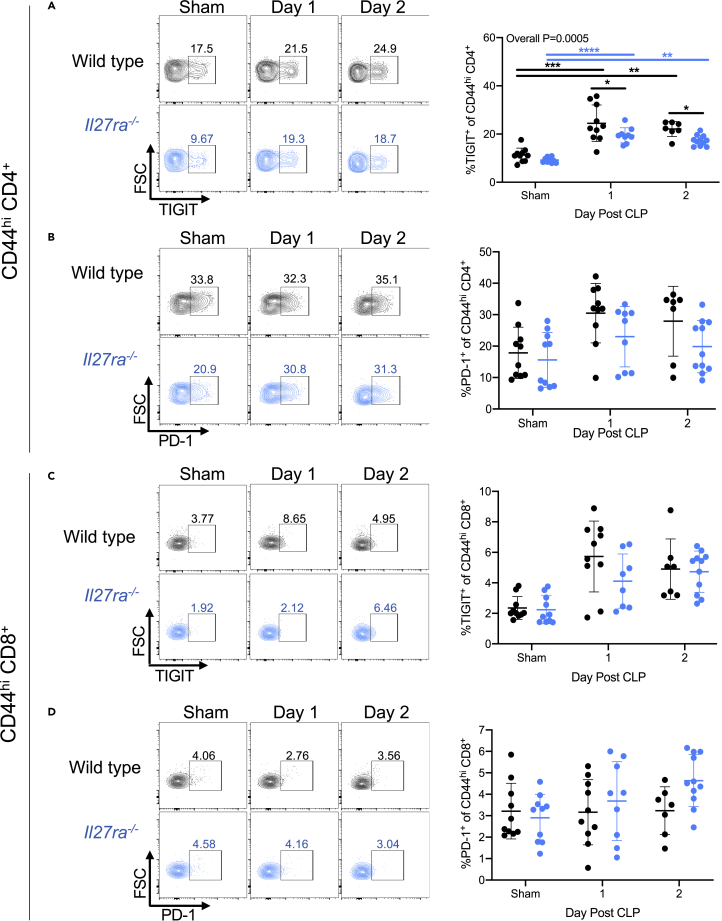
Septic mice lacking the IL-27Rα have a reduced frequency of TIGIT+ memory CD4+ T cells but a similar frequency of Treg compared with wild-type mice
After identifying an association between IL-27Rα positivity and TIGIT expression on CD4+ memory T cells, we assessed TIGIT and PD-1 expression on memory T cells in Il27ra−/− mice to determine whether IL27Rα deficiency altered their expression. First, we compared the frequency of the co-inhibitory molecules TIGIT and PD-1 in the CD44hi T cell compartment of Il27ra−/− with wild-type (WT) mice. We found that frequencies of TIGIT+ cells among the memory CD4+ T cell population increased following CLP in both WT and Il27ra−/− mice compared with the sham group (Figure 5A). However, on days 1 and 2 after CLP, Il27ra−/− mice had a significantly reduced frequency of TIGIT+ memory CD4+ T cells compared with WT mice (Figure 5A). We found that the frequency of PD-1+ cells among CD44hi CD4+ T cells increased in both WT and Il27ra−/− mice following CLP compared with the sham group; however, there was no difference between WT versus Il27ra−/− mice in the sham or CLP groups (Figure 5B). In contrast to the memory CD4+ T cell compartment, the memory CD8+ T cell compartment of the WT versus Il27ra−/− mice showed no difference in the frequency of TIGIT+ cells (Figure 5C) or PD-1+ cells (Figure 5D) among CD44hi CD8+ T cells. These results further support the idea that IL-27 signaling enhances TIGIT expression on CD4+ memory T cells.
Figure 5.
Il27ra−/− septic mice have a reduced frequency of TIGIT+ memory CD4+ T cells
Wild-type and Il27ra−/− mice underwent sham surgery (“sham”) or CLP surgery. On days 1 and 2 following surgery, splenocytes were collected and used for flow cytometric analysis.
(A) Representative flow cytometric plots (left) and summary graphs (right) showing TIGIT expression (x axis) in CD44hi memory CD4+ T cells of wild-type (top) and Il27ra−/− (bottom) mice.
(B) Representative flow cytometric plots (left) and summary graphs (right) showing PD-1 expression (x axis) in CD44hi memory CD4+ T cells of wild-type (top) and Il27ra−/− (bottom) mice.
(C) Representative flow cytometric plots (left) showing TIGIT expression (x axis) in CD44hi memory CD8+ T cells of wild-type (top) and Il27ra−/− (bottom) mice.
(D) Representative flow cytometric plots (left) and summary graphs (right) showing PD-1 expression (x axis) in CD44hi memory CD8+ T cells of wild-type (top) and Il27ra−/− (bottom) mice. Data were pooled from two independent experiments, with n = 7–11 mice per group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Error bars represent the mean ± the standard deviation.
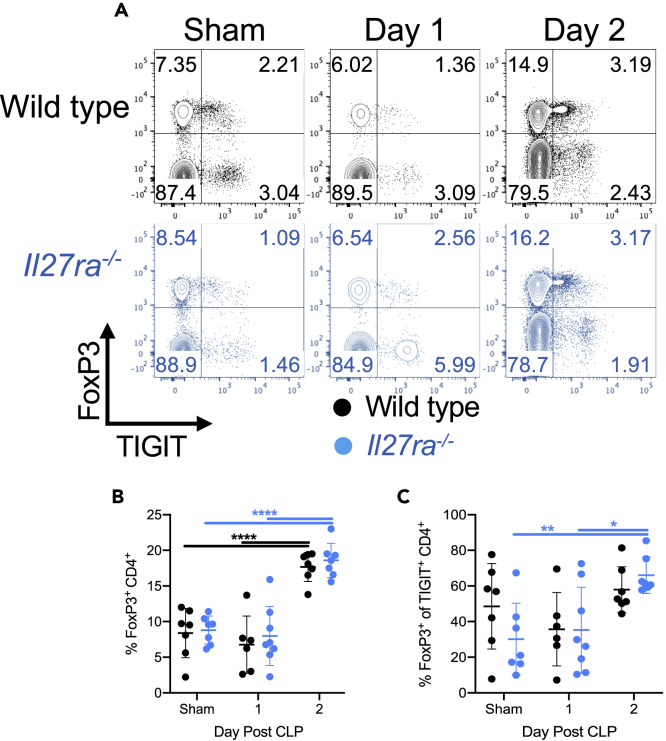
Previous studies showed that IL-27 promotes the development and effector function of T regulatory cells (Tregs) (Do et al., 2017; Kim et al., 2019; Moon et al., 2013; Nguyen et al., 2019; Wehrens et al., 2018). Because many Tregs express the co-inhibitory receptor TIGIT, we next looked to determine the frequency of Tregs in wild-type and Il27ra−/− mice. The frequency of FoxP3+ Tregs significantly increased in both wild-type and Il27ra−/− mice following CLP, but there was no difference in Treg frequency between these groups (Figures 6A and 6B). Within the TIGIT+ CD4+ T cell population, Il27ra−/− mice had a higher frequency of FoxP3+ expression following CLP compared with mice that underwent sham surgery (Figure 6C). However, the frequency of Tregs within the TIGIT+ population was unchanged in wild-type septic mice (Figure 6C).
Figure 6.
IL-27 signaling does not impact the frequency of Tregs during sepsis
One and two days after sham or CLP surgery on wild-type and Il27ra−/− mice, splenocytes were collected and used for flow cytometric analysis.
(A) Representative flow plots showing TIGIT (x axis) versus FoxP3 (y axis) in wild-type (black) and Il27ra−/− (blue) mice that underwent sham surgery (sham) or CLP 1 or 2 days prior.
(B) Summary graph showing the frequency of FoxP3+ CD4+ T cells within wild-type (black) or Il27ra−/− (blue) mice following surgery.
(C) Summary graph showing the frequency of FoxP3+ cells within the TIGIT+ CD4+ compartments of wild-type (black) and Il27ra−/− (blue) mice following sham or CLP surgery. Data were pooled from two independent experiments with n = 6–8 per group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. Error bars represent the mean ± the standard deviation.
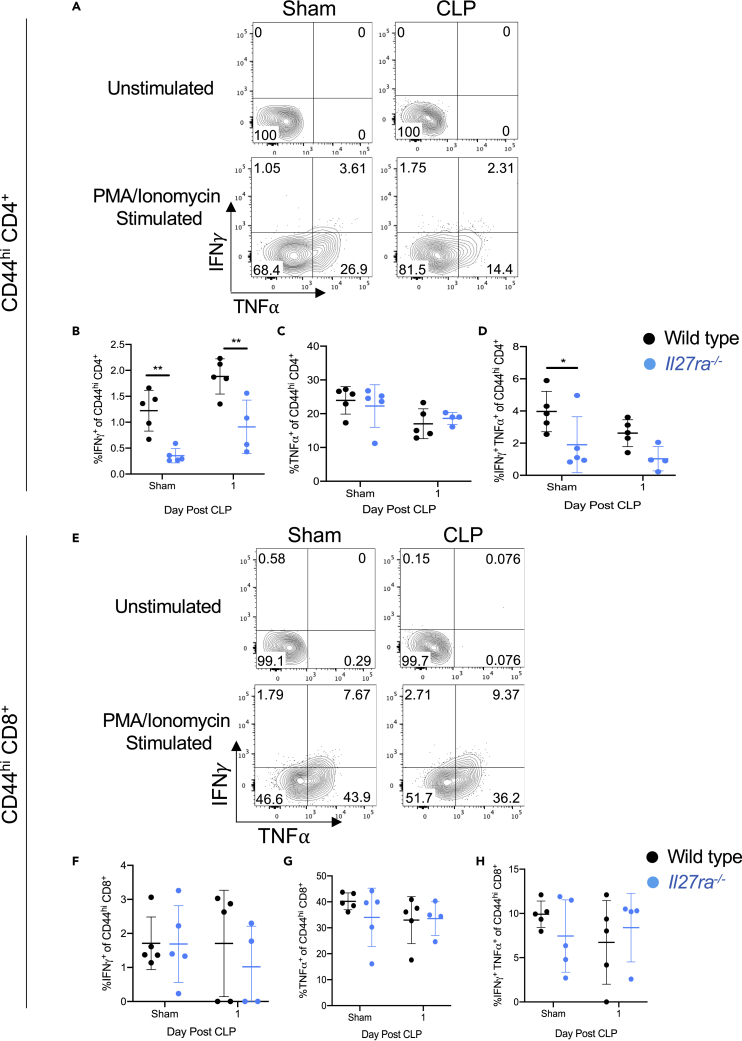
Memory CD4+ T cells in Il27ra−/− mice exhibit impaired production of IFN-γ, but this is not linked to TIGIT expression
To assess whether the IL-27-driven signals during sepsis impair the effector cytokine production, we measured IFN-γ and TNF⍺ production by memory CD4+ and CD8+ T cells in wild-type compared with Il27ra−/− mice following CLP-induced sepsis. Il27ra−/− mice exhibited a reduced frequency of IFN-γ-producing CD44hi CD4+ T cells compared with WT in mice receiving sham surgery and day 1 after CLP-induced sepsis (Figures 7A and 7B). The frequency of TNF⍺-producing CD4+ cells was similar in sham and CLP mice (Figures 7A and 7C). Assessment of the CD44hi CD4+ T cells producing both IFN-γ and TNF⍺ showed reduced frequencies of double-positive cells in the Il27ra−/− mice compared with the WT mice following sham surgery, but this difference did not persist after CLP-induced sepsis (Figure 7D). In the memory CD44hi CD8+ T cell compartment, WT and Il27ra−/− mice exhibited similar frequencies of IFN-γ+ (Figures 7E and 7F), TNF⍺+ (Figures 7E and 7G), and IFN-γ+ TNF⍺+ (Figures 7E and 7H) cells following CLP-induced sepsis. Circulating (plasma) IFN-γ was unchanged between wild-type versus Il27ra−/− mice that underwent sham surgery and CLP (Figure S3). Interestingly, circulating IFN-γ was reduced one and two days after CLP in wild-type mice (Figure S3).
Figure 7.
Memory CD4+ T cells in Il27ra−/– mice exhibit impaired production of IFN-γ at baseline and during sepsis
CLP and sham surgery (“sham”) were performed on wild-type and Il27ra−/− mice. One day after surgery, splenocytes were harvested for stimulation with PMA/Ionomycin or incubated without stimulation. Following incubation, samples were stained for IFN-γ and TNF-α and assessed by flow cytometry.
(A) Representative flow cytometric plots of IFN-γ (y axis) and TNF-α (x axis) production by the CD44hi memory CD4+ T cells of wild-type sham (left) and wild-type CLP mice (right) one day following surgery. The top row shows unstimulated controls, and bottom row shows stimulated samples.
(B) The frequency of IFN-γ producing CD44hi memory CD4+ T cells of wild-type (black) and Il27ra−/− (blue) mice following surgery.
(C) The frequency of TNF-α-producing CD44hi memory CD4+ T cells of wild-type (black) and Il27ra−/− (blue) mice following surgery.
(D) The frequency of IFN-γ and TNF-α co-producing CD44hi memory CD4+ T cells of wild-type (black) and Il27ra−/− (blue) mice following surgery.
(E) Representative flow cytometric plots of IFN-γ (y axis) and TNF-α (x axis) production by the CD44hi memory CD8+ T cells of wild-type sham (left) and wild-type CLP mice (right) one day following surgery. The top row shows unstimulated controls, and bottom row shows stimulated samples.
(F) The frequency of IFN-γ-producing CD44hi memory CD8+ T cells of wild-type (black) and Il27ra−/− (blue) mice following surgery.
(G) The frequency of TNF-α-producing CD44hi memory CD8+ T cells of wild-type (black) and Il27ra−/− (blue) mice following surgery.
(H) The frequency of IFN-γ and TNF-α co-producing CD44hi memory CD8+ T cells of wild-type (black) and Il27ra−/− (blue) mice following surgery. Data are representative of one experiment with n = 4–5 mice per group. ∗p < 0.05, ∗∗p < 0.01. Error bars represent the mean ± the standard deviation.
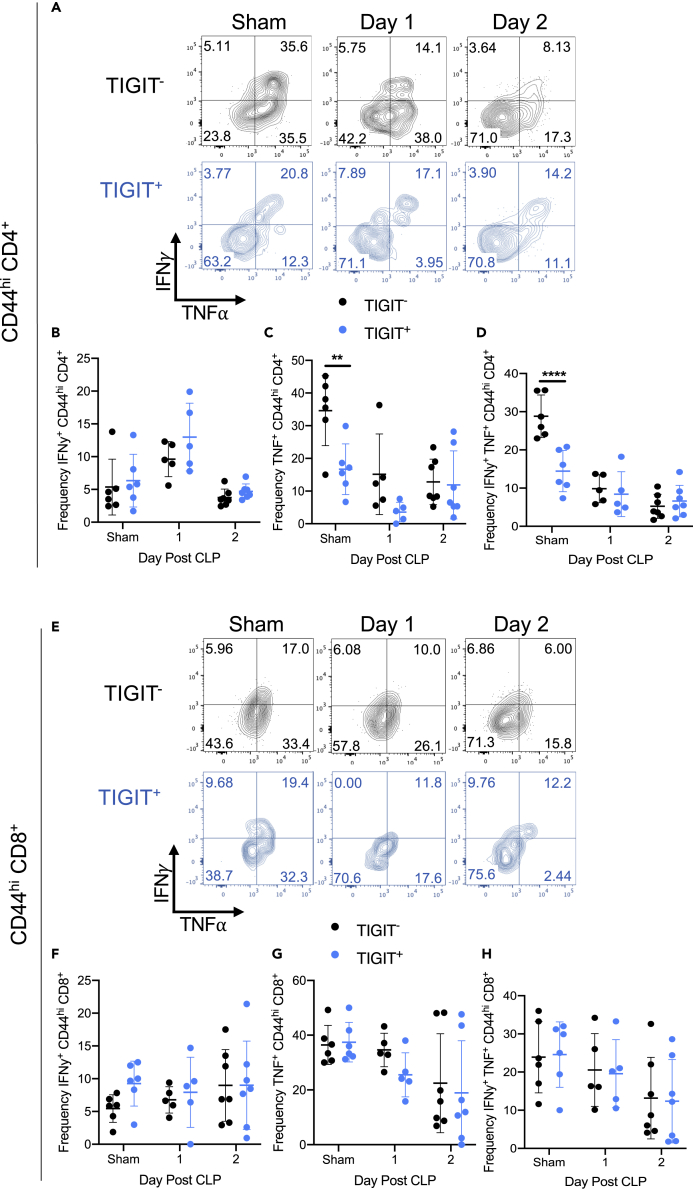
To determine the impact of IL-27-induced TIGIT expression on the production of IFN-γ and TNF⍺, we assessed cytokine production in wild-type IL-27Rα+ CD44hi T cells. TIGIT expression was not associated with a difference in the frequency of IFNγ production by memory CD4+ T cells in non-septic mice that underwent sham surgery or in septic mice (Figures 8A and 8B). However, the TIGIT− memory CD4+ T cells in non-septic mice produced significantly more TNF than did TIGIT+ cells (Figure 8C). The frequency of IFNγ+ TNF+ memory CD4+ T cells was also elevated in the TIGIT− population of non-septic animals (Figure 8D). In the memory CD8+ T cell compartment (Figure 8E), TIGIT expression was not associated with the frequency of IFNγ+- (Figure 8F), TNF+- (Figure 8G), or IFNγ+ TNF+ (Figure 8H)-producing cells. These data indicate that TIGIT expression is not associated with the defect in IFN-γ production by CD44hi CD4+ T cells in Il27ra−/− versus wild-type mice.
Figure 8.
TIGIT is not associated with the impairments observed in cytokine production
CLP and sham surgery (“sham”) were performed on wild-type mice. One and two days after surgery, splenocytes were harvested for stimulation with PMA/Ionomycin or incubated without stimulation. Following incubation, samples were stained for TIGIT, IFN-γ, and TNF-α and assessed by flow cytometry.
(A) Representative flow cytometric plots of IFN-γ (y axis) and TNF-α (x axis) production by TIGIT− (black) and TIGIT+ (blue) CD44hi memory CD4+ T cells following surgery.
(B) The frequency of IFN-γ producing TIGIT− (black) and TIGIT+ (blue) CD44hi memory CD4+ T cells following surgery.
(C) The frequency of TNF-α producing TIGIT− (black) and TIGIT+ (blue) CD44hi memory CD4+ T cells following surgery.
(D) The frequency of IFN-γ and TNF-α co-producing TIGIT− (black) and TIGIT+ (blue) CD44hi memory CD4+ T cells following surgery.
(E) Representative flow cytometric plots of IFN-γ (y axis) and TNF-α (x axis) production by TIGIT− (black) and TIGIT+ (blue) CD44hi memory CD8+ T cells of wild-type mice following sham or CLP surgery.
(F) The frequency of IFN-γ-producing TIGIT− (black) and TIGIT+ (blue) CD44hi memory CD8+ T cells following surgery.
(G) The frequency of TNF-α-producing TIGIT− (black) and TIGIT+ (blue) CD44hi memory CD8+ T cells following surgery.
(H) The frequency of IFN-γ and TNF-α co-producing TIGIT− (black) and TIGIT+ (blue) CD44hi memory CD8+ T cells following surgery. Data are representative of two experiments with n = 5–7 mice per group. ∗∗p < 0.01, ∗∗∗∗p < 0.0001. Error bars represent the mean ± the standard deviation.
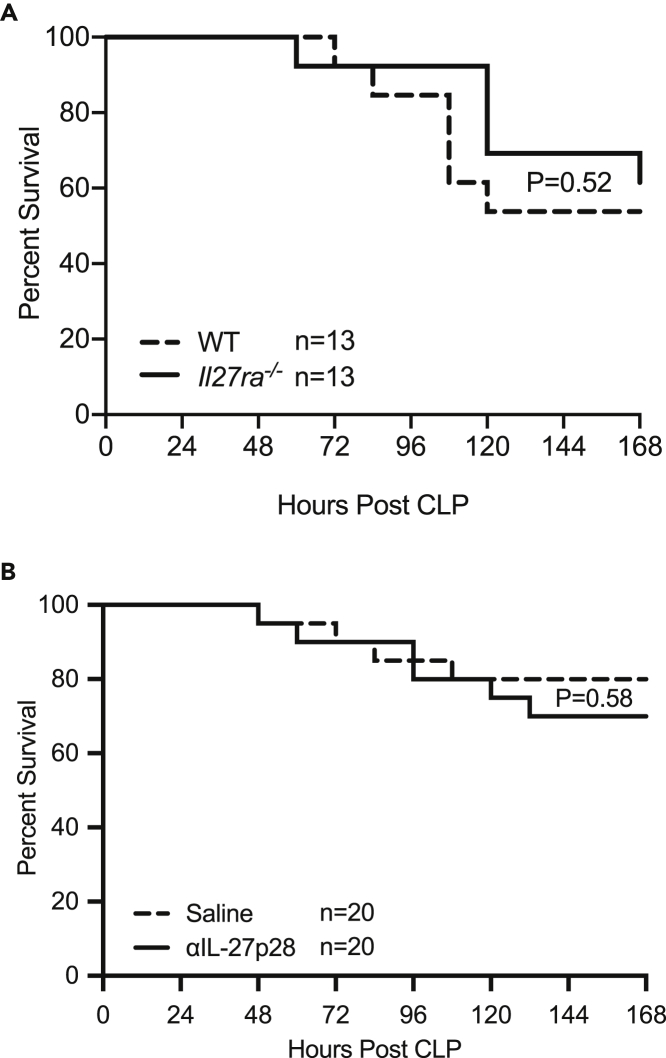
IL-27 signaling does not impact sepsis mortality
Our previous results indicated that IL-27 signaling is associated with an increase in TIGIT-expressing CD4+ memory T cells during sepsis. To determine if these differences resulted in a difference in sepsis survival, we first compared the survival of mice deficient in Il27ra with wild-type mice. In this setting, there was no difference in sepsis mortality between Il27ra−/− and wild-type mice (Figure 9A). To confirm these results, we next used a monoclonal antibody specific to the p28 subunit of IL-27 (α-IL-27p28) to pharmacologically disrupt IL-27 signaling in wild-type mice. Mice that received the α-p28 monoclonal antibody exhibited similar survival compared with saline treated mice over the course of 7 days following CLP (Figure 9B). These results indicate that in a moderate mortality model of sepsis, IL-27 is not associated with worsened survival.
Figure 9.
IL-27 signaling does not impact the survival of septic mice
(A) Wild-type (WT, dotted line) and Il27ra−/− (KO, solid line) mice underwent CLP and were followed for 7 days for survival. Each group contained 13 animals and was age and gender matched.
(B) Wild-type mice underwent CLP and received either saline (dotted line) or anti-IL-27 neutralizing mAb (solid line). Mice were followed for survival for 7 days following surgery. Each group contained 20 animals and was age and gender matched. All data shown are pooled from 2–3 independent experiments.
Discussion
Based on previous reports investigating the role of IL-27 in cancer and chronic infections, we hypothesized that IL-27 signaling upregulates PD-1 and TIGIT on memory T cells during sepsis. Although IL-27Rα was associated with increased TIGIT expression on memory CD4+ T cells following sepsis, this change did not correspond with a difference in T cell apoptosis, effector function, or sepsis survival.
Our results indicate that plasma IL-27 is significantly elevated following CLP, in agreement with previous work (Wirtz et al., 2006; Nelson et al., 2010; Wong et al., 2012, 2013, 2014; Bosmann et al., 2014b; Cao et al., 2014; Hanna et al., 2015; Gao et al., 2016; Yan et al., 2016). Because of previous studies linking IL-27 signaling to sepsis mortality, we sought to determine the impact of IL-27Rα signaling on memory T cells. Specifically, we sought to determine if there was an increase in IL-27Rα expression or changes in T cell apoptosis or cytokine production due to IL-27Rα. We found that the number of IL-27Rα+ CD4+ and CD8+ memory T cells decreased following CLP, but this was not associated with an increase in IL-27Rα expression, T cell apoptosis, or alterations in cytokine production.
The link we observed between IL-27Rα and the upregulation of TIGIT on CD4+ T cells during sepsis is consistent with previous papers that established a similar link in the setting of cancer and chronic infections (Chihara et al., 2018; Delong et al., 2019). In contrast to these studies, however, we did not find that IL-27 regulated TIGIT expression on CD8+ T cells. This suggests that mechanisms other than IL-27 are responsible for the induction of TIGIT on CD8+ T cells. Moreover, prior studies also reported a relationship between IL-27 and PD-1 expression on CD4+ and CD8+ T cells, which we also did not observe (Chihara et al., 2018; Delong et al., 2019). These studies highlight that the impact of IL-27 signaling on TIGIT expression may be disease state and context dependent.
Although our global knockout approach did not parse apart the cell-autonomous versus indirect effect of IL-27Rα signaling on TIGIT expression on CD4+ memory T cells, previous studies have found that TIGIT expression is induced directly by IL-27 on T cells through the action of the transcription factors PRDM1 and c-Maf (Chihara et al., 2018). Another possibility is that IL-27 signaling on other cells subsequently results in the upregulation of TIGIT on CD4+ T cells. As dendritic cells express IL-27Rα and also interact with T cells, it is possible that IL-27 signaling on DC may indirectly regulate TIGIT expression on T cells.
One possible explanation for the association between IL-27 signaling and TIGIT expression and the lack of association between IL-27 signaling and PD-1 expression is the difference in TCR requirements for their expression. DeLong et al. noted that TCR signaling is required in vitro for IL-27 to upregulate the expression of TIGIT, but that TCR signaling was not required for IL-27 to upregulate PD-L1 (Delong et al., 2019). This suggests that TCR signaling in the earliest stages of sepsis could play a role in the ability of IL-27 to upregulate TIGIT. In contrast, the factors regulating IL-27-dependent expression of PD-1 are not involved in the early stages of sepsis. First, multiple pathways regulate the transcription of coinhibitory molecules within T cells. Although no literature exists specifically for TIGIT, Boss and colleagues have shown that Pdcd1 expression is differentially regulated by the duration of antigen exposure (acute versus chronic) (Austin et al., 2014; Bally et al., 2016). In this sepsis model, we were only able to assess the T cell phenotype in settings of acute antigen exposure. It is possible that after longer periods, we would observe the changes noted in previous studies assessing the role of IL-27 in cancer and chronic infection. Despite little known information on the impact of antigen exposure and TIGIT, we observed an association between IL-27 signaling and TIGIT expression at early time points during sepsis. This indicates that acute antigen exposure allows a small but significant number of memory CD4+ T cells to upregulate the co-inhibitory molecule TIGIT. Surprisingly, these effects did not extend to memory CD8+ T cells, something that was previously seen in murine models of chronic infection and cancer. Because sepsis is primarily associated with the activation of CD4+ compared with CD8+ T cells, the difference in activation may explain why TIGIT was not upregulated on memory CD8+ T cells (Patenaude et al., 2005; Mcdunn et al., 2006).
Previous studies linked memory T cell apoptosis to sepsis mortality and the reactivation of latent viral infections in septic humans and mice. Because of the relationship between IL-27 signaling and TIGIT expression, we hypothesized that IL-27 is responsible for memory T cell apoptosis observed during sepsis. Surprisingly, we did not observe any difference in the frequency of apoptotic (Caspase 3/7+) memory T cells between wild-type and Il27ra−/− mice despite the differences in TIGIT expression. These results are not necessarily surprising, as TIGIT is upregulated in a TCR-signaling-dependent mechanism (Delong et al., 2019), and TCR signaling does not lead to the T cell apoptosis observed during sepsis (Unsinger et al., 2006).
In this study, we looked specifically at memory T cells due to the link between sepsis-impaired memory T cells and increased long-term mortality (Duong et al., 2014; Chen et al., 2017; Xie et al., 2019b). In previous studies, IL-27 limited the response of activated and memory T cells (Pflanz et al., 2002; Villarino et al., 2003; Yoshimura et al., 2006; Gwyer Findlay et al., 2014). In line with these results, we found that the frequency of TIGIT+ memory CD4+ T cells in Il27ra−/− mice was significantly reduced 1 and 2 days after CLP. However, the frequency of TIGIT-expressing memory CD4+ T cells in both wild-type and Il27ra−/− mice was similar in mice that underwent sham surgery, suggesting that IL-27 is not required for the induction of TIGIT on memory T cells. In addition, the changes in TIGIT expression induced by sepsis were not associated with alterations in T cell effector function, as the reduction in IFNγ seen in Il27ra−/− memory CD4+ T cells seen after CLP was also present in mice that underwent sham surgery. These findings are consistent with previous studies that found IL-27 signaling induced expression of IFNγ through the transcription factor T-bet (Mayer et al., 2008; Villarino et al., 2003). Interestingly, a more recent study found that IFNγ expression was no different in the Il27ra−/− mice with chronic LCMV infection (Harker et al., 2018), suggesting a disease-state- and context-dependent role of IL-27 signaling in mediating IFNy production.
Multiple previous studies reported a significant increase in sepsis mortality caused by IL-27 signaling. Similar to the present study, these studies used pharmacological inhibitors of IL-27 signaling to measure the impact IL-27 has on sepsis survival (Wirtz et al., 2006; Bosmann et al., 2014a, 2014b). The other studies reported impressive decreases in sepsis mortality following pharmacological inhibition of IL-27 signaling, suggesting a major role for IL-27 in sepsis mortality. We did not observe a similar decrease in mortality following IL-27 blockade. This may be linked to significant differences in the severity of the sepsis model used, as well as the reagents used. Although the previous studies used a high mortality CLP (where the vast majority of mice do not survive), our model used a mild-to-moderate mortality in which 50% or more mice are expected to survive. Thus, our data do not negate the possible role of IL-27 in high-mortality models of sepsis. Another difference in our study compared with the previous studies are the reagents used to interrupt IL-27 signaling: previous studies used a polyclonal α-IL-27 antibody (Bosmann et al., 2014a) or a soluble IL-27Rα (Wirtz et al., 2006). In contrast, we used a monoclonal α-IL-27 neutralizing antibody. Furthermore, our study compared the survival of Il27ra−/− mice with that of wild-type mice using the cecal ligation and puncture model of sepsis. In contrast, a previous study comparing wild-type with Il27ra−/− mice during sepsis used a model of severe endotoxemia (Bosmann et al., 2014b), which may not adequately replicate the inflammation and immunological response of septic patients (Opal et al., 2014; Osuchowski et al., 2018).
Our results indicate that sepsis decreases the frequency and number of IL-27Rα expressing memory T cells. We hypothesized that the decrease in the IL-27Rα+ memory T cell population could occur independently from apoptosis via a TIGIT-mediated inhibition of T cell proliferation (Joller et al., 2011). However, we did not observe a difference in the frequency of proliferating IL-27Rα− versus IL-27Rα+ memory CD4+ T cells, although TIGIT expression was associated with increased proliferation in IL-27Rα+ cells. IL-27Rα+ memory CD8+ T cells had a lower frequency of proliferating cells compared with the IL-27Rα− population, but TIGIT expression was again associated with a higher frequency of proliferation. These data suggest that TIGIT does not inhibit memory T cell proliferation in septic mice. One possibility for the reduction in the IL-27Rα+ memory T cell population is that these cells are leaving the circulation and going to the site of infection (Unsinger et al., 2010). In addition, IL-27Rα expression was associated with an increase in the expression of TIGIT on CD4+ memory T cells in septic mice. Differences in antigen chronicity, TCR signaling, and the cytokine milieu between sepsis and these models may explain this. Future studies investigating the role of each of these factors will be important to establish what is responsible for the upregulation of co-inhibitory markers during sepsis.
Limitations of the study
Our results indicate that plasma IL-27 is significantly elevated following CLP, in agreement with previous work (Wirtz et al., 2006; Nelson et al., 2010; Wong et al., 2012, 2013, 2014; Bosmann et al., 2014b; Cao et al., 2014; Hanna et al., 2015; Gao et al., 2016; Yan et al., 2016). However, this increase did not correlate with an increase in the number of T cells expressing IL-27Rα. We found that both the frequency and number of CD44lo naive and CD44hi memory CD4+ T cells expressing IL-27Rα were reduced following CLP compared with sham mice. We further found that the number of CD44hi CD8+ T cells expressing IL-27Rα+ decreased following sepsis. These findings are in line with published results showing that IL-27Rα can be shed from the cell surface of activated T cells through the action of metalloproteases (Dietrich et al., 2014). Thus, increased metalloprotease-mediated shedding of cell surface IL-27Rα during sepsis might underlie this observation. The reduction in surface expression of IL-27Rα is in contrast to previous research on the role of IL-27 during toxoplasma infection (Villarino et al., 2005). Villarino et al. found that mice infected with toxoplasma have a significantly higher frequency of both CD4+ and CD8+ T cells expressing IL-27Rα compared with uninfected mice (Villarino et al., 2005). Because the immune response to parasitic infection is very distinct from what occurs during sepsis, our results suggest that sepsis differentially regulates IL-27Rα expression.
Previous studies of co-inhibitory receptor expression on T cells following CLP have revealed that co-inhibitory receptor expression remains elevated far longer than expected from T cell activation and is associated with impairments in T cell effector function and ultimately apoptosis. However, TIGIT may also be acting as an activation marker. We cannot conclude whether it was acting as an activator or suppressor of T cell function. In addition, the changes in the frequency of TIGIT expression on T cells in this study were quite small, and the biological significance of these changes is unclear. Few studies have assessed the impact of TIGIT on T cell responses in the context of sepsis, but previous studies in our laboratory have found a similar induction of TIGIT expression in the CD4+ T cells of septic mice with solid tumors (Chen et al., 2019). However, future studies addressing the physiological impact of TIGIT on survival from sepsis are necessary to better understand the biological relevance of these findings.
Although T cell exhaustion is observed early after the onset of sepsis in both mouse models and human patients, it is unclear if the factors that regulate T cell exhaustion in early stages are the same that maintain T cell exhaustion in the weeks to months following sepsis resolution. Future studies that look at these factors will be necessary. Another limitation of this study was the use of immunologically naive mice, which have a lower frequency of memory T cells than do humans. We have previously published data indicating that mice sequentially infected with viral and bacterial infections have a memory compartment that better reflects what is observed in humans (Xie et al., 2019a). Although we failed to observe an association between IL-27 signaling and PD-1 expression in previously healthy septic mice, the findings in mice with pre-existing cancer may be different because there is an association between IL-27 signaling and PD-1 expression in the setting of cancer. In line with this idea, previous research in our laboratories has found that cancer septic mice differ in the expression of co-inhibitory markers on their T cells compared with previously healthy septic mice (Fox et al., 2010; Lyons et al., 2016; Xie et al., 2018; Chen et al., 2019).
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Mandy Ford (mandy.ford@emory.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze datasets or code.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Jacob Kohlmeier (Emory University) for providing IL-27Rα knockout mice and Dr. Jennifer Robertson for technical assistance. We also thank Kelsey Bennion, Dr. Anna Morris, and Dr. Marvi Tariq for critical reading of the manuscript. The study was funded by NIH R01s GM104323 GM095442, GM072808, and AI149724. The authors declare no competing interests.
Authors contribution
Conceptualization: K.N.M., C.M.C., and M.L.F.; Formal Analysis: K.N.M., C.M.C., and M.L.F.; Investigation: K.N.M., Z.L., M.X., D.B.C., Y.S., and C.C.; Writing—Original Draft: K.N.M.; Writing—Review and Editing: K.N.M., C.M.C., and M.L.F.; Visualization: K.N.M., C.M.C., and M.L.F.; Supervision: C.M.C. and M.L.F.; Funding Acquisition: C.M.C. and M.L.F.
Declaration of interests
The authors declare no competing interests.
Published: February 19, 2021
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2021.102093.
Contributor Information
Craig M. Coopersmith, Email: cmcoop3@emory.edu.
Mandy L. Ford, Email: mandy.ford@emory.edu.
Supplemental information
References
- Ammer-Herrmenau C., Kulkarni U., Andreas N., Ungelenk M., Ravens S., Hubner C., Kather A., Kurth I., Bauer M., Kamradt T. Sepsis induces long-lasting impairments in CD4+ T-cell responses despite rapid numerical recovery of T-lymphocyte populations. PLoS One. 2019;14:e0211716. doi: 10.1371/journal.pone.0211716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J.W., Lu P., Majumder P., Ahmed R., Boss J.M. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J. Immunol. 2014;192:4876–4886. doi: 10.4049/jimmunol.1302750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally A.P., Austin J.W., Boss J.M. Genetic and epigenetic regulation of PD-1 expression. J. Immunol. 2016;196:2431–2437. doi: 10.4049/jimmunol.1502643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomer J.S., Shuherk-Shaffer J., Hotchkiss R.S., Green J.M. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit. Care. 2012;16:R112. doi: 10.1186/cc11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomer J.S., To K., Chang K.C., Takasu O., Osborne D.F., Walton A.H., Bricker T.L., Jarman S.D., 2nd, Kreisel D., Krupnick A.S. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann M., Russkamp N.F., Strobl B., Roewe J., Balouzian L., Pache F., Radsak M.P., Van Rooijen N., Zetoune F.S., Sarma J.V. Interruption of macrophage-derived IL-27(p28) production by IL-10 during sepsis requires STAT3 but not SOCS3. J. Immunol. 2014;193:5668–5677. doi: 10.4049/jimmunol.1302280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann M., Strobl B., Kichler N., Rigler D., Grailer J.J., Pache F., Murray P.J., Muller M., Ward P.A. Tyrosine kinase 2 promotes sepsis-associated lethality by facilitating production of interleukin-27. J. Leukoc. Biol. 2014;96:123–131. doi: 10.1189/jlb.3A1013-541R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Xu F., Lin S., Song Z., Zhang L., Luo P., Xu H., Li D., Zheng K., Ren G., Yin Y. IL-27 controls sepsis-induced impairment of lung antibacterial host defence. Thorax. 2014;69:926–937. doi: 10.1136/thoraxjnl-2014-205777. [DOI] [PubMed] [Google Scholar]
- Chen C.W., Mittal R., Klingensmith N.J., Burd E.M., Terhorst C., Martin G.S., Coopersmith C.M., Ford M.L. Cutting edge: 2B4-mediated coinhibition of CD4(+) T cells underlies mortality in experimental sepsis. J. Immunol. 2017;199:1961–1966. doi: 10.4049/jimmunol.1700375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.W., Xue M., Zhang W., Xie J., Coopersmith C.M., Ford M.L. 2B4 but not PD-1 blockade improves mortality in septic animals with preexisting malignancy. JCI Insight. 2019;4:e127867. doi: 10.1172/jci.insight.127867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara N., Madi A., Kondo T., Zhang H., Acharya N., Singer M., Nyman J., Marjanovic N.D., Kowalczyk M.S., Wang C. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 2018;558:454–459. doi: 10.1038/s41586-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement M., Marsden M., Stacey M.A., Abdul-Karim J., Gimeno Brias S., Costa Bento D., Scurr M.J., Ghazal P., Weaver C.T., Carlesso G. Cytomegalovirus-specific IL-10-producing CD4+ T cells are governed by type-I IFN-induced IL-27 and promote virus persistence. PLoS Pathog. 2016;12:e1006050. doi: 10.1371/journal.ppat.1006050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong J.H., O'hara Hall A., Rausch M., Moodley D., Perry J., Park J., Phan A.T., Beiting D.P., Kedl R.M., Hill J.A., Hunter C.A. IL-27 and TCR stimulation promote T cell expression of multiple inhibitory receptors. Immunohorizons. 2019;3:13–25. doi: 10.4049/immunohorizons.1800083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C., Candon S., Ruemmele F.M., Devergne O. A soluble form of IL-27Ralpha is a natural IL-27 antagonist. J. Immunol. 2014;192:5382–5389. doi: 10.4049/jimmunol.1303435. [DOI] [PubMed] [Google Scholar]
- Do J., Kim D., Kim S., Valentin-Torres A., Dvorina N., Jang E., Nagarajavel V., Desilva T.M., Li X., Ting A.H. Treg-specific IL-27Ralpha deletion uncovers a key role for IL-27 in Treg function to control autoimmunity. Proc. Natl. Acad. Sci. U S A. 2017;114:10190–10195. doi: 10.1073/pnas.1703100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong S., Condotta S.A., Rai D., Martin M.D., Griffith T.S., Badovinac V.P. Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J. Immunol. 2014;192:3618–3625. doi: 10.4049/jimmunol.1303460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer R., Artigas A., Suarez D., Palencia E., Levy M.M., Arenzana A., Perez X.L., Sirvent J.M., Edusepsis Study G. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am. J. Respir. Crit. Care Med. 2009;180:861–866. doi: 10.1164/rccm.200812-1912OC. [DOI] [PubMed] [Google Scholar]
- Fox A.C., Robertson C.M., Belt B., Clark A.T., Chang K.C., Leathersich A.M., Dominguez J.A., Perrone E.E., Dunne W.M., Hotchkiss R.S. Cancer causes increased mortality and is associated with altered apoptosis in murine sepsis. Crit. Care Med. 2010;38:886–893. doi: 10.1097/CCM.0b013e3181c8fdb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Yang Y.Z., Feng X.Y., Fan T.T., Jiang L., Guo R., Liu Q. Interleukin-27 is elevated in sepsis-induced myocardial dysfunction and mediates inflammation. Cytokine. 2016;88:1–11. doi: 10.1016/j.cyto.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Guzzo C., Ayer A., Basta S., Banfield B.W., Gee K. IL-27 enhances LPS-induced proinflammatory cytokine production via upregulation of TLR4 expression and signaling in human monocytes. J. Immunol. 2012;188:864–873. doi: 10.4049/jimmunol.1101912. [DOI] [PubMed] [Google Scholar]
- Gwyer Findlay E., Villegas-Mendez A., O'regan N., De Souza J.B., Grady L.M., Saris C.J., Riley E.M., Couper K.N. IL-27 receptor signaling regulates memory CD4+ T cell populations and suppresses rapid inflammatory responses during secondary malaria infection. Infect. Immun. 2014;82:10–20. doi: 10.1128/IAI.01091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna W.J., Berrens Z., Langner T., Lahni P., Wong H.R. Interleukin-27: a novel biomarker in predicting bacterial infection among the critically ill. Crit. Care. 2015;19:378. doi: 10.1186/s13054-015-1095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker J.A., Wong K.A., Dallari S., Bao P., Dolgoter A., Jo Y., Wehrens E.J., Macal M., Zuniga E.I. Interleukin-27R signaling mediates early viral containment and impacts innate and adaptive immunity after chronic lymphocytic choriomeningitis virus infection. J. Virol. 2018;92 doi: 10.1128/JVI.02196-17. e02196–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joller N., Hafler J.P., Brynedal B., Kassam N., Spoerl S., Levin S.D., Sharpe A.H., Kuchroo V.K. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Le H.T., Nguyen Q.T., Kim S., Lee J., Min B. Cutting edge: IL-27 attenuates autoimmune neuroinflammation via regulatory T cell/Lag3-dependent but IL-10-independent mechanisms in vivo. J. Immunol. 2019;202:1680–1685. doi: 10.4049/jimmunol.1800898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J.D., Mittal R., Fay K.T., Chen C.W., Liang Z., Margoles L.M., Burd E.M., Farris A.B., Ford M.L., Coopersmith C.M. Murine lung cancer increases CD4+ T cell apoptosis and decreases gut proliferative capacity in sepsis. PLoS One. 2016;11:e0149069. doi: 10.1371/journal.pone.0149069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer K.D., Mohrs K., Reiley W., Wittmer S., Kohlmeier J.E., Pearl J.E., Cooper A.M., Johnson L.L., Woodland D.L., Mohrs M. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J. Immunol. 2008;180:693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- Mcdunn J.E., Turnbull I.R., Polpitiya A.D., Tong A., Macmillan S.K., Osborne D.F., Hotchkiss R.S., Colonna M., Cobb J.P. Splenic CD4+ T cells have a distinct transcriptional response six hours after the onset of sepsis. J. Am. Coll. Surg. 2006;203:365–375. doi: 10.1016/j.jamcollsurg.2006.05.304. [DOI] [PubMed] [Google Scholar]
- Moon S.J., Park J.S., Heo Y.J., Kang C.M., Kim E.K., Lim M.A., Ryu J.G., Park S.J., Park K.S., Sung Y.C. In vivo action of IL-27: reciprocal regulation of Th17 and Treg cells in collagen-induced arthritis. Exp. Mol. Med. 2013;45:e46. doi: 10.1038/emm.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D.A., Tolbert M.D., Clemens M.G., Bost K.L. Interleukin-27 expression following infection with the murine gammaherpesvirus 68. Cytokine. 2010;51:184–194. doi: 10.1016/j.cyto.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Nguyen Q.T., Jang E., Le H.T., Kim S., Kim D., Dvorina N., Aronica M.A., Baldwin W.M., Iii, Asosingh K., Comhair S., Min B. IL-27 targets Foxp3+ Tregs to mediate antiinflammatory functions during experimental allergic airway inflammation. JCI Insight. 2019;4:e123216. doi: 10.1172/jci.insight.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal S.M., Dellinger R.P., Vincent J.L., Masur H., Angus D.C. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C?∗. Crit. Care Med. 2014;42:1714–1721. doi: 10.1097/CCM.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuchowski M.F., Ayala A., Bahrami S., Bauer M., Boros M., Cavaillon J.M., Chaudry I.H., Coopersmith C.M., Deutschman C.S., Drechsler S. Minimum quality threshold in pre-clinical sepsis studies (MQTiPSS): an international expert consensus initiative for improvement of animal modeling in sepsis. Shock. 2018;50:377–380. doi: 10.1097/SHK.0000000000001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude J., D'elia M., Hamelin C., Garrel D., Bernier J. Burn injury induces a change in T cell homeostasis affecting preferentially CD4+ T cells. J. Leukoc. Biol. 2005;77:141–150. doi: 10.1189/jlb.0703314. [DOI] [PubMed] [Google Scholar]
- Patin E.C., Jones A.V., Thompson A., Clement M., Liao C.T., Griffiths J.S., Wallace L.E., Bryant C.E., Lang R., Rosenstiel P. IL-27 induced by select Candida spp. via TLR7/NOD2 signaling and IFN-beta production inhibits fungal clearance. J. Immunol. 2016;197:208–221. doi: 10.4049/jimmunol.1501204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflanz S., Timans J.C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Prescott H.C., Osterholzer J.J., Langa K.M., Angus D.C., Iwashyna T.J. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonell K.M., Zhang W., Hadley A., Chen C.W., Fay K.T., Lyons J.D., Klingensmith N.J., Mcconnell K.W., Coopersmith C.M., Ford M.L. CXCR4 blockade decreases CD4+ T cell exhaustion and improves survival in a murine model of polymicrobial sepsis. PLoS One. 2017;12:e0188882. doi: 10.1371/journal.pone.0188882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbanescu M.A., Ramonell K.M., Hadley A., Margoles L.M., Mittal R., Lyons J.D., Liang Z., Coopersmith C.M., Ford M.L., Mcconnell K.W. Attrition of memory CD8 T cells during sepsis requires LFA-1. J. Leukoc. Biol. 2016;100:1167–1180. doi: 10.1189/jlb.4A1215-563RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour C.W., Gesten F., Prescott H.C., Friedrich M.E., Iwashyna T.J., Phillips G.S., Lemeshow S., Osborn T., Terry K.M., Levy M.M. Time to treatment and mortality during mandated emergency care for sepsis. N. Engl. J. Med. 2017;376:2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjaastad F.V., Condotta S.A., Kotov J.A., Pape K.A., Dail C., Danahy D.B., Kucaba T.A., Tygrett L.T., Murphy K.A., Cabrera-Perez J. Polymicrobial sepsis chronic immunoparalysis is defined by diminished Ag-specific T cell-dependent B cell responses. Front. Immunol. 2018;9:2532. doi: 10.3389/fimmu.2018.02532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowrirajan B., Saito Y., Poudyal D., Chen Q., Sui H., Deravin S.S., Imamichi H., Sato T., Kuhns D.B., Noguchi N. Interleukin-27 enhances the potential of reactive oxygen species generation from monocyte-derived macrophages and dendritic cells by induction of p47(phox) Sci. Rep. 2017;7:43441. doi: 10.1038/srep43441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsinger J., Herndon J.M., Davis C.G., Muenzer J.T., Hotchkiss R.S., Ferguson T.A. The role of TCR engagement and activation-induced cell death in sepsis-induced T cell apoptosis. J. Immunol. 2006;177:7968–7973. doi: 10.4049/jimmunol.177.11.7968. [DOI] [PubMed] [Google Scholar]
- Unsinger J., Mcglynn M., Kasten K.R., Hoekzema A.S., Watanabe E., Muenzer J.T., Mcdonough J.S., Tschoep J., Ferguson T.A., Mcdunn J.E. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino A., Hibbert L., Lieberman L., Wilson E., Mak T., Yoshida H., Kastelein R.A., Saris C., Hunter C.A. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Villarino A.V., Larkin J., 3rd, Saris C.J., Caton A.J., Lucas S., Wong T., De Sauvage F.J., Hunter C.A. Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J. Immunol. 2005;174:7684–7691. doi: 10.4049/jimmunol.174.12.7684. [DOI] [PubMed] [Google Scholar]
- Wehrens E.J., Wong K.A., Gupta A., Khan A., Benedict C.A., Zuniga E.I. IL-27 regulates the number, function and cytotoxic program of antiviral CD4 T cells and promotes cytomegalovirus persistence. PLoS One. 2018;13:e0201249. doi: 10.1371/journal.pone.0201249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S., Tubbe I., Galle P.R., Schild H.J., Birkenbach M., Blumberg R.S., Neurath M.F. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J. Exp. Med. 2006;203:1875–1881. doi: 10.1084/jem.20060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.R., Cvijanovich N.Z., Hall M., Allen G.L., Thomas N.J., Freishtat R.J., Anas N., Meyer K., Checchia P.A., Lin R. Interleukin-27 is a novel candidate diagnostic biomarker for bacterial infection in critically ill children. Crit. Care. 2012;16:R213. doi: 10.1186/cc11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.R., Lindsell C.J., Lahni P., Hart K.W., Gibot S. Interleukin 27 as a sepsis diagnostic biomarker in critically ill adults. Shock. 2013;40:382–386. doi: 10.1097/SHK.0b013e3182a67632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.R., Liu K.D., Kangelaris K.N., Lahni P., Calfee C.S. Performance of interleukin-27 as a sepsis diagnostic biomarker in critically ill adults. J. Crit. Care. 2014;29:718–722. doi: 10.1016/j.jcrc.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Chen C.W., Sun Y., Laurie S.J., Zhang W., Otani S., Martin G.S., Coopersmith C.M., Ford M.L. Increased attrition of memory T cells during sepsis requires 2B4. JCI Insight. 2019;4:e126030. doi: 10.1172/jci.insight.126030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Crepeau R.L., Chen C.W., Zhang W., Otani S., Coopersmith C.M., Ford M.L. Sepsis erodes CD8(+) memory T cell-protective immunity against an EBV homolog in a 2B4-dependent manner. J. Leukoc. Biol. 2019;105:565–575. doi: 10.1002/JLB.4A0718-292R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Robertson J.M., Chen C.W., Zhang W., Coopersmith C.M., Ford M.L. Pre-existing malignancy results in increased prevalence of distinct populations of CD4+ T cells during sepsis. PLoS One. 2018;13:e0191065. doi: 10.1371/journal.pone.0191065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Mitra A., Hu J., Cutrera J.J., Xia X., Doetschman T., Gagea M., Mishra L., Li S. Interleukin-30 (IL27p28) alleviates experimental sepsis by modulating cytokine profile in NKT cells. J. Hepatol. 2016;64:1128–1136. doi: 10.1016/j.jhep.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Takeda A., Hamano S., Miyazaki Y., Kinjyo I., Ishibashi T., Yoshimura A., Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J. Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets or code.