Abstract
Reactive oxygen species (ROS) are produced as a result of various environmental factors and cellular metabolism reactions creating oxidative stress. The reversible oxidative modification on proteins such as cysteine oxidation may be useful and can play positive role. ROS generated offer some benefits such as cellular signalling and tissue repair when present in low concentration. However, most of the times, these reactive species cause detrimental effects to cell components which leads to various pathological conditions which causes or aggravates diseases due to oxidative stress. The degenerative diseases due to oxidative stress are diabetes, cardiovascular diseases, epilepsy, cancer and aging. Antioxidants are the compounds which scavenge these free radicals and hence neutralize their effects. Research has enabled the use of natural antioxidants as therapeutic agent in the treatment of diseases. Safranal is one such natural agent which is a major volatile component of saffron. Saffron, Red gold is the most expensive spice found in limited region of the planet and is also reported to be used in traditional systems of medicine. Chemically, safranal is a monoterpene aldehyde possessing a sweet fragrance. While exploring for the photoprotective properties of safranal, we learnt about the immense antioxidant potential of safranal. Investigation by various research groups established safranal as an anti-inflammatory, antidepressant, anxiolytic, antiasthamatic, antihypertensive, anticonvulsant, anticancer and antitussive and antigenotoxic agent. It has brought researchers over the world to explore the antioxidant benefits of saffron for human health. In the present paper, potential of safranal and its related molecules as radical scavenger in combating oxidative stress, diseased conditions is collated and the underlying mechanisms have been explained. Various cell lines and animal models used for study of Safranal have been discussed.
Keywords: Safranal, Oxidative stress, Antioxidant, Diseases, Mechanism
Safranal; Oxidative stress; Antioxidant; Diseases; Mechanism
1. Introduction
Reactive oxygen species (ROS) is generated in various metabolic reactions in the forms of superoxide anion radical (·O2−), hydroxyl radical (·OH) and hydrogen peroxide (H2O2). These free radicals being unstable are highly reactive and consequently cause potential detrimental effects (Hel and Schröder, 2018). ROS generated function as a key secondary messengers in numerous signalling pathways, including transcriptional regulation, differentiation and cell apoptosis (Paik et al., 2011).
Although detrimental in nature, but few studies of their beneficial effects have been reported. ROS appeared to play an important role in proapoptotic pathway in Cutaneous T-cell lymphoma (CTCL) when treated with the indirubin derivative DKP-071 (Soltan et al., 2019). In a search for newer antitumor strategy, a sharp decrease in cell proliferation and cell viability was observed with DKP-071. This plant based derivative activated the extrinsic apoptosis cascade via caspase-8 and caspase-3 by down regulating caspase antagonistic proteins (c-FLIP and XIAP). A hike in level of reactive oxygen species (ROS) was observed as an immediate early effect explaining the mode of action of this indirubin derivative.
Free radicals are also involved in a number of cellular signaling pathways (Pizzino et al., 2017). They play multiple beneficial roles when present in less or moderate amount. They are required for synthesis of some cellular structures which constitutes part of defense system of the body. Phagocytes synthesize as well as store free radicals and release them when invading pathogenic micro-organisms are to be killed.
Low concentrations of ROS is also reported to enhance cell survival and proliferation (Serras, 2016). Intracellular reduction–oxidation state which in turn depends on balance between reducing and oxidizing equivalents regulate various molecules entailed in ROS-induced cellular responses.
However, Imbalance between ROS and antioxidant defense system of the body damage organic molecules of biological importance such as DNA, RNA, lipids and proteins and are considered to cause various pathological states in humans (Sarangarajan et al., 2017). Human bodies have their own antioxidant defence system which is capable of adapting to the varying levels of oxidants in order to maintain the oxidant-antioxidant balance. This redox homeostasis in the body includes various antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase. Oxidative stress arises when balance between oxidants and antioxidants disrupts which lead to deleterious effects responsible for diseases such as cancer, cardiovascular diseases, sunburn, and other neurodegenerative diseases (Dunaway et al., 2018).
The pathological conditions of these diseases are reported to reduce through intake of antioxidants. Antioxidants are the compounds which act as first line of defence against free radical damage and critical for maintaining good health (Anuj Yadav et al., 2016). These compounds inhibit oxidation, scavenge free radicals, turn them into less active forms, prevent oxidative stress diseases and serve as critical component in maintaining optimum health and well being (Anuj Yadav et al., 2016).
Antioxidant compounds of natural origin have the capability to provide protection against ROS species. These components have been shown to reduce incidence of photocarcinogenesis and photoaging (Serafini et al., 2015). One such natural biomolecule is Safranal which has great antioxidant potential and reported to be a good therapeutic agent in diseases arising due to oxidative stress. It is the most abundant volatile compound obtained from stigmas of the flowers of saffron, Crocus sativus L. (Family Iridaceae). It represents about 30–70 % of volatile components and 0.001–0.006 % of dry matter of saffron. It is a product of natural deglycosylation of picrocrocin and chemically it is 2,6,6-trimethyl-1,3-cyclohexadien-1-carboxaldehyde. It is present in low amount in fresh stigmas and after drying of the stigmas or the passage of time, it is produced in high amount from its precursor (Rahaiee et al., 2015).
Saffron is found in Iran, Italy, India, Spain and Greece and is mainly valued as an additive for its property of tasting, flavoring and coloring food (Heydari and Haghayegh, 2014). Besides, It is widely cultivated in Azerbaijan, China, Egypt, France, Israel, Mexico, Morocco and Turkey (Patel et al., 2017). In India, the crop is cultivated in the valley of Kashmir. Saffron is bitter in taste but still used as food additive due to its antioxidant properties (Christodoulou et al., 2017). The cultivation of this plant dates back to almost 2300 B.C. by Sargon; in village Azupirano meaning thereby Saffron town (Srivastava et al., 2010). In Islamic period, It was mentioned in “Al-Hawi”, a 25 volume medical encyclopedia written by Zakariya Razi known as Rhazes in 865–925 A. D (Mollazadeh et al., 2015). It is one of the ancient medicinal plants with miracle effect in the treatment of various disorders (Khorasany and Hosseinzadeh, 2016).
C. sativus has been used traditionally in alternative system of medicine for various diseases. These medicinal effects are due to its major constituents like crocins, crocetin and safranal.
In addition to this, many non-volatile components also exist such as carotenoids - α- and β- carotene, zeaxanthin and lycopene.
The literature reveals use of saffron in treatment of coronary artery diseases, neurodegenerative disorders, asthma, diabetes and bronchitis. It has also been used as a food supplement, in drinks and beverages, in pharmaceutical preparations and cosmetic formulations due to beneficial properties (Assimopoulou et al., 2005a). These medicinal properties of saffron indicate that saffron has a potent antioxidant activity which is mostly due to the presence of unique carotenoids. Various parts of flowers of saffron including tepals, stigmas, styles and stamens have been explored and established to have antioxidant potential when tested using in-vitro assays namely lipid peroxidation, trolox equivalent antioxidant capacity and deoxyribose assay (Am et al., 2012). The other mechanisms of action proposed are its anti inflammatory and immunomodulatory properties.
Almost all allies of genus Crocus are diploid with an exception to C. sativus. This is triploid in genetic makeup with highly erratic meiosis which results into unbalanced or sterile gametes with no seed formation (Ghaffari and Bagheri, 2009). Since propagation by vegetative means through corms give poor yield, various breeding and biotechnological techniques like clonal selection, mutagenesis, micropropogation, molecular markers have been used as alternative method for saffron propagation (Mir et al., 2015). Thus, saffron cultivation is affected by many such factors which are responsible for making it the world's most expensive spice. Due to this, it has been named as ‘Red Gold’ in Iran. It is also prone to adulteration because of the cost factor.
2. Extraction and identification of safranal
Saffron consists of more than 150 chemicals with different properties that make its extraction, separation and quantification a challenging task.
Soxhlet extraction is the technique used since ages for isolating natural compounds. However, the technique is limited to thermally stable and volatile components (Wang and Weller, 2006). Solvent based extraction is another conventional technique used for procurement of bioactive constituents from saffron where stigmas of the flowers are extracted with solvents like methanol or ethanol after defatting. This process is repeated 2–3 times and extract is evaporated to dryness and purified for elution of different components using silica gel column chromatography. Large amount of solvent alongwith poor yield led to newer techniques like ultrasound extraction. This technique was used initially for extraction of Iranian saffron. The different extraction factors involved such as sample amount, solvent ratio, solvent volume, extraction time have been optimized by Heravi et al. The volatile components extracted were further separated with GC-MS and characterized (Jalali-Heravi et al., 2009). The isolation of safranal has also been achieved using the techniques of steam distillation, micro-steam distillation extraction and vacuum head space method and the various volatile components were further identified similarly with help of GC-MS (Carmona et al., 2007). A novel non destructive method was designed to extract the volatile compounds of saffron using supercritical carbon dioxide which further employed HPLC and GC for assay (Lozano et al., 2000).
Besides saffron, Safranal has also been successfully extracted from cumin seeds (Cuminum cyminum L.) using various extraction techniques (Kulkarni et al., 2014). Highest yield (44.8679 μg/g of cumin) was obtained with ultrasound assisted extraction where drug was given pre-treatment with microwave. In another study, a simple, rapid and reproducible UV spectrophotometric method of identification and estimation of safranal was developed by Sinica et al. (2013). The method was validated with respect to range, accuracy, precision, linearity and specificity. The drug followed Lambert beer's law in the concentration range of 0.001–0.01 μg/ml with 0.995 as regression coefficient. Bononi et al. applied and proposed Gas Chromatography as a more accurate and useful technique to quantify safranal (Bononi et al., 2015). The research group used ethyl alcohol as solvent in this new extraction method and obtained a linear response with R(2) = 0.995 and a reproducibility standard deviation of 4.7–6.0%. However, absorbance values of safranal did not correlate with the one obtained in UV.
HPTLC was also explored as a novel quantitative and qualitative technique of determination of safranal in saffron and safranal loaded nanoparticles. This method developed was selective for drug and proved to be accurate (recovery 97.4–102.0%) and linear (Pathan et al., 2009). Another technique to quantify safranal was carried out by Mohammad et al. He coined term “Safranal value”, a widely used quality measure determined by hydrolysis reaction carried out in dynamic supercritical-CO2 environment.
Heydari and Haghayegh have also compared the advantages and limitations of various techniques of extraction (Heydari and Haghayegh, 2014). They have proposed microextraction techniques namely solid phase microextraction (SPME), stir-bar sorptive extraction (SBSE), and liquid phase microextraction (LPME) as better for the analysis of saffron metabolites.
Besides safranal, saffron contains primary metabolites such as carbohydrates, minerals, fats vitamins (Melnyk et al., 2010), and more than other 150 volatile compounds chiefly crocin (mono-glycosyl or di-glycosyl polyene esters) and picrocrocin (mono-glycosyl or di-glycosyl polyene esters) (Srivastava et al., 2010). These compounds are together responsible not only for sensory profile of saffron but also for their health-promoting properties.
3. Antioxidant effect of saffron
Antioxidants are the molecules which scavenge free radicals arising out of oxidative stress. Most of the antioxidants taken exogenously are phytochemicals obtained from plants (Sarangarajan et al., 2017). They belong to different classes of secondary metabolites including alkaloids, isothiocyanates, carotenoids and flavonoids (Dinkova-Kostova, 2008). These antioxidants are tested for their antioxidant potential. A large number of in-vitro and in vivo methods of determination of antioxidant capacity of natural compounds have been developed. The choice of in vitro method depends upon the presence of functional group present in the antioxidant molecule and the solvent which can extract this molecule and show its antioxidant property. The methods are divided into two broad categories. Hydrogen atom transfer reactions and electron transfer reactions. The first category of reactions involves the transfer of hydrogen atom present in the molecule such as polyphenols and flavonoids and the latter involve the transfer of electrons. This category includes methods like diphenyl-picryl-hydrazyl radical scavenging assay (DPPH) method and ferric reducing antioxidant power (FRAP). An overview on various antioxidant methods with their respective models and standards is given by S. Chanda and R. Dave (Chanda and Dave, 2009). A comparison of various antioxidant methods has also been done by Badarinath et al. .He provided general aspects of various in-vitro antioxidant methods like superoxide radical scavenging activity (SOD), reducing power method (RP), hdroxyl radical scavenging activity, hydroxyl radical averting capacity (HORAC), ferric thiocyanate (FTC), Thiobarbituric acid (TBA) method, oxygen radical absorbance capacity (ORAC) and phosphomolybdenum method (Badarinath et al., 2010).
In-vivo methods involve use of test animals such as mice and rats where these animals are administered an antioxidant in a proper dosage regimen and thereafter sampling their blood or tissue for assay (Alam et al., 2013). The various in vivo methods rely on the presence of either enzymes such as catalase, glutathione reductase or molecules prone to oxidation thereby such as low density lipoproteins (LDL). These include ferric reducing ability of plasma, glutathione peroxidase (GSHPx) estimation, reduced glutathione (GSH) estimation, lipid peroxidation (LPO) assay, superoxide dismutase (SOD) method, catalase (CAT), c-Glutamyl transpeptidase activity (GGT) assay (Badarinath et al., 2010).
The antioxidant capacity of saffron has also been established by various in-vitro techniques. Methanolic extract of Crocus sativus flowers was prepared and evaluated for its total phenolic contents (TPC), 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity (ABTS) (Sariri et al., 2011). The TPC value of 86.65 mg/g gallic acid equivalents, while DPPH and ABTS for 1 mg/ml concentration were 92.41 and 86.87 were obtained respectively. However, the reducing power as measured by the IC50 value was found to be 231.75, which is 11 times lower than that of ascorbic acid.
Stigma of the flowers exhibited higher free radical scavenging activity and higher ferric reducing power. This antioxidant action was attributed to the presence of pyrogallol and gallic acid present in the stigma. Safranal exhibited concentration dependent radical scavenging property as proved by its action of donating a hydrogen atom to the DPPH radical (Assimopoulou et al., 2005b). However, crocin showed high radical scavenging activity (50%) for 500 ppm in comparison to safranal (34%).
In-vivo studies also established antioxidant capacity of safranal. Malondialdehyde (MDA) is an index of lipid peroxidation which is quantified by Thiobarbituric Acid Reactive Species (TBARS) Measurement. In this assay, MDA reacts with thio- barbituric acid (TBA) as a thiobarbituric acid reactive substance (TBARS) to produce a red colored complex with a peak absorbance at 532nm. Male wistar rats when pretreated with safranal (0.1, 0.25 and 0.5 ml kg−1) showed considerable reduction in the free radical-mediated lipid peroxidation which could be seen by a decrease in the MDA levels (Hosseinzadeh et al., 2009). This study was also done using saffron and its other constituents and concluded their protective effect against lower limb ischemia-reperfusion (I/R) in rats.
Chemically, safranal is a cyclical terpenic aldehyde with formula C10H14O. The structural formula is 2, 6, 6-trimethyl-1, 3-cyclohexadien-1-carboxaldehyde. It is a free radical scavenger and useful as an antioxidant molecule.
Although exact reason for its antioxidant behaviour is not clear, this feature is probably due to the extended conjugation present in its structure which quenches singlet oxygen in a reaction that involves transfer of excitation energy from 1O2 to safranal molecule. This has been proved by the authors through in silico studies of Safranal done on various enzymes.
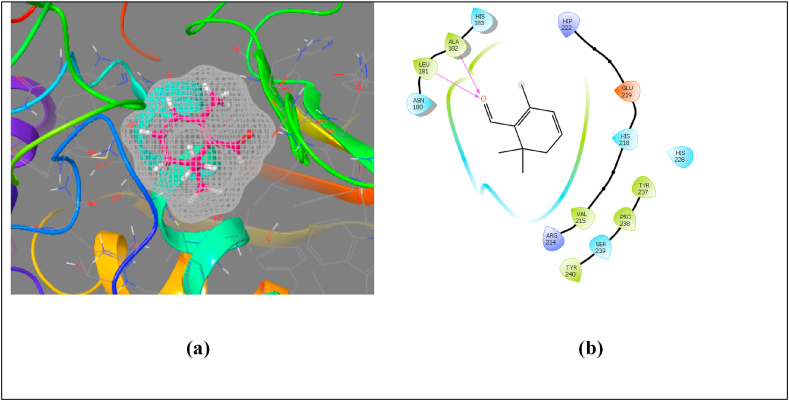
Molecular docking studies of the Safranal with different enzymes namely elastase, hyaluronidase and collagenase were performed by the authors using computational software Schrodinger v11.5. The purpose of the docking was to explore the antiphotoaging activity of Safranal and its possible mechanism. ADME studies of the co-crystallized structures were done using online software Swiss ADME. As per the molecular docking studies done, hydroxyl group help in hydrogen bond formation which results in the better binding with the amino acids residues present in the receptor as shown in the enzyme collagenase. (See Figure 1).
Figure 1.
Pictorial presentation (a) and Ligand interaction diagram (b) of safranal with the binding pocket of collagenase.
Due to its antioxidant behaviour, it has been used therapeutically in diseases arising due to oxidative stress. The application of safranal in these pathophysiological conditions alongwith its mechanism has been discussed.
4. Safranal in neurodegerative diseases
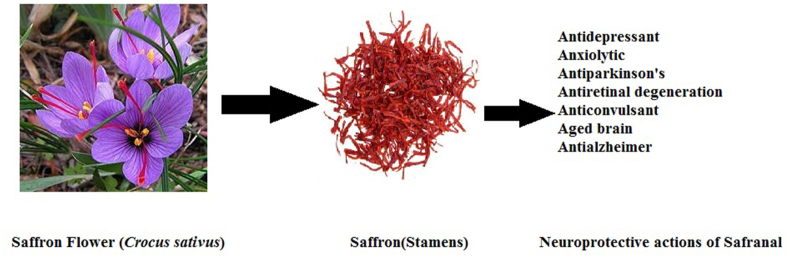
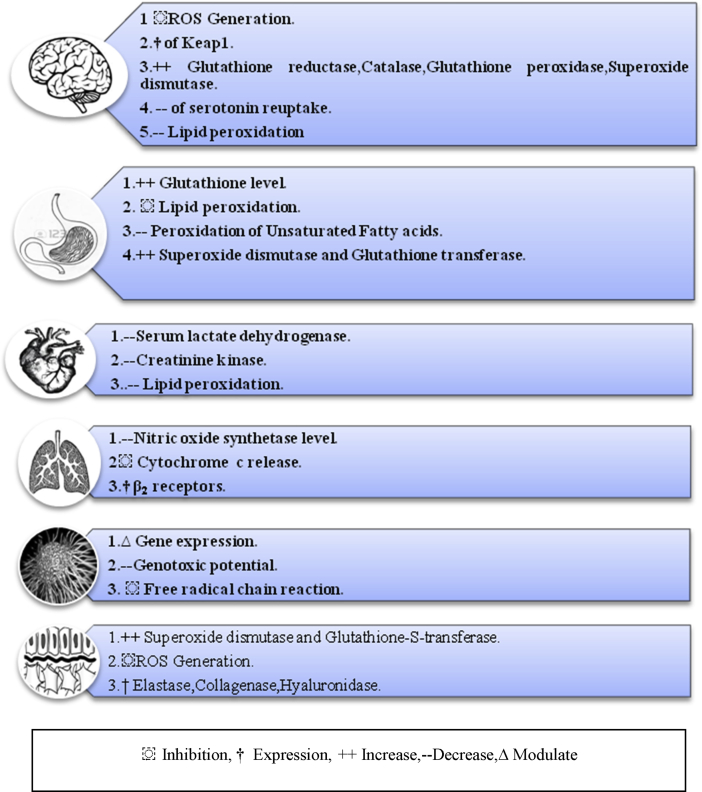
Safranal obtained from Saffron flower offers a large number of neuroprotective actions. (See Figure 2). Brain is rich in lipids and polyunsaturated fatty acids (PUFA) which is sensitive to peroxidation and hence this centre of nervous system is susceptible to oxidative stress conditions where metabolic demands of oxygen are high. Rise in free radical production in mitochondria causes degeneration of brain tissue leading to aging of brain. In a research study, protective effect of Safranal against oxidative damage by down regulation phenomenon was reported (Samarghandian et al., 2015a). This antioxidant biomolecule when administered to 10–20 months old rats amended the augmented lipid peroxidation and decreased GSH content in the brain which further restored GST and SOD activities in brain. In this research study, aged rats (10 and 20 months) were given injections of safranal (0.5 mg/kg day) intraperitoneally continuously over a period of month. Safranal was found to be effective in protection of susceptible aged brain from oxidative damage by increasing antioxidant defences as seen by decreased levels of GSH and activities of SOD and GST in the brain.
Figure 2.
Saffron Flower and its Stamens yield safranal with neuroprotective actions.
Similarly, excessive production of ROS is responsible for various neurodegenerative diseases. It leads to neuronal hyperexcitability and oxidative injury which plays a major role in initiation and progression of epilepsy (Geronzi et al., 2018). Therapeutic involvement of antioxidants may reduce the deleterious effect of epilepsy-related neurodegenerative diseases. Potential role of Safranal as neuroprotective agent has also been studied in an inflammation-associated neuronal apoptosis model where Safranal promoted the recovery of neuronal cells after Spinal cord injury (Zhang et al., 2015). In this study, a moderate damage to spinal cord of adult male Sprague-Dawley rat was made and the effect of safranal on neurologic functions and histopathologic changes after injury was observed. Function in hind limbs was evaluated using the Basso, Beattie, and Bresnahan locomotor rating scale and histopathologic changes were evaluated by performing Nissl staining, which indicated an increased number of neurons after safranal administration. This neuroprotection was observed due to anti-apoptotic effect, down regulation of inflammatory reactions, and edema weakening in spinal cord of rats. Its nerve growth stimulation was also verified in dopamine cells and this indicated use of Safranal as a novel drug for the treatment of Parkinson's disease (PD) (Zhao and Xi, 2018). This neurodegenerative disease is characterized by slow progress of dopamine (DA) neuron degeneration. In this study, neural stem cells (NSC) of rats were treated with varied doses of safranal (1, 20, or 100 ng/mL) and the expression levels of enzyme tyrosine hydroxylase (TH) and dopamine transporter (DAT) were assayed by flow cytometry. ELISA was used to study the secretion levels of dopamine. Safranal treated cells were introduced into PD rat model where increase in TH and DA cells was observed.
The potential effect of safranal on Parkinson's disease (PD) has also been investigated in an in-vitro model of PD induced by rotenone (Pk et al., 2016). Safranal markedly suppressed the reactive oxygen species (ROS) generation and cell apoptosis induced by rotenone. It leads to inhibition of expression of kelch-like ECH associated protein 1 (Keap1). In addition to this, it also lead to induction of antioxidant enymes like NADPH-quinone oxidoreductase 1, glutathione-S-transferase (GST), heme-oxygenase 1 (HO-1) and NADPH-quinone oxidoreductase 1 (NQO1). This study suggested that safranal may act as promising antiparkinson's drug in rotenone-induced neurotoxicity related to Nrf2 signalling pathway.
Anticonvulsant activity of safranal was evaluated in mice using pentylenetetrazole (PTZ) as a convulsion inducer. When given intraperitoneally in the dose of 0.15 and 0.35 ml/kg, it delayed the onset of tonic convulsions, reduced the duration of seizure and proved to be a good anticonvulsant drug (Hosseinzadeh and Talebzadeh, 2005). The efficacy of the drug as an anticonvulsant agent was further validated by the same research group (Hosseinzadeh and Sadeghnia, 2007a, Hosseinzadeh and Sadeghnia, 2007b). In a similar study, intracerebroventricular microinjection of safranal when administered to mice did not show any effect on the two major phases of epilepsy. However, peripheral administration reduced incidence of both generalized tonic-clonic seizures (GTCS) and minimal clonic seizures (MCS) in a dose dependent manner. A significant increase in latency of these phases was also observed. The dose of 72.75, 145.5 and 291 mg per kg of body weight provided 30%, 100% and 100% improvement in mortality rate respectively. This study established that this effect was mediated partly through GABA (A)-benzodiazepine receptor complex as the protective action was abolished when flumazenil was given (Hosseinzadeh and Sadeghnia, 2007a, Hosseinzadeh and Sadeghnia, 2007b). Another study also supported the involvement of Safranal in modification of benzodiazepine binding sites of GABA(A) receptor complex (Sadeghnia et al., 2008). An experimental animal model of generalized absence seizures revealed that safranal significantly decreased spike and wave discharges (SWD) duration when administered into systemic circulation. It is well documented that agents which affect the clonic phase of PTZ induced seizure may have anti-absence seizure activity and Safranal was found to exhibit similar effects.
In order to accomplish rapid delivery of safranal to the brain via nasal route, chitosan coated mucoadhesive nanoemulsion of safranal were prepared by G. K. Jain et al. (Jain and Pathan, 2012). The nanoemulsion was made by aqueous titration method. A higher brain/blood uptake ratio was observed with this delivery system through nasal route in comparison to the plain drug delivery via same route as seen by scintigraphic images. These studies demonstrated larger and faster transport of safranal via nasal route through this novel drug delivery system. Thus, it can be inferred from this study that a desirable modern delivery system can be designed to enhance the efficacy of Safranal.
The protective effect of safranal in neurodegenerative disorders have been identified in a quinolic acid excitotoxicity model. Quinolic acid is generally correlated to the pathogenesis of a range of neurodegenerative as well as non-inflammatory and inflammatory diseases related to neurons (Sadeghnia et al., 2013). It leads to rise in thiobarbituric acid reactive substance (TBARS) which is an indication of lipid peroxidation and is also responsible for oxidative DNA damage. The treatment of rats with safranal half hour before quinolic acid prevented the reduction of hippocampal thiol redox reaction and improved antioxidant status in hippocampal tissue of the brain.
A research study by H. Hosseinzadeh and N. B. Noraei established anxiolytic and hypnotic effect of safranal. The drug increased the total sleep time in a dose dependent manner when administered intraperitoneally in mice (Hosseinzadeh and Noraei, 2009). In lower doses (0.05 and 0.15 mL/kg), it decreased some locomotion activity parameters as observed in open field test whereas, in higher doses, (0.15 and 0.35 mL/kg), it showed anxiolytic effects as proved in elevated plus maze test. However, no effect was observed on motor coordination. In a similar study, the antidepressant activity of Crocin and Safranal was determined using forced swimming test in mice. Both biomolecules exhibited antidepressant activity due to the activation of serotonergic, noradrenergic and dopaminergic system (Shafiee et al., 2018).
Safranal also proved its efficacy in offering protective action on oxidative damage induced by beta-amyloid (Aβ) and hydrogen peroxide (H2O2) on in PC12 cells lines study (Rafieipour et al., 2019). The use of drug in this model of Alzheimer's cell damage proved its anti apoptotic effect for prevention of this neurodegerative disease.
The beneficial effects of safranal and its related components in neurodegenerative diseases is due to its interaction with cholinergic, glutamatergic and dopaminergic systems (Khazdair et al., 2015). Neuroprotective action of safranal is also extended to photoreceptor cells and retinal cells.
Besides, Safranal, the beneficial effects of crocetin esters have also been demonstrated on many body systems including endocrine, reproductive, gastrointestinal, and cardiovascular and CNS (Luis et al., 2018). Although many of these studies are related to the antioxidant-rich nature of the carotenoids in saffron, the action mechanisms is not clear. Research study done by Nam and coworkers on rat brains suggested that crocin and crocetin exert a neuroprotective effect because of their anti-inflammatory action in microglial cells, accompanied by a reduction in neurotoxic molecules such as interleukin-1,TNF, and intracellular ROS (Nyon et al., 2010). These compounds also reduced the lipo polysaccharide stimulated NF-κB activation and provided neuroprotection.
Administration of safranal to P23H (an animal model of retinitis pigmentosa) rats, maintained morphology and number of photoreceptors. The capillary network analysis has shown that safranal is able to prevent the loss of retinal vessels occurred in the P23H rat retina in comparison to untreated animals (Fernández-sánchez et al., 2015). The efficacy of Safranal has been reported in a research study done on aged rats where this antioxidant molecule protected susceptible aged brain from imbalance between ROS and antioxidant defence system of the body (Samarghandian et al., 2015b). This study was in accordance with previous study where Safranal ameliorated increased lipid peroxidation level, decreased GSH content of the aged brain and in turn restored GST and SOD activities.
Oxidative stress is the major cause of neurodegenerative diseases. Antioxidant,antiapototic and anti-inflammatory properties of Safranal has indicated its use as prophylactic agent in the diseases like Parkinson's, epilepsy, alzheimer, anxiety and retinal degeneration. This neuroprotective effect is an outcome of stimulation of activity of antioxidant enzymes when Safranal binds specific receptors and inhibit or prevent generation of reactive species. Further, clinical studies need to be carried for exploitation of this natural biomolecule as a neuroprotective agent.
5. Safranal in GIT diseases
Oxidative damage with ROS species is the major causative factor for mucosal lesions. Many anti-ulcer drugs are known to exert their action via anti-oxidative activity (Biswas et al., 2003). Thus, in view of the significant antioxidant effects of safranal, a study was designed to assess the possibility of the drug as a natural and novel antiulcer agent. In this study, male wistar rats were administered streptozocin for diabetic induction. Both diabetic and non diabetic rats were administered safranal prior to indomethacin (as ulcerogenic agent). The effect of safranal on lipid peroxidation and gastric mucosal lesion was observed by the levels of thiobarbituric acid reactants (TBARS) and Glutathione (GSH) content respectively. It lead to rise in glutathione level and reduced lipid peroxidation. It also reduced the peroxidation of unsaturated fatty acids. This is due to inhibitory effect of safranal on free radical generation. Thus, safranal prevented gastric mucosa and claims to be a good antiulcer agent (Kianbakht and Mozafari, 2009).
A research study by Farahmand et al. demonstrated the significance of safranal in augmentation in antioxidant enzymes namely superoxide dismutase, catalase and glutathione-S-transferase. According to this study, the animals (male wistar rats) were treated with safranal for 30 days after which they were sacrificed and suitable cellular homogenates were prepared for estimation of antioxidant enzymes. The catalase activity was assayed by Aebi's method (Aebi, 1984) and level of lipid peroxidation was measured by using a thiobarbituric acid reactive substance (TBARS). Safranal inhibited lipid peroxidation and increased antioxidant defences. It acted as a hormetin by inducing mild oxidative damage which further lead to antioxidative enzymes activation as suggested by this research group (Seyed Kazem Farahmand and Mohammad Samini Samarghandian, 2013).
6. Safranal as antidiabetic agent
The implication of oxidative stress is well suggested in the pathogenesis of diabetes and its related major complications such as diabetic retinopathy, neuropathy, accelerated coronary artery disease, respiratory diseases and nephropathy.
Safranal by virtue of its antioxidant properties was tested for its antidiabetic effect in a research work done by Tahereh Farkhondeh, Saeed Samarghandian and Azimi-Nezhad. The test animals were induced diabetes by streptozocin administration. The intraperitoneal administration of saffron extract significantly decreased blood glucose levels, lipids, nitric oxide, triglycerides concentration and the level of cholesterol. It also lead to increase in antioxidant enzymes level. This action of saffron extract is thought to be due to safranal as suggested by this study (Samarghandiana et al., 2016). Another similar clinical research work confirmed the efficacy of safranal in improvement of complications of diabetes due to oxidative stress. In this study, pro- oxidant-antioxidant balance was estimated by measuring serum Malonealdehyde (MDA) and Nitric oxide (NO) levels and presence of enzymatic antioxidants in the diabetes induced rats. The decreased level of antioxidant enzymes and increased NO and MDA pointed that the balance changed towards pro- oxidation in STZ-induced diabetic rats. However, treatment with safranal restored CAT, SOD and GST actions. It also lead to lower serum glucose levels, and improved lipid profile in comparison to rats treated with STZ alone (Samarghandian et al., 2013).
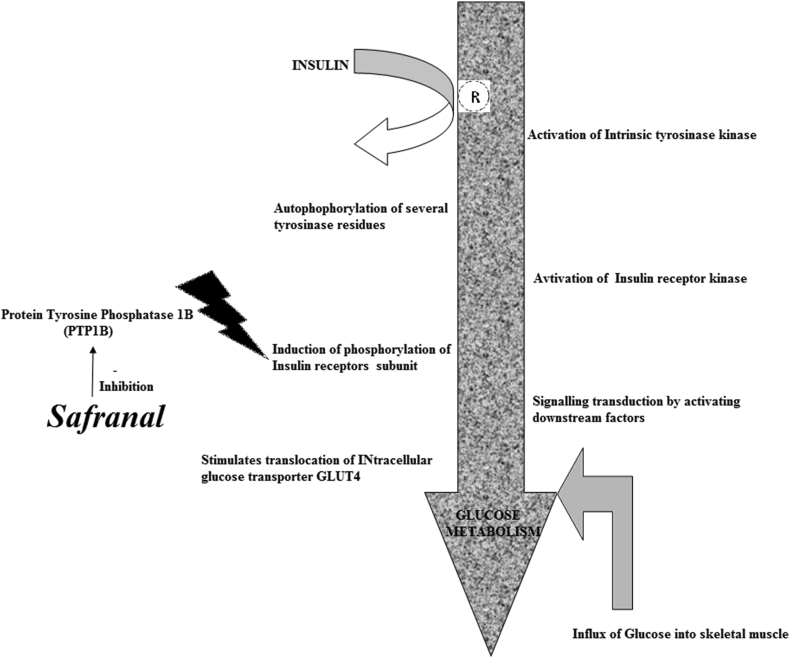
Safranal was also found to be effective in improving glucose tolerance in Type 2 Diabetes Mellitus (T2DM) (Maeda et al., 2014). The study done on mouse myoblast cell line (C2C12) revealed that Safranal is a Protein tyrosine phosphatase 1B (PTP1B) inhibitor which induces the ligand-independent activation of insulin signalling. This fact was also confirmed in associated animal experimentation when Safranal was given orally to the six week old male type 2 diabetic mice (KK-Ay/Kwl (KK-Ay)) and an oral glucose tolerance test (OGTT) was conducted. The study confirmed that safranal stimulates insulin signaling and mitigates impaired glucose metabolism in T2DM by inhibiting tyrosine phosphatase 1B.
Safranal greatly facilitates cellular glucose uptake through GLUT4 translocation in C2C12 myotubes. These findings suggested that safranal stimulates insulin signaling and ameliorates impaired glucose metabolism in T2DM by inhibiting PTP1B (Figure 3).
Figure 3.
Safranal as tyrosine phosphatise 1 B inhibitor.
Not only this, Safranal has been recommended for management of diabetes (Delkhosh-kasmaie et al., 2018). A research study was planned to investigate the separate and combined treatment effects of metformin and safranal on diabetes-induced learning and memory impairments. Streptozocin was administered to induce diabetes and thereafter different doses of Safranal, metformin and their combination was administered. Histopathological and biochemical evaluations were made. Four days learning abilities including escape latency and distance swum increased, where as memory activities including platform crossing number and time spent and distance travelled in target quadrant (Q2) decreased in STZ-induced diabetic rats. Safranal improved learning and memory impairments in STZ-induced diabetic rats. The positive effect of Safranal on memory diabetes enhanced the spectrum of therapeutic efficiency of drug.
Inflammatory mediators and oxidative stress are responsible mechanisms for induction of the pulmonary distress. Safranal has been reported to exert protective effect in lung oxidative damage in diabetic rats (Samarghandian et al., 2014). In this study, Safranal was administered to streptozocin induced diabetic rats and MDA, NO and GSH contents were measured along with SOD and CAT activity. In addition to these parameters, MDA and NO in bronchoalveolar lavage fluid (BALF) from lung tissue was also estimated. Safranal inhibited the content of NO and MDA, BALF supernatant and lung homogenate. Thus, it proved to be a natural drug which controls diabetes and its related complications.
Safranal has proven to be a safer antidiabetic drug than other drugs used clinically. Its protective effect in T1DM as well as T2DM has indicated its potential role in glucose tolerance as well as management of disease. However, selection of a particular model for diabetic study depends upon the purpose of the study. In various studies related to effect of Safanal in diabetes, same model of Streptozocin has been used, be the indication is diabetic peripheral neuropathy or glucose intolerance. Therefore, antidiabetic studies need to be done on other models too such as monobasic obese models.
7. Safranal in cardiovascular diseases (CVDs)
CVDs has been reported as the major cause of death in 2012 (Toledo-ibelles and Mas-oliva, 2018). These pathophysiological conditions results due to accumulation of the oxidised hydroxide and ketone forms of lipid in the blood vessel along with vascular inflammation. The vascular inflammation and oxidative stress and are very much interconnected to endothelial dysfunction leading further to vascular damage (Siti et al., 2015). Oxidative stress plays a key role in atherosclerosis by oxidising low density lipoproteins (LDLs) (Sarangarajan et al., 2017). Evidence regarding oxidation as a proatherogenic factor showed that biochemical events involved in atherosclerosis or degenerative cardiovascular diseases are undeniably a good target for the management of cardiovascular diseases (Toledo-ibelles and Mas-oliva, 2018). Safranal is shown to exhibit cardioprotective activity by modulating oxidative stress. The action has been studied in Isoproterenol (ISO) induced myocardial infarction (MI) in wistar rats. A significant decrease in the serum lactate dehydrogenase (LDH) and creatine kinase –muscle brain was observed when pretreatment with safranal for 8 days was given intraperitoneally to the rats. The drug also reduced lipid peroxidation in tissues of the heart and exhibited cardioprotective effect (Mehdizadeh et al., 2013).
Antioxidant property of Safranal has been found to be useful in hypertension (Imenshahidi et al., 2015). In an investigative study, the effects of safranal on blood pressure of normotensive and desoxycorticosterone acetate (DOCA) salt induced hypertensive rats was observed when the drug was administered to male wistar rats for a long period. Safranal decreased systolic blood pressure in hypertensive rats which showed that the vasodilatory effect of safranal was endothelium dependent. The antihypertensive effect of safranal at the highest dose was as much as spironolactone by the end of nine weeks. The study concluded that hypotensive effect of safranal was attributed to the inhibitory effect on smooth muscles by blocking of calcium channel or inhibition of sarcoplasmic reticulum Ca2+ release into cytosol.
The role of free radicals is well illustrated in hypertension. RONS activate the activation of specific molecular mechanisms which increase blood pressure level (Sorriento et al., 2018). Safranal is also known to exhibit vasodilatory effects. A study done by Imenshahidi et al. evaluated this effect both on intact and denuded endothelium aortic rings (Razavi et al., 2016). The research group contracted isolated rat thoracic aorta rings by using 80-mM KCl and 10−6M phenylephrine (PE). Dimethyl sulfoxide (DMSO) was taken as a control. The drug induced relaxation in concentration-dependent manner with a maximum relaxation of more than 100%. This implication of Safranal for vasodilatory effects decreases total peripheral resistance and useful in hypertension.
8. Safranal in cancer
Cancer is a disease that results due to uncontrolled division and growth of cells.Redox regulation controls various aspects of carcinogenesis including cancer cell growth, migration, and metastasis and cancer vascularization. The major source of oxidants in cancer is nicotinamide adenine dinucleotide phosphate oxidase (NOXs) which is multicomponent membrane bound enzyme that intercede electron transfer from NADPH to molecular oxygen, leading to the production of ROS; superoxide ion (Paik et al., 2011). Activation of signalling pathways leads to gene transcription, cell cycle proliferation and apoptosis leading to carcinogenesis (Dunaway et al., 2018).
The conventional anticancer treatment includes the use of chemotherapeutic agents, radiotherapy or photodynamic therapy. Singlet oxygen and hydroxyl radical production is common mechanism of various therapeutic approaches and is also responsible for its adverse effects. These therapies involve the creation of species which are coupled with damage instead of maintaining redox signalling. For a long time, antioxidants have been claimed to protect from cancer by inhibiting oxidative stress (Hel and Schröder, 2018). Plants, vegetables and herbs used in traditional medicine have been accepted presently as one of the main sources of cancer chemopreventive drug discovery and development due to their potential to inhibit oxidative stress (Chermahini et al., 2010).
Saffron has also been found to be active against various malignancies (Butt et al., 2013). Oral administration of saffron extract inhibited the growth of mouse tumours and significantly increased the life spans of treated tumour-bearing mice. Various animal models and cultured human malignant cell lines have also demonstrated antitumor and cancer preventive activities of saffron and its main ingredients namely crocin, crocetin and safranal (Samarghandian and Borji, 2014). In a research work done by Das et al., chemopreventive effect of saffron on DMBA-induced skin carcinogenesis was demonstrated in female Swiss albino mice (Das et al., 2004). Aqueous infusion of saffron was found to produce a remarkable reduction in the incidence of mouse skin papilloma (a pre-neoplastic benign growth) when administered orally.
The effect of ethanolic extract of saffron as a chemotherapeutic agent in lung cancer is established by a study where it decreased cell viability in malignant cells in a dose- dependent manner (Samarghandian et al., 2010). In a study,a modified 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium (MTT) assay was used to determine cell viability and the morphological changes of cell death in non-malignant and malignant cell lines were studied using inverted microscope. Saffron-induced cytotoxicity was involved in the induction of morphological changes and the study also confirmed that saffron exerts pro-apoptotic effects in a lung cancer-derived cell line and is safer even at higher doses. In another preclinical study, the major volatile component of saffron, Safranal was identified as the principle responsible for antitumor activity was established as safranal (Farahzad et al., 2014).
Saffron was also reported to prevent the oxidative stress by reduction of the lipid peroxidation with a concurrent increase of liver enzymes such as the catalase and superoxidedismutase. A nonenzymatic action is also suggested for these chemopreventive effects. It states that saffron modulates lipid peroxidation and antioxidants systems of the body (Bhargava, 2011).
In another study, neuroblastoma cells were exposed to safranal in the concentration of 0, 10, 15, 20, 50 μg/ml and the cell proliferation was observed using MTT assay. Safranal caused the inhibition of malignant cells in a time and dose dependent manner as analysed using flow cytometric analysis. However, exact molecular mechanism behind this anticancer activity could not be explained. Another study also established anticancer activity of safranal without explaining the mode of its action. Safranal curbed genotoxic potential of methyl methanesulfonate (MMS) when studied in single cell gel electrophoresis assay done on multiple organs of the mice such as liver, lung, kidney and spleen (Hosseinzadeh and Sadeghnia, 2007a, Hosseinzadeh and Sadeghnia, 2007b).
Safranal and its liposomes have also been investigated for their anticancer activity (Malaekeh-Nikouei et al., 2013). MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium) assay was taken as an assessing parameter for cytotoxic studies when studied on cultured HeLa, MCF7 and L929 cell lines. This in vitro anti-tumor activity was probably due to involvement of safranal in saffron induced cell death where it killed HeLa and MCF7 cells. (See Table 1)
Table 1.
Cell lines study and animal models used for Safranal study and their implication.
| S.No. | Year | Cell Line Study/Animal Model | Disease/Disorder | Ref. |
|---|---|---|---|---|
| 1 | 2004 | DMBA Induced model | Carcinogenesis | (Das et al., 2004) |
| 2 | 2006 | Methacholine mouse model | Asthma | (Boskabady et al., 2006) |
| 3 | 2014 | Neuroblastoma cells | Cancer | (Farahzad et al., 2014) |
| 4 | 2013 | Endothelin 1 mouse model | Inflammation | (Gholamnezhad et al., 2013) |
| 5 | 2013 | Isoproterenol mouse model | Cardiovascular diseases | (Mehdizadeh et al., 2013) |
| 6 | 2013 | HeLa, MCF7 and L929 cell lines | Cancer | (Malaekeh-Nikouei et al., 2013) |
| 7 | 2013 | Acetic acid mouse model | Inflammation | (Tamaddonfard et al., 2013) |
| 8 | 2014 | Ovalbumin sensitized model | Asthma | (Boskabady et al., 2014) |
| 9 | 2014 | Mouse myoblast cell line (C2C12) | Type 2 Diabetes Mellitus | (Maeda et al., 2014) |
| 10 | 2014 | KK-Ay/Kwl (KK-Ay) | Type 2 Diabetes Mellitus | (Maeda et al., 2014) |
| 11 | 2014 | MTT mouse model | Lung cancer | (Farahzad et al., 2014) |
| 12 | 2015 | P23H | Retinitis pigmentosa | (Fernández-sánchez et al., 2015) |
| 13 | 2015 | DOCA mouse model | Hypertension | (Imenshahidi et al., 2015) |
| 14 | 2016 | Phenylephrine mouse model | Hypertension | (Razavi et al., 2016) |
| 15 | 2016 | Peripheral blood mononuclear cells | Inflammation | (Feyzi et al., 2016) |
| 16 | 2017 | HeLa cell | Cancer | (Cheriyamundath et al., 2017) |
| 17 | 2017 | KB and NIH 3T3 cell lines | Oral squamous cell carcinoma | (Jabini et al., 2017) |
| 18 | 2018 | Neural Stem Cells (NSC) | Parkinson's Disease | (Zhao and Xi, 2018) |
| 19 | 2018 | Streptozocin mouse model | Diabetes | (Delkhosh-kasmaie et al., 2018) |
| 20 | 2018 | HepG2 Cell Lines | Liver Cancer | (Al-hrout et al., 2018) |
| 21 | 2019 | PC12 Cells | Alzheimer Disease | (Rafieipour et al., 2019) |
Antioxidant effect of Safranal has been proposed as a possible mechanism for its anticancer activity. Cytotoxic activities of different safranal concentrations (0.2–3.2 mM) were tested on oral squamous cell carcinoma (KB) and NIH 3T3 as nonmalignant cell lines using MTT assay (Jabini et al., 2017). This study demonstrated that safranal has >50% cytotoxicity on KB cells at 0.2 mM while showed <50% cytotoxic activity on normal cells (NIH 3T3 cells) at the same concentration after 72 hrs. Hence, it was proved that Safranal is selective and target cancer cells more efficiently than normal cells. These findings correlate with previous studies which demonstrated high lethal dose of Safranal for normal cells and proved its safety.
Anticancer activity of Safranal was also seen in hepatic carcinoma cells using in vitro, in silico, and network analyses (Al-hrout et al., 2018). Safranal arrested cell cycle at G2/M phase as observed by immunoblot analyses and apoptosis using liver cancer cell lines (HepG2). This marked effect of Safranal on DNA damage machinery indicated ER stress-mediated apoptosis.
A review by Chermahini et al. (2010) provided various hypothesis for the modes of its anticarcinogenic and antitumor actions. The inhibitory effect on free radical chain reactions is one of the proposed mechanisms for its antitumor effects. An attempt was made to study the mechanism of Safranal in cancer therapy by estimating the effect of Safranal on microtubule organisation obtained from the brain of sheep (Naghshineh et al., 2015). In this study, anti-microtubule activity of Safranal was observed by turbidimetric method and technique of transmission electron microscopy (TEM) where it considerably reduced microtubule polymerization. Docking studies done simultaneously also supported and estimated the extent of tubulin binding of Safranal. Safranal was found to be situated between a and b tubulin and formed hydrogen bond with amino acid glycine 142. The hydrophobic interactions stabilized Safranal in binding site This tubulin targeted antiproliferative mechanism was confirmed by another research group through an in vitro study (Cheriyamundath et al., 2017). In this study, Safranal disturbed secondary structure of tubulin without changing the microtubule polymer mass. It inhibited HeLa cell viability in a dose dependent manner, without causing any damage to cellular microtubules. This unusual, tubulin-targeting pattern of safranal was elucidated as one of the mechanisms of its anticancer activity.
Crocin has also been suggested as therapeutic anticancer component of Saffron. Studies on various cell lines such as Tca8113, HeLa, A549, VA13, K569 and promyelocytic leukaemia HL-60 cells has demonstrated its inhibitory action on synthesis of DNA and RNA as one of the possible mechanisms. Interaction with enzyme DNA topoisomerase has also been proposed as an anticancer mechanism while studying on p53 colorectal cancer cell lines(Hoshyar and Mollaei, 2017). The other machanisms proposed for anticancer activity include targeting of microtubules and suppression of the activity of enzyme telomerase. Moreover, It has also been found that crocin reverses the epithelial–mesenchymal transition and thus inhibit metastasis.
9. Safranal in skin photoaging
Aging is a natural, complex and progressive phenomenon which leads to aesthetic and functional changes in the skin. Aging is generally of two types, intrinsic (i.e., genetically determined) or extrinsic (due to environmental factors) (Kaur et al., 2014). The latter is termed as photoaging and depends upon the degree of sun exposure as well as on amount of melanin present in the skin. Skin aging varies with genetic makeup of an individual and their lifestyles. Photoaging and chronological aging vary in their histological and clinical changes.
Accumulation of ROS with time is a major contributor to aging process (Rinnerthaler et al., 2015). As per the oxidative stress theory, oxidative cellular damage plays a major role in aging process. There is substantial evidence showing that aging is associated with damage from free radicals represented by various reactive oxygen species (ROS). Skin aging results due to diminished endogenous antioxidant system and ROS formation which causes DNA damage (Gragnani et al., 2014) ROS causes irreversible damages like cross linking of proteins, DNA damage, cell apoptosis and formation of aging pigments (Singh, 2009). The disruption of extracellular matrix plays the apparent role in intrinsic as well as extrinsic aging (Rinnerthaler et al., 2015). The ROS producing enzymes present in the skin include the mitochondrial electron transport chain, NADPH oxidases, xanthine oxidoreductase (XOR), lipoxygenases, several peroxisomal oxidases, cyclooxygenases and cytochrome P450 family.
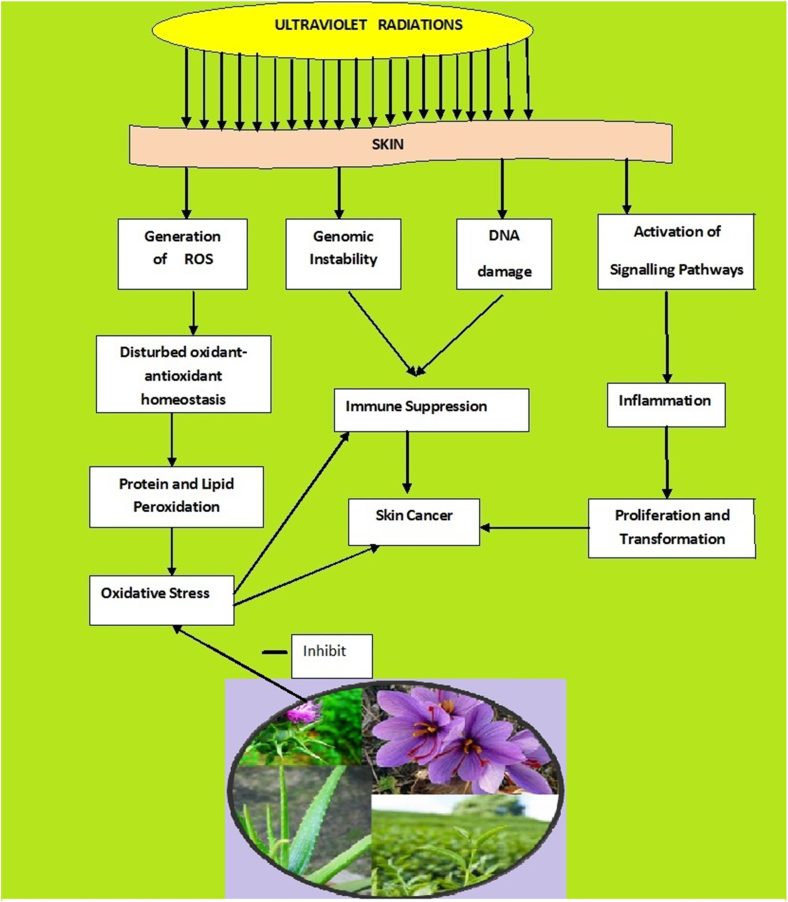
Photoaging differs from aging. The people with intensive sun exposure or those living in geographical areas which receives the most sunshine experience the greatest amount of load of ultraviolet radiation and hence are more prone to severe photoaging (Pandel et al., 2013). In this, the UVA rays penetrate the skin and are absorbed by cellular chromophores like urocanic acid, riboflavins, melanin, bilirubin, heme, porphyrin, and pterins (Chen et al., 2012). These photosensitizers absorb photons leading to their excited ‘singlet’ state which is short lived and converts into ‘triplet’ excited state. This excited state can react with both DNA and molecular oxygen, resulting in DNA modification and production of reactive oxygen species (ROS) respectively (Prasad and Pospísil, 2012). The ROS species such as hydrogen peroxide, superoxide, singlet oxygen or hydroxyl radical acts as secondary messenger leading to an activation of MAP-kinasep38, ERK (extracellular signal-regulated kinase) and JNK (c-Jun amino-terminal kinase). This leads to the expression of the transcription factor activator protein 1 (AP-1) resulting in the expression of matrix metalloproteinases (MMPs). These endopeptidases are secreted by keratinocytes and dermal fibroblasts in response to multiple stimuli such as cytokines, oxidative stress and UV radiation. As a result of this, the production of collagenase, gelatinase and stromelysin-1 is stimulated leading to the deterioration of collagen, elastin and other components of the dermal extracellular matrix (Farage et al., 2008). AP-1 also inhibits transforming growth factor-β (TGF- β) which is a major regulator for the production of procollagen type I in human skin (Pittayapruek et al., 2016). The AP-1 mediated MMP expression also leads to cellular damage (Rinnerthaler et al., 2015). Alteration in the structure of elastic fibres, subsequently reduces elastic properties of the skin and results in wrinkle formation (Imokawa, 2008) (See Figure 4)
Figure 4.
Safranal from Crocus sativus flower and implication in oxidative stress in skin.
The rising cases of skin disorders have demanded development of natural antioxidants for skin. These antioxidants have capacity to increase the endogenous antioxidant potential of the skin and neutralizing ROS induced by other extraneous factors such as ultraviolet radiations and air pollutants (Pai et al., 2014). Many natural agents like Vitamin A, Vitamin C, coenzyme Q 10,beta- carotene, silymarin, pycnogenol, procyanidins have been in use in antiaging therapies (Singh, 2009). A research study was carried out by Golmohammadzadeh et al. where different concentration of saffron were incorporated into sunscreen formula and compared with USFDA standard homosalate (Golmohammadzadeh et al., 2010). The lotion with 4% saffron showed an SPF value equivalent to the 8% homosalate lotion reference by an in vitro absorption method. The formulation also exhibited moisturising properties.
Saffron cream has been reported to exhibit significant depigmentation effect in a study done by Akhtar et al. (2014). Dried stigma was extracted using maceration and this extract was used to formulate a cream. This cream was tested for erythema and its effect on skin melanin. For this study, ten healthy human volunteers (all males) were chosen between age group of 25–40 years. Saffron cream and base cream was applied to their forearms or 8 weeks. At the end of this period, significant decrease in level of melanin was observed with cream containing saffron in comparison to base cream. The formulations also lead to reduction in the erythema level steadily over the period of 8 weeks. Antioxidant effect was proposed as one of the mechanisms for this action. Saffron has also been investigated as a natural moisturizer with antisolar properties. Its UV absorbing property is determined to be as equivalent as homosalate (USFDA sunscreen standard). This photoprotective effect is attributed to various constituents present in it (Golmohammadzadeh et al., 2010).
Safranal has great potential as an antiaging compound due to its property of suppression of oxidation stress. In a study, the aged rats were administered safranal intraperitoneally to assess the changes in activities of antioxidant enzymes in brain; superoxide dismutase (SOD), glutathione-S-transferase (GST) and catalase (Samarghandian et al., 2015b). The results demonstrated that aging caused significant increase in lipid peroxidation and decrease in GSH levels and activities of SOD and catalase. Safranal act by decreasing free radicals generation and restored levels of superoxide dismutase (SOD) and glutathione-S-transferase (GST) levels. Administration of safranal regulated and increased the expression of antioxidant enzymes, longevity related genes, and consequently levels of oxidants in aging animals.
Besides intrinsic aging, safranal has been claimed as a valuable component to be incorporated for herbal formulations meant to prevent photoaging. Photoaging results due to breakdown of collagen and elastin which causes significant changes in the integrity of skin. Repeated exposure of the skin to UV radiations generates ROS which are responsible for such changes. These changes be manifested as irregular epidermal thickness, coarse wrinkles, atrophic and leathery skin (Poon et al., 2015). In a research study, significant inhibitory activity of safranal on enzymes responsible for aging such as elastase, collagenase and hyaluronidase have been explored recently (Madan and Nanda, 2018). The basis of this research is sunprotective action of safranal. In a study, Golmohammadzadeh et al. formulated safranal nanoliposomes and calculated its sun protection factor (SPF) by using transpore tape method. Safranal encapsulated in liposomes in a concentration of 8% gave significant sunscreen properties (Golmohammadzadeh et al., 2011). The sunprotectant activity of safranal was also exhibited in another delivery system; Solid lipid Nanoparticles (SLN). SLN of safranal were formulated using glyceryl monostearate and tween -80 with high shear homogenization, and high pressure homogenization (HPH) methods. SLN containing 4% safranal showed higher SPF values in comparison with other SLN formulations and 8% homosalate reference. This better UV blocking property of safranal was found due to presence of lipid in solid state (Khameneh et al., 2015). The in vitro biochemical tests done along with its sun protective and antioxidant potential have claimed safranal a valuable component to be incorporated for antiaging herbal formulations.
However, antiaging or sunprotection properties are determined by in vitro assays only. There is no study proving efficacy of Safranal done ex-vivo or in vivo. Antiphotoaging studies are done by authors themselves and they purport to carry it further. For this, this molecule needs to be stabilized by suitable delivery system and the work is in progress.
10. Safranal in asthama
Smooth muscle relaxant effect of safranal have also been studied by Boskabady et al. (2006). This relaxant effect was examined in tracheal chains of guinea pigs. Drug was administered in the concentration of 0.15, 0.30, 0.45, and 0.60 mL 0.2 mg/mL where it showed significant relaxant effect compared with saline or theophylline in a group of animals whose tracheal chains were contracted using 10 microM methacholine. In another group whose tracheal chains were contracted with 60 mM KCl, it exhibited concentration-dependent relaxant effects. This research work justified its use in cough and asthma in traditional medicine where saffron extract was used and indicated that safranal was responsible for this muscle relaxant activity probably due to activation of β2-adrenoceptors. However, the saffron extract and theophylline showed higher relaxant effect. Reduction in number of cough observed in guinea pig also explained its application as an antitussive agent (Hosseinzadeh and Ghenaati, 2006). The Figure 5 below symbolizes the action of Safranal in various body systems.
Figure 5.
Mechanism of protectant action of safranal on various systems.
Safranal has also been tested in bronchial epithelial cells. It decreased NO(Nitric oxide), iNOS induced nitric oxide synthetase levels, peroxynitrite ion generation, and prevented cytochrome c release and hence proved to be a prospective candidate in treatment of asthma (Bukhari et al., 2015). This use of safranal is attributed to its antioxidant and anti-inflammatory potential. A study done on ovalbumin sensitized guinea pigs confirmed the protective effect of Safranal on tracheal responsiveness (Boskabady et al., 2014). The serum levels of NO and nitrite were also found to be decreased when Safranal was administered as reported earlier suggesting its antioxidant effect. The study also showed reduction of IL-4 and increasing IFN-g levels in the serum indicating the effect of Safranal on regulation of T helper 1 (Th1) and T helper 2 (Th2) balance. This immunoregulatory effect of safranal can be of therapeutic significance in Asthma. A review proposed various mechanisms for relaxing effect of Safranal and its associated components on smooth muscle (Mokhtari-Zaer et al., 2015). These include activation of ß2-adrenocepors, modulation of nitric oxide (NO), inhibition of histamine and muscarinic receptors. Another study done on inflammatory markers in sensitized guinea pigs also confirmed preventive effect of Safranal in asthma (Gholamnezhad et al., 2013). In this study, effect of the extract of the plant Crocus sativus and safranal on total protein and endotheline-1 of guinea pigs was seen which were previously sensitized with ovalbumin. Serum endothelin level was measured using the enzyme-linked immunosorbent assay (ELISA) and photometric method was used to determine the protein contents with Bio-Rad protein assay kit (Bio- Rad Laboratories, US). Safranal prevented increased endothelin and total protein levels of sensitized guinea pigs indicating its potential as prophylactic agent for treatment of asthma. The effect of Safranal was more than the plant extract alone proving that the plant extract shows similar effect due to presence of this molecule.
11. Safranal in inflammation
Thermal and chemical methods of nociception in mice have proven safranal to be a good antinociceptive agent (Hosseinzadeh and Shariaty, 2007). Inhibition of the acetic acid induced abdominal constrictions was observed when safranal was given at doses 0.1, 0.3 and 0.5 ml/kg/ip. Similarly, it increased the pain threshold of mice at a dose of 0.5 ml/kg/ip in hot plate test. These actions were however peripheral and not mediated through opiod receptors. Its anti-inflammatory effect equivalent to diclofenac is also proven in carrageen an model of inflammation which is possibly due to inhibition of cyclooxygenase enzyme (Tamaddonfard et al., 2013). Anti-inflammatory action of Safranal has been demonstrated in diseases linked to imbalance between T helper 1 (Th1) and T helper 2 (Th2) cells (Feyzi et al., 2016). Th2 and other inflammatory cells are activated in asthma and are responsible for airway hyper responsiveness, tissue inflammation and mucus secretion. Th1 cells are known to inhibit responses of Th 2 and therefore Th1 cells need to be stimulated to cure asthma. This protective effect of Safranal was seen by examining cell viability and cytokine profile of peripheral blood mononuclear cells (PBMCs). Safranal at higher concentrations inhibited cell viability of non-stimulated cultured human PBMCs. The similar effect was seen on stimulated cells with PHA at all concentrations. It also raised IFN-γ/IL-4 ratio in both stimulated and not-stimulated human PBMCs indicating its role in stimulation of Th1 and suppression of Th2 cells.
12. Safety of safranal administration
Little is known about the toxicological profile of saffron. A study has been carried out to estimate acute and subacute toxicity of safranal in rodents through oral and intraperitoneal route (Hosseinzadeh et al., 2013). In this study, the calculated safranal LD50 values of intraperitoneal route in male and female mice and male wistar rats were in the range of 1–5 g/kg and hence safranal should be considered as practically low-toxic in acute intraperitoneal route. Histological studies proved that safranal is free from toxic effects on the heart, liver and spleen. Similarly, the immunotoxic effect of safranal has been investigated (Riahi-zanjani et al., 2015). The drug was administered intraperitoneally at doses of 0.1, 0.5 and 1 ml/kg for 3 weeks. Histopathological examination of spleen and bone marrow in various groups of these experimental Balb/c mice proved its safety in to mice immune system in contrary to toxicological studies which have indicated that safranal is more toxic than other constituents of saffron. Safranal is non toxic to humoral and cellular immunity. Various cell lines or models studied for therapeutic efficacy of Safranal in various diseases have been tabulated below:
13. Conclusion
A wide range of factors such as modern lifestyle, drugs, smoking, psychological stress and toxins has lead to unusually high levels of oxidative stress in humans. Free radicals and reactive oxygen and nitrogen species so generated have been considered as catalyst to upset the antioxidant defence system of the body and have been reportedly known to be involved in large number of diseases including neurodegenerative diseases (Alzheimer's disease and Parkinson's disease), cardiovascular diseases, cancer and diabetes. Naturally occurring therapeutic agents are being resorted for most of the maladies, including stress related health problems. Safranal, a natural antioxidant chemical from saffron (Crocus sativus) has been reviewed in this paper. This volatile terpene aldehyde, also known for its aroma also possesses strong antioxidant properties. The potential role of this molecule in diseases arising due to oxidative stress has been established by various research groups. In this article, its therapeutic benefit in diabetes, asthma, cancer, GIT diseases. neurodegerative diseases and cardiovascular diseases has been extensively reviewed. A discussion on the of use of safranal as a skin antioxidant and as antiphotoaging molecule is also made. Besides Safranal, crocin and other apocarotenoids also offer therapeutic use in oxidative stress diseases as given by various clinical and non clinical studies. They also owe their action to their antioxidant and anti-inflammatory properties.
The administration of safranal in treatment of a range of pathologies and mechanism supporting its application is explored. Various studies have. proved that safranal shows its antioxidant impact by stabilizing the membranes in various biological systems. It reduces peroxidation of unsaturated membrane fatty acids and restores the diminished activities of antioxidant enzymes present in the body. Its efficient scavenging of singlet oxygen and peroxy radical is thought to be due to presence of unsaturation in this cyclic terpene aldehyde. The authors have attempted to provide an updated overview on the antioxidant properties of Safranal and its underlying mechanisms. Although in silico, in vitro and in vivo studies have indicated the use of this biomolecule in prevention and management of diseases arising out of oxidative stress but the authors feel that well designed clinical investigations are essential to finally confirm the potential of Safranal as a therapeutic agent. However, the other sensory molecules of Saffron are equally efficacious and has been established.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aebi H. Methods in enzymology. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Akhtar N., Khan H.M.S., Ashraf S., Mohammad I.S., Ali A. Skin depigmentation activity of crocus sativus extract cream. Trop. J. Pharmaceut. Res. 2014;13:1803–1808. [Google Scholar]
- Al-hrout A., Chaiboonchoe A., Khraiwesh B., Murali C., Greish Y.E., Ramadan W., Salehi-ashtiani K., Amin A. Safranal induces DNA double- strand breakage and ER- stress-mediated cell death in hepatocellular carcinoma cells. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-34855-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Am S., Maggi L., Ma M., Gl A. Increasing the applications of Crocus sativus flowers as natural antioxidants. J. Food Sci. 2012;77:C1162–C1168. doi: 10.1111/j.1750-3841.2012.02926.x. [DOI] [PubMed] [Google Scholar]
- Anuj Yadav K.R., Ashwani Yadav J.P., Mishra S.S., Prabha S. Antioxidants and its functions in human body - a Review. Res. Environ. Life Sci. 2016;9:1328–1331. [Google Scholar]
- Assimopoulou A.N., Sinakos Z., Papageorgiou V.P. Radical scavenging activity of Crocus sativus L . Extract and its bioactive constituents. Phyther. Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- Assimopoulou A.N., Sinakos Z., Papageorgiou V.P. Radical scavenging activity of crocus sativus L . Extract and its bioactive constituents. Phyther. Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- Badarinath A.V., Mallikarjuna Rao K., Madhu Sudhana Chetty C., Ramkanth S., Rajan T.V.S., Gnanaprakash K. A review on In-vitro antioxidant methods: comparisions, correlations and considerations. Int. J. Pharm. Tech. Res. 2010;2:1276–1285. [Google Scholar]
- Bhargava V. Medicinal uses and pharmacological properties of crocus sativus Linn (saffron) Int. J. Pharm. Pharm. Sci. 2011;3:1–5. [Google Scholar]
- Biswas K., Bandyopadhyay U., Chattopadhyay I., Varadaraj A., Ali E., Banerjee R.K. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J. Biol. Chem. 2003;278:10993–11001. doi: 10.1074/jbc.M210328200. [DOI] [PubMed] [Google Scholar]
- Bononi M., Milella P., Tateo F. Gas chromatography of safranal as preferable method for the commercial grading of saffron (Crocus sativus L.) Food Chem. 2015;176:17–21. doi: 10.1016/j.foodchem.2014.12.047. [DOI] [PubMed] [Google Scholar]
- Boskabady M.H., Kiani S., Rakhshandah H., Relaxant effects of Rosa damascena on Guinea pig tracheal chains and its possible mechanism(s) J. Pharm. Pharmacol. 2006;58:1385–1390. doi: 10.1016/j.jep.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Boskabady M.H., Byrami G., Feizpour A. Pharmacological Reports the effect of safranal , a constituent of Crocus sativus ( saffron ), on tracheal responsiveness , serum levels of cytokines , total NO and nitrite in sensitized Guinea pigs. Pharmacol. Rep. 2014;66:56–61. doi: 10.1016/j.pharep.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Bukhari S.I., Pattnaik B., Rayees S., Kaul S., Dhar M.K. Safranal of crocus sativus L. Inhibits inducible nitric oxide synthase and attenuates asthma in a mouse model of asthma. Phyther. Res. 2015;29:617–627. doi: 10.1002/ptr.5315. [DOI] [PubMed] [Google Scholar]
- Butt M.S., Naz A., Sultan M.T., Qayyum M.M.N. Anti-oncogenic perspectives of spices/herbs: a comprehensive review. EXCLI J. 2013;12:1043–1065. [PMC free article] [PubMed] [Google Scholar]
- Carmona M., Zalacain A., Salinas M.R., Alonso G.L., Zalacain A., Salinas M.R., New G.L.A.A. A new approach to saffron aroma. Crit. Rev. Food Sci. Nutr. 2007;47:145–159. doi: 10.1080/10408390600626511. [DOI] [PubMed] [Google Scholar]
- Chanda S., Dave R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties : an overview. Afr. J. Microbiol. Res. 2009;3:981–996. [Google Scholar]
- Chen L., Hu J.Y., Wang S.Q. The role of antioxidants in photoprotection: a critical review. J. Am. Acad. Dermatol. 2012;67:1013–1024. doi: 10.1016/j.jaad.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Cheriyamundath S., Choudhary S., Lopus M. Safranal inhibits HeLa cell viability by perturbing the reassembly potential of microtubules. Phyther. Res. 2017 doi: 10.1002/ptr.5938. [DOI] [PubMed] [Google Scholar]
- Chermahini S.H., Fadzilah Adibah a M., Sarmidi M.R., Taghizadeh E., Salehnezhad S. Impact of saffron as an anti-cancer and anti-tumor herb. Afric. J. Pharm. Pharmacol. 2010;4:834–840. [Google Scholar]
- Christodoulou E., Np K., Kostomitsopoulos N., Valsami G. 2017. Saffron : a Natural Product with Potential Pharmaceutical Applications. PubMed Commons 7–8. [DOI] [PubMed] [Google Scholar]
- Das I., Chakrabarty R.N., Das S. Saffron can prevent chemically induced skin carcinogenesis in Swiss albino mice. Asian Pac. J. Cancer Prev. 2004;5:70–76. [PubMed] [Google Scholar]
- Delkhosh-kasmaie F., Abbas A., Tamaddonfard E., Imani M. The e ff ects of safranal , a constitute of sa ff ron , and metformin on spatial learning and memory impairments in type-1 diabetic rats : behavioral and hippocampal histopathological and biochemical evaluations. Biomed. Pharmacother. 2018;107:203–211. doi: 10.1016/j.biopha.2018.07.165. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova A.T. Phytochemicals as protectors against ultraviolet radiation: versatility of effects and mechanisms. Planta Med. 2008;74:1548–1559. doi: 10.1055/s-2008-1081296. [DOI] [PubMed] [Google Scholar]
- Dunaway S., Odin R., Zhou L., Ji L., Zhang Y. Natural Antioxidants : multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018;9:1–14. doi: 10.3389/fphar.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage M.A., Miller K.W., Elsner P., Maibach H.I. Intrinsic and extrinsic factors in skin ageing: a review. Int. J. Cosmet. Sci. 2008;30:87–95. doi: 10.1111/j.1468-2494.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- Farahzad J., Samarghandian S., Shoshtari M., Sargolzaei J., Hossinimoghadam H. Anti-tumor activity of safranal against neuroblastoma cells. Phcog. Mag. 2014;10:S419–S424. doi: 10.4103/0973-1296.133296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-sánchez L., Lax P., Noailles A., Angulo A., Maneu V., Cuenca N. Natural compounds from saffron and bear bile prevent vision loss and retinal degeneration. Molecules. 2015;20:13875–13893. doi: 10.3390/molecules200813875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyzi R., Boskabady M.H., Seyed S.M. The effect of safranal on Th 1/Th 2 cytokine balance. Iran J. Immunol. 2016;13:263–273. doi: 10.1002/jemt.20944. [DOI] [PubMed] [Google Scholar]
- Geronzi U., Lotti F., Grosso S. Oxidative stress in epilepsy. Expert Rev. Neurother. 2018 doi: 10.1080/14737175.2018.1465410. [DOI] [PubMed] [Google Scholar]
- Ghaffari S.M., Bagheri A. Stigma variability in saffron (Crocus sativus L.) Afr. J. Biotechnol. 2009;8:601–604. [Google Scholar]
- Gholamnezhad Z., Koushyar H., Byrami G., Boskabady M.H. The extract of crocus sativus and its constituent safranal , affect serum levels of endothelin and total protein in sensitized Guinea pigs. Iran. J. Basic Med. Sci. 2013;16:1022–1026. [PMC free article] [PubMed] [Google Scholar]
- Golmohammadzadeh S., Jaafari M.R., Hosseinzadeh H. Does saffron have antisolar and moisturizing Effects ? Iran. J. Pharm. Res. (IJPR) 2010;9:133–140. [PMC free article] [PubMed] [Google Scholar]
- Golmohammadzadeh S., Imani F., Hosseinzadeh H., Jaafari M.R. Preparation, characterization and evaluation of sun protective and moisturizing effects of nanoliposomes containing safranal. Iran. J. Basic Med. Sci. 2011;14:521–533. [PMC free article] [PubMed] [Google Scholar]
- Gragnani A., Mac Cornick S., Chominski V., Ribeiro de Noronha S.M., Alves Corrêa de Noronha S.A., Ferreira L.M. Review of major theories of skin aging. Adv. Aging Res. 2014;3:265–284. [Google Scholar]
- Hel V., Schröder K. Redox control in cancer development and progression. Mol. Aspects Med. 2018;1 doi: 10.1016/j.mam.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Heydari S., Haghayegh G.H. Extraction and microextraction techniques for the determination of compounds from saffron. Can. Chem. Trans. 2014;2:221–247. [Google Scholar]
- Hoshyar R., Mollaei H. A comprehensive review on anticancer mechanisms of the main carotenoid of saffron , crocin. J. Pharm. Pharmacol. 2017;69:1419–1427. doi: 10.1111/jphp.12776. Nov. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Noraei N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother Res. 2009;23:768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Ghenaati J. Evaluation of the antitussive effect of stigma and petals of saffron (Crocus sativus) and its components, safranal and crocin in Guinea pigs. Fitoterapia. 2006;77:446–448. doi: 10.1016/j.fitote.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Sadeghnia H.R. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: involvement of GABAergic and opioids systems. Phytomedicine. 2007;14:256–262. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh Hossein, Sadeghnia H.R. Effect of safranal, a constituent of Crocus sativus (saffron), on methyl methanesulfonate (MMS)-induced DNA damage in mouse organs: an alkaline single-cell gel electrophoresis (comet) assay. DNA Cell Biol. 2007;26:841–846. doi: 10.1089/dna.2007.0631. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Shariaty V.M. Anti-nociceptive effect of safranal, a constituent of Crocus sativus (saffron), in mice. Pharmacologyonline. 2007;2:498–503. [Google Scholar]
- Hosseinzadeh H., Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–724. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H., Modaghegh M.H., Saffari Z. Crocus sativus L. ( saffron ) extract and its active constituents ( crocin and safranal ) on ischemia-reperfusion in rat skeletal muscle. eCAM. 2009;6:343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinzadeh H., Shakib S.S., Sameni A.K., Taghiabadi E. Acute and subacute toxicity of safranal, a constituent of saffron, in mice and rats. Iran. J. Pharm. Res. 2013;12:93–99. [PMC free article] [PubMed] [Google Scholar]
- Imenshahidi M., Marjan B., Faal A., Gholampoor A. The effect of chronic administration of safranal on systolic blood pressure in rats. Iran. J. Pharm. Res. 2015;14:585–590. [PMC free article] [PubMed] [Google Scholar]
- Imokawa G. Recent advances in characterizing biological mechanisms underlying UV-induced wrinkles: a pivotal role of fibrobrast-derived elastase. Arch. Dermatol. Res. 2008;300 doi: 10.1007/s00403-007-0798-x. [DOI] [PubMed] [Google Scholar]
- Jabini R., Ehtesham-gharaee M., Dalirsani Z., Delavarian Z., Behravan J. Evaluation of the cytotoxic activity of crocin and safranal , constituents of saffron, in oral squamous cell carcinoma (KB cell line) Nutr. Cancer. 2017:1–9. doi: 10.1080/01635581.2017.1339816. [DOI] [PubMed] [Google Scholar]
- Jain G.K., Pathan S.A. vol. 1. 2012. Mucoadhesive Nanoemulsion of Safranal for the Treatment of Epilepsy; p. 1307620. [Google Scholar]
- Jalali-Heravi M., Parastar H., Ebrahimi-Najafabadi H. Characterization of volatile components of Iranian saffron using factorial-based response surface modeling of ultrasonic extraction combined with gas chromatography-mass spectrometry analysis. J. Chromatogr. A. 2009;1216:6088–6097. doi: 10.1016/j.chroma.2009.06.067. [DOI] [PubMed] [Google Scholar]
- Kaur A., Thatai P., Sapra B. Need of UV protection and evaluation of efficacy of sunscreens. J. Cosmet. Sci. 2014;65:315–345. [PubMed] [Google Scholar]
- Khameneh B., Halimi V., Jaafari M.R., Golmohammadzadeh S. Safranal – loaded solid lipid nanoparticles: evaluation of sunscreen and moisturizing potential for topical applications. Iran. J. Basic Med. Sci. 2015;18:58–63. [PMC free article] [PubMed] [Google Scholar]
- Khazdair M.R., Boskabady M.H., Hosseini M., Rezaee R., M Tsatsakis A. The effects of Crocus sativus (saffron) and its constituents on nervous system: a review. Avicenna J. Phytomed. 2015;5:376–391. [PMC free article] [PubMed] [Google Scholar]
- Khorasany A.R., Hosseinzadeh H. Therapeutic effects of saffron (Crocus sativus L.) in digestive disorders: a review. Iran. J. Basic Med. Sci. 2016;19:455–469. [PMC free article] [PubMed] [Google Scholar]
- Kianbakht S., Mozafari K. Effects of saffron and its active constituents , crocin and safranal , on prevention of indomethacin induced gastric ulcers in diabetic and nondiabetic rats. J. Med. Plants. 2009;8:30–38. [Google Scholar]
- Kulkarni S., Sane A., Bhise K., Patil A., Dhamole P., Desai S. International Congress on Environmental, Biotechnology, and Chemistry Engineering IPCBEE. 2014. Development of extraction methods and quantification of safranal by high performance liquid chromatography from cuminum cyminum L . And studying its antimicrobial properties; pp. 5–9. [Google Scholar]
- Lozano P., Delgado D., Gomez D., Rubio M., Iborra J.L. A non-destructive method to determine the safranal content of saffron ( Crocus sativus L .) by supercritical carbon dioxide extraction combined with high-performance liquid chromatography and gas chromatography. Format Abstr. Full Text links J. Biochem. Biophys. Methods. 2000;43:367–378. doi: 10.1016/s0165-022x(00)00090-7. [DOI] [PubMed] [Google Scholar]
- Luis G., Salinas A., Jim A.M., Chaouqi S., Llorens S., Mart M., Alonso G.L. Saffron : an old medicinal plant and a potential novel functional food. Molecules. 2018;23:1–21. doi: 10.3390/molecules23010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan K., Nanda S. In-vitro evaluation of antioxidant , anti-elastase , anti- collagenase , anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorg. Chem. 2018;77:159–167. doi: 10.1016/j.bioorg.2017.12.030. [DOI] [PubMed] [Google Scholar]
- Maeda A., Kai K., Ishii M., Ishii T., Akagawa M. Safranal , a novel protein tyrosine phosphatase 1B inhibitor , activates insulin signaling in C2C12 myotubes and improves glucose tolerance in diabetic KK- A y mice. Mol. Nutr. Food Res. 2014;58:1177–1189. doi: 10.1002/mnfr.201300675. [DOI] [PubMed] [Google Scholar]
- Malaekeh-Nikouei B., Mousavi S.H., Shahsavand S., Mehri S., Nassirli H., Mehri S. Assessment of cytotoxic properties of safranal and nanoliposomal safranal in various cancer cell lines. Phytother Res. 2013;27:1868–1873. doi: 10.1002/ptr.4945. [DOI] [PubMed] [Google Scholar]
- Mehdizadeh R., Parizadeh M.R., Khooei A.R., Mehri S., Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran. J. Basic Med. Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- Melnyk J.P., Wang S., Marcone M.F. Chemical and biological properties of the world ’ s most expensive spice : Saffron. Food Res. Int. 2010;43:1981–1989. [Google Scholar]
- Mir J.I., Ahmed N., Singh D.B., Khan M.H., Zaffer S., Shafi W. Breeding and biotechnological opportunities in saffron crop improvement. Afr. J. Agric. Res. 2015;10:970–974. [Google Scholar]
- Mokhtari-Zaer A., Khazdair M.R., Boskabady M.H. Smooth muscle relaxant activity of Crocus sativus (saffron) and its constituents: possible mechanisms. Avicenna J. Phytomed. 2015;5:365–375. [PMC free article] [PubMed] [Google Scholar]
- Mollazadeh H., Emami S.A., Hosseinzadeh H. Razi ’ s Al – Hawi– and saffron ( Crocus sativus ): a review. Iran J. Basic Med. Sci. 2015;18:1153–1166. [PMC free article] [PubMed] [Google Scholar]
- Naghshineh A., Dadras A., Ghalandari B., Hossein G., Mohamad S., Modaresi S., Afrasiabi A., Kiani M. Chemico-Biological Interactions Safranal as a novel anti-tubulin binding agent with potential use in cancer therapy : an in vitro study. Chem. Biol. Interact. 2015;238:151–160. doi: 10.1016/j.cbi.2015.06.023. [DOI] [PubMed] [Google Scholar]
- Nyon K., Park Y., Jung H., Yeon J., Duk B., Park S., Jung W., Cho K., Park J. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Neuropharmacol. Analg. 2010;68:9–11. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Pai V.V., Shukla P., Kikkeri N.N. Antioxidants in dermatology. 2014;5:210–214. doi: 10.4103/2229-5178.131127. Varadraj V. Pai, Pankaj Shukla, Naveen Narayanshetty Kikker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik Y.-H., Iwaisako K., Seki E., Inokuchi S., Schnabl B., Osterreicher C.H., Kisseleva T., Brenner D. a. The nicotinamide adenine dinucleotide phosphate oxidase homologues {NOX1} and {NOX2/gp91phox} mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730–1741. doi: 10.1002/hep.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandel R., Poljšak B., Godic A., Dahmane R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:1–11. doi: 10.1155/2013/930164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Sarwat M., Khan T.H. Mechanism behind the anti-tumour potential of saffron ( crocus sativus L . ): the molecular perspective. Crit. Rev. Oncol./Hematol. 2017:27–35. doi: 10.1016/j.critrevonc.2017.04.010. Jul. [DOI] [PubMed] [Google Scholar]
- Pathan S.A., Alam S., Jain G.K., Zaidi S.M.A., Akhter S., Vohora D., Khar K., Ahmad F.J. Quantitative analysis of safranal in saffron extract and nanoparticle formulation by a validated high-performance thin-layer chromatographic method. Phytochem. Anal. 2009 doi: 10.1002/pca.1184. [DOI] [PubMed] [Google Scholar]
- Pittayapruek P., Meephansan J., Prapapan O., Komine M., Ohtsuki M. Role of matrix metalloproteinases in Photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017;1–13 doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pk P., Ly Q., Xn W. Safranal prevents rotenone-induced oxidative stress and apoptosis in an in vitro model of Parkinson ’ s disease through regulating Keap1/Nrf2 signaling pathway. Cell. Mol. Biol. 2016;62:11–17. [PubMed] [Google Scholar]
- Poon F., Kang S., Chien A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015;31:65–74. doi: 10.1111/phpp.12145. [DOI] [PubMed] [Google Scholar]
- Prasad A., Pospísil P. Ultraweak photon emission induced by visible light and ultraviolet A radiation via photoactivated skin chromophores: in vivo charge coupled device imaging. J. Biomed. Opt. 2012;17 doi: 10.1117/1.JBO.17.8.085004. [DOI] [PubMed] [Google Scholar]
- Rafieipour F., Hadipour E., Emami S.A., Asili J., Tayarani-najaran Z. Safranal protects against beta-amyloid peptide-induced cell toxicity in PC12 cells via MAPK and PI3 K pathways. Metab. Brain Dis. 2019;34:165–172. doi: 10.1007/s11011-018-0329-9. [DOI] [PubMed] [Google Scholar]
- Rahaiee S., Moini S., Hashemi M., Shojaosadati S.A. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): a review. J. Food Sci. Technol. 2015;52:1881–1888. doi: 10.1007/s13197-013-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razavi B.M., Amanloo Mojtaba Alipoor, Mohsen Imenshahidi H., Hosseinzadeh The relaxant activity of safranal in isolated rat aortas is mediated predominantly via an endothelium-independent mechanism - vasodilatory mechanism of safranal. J. Pharmacopuncture. 2016;19:329–335. doi: 10.3831/KPI.2016.19.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riahi-zanjani B., Balali-mood M., Mohammadi E., Badie H. Safranal as a safe compound to mice immune system. Avicenna J. Phytomed. 2015;5:441–449. [PMC free article] [PubMed] [Google Scholar]
- Rinnerthaler M., Bischof J., Streubel M.K., Trost A., Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5:545–589. doi: 10.3390/biom5020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghnia H.R., Cortez M.A., Liu D., Hosseinzadeh H. Antiabsence effects of safranal in acute experimental seizure Models : EEG and autoradiography. J. Pharm. Pharm. Sci. 2008;11:1–14. doi: 10.18433/j38g6j. [DOI] [PubMed] [Google Scholar]
- Sadeghnia H.R., Kamkar M., Assadpour E., Boroushaki M.T., Ghorbani A. Protective effect of safranal, a constituent of crocus sativus, on quinolinic acid-induced oxidative damage in rat hippocampus. Iran. J. Basic Med. Sci. 2013;16:73–82. [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S., Borji A. Anticarcinogenic effect of saffron ( Crocus sativus L .) and its ingredients. Pharmacogn. Res. 2014;6:99–107. doi: 10.4103/0974-8490.128963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S., Boskabady M., Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Phcog. Mag. 2010;6:309–314. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S., Borji A., Delkhosh M.B., Samini F. Safranal treatment improves hyperglcemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. J. Pharm. Pharm. Sci. 2013;16:352–362. doi: 10.18433/j3zs3q. [DOI] [PubMed] [Google Scholar]
- Samarghandian S., Afshari R., Sadati A. Evaluation of lung and bronchoalveolar lavage fluid oxidative stress indices for assessing the preventing effects of safranal on respiratory distress in diabetic rats. Sci. World J. 2014:6. doi: 10.1155/2014/251378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S., Azimi-nezhad M., Samini F. Preventive effect of safranal against oxidative damage in aged male rat brain. Exp. Anim. 2015;64:65–71. doi: 10.1538/expanim.14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S., Azimi-Nezhad M., Samini F. Preventive effect of safranal against oxidative damage in aged male rat brain. Exp. Anim. 2015;64:65–71. doi: 10.1538/expanim.14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarghandiana Saeed, Azimi-Nezhad Mohsen, Farkhondeh T. Immunomodulatory and antioxidant effects of saffron aqueous extract ( Crocus sativus L .) on streptozotocin-induced diabetes in rats. Indian Heart J. 2016;1–11 doi: 10.1016/j.ihj.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangarajan R., Meera S., Rukkumani R., Sankar P., Anuradha G. Antioxidants:Friend or foe? Asian Pac. J. Trop. Med. 2017;10:1111–1116. doi: 10.1016/j.apjtm.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Sariri R., Sabbaghzadeh R., Poumohamad F. In-vitro antioxidant and anti-tyrosinase activity of methanol extracts from crocus sativus flowers. Pharmacologyonline. 2011;3:1–11. [Google Scholar]
- Serafini M.R., Guimarães A.G., Quintans J.S., Araújo A.A., Nunes P.S., Quintans-Júnior L.J. Natural compounds for solar photoprotection: a patent review. Expert Opin. Ther. Pat. 2015;25:467–478. doi: 10.1517/13543776.2014.1000863. [DOI] [PubMed] [Google Scholar]
- Serras F. The benefits of oxidative stress for tissue repair and regeneration. Fly (Austin) 2016;10:128–133. doi: 10.1080/19336934.2016.1188232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyed Kazem Farahmand F.S., Mohammad Samini Samarghandian S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat river. Biogerontology. 2013;14:63–71. doi: 10.1007/s10522-012-9409-0. [DOI] [PubMed] [Google Scholar]
- Shafiee Mojtaba, Arekhi Soheil, Alireza Omranzadeh A.S. Saffron in the treatment of depression, anxiety and other mental disorders: current evidence and potential mechanisms of action. J. Affect. Disord. 2018:330–337. doi: 10.1016/j.jad.2017.11.020. Feb. [DOI] [PubMed] [Google Scholar]
- Singh G. Can we prevent skin aging ? Indian J. Dermatol. Venereol. Leprol. 2009;75:447–451. doi: 10.4103/0378-6323.55386. [DOI] [PubMed] [Google Scholar]
- Sinica D.P., Chaudhari S.P., Bhandurge N.B., Ratnaparkhi M.P. Development and validation of UV spectrophotometric method of safranal. Der Pharm. Sin. 2013;4:136–140. [Google Scholar]
- Siti H.N., Kamisah Y., Kamsiah J. The role of oxidative stress , antioxidants and vascular in fl ammation in cardiovascular disease ( a review ) Vasc. Pharmacol. 2015;71:40–56. doi: 10.1016/j.vph.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Soltan M.Y., Sumarni U., Assaf C., Langer P., Reidel U., Eberle J. Key role of reactive oxygen species ( ROS ) in indirubin derivative-induced cell death in cutaneous T-cell lymphoma cells. Int. J. Mol. Sci. Artic. 2019;20:1–14. doi: 10.3390/ijms20051158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorriento D., De Luca N., Trimarco B., Iaccarino G. The antioxidant Therapy : new insights in the treatment of hypertension. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Ahmed H., Dixit R.K., Saraf S.A. Crocus sativus L.: a comprehensive review. Pharmacogn. Rev. 2010;4:200–208. doi: 10.4103/0973-7847.70919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaddonfard E., Farshid A.A., Eghdami K., Samadi F., Erfanparast A. Comparison of the effects of crocin, safranal and diclofenac on local inflammation and inflammatory pain responses induced by carrageenan in rats. Pharmacol. Rep. 2013 doi: 10.1016/s1734-1140(13)71485-3. [DOI] [PubMed] [Google Scholar]
- Toledo-ibelles P., Mas-oliva J. Antioxidants in the fight against Atherosclerosis : is this a dead End ? Curr. Atheroscler. Rep. 2018;20 doi: 10.1007/s11883-018-0737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Weller C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006;17:300–312. [Google Scholar]
- Zhang C., Ma J., Fan L., Zou Y., Dang X., Wang K. Neuroprotective effects of safranal in a rat model of traumatic injury to the spinal cord by anti-apoptotic, anti-inflammatory and edema-attenuating. Tissue Cell. 2015;47:291–300. doi: 10.1016/j.tice.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Xi G. Safranal-promoted differentiation and survival of dopaminergic neurons in an animal model of Parkinson ’ s disease. Pharm. Biol. 2018;6:450–454. doi: 10.1080/13880209.2018.1501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.