Abstract
Objective
To evaluate efficacy and safety of fresh Salvia officinalis extract tablets in relieving typical symptoms in menopausal women and to gain insight in the mode of action by measuring altered cerebral wave intensities.
Methods
Randomized 80 menopausal women from 48 – 65 years of age received Menosan® tablets [3′400 mg ethanolic extract of freshly harvested Salvia officinalis L.] or placebo under double-blind conditions for 4 weeks. An efficacy analysis evaluated the developments of the menopausal rating scale [MRS], hot flush severity score [HFS] and quantitative electroencephalography [qEEG] intensities in the per protocol population. Results were further corroborated by data from the intention to treat population including late postmenopausal women.
Results
Salvia off. distinctly reduced MRS by 39.2% from 15.3 ± 6.87 to 9.3 ± 5.75 and significantly in comparison to placebo (p = 0.002). The HFS score decreased by 55.3% from 15.9 ± 13.77 to 7.1 ± 7.41, reaching significance on week 3 onwards (p = 0.028). Clinical effects of Salvia off. correlated with relevant reduction of frontal lobe beta2 wave qEEG intensities at electrodes F3/4/7/8 and are underpinned by secondary parameters and ITT analysis. Salvia off. within 4 weeks significantly reduced the somato-vegetative (e.g. hot flushes) and psychological MRS subscale (e.g. physical and mental exhaustion) subscale (p < 0.05) without a significant effect on the genito-urinary subscale. A positive impact of Salvia off. compared to placebo was furthermore seen on sleep quality, discontent and fatigue (p < 0.05) as evidenced by sleep and profile of mood state questionnaires.
Tolerability was uniformly rated as very good for Salvia off. extract and placebo, with an overall incidence of three adverse events in total, none of which treatment-related.
Conclusion
The results support the use of Salvia off. for the specific treatment of a wide range of somato-vegetative and psychological symptoms as experienced by menopausal women and correlate this effect to a restoration of associated dysbalanced brain waves.
The study was registered as EudraCT-No 2016-005033-77.
Keywords: Salvia officinalis, Menosan®, Menopause, Menopausal rating scale, Quantitative EEG, Hot flash, Hyperhidrosis, Cognition, Relaxation
Salvia officinalis; Menosan®; Menopause; Menopausal rating scale; Quantitative EEG; Hot flash; Hyperhidrosis; Cognition; Relaxation.
1. Introduction
A majority of perimenopausal women complain about challenging vasomotor symptoms like hot flushes, as well as fatigue, cognitive and mood alterations. It is widely known that estrogens neuro-modulate central neural circuits and exert an intricate influence on thermoregulation where the loss of a reliable estrogen rhythm weakens temperature homeostasis via dysmodulation of serotonergic and noradrenergic receptors (Rossmanith and Ruebberdt, 2009). Whilst standard therapy is still considered to be hormone replacement [HRT], its time restriction of use and association with a wide range of side effects including tumor growth, lead many women to seek effective and safe alternatives. Salvia off. has been shown to be depending on mechanisms of action other than estrogenicity and to regulate neurotransmitters implicated in hot flushes as well as state of mood and cognition. Salvia off. in the form of a hydroalcoholic extract has demonstrated inhibitory action on serotonergic, adrenergic, muscarinic and μ-opioid receptors in-vitro (Tober and Schoop, 2019).
Salvia off. furthermore has traditionally been used for the treatment of hyperhidrosis and recent studies indicate wider benefits. Hence, the present study aimed to provide placebo controlled efficacy confirmation for Salvia off. in the treatment of climacteric symptoms as observed in a prior uncontrolled study (Bommer et al., 2011), and to confirm in-vitro data concerning neurotransmitter activity targeted by Salvia off. by testing associated brain waves. Quantitative electroencephalograms already explored pharmacological effects through neuro-modulation to allow for a correlation of brain activity changes with menopausal clinical symptoms (Staikou et al., 2012; Campbell et al., 2011). Based on these findings and the in vitro data obtained for the specific Salvia off. extract under investigation, examination of brain waves under therapy have been undertaken to elucidate the mode of action of Salvia off..
Altered cerebral beta2 wave intensities at F3/4/7/8 positions had been proposed as physiological markers for distinction of pre-from menopausal and early postmenopausal women, while excluding late postmenopausal women. Testing a putative adaptogenic effect of Salvia off. by modulating brain wave dysregulation associated with the menopausal transition as seen most prominently in perimenopause and especially early postmenopause, we aimed to investigate efficacy of a proprietary Salvia off. extract in a confirmatory efficacy analysis in this latter group (per protocol, PP) to more specifically address benefits of Salvia off. for typical menopausal ailments. Results were finally backed up with data including women in the late postmenopause with additional impact of age-related symptomatology to test for generalizability of treatment benefits (intention to treat, ITT). The definition of the per protocol group essentially correlates with the age definitions provided by the Staging of Reproductive Aging Workshop/STRAW+10, establishing the marking line between early and late postmenopause at 5 years after the final menstrual period (Harlow et al., 2012).
2. Materials and methods
This double-blind, randomized and controlled clinical study (EudraCT-No 2016-005033-77) was authorized by the Federal Institute for Drugs and Medical Devices (BfArM), Bonn/Germany, on April 18, 2017, approved by the ethics committee of the Medical Association of Hessen (Landesärztekammer) on April 27, 2017, and conducted at NeuroCode, Wetzlar/Germany in accordance with the Declaration of Helsinki.
Verum tablets Menosan® (Swiss authorization no. 61664, held by A.Vogel AG, Roggwil/Switzerland) contained 280 mg thujone-free spissum extract of freshly harvested sage tips (Salvia off. Lamiaceae) equivalent to 3400 mg tincture. Inert placebo tablets were identically sized and colored to match verum tablets.
The randomization list was prepared by Hans Carlos Hoffmann (Asslar-Berghausen/Germany), using Kernel's random number generator as entropy source with a block size of 8. Sealed emergency envelopes containing the randomization codes were retained by the statistician and by the investigator.
Informed consent was obtained from patients screened for eligibility by the following inclusion criteria: women between 48 and 65 years (both included) menopausal since ≥12 months, Menopausal Rating Scale [MRS] total score ≥10, at least 5 hot flushes/24 h or intense hot flushes daily, disturbing sweating (Visual Analogue Scale [VAS] 0–4: >1) and inconspicuous examination (i.e. no study relevant pathological findings, acute or chronic diseases and allergies). Exclusion criteria were ovariectomy, chronic resistance to therapy in the indication area, epilepsy or severe depression (Hamilton Depression rating scale [HAMD] ≥ 30), anamnestic or current alcohol, drug, nicotine, coffee or tea abuse, intake of clinically relevant medications during the last two weeks before screening as well as during the active study period, particularly of Hormone Replacement Therapy [HRT], menopause- or cognitive performance-related herbal preparations and medication with central nervous action (e.g. psychotropics or antiepileptics), participation in another clinical study within the last 60 days, Body Mass Index/BMI ≤17.9 or ≥35.1, pregnancy, lactation, negative or invalid menopause quick test, noncompliance and cancellation of informed consent.
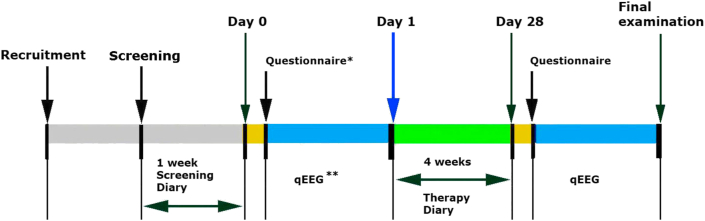
On screening day -7, demographic data were obtained and eligible patients were allocated to Salvia off. or placebo groups according to randomization list and in ascending order of patient's appearance. Vital signs were checked during clinical examination, a 12 channel qEEG was performed and samples were collected for laboratory tests (hematology, urine status). Quick tests for drugs, alcohol (repeated on day 28), menopause and absence of pregnancy were performed. During the following 7 day baseline and throughout the four treatment weeks, participants recorded hot flushes' number and intensity (ranging from 1 to 4 according to the definition of Sloan et al., 2001) and yielding by multiplication the Hot flush severity [HFS] score. First exposure to study medication was on day 0 under supervision when also different questionnaires were completed and participants underwent extensive qEEG examination before and after intake of study medication. Completed was the Menopause Rating Scale [MRS] calculated as the sum of the scores of the somato-vegetative subscale with the 4 symptoms hot flushes, heart discomfort, sleep problems, and joint and muscular discomfort, the psychological subscale with the 4 symptoms depressive mood, irritability, anxiety, and physical and mental exhaustion, and the genitourinary subscale with the 3 symptoms sexual problems (change in sexual desire, in sexual activity and satisfaction), bladder problems, and vaginal dryness, furthermore the Hamilton Anxiety Rating Scale [HAMA], Profile of Mood State [POMS], and sleep quality questionnaire [SF-B/R]. The same qEEG and filling of questionnaires were repeated on day 28 after self-recorded daily intake of study medication. Returned tablets were counted to measure compliance. A closure visit with assessment of physical and safety parameters was conducted within 2 days after day 28 (Figure 1).
Figure 1.
Study course including examination days 0 and 28. ∗Questionnaires: MRS, HAMA, POMS,SF-B/R. ∗∗qEEG recording conditions and tests: eyes open [EO], reading [RE], concentration d2-test [d2], memory test [ME], concentration performance test [CPT], reaction time test [RT], number identification test [NIT] and number connection test [NCT].
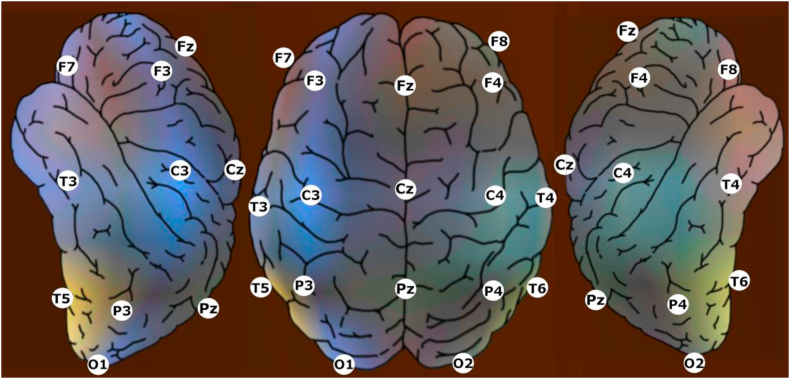
Estimation of the required sample size was established using archived qEEG data of pre-menopausal women free of complaints. The spectral power of frontal beta2 waves recorded “eyes open” at electrodes F3/4/7/8 (Figure 2) of menopausal women was found to be related to hot flushes (Tegeler et al., 2015) and significantly higher than for fertile women aged 38 to 47. A 50 % reduction of the spectral power by verum treatment was targeted. Taking into account the data distribution of previous studies, a sample size of n = 40 was regarded sufficient for PP analysis excluding late postmenopausal women, i.e. participants above age 56 years (unilaterally tested on the level of alpha = 0.1).
Figure 2.
Standard positions for electrodes during qEEG measurements.
Statistical analysis encompassed the primary clinical endpoints predefined as reductions of MRS (days 0–28) and of HFS Score (from baseline week to day 28) as well as the pharmacological endpoint change in frontal beta2 brain waves “eyes open” (F3/4/7/8) from days 0–28. Evaluated secondary endpoints were the courses of the scores of HAMA, POMS, and SF-B/R and the changes of brain waves within 6 distinct frequency bands (alpha1 and 2, beta1 and 2, delta, theta) “eyes open” as well as during different cognitive tests including reading [RE], d2-concentration test [d2], memory test [ME], concentration performance test [CPT], reaction time test [RT], number identification test [NIT], and number connection test [NCT] from day 0 to day 28. The exploratory EEG data were conservatively interpreted in a qualitative way. Treatment group comparisons were performed with non-parametric testing using SAS Version 9.3 and statistical significance evaluated, principally on basis of a = 0.05. The differences between Salvia off. and placebo and their 95% CI in the course of the MRS from days 0–28 were calculated with Anova, as well as the HFS score differences between Salvia off. and placebo and their 95% CI from baseline to day 28 and to weeks 3 and 4, with baseline adjustment. The relative risk ratio for % reduction of MRS (total, subgroups, single symptoms) by Salvia off. compared to placebo were assessed using Chi2 test. qEEG data and questionnaires were analyzed with Chi2, Anova and the non-parametric Wilcoxon test while pharmacodynamic parameters were unilaterally t-tested. According to protocol, patients were evaluated for the primary analysis with the PP collective for which data is given in the results section unless otherwise stated. For demonstration of sensitivity, ITT analysis was performed as applicable. The safety collective encompassed all patients with administration of at least one tablet of the study medication.
3. Results
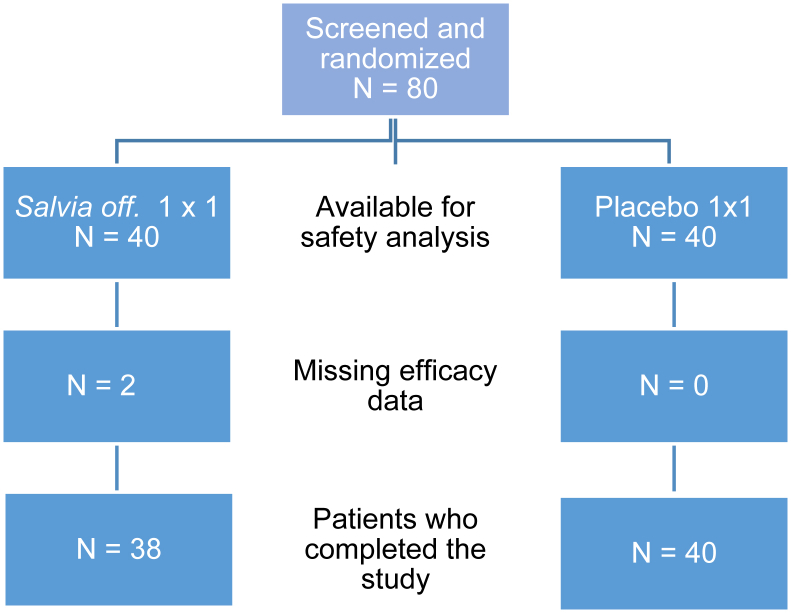
In total 80 Caucasian women suffering from menopause distress were enrolled (safety population) and randomly allocated to the Salvia off. or placebo treatment group. Two patients dropped out from ITT analysis with lacking efficacy data. 38 and 40 patients in Salvia off. and placebo groups, respectively, completed the study. Thereof 48 menopausal patients aged 48 to 56 (mean 53 ± 2.19 years) met the PP analysis criteria as set out in the statistical sample size calculation/case definition (Figure 3).
Figure 3.
Patient flow diagram.
Baseline demographics (age, BMI, body weight and height) were comparable for Salvia off. and placebo groups. Table 1a, Table 1ba, b displays PP and ITT population. Overall compliance was 97.2 % for Salvia off. and 96.1% for placebo, respectively.
Table 1a.
Patient characteristics in the per protocol population.
| Characteristics∗ | Placebo (n = 26) | Salvia off. (n = 22) | P value (Wilcoxon) |
|---|---|---|---|
| Age | 53.1 (2.29) | 53.1 (2.11) | >0.05 |
| Body weight | 67.15 (9.93) | 70.70 (11.35) | >0.05 |
| Body height | 166.23 (5.62) | 168.00 (5.46) | >0.05 |
| BMI (kg/m2) | 24.00 (4.18) | 24.32 (3.71) | >0.05 |
Values for age, body weight, height and body mass index are presented as mean (SD).
Table 1b.
Patient characteristics in the ITT population.
| Characteristics∗ | Placebo (n = 40) | Salvia off. (n = 38) | P value (Wilcoxon) |
|---|---|---|---|
| Age | 55.68 (4.30) | 55.71 (3.75) | >0.05 |
| Body weight | 68.98 (12.31) | 71.12 (12.13) | >0.05 |
| Body height | 165.68 (5.94) | 167.45 (5.57) | >0.05 |
| BMI (kg/m2) | 24.78 (4.57) | 24.71 (3.57) | >0.05 |
Values for age, body weight, height and body mass index are presented as mean (SD).
The primary efficacy analysis focused on the course of the clinical MRS and HFS scores under treatment. Table 2 shows a significant decrease of the MRS score for Salvia off. compared to placebo with a 39.2% decrease from 15.3 ± 6.87 to 9.3 ± 5.75 for Salvia off. and from 14.5 ± 6.04 to 13.0 ± 6.35 for placebo (p = 0.002) from day 0 to day 28. A very similar and significant reduction from 14.76 ± 6.18 to 11.13 ± 6.09 was seen for Salvia off. in the ITT analysis (p = 0.012) in contrast to placebo with a reduction from 14.63 ± 6.33 to 12.13 ± 5.86.
Table 2.
Menopause rating scale score day 0–28.
|
Salvia off. (A), n = 22 |
Placebo (B), n = 26 |
Difference |
||||
|---|---|---|---|---|---|---|
| Actual | %-Change | Actual | %-Change | B - A | ||
| Day 0 | Mean (SD) | 15.3 (6.87) | 14.5 (6.04) | |||
| Min-Max | 7.0–36.0 | 4.0–29.0 | ||||
| Day 28 | Mean (SD) | 9.3 (5.75) | -37.1 (29.90) | 13.0 (6.35) | -6.3 (34.34) | 30.8 (32.4) |
| 95% CI | [6.8; 11.9] | [-50.4; -23.9] | [10.4; 15.6] | [-20.2; 7.6] | P = 0.002 | |
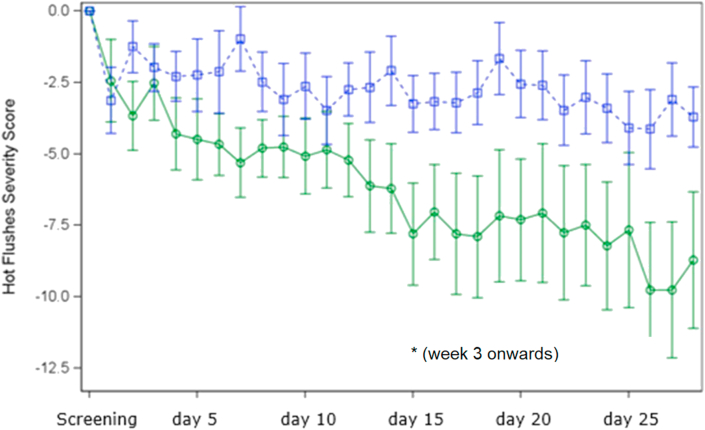
Figure 4 shows the courses of the HFS score for the two treatment groups. The obvious reduction of baseline HFS in favor of Salvia off. reaches statistical significance from week 3 onwards (p = 0.028) with a highly distinct reduction by 55% for Salvia off. from 15.9 ± 13.77 to 7.1 ± 7.41 and from 14.1 ± 9.33 to 10.3 ± 9.97 for placebo within four weeks, respectively. The percentage of patients, which experienced a clinically relevant, at least 50 % HSF score reduction from screening week to week 4 was significantly higher for Salvia off. compared to placebo with 59.1% versus 26.9% respectively (p = 0.024). Results were again confirmed for the ITT collective which showed a clinically relevant ≥50 % reduction of the HSF score for 55% of Salvia off. patients compared to 32.5% placebo patients (p = 0.043).
Figure 4.
Daily change of the Hot Flush Severity (HFS) Score over the 4 weeks treatment period compared to the arithmetic mean of the screening week for Salvia (green) vs Placebo (blue) ∗p ≤ 0.05.
Regarding the primary pharmacodynamic endpoint reduction of frontal beta2 power at electrode positions F3/4/7/8 from days 0–28, a relevant reduction was found for “eyes open” with significant differences between Salvia off. and placebo after 4 treatment weeks (Figure 5).
Figure 5.
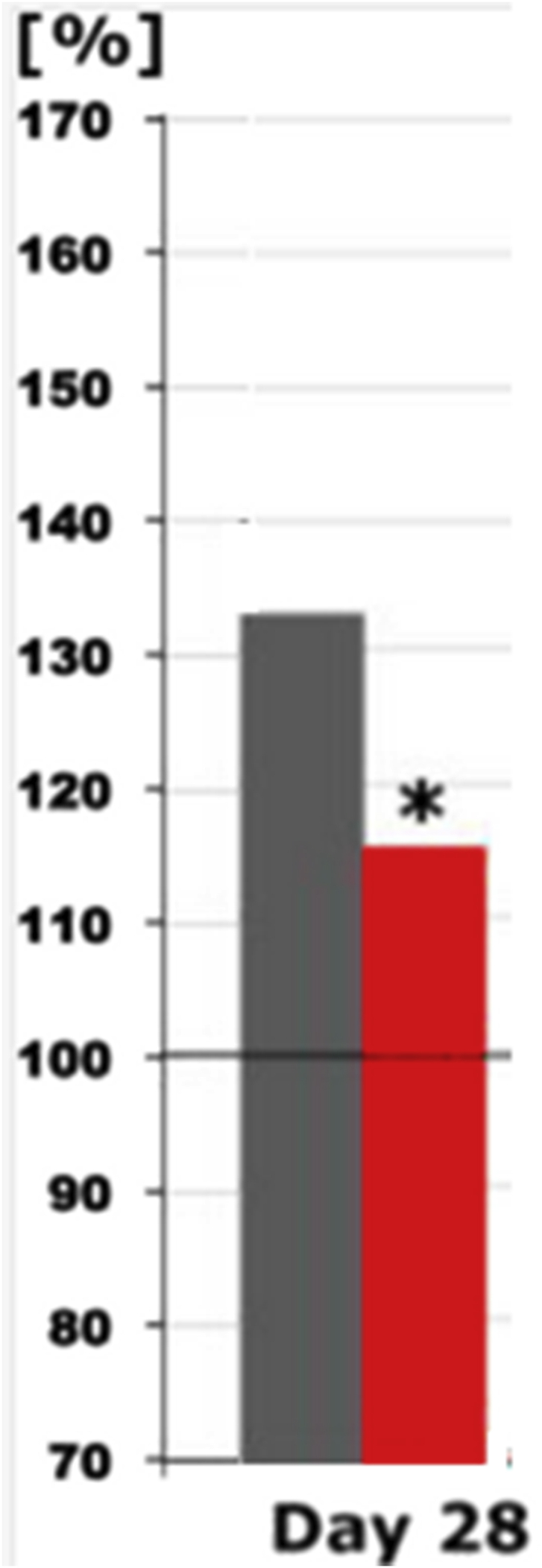
Beta 2 spectral power differences under the recording condition “eyes open” on day 28 in the presence of placebo (grey) or Salvia off. (red) with respect to F3/4/7/8 electrodes (one sided t-test). The spectral power (between 70 and 170% on the ordinate of the bar graph) is plotted against the pre-drug value (at baseline set to 100%), thus reflecting the effect of placebo or Salvia off.
The analysis of secondary endpoints revealed a highly significant (p = 0.006) difference between Salvia off. and placebo for a clinically relevant ≥50% reduction of the MRS score and p values ranging from 0.008 to 0.049 for a 0%, or min. 20% or 60% reduction, respectively (Figure 6).
Figure 6.
Treatment success for Salvia off. and placebo regarding reduction of MRS in % of responders for a ≥0%,20%,50% and 60% reduction, respectively. ∗∗p ≤ 0.01.
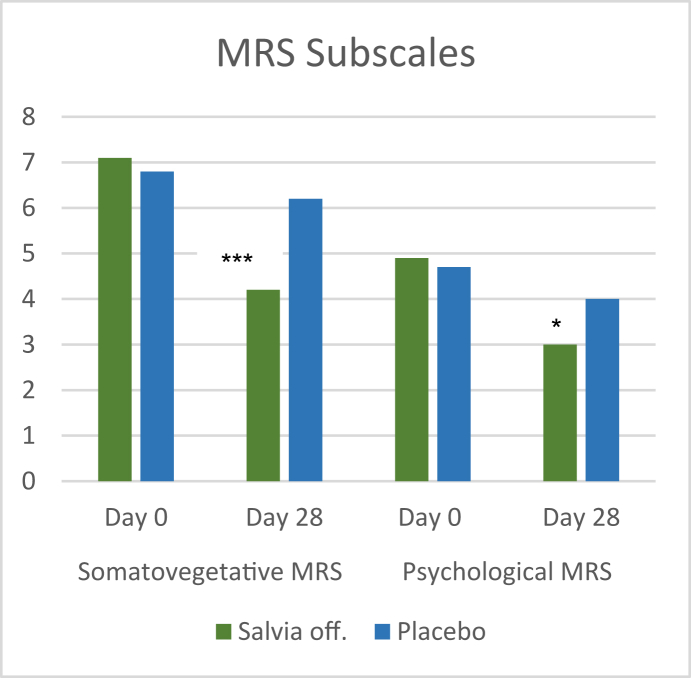
There was also a significant difference in reduction of the somato-vegetative and psychological MRS subscales (p = 0.0004 and p = 0.038, respectively) favoring Salvia off. (Figure 7), whilst the urogenital subscale showed only a trend regarding % of participants experiencing amelioration (data not shown). The single scores showed over the treatment period a significant difference (p < 0.05) in the reduction of the symptoms hot flushes, physical and mental exhaustion, sexual problems and joint and muscular discomfort whilst a trend was seen for sleep problems.
Figure 7.
Reduction of MRS somato-vegetative and psychological MRS subscales for Salvia off. and placebo. ∗∗∗p ≤ 0.001.
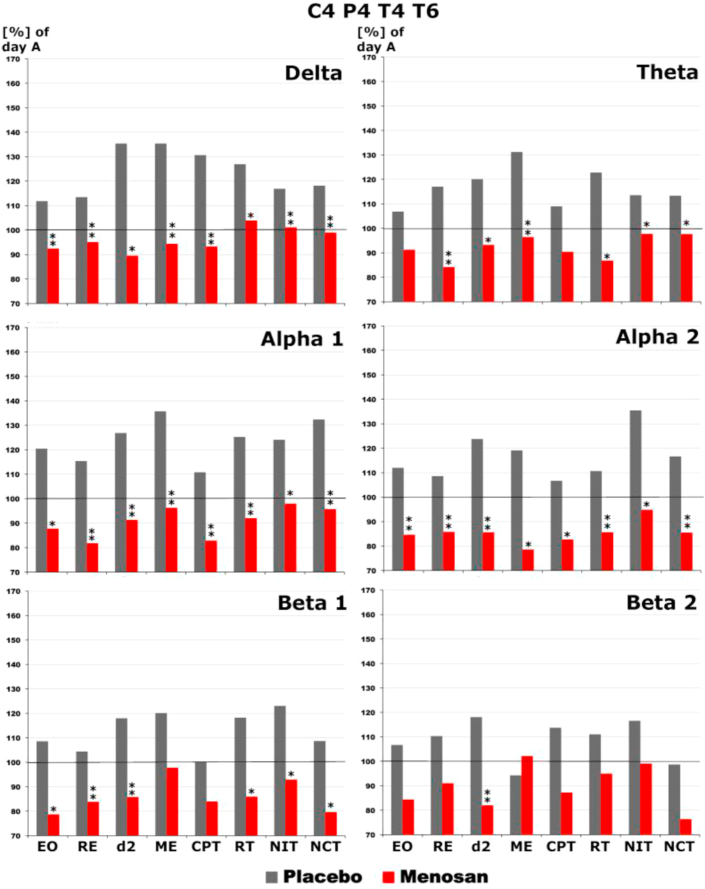
Electrical brain activity (days 0–28) in all brain areas within the 6 distinct frequency bands showed statistically significant spectral power differences for Salvia off. versus placebo at electrode positions C4, P4, T4 and T6 under test conditions “eyes open” [EO], concentration d2-test [D2], memory test [MT], concentration performance test [CPT], reaction time test [RT], number identification test [NIT] and number connection test [NCT] after 4 treatment weeks, mainly in the alpha1, alpha2, delta and theta bands (Figure 8).
Figure 8.
Change at the combination of C4P4T46 of brainwave activity of distinct frequency ranges (delta, theta, alpha1 and alpha2, beta1 and beta2) on Day 28 (after 4 weeks’ therapy) compared to baseline Day A = 0 (set to 100%) under qEEG recording conditions “eyes open” [EO] and cognitive tests reading [RE], d2-concentration test [d2], memory test [ME], concentration performance test [CPT], reaction time test [RT], number identification test [NIT], and number connection test [NCT] under treatment with Salvia off. and placebo.
The psychological POMS scale showed a significant difference between Salvia off. and placebo regarding discontent (-0.64 ± 0.65 to -0.27 ± 0.34, p = 0.019) and fatigue (2.19 ± 1.34 to 1.47 ± 0.99, p = 0.029). A statistical relevance compared to placebo could not be demonstrated for the remaining single items, albeit downheartedness showed a statistically relevant reduction (p = 0.021) from day 0–28 (0.66 ± 0.69 to 0.36 ± 0.43). No statistically significant changes were observed for the placebo patients. A relevant difference in treatment effect was seen for the sleep questionnaire S/F-BR for sleep quality and psychosomatic problems during sleep (p < 0.05) whilst the other items, i.e. mental balance before sleeping, mental exhaustion before sleeping, sleep wake regulation, dream recall and feeling refreshed after sleep, didn't change. A significant (p = 0.007) decrease in total HAMA score from 15.68 (day 0) to 9.82 (day 28) was observed for Salvia off. (p = 0.007), constituting a trend in favor of Salvia off. when compared with placebo where the total HAMA score was reduced from 15.27 to 11.19 (p = 0.02). According to protocol no analysis was performed on the level of HAMAS 14 single items, measuring psychic anxiety (mental agitation and psychological distress) as well as somatic anxiety (physical complaints related to anxiety), and encompassing anxious mood, tension, fears, insomnia, depressed mood, as well as intellectual, muscular, sensory, cardiovascular, respiratory, gastrointestinal, genitourinary, and autonomic symptoms and behaviour at interview, as the assay sensitivity for these particular parameters was judged not to be sufficiently precise and the intent was rather to gain an overall impression.
Tolerability was rated as very good by 100% of the patients of both treatment groups. Three adverse events in 3 patients (herpes zoster and road traffic accident in the Salvia off. and back pain in the placebo group) were reported and judged as unrelated and non-serious. No clinically relevant changes in laboratory parameters and vital signs were observed from day 0 to day 28.
4. Discussion
Salvia off. proved to be a valid option to fulfill the increasing demand of women reluctant or contraindicated to HRT who seek a natural and safe alternative treatment of their vasomotor and other typical menopausal symptoms like alterations of mood and cognition. This is the first time that in a placebo controlled, confirmatory clinical trial efficacy for a Salvia off. preparation was shown not only in reduction of hot flashes but of other climacteric symptoms as well.
As previously shown (Bommer et al., 2011) and demonstrated by the present study Salvia off. improves not only vasomotor symptoms (MRS, HFS scores) but simultaneously exerts a positive impact on accompanying somato-vegetative and psychological symptoms thus addressing a broad range of menopausal complaints. Indeed similar effects have been shown in this prior uncontrolled study for 2 months with the preparation under investigation. It is of interest to note that although this study demonstrated in the course of a 4 weeks application significant effects of Salvia off. on MRS and HSF against placebo, a further increase of treatment benefit can be expected with prolonged treatment based on the results of a 3 month placebo-controlled study with a Salvia off. preparation which showed increase of treatment effects in flushing frequency and severity compared to placebo with increase of application period (Zeidabadi, 2020). The same is however not to be anticipated for vaginal dryness which in contrast to sexual problems didn't yield significant effects compared to placebo in both studies, be it over a 4 or a 12 weeks period.
Results indicate a stronger effect of Salvia off. in the PP population compared to the ITT collective, which however still yielded significant results as evidenced by the data obtained for the clinical score of the MRS as well as for the HFS. Hence, Salvia off. clearly showed superior efficacy in women experiencing a typical menopause associated with symptoms lasting for up to 5 years after the final menstrual period (Bachmann, 2005).
With hot flushes and sleep problems constituting the most prevalent concerns in menopause, the impact of Salvia off. shown on improvement of sleep quality and psychosomatic problems during sleep constitutes a relevant asset, regardless of whether owed primarily to alleviation of night sweats and their detrimental impact on sleep or due to other, as yet to be elucidated intrinsic factors of a treatment with Salvia off. itself, even more so as life quality remains a core issue for postmenopausal women (Schneider, 2017; Conde et al., 2006; Ledesert et al., 1994). The relevant difference in reduction of discontent compared to placebo as well as the trend in favour of Salvia off. observed for reduction of downheartedness might further indicate a positive impact on perception of life quality as conferred by the treatment with Salvia off. The highly significant reduction of the HAMA score by Salvia off. over the course of 28 days showing a trend compared to placebo is backed up by former study results obtained for single dose applications of Salvia off. in young healthy adults showing an anxiolytic effect of the verum treatment (Kennedy, 2006).
Memory improvement has been shown for Salvia off. preclinically (Smach et al., 2015), and supports the clinical findings of enhanced cognition as seen in the present study. Such an effect, in contrast to vasomotor improvements (Imhof et al., 2018) was not confirmed in a recent clinical study with soy (Furlong et al., 2020). Clinical vasomotor improvements have furthermore been shown for a wide variety of phytopharmaceuticals, many relying on a phytoestrogenic way of action, including evening primrose oil (Farzaneh et al., 2013), red clover (Myers and Vigar, 2017; Ghazanfarpour et al., 2015, 2016; Shakeri et al., 2015), valerian (Ensiyeh et al., 2018), and black cohosh, the latter showing in a comparative trial of two non-proprietary preparations inferiority to Salvia off. in improving duration, severity and frequency of hot flushes (Masoumi et al., 2019).
As Tober (Tober et al., 2019) has shown convincing effects for Salvia off. not on estrogen but on neurotransmitter level, qEEG measurements have been chosen to track Salvia off. ‘s mode of action. This became consequently the first study to gain insight into the mode of action of Salvia off. in menopause. In vitro data show a distinct influence of the extract on serotonergic, adrenergic, muscarinic, GABA-ergic, and μ-opioid receptors, exempt from an estrogenic action (Rahte et al., 2013).
In this study we observed a reduction of the primary pharmacodynamic parameter frontal spectral beta2 power at electrodes F3/4/7/8 in the PP population after 4 treatment weeks with Salvia off. compared to placebo, indicative that less stress is produced and correlating with menopausal symptoms. Beta1 and 2 spectral power have been associated with glutamergic and GABA-ergic transmission, respectively, indicating that a normalization of the activity of these transmitters also might contribute to the action of Salvia off.
Mental rest is visualized by decreased delta power and mental endeavor by enhancement of theta power (Dimpfel et al., 2011) while in the current study, attenuation of both delta and theta power was measured at central, parietal and temporal electrode positions at “eyes open” and during mental challenges. Taking into consideration that delta waves are controlled by acetylcholine, such attenuation of central, parietal and temporal brain areas reflects an activation of cholinergic transmission, known to be implicated in the central thermoregulation while additionally changes of theta power have been related to noradrenergic transmission, two mechanisms matching in being involved in sweating and hot flushes both of which were shown to be controlled by Salvia off. also under in vitro conditions (Tober and Schoop, 2019).
Alpha1 spectral power reflects changes in serotoninergic transmission, whereas alpha2 waves seem to be under the control of dopamine. Attenuation of alpha waves, as experimentally shown in the current study, both acutely as well as after the course of four study weeks, probably indicate activation of these two transmitter systems, which themselves are known to be modulated by estrogens and reflects mental activation.
Hence Salvia off. induced both a state of mental activation as well as relaxation.
As for the safety analysis, the known excellent safety and tolerability profile of Salvia off. once more was demonstrated. Since the proprietary Salvia off. extract under investigation is devoid of thujone it is most suitable for long term therapy as usually requested by the natural course of the condition to be treated.
Overall, this double-blind, placebo controlled study proves the clinical efficacy of Salvia off. with respect to reduction of most of the typical menopausal complaints, in particular hot flushes and elucidates their association with altered brain waves as evidenced by qEEG spectral power intensities.
A significant change of general electrical brain activity indicated higher mental capacity and improved coping with cognitive challenges accompanied by a reduction of endogenous stress production. qEEG data visualized the impact on central nervous transmitter systems which are involved in required neuroadaptive processes as a consequence of menopausal estrogen decline.
Results confirmed the data of the prior uncontrolled study with Salvia off. and suggest that Salvia off. and more specifically the thujone-free proprietary extract from freshly harvested Salvia off. leaves under investigation, is an effective and safe herbal approach for the long-term treatment of hot flushes and other climacteric symptoms in menopause.
Declarations
Author contribution statement
D. Wilfried: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
C. D. G. Nina: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
B. Silvia: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by A.Vogel, Roggwil, Switzerland, manufacturer of the tested Salvia off. preparation Menosan® Salvia . S. Bommer is an A.Vogel employee.
Data availability statement
Data included in article.
Declaration of interests statement
The authors declare the following conflict of interests: This study was sponsored by A.Vogel, Roggwil, Switzerland, manufacturer of the tested Salvia off. preparation Menosan® Salvia . S. Bommer is an A.Vogel employee.
Additional information
No additional information is available for this paper.
Acknowledgements
We cordially thank Mrs. Leonie Schombert for qEEG analysis and documentation of the results.
References
- Bachmann G.A. Menopausal vasomotor symptoms. A review of causes, effects and evidence-based treatment options. J. Reprod. Med. 2005;50:155–165. [PubMed] [Google Scholar]
- Bommer S., Klein P., Suter A. First time proof of sage’s tolerability and efficacy in menopausal women with hot flushes. Adv. Ther. 2011 doi: 10.1007/s12325-011-0027-z. [DOI] [PubMed] [Google Scholar]
- Campbell I.G., Bromberger J.T., Buysse D.J. Evaluation of the association of menopausal status with delta and beta EEG activity during sleep. Sleep. 2011;34:1561–1568B. doi: 10.5665/sleep.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde D.M. Factors associated with quality of life in a cohort of postmenopausal women. Gynecol. Endocrinol. 2006;22:441–446. doi: 10.1080/09513590600890306. [DOI] [PubMed] [Google Scholar]
- Dimpfel W., Storni C., Verbruggen M. Ingested oat herb extract (Avena sativa) changes EEG spectral frequencies in healthy subjects. J. Alternative Compl. Med. 2011;17:427–434. doi: 10.1089/acm.2010.0143. [DOI] [PubMed] [Google Scholar]
- Ensiyeh J. The effect of Valerian on the severity and frequency of hot flashes : a triple –blind randomized clinical trial. Women Health. 2018;3:297–304. doi: 10.1080/03630242.2017.1296058. [DOI] [PubMed] [Google Scholar]
- Farzaneh F., Fatehi S., Sohrabi M.R. The effect of oral evening primrose oil on menopausal hot flushes: a randomized clinical trial. Arch. Gynecol. Obstet. 2013;288:1075–1079. doi: 10.1007/s00404-013-2852-6. [DOI] [PubMed] [Google Scholar]
- Furlong O.N., Parr H.J., Hodge S.J. Consumption of a soy drink has no effect on cognitive function but may alleviate vasomotor symptoms in post-menopausal women; a randomised trial. Z. Ernahrungswiss. 2020;59:755–766. doi: 10.1007/s00394-019-01942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazanfarpour M., Sadeghi R., Roudsari R.L. Effects of red clover on hot flush and circulating hormone concentrations in menopausal women: a systematic review and meta-analysis. Avicenna J. Phytomed. 2015;5:498–511. [PMC free article] [PubMed] [Google Scholar]
- Ghazanfarpour M., Sadeghi R., Roudsari R.L. Red clover for treatment of hot flushes and menopausal symptoms: a systematic review and meta-analysis. J. Obstet. Gynaecol. 2016;36:301–311. doi: 10.3109/01443615.2015.1049249. [DOI] [PubMed] [Google Scholar]
- Harlow S.D. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Climacteric. 2012;15(2):105–114. doi: 10.3109/13697137.2011.650656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof M., Gocan A., Imhof M. Soy germ extract alleviates menopausal hot flushes: placebo controlled double-blind trial. Eur. J. Clin. Nutr. 2018 doi: 10.1038/s41430-018-0173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.O. Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacology. 2006;31(4):845–852. doi: 10.1038/sj.npp.1300907. [DOI] [PubMed] [Google Scholar]
- Ledésert B. Menopause and perceived health status among the women of the French GAZEL cohort. Maturitas. 1994;20:113–120. doi: 10.1016/0378-5122(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Masoumi S.Z., Shayan A., Tabesh A. A comparative study on the effect of black cohosh and Salvia off.L. on hot flushes in postmenopausal women. Iranian J. Obstet. Gynecol. Infertility. 2019;22:1–12. [Google Scholar]
- Myers S.P., Vigar V. Effects of a standardised extract of Trifolium pratense (Promensil) at a dosage of 80 mg in the treatment of menopausal hot flushes: a systematic review and meta-analysis. Phytomedicine. 2017;24:141–147. doi: 10.1016/j.phymed.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Rahte S., Evans R., Eugster P.J., Marcourt L., Wolfender J.L., Kortenkamp A. Salvia officinalis for hot flushes: towards determination of mechanism of activity and active principles. Planta Med. 2013;79(9):753–760. doi: 10.1055/s-0032-1328552. [DOI] [PubMed] [Google Scholar]
- Rossmanith W.G., Ruebberdt W. What causes hot flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. Gynecol. Endocrinol. 2009;25:303–314. doi: 10.1080/09513590802632514. [DOI] [PubMed] [Google Scholar]
- Schneider H.P.G. Quality of life in climacteric women. Climacteric. 2017;20(3):187–194. doi: 10.1080/13697137.2017.1279599. [DOI] [PubMed] [Google Scholar]
- Shakeri F., Taavoni S., Goushegir A. Effectiveness of red clover in alleviating menopausal symptoms: a 12-week randomized, controlled trial. Climacteric. 2015;18:568–573. doi: 10.3109/13697137.2014.999660. [DOI] [PubMed] [Google Scholar]
- Sloan J.A. Methodologic lessons learned from hot flash studies. J. Clin. Oncol. 2001;19(23):4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- Smach M.A., Hafsa J., Charfeddine B., Dridi H., Limem K. Effects of sage extract on memory performance in mice and acetylcholinesterase activity. Ann. Pharm. Fr. 2015;73(4):281–288. doi: 10.1016/j.pharma.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Staikou C., Kyrozis A., Moschovos C., Fassoulaki A. Effects of morning melatonin administration on electroencephalographic theta to alpha power ratio in reproductive versus postmenopausal healthy female volunteers. Neurosci. Lett. 2012;507:90–93. doi: 10.1016/j.neulet.2011.11.061. [DOI] [PubMed] [Google Scholar]
- Tegeler C.H. Reduction in menopause-related symptoms associated with use of a noninvasive neurotechnology for autocalibration of neural oscillations. Menopause. 2015;22(6):650–655. doi: 10.1097/GME.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tober C., Schoop C. Modulation of neurological pathways by Salvia off.L. officinalis and its dependence on manufacturing process and plant parts used. BMC Compl. Alternative Med. 2019;19:128. doi: 10.1186/s12906-019-2549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidabadi A. The effect of Salvia officinalis extract on symptoms of flushing, night sweat, sleep disorders, and score of forgetfulness in postmenopausal women. J. Fam. Med. Prim. Care. 2020;9(2):1086–1092. doi: 10.4103/jfmpc.jfmpc_913_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article.