Abstract
Insecticides can have consequences for beneficial arthropods. Insect parasitoids can contact insecticides through direct exposure spray droplets or residues on crop foliage. Here, we focus on better understand the response of Meteorus pulchricornis (Wesmael), a parasitoid wasp of lepidopteran pests, and its detoxification mechanisms on stress caused by phoxim and cypermethrin. Hence, we determined the dose–mortality curves and estimating the sublethal concentrations (LC30 and LC50). Then, we applied the sublethal concentrations against adult parasitoids to assess its survival, parasitism efficacy, and also developmental and morphometric parameters of their offspring. Simultaneously, we check the activities of glutathione S-transferase (GST), acetylcholinesterase (AChE), and peroxidase (POD) after sublethal exposure of both insecticides, which has measured until 48 h after treatment. Overall, phoxim and cypermethrin exhibited acute lethal activity toward the parasitoid with LC50 values 4.608 and 8.570 mg/liter, respectively. Also, we detect that LC30 was able to trigger the enzymatic activity of GST, AChE, and POD, suggesting a potential detoxification mechanism. However, even when subjected to sublethal exposure, our results indicate strong negatives effects, in particular for phoxim, which has affected the parasitism efficacy and also the developmental and morphometric parameters of M. pulchricornis offspring. Therefore, it can be concluded that both phoxim and cypermethrin have negative impacts on M. pulchricornis and we suggest cautioning their use and the need for semifield and field assessments to confirm such an impact.
Keywords: Meteorus pulchricornis, parasitic wasps, mulberry pests, chemical control, detoxification mechanism
The unreasonable application of chemical insecticides has led to the selection of resistant populations among targeted pest species and has unavoidable negative effects on human and animal health, and even the environment (Desneux et al. 2006, Tingle et al. 2003, Khan and Ruberson 2017). Furthermore, the insecticides at nonlethal doses can cause sublethal impacts at different levels on beneficial arthropods, such as the natural enemies of insect pests (Desneux et al. 2007). Hence, it is necessary to evaluate the disadvantages arising from the use of insecticides. Several species of parasitoids, such as Trichogramma spp. (Hymenoptera: Trichogrammatidae), have been regularly reared and released for the control of insect pests with some success (Polaszek 2010, Zucchi 2010, Khan and Ruberson 2017). It is vital to reasonably combine biological control with chemical control in an optimal way in integrated pest management (IPM), which is more beneficial and effective for building sustainable agroecological systems (Preetha et al. 2009, Saber 2011, Zhao et al. 2012).
Several studies have demonstrated that pesticides can cause lethal effects on the parasitoids or give rise to sublethal effects on them (Desneux et al. 2007). For example, sublethal doses of imidacloprid and fenpyroximate can have negative effects on the adult emergence, fecundity, longevity, and developmental duration of Trichogramma cacoeciae, Trichogramma embryophagum (Hartig), and Trichogramma evanescens (Westwood) (Hymenoptera: Trichogrammatidae) (Saber 2011, Poorjavad et al. 2014).
After treatment with insecticides, it is crucial to assess the physiological responses of parasitoids and to understand the detoxification mechanism (Boily et al. 2013, Carvalho et al. 2013, Badawy et al. 2015). Among these, increases in the activities of detoxification enzymes such as glutathione S-transferase (GST) and acetylcholinesterase (AChE), and protective enzyme peroxidase (POD) are the most frequent type occurring in insects (Wang et al. 2016, Pavlidi et al. 2017, Zhou et al. 2019). The mulberry foliage is the exclusive food source of the silkworm, Bombyx mori (Linnaeus) (Lepidoptera: Bombycidae), which is an important resource insect because it is able to yield silk productions for human. Therefore, many kinds of mulberry pests, such as the common cutworm Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae), cause great damage to the sericulture industry. Due to a lack of more selective insecticides between the silkworm and mulberry pests, classical insecticides are still being applied in mulberry fields. To date, phoxim and cypermethrin are the two most commonly used insecticides to control Lepidoptera pests (Liu et al. 2018, Gu et al. 2020) including S. litura. For decades of mulberry cultivation, the sericultural farmers used to harvest the mulberry leaves to feed the silkworms several days (generally 7–10 d) after spraying these insecticides in the fields, and these insecticides can degrade until the residues on the leaves to reach a quite low level. This empirical approach can guarantee the security for the silkworm (Wu et al. 2007a); however, studies of the side effects of phoxim and cypermethrin on parasitoid wasps in the mulberry agroecosystem are still limited.
The solitary endoparasitoid Meteorus pulchricornis (Wesmael) is a dominant natural enemy of many lepidopteran pests, including S. litura (Sheng et al. 2014, 2017, Nishimura et al. 2015, Maeto 2018, Nakano et al. 2018). There have been many studies addressing the insecticide resistance of S. litura (Shi et al. 2019, Xu et al. 2020); however, there are few studies on the negative effects of insecticides on M. pulchricornis. Therefore, a better understanding of the interaction of this parasitoid wasp with insecticides and the effect of insecticides on the development of M. pulchricornis could provide new insights for integrated pest management in mulberry fields.
The main objective of this study was to determine the sublethal concentrations of phoxim and cypermethrin for M. pulchricornis, and to evaluate their effects on the oviposition performance of adult wasps and the developmental parameters of parasitoid offspring. Furthermore, we aimed to evaluate physiological disturbances by measuring the activities of main detoxifying enzymes in M. pulchricornis adults after treated with phoxim and cypermethrin. This study illuminated the understanding of side effects of phoxim and cypermethrin on M. pulchricornis.
Materials and Methods
Parasitoid and Host Insects
The laboratory colonies of M. pulchricornis was thelytokous and obtained from rearing parasitized S. litura larvae collected in the mulberry field of the Chinese Academy of Agricultural Sciences (Zhenjiang, Jiangsu, China). All of these parasitoids were not exposed to insecticides. Adults of M. pulchricornis were reared in the larvae of S. litura, which was collected from the mulberry field of the Chinese Academy of Agricultural Sciences (Zhenjiang, Jiangsu, China). These insects were fed in the insectary [25 ± 1°C, 60–80% relative humidity, and a photoperiod of 14:10 (L:D) h]. The parasitoids were fed with 10% (w/w) honey solution in glass tubes (2-cm diameter × 10-cm height) (Sheng et al. 2017), and the hosts were reared on artificial diets (Shen and Wu 1995) in plastic cases (length × width × height =13 × 13 × 6 cm). Adult moths were fed with a 10% honey solution (Sheng et al. 2017), supplied on strips of paper as a substrate for oviposition in organza-covered cages (length × width × height = 20 × 20 × 30 cm). Six to eight-day-old naive parasitoids were used for all experiments.
Insecticides
Phoxim (purity 99.0%) and cypermethrin (purity 99.7%) analytical standards were purchased from Aladdin (Shanghai, China). Then, they were dissolved in analytical–grade acetone to prepare five serial concentrations of insecticide solution (for phoxim, 2.0, 4.0, 6.0, 8.0, and 10.0 mg/liter were used and for cypermethrin, 1.0, 2.0, 4.0, 8.0, and 16.0 mg/liter were used).
Bioassay
Adults of M. pulchricornis were treated individually with 1.0 µl of insecticide solution. Briefly, the wasps were mildly anesthetized on ice for 20–30 s, and the test insecticide solution was then applied to the pronotum and mesoscutum of M. pulchricornis using a microsyringe (Sangon Biotech, Shanghai, China) under a stereomicroscope (Olympus SMZ800N, Japan). Acetone was used as a control. After treatment, all parasitoids were reared separately in glass tubes (2-cm diameter × 10-cm height) and were fed with a 10% honey solution for 1 d. Each concentration of insecticide and control was replicated three times and each replicates involved 30 individual wasps. The mortality of M. pulchricornis was recorded after 24 h of treatment with acetone and insecticide solution.
Analysis of GST, AChE, and POD Activities
The parasitoids that remained alive after exposure were tested at the LC30 at 1, 6, 12, 24, and 48 h. The optical density (OD) values of GST, AChE, and POD activities were respectively measured using spectrophotometry (Rayleigh, Beijing, China) at 412, 412, and 420 nm according to the specifications of the commercially available enzyme assay kits (Nanjing Jiancheng Bioengineering Institute, China) (Kang et al. 2018). Briefly, 5–10 wasps were pooled together as a replicate and homogenized in normal saline (weight of wasps: volume of saline = 1:99) on ice using a motor-driven homogenizer, followed by centrifugation at 2,500 g for 10 min at 4°C. The 1% supernatant solution was used for protein concentration quantification using the Coomassie blue assay kit (Nanjing Jiancheng Bioengineering Institute), and the activities of three enzymes were measured. Each determination of enzyme activity was repeated in triplicate.
Effect of Insecticides on the Oviposition Performance and Developmental Parameters of M. pulchricornis
After treatment with LC30 and LC50 of each insecticide and the solvent acetone for 24 h, the survivors were allowed to parasitize the larvae (L3) of S. litura. In each parasitization test, a disc of the mulberry leaf (9-cm diameter) was laid at the bottom of a transparent plastic box (10-cm diameter × 8-cm height) containing a layer of 1% agar (w/w). Twenty L3 hosts were exposed to a parasitoid wasp for eight hours. Then, the wasps were removed in glass tubes and supplied with a 10% honey solution, and the hosts were collected individually and maintained with an artificial diet in plastic culture dishes (6-cm diameter × 1-cm height). After the pupation of parasitoid wasp larvae, the cocoons were collected and weighed by an electronic balance (Ohaus, AR224CN). Meanwhile, development times from eggs to cocoons and durations from cocoons to adults were also recorded. The parasitism efficiency was denoted as the number of cocoons among parasitoid offspring, whose body size was determined by the length of the hind tibiae (mm) measured using a stereomicroscope (Olympus SMZ800N, Japan). Fifteen to seventeen maternal wasps were performed for the control and each concentration of insecticide-treated group, respectively.
Statistical Analysis
The LC50 and LC30 values and confidence limits were determined by regression based on the probit mortality concentration with the PROBIT procedure of SPSS16.0 software (SPSS Inc., Chicago, IL). A two-way analysis of variance (ANOVA) was performed to test the effects of insecticide exposure and durations posttreatment on GST, AChE, POD activities, and interactions between these two variables were also identified. The TukeyHSD multiple–range test for multiple comparisons was applied. The effect of insecticide exposure on the longevity of parasitoid offspring was examined using Cox proportional hazards modeling, which estimates the hazard rate at time t, and can be interpreted biologically as a death risk (Collett 1994). The hazard rate h(t) at time t is given by
where h0(t) is the baseline hazard function to die depending only on the survival time when all covariates zi are set to zero, and βi are the regression coefficients that give the relative contributions of the n (n = 1 in the present analysis) covariates zi(t) (Cox 1972, Sheng et al. 2019). A simultaneous test for general linear hypotheses was used to account for each of the pair-wise comparisons from the ‘multcomp’ package.
Cocoon number, cocoon weight, development times from egg to cocoon, durations from cocoons to adults, rate of emergence (percentage of the number of adult wasps to the total number of cocoons), and hind tibia length data of the parasitoid offspring were analyzed using ANOVA and the Tukey HSD multiple-range test for multiple comparisons. Log transformations were necessary to stabilize variances concerning the insecticide treatment. We performed logistic model with a Poisson distribution to analyze the effects of insecticide exposure on offspring development times from eggs to cocoons and durations from cocoons to adults. In the process of detecting a significant effect, a simultaneous test for general linear hypotheses was used to account for each of the pair-wise comparisons from the ‘multcomp’ package.
All statistical tests were performed at a 5% significance level. Analyses were carried out using R 3.3.3 software (R Development Core Team 2017).
Results
Susceptibility of Parasitoids Exposed to Phoxim and Cypermethrin
We evaluated the susceptibility of M. pulchricornis adult wasps to phoxim and cypermethrin in terms of the determination of the sublethal concentration of each insecticide (Table 1). All of the parasitoids remained alive in the control group. The estimated LC50 values of phoxim and cypermethrin were 4.608 and 8.570 mg/liter after 24 h, respectively. The theoretical LC30 values of phoxim and cypermethrin were 3.475 and 5.904 mg/liter after 24 h, respectively.
Table 1.
Susceptibility of Meteorus pulchricornis to phoxim and cypermethrin
| Insecticide | Regression equation | Slope ± SE | P-value | LC50a (mg/liter) | 95% CI | df |
|---|---|---|---|---|---|---|
| Phoxim | Y = –2.13 + 0.46X | 0.46 ± 0.041 | <0.001 | 4.608 | 3.710–5.397 | 4, 85 |
| Cypermethrin | Y = –1.69 + 0.20X | 0.20 ± 0.016 | <0.001 | 8.570 | 7.263–10.224 | 4, 85 |
aLC50 , lethal concentration for 50%.
Determination of GST, AChE, and POD Activities
For phoxim, both insecticide exposure (F1, 20 = 63.28, P < 0.001) and time posttreatment (F4, 20 = 272.29, P < 0.001) influenced the GST activity. Interaction between these two variables was also detected (F4, 20 = 80.87, P < 0.001). GST activity was increased significantly at 12 h (Fig. 1A). For cypermethrin, insecticide exposure (F1, 17 = 198.40, P < 0.001), time posttreatment (F4, 17 = 519.40, P < 0.001) and their interaction (F4, 17 = 247.70, P < 0.001) also had an impact on GST activity. The activity of GST increased significantly after 1 h of exposure to cypermethrin but decreased notably at 6 and 24 h (Fig. 1B).
Fig. 1.
Effect of the LC30 of phoxim (A) and cypermethrin (B) on GST activity in Meteorus pulchricornis. The specific activity of GST in newly emerged adults was examined after exposure to acetone and two insecticides at LC30 for 1, 6, 12, 24, and 48 h. Vertical bars represent the standard error of the mean of three independent replicates. Asterisks above the error bars indicate significant differences in GST activity at different time points after phoxim and cypermethrin treatment.
For phoxim, both insecticide exposure (F1, 18 = 5.79, P = 0.027) and time posttreatment (F4, 18 = 163.57, P < 0.001) had a significant impact on AChE activity, the interaction between these two variables was also significant (F4, 18= 27.80, P < 0.001). AChE activity was increased significantly at 1, 6, and 12 h after exposure to phoxim at the LC30. Thereafter, there was a significant decrease in AChE activity at 24 h (Fig. 2A). Cypermethrin, insecticide exposure (F1, 20 = 12.05, P < 0.01), time posttreatment (F4, 20 = 34.79, P < 0.001) and their interaction (F4, 20 = 16.36, P < 0.001) had impacts on AChE activity. AChE activity was significantly downregulated at 6 h (Fig. 2B).
Fig. 2.
Effect of the LC30 of phoxim (A) and cypermethrin (B) on AChE activity in Meteorus pulchricornis. The specific activity of GST in newly emerged adults was examined after exposure to acetone and two insecticides at the LC30 for 1, 6, 12, 24, and 48 h. Vertical bars represent the standard error of the mean of three independent replicates. Asterisks above the error bars indicate significant differences in AChE activity at different time points after phoxim and cypermethrin treatment.
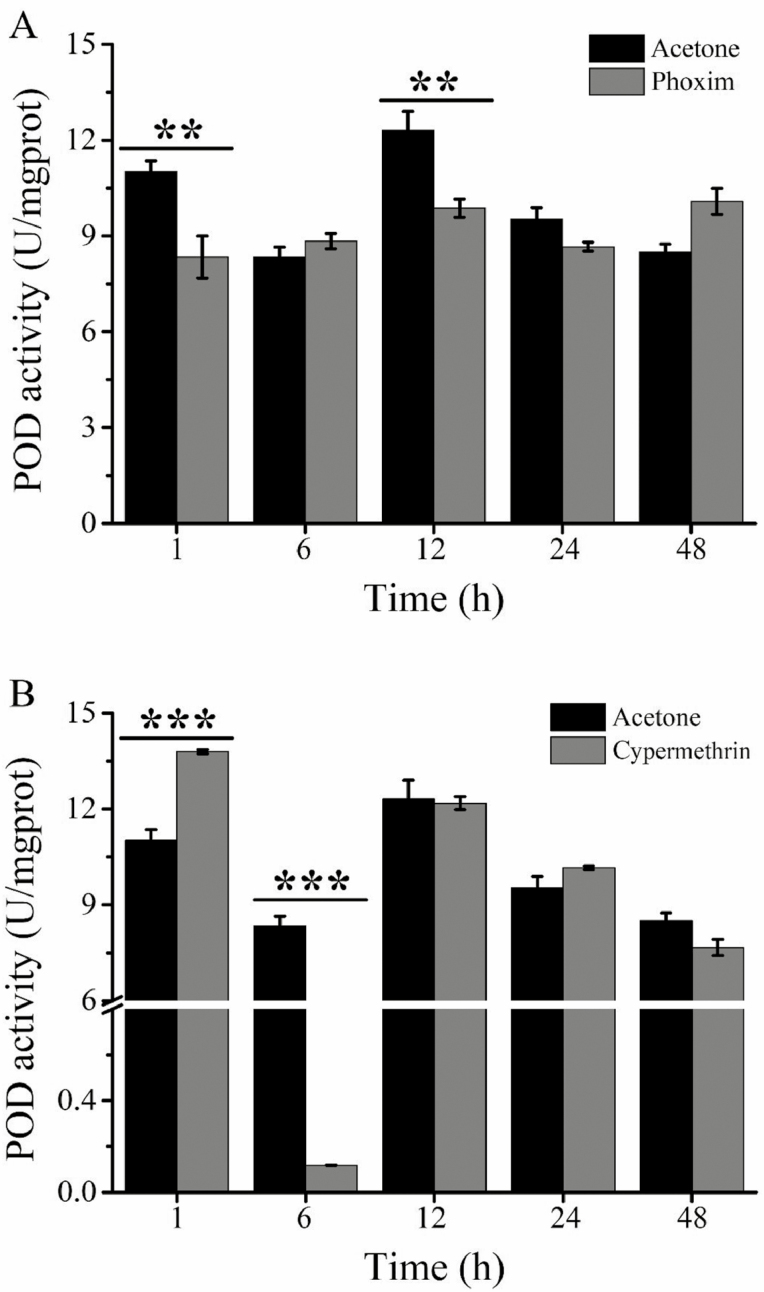
Under treatment with phoxim at the LC30, insecticide exposure (F1, 19 = 9.89, P < 0.01), time posttreatment (F4, 19 = 14.42, P < 0.001) and their interaction (F4, 19 = 12.66, P < 0.001) had an impact on POD activity. POD activity was significantly decreased at 1 and 12 h (Fig. 3A). For cypermethrin, insecticide exposure (F1, 18 = 14.26, P < 0.01), time posttreatment (F4, 18 = 184.22, P < 0.001), and their interaction (F4, 18 = 80.99, P < 0.001) significantly influenced POD activity. The activity of POD was only increased significantly at 1 h of exposure to cypermethrin and then showed a prominent inhibition at 6 h compared with the control level (Fig. 3B).
Fig. 3.
Effect of the LC30 of phoxim (A) and cypermethrin (B) on POD activity in Meteorus pulchricornis. The specific activity of GST in newly emerged adults was examined after exposure to acetone and two insecticides at the LC30 for 1, 6, 12, 24, and 48 h. Vertical bars represent the standard error of the mean of three independent replicates. Asterisks above the error bars indicate significant differences in POD activity at different time points after phoxim and cypermethrin treatment.
The Effect of Insecticides on the Oviposition Performance and Developmental Parameters of M. pulchricornis
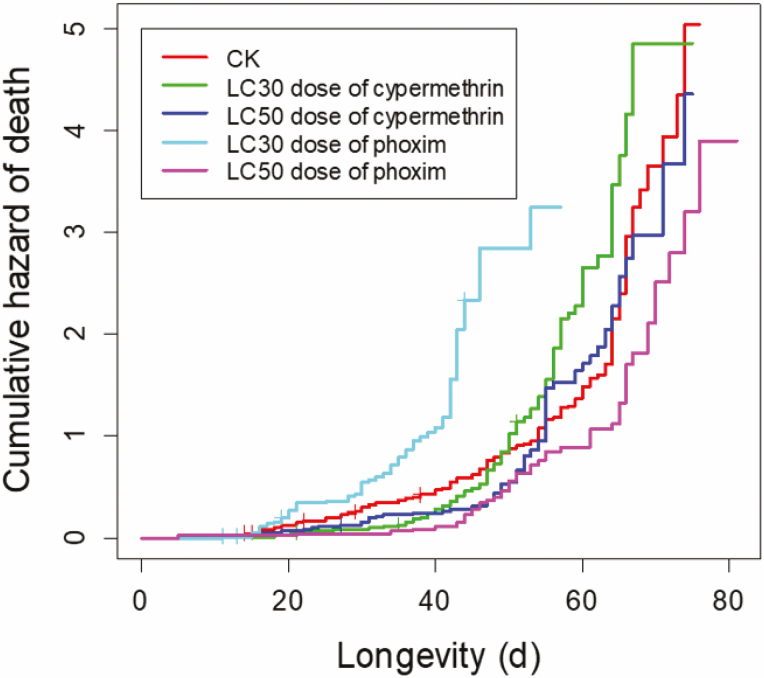
The cocoon numbers produced by M. pulchricornis after exposure to insecticides were affected (F4, 73 = 6.43, P < 0.001; Table 2). In comparison with the acetone control, the numbers of cocoons were significantly decreased when the maternal wasps were treated with LC30 and LC50 concentrations of phoxim. There were no significant differences in the groups treated with cypermethrin, compared with the acetone group. The cocoon weights of M. pulchricornis offspring were affected by insecticides exposure (F4, 537 = 17.85, P < 0.001, Table 2). Offspring emergence rates were also affected by insecticide exposure of adult female wasps (F4, 73 = 5.72, P < 0.001) and these parameters’ correlates were comparable except for the cohorts derived from phoxim LC50 treatments (Table 2). Development times from the egg to cocoon stages were not affected by insecticide exposure to adult maternal parasitoids (χ 2 = 7.50, df = 4, 537; P = 0.11, Table 2) but the durations of cocoon stages were affected by insecticide exposure (χ 2 = 41.60, df = 4, 491; P < 0.001, Table 2). Insecticide treatment had a significant impact on offspring longevity (χ 2 = 83.78, df = 4, 481; P < 0.001, Table 3). It is worth noting that the longevity of M. pulchricornis offspring was significantly greater after application of the LC50 concentration of phoxim compared with the acetone control, while the shortest survival duration was observed after exposure to phoxim at the LC30. However, it was not affected by the concentration of cypermethrin (Tables 2 and 3, Fig. 4). Offspring body size, assessed based on hind tibia length, was affected slightly by insecticide exposure (F4, 455= 2.60, P = 0.036, Table 2).
Table 2.
Estimated regression coefficient (β) of a Cox proportional hazard model for tested covariates that may have an effect on the death risk of Meteorus pulchricornis offspring when the maternal wasps were treated with insecticidesa
| Treatments | β | SE (β) | exp (β) | Z-value | P |
|---|---|---|---|---|---|
| Acetone control | 0 | – | 1 | ||
| Phoxim (LC30) | 1.31 | 0.16 | 3.71 | 8.10 | <0.001 |
| Phoxim (LC50) | −0.56 | 0.17 | 0.57 | −3.35 | <0.001 |
| Cypermethrin (LC30) | 0.20 | 0.12 | 1.22 | 1.66 | 0.097 |
| Cypermethrin (LC50) | −0.11 | 0.14 | 0.90 | −0.75 | 0.45 |
aThe overall significance of Cox proportional hazard model was assessed by likelihood ratio test (χ 2 = 83.78, df = 4, 481; P < 0.001).
Table 3.
Effects of insecticides on the cocoon numbers, cocoon weight, development times from eggs to cocoons, durations from cocoons to adults, emergence rates, longevity and hind tibia length of Meteorus pulchricornis
| Treatments | Cocoon number | Cocoon weight (mg) | Development times from eggs to cocoons (d)a | Durations from cocoons to adults (d)a | Emergence rate (%) | Longevity (d)a | Hind tibia length (mm) |
|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Median ± SE | Mean ± SE | |
| Acetone control | 10.06 ± 1.01 a (n = 17) | 8.86 ± 0.05 a (n = 171) | 8.53 ± 0.040 a (n = 171) | 6.62 ± 0.040 c (n = 162) | 93.96 ± 2.24 a (n = 17) | 46.0 ± 1.37 b (n = 161) | 1.651 ± 0.004 a (n = 161) |
| Phoxim (LC30) | 4.73 ± 1.15 b (n = 15) | 8.38 ± 0.11 bc (n = 71) | 8.22 ± 0.052 a (n = 71) | 6.80 ± 0.064 b (n = 66) | 92.15 ± 3.18 a (n = 15) | 34.0 ± 1.47 c (n = 64) | 1.635 ± 0.007 a (n = 64) |
| Phoxim (LC50) | 4.20 ± 0.64 b (n = 15) | 8.10 ± 0.15 c (n = 63) | 8.06 ± 0.035 a (n = 63) | 6.98 ± 0.036 b (n = 49) | 81.19 ± 6.19 b (n = 15) | 53.0 ± 2.03 a (n = 49) | 1.629 ± 0.009 b (n = 49) |
| Cypermethrin (LC30) | 9.06 ± 1.15 a (n = 16) | 8.96 ± 0.08 a (n = 145) | 8.88 ± 0.051 a (n = 145) | 6.90 ± 0.057b (n = 136) | 91.30 ± 2.62 a (n = 16) | 48.0 ± 0.99 b (n = 133) | 1.644 ± 0.004 a (n = 129) |
| Cypermethrin (LC50) | 6.13 ± 1.14 ab (n = 15) | 8.76 ± 0.12 ab (n = 92) | 9.29 ± 0.28 a (n = 92) | 7.43 ± 0.24 a (n = 83) | 90.86 ± 3.27 a (n = 15) | 52.0 ± 1.67 ab (n = 79) | 1.639 ± 0.006 a (n = 57) |
Means within a column followed by different letters are significantly different (by Tukey’s test, P < 0.05).
aComparison within this column was based on the Tukey Contrasts of multiple comparisons for general linear hypotheses.
Fig. 4.
Cumulative death hazard of Meteorus pulchricornis offspring when the maternal wasps were treated with LC30 and LC50 of phoxim and cypermethrin.
Discussion
In the present study, both phoxim and cypermethrin was found to be detrimental to M. pulchricornis through the topical application method. Previous studies have shown an unfavorable effect of cypermethrin exposure on several other parasitoids, such as Cotesia vestalis (Haliday) (Hymenoptera: Braconidae) (Stemele et al. 2017), Trichogramma brassicae (Parsaeyan et al. 2017) (Hymenoptera: Trichogrammatidae), and Eretmocerus mundus (Mercet) (Hymenoptera: Aphelinidae) (Francesena et al. 2017). However, rare studies have shown the toxicity of phoxim to other parasitoid wasps, such as Peristenus spretus (Hymenoptera: Braconidae) (Chen & van Achterberg) adults with an LC50 value of 0.06 mg a.i./liter (Liu et al. 2015b) and Trichogramma japonicum (Ahmead) (Hymenoptera: Trichogrammatidae) with an LC50 of 0.11 mg a.i./liter (Zhao et al. 2012). To date, the information available on the toxicity of insecticides to parasitoid wasps in the Braconinae family has been limited. This is, to our knowledge, the first investigation into the effects of phoxim and cypermethrin on the development of M. pulchricornis after exposure in adult stages.
Research into insecticide sensitivity mechanisms in insects has mainly focused on target site modification and the metabolic disturbance of detoxification enzymes (enhancement as a result of the activities of detoxification enzymes, such as GST, CXE, and/or cytochrome P450s) (Perry et al. 2011, Liu et al. 2015a, Wang et al. 2016, Pavlidi et al. 2017). The increases in the activities of these detoxification enzymes, especially GST and AChE, have been correlated with resistance to some insecticides in other insects. For example, in the larvae of H. armigera, another host of M. pulchricornis females, exposure to a pesticide mixture consisting of chlorpyrifos, dichlorvos, and cypermethrin can significantly result in a dose-dependent upregulation of GST activity (Labade et al. 2018). Nevertheless, fewer studies are addressing the variation in enzyme activities upon exposure to insecticides in parasitoid wasps than in insect pests. In the present study, both phoxim and cypermethrinc disrupted the physiology of the wasps by modifying the activity of GST, AChE, and POD, when the wasps were treated at the LC30.
Many studies have suggested that the activity of GST in insects can be inhibited and/or induced by insecticides. For instance, significantly elevated enzymatic activity of GST has been detected in the silk gland of B. mori exposed to phoxim (Cheng et al. 2018). In the present study, the GST activity in M. pulchricornis showed a significant increase at 12 and 1 h after exposure to phoxim and cypermethrin, respectively. Furthermore, our previous work had indicated that exposure to phoxim and cypermethrin significantly increased the relative expression levels of several MpulGSTs in M. pulchricornis (Zhang et al. 2019), suggesting GSTs may play essential roles in overcoming insecticides stress. Meanwhile, the repressive activity of GST in M. pulchricornis treated with cypermethrin was detected at 6 and 24 h. Likewise, the activity of GST was found to decrease in B. mori treated with imidacloprid (Chen et al. 2015). Diethyl maleate, a GST inhibitor and intermediate of the organophosphorus insecticide malathion, strongly suppresses the activity of GST in C. vestalis and Diaeretiella rapae (MacIntosh) (Hymenoptera: Braconidae) (Wu et al. 2007b).It has been reported that the activity of AChE in C. plutellae and D. rapae is slightly induced by diethyl maleate at high concentrations (Wu et al. 2007b). In the present study, AChE activity in M. pulchricornis exhibited a remarkable increase at 1, 6, and 12 h after exposure to phoxim. It has been proven that higher enzymatic activities of AChE might be the underlying mechanism of higher phoxim resistance in Bombyx mandarina (Moore) (Lepidoptera: Bombycidae) (Li et al. 2010). However, significantly increased AChE activity was not detected after exposure to cypermethrin in M. pulchricornis. Similarly, the absence of an AChE response has been observed after exposure to deltamethrin in A. mellifera (Carvalho et al. 2013). This result indicated that the stimulatory effect of cypermethrin on AChE may only be detectable after longer exposure periods. Besides, treatment with an insecticide such as deltamethrin induced a decrease in AChE activity in A. mellifera compared with the control (Badiou et al. 2008). Similarly, in the present study, the activity of AChE in M. pulchricornis was significantly repressed after treatment with phoxim at 24 h, and cypermethrin at 6 h. Different mechanisms may account for the variation in AChE activity over time in M. pulchricornis treated with organophosphate and pyrethroid insecticides.
At present, research into the POD enzyme has mainly focused on their protective effect on plants, which contributes to resisting insect attack. However, insect pests have evolved POD enzymes that reduce the toxicity of plant metabolites (Cui et al. 2019), followed by evolution in the parasitoid wasps of insect pests (Kang et al. 2018). Thus, the increase in POD activity in insects might be a biological response to detoxifying chemical insecticides. In this study, the biochemical assays showed that POD activity in M. pulchricornis was induced by phoxim at 6 and 48 h, and cypermethrin at 1 and 24 h. A previous study revealed that exposure to sublethal doses of imidacloprid also significantly increased the activity of POD in A. gifuensis (Kang et al. 2018). However, the activity of POD in M. pulchricornis decreased after treatment with phoxim at 1 and 12 h, and cypermethrin at 6 and 48 h. Notably, there was a sharp reduction in POD activity in M. pulchricornis after 6-h exposure to cypermethrin, which may be related to the inflexibility of insecticide-treated inactive wasps.
It has been reported that many parasitoids are affected detrimentally after exposure to insecticides, including a reduction in parasitism efficiency or viability. For instance, the parasitism rates of Aphidius colemani (Viereck) (Hymenoptera: Braconidae) and C. vestalis treated separately with lambda-cyhalothrin (D’Ávila et al. 2018) and cypermethrin (Stemele et al. 2017) were lower compared with the control groups. Fecundity and mean longevity were significantly reduced after the exposure of Habrobracon hebetor (Say) (Hymenoptera: Braconidae) adults to sublethal concentrations of cypermethrin in comparison with the control group (Abedi et al. 2014). The organophosphates dimethoate and chlorpyrifos are harmful to the adult and pupal stages of Tamarixia radiate (Waterston) (Hymenoptera: Eulophidae), which negatively affects parasitoid emergence (Beloti et al. 2015). In the present study, the oviposition performance and developmental parameters of M. pulchricornis offspring were consistently detrimentally affected when the maternal wasps were treated with phoxim and cypermethrin. The cocoon numbers and weight, emergence rates, longevity, and body size of emerged M. pulchricornis offspring were significantly affected when the maternal wasps were treated with phoxim. In general, insecticides usually shorten the longevity of parasitoid offspring. In contrast, the survival duration was prolonged when exposed to high concentrations of phoxim in M. pulchricornis offspring. Prolonged longevity has also been observed in adults of Diaeretiella rapae (M’Intosh) (Hymenoptera: Aphidiidae) treated with an organophosphorus insecticide methamidophos (Lin et al. 2007). It is predicted that parasitoid wasps may adopt a balanced strategy that prolongs their lifespan when optimal progeny are not obtained (Harvey and Malcicka 2016). Thus, we speculate that the surviving parasitoid wasps showed prolonged longevity to balance parasitism and reproduction under the stress of insecticide treatments. Further research is required to examine this hypothesis. Also, the assessed parameters, except for durations from cocoons to adults, were not found to be significantly affected when the maternal wasps were treated with cypermethrin compared with the acetone control treatment. Thus, the effect of phoxim was more severe than the effect of cypermethrin on the oviposition performance and developmental parameters of M. pulchricornis offspring. To obtain more information about the effects of insecticides on parasitoids, some investigations, such as the interaction between pesticide and temperature (Abbes et al. 2015, Ricupero et al. 2020) or pesticide impacts at the population level of parasitoids, could be conducted in the future research.
In conclusion, the present results showed that both phoxim and cypermethrin could give rise to a great death risk for M. pulchricornis and this wasp can protect itself by activating the GST, AChE, and POD enzymes under laboratory conditions. According to the sublethal effects on the oviposition performance and developmental parameters of the parasitoid offspring of M. pulchricornis after exposure to both insecticides, it is reasonable to conclude that these chemical stresses may jeopardize the survival of M. pulchricornis populations against agricultural pests. These results are of great concern for the application of insecticides through integrated pest control.
Acknowledgments
This study was supported by the Key Research and Development program (Modern Agriculture) of Zhenjiang City (NY2019021), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX20_1492), the National Natural Science Foundation of China (31500312), and the Special Fund for China Agriculture Research System (CARS-18).
Author Contributions
SS conceived, designed and performed the experiments; Jiao-W, X-RZ and M-WY performed the experiments; YS analyzed the data; J-CZ, Jun-W and F-AW contributed reagents/materials/analysis tools; SS, JW (Jiao Wang) and X-RZ wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References Cited
- Abbes, K., A. Biondi, A. Kurtulus, M. Ricupero, A. Russo, G. Siscaro, B. Chermiti, and L. Zappalà. . 2015. Combined non-target effects of insecticide and high temperature on the parasitoid Bracon nigricans. PLoS One. 10: e0138411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedi, Z., M. Saber, G. Gharekhani, A. Mehrvar, and S. G. Kamita. . 2014. Lethal and sublethal effects of azadirachtin and cypermethrin on Habrobracon hebetor (Hymenoptera: Braconidae). J. Econ. Entomol. 107: 638–645. [DOI] [PubMed] [Google Scholar]
- Badawy, M. E. I., H. M. Nasr, and E. I. Rabea. 2015. Toxicity and biochemical changes in the honey bee Apis mellifera exposed to four insecticides under laboratory conditions. Apidologie 46: 177–193. [Google Scholar]
- Badiou, A., M. Meled, and L. P. Belzunces. . 2008. Honeybee Apis mellifera acetylcholinesterase—a biomarker to detect deltamethrin exposure. Ecotox. Environ. Safe 69: 246–253. [DOI] [PubMed] [Google Scholar]
- Beloti, V. H., G. R. Alves, D. F. Araújo, M. M. Picoli, R. d. e. A. Moral, C. G. Demétrio, and P. T. Yamamoto. . 2015. Lethal and sublethal effects of insecticides used on citrus, on the ectoparasitoid Tamarixia radiata. PLoS One. 10: e0132128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boily, M., B. Sarrasin, C. DeBlois, P. Aras, and M. Chagnon. . 2013. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: laboratory and field; experiments. Environ. Sci. Pollut. R. 20: 5603–5614. [DOI] [PubMed] [Google Scholar]
- Carvalho, S. M., L. P. Belzunces, G. A. Carvalho, J. L. Brunet, and A. Badiou-Beneteau. . 2013. Enzymatic biomarkers as tools to assess environmental quality: a case study of exposure of the honeybee Apis mellifera to insecticides. Environ. Toxicol. Chem. 32: 2117–2124. [DOI] [PubMed] [Google Scholar]
- Chen, X.Y., J. Liu, C.D. Zhang, Y.F. Li, T.H. Liu, L. Wang, Q.Y. Yu, Y.H. Zhang, C. Lu, M.H. Pan. . 2015. The silkworm GSTe4 is sensitive to phoxim and protects HEK293 cells against UV-induced cell apoptosis. B. Entomol. Res. 105: 339–407. [DOI] [PubMed] [Google Scholar]
- Cheng, X. Y., J. H. Hu, J. X. Li, J. Chen, H. Wang, T. T. Mao, B. Xue, and B. Li. . 2018. The silk gland damage and the transcriptional response to detoxifying enzymes–related genes of Bombyx mori under phoxim exposure. Chemosphere 209: 964–971. [DOI] [PubMed] [Google Scholar]
- Collett, D 1994. Modeling survival data in medical research. Chapman and Hall Press, London, United Kingdom. [Google Scholar]
- Cox, D. R 1972. Regression models and life-tables. J. Roy. Stat. Soc. 34: 187–220. [Google Scholar]
- Cui, B. Y., X. B. Huang, S. Li, K. Hao, B. H. Chang, X. B. Tu, B. P. Pang, and Z. H. Zhang. . 2019. Quercetin affects the growth and development of the grasshopper Oedaleus asiaticus (Orthoptera: Acrididae). J. Econ. Entomol. 112: 1175–1182. [DOI] [PubMed] [Google Scholar]
- D’Ávila, V. A., W. F. Barbosa, R. N. C. Guedes, and G. C. Cutler. . 2018. Effects of Spinosad, Imidacloprid, and Lambda–cyhalothrin on Survival, Parasitism, and Reproduction of the Aphid Parasitoid Aphidius colemani. J. Econ. Entomol. 111: 1096–1103. [DOI] [PubMed] [Google Scholar]
- Desneux, N., R. Denoyelle, and L. Kaiser. . 2006. A multi-step bioassay to assess the effect of the deltamethrin on the parasitic wasp Aphidius ervi. Chemosphere. 65: 1697–1706. [DOI] [PubMed] [Google Scholar]
- Desneux, N., A. Decourtye, and J. M. Delpuech. . 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52: 81–106. [DOI] [PubMed] [Google Scholar]
- Francesena, N., N. Desneux, M. R. de Campos, and M. I. Schneider. . 2017. Side effects of spirotetramat on pupae and adults of a Neotropical strain of Eretmocerus mundus (Hymenoptera: Aphelinidae): effects on the life parameters and demography. Environ. Sci. Pollut. Res. Int. 24: 17719–17730. [DOI] [PubMed] [Google Scholar]
- Gu, Z. Y., M.X. Li, S. X. Xia, T. T. Mao, Z. T. Lu, J. Chen, H. Wang, J. W. Qu, Y. L. Fang, F. C. Li, . et al. 2020. Effects of sublethal phoxim exposure and lower food intake on nutrient metabolism in the midguts of Bombyx mori. Pestic. Biochem. Phys. 167: 104593. [DOI] [PubMed] [Google Scholar]
- Harvey, J. A., and M. Malcicka. . 2016. Nutritional integration between insect hosts and koinobiont parasitoids in an evolutionary framework. Entomol. Exp. Et. Appl. 159: 181–188. [Google Scholar]
- Kang, Z. W., F. H. Liu, R. P. Pang, H. G. Tian, and T. X. Liu. . 2018. Effect of sublethal doses of imidacloprid on the biological performance of aphid endoparasitoid Aphidius gifuensis (Hymenoptera: Aphidiidae) and influence on its related gene expression. Front. Physiol. 9: 1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. A., and J. R. Ruberson. . 2017. Lethal effects of selected novel pesticides on immature stages of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Pest Manag. Sci. 73: 2465–2472. [DOI] [PubMed] [Google Scholar]
- Labade, C. P., A. R. Jadhav, M. Ahire, S. S. Zinjarde, and V. A. Tamhane, . 2018. Rote of induced glutathione-S-transferase from Helicoverpa armigera (Lepidoptera: Noctuidae) HaGST-8 in detoxification of pesticides. Ecotox. Environ. Safe. 147: 612–621. [DOI] [PubMed] [Google Scholar]
- Li, B., Y. H. Wang, H. T. Liu, Y. X. Xu, Z. G. Wei, Y. H. Chen, and W. D. Shen. . 2010. Resistance comparison of domesticated silkworm (Bombyx mori L.) and wild silkworm (Bombyx mandarina M.) to phoxim insecticide. Afr. J. Biotechnol. 9: 1771–1775. [Google Scholar]
- Lin, Y. W., G. Wu, and T. Miyata. . 2007. Insecticide susceptibility of surviving Cotesia plutellae (Hym: Braconidae) and Diaeretiella rapae (M’Intosh) (Hym: Aphidiidae) as affected by sublethal insecticide dosages on host insects. Pest Manag. Sci. 63: 841–850. [DOI] [PubMed] [Google Scholar]
- Liu, N. N., M. Li, Y. H. Gong, F. Liu, and T. Li. . 2015a. Cytochrome P450s—their expression, regulation, and role in insecticide resistance. Pestic Biochem. Phys. 120: 77–81. [DOI] [PubMed] [Google Scholar]
- Liu, Y. Q., B. Liu, A. Ali, S. P. Luo, Y. H. Lu, and G. M. Liang. . 2015b. Insecticide Toxicity to Adelphocoris lineolatus (Hemiptera: Miridae) and its Nymphal Parasitoid Peristenus spretus (Hymenoptera: Braconidae). J. Econ. Entomol. 108: 1779–1785. [DOI] [PubMed] [Google Scholar]
- Liu, Y., H. Zhang, F. He, X. Li, H. Tan, and D. Zeng. . 2018. Combined toxicity of chlorantraniliprole, lambda-cyhalothrin, and imidacloprid to the silkworm Bombyx mori (Lepidoptera: Bombycidae). Environ. Sci. Pollut. Res. Int. 25: 22598–22605. [DOI] [PubMed] [Google Scholar]
- Maeto, K 2018. Polyphagous koinobiosis: the biology and biocontrol potential of a braconid endoparasitoid of exophytic caterpillars. Appl. Entomol. Zool. 53: 433–446. [Google Scholar]
- Nakano, S., J. J. Gau, and K. Maeto. . 2018. Host suitability of the Mediterranean flour moth for rearing Meteorus pulchricornis (Hymenoptera: Braconidae), a polyphagous endoparasitoid of pest lepidopteran larvae. Appl. Entomol. Zool. 53: 291–296. [Google Scholar]
- Nishimura, T., T. Fujii, K. Sakamoto, and K. Maeto. . 2015. Daily locomotor activity of the parasitoid wasp Meteorus pulchricornis (Hymenoptera: Braconidae) that attacks exposed lepidopteran larvae. Appl. Entomol. Zool. 50: 525–531. [Google Scholar]
- Parsaeyan, E., S. A. Safavi, M. Saber, and N. Poorjavad. . 2017. Effects of emamectin benzoate and cypermethrin on the demography of Trichogramma brassicae Bezdenko. Crop Prot. 110: 269–274. [Google Scholar]
- Pavlidi, N., M. Khalighi, A. Myridakis, W. Dermauw, N. Wybouw, D. Tsakireli, E. G. Stephanou, N. E. Labrou, J. Vontas, and L. T. Van, . 2017. A glutathione–S–transferase (TuGSTd05) associated with acaricide resistance in Tetranychus urticae directly metabolizes the complex II inhibitor cyflumetofen. Insect Biochem. Molec. 80: 101–115. [DOI] [PubMed] [Google Scholar]
- Perry, T., P. Batterham, and P. J. Daborn. . 2011. The biology of insecticidal activity and resistance. Insect Biochem. Mol. Biol. 41: 411–422. [DOI] [PubMed] [Google Scholar]
- Polaszek, A 2010. Species diversity and host associations of Trichogramma in Eurasia, pp. 237–266. In F.L. Cônsoli, J.R.P. Parra, R.A. Zucchi (eds.), Egg parasitoids in agroecosystems with emphasis on trichogramma. Springer, Dordrecht, the Netherlands. [Google Scholar]
- Poorjavad, N., S. H. Goldansaz, H. Dadpour, and J. Khajehali, . 2014. Effect of Ferula assafoetida essential oil on some biological and behavioral traits of Trichogramma embryophagum and T. evanescens. Biocontrol 59: 403–413. [Google Scholar]
- Preetha, G., J. Stanley, S. Suresh, S. Kuttalam, and R. Samiyappan. . 2009. Toxicity of selected insecticides to Trichogramma chilonis: assessing their safety in the rice ecosystem. Phytoparasitica 37: 209–215. [Google Scholar]
- R Development Core Team 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07–0. Available from http://www.R–project.org/ [Google Scholar]
- Ricupero, M., K. Abbes, K. Haddi, A. Kurtulus, N. Desneux, A. Russo, G. Siscaro, A. Biondi, and L. Zappalà. . 2020. Combined thermal and insecticidal stresses on the generalist predator Macrolophus pygmaeus. Sci. Total Environ. 729: 138922. [DOI] [PubMed] [Google Scholar]
- Saber, M 2011. Acute and population level toxicity of imidacloprid and fenpyroximate on an important egg parasitoid, Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Ecotoxicology. 20: 1476–1484. [DOI] [PubMed] [Google Scholar]
- Shen, J. L., and Y. D. Wu. . 1995. Pesticide resistance and management of Helicoverpa armigera. China Agricultural Press, Beijing, China, pp. 91–94. [Google Scholar]
- Sheng, S., S. F. Feng, L. Meng, and B. P. Li. . 2014. Departure mechanisms for host search on high–density patches by the parasitoid, Meteorus pulchricornis. J. Insect Sci. 14: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, S., C. W. Liao, Y. Zheng, Y. Zhou, Y. Xu, W. M. Song, P. He, J. Zhang, and F. A. Wu. . 2017. Candidate chemosensory genes identified in the endoparasitoid Meteorus pulchricornis (Hymenoptera: Braconidae) by antennal transcriptome analysis. Comp. Biochem. Physiol. Part D. Genomics Proteomics. 22: 20–31. [DOI] [PubMed] [Google Scholar]
- Sheng, S., X. R. Zhang, Y. Zheng, J. Wang, Y. Zhou, C. W. Liao, J. Wang, and F. A. Wu. . 2019. Effect of six sugars on the longevity, oviposition performance and nutrition accumulation in an endoparasitoid, Meteorus pulchricornis (hymenoptera: braconidae). J. Asia-Pac. Entomol. 22: 263–268. [Google Scholar]
- Shi, L., Y. Shi, Y. Zhang, and X. Liao. . 2019. A systemic study of indoxacarb resistance in Spodoptera litura revealed complex expression profiles and regulatory mechanism. Sci. Rep. 9: 14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemele, M. A 2017. Comparative effects of a selective insecticide, Bacillus thuringiensis var. kurstaki and the broad–spectrum insecticide cypermethrin on diamondback moth and its parasitoid Cotesia vestalis (Hymenoptera: Braconidae). Crop Prot. 101: 35–42. [Google Scholar]
- Tingle, C. C., J. A. Rother, C. F. Dewhurst, S. Lauer, and W. J. King. . 2003. Fipronil: environmental fate, ecotoxicology, and human health concerns. Rev. Environ. Contam. Toxicol. 176: 1–66. [DOI] [PubMed] [Google Scholar]
- Wang, L. L., X. P. Lu, L. W. Meng, Y. Huang, D. Wei, H. B. Jiang, G. Smagghe, and J. J. Wang. . 2016. Functional characterization of an α–esterase gene involving malathion detoxification in Bactrocera dorsalis (Hendel). Pestic. Biochem. Phys. 130: 44–51. [DOI] [PubMed] [Google Scholar]
- Wu, F. A., Y. R. Li, Y. L. Pan, J. L. Cheng, Z. S. Xia, L. Liu, and J. X. Wang. . 2007a. Establishment of the technical standard of chemical application in mulberry proclaimed by the Ministry of Agriculture. Sci. Sericulture. 33: 151–154. (In Chinese). [Google Scholar]
- Wu, G., T. Miyata, C. Y. Kang, and L. H. Xie. . 2007b. Insecticide toxicity and synergism by enzyme inhibitors in 18 species of pest insect and natural enemies in crucifer vegetable crops. Pest Manag. Sci. 63: 500–510. [DOI] [PubMed] [Google Scholar]
- Xu, L., Y. Mei, R. Liu, X. Chen, D. Li, and C. Wang. . 2020. Transcriptome analysis of Spodoptera litura reveals the molecular mechanism to pyrethroids resistance. Pestic. Biochem. Physiol. 169: 104649. [DOI] [PubMed] [Google Scholar]
- Zhang, X. R., J. Q. Zhang, Y. Y. Shao, X. R. Xing, J. Wang, Z. X. Liu, Y. J. C. Li, D.O. Andrews, Q. B. Tu, J. Wang, S. Sheng, and F. A. Wu. 2019. Identification of glutathione-S-transferase genes by transcriptome analysis in Meteorus pulchricornis (Hymenoptera: Braconidae) and their expression patterns under stress of phoxim and cypermethrin. Comp. Biochem. Phys. D. 31: 100607. [DOI] [PubMed] [Google Scholar]
- Zhao, X. P., C. X. Wu, Y. H. Wang, T. Cang, L. P. Chen, R. X. Yu, and Q. Wang. . 2012. Assessment of toxicity risk of insecticides used in rice ecosystem on Trichogramma japonicum, an egg parasitoid of rice Lepidopterans. J. Econ. Entomol. 105: 92–101. [DOI] [PubMed] [Google Scholar]
- Zhou, C., H. Yang, Z. Wang, G. Y. Long, and D. C. Jin. . 2018. Protective and detoxifying enzyme activity and ABCG subfamily gene expression in Sogatella furcifera under insecticide stress. Front. Physiol. 9: 1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi, R.A., R.B. Querino, and R.C. Monteiro. . 2010. Diversity and hosts of Trichogramma in the New World, with emphasis in South America, pp. 219–236. In F.L. Cônsoli, J.R.P. Parra, R.A. Zucchi, Egg parasitoids in agroecosystems with emphasis on trichogramma. Springer, Dordrecht, the Netherlands. [Google Scholar]