Highlights
-
•
A landmark synthesis method of silica xerogel was proposed by ultrasonic irradiation.
-
•
Semi-solid hydrogel was irradiated ultrasonic for aging and hydrophobization.
-
•
Ultrasonic cavitation greatly accelerates aging and hydrophobization reactions.
-
•
The pore structure and density can be easily controlled by ultrasonic irradiation.
-
•
Low-density xerogels were prepared in less than 1/5 of the usual time by ultrasonic.
Keywords: Silica xerogel, Aging, Hydrophobization, Ultrasonic irradiation, Ambient pressure drying
Abstract
Silica xerogels were prepared by the sol-gel method under ultrasonic irradiation, using tetraethylorthosilicate (TEOS) as the starting material. Hexamethyldisiloxane (HMDSO) was used as the hydrophobizing agent. When preparing silica xerogel, it is necessary to perform aging and hydrophobization to suppress shrinkage during ambient pressure drying, however, such treatments are time-consuming. In this study, the semi-solid hydrogel was irradiated with ultrasonic for the first time in order to accelerate aging and hydrophobic treatment, and the effect of ultrasonic frequency on structure was investigated. Firstly, ultrasonic irradiation was performed at frequencies of 100 kHz and 500 kHz, followed by hydrophobic treatment at a frequency of 500 kHz, in order to promote aging. The results identify optimum conditions for ultrasonic irradiation to promote aging and hydrophobization reactions, and it was found to be possible to prepare silica xerogels in less than 1/5 of the conventional time. The silica xerogels had a low density and the shrinkage was suppressed. In this study, it was found that ultrasonic irradiation of semi-solid hydrogel was very effective for promoting the reaction.
1. Introduction
Silica aerogels are porous materials, typically obtained by subjecting wet silica gels to supercritical drying. They have a highly crosslinked network structure of low-density material comprising more than 90% air and less than 10% silica skeleton. Aerogels have many unique properties, such as high transparency in visible light, high surface area, and low thermal conductivity [1]. Utilizing these properties, applications to high-performance insulated windows, Cherenkov photodetectors, and space dust collection have been demonstrated [2], [3], [4], [5]. In particular, silica xerogel with high thermal insulation properties are increasingly used in home appliances, housing, and automobiles [6].
In the past, silica aerogels have been obtained by removing solvents such, as water and alcohol, via supercritical drying [7]. While high quality materials can be obtained by supercritical drying, this drying method requires a long time and a large amount of solvent, thus it is expensive and include complex process [8].
In the past, several studies have reported silica xerogels obtained by drying under atmospheric pressure [9], [10], [11]. In atmospheric pressure drying, the gel tends to shrink due to the capillary force during drying [12], so aging of the gel and hydrophobic treatment before drying are required to suppress shrinkage. Generally, trimethylchlorosilane (TMCS) is used as a hydrophobizing agent [13], [14], [15], [16], but this is potentially harmful. Oikawa et al. succeeded in hydrophobizing a silica particle surface using hexamethyldisiloxane (HMDSO), i.e., without using TMCS [17]. HMDSO was used as the hydrophobizing agent in this study; however, the aging and hydrophobic treatment described in previous studies is time-consuming. In the preparation of silica xerogels, the production of low-density xerogels with low thermal conductivity on a large scale at reasonable cost in a short time remains a great challenge.
Ultrasonic irradiation has recently been employed in the synthesis of nanomaterials [18], [19], [20], and the physical and chemical effects of the collapse of acoustic cavitation bubbles resulting in unusual materials properties and reactions [21], [22], [23]. Physical action is caused by the shock wave at the time of acoustic cavitation crushing, and enables cleaning, dispersing, and stirring functions [24]. On the other hand, the chemical effect promotes chemical reactions through the generation of radicals and the thermal effect caused by the local hot spot at the time of acoustic cavitation collapse [25].
Although there is no example of ultrasonic irradiation of the semi-solid hydrogel, it has been reported that hydrolysis and condensation rates were improved when using ultrasonic irradiation in a solution in sol–gel synthesis [26], [27]. It is known that the hydrogels are not completely solid and have some degree of fluidity, and that the reactions that affect the structure and properties of the gel occur inside the gel [28]. Ultrasonic irradiation of the semi-solid hydrogel may accelerate aging and hydrophobic reactions in the preparation of silica xerogels, which may enable more rapid preparation of xerogels and even tunning of the pore structure by acoustic cavitation bubbles. Therefore, the semi-solid hydrogel was irradiated with ultrasonic for the first try and we reported the effects of ultrasonic irradiation conditions on the pore structure of silica xerogels and optimal conditions of ultrasonic irradiation.
2. Experimental procedures
2.1. Equipment
Fig. 1 shows a schematic representation of experimental equipment used for synthesizing silica xerogels by ultrasonic irradiation. The water temperature is maintained at 60 °C using a circulating cooling device (CTP-3000, Tokyo Rikakikai Co., Ltd), and 100 ml Erlenmeyer flask was fixed in a water bath. The water bath is equipped with an ultrasonic transducer (Honda Electronics Co., Ltd.), which can be controlled using a Multifunction synthsizer (WF 19458, NF Corp.), a RF power amplifier (Electronics and Innovation Ltd.) and a matching box(Honda Electronics Co., Ltd.) to expose silica sol or gel to ultrasonic irradiation. The size of the water bath was 35 cm × 22 cm and the ultrasonic transducer was 15 cm × 15 cm. The distance between the ultrasonic transducer and 100 ml Erlenmeyer flask was set at approximately 3 cm.
Fig. 1.
Schematic representation of experimental equipment.
2.2. Preparation of silica xerogels and examination of aging conditions
Silica xerogel was prepared in two steps following methods reported in A. V. Rao et al [29]. Tetraethylorthosilicate (TEOS, 95.0%, Fujifilm Wako Pure Chemical, Co., Ltd.) was used as the starting material, and the hydrogel was made from TEOS, distilled water (H2O), ethanol (EtOH, 99.0%, Japan Alcohol Trading Co., Ltd.), hydrochloric acid (HCl, 4 wt%, Fujifilm Wako Pure Chemical, Co., Ltd.), and ammonia (NH4OH, 25 wt%, Fujifilm Wako Pure Chemical, Co., Ltd.) in the molar ratio of 1:3.5:3.9:7.8 × 10-4:1.7 × 10-2. In the first step, 13.89 g of TEOS, 11.96 g of EtOH, 1.18 g of H2O, and 0.0474 g of HCl were added to a 100 ml Erlenmeyer flask, irradiated with ultrasonic irradiation for 5 min, and maintained at 60 ˚C for 1 h to obtain a hydrolysis solution. In the second step, 2.95 g of H2O and 0.0776 g of NH4OH as a base catalyst was dropped, and subjected to ultrasonic irradiation for 5 min to obtain a silica sol. Thereafter, in order to evaluate the effect of ultrasonic irradiation on aging treatment, the reaction solution was gelated and aged using ultrasonic irradiation of hydrogel at frequencies of 100, 500 kHz and an output of 45 W at 60 °C for 3 to 12 h, and each gel was compared with that from the standing condition without ultrasonic irradiation. Next, a hydrophobic treatment was performed following the method of Oikawa et al [17]. First, the hydrogel was pulverized with a spatula and immersed in 6 N HCl (Fujifilm Wako Pure Chemical, Co., Ltd.) for 60 min. Approximately 10 g of the hydrogel and the amount of HCl to completely immersed the gel was added. Thereafter, the gel was removed from the HCl solution, immersed in a mixture with a mass ratio of hexamethyldisiloxane (HMDSO, 97.0%, Tokyo Chemical Industry Co., Ltd.): isopropanol (IPA, 99.7%, Fujifilm Wako Pure Chemical, Co., Ltd.) = 10:1, and heated at 60 °C for 3 h to perform a hydrophobic treatment. The silica xerogel was prepared by drying overnight at 120 ˚C under atmospheric pressure.
2.3. Preparation of silica xerogels and examination of hydrophobic conditions
Aging used ultrasonic irradiation at a frequency of 500 kHz and an output of 45 W at 60 °C for 6 h. Following this, in order to evaluate the effect of ultrasonic irradiation on hydrophobic treatment, hydrophobization was performed by ultrasonic irradiation at a frequency of 500 kHz and an output of 45 W at 60 °C for 5 min to 3 h, and each result was compared with the standing condition without ultrasonic irradiation. The silica xerogel was prepared by drying overnight at 120 ˚C under atmospheric pressure.
2.4. Characterization
The gel obtained in each synthesis was cut into size of 1 cm square, bulk density was determined by weighing samples of known dimensions. The surface morphology of the xerogel samples was observed using field emission scanning electron microscope (FE-SEM, JSM-7610F, JEOL Ltd.). For FE-SEM analysis, xerogel samples were pulverized and sputter-coated with osmium to prevent charge accumulation during FE-SEM observation. Micrstructural features of the silica xerogel, such as specific surface area and pore volume, were investigated using nitrogen gas adsorption BET measurements (BELSORP-mini, MicrotracBEL Co., Ltd). Fourier transform infrared spectroscopy (FT-IR, Nicolet6700, Thermo Fisher Scientific Inc.) was used to investigate the functional groups present at the surface of hydrophobic silica xerogel. The thermal stability of surface hydrophobic functional groups with temperature was estimated from thermogravimetric analysis (TG-DTA, SDTQ600, TA Instruments Inc.). Thermal analysis was performed in air at temperatures between 50 °C and 600 °C (heating rate = 10 °C/min, flow rate = 100 ml/min).
3. Results & discussions
3.1. Examination of aging conditions
Fig. 2 shows the dependence of apparent density on aging time. As shown, xerogels aged at a frequency of 100 kHz and had higher apparent densities than xerogels aged under standing conditions without ultrasonic irradiation at all treatment times. This suggests that the gel shrank during drying. On the other hand, the xerogel obtained by aging at a frequency of 500 kHz had a lower density after treatment times of 6 h and 12 h. This suggests that aging progressed sufficiently at 6 h at 500 kHz and gel shrinkage was suppressed during drying. This suggests that shrinkage during drying was most suppressed when aged at 500 kHz.
Fig. 2.
The dependence of apparent density on aging time.
Fig. 3(a–c) show FE-SEM images of silica xerogel obtained by aging for 6 h at 100 kHz, 500 kHz and under standing conditions, respectively. Almost all silica xerogel particles were spherical, and their particle size was approximately 10 nm, i.e., small. The pore structure of the xerogel aged at 100 kHz was somewhat coarser and had relatively large pores and a densely packed silica skeleton, as shown in Fig. 3(a). This skeletal structure arises from gel shrinkage during drying. The xerogel aged at 500 kHz showed a large number of pores (approximately 20–100 nm) and a coarse pore structure, as shown in Fig. 3(b). This suggests that gel shrinkage was suppressed during drying. The xerogel aged in standing conditions had relatively small pores and the most densely packed skeleton, as shown in Fig. 3(c). As highlighted with circles, skeletons in which particles were densely aggregated are present and, in some places, pores could not be confirmed. This aggregate is due to gel shrinkage during drying. These FE-SEM images also suggest that dry shrinkage is most suppressed when aged at 500 kHz. Such inhomogeneous pore structures result in the opaque appearance of the xerogel.
Fig. 3.
FE-SEM micrographs of silica xerogels aged for 6 h (a) at 100 kHz, (b) at 500 kHz, (c) under standing conditions.
The pore characteristics of each silica xerogel were investigated based on adsorption/desorption isotherm lines of nitrogen gas and pore distribution. Fig. 4(a) shows the adsorption/desorption isotherm lines of nitrogen gas for xerogels aged under various conditions. Fig. 4(a) shows that nitrogen was absorbed according to the pore size distribution and amount in each xerogel. The adsorption/desorption isotherms of each xerogel were typical of type IV. These isotherm lines were belonging to the hysteresis loops of the type H2, and the pore shape was difficult to identify. Fig. 4(b) shows the pore size distribution calculated from the adsorption surface of the xerogel aged under various conditions. This shows the most probable pore diameters for various xerogels, revealing a very broad pore distribution curve. The synthesized xerogel had a mesopore region (2 to 50 nm) and a macropore region (50 nm or more), corresponding reasonably well to FE-SEM observations, and indicating a non-uniform pore structure. This is probably because the gelation time was too short to form a uniform three-dimensional network.
Fig. 4.
(a) N2 adsorption/desorption isotherm and (b) pore size distributions of silica xerogels aged for 6 h at 100 kHz, 500 kHz, and under standing conditions. N2 adsorption/desorption data were calculated using the BJH method.
Table 1 shows the calculated specific surface area, pore volume, and average pore diameter of each xerogel synthesized herein. The specific surface area of each xerogel was 649–677 g/cm3, i.e., was not significantly different depending on aging conditions. The pore volumes of the silica xerogels aged at 100 kHz, 500 kHz, and in standing conditions were 3.53 cm3/g, 3.88 cm3/g and 2.97 cm3/g, respectively. Also, it was found that silica xerogels aged at 500 kHz showed relatively higher average pore diameters of 51.6 nm, whereas those aged at 100 kHz and at standing conditions possessed lower average pore diameters of 29.8 nm and 22.4 nm, respectively. It is considered that this reflects the promotion of the aging reaction by ultrasonic irradiation of hydrogel at 500 kHz. In comparison, the xerogels aged at 100 kHz and under standing conditions were aged insufficiently, and thses gels contracted due to capillary forces during drying. Consequently, the shrinkage of the gel resulted in a smaller pore volume and average pore size. On the other hand, the xerogel aged at 500 kHz underwent an accelerated aging reaction and developed a strengthened silica skeleton in a short time. As a result, gel shrinkage was suppressed and the pore volume and average pore size increased.
Table 1.
Physical properties of silica xerogels for each aging condition.
| Aging condition | Surface area [m2/g] | Pore volume [cm3/g] | Average size pore [nm] |
|---|---|---|---|
| 100 kHz, 6h | 677 | 3.53 | 29.8 |
| 500 kHz, 6h | 649 | 3.88 | 51.6 |
| standing, 6h | 652 | 2.97 | 22.4 |
FT-IR spectroscopy was used to investigate the functional groups present at the surface of hydrophobic silica xerogel. Fig. 5 shows the FT-IR spectra of each synthesized silica xerogel. For all xerogels synthesized in this study, absorption due to C–H bonding was observed at 1360 cm−1 and 2960 cm−1, while absorption due to Si-C bonding was observed at 1250 cm−1 and 839–757 cm−1. Also, absorption corresponding to Si-O bonding was confirmed at 1005 cm−1. On the other hand, the absorption features corresponding to O–H bonding at 1620 and 3660 cm−1 were found to be weaker in the xerogel aged at 500 kHz than in the xerogels aged at 100 kHz and under standing conditions. It was found that the xerogel aged at 500 kHz had a low number of residual silanol groups. This is because ultrasonic irradiation of hydrogel at 500 kHz promoted the condensation of silanol groups between particles and shows that the aging reaction proceeded and interparticle bonding was strengthened.
Fig. 5.
FT-IR spectra of silica xerogels synthesized using each aging condition.
The differences between ultrasonic frequencies and the corresponding effects on silica xerogel are discussed later.
3.2. Examination of hydrophobic conditions
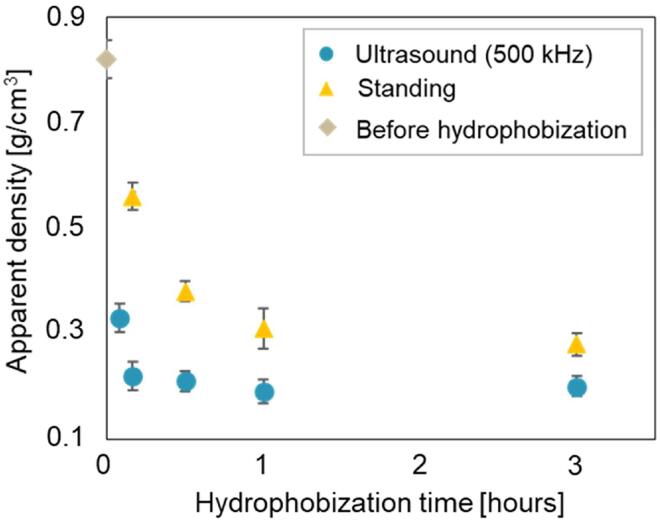
Fig. 6 shows the dependence of apparent density on hydrophobization time. The xerogel hydrophobized under standing conditions without ultrasonic irradiation had a higher apparent density at any treatment time. This suggests that the gel shrank during drying. On the other hand, the density of the xerogel hydrophobized at a frequency of 500 kHz is less than that of the xerogel hydrophobized in standing conditions. When hydrophobized at a frequency of 500 kHz, the apparent density became as low as 0.21 g/cm 3 in 10 min, suggesting that gel shrinkage was suppressed during drying under such conditions.
Fig. 6.
Dependence of apparent density on hydrophobization time.
Fig. 7(a-b) show FE-SEM images of silica xerogel hydrophobized for 10 min and 1 h at 500 kHz, respectively, and Fig. 7(c-d) show FE-SEM images of silica xerogel hydrophobized for 10 min or 1 h under standing conditions, respectively. Similar to the FE-SEM images in Fig. 3(a-c), almost all particles of the silica xerogels were spherical, with sizes around 10 nm, i.e., small. The xerogel hydrophobized at 500 kHz had a large number of pores (approximately 20–100 nm) and a coarse pore structure after treatment for 10 min and 1 h, as shown in Fig. 7(a-b). This is because the surface of silica particles was made hydrophobic, which suppressed gel shrinkage during drying. On the other hand, the xerogel hydrophobized for 10 min in standing conditions had densely aggregated particles with very small pore sizes, as shown in Fig. 7(c). The xerogel hydrophobized for 1 h under standing had relatively small pores and a more densely packed skeleton, as shown in Fig. 7(d). This aggregate is due to insufficient hydrophobization and shrinkage of the gel during drying.
Fig. 7.
FE-SEM micrographs of silica xerogels hydrophized (a) for 10 min at 500 kHz, (b) for 1 h at 500 kHz, (c) for 10 min under standing conditions, and (d) for 1 h under standing conditions.
The pore characteristics of each silica xerogel were investigated based on the adsorption/desorption isotherm lines of nitrogen gas and pore distribution. Fig. 8(a) shows the adsorption/desorption isotherm lines for xerogels hydrophobized under various conditions. Fig. 8(a) indicates that nitrogen absorbed into the pores of each xerogel. Similar to Fig. 4(a), the adsorption–desorption isotherms of each xerogel were of the typical type IV. Fig. 8(b) shows the pore size distribution calculated from the adsorption surface of xerogels aged under various conditions and shows the most probable pore diameters for various xerogels, revealing a very broad pore distribution curve for all xerogels. Furthermore, the pore distribution of the xerogel hydrophobized in standing conditions was shifted to the left side compared to the xerogel hydrophobized at 500 kHz.
Fig. 8.
(a) N2 adsorption/desorption isotherm and (b) pore size distributions of silica xerogels hydrophobized for 10 min and 1 h at 100 kHz and 500 kHz, and silica xerogels hydrophobized for 10 min and 1 h under standing conditions. N2 adsorption/desorption data were calculated using the BJH method.
Table 2 shows calculated specific surface areas, pore volumes, and average pore diameters for each xerogel synthesized under hydrophobic conditions. The specific surface area of each xerogel was 683–753 g/cm3, which was not significantly different depending on the hydrophobic condition emplyed. The pore volumes of the xerogel hydrophobized for 10 min and 1 h at 500 kHz, and the xerogel hydrophobized for 10 min and 1 h in standing conditions were 3.50 cm3/g, 4.68 cm3/g, 2.38 cm3/g, and 3.95 cm3/g, respectively. The fact that the pore volume for 500 kHz at 1 h is larger than the pore volume for 500 kHz at 10 min is due to the presence of a large number of pores about 100 nm in the pore size distribution in Fig. 8(b) for 1 h at 500 kHz. It was also found that silica xerogel hydrophobized at 500 kHz had high average pore diameters of 40.3 nm, whereas the xerogel hydrophobized for 10 min or 1 h in standing conditions exhibited lower average pore diameters of 20.1 nm and 29.8 nm, respectively, compared to the xerogel aged at 500 kHz. This may arise from the promotion of the hydrophobic reaction by ultrasonic irradiation. The xerogel hydrophobized in standing conditions, however, still did not exhibit sufficient hydrophobized reaction, and the gel contracted due to capillary forces during drying. Consequently, the shrinkage of the gel resulted in smaller pore volumes and average pore sizes. On the other hand, the xerogel hydrophobized at 500 kHz demonstrated an accelerated reaction and its particle surface was modified in a short time. As a result, gel shrinkage was suppressed and the pore volume and average pore size increased.
Table 2.
Physical properties of silica xerogels for each aging condition.
| Hydrophobization Condition | Surface area [m2/g] | Pore volume [cm3/g] | Average pore size [nm] |
|---|---|---|---|
| 500 kHz, 10 min | 683 | 3.50 | 40.3 |
| 500 kHz, 1 h | 753 | 4.68 | 40.3 |
| Standing, 10 min | 710 | 2.38 | 20.1 |
| Standing, 1 h | 694 | 3.95 | 29.8 |
FT-IR spectroscopy was used to investigate the functional groups present at the surface of hydrophobic silica xerogels. Fig. 9 shows the FT-IR spectra of each synthesized silica xerogel. For all xerogels synthesized in this study, absorption due to Si-O bonding was observed at 1005 cm−1 and O–H bonding at 1620 and 3680 cm−1. On the other hand, the absorption corresponding to the C–H bonding at 1360 and 2960 cm−1 and Si-C bonding at 1250 cm−1 and 839–757 cm−1 were stronger in the xerogel hydrophobized at 500 kHz than in the xerogel hydrophobized under standing conditions. This suggests that the xerogel hydrophobized using ultrasonic irradiation at 500 kHz has many Si-C bonds as a result of the promotion of the hydrophobic reaction by ultrasonic irradiation.
Fig. 9.
FT-IR spectra of silica xerogels synthesized under different hydrophobization conditions.
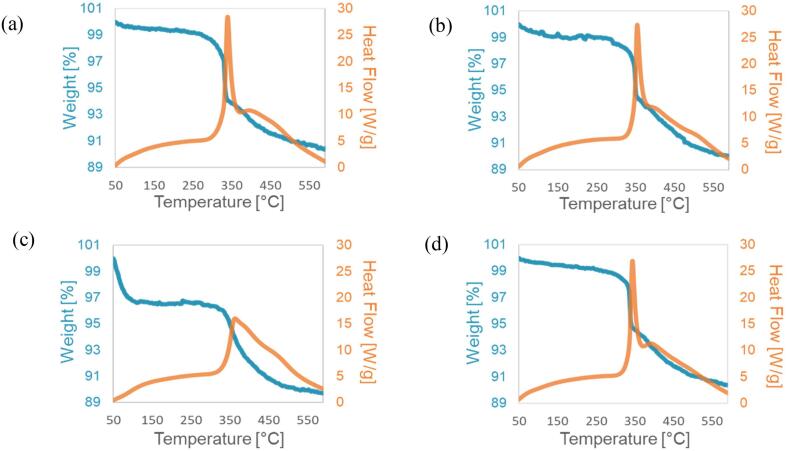
The thermal stability of surface hydrophobic functional groups according to temperature was estimated using thermogravimetric analysis performed in air at temperatures between 50 °C and 600 °C (heating rate = 10 °C/min, flow rate = 100 ml/min). Fig. 10(a-b) show TG-DTA analyses of silica xerogel hydrophobized for 10 min and 1 h at 500 kHz, whereas Fig. 10(c-d) show TG-DTA analyses of silica xerogel hydrophobized for 10 min and 1 h in standing conditions, respectively. The TG curves of the xerogel hydrophobized at 500 kHz and the xerogel hydrophobized for 1 h in standing conditions were stable up to approximately 350 °C, and their weight decreased when the temperature exceeded 350 °C due to thermal decomposition of the methyl group. This fact is clear from the fact that a sharp exothermic peak was confirmed around 350 °C in the DTA curve. The hydrophobic organic functional group became hydrophilic due to thermal decomposition at approximately 350 °C and lost its hydrophobicity. On the other hand, the TG curves of the xerogel hydrophobized for 10 min in standing conditions lost weight twice. The first weight loss occurred due to evaporation of residual solvent and adsorbed water at temperatures below 100 °C [30]. The second weight loss occurred due to the thermal decomposition of the methyl group or the condensation of silanol groups remaining on the silica particle surface around 360 °C [31]. This is also clear from the difference in the behavior of the exothermic peak relative to of other xerogels. The sharpness of the exothermic peaks in the xerogels hydrophobized for 10 min and 1 h at 500 kHz and the xerogel hydrophobized for 1 h in standing conditions were larger than that of the xerogel hydrophobized for 10 min in standing conditions. It is considered that this depends on the amount of attached methyl groups. Also, in the TG curve, the weight loss around 360 °C of the xerogel hydrophobized for 10 min under standing conditions was small, suggesting that the treatment was clearly insufficient in the xerogel hydrophobized for 10 min under standing.
Fig. 10.
Thermogravimetric (TG-DTA) curves of silica xerogel hydrophobized (a) for 10 min at 500 kHz, (b) for 1 h at 500 kHz, (c) for 10 min under standing, and (d) for 1 h under standing conditions.
3.3. Mechanism consideration by ultrasonic irradiation
Finally, we discuss the differences between ultrasonic frequencies and their effects on silica xerogel.
First, irradiating liquids with ultrasonic waves create acoustic cavitation bubbles. The sources of such bubbles are usually either existing stable bubbles present in the liquid or acoustic cavitation bubbles formed when the pressure falls below the vapor pressure of the liquid.
For bubbles that vibrate ultrasonically in liquid, the diameter of the bubbles that resonate at a specific frequency is fixed. Here, we will consider the simplest model that ignores the viscosity of the liquid. The resonant radius of the bubble ( in mm) can be estimated using the following equation [32]:
| (1) |
where is the heat capacity ratio in Cp/CV, is the liquid density in kg/m3, is the hydrostatic pressure in Pa and is the ultrasonic frequency in Hz.
Eq. (1) shows that the resonant radius of the bubble is highly frequency-dependent. Also, when the expanded size of a bubble exceeds the limit value termed the resonant radius, the bubble grows rapidly then collapses violently. A local high temperature and high pressure region (hot spot) occurs when bubbles collapse.
Fig. 11(a–c) show the reaction field under each condition in this study. Fig. 11(a) shows the standing conditions without ultrasonic irradiation. At this time, the reaction field is uniformly maintained at the reaction temperature of 60 °C, suggesting that aging and hydrophobization reactions proceed slowly over time under standing conditions.
Fig. 11.
Reaction fields (a) under standing conditions, (b) at 100 kHz, and (c) at 500 kHz.
Fig. 11(b–c) show the reaction fields at frequencies of 100 kHz and 500 kHz, respectively. Comparing the acoustic cavitation bubble sizes when crushing with reference to Eq. (1), bubble sizes become smaller at high frequencies.
Therefore, the hot spot size is smaller in the reaction field at 500 kHz. On the other hand, the number of hot spots is higher at high frequencies characterized by short wavelengths, indicating that there are sparse large hot spots in the reaction field at 100 kHz. As the size of the acoustic cavitation bubble increases, physical shock also increases due to the shock wave caused by the crushing of the bubble. In this study, the gel aged at 100 kHz cracked due to shock waves. Therefore, in the reaction field of 100 kHz, it is thought that while the physical impact is large, the chemical effect promoting chemical reactions due to thermal effects is small. On the other hand, the reaction field at 500 kHz has many small hot spots and is more evenly distributed. Therefore, compared with the reaction field at 100 kHz, it is thought that the reaction field at 500 kHz has a smaller physical impact, but a larger chemical effect promoting chemical reactions due to the thermal effect.
Combined, this suggests that a frequency of 500 kHz, which had a large chemical effect, promoted aging and hydrophobization reactions more effectively than a frequency of 100 kHz.
4. Summary and conclusions
In this study, the semi-solid hydrogel was irradiated with ultrasonic for the first time in order to accelerate aging and hydrophobic treatment in preparing silica xerogels. First, we examined the aging conditions. The xerogels aged at a frequency of 100 kHz and under standing conditions without ultrasonic irradiation had a higher apparent density after drying. Also, their pore volumes and average pore diameters were smaller than those of xerogels aged at a frequency of 500 kHz. FT-IR analyses suggested that there were many residual silanol groups. These results indicate that both the xerogel aged under standing conditions and the xerogel aged at 100 kHz shrunk due to insufficient aging reactions. On the other hand, the xerogel aged at 500 kHz had a smaller apparent density after drying, a larger pore volume, and a larger average pore diameter because the ultrasonic irradiation of hydrogel at 500 kHz accelerated the aging reaction and rapidly suppressed the shrinkage of the gel.
Next, we examined the hydrophobic conditions. Similar to aging, xerogels hydrophobized in standing conditions had higher apparent densities after drying. Also, their pore volumes and average pore diameters were smaller than those of xerogels hydrophobized at 500 kHz. FT-IR and TG-DTA suggested that there were few methyl groups present at the surfaces of these silica particles indicating that the xerogel hydrophobized in standing conditions shrunk due to an insufficient hydrophobization reaction. On the other hand, the xerogel hydrophobized at 500 kHz had a smaller apparent density after drying, but larger pore volume and average pore diameter. This is because ultrasonic irradiation at 500 kHz accelerated hydrophobization and rapidly suppressed the shrinkage of the gel.
The present study has made it possible to synthesize silica xerogel by ultrasonic irradiation of the semi-solid hydrogel in a very short time, less than 1/5 of the time required for conventional methods. Ultrasonic irradiation of hydrogel accelerates the reaction and can easily control of the pore structure and density of silica xerogels. This research can be expected to improve the productivity of xerogels, and aid their application as excellent thermal insulation materials in various fields, such as the building of residential structures and automobiles. Furthermore, ultrasonic irradiation of semi-solid hydrogel may be effective in accelerating the reactions not only in xerogel synthesis but also in the synthesis of other materials.
CRediT authorship contribution statement
Yuki Maeda: Writing - original draft, Formal analysis. Yamato Hayashi: Conceptualization, Methodology, Investigation, Funding acquisition, Writing - review & editing, Funding acquisition, Project administration. Jun Fukushima: Investigation. Hirotsugu Takizawa: Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to thank Professor D. Nagao of Tohoku University for his help in nitrogen gas adsorption BET measurements.
References
- 1.Pierre A.C., Pajonk G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002;102:4243–4265. doi: 10.1021/cr0101306. [DOI] [PubMed] [Google Scholar]
- 2.Jensen K.I., Schultz J.M., Kristiansen F.H. Development of windows based on highly insulating aerogel glazings. J. Non-Cryst. Solids. 2004;350:351–357. [Google Scholar]
- 3.Baetens R., Jelle B.P., Gustavsen A. Aerogel insulation for building applications: a state-of-the-art review. Energy Build. 2011;43:761–769. [Google Scholar]
- 4.Tabata M., Adachi I., Ishii Y., Kawai H., Sumiyoshi T., Yokogawa H. Development of transparent silica aerogel over a wide range of densities. Nucl. Instrum. Methods Phys. Res., Sect. A. 2010;623:339–341. [Google Scholar]
- 5.Tsou P. Silica aerogel captures cosmic dust intact. J. Non-Cryst. Solids. 1995;186:415–427. [Google Scholar]
- 6.Riffat S.B., Qiu G. A review of state-of-the-art aerogel applications in buildings. Int. J. Low. Carb. Technol. 2012;8:1–6. [Google Scholar]
- 7.Ru Y., Guoqiang L., Min L. Analysis of the effect of drying conditions on the structural and surface heterogeneity of silica aerogels and xerogel by using cryogenic nitrogen adsorption characterization. Micropor. Mesopor. Mater. 2010;129:1–10. [Google Scholar]
- 8.Fricke J., Emmerling A. Aerogels—recent progress in production techniques and novel applications. J. Sol-Gel. Sci. Technol. 1998;13:299–303. [Google Scholar]
- 9.Kanamori K., Aizawa M., Nakanishi K., Hanada T. New transparent methylsilsequioxane aerogels and xerogels with improved mechanical properties. Adv. Mater. 2007;19:1589–1593. [Google Scholar]
- 10.Bangi U.K.H., Rao A.V., Rao A.P. A new route for preparation of sodium silicate-based hydrophobic silica aerogels via ambient pressure drying. Sci. Technol. Adv. Mater. 2008;9 doi: 10.1088/1468-6996/9/3/035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maleki H., Duraes L., Portugal A. Development of mechanically strong ambient pressure dried silica aerogels with optimized properties. J. Phys. Chem. C. 2015;119:7689–7703. [Google Scholar]
- 12.Scherer G.W. Theory of drying. J. AM. Ceram. Soc. 1990;73:3–14. [Google Scholar]
- 13.Lee C.L., Kim G.S., Hyun S.H. Synthesis of silica aerogels from waterglass via new modified ambient drying. J. Mater. Sci. 2002;37(11):2237–2241. [Google Scholar]
- 14.Schwertfeger F., Frank D., Schmidt M. Hydrophobic waterglass based aerogels without solvent exchange or supercritical drying. J. Non-Cryst. Solids. 1998;225:24–29. [Google Scholar]
- 15.Hwang S.W., Kim T.Y., Hyun S.H. Optimization of instantaneous solvent exchange/surface modification process for ambient synthesis of monolithic silica aerogels. J. Colloid Interface Sci. 2008;322(1):224–230. doi: 10.1016/j.jcis.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 16.Mahadik D.B., Rao A.V., Rao A.P., Wagh P.B., Ingale S.V., Gupta S.C. Effect of concentration of trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDZ) silylating agents on surface free energy of silica aerogels. J. Colloid Interface Sci. 2011;356(1):298–302. doi: 10.1016/j.jcis.2010.12.088. [DOI] [PubMed] [Google Scholar]
- 17.Oikawa K., Toyota K., Sakatani S., Hayashi Y., Takizawa H. Effect of organic hydrophobic groups on the pore structure and thermal properties of waterglass-based silica xerogels. J. Ceram. Soc. Jpn. 2017;125(12):906–912. [Google Scholar]
- 18.Gedanken A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004;11:47–55. doi: 10.1016/j.ultsonch.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Dang F., Enomoto N., Hojo J., Enpuku K. Sonochemical coating of magnetite nanoparticles with silica. Ultrason. Sonochem. 2010;17:193–199. doi: 10.1016/j.ultsonch.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y.Q., Tang X.H., Yin L.X., Huang W.P., Hacohen Y.R., Gedanken A. Sonochemical synthesis of mesoporous titanium oxide with wormhole-like framework structures. A. Adv. Mater. 2000;12:1183–1186. [Google Scholar]
- 21.Yasui K. Influence of ultrasonic frequency on multibubble sonoluminescence. J. Acoust. Soc. Am. 2002;112:1405–1413. doi: 10.1121/1.1502898. [DOI] [PubMed] [Google Scholar]
- 22.Edrissi M., Soleymani M., Adinehnia M. Synthesis of silica nanoparticles by ultrasound-assisted sol-gel method: optimized by taguchi robust design. Chem. Eng. Technol. 2011;34(11):1813–1819. [Google Scholar]
- 23.Bang J.H., Suslick K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010;22:1039–1059. doi: 10.1002/adma.200904093. [DOI] [PubMed] [Google Scholar]
- 24.Shchukin D.G., Skorb E., Belova V., Mohwald H. Ultrasonic cavitation at solid surfaces. Adv. Mater. 2011;23:1922–1934. doi: 10.1002/adma.201004494. [DOI] [PubMed] [Google Scholar]
- 25.Enomoto N., Sung T., Nakagawa Z. Effect of ultrasonic waves on crystallization from a supersaturated solution of alum. J. Mater. Sci. 1992;27:5239–5243. [Google Scholar]
- 26.Morita K., Hu Y., Mackenzie J.D. The effects of ultrasonic irradiation on the preparation and properties of ormosils. J. Sol-Gel. Sci. Technol. 1994;3:109–116. [Google Scholar]
- 27.Yasuda K., Takahashi Y., Asakura Y. Effect of ultrasonication on polymerization of silicic acid in geothermal water. Jpn. J. Appl. Phys. 2014;53:07KE08. [Google Scholar]
- 28.Soleimani Dorcheh A., Abbasi M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008;199(1-3):10–26. doi: 10.1016/j.jmatprotec.2007.10.060. [DOI] [Google Scholar]
- 29.Rao A.V., Nilsen E., Einarsrud M.-A. Effect of precursors, methylation agents and solvents on the physicochemical properties of silica aerogels prepared by atmospheric pressure drying method. J. Non-Cryst. Solids. 1998;225:24–29. [Google Scholar]
- 30.Liu H., Sha W., Cooper A.T., Fan M. Preparation and characterization of a novel silica aerogel as adsorbent for toxic organic compounds. Colloids Surf. A: Physicochem. Eng. Asp. 2009;347:38–44. [Google Scholar]
- 31.Zhou T., Cheng X., Pan Y., Li C., Gong L., Zhang H. Mechanical performance and thermal stability of glass fiber reinforced silica aerogel composites based on co-precursor method by freeze drying. Appl. Surf. Sci. 2018;437:321–328. [Google Scholar]
- 32.Schroeder A., Kost J., Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem. Phis. Lipids. 2009;162:1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]