Graphical abstract
Abbreviations: nm/μm, Nanometer/Micrometer; G/GO/Gt, Graphene/Graphene Oxide/Graphite; SW/MW/CNT, Single wall/Multi wall/Carbon Nanotubes; TiO2, Titanium oxide; Fe3O4, Iron oxide; UV–Vis, Ultraviolet visible; SEM, Scanning Electron Microscope; TEM, Transmission Electron Microscope; FTIR, Fourier transform infrared; TGA, Thermogravimetric analysis; AFM, Atomic Force Microscopy; FLG, Few layer Graphene; XRD, X-ray powder diffraction; EG, Ethylene Glycol; NPs, Nanoparticles; DW, Distilled water; SDS/SDBS, Sodium Dodecyl Sulfate/Sodium Dodecyl Benzene Sulfonate; CTAB, Cetrimonium bromide; mV, millivolts; kHz, kilohertz; w, weight of nanoparticle; wbf, Weight of base fluid; ρbf, Density of base fluid (g/cm3); µ, Dynamic viscosity (kg/ms); φ, Volumetric concentration (%); ρp, Density of Nanoparticle (g/cm3
Keywords: Probe/bath sonicator, Graphene-Nanofluid, Stability, Thermal properties, Heat Transfer
Highlights
-
•
Ultrasonication period is most crucial aspect in heat transfer via nanofluids.
-
•
Effect of ultrasonication time through microscopic observations is reviewed.
-
•
Retentive sonication period is suitable for proper scattering of particles.
-
•
Smaller particle size is influential on higher stability and reduced viscosity.
Abstract
Optimum ultrasonication time will lead to the better performance for heat transfer in addition to preparation methods and thermal properties of the nanofluids. Nano particles are dispersed in base fluids like water (water-based fluids), glycols (glycol base fluids) &oils at different mass or volume fraction by using different preparation techniques. Significant preparation technique can enhance the stability, effects various parameters & thermo-physical properties of fluids. Agglomeration of the dispersed nano particles will lead to declined thermal performance, thermal conductivity, and viscosity. For better dispersion and breaking down the clusters, Ultrasonication method is the highly influential approach. Sonication hour is unique for different nano fluids depending on their response to several considerations. In this review, systematic investigations showing effect on various physical and thermal properties based on ultrasonication/ sonication time are illustrated. In this analysis it is found that increased power or time of ideal sonication increases the dispersion, leading to higher stable fluids, decreased particle size, higher thermal conductivity, and lower viscosity values. Employing the ultrasonic probe is substantially more effective than ultrasonic bath devices. Low ultrasonication power and time provides best outcome. Various sonication time periods by various research are summarized with respect to the different thermophysical properties. This is first review explaining sonication period influence on thermophysical properties of graphene nanofluids.
1. Introduction
Nanofluids comprise particles that exist in nanometer range recognized as nanoparticles scattered in the liquid [1], [2]. These nanoparticles are generally metallic element, carbides, oxides, graphite, carbon nanotubes and Graphene etc. Water, glycerol, oil, and ethylene glycol (EG) are being used as base fluids to dissolve the nanoparticles [3], [4]. Graphene has taken all the attentions of researchers due to multiple applications. Graphene Nanofluids take potential applications in fuel cells, heat exchangers for heat transfer, electronics cooling system refrigeration systems, solar collectors, cosmetics, defect sensor, antagonistic–infection therapy, power systems and biomedical [5], [6], [7], [8], [9], [10], [11], [12]. Equal dissemination of graphene nanoparticles in the base fluid is a crucial factor in determining the performance of nanofluid. In the base fluids the nanoparticles have tendency to cluster [13] and form masses, which is an obstacle in functioning of the nanofluids [14]. The reason expected is of due to the high cohesive energy of van der Waals and heavy π-π stacking. Graphene exhibits a lattice of honeycomb, the sp2 attachment of which is significantly stronger than the diamond sp3 bond. Between the covalently bonded px and py orbitals, a σ-bond is formed and the pz orbital angular momentum form π-bonds [15] with partially filled bands that permit electron free movement [16]. The carbon atoms are introduced into vacancies when graphene is bombarded with pure carbon atoms, hydrocarbons, or other carbon-containing molecules, thereby auto-repairing spaces in the graphene layer and high outward energy on the surface of nanoparticles that leads to depositing and added affects in thermophysical properties as thermal conductivity, viscosity, and pressure drop capability that depend on dynamic Brownian motion of NPs [17], also causing blockage and abrasion problems in specific for channels and tubes in heat transfer and in micro automatic organizations. The surface density of the nanoparticles, existence of ionic adulterations in base fluid, at a low-level pH., and non-effective reaction or functionalization refer to the hydrophobic nature of the graphene nanoparticles.Table 1.
Table 1.
Water/Glycol Based Graphene Nano Fluids sonication time and observation details.
| Nano particle | Size | Concentration | Type of sonicator | Sonication time | Surfactant | Characterization technique | Observations | Ref. |
|---|---|---|---|---|---|---|---|---|
| Graphene + EG | 0.7–1.3 nm | 0.01–0.05% | Magnetic stirrer & ultrasonic washing machine | greater than5 mins | SDBS | TEM FTIR TG |
Weak non-covalent bond. Prevention of agglomeration by SDBS Increased thermal conductivity with graphene nanofluid. Since the obtained graphene is synthesized by reduction of graphene oxide, author dispersed graphene in<5 mins. | [66] |
| Graphene + DW | <100 nm | 0.01–0.05% | Magnetic stirrer & Sonication bath and vibrator | 16hrs stirring &1hr-sonication bath. | – | TEM Raman |
SDS unable to disperse graphene and settled after 5 minutes TEM showed little traces of agglomeration Low concentrations have good dispersions and less agglomerations |
[67] |
| Graphene | 500–1.5 μm | 0.055% (~4mg/ml) | Sonication bath, ultrasonicator, centrifuge | 24hrs sonication | Sodium cholate, sodium taurocholate, Polyvinylpyrrolidone | TGA UV–Vis |
Polymer showed the highest thermal conductivity with an enhancement of 25%. The highest stability and dispersion have been obtained by the polymer surfactant. | [68] |
| Graphene | 1 μm | 0.3 mg/ml | Sonication Centrifugation (500 – 2000 rpm) |
430hrs of mild sonication | Sodium cholate | Raman TEM |
Raman spectroscopy proved below 2000 rpm there is defect free flakes, and the flake size has been decreased to 500 nm TEM Results showed effective dispersion |
[69] |
| Graphene nanoplatelets-W + EG | – | 0–0.2% | Probe sonicator | 25mins | – | FTIR TGA TEM |
The hydroxyl groups are attached with graphene particles. TEM structure shows wrinkles, as a result of sonication. |
[70] |
| Layer Graphene + water | <200 nm | – | Probe sonicator (36 mm dia)180W Centrifuge |
0-200mins sonication, 45 mins of centrifugation | used | Raman TEM FLG |
Sonication generated smaller size flakes To avoid bubbles or foam during sonication, proper surfactant has to be used. Sonication process proved to be effective. |
[71] |
| Graphene | – | 0–0.035% | Sonication bath Probe sonicator |
120 mins bath sonication. 2hrs-sonication |
DMF (dimethyl formamide) | TEM UV–Vis AFM |
High power yields high graphene concentration and vice versa by UV–Vis. Sonication followed by bath sonicator yielded better results for stability. |
[72] |
| Graphene – DI Water + EG | <100 nm | 0.005–0.01% | Probe sonicator | 45 mins | Not used | TEM Raman FTIR |
60 days no sedimentation observed.Graphene functional groups interacted with base fluid. Heat transfer and thermal conductivity enhancements due to stable nanofluid obtained after sonication |
[73] |
| Graphene oxide- Water + EG | – | 0.0001–0.0007 | Ultrasonic vibrator (50 W power) | 15mins | Not used | TEM UV–Vis FTIR Zeta Potential |
UV–Vis and zeta potential analysis showed the stable nanofluids 30 % of enhancement observed in thermal conductivity for EG sample |
[74] |
| Graphene oxide | – | 0.01–0.25 wt% | 750 W sonic mixer | 40 mins ultrasonication | CTAB, SDS, & Triton X-100 |
UV–Vis Zeta Thermal conductivity |
Zeta and UV–Vis showed good dispersion stability SDS showed more stability than other surfactant used here. CTAB has no low stability Thermal conductivity and stability are high when compared with surfactants sample |
[75] |
| Functionalized Graphene- EG + Water | <100 nm | 0.041, 0.124, 0.207, and 0.395 vol%) | Ultrasonicator | 45 mins | Not used | Thermal conductivity Raman |
Stable graphene nano fluids for more than 150 days Viscosity result showed non-Newtonian behavior of the fluid and enhanced thermal conductivity with stable nanofluid without surfactant. |
[76] |
| Graphene oxide/Co3O4- water/EG | <50 nm | 0.2% | Sonication bath and vibrator | 2hrs | Not used | Thermal conductivity viscosity |
Size of the particle influenced the thermal conductivity and viscosity enhancements Water based nanofluids have |
[77] |
| Carboxyl graphene |
<100 nm | 0.02–0.04% | Sonicator | 9hrs | Not used | Visual photographic observation | 72 h of stable nanofluid obtained Stability and performance characteristics are increased with fluid |
[78] |
| graphene quantum dots nanofluids-W/EG/W + EG | – | 0.05–0.5 | Ultra sonicator | 15 mins | Not used | UV–Vis | The strong peak in UV shows the stable nanofluid prepared More than 30 days stable nano fluid achieved |
[79] |
| Graphene nanoplatelet-Water | <500 nm | 0.50, 0.75 and 1.0 wt% | Ultrasounds ultrasonic bath- 200 W | 240mins | SDBS | Zeta potential | Thermal conductivity enhancements achieved with Newtonian behavior of the samples. To stabilize the nanofluid the surfactant was added with base fluids |
[80] |
| Graphene-DW | 7-nm & 40-nm size | 0.1 wt% | Ultrasound vibrator | 40mins | SDS & SDBS | UV spectroscopy zeta potential |
SDBS showed stable fluid with zeta analyzer, while SDS showed high thermal conductivity. Thermal conductivity value decreased with amount of surfactant increased. Stable nanofluid achieved |
[81] |
| functionalized graphene nanoplatelets-PEG(poly ethylene glycol) | – | 0.5 wt% | Probe sonicators Magnetic stirrer |
70mins | Not used | Zeta potential | Increased sonication time increased the aggregation dispersion with 240 mins of bath and 45 mins vibrator. Size of the nanoparticle was not changing in between 45 and 70mins, but it showed higher stability. |
[82] |
| Graphene platelet-DI Water | – | 0.02–0.1 wt% | Magnetic stirrer(750 rpm) sonicator |
Stirring −10hrs Sonicated −5hrs |
Not used | Visual observation | Minimum sedimentation observed for 30 days Graphene nanoplatelets showed enhanced thermal conductivity. |
[83] |
| Graphene- Water | 54 nm | 0.4%-0.6% | Ultrasonic vibrator | 30 mins | Not used | Zeta potential | Heat transfer capacity increases with the increase in amount of the nano particle | [84] |
| Graphene nanoplatelets- water + EG | 123–424 nm. | 0.1% | Magnetic stirrer(300 rpm) ultrasonic homogenizer (20 kHz and 150Watts) |
20 mins stirrer 10 mins sonication(400 W) |
Time settle observations | 21 days of stability obtained. Visual identification technique confirmed the stability Minimum sonication time chosen to avoid damage of particle Thermal conductivity increased with increase in mass and temperature |
[85] | |
| Exfoliated Graphene-ionic liquids | 40–50 nm | – | Ultrasonicator & Centrifugation (10000 rpm) | 24hrs | perfluorinated aromatic solvents | UV vis Raman XRD |
Graphene in high concentrations with ionic liquid as solvent have lengthy side chains by the groups of benzyls. Stable large concentrations of the graphene can be produced with acceptable density and surface ratio. | [86] |
| FLG (exfoliate few layer Graphene) | 350 nm −35 µm | – | Sonication Microwaving 5sec |
Not mentioned | Polypropylene Carbonate (PPC) | XRD, HR-SEM, HR-TEM | Sonication caused the bonding of non-covalent and there is efficient transmission in the graphene sheet alignment with cavitation exfoliation and pressure by heat treatment, Graphene layers are separated by vibrational influences with no change in the properties of surface. | [87] |
Consequently, to lower the group and sedimentation of graphene nanoparticles, they must be well spread, dispersed, and steadied. Stability of a graphene nanofluid is the most important concern prior to preparing it. The stability of the nanofluid is the capability of particle to remain distributed in the base fluid with no developed clusters. Numerous factors like size of particle, type of surfactant used, sonication time/hour, volume/weight concentration, power, and type of sonication (pulse or nonplused) effects the stability of graphene based nano fluid. The higher agglomeration size leads to change in density and they tend to settle down, hence reducing stability. Agglomeration also effects thermal conductivity of the graphene nanofluid [18], [19], [20]. Consequently, the agglomeration is to be restrained as considerably as possible. The added Graphene NPs, nevertheless, usually give inadequate compatibility with the base fluids because of the phobic nature and have the strong bias to produce clustered aggregation. Studies have previously assumed that a special type of carbon, a property known as being hydrophobic, is repelled in water. A recent research published however, has disclosed that it is particularly attracted by graphene floating over water, indicating that graphene is indeed hydrophilic, Yet previous studies have demonstrated conflicting results on graphene-water interactions being hydrophobic [21], [22]. Amalgamation of nanoparticles is due to particle adhesion to each other facilitated by weak intermolecular forces leading to micron size. It has been reported that the excess loading of GO has a negative effect on the microstructure of the composite, and even forms an aggregate because of the inadequate compatibility between the GO and polymer chain [23]. From the other end, nanoparticle aggregates are due to the creation of van der Waals forces or metal bonds that cannot easily be separated. To focus on this challenge, terrific attempts have been allocated to upgrade the diffusion stability of thermal nanofluids by adapting the methodologies such as mechanical stirring, ultrasonic therapy, surface accusations or employing surface modification techniques with surfactants [24], [25]. Various techniques are equipped to evaluate the stability, like quantitative and qualitative technique which includes photograph capturing method for sedimentation, zetapotential, centrifugation, spectral analysis, and 3ω-method for graphene based nano fluids [26], [27], [28]. Zeta potential creates an amount of useful electrifying charge on the exterior surface of the nanoparticle in fluid. The magnitude gives knowledge regarding stability of the particle. Particles with higher magnitude zeta potentials exhibit increased stability due to a larger electrostatic repulsion between particles. A zeta potential range additional to − 30 mV to + 30 mV believed to get appropriate repellent strength to achieve superior colloidal steadiness. Ultrasonication is one of the well-known highly practiced methods in stable nanofluids preparation.
Ultrasonication is unique homogenization technique utilized in variety of applications. It is a process which break large particle into smaller fragment or better uniform sized particles in the base fluid [29], [30]. Sonication of nanofluid is achieved by providing sound energy to agitate the nanoparticles in the suspension [31], [32], [33]. Ultrasonication is a process where above 20 kHz of ultrasonic rates/frequencies are utilized for homogenization. Two commonly equipped sonicators are Bath-type and probe-type. Probe type sonicators having high intensity is noticed to be more effective than bath-type [34], [35]. The stability of a nanofluid can be dependent on concentrations, viscosity (low viscous liquids) and can be improved by using surfactants but in most cases, surfactants increased the viscosity of nanofluid. The ultrasound effect will increase the heat transfer in nanofluids. The heat flux will be decreased due to enhanced efficiency of heat transfer. When the viscosity of nanofluid increases with increase of volume concentration, the ultrasonic velocity decreases gradually. This review is focused on method of preparation, most specifically on ultrasonication time and its effect. This is the first unique review explaining the importance of sonication time period with respect to power to produce the efficiently dispersed and stable graphene nanofluid. However, surfactant effects with ultrasonication time/power are counted here. The summary of this analysis is conveyed in Fig. 1. In detail, review condenses the scattering approaches to stabilize the carbon-based/graphene based nanofluids for stable outcome. Summarized analysis of the existing information has shown that, ultrasonicator usage for preparing nanofluids, the sonication bath type leads to high temperature progressively rises up with point in time, and the peak temperature is restricted by the surrounding atmosphere. There is no definite clarification and data available on exactly how considerable ultrasonication time/power is essential to standardize the graphene suspensions. A few scholars attained clearer constancy with a particular period of ultrasonication and subsequently decreased stability parameters of graphene based nanofluids. Hence the systematic review intended to present the time period of the sonication needed for graphene nanofluids which shows the effect on thermal, physical and chemical properties reported by several researchers. Each segment includes knowledge regarding the impact of ultrasonication on colloidal dispersion and thermophysical properties of that type of nanofluids. The following subsequent sections include information of the graphene nanofluid preparation methods, experimental works about ultrasonication effect on nanofluids/graphene based nanofluids.
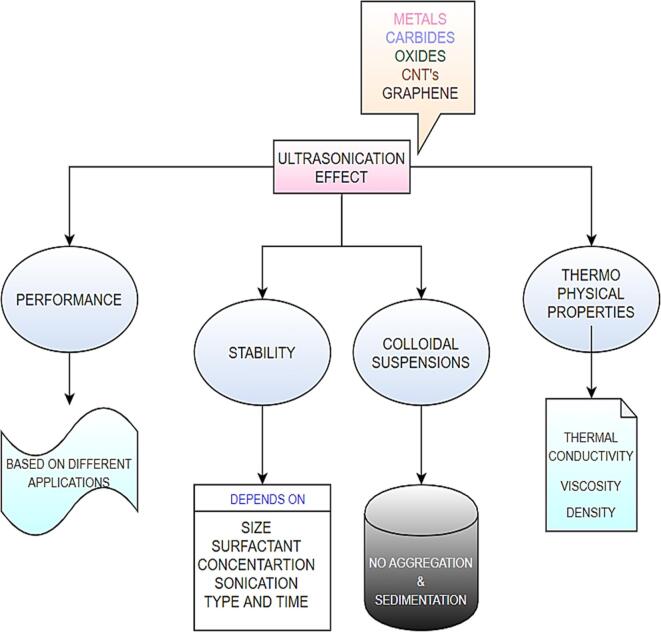
Fig. 1.
Effect of ultrasonication time with respect to performance, stability and thermophysical properties of graphene nanofluid.
2. Preparation of graphene-nanofluid
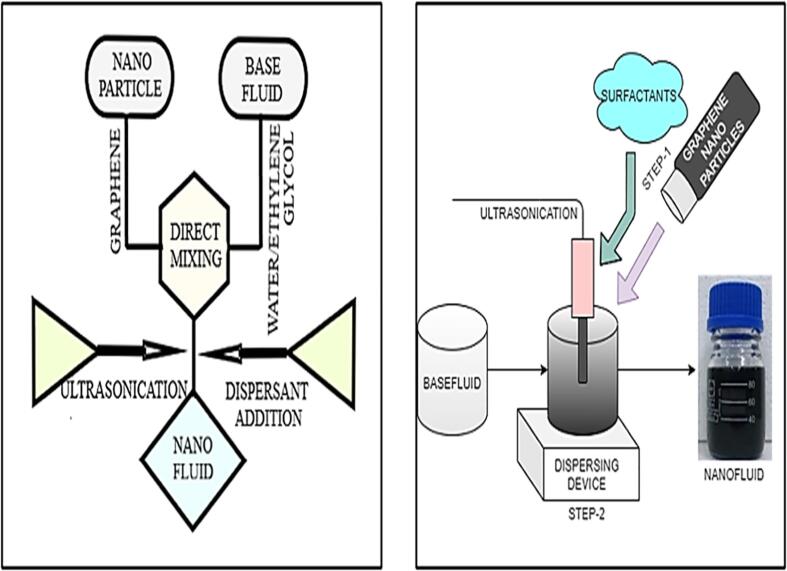
The preparation of graphene nanofluids is the initial and key element of the experimental study on nanofluids. It is not only the process of mixing the graphene nanoparticles into the base fluid but important to minimize the particle agglomeration which is possible by using different techniques. One step and two step are the most commonly and broadly used techniques to mixing operation [36], [37]. Though single or one step method can minimize the particle agglomeration it requires stringent restrictions for this technique. The effortlessness technique which is prevalent for commercial production is the twostep procedure which is extremely widespread in research articles. Strong van der Waals strength amongst nanoparticles require proper mixing and stabilization to get homogeneously dispersed nano sized graphene particles [38], [39]. The highly popular practice for preparing graphene nanofluid is shown in Fig. 2. A sophisticated shear blending, or ultrasonic vibrator is usually used to combine Nano residues with Base fluids. Most commonly used base fluids are water, oil, or water with ethylene glycols in scholarly articles. Particle agglomeration can be reduced by using techniques like stirring, ultrasonication or both [40], [41]. Dynamic stability, Diffusion stability, and Chemical/Biological stability are the methods described for preparation of graphene based nanofluids [42]. Several parameters including the base fluid selection and determination of nano particles are the considering factors to avoid sedimentation/agglomeration for long durations and obtain stable graphene nanofluid.
Fig. 2.
Schematic representation of twostep preparation of Graphene nanofluid.
Currently, Nanoparticles availability makes two-step method legitimately attractive to the investigators. Two-step approach performs effectively for several oxide and carbon-based nanoparticles. An ultrasonicator is used in this twostep procedure to disperse nanoparticles, initially mass/volume fraction (concentrations) are calculated [43] using the following eq (1)
| (1) |
where ‘φ’ is the volume concentration ‘w’ is the weight of nanoparticle, ‘ρp’ is the density of nanoparticles, ‘wbf’ and ‘ρbf’ are weight and density of base fluid, respectively.
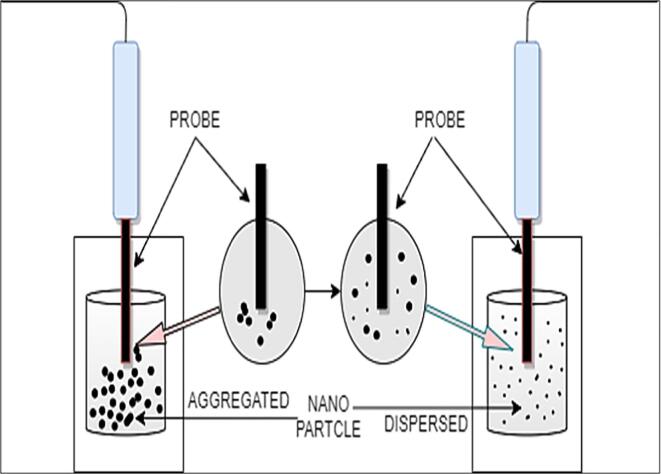
Possessing to the advantage of having higher thermal conductivity, thermal capacity and low density carbon based nano particles like Graphite (Gt), carbon nanotube (CNT) , Graphene (G), Graphene oxide (GO) and other carbon based allotropes are considered for thermal applications [44], [45], [46], [47]. Amongst all of them, in current decade graphene nanofluids have got growing study interest for the reason that of its quickly built production process and superior thermophysical properties. Graphene nanoparticles, on the other hand, typically come up with clearer tendency to develop aggregation since larger constants and more crucially their physical appearance and nature [48], [49], [50]. In specific, the larger superficial interaction region among adjacent graphene particles take the lead to strong inter-plane van der Waals attraction and irrevocable accumulation. Below Table (1) provide the information of sonication time and important findings of the authors research to prevent the aggregation of graphene nanoparticles via stirring and vibrations to maintain long term stability and dispersion for better performance of the fluids [51]. Including the practices, ultrasonication is the highly widespread procedure showing the good possibility in shattering down the groups of particles [52], [53], [54], which in turn increases the constancy of the suspension [55]. Fig. 3 shows the schematic view of breaking the nanoparticles and dispersing in the fluid. Ultrasonication is vital procedure which demonstrated incredible potential in separating the group of particles, which prompts to increase the stability of the suspension. The ultrasonic treatment utilizes for various purposes, like dispersion of the nanoparticles into the base liquids, de-agglomeration of particles, decreasing particle size, molecule amalgamation and precipitation, and surface functionalization. Sound energy is a wave comprised of high and low pressures. The sound waves generated from the probe effect on the particles to get segregated throughout the liquid. An implosion is the contrary of a detonation, quiet . Subatomic particles travel outwards in an eruption, but matter and energy implode inwards in an implosion. Implosions will be attributed to higher pressure on an object's outside than that on the inside. The sound waves utilized in sonication are typically ultrasound waves with frequencies over 20 kHz, that is 20,000 cycles for each second and as recurrence increases the strength of the agitation increases. The particles vibrate as they experience pressure cycles, microscopic vacuum bubbles structure is formed and afterward breakdown into solution, it is known as cavitation process [56]. These vibrations can disrupt atomic interactions (for example between atoms of water), split clusters of particles up, and lead to blending. In case of disintegrated gas, these vibrations can allow the gas bubbles to come together and more easily leave the solution. Sonicator either produce sound waves into a water shower, samples are put, or can be probes that are put straightforwardly into the example to be sonicated [57]. The desperation rate may vary time to time and power being utilized and pulse rate may count.Fig. 4.
Fig. 3.
A schematic view of ultrasonication breaking agglomerations of nanoparticles.
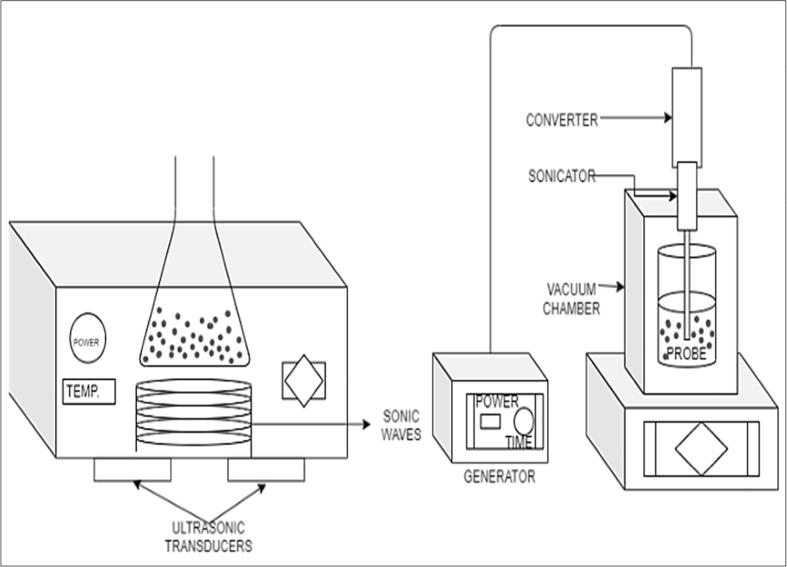
Fig. 4.
Types of Sonication instruments bath type (left) and probe sonicator (right).
2.1. Types of ultrasonication apparatus
Ultrasonicator is an intensifying tool in homogenization techniques, which serves efficient and collaborative breaking of particles that to be dispersed into the liquid. Generally, ultrasonicator serves for various objectives like nanoparticles to be dispersed in the base fluids, to avoid the agglomeration [58], to decrease the size of the nanoparticle in the fluid or during the synthesization of nanoparticles and functionalization of surface [59]. There are two types of sonicator are being used in various applications such as a) bath type sonication b) probe type sonication can be done using a probe-type ultrasonic [60]. In the bath type ultrasonic devices there is minimal [61] intensity (10–40 W/L) and effect on the particles because of lower efficient way. In contrast to this, the probe sonicator (>=20000 W/L) is higher intensified sector with more focused influence with uniform concentration in the fluid [62]. Since ultrasonic probe is able to create energy more efficiently, area-intense and focus on nature, it is relatively more suitable for thermal-based applications. Obviously, larger diameter probe having greater thermal features. Different types probes have different intensities at the point of action. The larger probe point produces high number of disturbances than the pinpoint probe. As for rapid and localize applications such as particle size reduction and emulsification probe are appropriate and for the applications such as cleaning apparatus and degassing, bath fits well. In the summary the probe type is the best utilized type in nanofluids. The current work is to identify the sonication effect on heat transfer, thermophysical properties, and stability/density.
2.2. Ultrasonication time and energy influences
The time duration and power implied by the sonicator gives various impact on the respective nano fluids and the most critical concern of researchers is to achieve the enhanced thermal conductivity and stability with lower viscosity [63], [64], [65]. There are different methods to determine the stability of the nanofluids like zeta potential, apart from this, the size of the cluster, distribution of the particle in the fluid, morphology and crystallinity, light scattering technique to find the agglomeration of the particles, agglomeration size and structure of the particle [66], [67]. The effects of sonication time and power on the stability by various researchers are investigated and listed with most critical conclusions on the stability of graphene based nano fluids. Sonication power is one of the critical components and helps in improving the LPE process efficiency [51], [68]. Exfoliation yield is majorly dependent on sonication power. For investigating the impact of sonication power, graphene is subjected to various power levels of ultrasonic treatment.
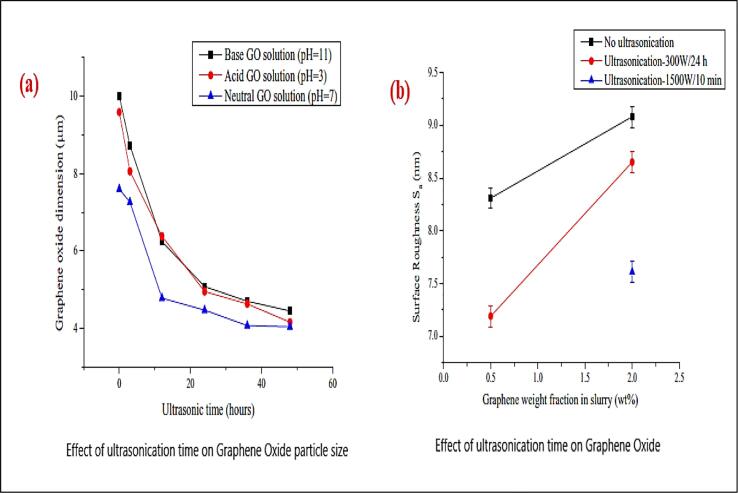
According to author Liu, Chen [69] studies, ultrasonication process is potential in breaking the particles and be able to disperse in a better way, the Fig. 5. indicates that the particle size has been decreased in increasing the sonication time and the smallest particle size of graphene oxide is obtained at 48 hrs. The Fig. 5a showing the GO particles reducing their size as the sonication time increases. It was also observed that the neutral pH nanofluids have better dispersion compared to acidic and basic nanofluids. The Fig. 5b. also depicts the surface roughness influenced by sonication. further, ultrasonication power effect also presented in Fig. 5(b) showed the better mixing with respect to nanoparticle roughness with 10 mins and 1500 W power. also, the conclusion is made that material removal rate is directly proportional to ultrasonication time and it influences the particle size reduction.
Fig. 5.
An effect of ultrasonication time and energy on particle size Liu, Chen [59]
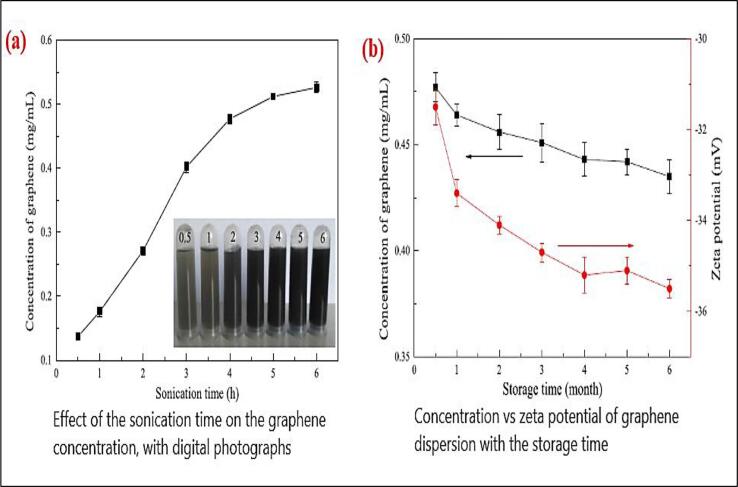
The effect on dispersions of graphene concentration due to ultrasonication time are presented in Fig. 6. Concentration and sonication time have direct relation until reached 4 h, consequently the concentration decreased [70]. The graphene flakes suspended in water, at 30 mins to 4 hrs the concentration values are 0.137–0.4 mg/ml. Also mentioned that long ultrasonication time decreased the size of the flakes. The stability of graphene nanofluid is observed for one month and evaluate as stable with little agglomeration at the bottom after several days as shown in Fig. 6(b). and this stability was confirmed by the zeta potential analysis by the value as −35.5mv after 180 days, medium or more sized flakes were unstable after 6 months with −25mv of zeta value. This kind of similar trend is also observed by Lotya, Hernandez [71]. Hadi, Zahirifar [72] experimentally performed ultrasonic treatment between 240 w and 600 w and exfoliated yield obtained is about 8.07% and 19.63%. High sonication power has resulted in low quality exfoliated graphene which may affect the NPs. Skaltsas, Ke [73] studied the effect of Graphite flakes addition with dichlorobenzene and N-methylpyrrolidone solvent and sonicated at various times (5mins to 60mins) at diverse sonication powers 20 W & 40 W. Sonication power and time has impact on exfoliated graphene output as a prolonged Ultrasonication resulted in loss of Oxygen. Baig, Mamat [74] in his study evaluated tip sonication influence on the carbon structure of GNP characterization by varying sonication time 1 to 120 mins and sonication power. With increase of sonication time, GNP size is reduced. The imperfection proportion and horizontal size of sonicated test samples affirm that GNPs experience a change from change state to nano-crystalline stage up to 60 min sonication time at all amplitudes. Yu, Hermann [75] found that sonication power impacted SWCNT scattering than sonication time. SWCNT with water sodium deoxycholate is used as surfactant.
Fig. 6.
a) An effect of ultrasonication time on concentration of graphene b) stability over a month w.r.t concentration of graphene Zhang, Zhang [60]
3. Effect of ultrasonication on graphene nanofluids
3.1. Ultrasonication effect on UV–Vis spectroscopy and zeta potential
Xian, Sidik [55] investigated the optimal impacts of several surface-active agent and ultrasonication time by selecting the highly stable nanofluid with possible less sonication time to evaluate the thermo-physical properties of GnP-TiO2 particles and concluded that 90 mins of the sonication procedure with surfactants CTAB/SDBS resulted in stable nanofluid with UV–Vis and visual analysis. Krishnamoorthy, Kim [76] used ultrasound irradiation for exfoliating graphite oxide to graphene oxide and sonication was performed for 30mins. In this research, author characterized the synthesized graphene sheets using FTIR, UV–Vis, Raman spectroscopy, TEM and XPS techniques. Graphene nanosheets formation was confirmed by UV–vis spectroscopy when absorption peak was shifted due to reduced GO. XPS and FT-IR techniques confirmed the oxygenated functional groups removal. Raman helped to study new SP2 carbon atoms in graphene sheets. Hadi, Zahirifar [72] Sonication duration has been varied between 30 and 120 mins. With increase in ultrasonication, the yield enhanced from 17.21 to 20.84%. Graphene exfoliation yield increased by 19.63% when sonication power raised to 600 W. In TEM images, Fe3O4 is observed on graphene surface. Raman spectroscopy, XRD, FTIR confirmed less impurities and presence of Fe3O4 affected exfoliation of graphite flakes. Durge, Kshirsagar [77] authors performed bath and probe sonication and prepared graphene using liquid phase exfoliation by around 60–120 mins sonication. Graphene suspension was stable for more than 30 days using low power and high power sonicators. UV–vis spectroscopy results confirmed high power probe sonication yielded high exfoliation. TEM spectroscopy confirmed the hexagonal structure of graphene.
Mehrali, Sadeghinezhad [78] experimentally investigated and confirmed that prepared NDG nanofluid is stable for 60 mins ultrasonication. Triton X-100 is used as surfactant which were stable for 6 months. UV–vis is performed to identify the stability and FESEM used for identification of NDG microstructures. XPS spectrum values showed peaks values at 284.2, 399.3, and 532 eV showing the incorporation of nitrogen within the graphene. Arao and Kubouchi [79] Triton x100 surfactant is used for stabilizing FLG. High power (600 W) ultrasonication has been performed for 1hr and centrifugation (1500 rpm) is used for dispersing the thick flakes. Raman spectroscopy and TEM are performed and confirmed FLG presence in Graphene. GNPs dispersion performance has been observed by Wang, Jiang [80] performing UV vis absorbance test and found that for minimum sonication time for dispersion of GNP is 20 mins and it provided best result at 80 mins. It is also noticed that the concentration of GNP is decreased gradually with increase in sedimentation time.
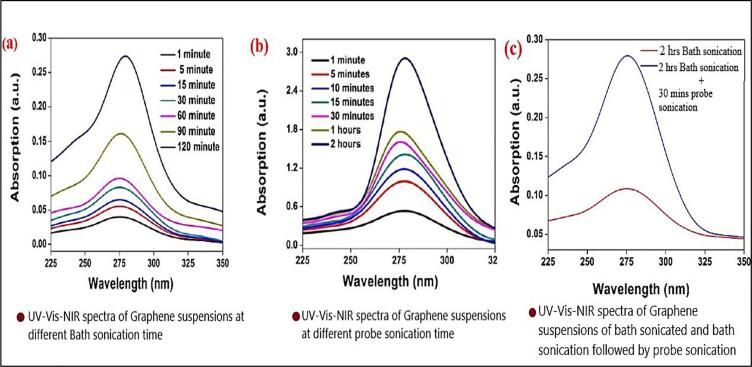
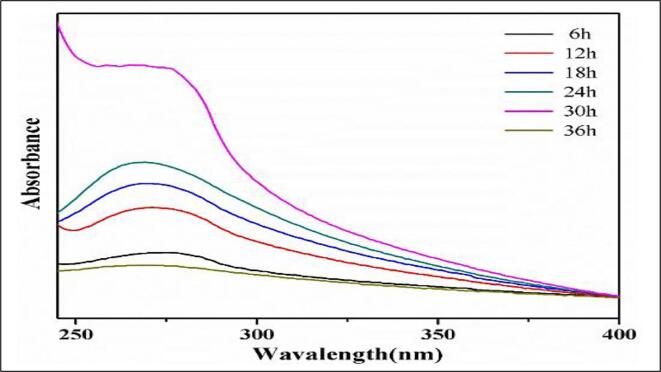
The large flake size of the graphite is reduced with the sonication effect and the graphene is properly dispersed in the base fluid by increasing the power of the sonicator [77]. The author used different sonication methods such as bath and probe sonication to disperse the graphene fluids in the solvent as shown in Fig. 7. and confirmed that the best way to obtain greater than 30 days stable nanofluid is bath sonication later followed by probe sonication process. Fig. 8. shows the absorption spectra of graphene at different sonication time ranging from 6 to 36 h, for the reason of heavy ultraviolet absorption there is a different level of absorbance graph seen at 30 hr timed sonication, Author [81] confirmed the increased thermal stability with the 24hrs sonicated graphene nano fluid. UV–visible spectrophotometry proved the formation of the nanocomposite along with TEM images that confirmed the decoration of the GO sheets with the rod-like and spherical CuO nanoparticles. Augmentation in the thermal conductivity of the GO-CuO nanocomposite based nanofluid prepared in water was found which is due to the advantageous effects of the ultrasound on the nanocomposite structure [53].
Fig. 7.
UV–Vis Observations of Graphene nanofluids with Sonication time Durge, Kshirsagar [72]
Fig. 8.
UV–vis absorption spectra image with ultrasonic time of Graphene Han, Li [91]
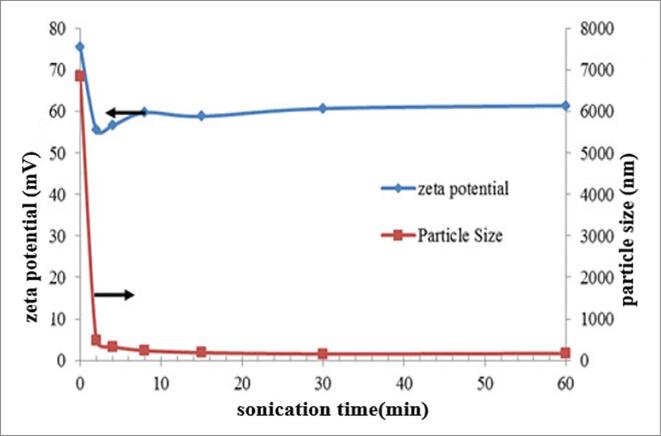
Fig. 9. shows zeta potential and size at various sonication times of Graphene Oxide. The graph represents the zeta potential value of more than 60 mV magnitude indicating high stability. There is a decrease in the value of zetapotential after probe sonication for 120 sec. The colloidal stability depends on the different size of the particles. Further, sonication with same power have minimum impact because of the smaller size on the Graphene oxide particle on stability. During the sonication process Graphene oxide particles break and easily disperse in the solvent and the particles charge density also drops in zetapotential [82]. Ramis [83] has studied the ultrasonication effect on stability of Graphite Nanofluids using Zeta potential Conducted two experiments by varying the ultrasonication time by 2hrs and 4hrs they have found that graphite ethylene glycol based nanofluid has obtained zeta potential –33.4 and −66.2 mV values. Larger duration of ultrasonication is required for higher stability.
Fig. 9.
Zeta potential and particle size effect with sonication time Kazi, Badarudin [93]
3.2. Analysis of ultrasonication effect by SEM
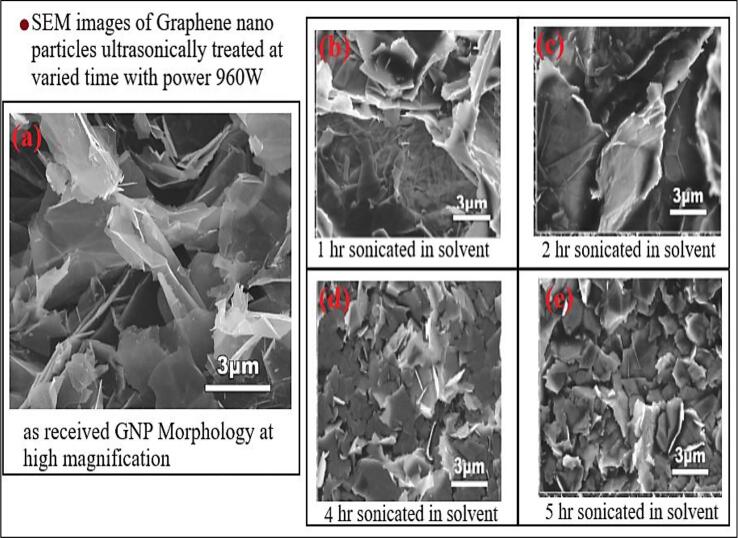
The effects of ultrasonication time, power, solvent and temperature are studied by the author Zhang and Chen [84] and the Fig. 10 (a). gives the images of SEM showing the graphene nanoparticles morphology before and after sonication, the amount of the agglomeration and size of the agglomerates are seen in the Fig. 10 (b) and started reducing in terms of agglomeration until the sonication time reaches 4 hrs, later to the prolonged time of sonication (5hrs), there is no change in the size of the particle but still can observe that the particles are more disturbed in comparison with Fig. 10 (d) & (e). To improve the dispersion of the particles in the fluid there is a need to maintain low temperature and viscosity. Ghozatloo, Rashidi [85] sonicated the nanofluid suspensions for 15 mins at 30 ˚C and investigated the flow and heat transfer enhancements. It was identified from the SEM analysis that graphene nanoparticles have a large surface area, and the morphology of the particle is affected by the concentration and the temperature of fluids in laminar flow conditions [74]. For determining the ultrasonication effect on the nanostructure, fracture surfaces and films were studied and analyzed using SEM analysis. GO/PVA composites ultrasonication effect is studied by and confirmed that ultrasonication pretreatment of GO/PVA effect has significant impact on GO/PVA mechanical properties [86]. Qi, Zhou [87] performed SEM analysis for studying the effect of ultrasonication of Graphene Oxide. SEM images are captured with and without sonication effect. After detailed investigation of SEM pictures author demonstrated that the normal size of the synthetically shed GO sheets can be constrained by changing the ultrasonication time to huge size GO sheets.
Fig. 10.
SEM images of Graphene particles in solvent with respect to ultrasonication time Zhang and Chen [95].
Xu, Wang [88] analyzed Graphene oxide Crystal Nanofibril treated films SEM analysis has been carried out after 45 mins of ultrasonication and it has been observed that SEM picture examinations delineated that the GO was scattered generally inside the CNF grid after ultrasonication and that the GO communicated with CNF through intermolecular bonds. Durge, Kshirsagar [77] studied that graphene suspension probe which was sonicated for 120 mins displayed less layer of graphene nanosheets whereas bath sonicated for 60 mins showed more graphene nanosheets. They clearly obtained the graphene flakes on the SEM image of graphene nanosheets. Wang, Wu [89] studied the graphene nanoplatelets with water and ethylene glycol at different concentrations and stirred for 30 mins & sonicated for 2 h and studied the shape and distribution of particles and validated the stability of the nanofluids, with no agglomeration in visual inspection.
3.3. Analysis of ultrasonication effect by TEM
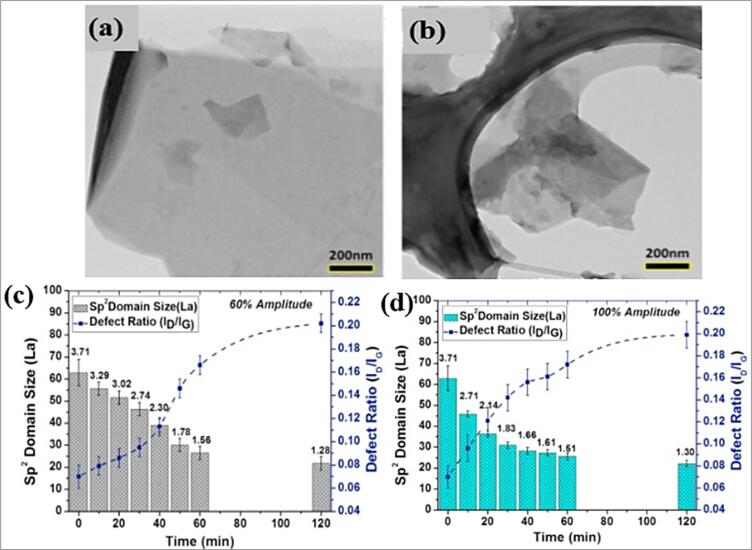
To understand the graphene nanoplatelets quality after the sonication, the TEM analysis is performed with the samples of different amplitude from 60 to 100%, the Fig. 11. show the smooth edges with quality at different amplitudes with no visible damage Fig. 11 (c & d) explains the defect ratio at different amplitudes and the flake size is also reduced with sonication time and power, but the author suggested with 60% amplitude to avoid the cavitation in higher level and to produce controlled size of particles in base fluid [74]. Durge, Kshirsagar [77] studied the effect of bath and probe sonication of Graphene nanosheets by TEM analysis and confirmed that exfoliation of Graphene nanosheets took place and wrinkles are observed on the graphene nanosheets. synthesized multilayer graphene and sonicated at 15,30 and 45 mins and observed the effects of Sonication time on Graphene properties. High resolution TEM image has been captured at 10 k and 50 k magnitude and observed multilayer graphene sonicated at 45mins appeared to have semi-transparent and thin sheets and confirmed that with increase of sonication time graphene sheets produced became more thinner due to continuous exposure of solution at constant shear stress. Ling, Yee [90] synthesized multilayer graphene and sonicated at 15,30 and 45 mins and observed the effects of Sonication time on Graphene properties. High resolution TEM image has been captured at 10 k and 50 k magnitude and observed multilayer graphene sonicated at 45mins appeared to have semi-transparent and thin sheets and confirmed that with increase of sonication time graphene sheets produced became more thinner due to continuous exposure of solution at constant shear stress. Hadi, Zahirifar [72] exfoliated graphene at 240w to 600w of sonication power and observed TEM image of FE3O4 particles. Due to high sonication power presence, the produced yield has good quality of graphene layers and TEM image confirmed the exfoliation of graphite using ultrasonication via NMP solvent. RGO has been ultrasonicated and data exhibit acquired displayed that RGO sheets comprise of scarcely any layers (n < 6) stacked each other with less wrinkles and collapsing [91]. HRTEM images did not show the COOH functional group but did show a few variations in surface deterioration, which may be evidence of successful functionalization of covalent. Some transparent flakes in the HRTEM images have shown that the GNPs have few layered structures [30]. The volume concentration is highly dependent as it increases the temperature relating thermal conductivity rises. Denser the nanofluid concentration number of intermolecular collisions is increasing accordingly. The maximum recorded thermal conductivity was 33.9% with 1% volume fraction and 50˚c [92]. Graphene nanoplatelets are dispersed in 30 PPT saline media using SDS as a stabilizer agent and the ultrasonic homogenization is done by using probe sonicator for 1 h and the TEM analysis revealed that big sized agglomeration is decreased to small size [93].
Fig. 11.
TEM of Graphene particles after sonication (a)60%, (b)100% amplitude, (c) & (d) Analysis of size vs defect density ratio (ID/IG) with sonication time and amplitude (60% & 100%) [64].
4. Ultrasonication effect on thermophysical properties & heat transfer of graphene nanofluids
4.1. Thermal conductivity
Generally, the effective thermal conductivity value increases with increase of nanoparticles volume concentration. As the nanoparticle concentration is increased, the molecular distance among the nanoparticles decreases and this helps to enhance the value of thermal conductivity. In the case of Graphene nanofluids. The Higher sonication time would help in nanoparticles aggregation having small size. This is the main reason higher the sonication time and higher the thermal conductivity of the nanoparticle. Yashawantha, Afzal [94] examined the Graphite nanofluid properties by performing ultrasonication for 5 hrs. for various concentrations. Thermal conductivity calculated for < 100 nm and < 50 nm and obtained 9.02% and 24.46% enhancement. As the particle size is less, higher the Thermal conductivity and with more ultrasonication time, the prepared nanofluid is more stable. Thermal conductivity of developed rGO-Fe3O4 nanoparticles derived nanofluids with help of ultra sound reported an 83% enhancement with 0.2% concentration of the rGO-Fe3O4 at 40 °C [95]. The processing of water-based nanofluid comprising reduced nanocomposite fragments of graphene oxide-zinc oxide (rGO-ZnO) and rGO–TiO2 are synthesized by the ultrasound-assisted approach and concluded that the thermal conductivity was found to increase with both the concentration and temperature of the nanofluid and useful for high heat transfer rate applications [96], [97], [98].
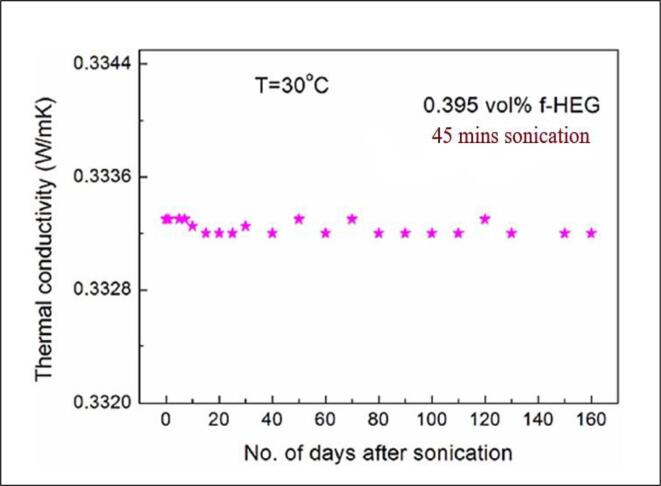
GNP nanofluids stability and thermal conductivity prepared by high power ultrasonication is experimentally investigated by Mehrali, Sadeghinezhad [99] and obtained 27.64% maximum enhancement at 0.1 wt% at 30C. Below Fig. 12. shows the Graphene thermal conductivity enhancement due to the result of ultrasonication time. 45mins of sonication time of graphene nanoparticles resulted in the thermal conductivity increase. The value decreased gradually as observed for period of 1–160 days. Arzani, Amiri [100] using sonicator for 1 h prepared functionalized GNPs nanofluid and observed that thermal conductivity increased with Ethylene glycol treated graphene nanoparticle. Zhang, Zhang [101] studied effects of ultrasonic power at 50 W,40Khz of electrodeposited nickel and graphene oxide at various concentrations and identified that hardness increased with increase of ultrasonication power by 4.4times and thermal conductivity of nanofluid increased.
Fig. 12.
Thermal Conductivity of the graphene stability after sonication, Kole and Dey [76]
The thermal conductivity of the nanofluids according to the research available, have a direct effect with the sonication time and there is a desirable point to achieve good stable dispersion, later the particle starts deteriorating. The sequence of experimentation has been conducted by few authors by different sonication techniques like probe sonicator and bath sonicator by adding surfactant to find out the effective thermal conductivity on graphene nanoparticles by different solvents and concluded that by using the ultrasonic probe there will be improved thermal conductivity when compared with the ultrasonication bath. Few sets of studies in the literature listed below in Table 2 reveals that the thermal conductivity increases with a limit in sonication time and later the thermal conductivity reduction takes place. With the limited conducted research on the sonication time of graphene there is a need to conduct series of experiments to get the optimum sonication time for graphene based nanofluids to deliver a conclusion to apply the ultrasonication process.
Table 2.
Thermal conductivity of graphene base nanofluids with respect to ultrasonication period.
| NANOPARTICLE | CONCENTRATION | SONICATION DETAILS | SURFACTANTS | THERMAL CONDUCTIVITY | REFERENCES | |||
|---|---|---|---|---|---|---|---|---|
| 30 ˚C | 40 ˚C | 50 ˚C | 60 ˚C | |||||
| Graphene | 0.05% | Stirring & 30 mins ultrasonication vibration | SDBS | 0.705 | 0.727 | 0.76 | N/A | [109] |
| 0.1% | 0.725 | 0.765 | 0.805 | |||||
| 0.15% | 0.785 | 0.825 | 0.877 | |||||
| Graphene nanoplatelets | 0.1 wt% | 60 mins ultrasonication | SDS | 0.559 | 0.618 | N/A | [110] | |
| CTAB | 0.635 | 0.648 | ||||||
| SDBS | 0.64 | 0.66 | ||||||
| Gum Arabic | 0.645 | 0.676 | ||||||
| Graphene nanoplatelets | 0.01 wt% | 10 mins sonication | – | 0.31 | 0.34 | 0.36 | 0.37 | [70] |
| 0.05 wt% | 0.38 | 0.4 | 0.41 | 0.42 | ||||
| 0.1 wt% | 0.43 | 0.44 | 0.45 | 0.46 | ||||
| 0.2 wt% | 0.46 | 0.48 | 0.5 | 0.52 | ||||
| Graphene | 0.05% | 30–45 mins | – | 1.02 | 1.019 | 1.03 | N/A | [73] |
| 0.08% | 1.052 | 1.066 | 1.078 | |||||
| Graphene oxide | 0.1 wt% | 40mins sonication | SDS | 0.63 | 0.65 | N/A | [75] | |
| Triton X-100 | 0.62 | 0.64 | ||||||
| Graphene | 0.124% | 45mins sonication | Not used | 0.315 | 0.318 | 0.319 | 0.325 | [76] |
| 0.207% | 0.324 | 0.327 | 0.33 | 0.339 | ||||
| 0.395% | 0.335 | 0.339 | 0.342 | 0.345 | ||||
| Graphene nanoplatelets | 0.02 wt% | Magnetic stirring 10hrs, 5hrs sonication | Gum acacia | 0.63 | 0.66 | N/A | [83] | |
| 0.1 wt% | 0.72 | 0.77 | ||||||
| Carboxyl graphene | 0.04% | 40 mins stirring, 9 h sonication | SDS | N/A | 0.373 | 0.395 | N/A | [1 1 1] |
| Graphene nanoparticles (750 m2/g) | 0.025 wt% | Probe sonicator 1200 W ,20 kHz | Not used | 0.68 | 0.71 | N/A | [106] | |
| 0.05 wt% | 0.71 | 0.75 | ||||||
| 0.1 wt% | 0.75 | 0.8 | ||||||
| Graphene NP-Ag | 0.2% | Ultrasonication bath-3hrs | Not used/Acid treatment | 0.63 | 0.651 | N/A | [1 1 2] | |
| 1.0% | 0.72 | 0.77 | ||||||
| Graphene nano-platelets | 0.1% | 20 mins stirring, 10mins sonication | NPE 400 (ionic) | 0.5 | 0.51 | 0.525 | N/A | [85] |
| 0.2% | 0.54 | 0.55 | 0.565 | |||||
| 0.3% | 0.62 | 0.64 | 0.66 | |||||
| Graphene nanoplatelets | 0.01% | 30mins sonication | Gum Arabic | 0.63 | 0.64 | 0.657 | 0.663 | [1 1 3] |
| 0.05% | 0.64 | 0.642 | 0.67 | 0.682 | ||||
| 0.1% | 0.641 | 0.68 | 0.7 | 0.712 | ||||
| Graphene nanoplatelets | 0.1% | 70 mins | Not used | 0.187 | 0.18 | 0.179 | 0.17 | [82] |
| 0.25% | 0.20 | 0.20 | 0.199 | 0.19 | ||||
| 0.5% | 0.215 | 0.213 | 0.21 | 0.209 | ||||
| Graphene | 0.3% | 40 mins | SDS | 0.586 | 0.606 | N/A | [81] | |
| 0.05% | 0.626 | 0.636 | ||||||
| 0.611 | 0.62 | |||||||
| 0.1% | SDBS | 0.605 | 0.613 | |||||
| 0.613 | 0.617 | |||||||
| 0.610 | 0.6175 | |||||||
| Graphene nanoparticles | 0.25 wt% | 240mins sonication bath with vibrations | SDBS | 0.40 | 0.405 | 0.419 | 0.42 | [80] |
| 0.5 wt% | 0.41 | 0.415 | 0.421 | 0.43 | ||||
| 1.0 wt% | 0.42 | 0.425 | 0.435 | 0.44 | ||||
4.2. Viscosity & density
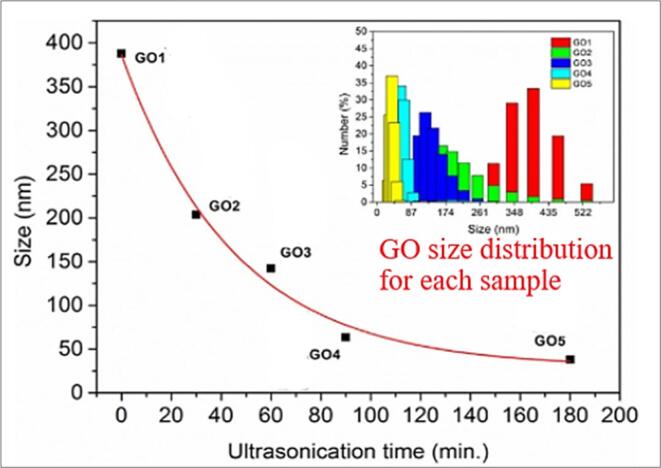
Effects of the sonication time on the viscosity of graphene nanofluids are reported by very few authors that increase in sonication time is decrease in viscosity gradually, the main conclusion towards this article leads to decrease in viscosity is possible by sonication time and size of the particle. According to the engineering applications the increase in viscosity will have the effect directly on the pressure drop and pumping power. Increase in the time of sonication can lead to the increase in thermal conductivity can in-turn increase the heat transfer performance of the applications, however the reduction in size of the particle due to the sonication process can vary the relative viscosity and the finding of the authors are discussed. The author [102] prepared the nano graphene oxide by using mechanical stirring and ultrasonication process to reduce the size of the sheets with respect to the time. The average size of the graphene oxide is reduced from 390 nm to 38 nm in Fig. 13. from GO1 to GO5, this was determined by using the DLS Dynamic light scattering procedure by considering the dynamic size of the particles. The analysis has revealed that over the treatment of time the size of the graphene sheets has been decreased. The reduction of the size over the sonication time from 20mins to 180 mins is discussed in the graph below.
Fig. 13.
Graphene Oxide sheets mean size vs ultra-sonication time, Gonçalves, Vila [1 1 4]
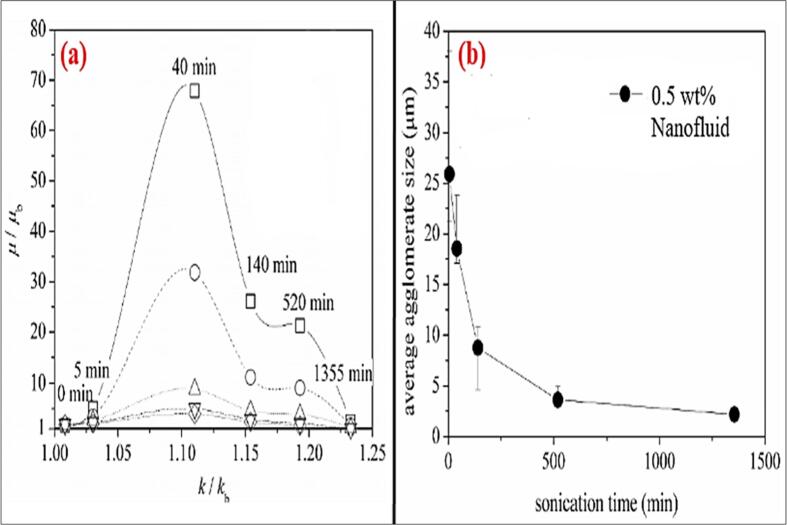
The effects of viscosity on the graphene nanoplatelets dispersion in the solvent is discussed by Zhang and Chen [84] and indicated that low viscosity of the solvent is also beneficial to the graphene nanoplatelets dispersions. The solvent or Graphene nanofluid with lower viscosity and temperatures benefits in better dispersion and can enhance the exfoliation. The role of a solvent in transferring the vibrational waves will increase with increase in the viscosity and results in the bubbles or cavitation formation [103]. Bahaya, Johnson [104] sonicated the graphene nano particles in the water solvent for 60 mins and measured the thermal conductivity and viscosity of the fluid with high volume concentration and concluded that viscosity plays a major role along with thermal conductivity in heat transfer applications for enhancement in heat transfer and stability. The rheological behavior of the carbon based nanofluid (MWCNT)is studied [64] at different sonication time ranging from 5 mins to 1355 mins. The Fig. 14(a). Shows that the viscosity started decreasing after 40 mins of sonication time with thermal conductivity, the higher thermal conductivity value and the lower viscosity value is able to be achieved with longest sonication time which are key to heat transfer applications with low agglomeration. In the Fig. 14(b). the agglomeration effect due to the sonication time is presented that the uniform distribution or the low agglomeration dispersion of nanofluids effects the thermal conductivity to increase. Graphene oxide nanofluids are prepared at different concentrations by dispersing in de-ionized water by 5 h of stirring and sonicating at a frequency of 42khz and power 130 W for 1–2 h and determined the flow properties of nanofluid and observed that there is an increase in the viscosity with increase in concentration to the fact of agglomeration formation and this can be broken into smaller agglomeration by sonication process resulting in less viscosity and higher thermal conductivity because of the reduced particle size. Thus, it is to conclude that to gain the nano suspension particles, ultrasonication time is important during the preparation process.
Fig. 14.
Increased viscosity of carbon based nanofluid with Thermal conductivity enhancement, Ruan and Jacobi [54]
There is a very limited study on the effect of ultrasonication time/power on density. In general, there will be a straight connection between density and sonication like increasing the ultrasonication time the nanofluid density increases [105] . The density can be measured for the fluids without any consideration to the agglomerated particles and the concentration of the particles are affected by the sonication time with regards to the flake size by the empirical law (C = Kt ½) [106]. Sundar, Ramanathan [107] have estimated the density of nanofluids at various temperatures and concluded that density decreased with temperature increase and decreased particle size [30]. The size, shape, density, concentration, and sort of nanoparticle including the ultrasonication will affect the nanofluids stability. Density of hybrid nanofluid increased with decrease in temperature & volume concentration increase. GnP/water hybrid nanofluid enhanced by 12.5% from base fluid [108]. Graphene nanoplatelets addition leads to increase in viscosity and density with 13.3% higher. Additionally, there is an increase in pressure drop of the nanofluids with density [109]. The inhomogeneity image of the particles concludes agglomeration of particles caused by density of nanoparticle. The graphene nanoparticles defect density was determined by the Raman spectroscopy at various time/power of sonication. The smaller the defect ratio smaller the density defect [110].
5. Conclusion
Ultrasonication parameters have various effects on the nanofluids dispersion, thermophysical properties and the heat transfer performance of the fluids. At the optimum sonication point the better dispersions are possible and accurate and later results in the agglomeration. This dispersion quality, agglomeration size and stability are dependent on various factors like nano particle size and properties, solvent/base fluid properties, weight or volume concentration, type, and power of ultrasonicator. Increase in power/time enhances the exfoliation/dispersion but can give impact on the carbon atom dissemination and degree. several characterization techniques to understand the stability and qualitative methods are identified and discussed. UV–Vis, Zeta potential, SEM, TEM, DLS, & FTIR are few among those techniques. Stability also dependent on the type of solvent and surfactant used for graphene nanoparticle. graphene nanoparticles are more stable over months with surfactants when compared with without surfactants, choosing of proper surfactant in accurate amounts decreases the foam formation, decrease the viscosity, pressure drop & increase the stability.
-
•
This literature Survey indicated that thermophysical properties effects by sonication time and power, thermal conductivity, being one of the thermal properties of the nanofluid is proportional to the ultrasonication time and power. It is also reported that after prolonged time the thermal conductivity value starts decreasing dramatically. Several researchers reported that after reaching the optimum time, there is no change in particle size, but they might result in the better dispersion since the size of the particle is reduced. The review also indicated that heat transfer increases as an effect of increase in time and power of sonication (since direct correlation with thermal properties of the fluid).
-
•
The impact of the ultrasonication procedure is affects the size of nanoparticle and size of agglomerates in the base fluid, heavy cluster particles will break down during the direct sonication process and reduces the cluster size. Moreover, the stability of the particle depends on the size of the particle thus the vibrator effects are peculiar.
-
•
For viscosity according to the literature to graphene particle the trend is similar to thermal conductivity, that after reaching certain level of sonication point the viscosity have no change or no decreasing trend as there is no reduction in particle size, a very few research articles revealed density of the nanofluids increased with sonication hours.
However, the optimum points of sonication are different for various nanofluids and there is no particular report for graphene nanofluids stating the parameters of sonication process like type of sonicator, tip diameter and material of probe, power, and pulse etc., there is a lack of study in terms of dispersion and agglomeration with effect of sonicator on graphene nanofluids or hybrid nanoparticles in fluids which have more possibilities of phobic nature. Research can also be focused on pH, surfactants & temperature effect on the nanofluids to decide the optimum hour of sonication. The future work may consider on the sonicator probe tip size and material to be used for probes will be great impact for the readers.
CRediT authorship contribution statement
Madderla Sandhya: Conceptualization, Visualization, Investigation, Data curation, Writing - original draft, Writing - review & editing. D. Ramasamy: Supervision, Writing - review & editing. K. Sudhakar: Writing - review & editing. K. Kadirgama: Writing - review & editing. W.S.W. Harun: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank University Malaysia Pahang (UMP) for Grant RDU 190194, FRGS/1/2018/TK03/UMP/02/26, and UMP Flagship RDU192204 for financial assistance and facilities provided.
References
- 1.Choi S.U., Eastman J.A. Argonne National Lab; IL (United States): 1995. Enhancing thermal conductivity of fluids with nanoparticles. [Google Scholar]
- 2.Putra N., Roetzel W., Das S.K. Natural convection of nano-fluids. Heat Mass Transf. 2003;39(8–9):775–784. [Google Scholar]
- 3.Presser V., Heon M., Gogotsi Y. Carbide-derived carbons–from porous networks to nanotubes and graphene. Adv. Funct. Mater. 2011;21(5):810–833. [Google Scholar]
- 4.Mohanraj V., Chen Y. Nanoparticles-a review. Trop. J. Pharm. Res. 2006;5(1):561–573. [Google Scholar]
- 5.Sadeghinezhad E. A comprehensive review on graphene nanofluids: recent research, development and applications. Energy Convers. Manage. 2016;111:466–487. [Google Scholar]
- 6.Rueda-Garcia D. Battery and supercapacitor materials in flow cells. Electrochemical energy storage in a LiFePO4/reduced graphene oxide aqueous nanofluid. Electrochim. Acta. 2018;281:594–600. [Google Scholar]
- 7.Mehrali M., Ghatkesar M.K., Pecnik R. Full-spectrum volumetric solar thermal conversion via graphene/silver hybrid plasmonic nanofluids. Appl. Energy. 2018;224:103–115. [Google Scholar]
- 8.Bahiraei M., Heshmatian S. Graphene family nanofluids: a critical review and future research directions. Energy Convers. Manage. 2019;196:1222–1256. [Google Scholar]
- 9.Fu Y. Investigation on enhancing effects of Au nanoparticles on solar steam generation in graphene oxide nanofluids. Appl. Therm. Eng. 2017;114:961–968. [Google Scholar]
- 10.Rueda-García D. From thermal to electroactive graphene nanofluids. Energies. 2019;12(23):4545. [Google Scholar]
- 11.Feng L., Liu Z. Graphene in biomedicine: opportunities and challenges. Nanomedicine. 2011;6(2):317–324. doi: 10.2217/nnm.10.158. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar J., Ghosh P., Adil A. A review on hybrid nanofluids: recent research, development and applications. Renew. Sustain. Energy Rev. 2015;43:164–177. [Google Scholar]
- 13.Gao J. Experimental investigation of heat conduction mechanisms in nanofluids. Clue on clustering. Nano letters. 2009;9(12):4128–4132. doi: 10.1021/nl902358m. [DOI] [PubMed] [Google Scholar]
- 14.Kralj S., Makovec D. Magnetic assembly of superparamagnetic iron oxide nanoparticle clusters into nanochains and nanobundles. ACS Nano. 2015;9(10):9700–9707. doi: 10.1021/acsnano.5b02328. [DOI] [PubMed] [Google Scholar]
- 15.Atif R., Inam F. Reasons and remedies for the agglomeration of multilayered graphene and carbon nanotubes in polymers. Beilstein J. Nanotechnol. 2016;7(1):1174–1196. doi: 10.3762/bjnano.7.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsnelson, M.I. and M.I. Kat︠s︡nelʹson, Graphene: carbon in two dimensions. 2012: Cambridge university press.
- 17.Yesibolati M.N. Unhindered Brownian Motion of Individual Nanoparticles in Liquid-Phase Scanning Transmission Electron Microscopy. Nano Lett. 2020;20(10):7108–7115. doi: 10.1021/acs.nanolett.0c02352. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y. Particle agglomeration and properties of nanofluids. J. Nanopart. Res. 2012;14(5):852. [Google Scholar]
- 19.Esfahani M.R., Languri E.M., Nunna M.R. Effect of particle size and viscosity on thermal conductivity enhancement of graphene oxide nanofluid. Int. Commun. Heat Mass Transfer. 2016;76:308–315. [Google Scholar]
- 20.Sadri R. An experimental study on thermal conductivity and viscosity of nanofluids containing carbon nanotubes. Nanoscale Res. Lett. 2014;9(1):151. doi: 10.1186/1556-276X-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai, Y. Research on hydrophobicity of graphene composites. in AIP Conference Proceedings. 2017. AIP Publishing LLC.
- 22.Hong G. On the mechanism of hydrophilicity of graphene. Nano Lett. 2016;16(7):4447–4453. doi: 10.1021/acs.nanolett.6b01594. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed J., Mulla M., Arfat Y.A. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocolloids. 2017;71:141–148. [Google Scholar]
- 24.Chen L. Enhanced epoxy/silica composites mechanical properties by introducing graphene oxide to the interface. ACS Appl. Mater. Interfaces. 2012;4(8):4398–4404. doi: 10.1021/am3010576. [DOI] [PubMed] [Google Scholar]
- 25.Gao X. Mechanical properties and thermal conductivity of graphene reinforced copper matrix composites. Powder Technol. 2016;301:601–607. [Google Scholar]
- 26.Rodríguez-Laguna M.D.R. Modification of the Raman spectra in graphene-based nanofluids and its correlation with thermal properties. Nanomaterials. 2019;9(5):804. doi: 10.3390/nano9050804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty S., Panigrahi P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020 [Google Scholar]
- 28.Sharafeldin, M., Effects of nanofluids on the performance of solar collectors. 2019.
- 29.Liu W. A novel comprehensive experimental study concerned graphene oxide nanoparticles dispersed in water: synthesise, characterisation, thermal conductivity measurement and present a new approach of RLSF neural network. Int. Commun. Heat Mass Transfer. 2019;109 [Google Scholar]
- 30.Karami H. The thermophysical properties and the stability of nanofluids containing carboxyl-functionalized graphene nano-platelets and multi-walled carbon nanotubes. Int. Commun. Heat Mass Transfer. 2019;108 [Google Scholar]
- 31.Mahbubul I. Influence of ultrasonication duration on rheological properties of nanofluid: an experimental study with alumina–water nanofluid. Int. Commun. Heat Mass Transfer. 2016;76:33–40. [Google Scholar]
- 32.Noroozi M., Radiman S., Zakaria A. Influence of sonication on the stability and thermal properties of Al2O3 nanofluids. Journal of Nanomaterials. 2014;2014 [Google Scholar]
- 33.Leena M., Srinivasan S. Synthesis and ultrasonic investigations of titanium oxide nanofluids. J. Mol. Liq. 2015;206:103–109. [Google Scholar]
- 34.Green A.A., Hersam M.C. Emerging methods for producing monodisperse graphene dispersions. The journal of physical chemistry letters. 2010;1(2):544–549. doi: 10.1021/jz900235f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Son Y., No Y., Kim J. Geometric and operational optimization of 20-kHz probe-type sonoreactor for enhancing sonochemical activity. Ultrason. Sonochem. 2020 doi: 10.1016/j.ultsonch.2020.105065. [DOI] [PubMed] [Google Scholar]
- 36.Toh S.Y. Graphene production via electrochemical reduction of graphene oxide: synthesis and characterisation. Chem. Eng. J. 2014;251:422–434. [Google Scholar]
- 37.Zhang W., He W., Jing X. Preparation of a stable graphene dispersion with high concentration by ultrasound. J. Phys. Chem. B. 2010;114(32):10368–10373. doi: 10.1021/jp1037443. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y.Y., Terentjev E.M. Dispersion of carbon nanotubes: mixing, sonication, stabilization, and composite properties. Polymers. 2012;4(1):275–295. [Google Scholar]
- 39.Szabo T., Maroni P., Szilagyi I. Size-dependent aggregation of graphene oxide. Carbon. 2020;160:145–155. [Google Scholar]
- 40.Patel C.M., Chakraborty M., Murthy Z. Preparation of fenofibrate nanoparticles by combined stirred media milling and ultrasonication method. Ultrason. Sonochem. 2014;21(3):1100–1107. doi: 10.1016/j.ultsonch.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Ilyas S.U., Pendyala R., Marneni N. Preparation, sedimentation, and agglomeration of nanofluids. Chem. Eng. Technol. 2014;37(12):2011–2021. [Google Scholar]
- 42.Hasan M. Biological entities as chemical reactors for synthesis of nanomaterials: Progress, challenges and future perspective. Mater. Today Chem. 2018;8:13–28. [Google Scholar]
- 43.Ponangi, B.R., et al. Effect of Carboxyl Graphene Nano-Coolant on the Performance of Radiator− A Numerical Study. in Proceedings of the 25th National and 3rd International ISHMT-ASTFE Heat and Mass Transfer Conference (IHMTC-2019). 2019. Begel House Inc.
- 44.Malekpour H. Thermal conductivity of graphene laminate. Nano Lett. 2014;14(9):5155–5161. doi: 10.1021/nl501996v. [DOI] [PubMed] [Google Scholar]
- 45.Teng C.-C. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon. 2011;49(15):5107–5116. [Google Scholar]
- 46.Im H., Kim J. Thermal conductivity of a graphene oxide–carbon nanotube hybrid/epoxy composite. Carbon. 2012;50(15):5429–5440. [Google Scholar]
- 47.Chen S. Thermal conductivity of isotopically modified graphene. Nat. Mater. 2012;11(3):203–207. doi: 10.1038/nmat3207. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh A.G. Dispersion stability of functionalized graphene in aqueous sodium dodecyl sulfate solutions. Langmuir. 2013;29(48):14831–14838. doi: 10.1021/la4035326. [DOI] [PubMed] [Google Scholar]
- 49.Tung V.C. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009;4(1):25. doi: 10.1038/nnano.2008.329. [DOI] [PubMed] [Google Scholar]
- 50.Lin L.-S. A practical characterisation protocol for liquid-phase synthesised heterogeneous graphene. Carbon. 2020 [Google Scholar]
- 51.Ciesielski A., Samori P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014;43(1):381–398. doi: 10.1039/c3cs60217f. [DOI] [PubMed] [Google Scholar]
- 52.Barai, D.P., B.A. Bhanvase, and V.K. Saharan, Reduced Graphene Oxide-Fe3O4 Nanocomposite Based Nanofluids: Study on Ultrasonic Assisted Synthesis, Thermal Conductivity, Rheology, and Convective Heat Transfer. Industrial & engineering chemistry process design and development, 2019.
- 53.Sarode H. Investigation on preparation of graphene oxide-CuO nanocomposite based nanofluids with the aid of ultrasound assisted method for intensified heat transfer properties. Mater. Chem. Phys. 2020 [Google Scholar]
- 54.Chawhan S.S., Barai D.P., Bhanvase B.A. Sonochemical preparation of rGO-SnO2 nanocomposite and its nanofluids: Characterization, thermal conductivity, rheological and convective heat transfer investigation. Materials Today. Communications. 2020 [Google Scholar]
- 55.Xian H.W., Sidik N.A.C., Saidur R. Impact of different surfactants and ultrasonication time on the stability and thermophysical properties of hybrid nanofluids. Int. Commun. Heat Mass Transfer. 2020;110 [Google Scholar]
- 56.Abd Malek M.N.F. Ultrasonication: a process intensification tool for methyl ester synthesis: a mini review. Biomass Convers. Biorefin. 2020:1–11. [Google Scholar]
- 57.Tyurnina A.V. Ultrasonic exfoliation of graphene in water: A key parameter study. Carbon. 2020;168:737–747. [Google Scholar]
- 58.Zakaria I. Experimental investigation of thermal conductivity and electrical conductivity of Al2O3 nanofluid in water-ethylene glycol mixture for proton exchange membrane fuel cell application. Int. Commun. Heat Mass Transfer. 2015;61:61–68. [Google Scholar]
- 59.Zhang Y. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008;42(8–9):2204–2212. doi: 10.1016/j.watres.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 60.Malek M.N.F.A. Ultrasonication: a process intensification tool for methyl ester synthesis: a mini review. Biomass Convers. Biorefin. 2020 [Google Scholar]
- 61.Bermúdez-Aguirre D., Mobbs T., Barbosa-Cánovas G.V. Ultrasound technologies for food and bioprocessing. Springer; 2011. Ultrasound applications in food processing; pp. 65–105. [Google Scholar]
- 62.Mouri S., Miyauchi Y., Matsuda K. Dispersion-process effects on the photoluminescence quantum yields of single-walled carbon nanotubes dispersed using aromatic polymers. The Journal of Physical Chemistry C. 2012;116(18):10282–10286. [Google Scholar]
- 63.Mahbubul I. Effect of ultrasonication duration on colloidal structure and viscosity of alumina–water nanofluid. Ind. Eng. Chem. Res. 2014;53(16):6677–6684. [Google Scholar]
- 64.Ruan B., Jacobi A.M. Ultrasonication effects on thermal and rheological properties of carbon nanotube suspensions. Nanoscale Res. Lett. 2012;7(1):1–14. doi: 10.1186/1556-276X-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daungthongsuk W., Wongwises S. A critical review of convective heat transfer of nanofluids. Renew. Sustain. Energy Rev. 2007;11(5):797–817. [Google Scholar]
- 66.Sun R. Graphene quantum dots and the resonance light scattering technique for trace analysis of phenol in different water samples. Talanta. 2014;125:341–346. doi: 10.1016/j.talanta.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Peres N. Light scattering by a medium with a spatially modulated optical conductivity: the case of graphene. J. Phys.: Condens. Matter. 2012;24(24) doi: 10.1088/0953-8984/24/24/245303. [DOI] [PubMed] [Google Scholar]
- 68.Arao Y., Mori F., Kubouchi M. Efficient solvent systems for improving production of few-layer graphene in liquid phase exfoliation. Carbon. 2017;118:18–24. [Google Scholar]
- 69.Liu H.-K., Chen C.-C.A., Chen W.-C. Diamond lapping of sapphire wafer with addition of graphene in slurry. Procedia Eng. 2017;184:156–162. [Google Scholar]
- 70.Zhang K. Direct exfoliation of graphite into graphene in aqueous solution using a novel surfactant obtained from used engine oil. J. Mater. Sci. 2018;53(4):2484–2496. [Google Scholar]
- 71.Lotya M. Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J. Am. Chem. Soc. 2009;131(10):3611–3620. doi: 10.1021/ja807449u. [DOI] [PubMed] [Google Scholar]
- 72.Hadi A. Graphene nanosheets preparation using magnetic nanoparticle assisted liquid phase exfoliation of graphite: the coupled effect of ultrasound and wedging nanoparticles. Ultrason. Sonochem. 2018;44:204–214. doi: 10.1016/j.ultsonch.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 73.Skaltsas T. Ultrasonication induces oxygenated species and defects onto exfoliated graphene. The Journal of Physical Chemistry C. 2013;117(44):23272–23278. [Google Scholar]
- 74.Baig Z. Investigation of tip sonication effects on structural quality of graphene nanoplatelets (GNPs) for superior solvent dispersion. Ultrason. Sonochem. 2018;45:133–149. doi: 10.1016/j.ultsonch.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Yu H. Optimizing sonication parameters for dispersion of single-walled carbon nanotubes. Chem. Phys. 2012;408:11–16. [Google Scholar]
- 76.Krishnamoorthy K., Kim G.-S., Kim S.J. Graphene nanosheets: ultrasound assisted synthesis and characterization. Ultrason. Sonochem. 2013;20(2):644–649. doi: 10.1016/j.ultsonch.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 77.Durge R., Kshirsagar R., Tambe P. Effect of sonication energy on the yield of graphene nanosheets by liquid-phase exfoliation of graphite. Procedia Eng. 2014;97:1457–1465. [Google Scholar]
- 78.Mehrali M. Preparation, characterization, viscosity, and thermal conductivity of nitrogen-doped graphene aqueous nanofluids. J. Mater. Sci. 2014;49(20):7156–7171. [Google Scholar]
- 79.Arao Y., Kubouchi M. High-rate production of few-layer graphene by high-power probe sonication. Carbon. 2015;95:802–808. [Google Scholar]
- 80.Wang B. Controlling dispersion of graphene nanoplatelets in aqueous solution by ultrasonic technique. Russ. J. Phys. Chem. A. 2017;91(8):1517–1526. [Google Scholar]
- 81.Han Y. Properties of soy protein isolate biopolymer film modified by graphene. Polymers. 2017;9(8):312. doi: 10.3390/polym9080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kazi S.N. Investigation on the use of graphene oxide as novel surfactant to stabilize weakly charged graphene nanoplatelets. Nanoscale Res. Lett. 2015;10(1):212. doi: 10.1186/s11671-015-0882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramis M.K., Yashawantha K.M., Asif A., Faisal U. Effect of ultrasonication duration on stability of Graphite nanofluids. International Journal of Mechanical and Production Engineering. 2018;6(2):2320–2392. [Google Scholar]
- 84.Zhang B., Chen T. Study of Ultrasonic Dispersion of Graphene Nanoplatelets. Materials. 2019;12(11):1757. doi: 10.3390/ma12111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghozatloo A., Rashidi A., Shariaty-Niassar M. Convective heat transfer enhancement of graphene nanofluids in shell and tube heat exchanger. Exp. Therm Fluid Sci. 2014;53:136–141. [Google Scholar]
- 86.Li Y. The effect of the ultrasonication pre-treatment of graphene oxide (GO) on the mechanical properties of GO/polyvinyl alcohol composites. Carbon. 2013;55:321–327. [Google Scholar]
- 87.Qi X. Size-specified graphene oxide sheets: ultrasonication assisted preparation and characterization. J. Mater. Sci. 2014;49(4):1785–1793. [Google Scholar]
- 88.Xu C. Effect of graphene oxide treatment on the properties of cellulose nanofibril films made of banana petiole fibers. BioResources. 2015;10(2):2809–2822. [Google Scholar]
- 89.Wang Z. Experimental comparative evaluation of a graphene nanofluid coolant in miniature plate heat exchanger. Int. J. Therm. Sci. 2018;130:148–156. [Google Scholar]
- 90.Ling, L.J., C.S. Yee, and M. Jaafar. Effect of sonication time on the properties of multilayer graphene. in AIP Conference Proceedings. 2017. AIP Publishing LLC.
- 91.Shalaby A. Structural analysis of reduced graphene oxide by transmission electron microscopy. Bul. Chem. Commun. 2015;47(1):291–295. [Google Scholar]
- 92.Taherialekouhi R., Rasouli S., Khosravi A. An experimental study on stability and thermal conductivity of water-graphene oxide/aluminum oxide nanoparticles as a cooling hybrid nanofluid. Int. J. Heat Mass Transf. 2019;145 [Google Scholar]
- 93.Ilyas S.U., Ridha S., Kareem F.A.A. Dispersion stability and surface tension of SDS-Stabilized saline nanofluids with graphene nanoplatelets. Colloids Surf., A. 2020;592 [Google Scholar]
- 94.Yashawantha K. Experimental Investigation on Physical and Thermal Properties of Graphite Nanofluids. AIP Conf. Proc. 2018 [Google Scholar]
- 95.Barai D.P., Bhanvase B.A., Saharan V.K. Reduced graphene oxide-Fe3O4 nanocomposite based nanofluids: study on ultrasonic assisted synthesis, thermal conductivity, rheology, and convective heat transfer. Ind. Eng. Chem. Res. 2019;58(19):8349–8369. [Google Scholar]
- 96.Mandhare H. Preparation and thermal conductivity investigation of reduced graphene oxide-ZnO nanocomposite-based nanofluid synthesised by ultrasound-assisted method. Mater. Res. Innovations. 2020:1–9. [Google Scholar]
- 97.Barai D., Bhanvase B.A., Sonawane S.H. A review on graphene derivatives based nanofluids: Investigation on properties and heat transfer characteristics. Ind. Eng. Chem. Res. 2020 [Google Scholar]
- 98.Koshta N.R. Investigation on the thermal conductivity and convective heat transfer enhancement in helical coiled heat exchanger using ultrasonically prepared rGO–TiO2 nanocomposite-based nanofluids. Indian Chem. Eng. 2020;62(2):202–215. [Google Scholar]
- 99.Mehrali M. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014;9(1):15. doi: 10.1186/1556-276X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arzani H.K. Toward improved heat transfer performance of annular heat exchangers with water/ethylene glycol-based nanofluids containing graphene nanoplatelets. J. Therm. Anal. Calorim. 2016;126(3):1427–1436. [Google Scholar]
- 101.Zhang H., Zhang N., Fang F. Fabrication of high-performance nickel/graphene oxide composite coatings using ultrasonic-assisted electrodeposition. Ultrason. Sonochem. 2020;62 doi: 10.1016/j.ultsonch.2019.104858. [DOI] [PubMed] [Google Scholar]
- 102.Gonçalves G. Breakdown into nanoscale of graphene oxide: confined hot spot atomic reduction and fragmentation. Sci. Rep. 2014;4(1):1–8. doi: 10.1038/srep06735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oroian M. Measurement, prediction and correlation of density, viscosity, surface tension and ultrasonic velocity of different honey types at different temperatures. J. Food Eng. 2013;119(1):167–172. [Google Scholar]
- 104.Bahaya B., Johnson D., Yavuzturk C. On the effect of graphene nanoplatelets on water–graphene nanofluid thermal conductivity, viscosity, and heat transfer under laminar external flow conditions. J. Heat Transfer. 2018;140(6) [Google Scholar]
- 105.Turner P. Controlled sonication as a route to in-situ graphene flake size control. Sci. Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-45059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bracamonte M.V. On the nature of defects in liquid-phase exfoliated graphene. The Journal of Physical Chemistry C. 2014;118(28):15455–15459. [Google Scholar]
- 107.Sundar L.S. Temperature dependent flow characteristics of Al2O3 nanofluid. International Journal of Nanotechnology and Applications. 2007;1(2):35–44. [Google Scholar]
- 108.Kishore P. Preparation, characterization and thermo-physical properties of Cu-graphene nanoplatelets hybrid nanofluids. Mater. Today:. Proc. 2020 [Google Scholar]
- 109.Balaji T. Enhanced heat transport behavior of micro channel heat sink with graphene based nanofluids. Int. Commun. Heat Mass Transfer. 2020;117 [Google Scholar]
- 110.Cai X. Effects of tip sonication parameters on liquid phase exfoliation of graphite into graphene nanoplatelets. Nanoscale Res. Lett. 2018;13(1):241. doi: 10.1186/s11671-018-2648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]