Abstract
Background and Objectives:
With obesity rates rising in the United States, bariatric surgery has become a well-established and effective treatment for morbid obesity and its comorbid conditions. Laparoscopic Roux-en-Y gastric bypass and laparoscopic Sleeve Gastrectomy are two of the more common bariatric procedures. This study analyzes whether gender differences play a role in procedure selection and outcomes following either procedure.
Methods:
Using the American College of Surgeons National Surgical Quality Improvement Program database for years 2015 to 2017, we assessed demographics, postoperative complications, and readmission rates. Chi-square analysis, student t-test, and propensity analyses were performed appropriately.
Results:
Data review found that men presenting for bariatric surgery had a higher incidence of comorbidities and higher body mass index than women. More men than women underwent Sleeve Gastrectomy (68.5% vs 63.0%, P <0.0001), while more women than men underwent Laparoscopic Roux-en-Y gastric bypass (37.0% vs 31.5%, P < 0.0001). In the Laparoscopic Roux-en-Y group, men experienced more postoperative complications, including cardiac arrest (0.2% vs 0.1%, P = 0.02) and unplanned intubation (0.4% vs 0.2%, P = 0.02). In the Sleeve Gastrectomy group, men experienced more postoperative complications, including myocardial infarction (0.2% vs 0.1%, P = 0.006). In both groups, women experienced higher rates of unplanned readmissions (3.5% vs 2.8%, P = 0.0012).
Conclusions:
This study found that men are more likely to undergo Sleeve Gastrectomy than Laparoscopic Roux-en-Y gastric bypass, despite higher complication rates for both. Women have higher rates of unplanned readmission rates regardless of procedure, despite lower postoperative morbidity.
Keywords: Bariatric surgery, Gender, Patient readmission, Complications
INTRODUCTION
As obesity prevalence continue to rise in the United States, bariatric surgery has become a well-established and effective option for the treatment of morbid obesity and its comorbid conditions. Laparoscopic Roux-en-Y gastric bypass (LRYGB) and laparoscopic sleeve gastrectomy (LSG) are two of the more common types of bariatric surgical procedures available to patients. During LRYGB, the duodenum, gastric fundus, and body of the stomach are bypassed, which results in restricted gastric capacity and reduced nutrient and caloric absorption.1 During LSG the greater curvature and fundus of the stomach are resected, essentially creating a tube of the stomach. In addition to the restriction in stomach size, the ghrelin-producing cells are resected during removal of the fundus of the stomach. LSG involves the resection of the greater curvature and fundus of the stomach, which eliminates ghrelin-producing cells and restricts the size of the stomach. As a result, patients experience weight loss due to both anatomical and endocrine changes.2
While there is extensive literature analyzing outcomes after bariatric surgery, including a well-established health profile of patients based upon gender,3–5 this study aims to examine the influence of gender upon procedure choice as well as postoperative complications and readmission rates.
METHODOLOGY
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) Participant User File database was used to identify patients who underwent either laparoscopic Roux-en-Y gastric bypass or laparoscopic sleeve gastrectomy between January 2015 and December 2017. Current Procedural Terminology codes for laparoscopic Roux-en-Y gastric bypass (43846, 43644, and 43645) and laparoscopic sleeve gastrectomy (43775) were used to identify patients who underwent primary bariatric surgery for obesity. Clinically relevant pre-operative comorbidities and postoperative events including complications and readmission related to primary operation were noted and reviewed. Major complications were defined as an occurrence of one of the following events: superficial, deep and organ space infection, wound dehiscence, renal insufficiency, renal failure, prolonged ventilation, pulmonary embolism, deep vein thrombosis, cardiac arrest, stroke, myocardial infarction, urinary tract infection, pneumonia, sepsis, and septic shock. All clinical factors in the ACS-NSQIP database are defined in the user guide. The ACS-NSQIP and participating hospitals are the sources of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Patients were grouped by gender into males and females. Categorical variables were analyzed between the two groups by chi-square test where appropriate. Propensity matching was used to determine selection of surgical procedure. Additionally, propensity score matching was used to evaluate 30-day hospital readmission rates and postoperative complications as independent variables with other clinically relevant pre-operative characteristics. This allowed us to make comparison groups that were otherwise similar. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
This study was reviewed and approved by the Institutional Review Board. A waiver of patient consent was granted.
RESULTS
Fewer Male Patients Underwent LRYGB
We identified 26,238 patients who underwent LRYGB and 51,830 patients who underwent LSG for morbid obesity during a three-year period. Overall, more men than women underwent LSG (68.5% vs 63.0%, P < 0.0001), while more women than men underwent LRYGB (37.0% vs 31.5%, P < 0.000; Figure 1). For patients with a body mass index (BMI) between 35 and 39 kg/m,2 more females than males underwent LRYGB (13.9% vs 11.7%, P < 0.0001) and LSG (16.3% vs 13.7%, P < 0.0001). This was also observed for patients with BMI of 40 to 49 kg/m,2 with more females than males undergoing LRYGB (47.8% vs 45.0%, P < 0.0003) and LSG (49.9% vs 46.5%, P < 0.0001). However, for patients with BMI ≥ 50 kg/m,2 more males than females underwent LRYGB (30.8% vs 24.6%, P < 0.0001) and LSG (27.6% vs 20.7%, P < 0.0001; Table 1 and Table 2).
Figure 1.
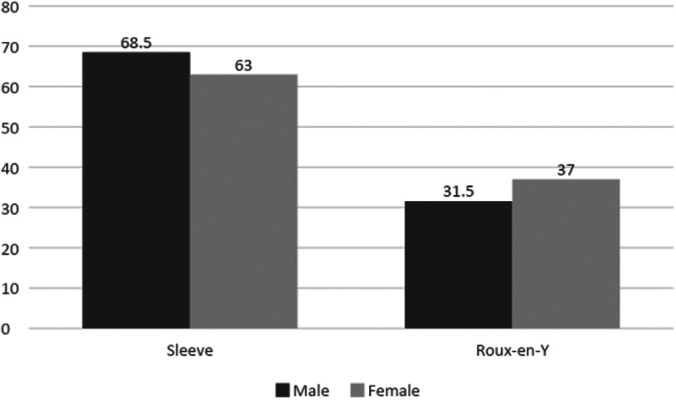
Percentage of Patients Undergoing Roux-en-Y Gastric Bypass (P < 0.0001) and Sleeve Gastrectomy (P < 0.0001) Based Upon Gender.
Table 1.
Demographics and Comorbidities of Patients Undergoing Laparoscopic Sleeve Gastrectomy
| Variable | Total (n = 51830) | Male (n = 10797) | Female (n = 41033) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age ≥ 65 years | 2690 (5.20%) | 835 (7.70%) | 1855 (4.50%) | <0.0001 |
| BMI 35 – 39 | 8151 (15.70%) | 1482 (13.70%) | 6669 (16.30%) | <0.0001 |
| BMI 40 – 49 | 25502 (49.20%) | 5025 (46.50%) | 20477 (49.90%) | <0.0001 |
| BMI ≥ 50 | 11452 (22.10%) | 2979 (27.60%) | 8473 (20.70%) | <0.0001 |
| Black | 9541 (18.40%) | 1245 (11.50%) | 8296 (20.20%) | <0.0001 |
| Native American | 186 (0.40%) | 36 (0.30%) | 150 (0.40%) | 0.62 |
| White | 34901 (67.30%) | 7876 (73.00%) | 27025 (65.9%) | <0.0001 |
| Hispanic | 5890 (11.40%) | 1114 (10.30%) | 4776 (11.60%) | 0.0001 |
| Smoker | 4776 (9.20%) | 1139 (10.60%) | 3637 (8.90%) | <0.0001 |
| Asian | 376 (0.70%) | 95 (0.90%) | 281 (0.70%) | 0.03 |
| Pacific Islander | 143 (0.30%) | 38 (0.40%) | 105 (0.30%) | 0.09 |
| Comorbidities | ||||
| Dyspnea | 6342 (12.20%) | 1412 (13.10%) | 4930 (12.00%) | 0.003 |
| Hypertension requiring medication | 23607 (45.60%) | 6455 (59.8%) | 17152 (41.80%) | <0.0001 |
| Diabetes | 11869 (22.90%) | 3313 (30.7%) | 8556 (20.90%) | <0.0001 |
| Chronic Obstructive Pulmonary Disease | 845 (1.60%) | 229 (2.1%) | 619 (1.50%) | <0.0001 |
| Ventilation | 8 (0.02%) | 2 (0.02%) | 6 (0.01%) | 0.77 |
| Ascites | 3 (0.01%) | 2 (0.02%) | 1 (0.01%) | 0.05 |
| Congestive Heart Failure | 194 (0.40%) | 88 (0.80%) | 106 (0.30%) | <0.0001 |
| Acute Renal Failure | 28 (0.10%) | 13 (0.10%) | 15 (0.04%) | 0.0008 |
| Currently on Dialysis | 240 (0.50%) | 98 (0.90%) | 142 (0.40%) | <0.0001 |
| Disseminated Cancer | 5 (0.01%) | 1 (0.01%) | 4 (0.01%) | 0.96 |
| Open Wound Infection | 116 (0.20%) | 57 (0.50%) | 59 (0.10%) | <0.0001 |
| Steroid Use (for chronic condition) | 1072 (2.10%) | 203 (1.90%) | 869 (2.10%) | 0.12 |
| >10% body weight loss | 26 (0.10%) | 7 (0.10%) | 19 (0.10%) | 0.44 |
| Bleeding Disorder | 503 (1.00%) | 175 (1.60%) | 328 (0.80%) | <0.0001 |
| Pre-operative Blood Transfusion | 6 (0.01%) | 1 (0.01%) | 5 (0.01%) | 0.80 |
BMI, body mass index in kg/m2; Smoker, current smoker within one year; Diabetic, diabetes mellitus requiring therapy with non-insulin agents or insulin; Ascites, ascites within 30 days prior to surgery; History of congestive heart failure within 30 days prior surgery; >10% body weight loss, greater than 10% loss in body weight in last 6 months; Pre-operative Blood Transfusion, pre-operative transfusion of greater than or equal to 1 unit of whole/packed red blood cells in 72 h prior to surgery.
Table 2.
Demographics and Comorbidities of Patients Undergoing Laparoscopic Roux-en-Y Gastric Bypass
| Variable | Total (n = 26238) | Male (n = 4962) | Female (n = 21276) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age ≥ 65 years | 1302 (5.00%) | 360 (7.30%) | 942 (4.40%) | <0.0001 |
| BMI 35 – 39 | 3533 (13.50%) | 578 (11.70%) | 2955 (13.90%) | <0.0001 |
| BMI 40 – 49 | 12405 (47.30%) | 2232 (45.00%) | 10173 (47.80%) | 0.0003 |
| BMI ≥ 50 | 6769 (25.80%) | 1526 (30.80%) | 5243 (24.60%) | <0.0001 |
| Black | 3428 (13.10%) | 500 (10.10%) | 2928 (13.80%) | <0.0001 |
| Native American | 92 (0.40%) | 7 (0.10%) | 85 (0.40%) | 0.01 |
| White | 17920 (68.30%) | 3553 (71.60%) | 14367 (67.50%) | <0.0001 |
| Hispanic | 2915 (11.10%) | 514 (10.40%) | 2401 (11.30%) | 0.06 |
| Smoker | 2189 (8.30%) | 413 (8.30%) | 1776 (8.40%) | 0.96 |
| Asian | 200 (0.80%) | 46 (0.90%) | 154 (0.70%) | 0.14 |
| Pacific Islander | 233 (0.90%) | 59 (1.20%) | 174 (0.80%) | 0.01 |
| Comorbidities | ||||
| Dyspnea | 3309 (12.60%) | 671 (13.50%) | 2638 (12.40%) | 0.03 |
| Hypertension requiring medication | 13018 (49.60%) | 3323 (67.00%) | 9695 (45.60%) | <0.0001 |
| Diabetes | 8473 (32.30%) | 2244 (45.20%) | 6229 (29.30%) | <0.0001 |
| Chronic Obstructive Pulmonary Disease | 520 (2.00%) | 131 (2.60%) | 389 (1.80%) | 0.0002 |
| Ventilation | 2 (0.01%) | 0 (0%) | 2 (0.01%) | 0.49 |
| Ascites | 4 (0.02%) | 1 (0.02%) | 3 (0.01%) | 0.76 |
| CHF | 85 (0.30%) | 34 (0.70%) | 51 (0.20%) | <0.0001 |
| Acute Renal Failure | 3 (0.01%) | 1 (0.02%) | 2 (0.01%) | 0.52 |
| Currently on Dialysis | 44 (0.20%) | 15 (0.30%) | 29 (0.1%) | 0.01 |
| Disseminated Cancer | 16 (0.10%) | 7 (0.10%) | 9 (0.04%) | 0.01 |
| Open Wound Infection | 83 (0.30%) | 37 (0.80%) | 46 (0.20%) | <0.0001 |
| Steroid Use (for chronic condition) | 429 (1.60%) | 58 (1.20%) | 371 (1.70%) | 0.004 |
| >10% Body Weight Loss | 36 (0.10%) | 12 (0.20%) | 24 (0.10%) | 0.03 |
| Bleeding Disorder | 258 (1.00%) | 69 (1.4%) | 189 (0.90%) | 0.0012 |
| Pre-operative Blood Transfusion | 6 (0.02%) | 3 (0.06%) | 3 (0.01%) | 0.05 |
BMI, body mass index in kg/m2; Smoker, current smoker within one year; Diabetic, diabetes mellitus requiring therapy with non-insulin agents or insulin; Ascites, ascites within 30 days prior to surgery; History of congestive heart failure within 30 days prior surgery; >10% body weight loss, greater than 10% loss in body weight in last 6 months; Pre-operative Blood Transfusion, pre-operative transfusion of greater than or equal to 1 unit of whole/packed red blood cells in 72 h prior to surgery.
Increased Comorbidities in the Male Population
In the LRYGB group, male patients had a higher incidence of comorbidities than female patients, including dyspnea (13.5% vs 12.4%, P = 0.03), hypertension (67.0% vs 45.6%, P < 0.0001), diabetes (45.2% vs 29.3%, P < 0.0001), chronic obstructive pulmonary disease (2.6% vs 1.8%, P 0.0002), congestive heart failure (0.7% vs 0.2%, P < 0.0001), dialysis (0.3% vs 0.1%, P = 0.01), cancer (0.1% vs 0.04%, P = 0.01), and bleeding disorders (1.4% vs 0.9%, P = 0.0012). Similarly for LSG, male patients had a higher incidence of comorbid conditions than female patients, including dyspnea (13.1% vs 12.0%, P = 0.003), hypertension (59.8% vs 41.8%, P < 0.0001), diabetes (30.7% vs 20.9%, P < 0.0001), chronic obstructive pulmonary disease (2.1% vs 1.5%, P < 0.0001), congestive heart failure (0.8% vs 0.3%, P < 0.0001), renal failure (0.1% vs 0.04%, P = 0.0008), dialysis (0.9% vs 0.4%, P < 0.0001), and bleeding disorders (1.6% vs 0.8%, P < 0.0001; Table 3 and Table 4).
Table 3.
Demographics and Comorbidities of the Propensity Matched Cohort After Laparoscopic Sleeve Gastrectomy
| Total (n = 21602) | Men (n = 10788) | Women (n = 10814) | p-value | |
|---|---|---|---|---|
| BMI 35 – 39 | 2963 (13.72%) | 1481 (13.73%) | 1482 (13.70%) | 0.96 |
| BMI 40 – 49 | 10063 (46.58%) | 5023 (46.56%) | 5040 (46.61%) | 0.95 |
| BMI ≥ 50 | 5942 (27.51%) | 2973 (27.56%) | 2969 (27.46%) | 0.87 |
| Elderly (age > 65 years) | 1682 (7.79%) | 833 (7.72%) | 849 (7.85%) | 0.72 |
| Black | 2480 (11.48%) | 1244 (11.53%) | 1236 (11.43%) | 0.81 |
| Native American | 69 (0.32%) | 36 (0.33%) | 33 (0.31%) | 0.71 |
| White | 15801 (73.15%) | 7870 (72.95%) | 7931 (73.34%) | 0.52 |
| Hispanic | 2234 (10.34%) | 1114 (10.33%) | 1120 (10.36%) | 0.94 |
| Asian | 182 (0.84%) | 95 (0.88%) | 87 (0.80%) | 0.54 |
| Pacific-Islander | 81 (0.37%) | 38 (0.35%) | 43 (0.40%) | 0.58 |
| Smoker | 2310 (10.69%) | 1138 (10.55%) | 1172 (10.84%) | 0.49 |
| Dyspnea | 2821 (13.06%) | 1411 (13.08%) | 1410 (13.04%) | 0.93 |
| Hypertension requiring medication | 12937 (59.89%) | 6446 (59.75%) | 6491 (60.02%) | 0.68 |
| Diabetic | 6665 (30.85%) | 3304 (30.63%) | 3361 (31.08%) | 0.47 |
| Chronic Obstructive Pulmonary Disease | 443 (2.05%) | 229 (2.12%) | 214 (1.98%) | 0.46 |
| Ventilator Dependent | 2 (0.01%) | 2 (0.02%) | 0 (0%) | 0.15 |
| Ascites | 2 (0.01%) | 2 (0.02%) | 0 (0%) | 0.15 |
| Congestive Heart Failure | 151 (0.70%) | 83 (0.77%) | 68 (0.63%) | 0.215 |
| Acute Renal Failure | 23 (0.11%) | 13 (0.12%) | 10 (0.09%) | 0.53 |
| Currently on Dialysis | 176 (0.81%) | 96 (0.89%) | 80 (0.74%) | 0.22 |
| Disseminated Cancer | 4 (0.02%) | 1 (0.01%) | 3 (0.03%) | 0.32 |
| Open Wound Infection | 92 (0.43%) | 52 (0.48%) | 40 (0.37%) | 0.21 |
| Steroid Use (for chronic condition) | 397 (1.84%) | 202 (1.87%) | 195 (1.80%) | 0.70 |
| >10% Body Weight Loss | 12 (0.06%) | 7 (0.06%) | 5 (0.05%) | 0.56 |
| Bleeding Disorder | 324 (1.50%) | 173 (1.60%) | 151 (1.40%) | 0.21 |
| Pre-operative Blood Transfusion | 2 (0.01%) | 1 (0.01%) | 1 (0.01%) | 0.99 |
BMI, body mass index in kg/m2; Smoker, current smoker within one year; Diabetic, diabetes mellitus requiring therapy with non-insulin agents or insulin; Ascites, ascites within 30 days prior to surgery; History of congestive heart failure within 30 days prior surgery; >10% body weight loss, greater than 10% loss in body weight in last 6 months; Pre-operative Blood Transfusion, pre-operative transfusion of greater than or equal to 1 unit of whole/packed red blood cells in 72 h prior to surgery.
Table 4.
Demographics and Comorbidities of the Propensity Matched Cohort After Laparoscopic Roux-en-Y Gastric Bypass
| Total (n = 9896) | Men (n = 4961) | Women (n = 4935) | p-value | |
|---|---|---|---|---|
| BMI 30 – 39 | 1157 (11.69%) | 578 (11.65%) | 579 (11.73%) | 0.89 |
| BMI 40 – 49 | 4445 (44.92%) | 2232 (44.99%) | 2213 (44.84%) | 0.88 |
| BMI ≥ 50 | 3056 (30.88%) | 1525 (30.74%) | 1531 (31.02%) | 0.76 |
| Elderly (age > 65 years) | 712 (7.19%) | 360 (3.64%) | 352 (7.13%) | 0.81 |
| Black | 991 (10.01%) | 500 (10.08%) | 491 (9.95%) | 0.83 |
| Native American | 15 (0.15%) | 7 (0.14%) | 8 (0.16%) | 0.79 |
| White | 7102 (71.77%) | 3552 (71.60%) | 3550 (71.94%) | 0.71 |
| Hispanic | 1005 (10.16%) | 514 (10.36%) | 491 (9.95%) | 0.5 |
| Asian | 76 (0.77%) | 46 (0.93%) | 30 (0.61%) | 0.068 |
| Smoker | 824 (8.33%) | 413 (8.32%) | 411 (8.33%) | 0.995 |
| Pacific Islander | 112 (1.13%) | 59 (1.19%) | 53 (1.07%) | 0.59 |
| Dyspnea | 1330 (13.44%) | 671 (13.53%) | 659 (13.35%) | 0.8 |
| Hypertension requiring medication | 6629 (66.99%) | 3322 (66.96%) | 3307 (67.01%) | 0.96 |
| Diabetic | 4467 (45.14%) | 2243 (45.21%) | 2224 (45.07%) | 0.88 |
| Chronic Obstructive Pulmonary Disease | 249 (2.52%) | 131 (2.64%) | 118 (2.39%) | 0.43 |
| Ascites | 2 (0.02%) | 1 (0.02%) | 1 (0.02%) | 0.997 |
| Congestive Heart Failure | 60 (0.61%) | 33 (0.67%) | 27 (0.55%) | 0.45 |
| Acute Renal Failure | 2 (0.02%) | 1 (0.02%) | 1 (0.02%) | 0.997 |
| Currently on Dialysis | 26 (0.26%) | 15 (0.30%) | 11 (0.22%) | 0.44 |
| Disseminated Cancer | 10 (0.10%) | 7 (0.14%) | 3 (0.06%) | 0.21 |
| Open Wound Infection | 61 (0.62%) | 36 (0.73%) | 25 (0.51%) | 0.16 |
| Steroid Use (for chronic condition) | 108 (1.09%) | 58 (1.17%) | 50 (1.01%) | 0.46 |
| >10% body weight loss | 19 (0.19%) | 12 (0.24%) | 7 (0.14%) | 0.26 |
| Bleeding Disorder | 124 (1.25%) | 69 (1.39%) | 55 (1.11%) | 0.22 |
| Pre-operative Blood Transfusion | 4 (0.04%) | 3 (0.06%) | 1 (0.02%) | 0.32 |
BMI, body mass index in kg/m2; Smoker, current smoker within one year; Diabetic, diabetes mellitus requiring therapy with non-insulin agents or insulin; Ascites, ascites within 30 days prior to surgery; History of congestive heart failure within 30 days prior surgery; >10% body weight loss, greater than 10% loss in body weight in last 6 months; Pre-operative Blood Transfusion, pre-operative transfusion of greater than or equal to 1 unit of whole/packed red blood cells in 72 h prior to surgery.
Increased Mortality Rates and Severe Complications in the Male Population
Individual propensity matching analysis also revealed differences in certain postoperative complications between male and female patients. In the LSG group, males had a higher 30-day mortality rate than females (0.15% vs 0.05%, P = 0.02), while females had higher rates of urinary tract infections (0.55% vs 0.24%, P = 0.00003). In the LRYGB group, males were more likely to experience cardiac arrest requiring cardiopulmonary resuscitation (0.2% vs 0.1%, P = 0.02), were more likely to require mechanical ventilation for greater than 48 hours (0.4% vs 0.2%, P = 0.02), and exhibited higher 30-day mortality rates (0.24% vs 0.1%, P = 0.09). Females in the LRYGB group had higher rates of urinary tract infections (1.0% vs 0.3%, P < 0.0001) and superficial surgical site infection (1.6% vs 1.1%, P = 0.04).
Increased Readmission Rates in the Female Population
Readmission rates for women were found to be higher after both LRYGB (5.51% vs. 4.39%, P = 0.01) and LSG (2.52% vs. 1.05%, P = 0.04; Table 5 and Table 6). Additionally, unplanned readmissions related to the principal procedure for both procedures combined is independently associated with female gender (3.5% vs 2.8%, P = 0.0012; Figure 1 and Figure 2).
Table 5.
Postoperative 30-Day Outcomes After Propensity Analysis for Laparoscopic Sleeve Gastrectomy
| Total (n = 21602) | Men (n = 10788) | Women (n = 10814) | p-value | |
|---|---|---|---|---|
| Superficial surgical site infection | 108 (0.50%) | 55 (0.51%) | 53 (0.49%) | 0.83 |
| Deep incisional surgical site infection | 7 (0.03%) | 2 (0.02%) | 5 (0.02%) | 0.26 |
| Organ space infection | 69 (0.32%) | 34 (0.32%) | 35 (0.32%) | 0.91 |
| Wound dehiscence | 7 (0.03%) | 3 (0.03%) | 4 (0.04%) | 0.70 |
| Pneumonia | 45 (0.21%) | 21 (0.19%) | 24 (0.22%) | 0.66 |
| Unplanned intubation | 42 (0.19%) | 25 (0.23%) | 17 (0.16%) | 0.21 |
| Pulmonary embolism | 36 (0.17%) | 20 (0.19%) | 16 (0.15%) | 0.50 |
| Acute renal failure | 20 (0.09%) | 9 (0.08%) | 11 (0.10%) | 0.66 |
| Urinary tract infection | 85 (0.39%) | 26 (0.24%) | 59 (0.55%) | 0.0003 |
| Cerebrovascular accident | 4 (0.02%) | 2 (0.02%) | 2 (0.02%) | 0.99 |
| Cardiac arrest | 16 (0.07%) | 9 (0.08%) | 7 (0.06%) | 0.60 |
| Blood loss requiring transfusion | 144 (0.67%) | 79 (0.73%) | 65 (0.60%) | 0.24 |
| Deep vein thrombosis | 73 (0.34%) | 41 (0.38%) | 32 (0.30%) | 0.29 |
| Sepsis | 41 (0.19%) | 19 (0.18%) | 22 (0.20%) | 0.64 |
| Reoperation | 216 (1.00%) | 111 (1.03%) | 105 (0.97%) | 0.66 |
| Unplanned readmission | 500 (2.31%) | 227 (1.05%) | 273 (2.52%) | 0.04 |
| 30-day mortality | 21 (0.10%) | 16 (0.15%) | 5 (0.05%) | 0.02 |
Table 6.
Postoperative 30-Day Outcomes After Propensity Analysis for Laparoscopic Roux-en-Y Gastric Bypass
| Total (n = 9896) | Men (n = 4961) | Women (n = 4935) | p-value | |
|---|---|---|---|---|
| Superficial surgical site infection | 136 (1.37%) | 56 (1.13%) | 80 (1.62%) | 0.04 |
| Deep incisional surgical site infection | 13 (0.13%) | 3 (0.06%) | 10 (0.20%) | 0.05 |
| Organ space infection | 84 (0.85%) | 39 (0.79%) | 45 (0.91%) | 0.50 |
| Wound dehiscence | 21 (0.21%) | 10 (0.20%) | 11 (0.22%) | 0.81 |
| Pneumonia | 59 (0.60%) | 35 (0.71%) | 24 (0.49%) | 0.16 |
| Unplanned intubation | 25 (0.25%) | 15 (0.30%) | 10 (0.20%) | 0.32 |
| Pulmonary embolism | 29 (0.29%) | 16 (0.32%) | 13 (0.26%) | 0.59 |
| Ventilation | 29 (0.29%) | 21 (0.42%) | 8 (0.16%) | 0.02 |
| Acute renal failure | 13 (0.13%) | 10 (0.20%) | 3 (0.06%) | 0.05 |
| Urinary tract infection | 65 (0.66%) | 15 (0.30%) | 50 (1.01%) | <0.001 |
| Cerebrovascular accident | 2 (0.02%) | 0 (0%) | 2 (0.04%) | 0.16 |
| Cardiac arrest | 15 (0.15%) | 12 (0.24%) | 3 (0.06%) | 0.02 |
| Blood loss requiring transfusion | 135 (1.36%) | 71 (1.43%) | 64 (1.30%) | 0.56 |
| Deep vein thrombosis | 35 (0.35%) | 21 (0.42%) | 14 (0.28%) | 0.24 |
| Sepsis | 46 (0.46%) | 26 (0.52%) | 20 (0.41%) | 0.38 |
| Reoperation | 245 (2.48%) | 127 (2.56%) | 118 (2.39%) | 0.59 |
| Unplanned readmission | 490 (4.95%) | 218 (4.39%) | 272 (5.51%) | 0.01 |
| 30-day mortality | 17 (0.17%) | 12 (0.24%) | 5 (0.10%) | 0.09 |
Figure 2.
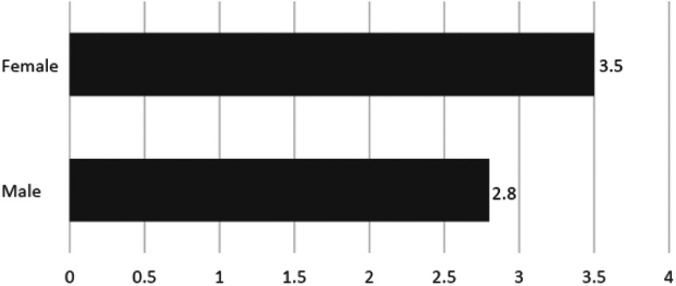
Percentage of Patients with Unplanned Readmission Related to Initial Procedure (P = 0.0012) Based Upon Gender.
DISCUSSION
This study shows that males undergoing bariatric surgery have more comorbid conditions and are less likely to undergo LRYGB. They have an increased incidence of postoperative cardiac arrest, prolonged ventilation, and 30-day mortality. Despite this, females are more likely to be readmitted after bariatric surgery. This paradox has not been demonstrated in previous studies.
Although men account for nearly half of the obese population in the United States, it is well established that significantly fewer men undergo bariatric surgery overall.6 Our study shows that this is true for bariatric patients with BMI between 35 kg/m2 and 49 kg/m.2 However, for BMI ≥ 50 kg/m,2 males make up a greater percentage of bariatric patients. Prior publications examining gender distribution in bariatric surgery have shown that patients are more likely to be female if from a lower-income neighborhood or if African American or Hispanic.6 The present study identifies BMI as a factor which significantly impacts gender distribution among bariatric patients.
The literature has shown that there is increasing popularity of LSG over other bariatric surgical procedures given the lower rate of complications when compared with LRYGB.7–9 Thirty day postoperative mortality and composite adverse events after LSG have been reported to be as low as 0.05% and 2.4% respectively.10 It has also been reported that patients experience less frequent hospital readmissions after LSG than LRYGB.7–9,11,12 The decision to recommend LSG to male patients is well documented in the current literature and after matching for BMI and comorbidities across both genders, men are more likely to undergo LSG than LRYGB than females.11 However, despite more males undergoing LSG, higher rates of morbidity and mortality persist in this population when compared to female patients.7,10,13,14 In fact, when determining baseline variables to be included in a risk calculator for serious adverse events following LSG, Aminian et al. found male gender to be independently associated with higher morbidity and mortality (odds ratio [OR] 1.68; 95% confidence interval [CI] 1.03 – 2.72).10 After controlling for comorbidities, male gender is still an independent risk factor for both LSG and RYBG.15 Thus, these worse outcomes in males cannot be attributed to age and preoperative co-morbidities. Some studies have shown that male gender is not an independent risk factor specifically for bariatric-related mortality.16 Despite more frequently undergoing LSG, a procedure independently associated with lower risks than LRYGB, this might not be enough to mitigate the postoperative morbidity and mortality associated with bariatric surgery for males. Using this information, it may be more prudent to place greater emphasis on modifiable risk factors such as pre-operative BMI, cardiovascular disease, smoking, diabetes, and limited ambulation during pre-operative patient counselling regarding severe adverse events following bariatric surgery.17
This study demonstrates a higher readmission rate for LRYGB compared to LSG, which is consistent with the current literature.9 We also show that more females are undergoing LRYGB, and this correlates with the higher rates of readmission among female patients after matching for all peri-operative variables. This is consistent with the prior reports, which identifies female gender as a risk factor for increased early hospital readmission after bariatric surgery.18–20 In a study by Aman et al.,20 female gender made up 78.9% of all 30-day readmissions following bariatric surgery over a two-year period from 2012 to 2013.21 The present study demonstrates that females made up 55.5% of 30-day readmissions over a two-year period from 2015 to 2017. It is important to note that although female patients continue to make up the majority of 30-day readmissions, this has decreased substantially. It is possible that technological advances, improvements in surgical techniques, and closer outpatient monitoring have contributed to the overall lower rates of readmission seen in later years. Interestingly, this phenomenon of higher female readmission rates persists beyond the 30-day benchmark. According to Bruze et al., when compared to the general population, female patients continue to exhibit higher all-cause readmission rates even six years following surgery.21 Prior studies have demonstrated a relationship between independent pre-operative psychosocial factors which affect the likelihood of readmission following bariatric surgery. Such factors have little to do with the surgical procedure itself, and more to do with unrelated demographic characteristics that may not be immediately obvious during pre-operative assessment. Readmitted patients have been found to present themselves more positively pre-operatively, and are less likely to be receiving outpatient psychiatric care.19 Thus, one must look beyond established surgical comorbidities such as hypertension and coronary artery disease when examining modifiable pre-operative risk factors that might decrease the risk of readmission in the long term.
The paradox between lower readmission rates in men despite higher postoperative complications is concerning given how severe these complications can be once they occur. Our study found a 0.39% mortality rate in men after LSG and LRYGB, more than double the 0.15% mortality rate seen in women. When examining the percentage of patients who died after experiencing a postoperative complication requiring re-operation, i.e., failure to rescue, male gender was identified as an independent risk factor for higher failure to rescue rates (OR 2.81, CI 1.86 – 4.24).22 These trends could provide insight that men have worse follow-up overall following bariatric surgery. Thereaux et al. reported risk factors for poor follow-up for patients five years after the index bariatric procedure. Multivariate analysis showed that male gender was an independent risk factor for poor follow-up, in addition to young age, and type II diabetes.23
Although the NSQIP dataset offers a large national population for analysis, it is limited by the retrospective data collection which has inherent selection bias by select participating centers. Additionally, it is limited to 30-day follow up. Further long-term analysis is needed to more fully determine postoperative complications and readmission rates.
CONCLUSIONS
Although fewer men undergo bariatric surgery than women, they present with greater pre-operative comorbidities and make up a greater percentage of patients with BMI greater than 50 kg/m.2 They also experience more severe postoperative complications following bariatric surgery, despite favoring LSG, which has been associated with lesser operative risks.
These results demonstrate that despite controlling for comorbidities, complications, and procedure type, females are more likely to be readmitted than males. These findings also indicate the need to examine healthcare barriers faced by male patients in the context of follow-up care, as well as guide discussions about the need for close postoperative monitoring given the higher number of severe complications in this demographic.
Contributor Information
Japjot Bal, Department of Surgery, Icahn School of Medicine at Mount Sinai, New York, NY..
Nicole Ilonzo, Department of Surgery, The Mount Sinai Hospital, New York, NY..
Tiwalade Adediji, Department of Surgery, Mount Sinai Morningside, New York, NY..
I. Michael Leitman, Department of Surgery, Icahn School of Medicine at Mount Sinai, New York, NY..
References:
- 1.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–785. [DOI] [PubMed] [Google Scholar]
- 2.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401–407. [DOI] [PubMed] [Google Scholar]
- 3.Patterson WL, Peoples BD, Gesten FC. Predicting potentially preventable hospital readmissions following bariatric surgery. Surg Obes Relat Dis. 2015;11(4):866–872. [DOI] [PubMed] [Google Scholar]
- 4.Perrone F, Bianciardi E, Benavoli D, et al. Gender influence on long-term weight loss and comorbidities after laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass: a prospective study with a 5-year follow-up. Obes Surg. 2016;26(2):276–281. [DOI] [PubMed] [Google Scholar]
- 5.Lager CJ, Esfandiari NH, Subauste AR, et al. Roux-En-Y gastric bypass vs. sleeve gastrectomy: balancing the risks of surgery with the benefits of weight loss. Obes Surg. 2017;27(1):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchs HF, Broderick RC, Harnsberger CR, et al. Benefits of bariatric surgery do not reach obese men. J Laparoendosc Adv Surg Tech A. 2015;25(3):196–201. [DOI] [PubMed] [Google Scholar]
- 7.Celio AC, Wu Q, Kasten KR, Manwaring ML, Pories WJ, Spaniolas K. Comparative effectiveness of Roux-en-Y gastric bypass and sleeve gastrectomy in super obese patients. Surg Endosc. 2017;31(1):317–323. [DOI] [PubMed] [Google Scholar]
- 8.Sippey M, Kasten KR, Chapman WH, Pories WJ, Spaniolas K. 30-day readmissions after sleeve gastrectomy versus Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(5):991–996. [DOI] [PubMed] [Google Scholar]
- 9.Ignat M, Vix M, Imad I, et al. Randomized trial of Roux-en-Y gastric bypass versus sleeve gastrectomy in achieving excess weight loss. Br J Surg. 2017;104(3):248–256. [DOI] [PubMed] [Google Scholar]
- 10.Aminian A, Brethauer SA, Sharafkhah M, Schauer PR. Development of a sleeve gastrectomy risk calculator. Surg Obes Relat Dis. 2015;11(4):758–764. [DOI] [PubMed] [Google Scholar]
- 11.Kizy S, Jahansouz C, Downey MC, et al. National trends in bariatric surgery 2012-2015: demographics, procedure selection, readmissions, and cost. Obes Surg. 2017;27(11): 2933–2939. [DOI] [PubMed] [Google Scholar]
- 12.Garg T, Rosas U, Rogan D, et al. Characterizing readmissions after bariatric surgery. J Gastrointest Surg. 2016;20(11):1797–801. [DOI] [PubMed] [Google Scholar]
- 13.Benotti P, Wood GC, Winegar DA, et al. Risk factors associated with mortality after Roux-en-Y gastric bypass surgery. Ann Surg. 2014;259(1):123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan MA, Grinberg R, Johnson S, Afthinos JN, Gibbs KE. Perioperative risk factors for 30-day mortality after bariatric surgery: is functional status important? Surg Endosc. 2013;27(5):1772–1777. [DOI] [PubMed] [Google Scholar]
- 15.Dugan N, Thompson KJ, Barbat S, et al. Male gender is an independent risk factor for patients undergoing laparoscopic sleeve gastrectomy or Roux-en-Y gastric bypass: an MBSAQIP(R) database analysis. Surg Endosc. 2020;34(8):3574–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falvo A, Vacharathit V, Kuhn JE, et al. Comparison of short-term outcomes following Roux-en-Y gastric bypass in male and female patients using the MBSAQIP database. Surg Obes Relat Dis. 2020;16(9):1236–1241. [DOI] [PubMed] [Google Scholar]
- 17.El Chaar M, Stoltzfus J, Gersin K, Thompson K. A novel risk prediction model for 30-day severe adverse events and readmissions following bariatric surgery based on the MBSAQIP database. Surg Obes Relat Dis. 2019;15(7):1138–1145. [DOI] [PubMed] [Google Scholar]
- 18.Telem DA, Talamini M, Gesten F, et al. Hospital admissions greater than 30 days following bariatric surgery: patient and procedure matter. Surg Endosc. 2015;29(6):1310–1315. [DOI] [PubMed] [Google Scholar]
- 19.Heinberg LJ, Marek R, Haskins IN, Bucak E, Nor Hanipah Z, Brethauer S. 30-day readmission following weight loss surgery: can psychological factors predict nonspecific indications for readmission? Surg Obes Relat Dis. 2017;13(8):1376–1381. [DOI] [PubMed] [Google Scholar]
- 20.Aman MW, Stem M, Schweitzer MA, Magnuson TH, Lidor AO. Early hospital readmission after bariatric surgery. Surg Endosc. 2016;30(6):2231–2238. [DOI] [PubMed] [Google Scholar]
- 21.Bruze G, Ottosson J, Neovius M, Naslund I, Marsk R. Hospital admission after gastric bypass: a nationwide cohort study with up to 6 years follow-up. Surg Obes Relat Dis. 2017;13(6):962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gribben JL, Ilonzo N, Neifert S, Leitman IM. Predictors of reoperation and failure to rescue in bariatric surgery. JSLS. 2018;22(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thereaux J, Lesuffleur T, Paita M, et al. Long-term follow-up after bariatric surgery in a national cohort. Br J Surg. 2017;104(10):1362–1371. [DOI] [PubMed] [Google Scholar]


