Abstract
Among 275 patients with COVID-19, we found that median blood zinc level was significantly lower in patients with poor clinical outcome (N = 75) as compared to patients with good clinical outcome (N = 200) (840 μg/L versus 970 μg/L; p < 0.0001), suggesting that zinc supplementation could be useful for patients with severe COVID-19.
Keywords: Zinc, SARS-COV-2, Severe, Clinical outcome, Chloroquine, Azithromycin
Introduction
In the current situation of COVID-19 pandemic, drug repurposing may be the best option over the development of novel drugs to fight this new acute infectious disease. Among existing drugs that are currently under evaluation for treating COVID-19 infection, hydroxychloroquine (HCQ) in combination with azithromycin (AZ) has been shown to be effective and synergistic both in vitro1 and in vivo.2 Using this combination, we have recently treated 3737 COVID-19 patients and observed a lower mortality and better outcome as compared to other regimens in Marseille public hospitals.3 In parallel with our studies, an empiric regimen combining HCQ, AZ and zinc sulfate was recently proposed for treating outpatients with a reduced hospitalization risk.4 Zinc is an essential metal in human being and is involved in a wide range of biological processes as a cofactor and signaling molecule.5 In vitro, zinc demonstrated its antiviral activity through inhibition of Severe Acute Respiratory Syndrome 1 (SARS-CoV-1) RNA polymerase.6 Moreover, zinc is well known as an essential element for immune function and resistance to infections.5 Zinc deficiency is very common, even in developing countries and is associated to increased risk of infectious diseases (pneumonia, diarrhea, malaria).5 Conversely, zinc supplementation is associated with a significant decrease in mortality during severe pneumonia.7 Finally, it has also been demonstrated that chloroquine derivatives are ionophores for intracellular uptake of zinc into the lysosomes.5 Based on this knowledge and the fact that some of our patients from our series of 3737 patients had a poor clinical outcome, the purpose of this study was to assess whether zinc concentration in COVID-19 patients was associated with the clinical outcome of the disease.
The study
Patients were included as a part of a previous work3 and biochemical analysis were performed as standard care. Patients were included between 2020, March, 1st and 2020, April, 16th, with the following inclusion criteria; age >18 y.o., with at least one nasopharyngeal, specimen positive for SARS-CoV-2 and a blood sample available for the measurement of zinc concentration that was collected following the positive testing. Zinc administration before during the admission was an exclusion criterion. Patients were previously categorized according to their clinical outcome in two groups, as previously describe.3 Briefly, either death or transfer to ICU or hospitalization for 10 days or more defined poor clinical outcome (PClinO). Other patients were classified in the good clinical outcome (GO) group. The HCQ-AZ group includes patients that received at least a 3-day course of HCQ-AZ. The other regimens comprise shorter course of HCQ-AZ, AZ alone, HCQ alone, or none of these molecules. The assessment of plasmatic zinc level was performed by inductively coupled plasma-mass spectrometry (ICP-MS) using a Nexion ICP MS (PerkinElmer, Waltham, Massachusetts, USA). We performed Student t-test and Mann–Whitney U test, where applicable, using GraphPad Prism 7.0 (GraphPad Software, La Jolla, USA) while we performed the Chi-square test using the OpenEpi Platform (www.openepi.com). Logistic regression was performed using XLStat 2016 (Addinsoft, Paris, France). Data from patients were protected according to the European General Data Protection Regulation and follow the MR004 processing registered under N° MR 5010010520.
A total of 275 patients were included (Table 1 ) with 200 (75%) that had a good clinical outcome (GO group), and 75 (25%) with a poor clinical outcome (PClinO group) as previously defined.3 The median age was of 49.4 years old (standard deviation, sd, 16.2 y.o.) and 64.1 years old (sd, 14.58 y.o.) in the PClinO and the GO group, respectively (p < 0.0001).
Table 1.
Baseline characteristics according to clinical outcome of 275 COVID-19 patients at IHU Méditerranée infection Marseille, France between 2020, March, 1st and 2020, April, 16th.
| Good outcome |
Poor clinical outcome |
Total |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Groupe size | 200 (73%) | 75 (27%) | 275 (100%) |
| Age (years) | |||
| Mean (SD) | 49.4 (16.2) | 64.1 (14.6)∗∗∗ | 53.4 (17) |
| Median [Mean - Max] | 49 [18–89] | 63 [31–91] | 54 [18–91] |
| Sex ratio (M/F) | 0.74 | 1.34∗ | 0.87 |
| Time between diagnosis and Zn Level measurement | |||
| Mean (SD) | 2.61 (2.27) | 2.17 (2.4) | 2.5 (2.3) |
| Median [Mean - Max] | 2 [0–15] | 1 [0–9]∗ | 2 [0–15] |
| Clinical manifestations | |||
| Fever | 39 (19.5) | 28 (37.3)∗∗ | 67 |
| Cough | 71 (35.5) | 32 (42.7) | 103 |
| Rhinitis | 27 (13.5) | 8 (10.7) | 35 |
| Anosmia | 32 (16)∗ | 5 (6.67) | 37 |
| Agueusia | 26 (13) | 5 (6.67) | 31 |
| Dyspnea | 29 (14.5) | 14 (18.7) | 43 |
| Clinical classification (NEWS score) | |||
| 0–4 (low) | 182 (91)∗∗∗ | 31 (41.3) | 213 |
| 5–6 (medium) | 8 (4) | 14 (18.7)∗∗∗ | 22 |
| ≥7 (high) | 10 (5) | 30 (40)∗∗∗ | 40 |
| Evolution | |||
| Hospitalization | 63 (31.5) | 75 (100)∗∗∗∗ | 138 |
| Median days of hospitalization [Min - Max] | 4 [1–9] | 15 [1–40]∗∗∗∗ | |
| ICU | 0 (0) | 28 (37.3)∗∗∗∗ | 28 |
| Deaths | 0 (0) | 13 (17.3)∗∗∗∗ | 13 |
| Comorbidities | |||
| Hypertension | 45 (22.5) | 36 (48)∗∗∗ | 81 |
| Diabetes mellitus | 21 (10.5) | 21 (28)∗∗∗ | 42 |
| Obesity | 16 (8) | 13 (17.3)∗ | 29 |
| Cancer | 8 (4) | 12 (16)∗∗ | 20 |
| Coronary disease | 8 (4) | 12 (16)∗∗ | 20 |
| Zinc blood level | |||
| Mean (SD) | 1007 (240.8) | 841.1 (198.8) | 961.9 (241.4) |
| Median [Mean - Max] | 970.5 [627–3058]∗∗∗ | 840 [270–1326] | 947 [270–3058] |
| Therapeutics | |||
| HCQ+AZ | 178 (89)∗∗∗ | 46 (61.3) | 224 (81.5) |
| Other treatment | 22 (11) | 29 (38.7)∗∗∗ | 51 (18.5) |
The frequencies or the values found significantly higher when comparing the two groups are highlighted in bold.
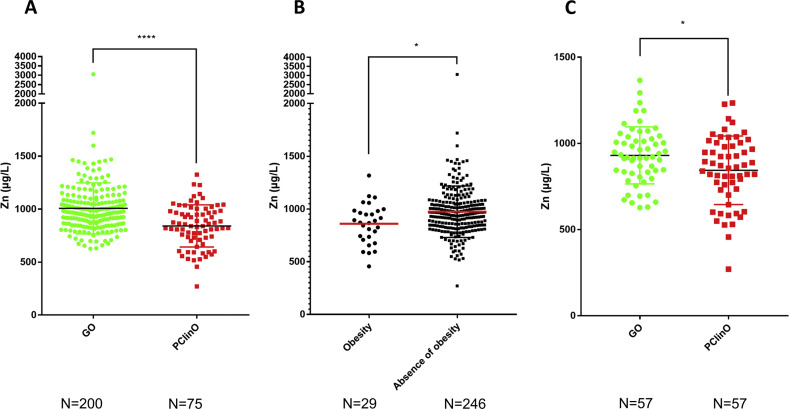
The mean plasmatic zinc level among the 275 patients was 961.9 μg/L (sd 241.4 μg/L). No difference was observed regarding the gender. The median plasmatic zinc level was significantly lower in the PClinO group (840 μg/L) as compared to the GO group (970 μg/L) (p < 0.0001) (Fig. 1 ). Two outliers (one belonging to each group) were excluded using the ROUT method (Q = 1%), resulting in a subsequent analysis of a total of 273 patients for which the level of zinc remains significantly lower in the PClinO group (p < 0.0001). Due to the significant difference in age between the PClinO and GO groups, we randomly paired by age patients from the GO group (mean 61.1 y.o.; sd, 13.9) with those from the PClinO group (mean, 61.1 y.o.; sd, 14.1) resulting a total of 114 patients (57 in each group) (Table S1). The mean plasmatic zinc level was significantly lower in the PClinO group (843.6 μg/mL, sd 198.9) than in the GO group (930.3 μg/mL, sd 165.6) (p = 0.013) (Fig. 1C). We also aimed to investigate if the therapeutics could affect zinc blood level. Of the 275 patients included, the 224 that complete the treatment combining hydroxychloroquine and azithromycin for at least 3 days exhibited higher but non-significant zinc blood levels (median 955.2 μg/L) when compared to those that experienced another treatment (median 890 μg/L) (p = 0.088). In the multivariate analysis we found that obesity (p = 0.01) (Fig. 1B) and age (p < 0.0001) were associated with the zinc status (Table S2). Only 25 subjects had zinc deficiency stricto sensu (i.e., zinc plasmatic level <700 μg/L). The proportions of patients with zinc deficiency was higher in the PClinO group (21%) when compared to the GO group (4.5%) (p < 0.0001). Their mean age was 69 y.o. (Sd = 14.5 y.o). We show by using both multivariate and univariate analysis that zinc deficiency is associated with older age (p < 0.0001) and obesity (p = 0.011) (Table S3). Finally, we failed to correlate zinc status with the clinical presentation or the symptoms recorded. Overall, our study demonstrates that patients with poor clinical outcomes, although treated by the combination of HCQ + AZ, exhibit lower levels of plasmatic zinc than those with good outcome during SARS-CoV-2 infection. This phenomenon is not only due to the fact that patients in the PClinO group were older, as these findings were comforted in a subgroup analysis with patients paired by age (Fig. 1C) (i.e., mean age of 61.1 y.o.). However, older patients are more likely to develop severe forms of SARS-CoV-2 infection (Table 1).3 Zinc deficiency has been associated with severity during SARS-CoV-2 infection.8 The elderly are more frequently affected by zinc deficiency5 but the diabetic patients and the obese people are also more likely to have a lower zinc status. In this study, we identified only 25 patients suffering from zinc deficiency stricto sensu, mostly belonging to the PClinO group (i.e., 64%). The zinc concentration was assessed from K2EDTA blood samples, which may overestimate zinc plasmatic level.9 In our study zinc deficiency was significantly associated with older age and obesity. These data raise the question about the zinc supplementation in these populations. To the best of our knowledge, impact of zinc supplementation in overweight individuals on the incidence or severity of respirations has not been assessed. There are however several studies regarding the elderly. Indeed, zinc supplementation was shown to significantly reduce the incidence of infections in the older subjects, in particular regarding the “common cold” and the upper respiratory tract infections.5 Besides, zinc appears to reduce the duration of common cold when administered 24 h following the onset of the symptoms.5 Moreover, a high zinc level is associated with a lower risk of pneumonia and a reduced duration of infection.5 Importantly, oral administration of zinc is safe as zinc intoxication is a very rare event. Taken together, these elements suggest that zinc supplementation could be an empiric adjuvant therapy for COVID-19 especially in high-risk patients. This proposal makes sense considering that chloroquine is in fact a zinc ionophore,5 inducing an increase in the uptake of zinc in cells and thus a higher zinc concentration in lysosomes. These observations have led some physicians to propose a regimen combining hydroxychloroquine, azithromycin and zinc for treating patients with SARS-CoV-2 infection.4 As a result, the authors report a significant reduction of hospitalization when compared to the untreated group, with no cardiac side effects. The conclusions are however difficult to interpret as the patient characteristics and clinical data were however not available for the untreated group and could affect the outcome. In addition, the impact of zinc supplementation on the clinical course remains unclear. However, a retrospective study has recently shown that the addition of zinc to the chloroquine + azithromycin regimen is associated with a higher rate of patients discharged home and a lower mortality in patients that did not require ICU when compared to chloroquine + azithromycin only.10 In vitro works are ongoing to demonstrate if the addition of zinc may enhance the efficacy of the combination of HCQ and AZ against SARS-CoV-2 viruses.1 Further clinical studies would allow to evaluate how the zinc supplementation could prevent severe forms of SARS-CoV-2 infections in high-risk patients, in particular the elderly and the obese individuals.
Figure 1.
Plasmatic zinc levels in COVID-19 patients according to the clinical outcome among the 275 patients (A) and among 114 patients paired by age (C). The figure B highlights the plasmatic zinc levels of the obese and non-obese patients among the 275 patients.
Funding
This work was supported by the French Government under the “Investments for the Future” program managed by the National Agency for Research (ANR), Méditerranée-Infection 10-IAHU-03.
Declaration of competing interest
None to declare.
Acknowledgments
We thank all clinical and paramedical staffs of hospitalization units and laboratories for their work in this critical situation, in particular Mrs Véronique Filosa and Mrs Séverine Guitton. We are grateful to Dr Yolande Obadia for the epidemiological data collection and to Pr Matthieu Million for his help regarding the statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmii.2021.01.012.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020 Apr 25:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Sevestre J. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Trav Med Infect Dis. 2020 Apr 11:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagier J.-C., Million M., Gautret P., Colson P., Cortaredona S., Giraud-Gatineau A. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: a retrospective analysis. Trav Med Infect Dis. 2020;36:101791. doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derwand R., Scholz M., Zelenko V. COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study. Int J Antimicrob Agents. 2020 Oct 26:106214. doi: 10.1016/j.ijantimicag.2020.106214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020:11. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010 Nov 4;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L., Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: a meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Res J. 2018 Mar;12(3):857–864. doi: 10.1111/crj.12646. [DOI] [PubMed] [Google Scholar]
- 8.Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P. COVID-19: poor outcomes in patients with Zinc deficiency. Int J Infect Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank E.L., Hughes M.P., Bankson D.D., Roberts W.L. Effects of anticoagulants and contemporary blood collection containers on aluminum, copper, and zinc results. Clin Chem. 2001;47(6):1109–1112. [PubMed] [Google Scholar]
- 10.Carlucci P.M., Ahuja T., Petrilli C., Rajagopalan H., Jones S., Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020 Oct;69(10):1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.