Abstract
Objective
The new coronavirus disease 2019 (COVID-19) is a major health problem worldwide. The surveillance of seropositive individuals serves as an indicator to the extent of infection spread and provides an estimation of herd immunity status among population. Reports from different countries investigated this issue among healthcare workers (HCWs) who are “at risk” and “sources of risk” for COVID-19. This study aims to investigate the seroprevalence of COVID-19 among HCWs in one of the COVID-19 referral centers in Makkah, Saudi Arabia using three different serological methods.
Methods
In-house developed enzyme-linked immunoassay (ELISA), commercially available electro-chemiluminescence immunoassay (ECLIA), and microneutralization (MN) assay were utilized to determine the seroprevalence rate among the study population. 204 HCWs participated in the study. Both physicians and nurses working in the COVID-19 and non COVID-19 areas were included. Twelve out of 204 were confirmed cases of COVID-19 with variable disease severity. Samples from recovered HCWs were collected four weeks post diagnosis.
Results
The overall seroprevalence rate was 6.3% (13 out of 204) using the in-house ELISA and MN assay and it was 5.8% (12 out of 204) using the commercial ECLIA. Among HCWs undiagnosed with COVID-19, the seroprevalence was 2% (4 out 192). Notably, neutralizing antibodies were not detected in 3 (25%) out 12 confirmed cases of COVID-19.
Conclusions
Our study, similar to the recent national multi-center study, showed a low seroprevalence of SARS-Cov-2 antibodies among HCWs. Concordance of results between the commercial electro-chemiluminescence immunoassay (ECLIA), in-house ELISA and MN assay was observed. The in-house ELISA is a promising tool for the serological diagnosis of SARS-CoV-2 infection. However, seroprevalence studies may underestimate the extent of COVID-19 infection as some cases with mild disease did not have detectable antibody responses.
Keywords: COVID-19, SARS-CoV-2, Seroprevalence, Healthcare workers, Saudi Arabia
1. Introduction
In December 2019, a viral-induced pneumonia outbreak caused by a novel betacoronavirus was reported in Wuhan, China. The virus and disease have been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the coronavirus disease of 2019 (COVID-19), respectively (WHO, 2020c, WHO, 2020d). The clinical spectrum of COVID-19 ranges widely in term of severity. While some infected individuals experience no or very mild symptoms, others develop life-threatening complications (Hoehl et al., 2020, Huang et al., 2020, Wang et al., 2020). Acute Respiratory Distress Syndrome (ARDS) is a common life-threatening complication of SARS-CoV-2 infection (Huang et al., 2020, Xu et al., 2020, Zhou et al., 2020). Extrapulmonary organ dysfunction is another major complication that may lead to death of COVID-19 patients (Cheng et al., 2020, Xiao et al., 2020). The rapid human-to-human transmission of the virus by respiratory droplets and direct contact with patients led to COVID-19 pandemic (Lam et al., 2020, WHO, 2020c, Wu et al., 2020a, Wu et al., 2020b). What intensifies the problem is that both symptomatic and asymptomatic individuals can be sources of infection (Nikolai et al., 2020). By the end of December 2020, Over 80 million COVID-19 cases and 1.7 million deaths have been reported (Who, 2020b).
Many countries, including Saudi Arabia, have initially implemented control measures to contain the spread of infection but they are now the process of returning to “normal” life (Alandijany et al., 2020b, Ebrahim et al., 2020, MOH-KSA, 2020). Hence, it is expected that SARS-CoV-2 will continue to spread among societies. Healthcare workers (HCWs) and family members of infected individuals are at increased risk of being exposed to SARS-CoV-2 infection (Jin et al., 2020). According to the WHO, 14% of total global cases are related to health workers (Who, 2020a). Systematic screening for the seroprevalence of anti-SARS-CoV-2 antibodies is a valuable indicator for the spread of the infection (Peto, 2020). Several recent studies have investigated the prevalence of antibodies specifically directed against SARS-CoV-2 among HCWs with variable findings (Chen et al., 2020b, Garcia-Basteiro et al., 2020, Gómez-Ochoa et al., 2020, Hunter et al., 2020, Korth et al., 2020). A study conducted on 734 HCWs at Indiana University Health, USA revealed 1.6% prevalence rate of SARS-CoV-2 IgG antibodies (Hunter et al., 2020). Similar rate was reported from a study conducted on 316 individuals working in the University Hospital Essen, Germany (Korth et al., 2020). Another assessment conducted on the Hospital Clinic in Barcelona demonstrated 6.23%, 7.61%, and 8.13% seroprevalence rates of IgM, IgG, and IgA, respectively, among 578 HCWs (Garcia-Basteiro et al., 2020). The prevalence rate was considerably higher (17.14%) in an independent study, which investigated the prevalence of neutralizing antibody to SARS-CoV-2 among 105 HCWs who have been in close contact with COVID-19 patients in Nanjing Drum Tower Hospital, China (Chen et al., 2020b). A systematic review and meta-analysis of 97 reports from different countries estimated 11% and 7% prevalence rates of SARS-CoV-2 infection and antibodies, respectively, among HCWs (Gómez-Ochoa et al., 2020). A recent national multi-center study in Saudi Arabia showed 2.36% seroprevalence of SARS-CoV-2 antibodies among HCWs. However, there was discordant results between the commercial electro-chemiluminescence immunoassay (ECLIA) and the pseudotyped viral particles neutralization assay (Alserehi et al., 2020).
We have developed and optimized an in-house indirect ELISA that enable sensitive and specific detection of SARS-CoV-2 IgG antibodies (27). Microneutralization (MN) assay is the gold standard for evaluating the presence of neutralizing antibody for SARS-CoV-2 infection (GeurtsvanKessel et al., 2020, Kohmer et al., 2021, Lisboa Bastos et al., 2020). We have recently applied these two valuable techniques to investigate the seroprevalence rate among healthy blood donors during COVID-19 lockdown in the country (Alandijany et al., 2021). In this study, in-house ELISA in addition to commercially available ECLIA and micro-neutralization (MN) assay were utilized to determine the seroprevalence rate among HCWs at the Security Forces Hospital (SFH), Makkah, Saudi Arabia. The study site is one of the COVID-19 referral hospitals in Makkah. Our data demonstrate three key findings: (1) a low prevalence rate of anti-SARS-CoV-2 neutralizing antibody among HCWs undiagnosed with COVID-19,(2) neutralizing antibodies were not detected among some HCWs diagnosed with COVID-19 and (3) concordant results between the three serological tests (in house ELISA, commercial ECLIA and MN assay)
2. Materials and methods
2.1. Ethic statement
This study was approved by the research institutional board at SFH in Makkah, Saudi Arabia (0369–24062). Written consent forms were obtained from participants.
2.2. Study population
The total number of HCWs participated in this study was 204 comprising 192 individuals undiagnosed with COVID-19 and 12 recovered patients. Serum samples were collected during June and July 2020. All samples were collected, transported to the Special Infectious Agents Unit (SIAU), King Fahd Medical Research Center (KFMRC), King Abdulaziz University (KAU), aliquoted, and frozen at –20 °C until utilized in the experiments.
2.3. Immunoassays
Sera were screened for the presence of antibody directed against SARS-CoV-2 by (1) a commercially available Elecsys® test system from Roche was performed according to the manufacturers' instructions. The assay principle is based on ECLIA utilizing a modified recombinant nucleocapsid protein. (2) a recently developed in-house ELISA was conducted as previously described (27). Briefly, antigen coating of flat bottom microtiter plates (Immulon® 2 HB, USA) was performed overnight at 4 °C with 100 ng per well of SARS-CoV-2 (2019-nCoV) spike S1 + S2 ECD-His recombinant protein (Sino Biological, China). Subsequently, the plates were subjected to three washes with phosphate buffer saline (PBS) containing 0.1% Tween 20 (PBST) prior to blocking in PBST containing 5% skimmed milk for 1 h at room temperature. This step was followed by three washes with PBST. Sera were diluted at 1:100 dilutions in PBST containing 5% skimmed milk, added at 100 µl volume, and incubated for an hour at 37 °C. Following three washes with PBST, 100 µl of secondary antibody (goat KPL peroxidase-labelled antibodies to human IgG; Seracare, USA) diluted at 1:64,000 in PBST were added and allowed to incubate for an hour at 37°. The plates were subjected to three washes with PBST. Then, 100 µl of substrate (3,3′,5,5′-Tetramethylbenzidine (TMB); Seracare, USA) were added for 5 min for color development prior to addition of 100 µl of 1 N hydrochloric acid (HCL) to stop the reaction. Using Elx 800 bioelisa Reader (Biokit, Spain), the optical density was measured at 450 nm (OD450). OD450 values of > 0.27 were considered positive. Negative and positive controls utilized in this assay were sera of a healthy blood donor and a recovered COVID-19 patient known to have neutralizing IgG antibodies, respectively. The in-house ELISA provides 100% sensitivity, 98.4% specificity, 98.8% agreement, and high overall accuracy (Alandijany et al., 2020a).
2.4. MN assay
MN assay was used as a confirmatory test to determine the presence of SARS-CoV-2-specific neutralizing antibodies. The local SARS-CoV-2 clinical isolate (SARS-CoV-2/human/SAU/85791C/2020) (Genbank accession number MT630432.1) were utilized in this assay. The virus stock was propagated and titrated by Median Tissue Culture Infectious Dose (TCID50) on African green monkey kidney cells Vero E6 (ATCC® CRL-1586™). Sera were subjected to heat inactivation at 56 °C for 30 min. Then, samples were serially diluted in Dulbecco's modified eagle medium containing 2% fetal calf serum (DMEM-FCS) and added with equal volume of DMEM-FCS containing 100 TCID50 of SARS-CoV-2 on confluent cells. The cells were allowed to incubate at 37 °C in 5% CO2 until extensive cytopathic effect was observed (typically for 3–4 days). Uninfected cells and SARS-CoV-2 infected cells in the absence of human serum were utilized as controls. MN titers of ≥ 1:20 were considered positive.
2.5. Data curation
Figure drawing, data processing and calculations were performed by GraphPad Prism software.
3. Results
3.1. Distribution of study population
The total number of participants enrolled in this study was 204 (n = 204) which comprises 192 (94.1%) undiagnosed with COVID-19 and 12 (5.9%) diagnosed with COVID-19 by reverse transcriptase polymerase chain reaction (RT-PCR). Serum samples of those diagnosed with COVID-19 were collected four weeks post diagnosis. The categorization of study population according to COVID-19 status (undiagnosed vs diagnosed), gender (male vs female), department (COVID-19 area vs other departments), and profession (physicians vs nurses vs other professions) are shown (Table 1 ).
Table 1.
Demographic data of study population (n = 204).
| Variable | n (%) |
|---|---|
| Demographics | |
| Male | 83 (40.7%) |
| Female | 121 (59.3%) |
| Age: Median (IQR) | 33.5 (9) |
| Occupation | |
| Physicians | 84 (41.2%) |
| Nurses | 119 (58.3%) |
| Others | 1 (0.5%) |
| Department | |
| COVID-19 area | 93 (45.6%) |
| Non-COVID-19 area | 36 (17.7%) |
| Emergency room | 41 (20.1%) |
| Radiology | 17 (8.3%) |
| Others | 17 (8.3%) |
| COVID-19 status | |
| Undiagnosed | 192 (94.1%) |
| Diagnosed | 12 (5.9%) |
3.2. The overall seroprevalence rate of anti-SARS-CoV-2 IgG antibody
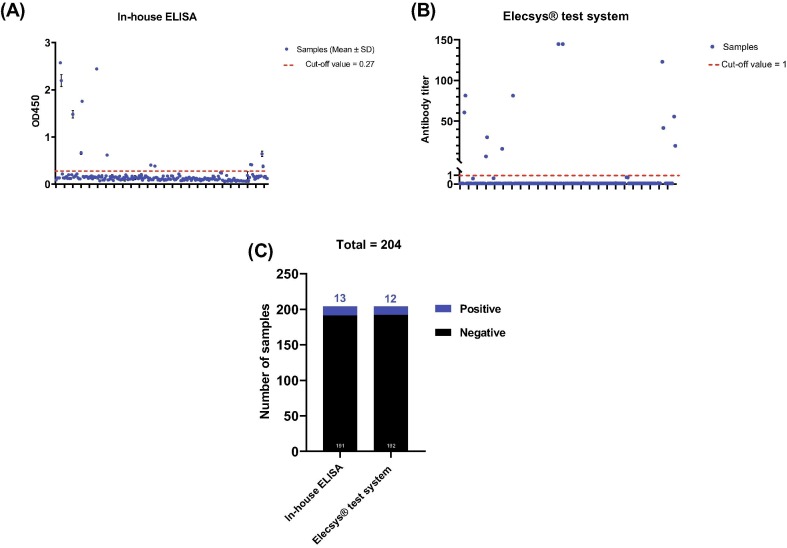
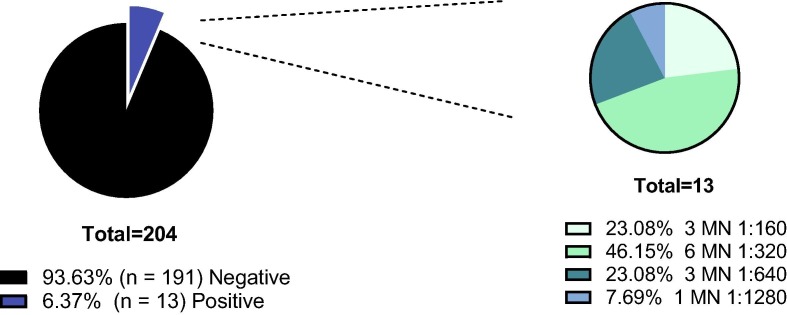
Utilizing in-house and commercially available immunoassays, serum samples from HCWs (n = 204) were assessed for the presence of IgG antibody to SARS-CoV-2 infection. The cut-off values for the in-house ELISA and Elecsys® test system are > 0.27 and ≥ 1, respectively. The in-house assay identified 13 sero-positive samples while 12 samples tested “reactive” by Elecsys® test system (Fig. 1 ). Next, all samples were subjected to MN assay which represents the gold standard method for evaluation of the presence of virus-specific IgG neutralizing antibodies with MN titer of ≥ 1:20 considered positive. There was a 100% match between the results of in-house ELISA and MN assay. The MN titer of positive samples (n = 13) is shown (Fig. 2 ). Collectively, this data demonstrates an overall seroprevalence rate of 6.3% among the study population.
Fig. 1.
The overall seroprevalence rate of anti-SARS-CoV-2 IgG antibody among healthcare workers by ELISAs. (A) Results obtained from in-house ELISA. The means and standard deviations of optical density values at 450 nm (OD450) are shown. The cut-off value of the assay is 0.27. (B) Results obtained from Elecsys® test system. The antibody titer for each sample is shown. The cut-off value of the assay is ≥ 1. (C) The numbers of positive (blue) and negative (black) samples obtained from in-house ELISA and Elecsys® test system.
Fig. 2.
Assessment of neutralizing antibody titers among healthcare workers by micro-neutralization (MN) assay. (A) The seroprevalence rate of anti-SARS-CoV-2 neutralizing antibody among healthcare workers by MN assay. (B) MN titers of sero-positive samples. The actual numbers and relative percentages (%) are shown.
3.3. Sero-positive samples categorized according to COVID-19 status
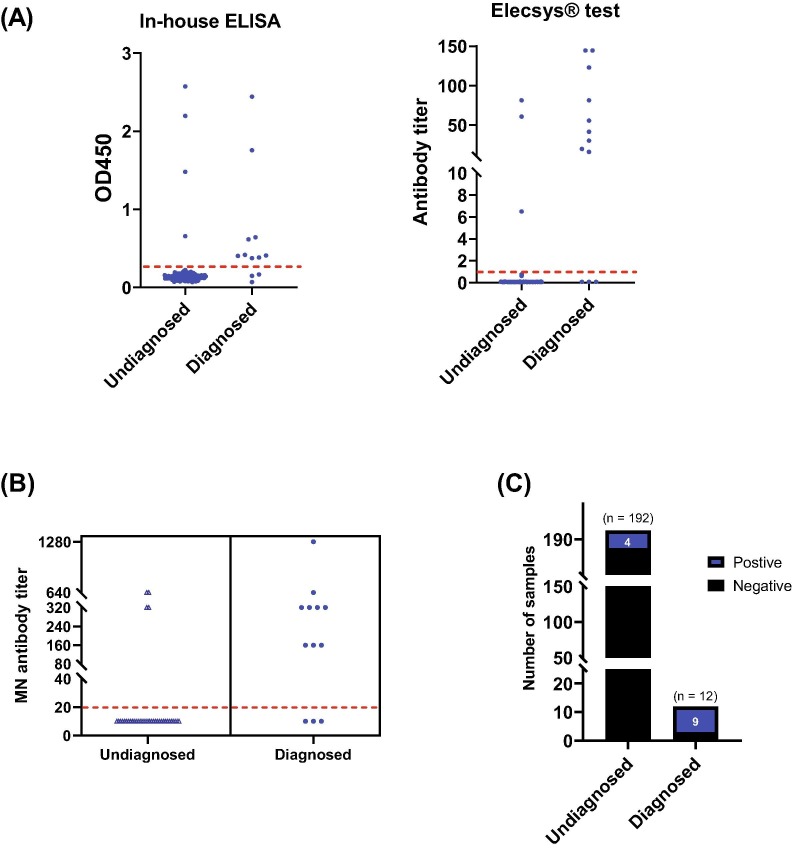
Among the 13 seropositive samples, 9 HCWs are confirmed COVID-19 cases and 4 cases are undiagnosed previously with COVID-19. Nine cases of confirmed COVID-19, whether they were asymptomatic or had pneumonia, developed neutralizing antibody titers between 1:160 and 1:1280. Three cases of confirmed COVID-19 who had upper respiratory tract infection did not mount a neutralizing antibody response. Among the 4 undiagnosed COVID-19 cases, 50% of them worked in the COVID-19 area and they had neutralizing antibody titers between 1:320 and 1:640 (Fig. 3 and Table 2 ).
Fig. 3.
Categorization of sero-positive healthcare workers according to their COVID-19 status. (A) Results obtained from in-house (left panel) and Elecsys® test system (right panel) ELISAs. Dashed red lines represent the cut-off values for each assay. (B) Results obtained from MN assay. MN titers of each positive sample is shown. (C) The number of positive (blue) and negative (black) samples categorized according to COVID-19 status.
Table 2.
Categorization of sero-positive healthcare workers according to their demographic data.
| COVID-19 status | Category | Sub-category | n (%) |
|---|---|---|---|
| Undiagnosed(n = 4 out of 192; 2%)) | Gender | Male | 1 (25%) |
| Female | 3 (75%) | ||
| Department | COVID-19 | 2 (50%) | |
| Non-COVID-19 | 2 (50%) | ||
| Profession | Physician | 1 (25%) | |
| Nurse | 2 (50%) | ||
| Other professions | 1 (25%) | ||
| Diagnosed*(n = 9 out of 12, 75%) | Gender | Male | 6 (66.6%) |
| Female | 3 (33.3%) | ||
| Clinical Manifestations | Asymptomatic | 5 (55.5%) | |
| URTI | 0 (0%) | ||
| Pneumonia | 4(44.4%) | ||
| Severe Pneumonia | 0 (0%) | ||
| Department | COVID-19 | 3 (33.3%) | |
| NON COVID-19 | 6 (66.6%) | ||
| Profession | Physician | 5 (55.5%) | |
| Nurse | 1 (11.1%) | ||
| Other professions | 3 (33.3%) |
4. Discussion
COVID-19 is a global crisis caused by SARS-CoV-2. Officials in Saudi Arabia have implemented timely and unprecedented measures to contain the spread of the infection (Alandijany et al., 2020b, Algaissi et al., 2020, Dalia et al., 2020). As of now (January 2021), many COVID-19 restrictions have been relaxed with enforcement of maintaining physical distancing and mask wearing (MOH-KSA, 2020). At this stage and through the post-pandemic phase, it is important to assess the virus circulation and evaluate the seroprevalence of neutralizing antibody among the community (Peto, 2020, Sunjaya and Sunjaya, 2020). Few reports addressed the seroprevalence status among the Saudi population (Alandijany et al., 2021, Alserehi et al., 2020). In this study, we have evaluated the seroprevalence rate among HCWs at the SFH, one of the COVID-19 referral centers in Makkah, Saudi Arabia.
The overall seroprevalence of SARS-CoV-2 antibodies among HCWs was 6.3% (13 out 204) and 2% among those undiagnosed previously with disease (4 out 192) (Fig. 1, Fig. 3 and Table 2 ). Other studies have described varied results depending on the inclusion or exclusion of confirmed COVID-19 cases in the analysis (Gómez-Ochoa et al., 2020). The low SARS-CoV-2 antibodies seroprevalence in our study was comparable to the national multi-center study showing 2.36% seropositivity and it is suggestive of effective infection control measures among HCW (Alserehi et al., 2020). Knowing these results, a significant number of HCWs will be at risk of COVID-19 infection. With the limited supply of vaccination, HCWs in Makkah who are exposed to pilgrims should have a priority in vaccination programs (Abd El Ghany et al., 2016).
The recent national multi-center study utilized a commercially available chemiluminescent microparticle assay to screen sera of HCWs (Alserehi et al., 2020). Only 100 positive samples were subjected to SARS-CoV-2 pseudotyped viral particles neutralization assay. Results showed that 8% of these samples lacked neutralizing activity (Alserehi et al., 2020). In our study, we performed three tests; in house ELISA, commercial ECLIA and MN assay in all 204 cases (Fig. 1 and Fig. 2). There were concordant results in all 13 seropositive cases using the in house ELISA and MN assay which corresponds to the reported sensitivity and specificity of our developed assay (Alandijany et al., 2020a). The in-house ELISA is a promising serological test for the diagnosis of COVID-19 infection. Twelve out of 13 cases were positive using the Elecsys® test system (Fig. 1 and Fig. 3). The single false negative result obtained from this assay, in addition to several recent reports, highlight the importance of evaluating the performance of commercially available kits prior to utilizing them for diagnostic and epidemiological applications (GeurtsvanKessel et al., 2020, Lisboa Bastos et al., 2020).
Among COVID-19 confirmed HCW, 9 out 12 cases had detectable neutralizing antibodies (Fig. 3 and Table 2). As previously reported, patients with pneumonia tended to have higher neutralizing antibodies compared to cases with asymptomatic infection (Ko et al., 2020, Kohmer et al., 2021). Three Patients with upper respiratory tract infections did not mount a neutralizing antibody response (Fig. 3 and Table 2). Some reports described lack of humeral response in cases of mild infection (Chen et al., 2020a, Gattinger et al., 2021, Melgaço et al., 2020). In addition, other reports showed waning of neutralizing antibodies over time (Beaudoin-Bussières et al., 2020, Robbiani et al., 2020). A real concern is the increasing reports of COVID-19 re-infection (AlFehaidi et al., 2020, Dao et al., 2020). HCWs working in Makkah who are exposed to pilgrims are at risk of respiratory tract infections (Yezli et al., 2019). Hence, further studies are required to assess their vulnerability to recurrent COVID-19 infection.
5. Conclusions
The seroprevalence of neutralizing antibodies to SARS-CoV-2 infection among HCWs in our study was low and it was comparable to the national multi-center study. Our findings are suggestive of effective infection control measures among HCW. However, a significant number of HCWs are still at risk of COVID-19 and should have the priority for future vaccination program, particularly those working in Makkah and frequently exposed to the pilgrims. In addition, our study showed that the performance of our in-house ELISA was comparable to the MN assay validating it as a valuable serological test for the diagnosis of COVID-19. The lack of antibody response among some HCWs with mild infection, raises the need for more studies to evaluate the risk of future recurrent infections.
6. Disclosure of funding
The Author Acknowledgments The Financial Support Provided By King Abdulaziz City for Science and Technology (General Directorate for Research & Innovation Support (GDRIS)) To King Abdulaziz University To Implement This Work Through Fast Track Program For COVID-19 Research Project No. 5–20-01-009-0080.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El Ghany M., Sharaf H., Hill-Cawthorne G.A. Hajj vaccinations-facts, challenges, and hope. Int. J. Infect. Diseas. IJID: Off. Publicat. Int. Soc. Infect. Dis. 2016;47:29–37. doi: 10.1016/j.ijid.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Alandijany T.A., El-Kafrawy S.A., Al-Ghamdi A.A., Qashqari F.S., Faizo A.A., Tolah A.M., Hassan A.M., Sohrab S.S., Hindawi S.I., Badawi M.A., Azhar E.I. Lack of Antibodies to SARS-CoV-2 among Blood Donors during COVID-19 Lockdown: A Study from Saudi Arabia. Healthcare. 2021;9:51. doi: 10.3390/healthcare9010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alandijany T.A., El-Kafrawy S.A., Tolah A.M., Sohrab S.S., Faizo A.A., Hassan A.M., Alsubhi T.L., Othman N.A., Azhar E.I. Development and Optimization of In-house ELISA for Detection of Human IgG Antibody to SARS-CoV-2 Full Length Spike Protein. Pathogens. 2020;9:803. doi: 10.3390/pathogens9100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alandijany T.A., Faizo A.A., Azhar E.I. Coronavirus disease of 2019 (COVID-19) in the Gulf Cooperation Council (GCC) countries: Current status and management practices. J. Infect. Public Health. 2020 doi: 10.1016/j.jiph.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlFehaidi A., Ahmad S.A., Hamed E. SARS-CoV-2 re-infection: a case report from Qatar. J. Infect. 2020 doi: 10.1016/j.jinf.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algaissi A.A., Alharbi N.K., Hassanain M., Hashem A.M. Preparedness and response to COVID-19 in Saudi Arabia: Building on MERS experience. J. Infect. Public Health. 2020 doi: 10.1016/j.jiph.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alserehi H.A., Alqunaibet A.M., Al-Tawfiq J.A., Alharbi N.K., Alshukairi A.N., Alanazi K.H., Saleh G.M.B., Alshehri A.M., Almasoud A., Hashem A.M., Alruwaily A.R., Alaswad R.H., Al-Mutlaq H.M., Almudaiheem A.A., Othman F.M., Aldakeel S.A., Ghararah M.R.A., Jokhdar H.A., Algwizani A.R., Almudarra S.S., Albarrag A.M. Seroprevalence of SARS-CoV-2 (COVID-19) among Healthcare Workers in Saudi Arabia: Comparing Case and Control Hospitals. Diagnost. Microbiol. Infect. Dis. 2020;115273 doi: 10.1016/j.diagmicrobio.2020.115273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin-Bussières G., Laumaea A., Anand S.P., Prévost J., Gasser R., Goyette G., Medjahed H., Perreault J., Tremblay T., Lewin A., Gokool L., Morrisseau C., Bégin P., Tremblay C., Martel-Laferrière V., Kaufmann D.E., Richard J., Bazin R., Finzi A. Decline of Humoral Responses against SARS-CoV-2 Spike in Convalescent Individuals. mBio. 2020;11:e02590–02520. doi: 10.1128/mBio.02590-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Pan Z., Yue S., Yu F., Zhang J., Yang Y., Li R., Liu B., Yang X., Gao L., Li Z., Lin Y., Huang Q., Xu L., Tang J., Hu L., Zhao J., Liu P., Zhang G., Chen Y., Deng K., Ye L. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Sign. Trans. Targeted Therapy. 2020;5:180. doi: 10.1038/s41392-020-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Tong X., Wang J., Huang W., Yin S., Huang R., Yang H., Chen Y., Huang A., Liu Y., Chen Y., Yuan L., Yan X., Shen H., Wu C. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J. Infect. 2020;81:420–426. doi: 10.1016/j.jinf.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia A.O., Fatimah S.A., Ahmed A.-A.-Q., Mohammed N.-A.-A. Containment of COVID-19: the unprecedented response of Saudi Arabia. J. Infect. Dev. Count. 2020;14 doi: 10.3855/jidc.13203. [DOI] [PubMed] [Google Scholar]
- Dao T.L., Hoang V.T., Gautret P. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur. J. Clin. Microbiol. Infect. Diseas. Off. Publicat. Eur. Soc. Clin. Microbiol. 2020:1–13. doi: 10.1007/s10096-020-04088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S.H., Ahmed Q.A., Gozzer E., Schlagenhauf P., Memish Z.A. Covid-19 and community mitigation strategies in a pandemic. BMJ (Clinical research ed.) 2020;368 doi: 10.1136/bmj.m1066. [DOI] [PubMed] [Google Scholar]
- Garcia-Basteiro A.L., Moncunill G., Tortajada M., Vidal M., Guinovart C., Jiménez A., Santano R., Sanz S., Méndez S., Llupià A., Aguilar R., Alonso S., Barrios D., Carolis C., Cisteró P., Chóliz E., Cruz A., Fochs S., Jairoce C., Hecht J., Lamoglia M., Martínez M.J., Mitchell R.A., Ortega N., Pey N., Puyol L., Ribes M., Rosell N., Sotomayor P., Torres S., Williams S., Barroso S., Vilella A., Muñoz J., Trilla A., Varela P., Mayor A., Dobaño C. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat. Commun. 2020;11:3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinger, P., Borochova, K., Dorofeeva, Y., Henning, R., Kiss, R., Kratzer, B., Mühl, B., Perkmann, T., Trapin, D., Trella, M., Ettel, P., Tulaeva, I., Pickl, W.F., Valenta, R., Antibodies in serum of convalescent patients following mild COVID-19 do not always prevent virus-receptor binding. Allergy n/a. [DOI] [PMC free article] [PubMed]
- GeurtsvanKessel C.H., Okba N.M.A., Igloi Z., Bogers S., Embregts C.W.E., Laksono B.M., Leijten L., Rokx C., Rijnders B., Rahamat-Langendoen J., van den Akker J.P.C., van Kampen J.J.A., van der Eijk A.A., van Binnendijk R.S., Haagmans B., Koopmans M. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat. Commun. 2020;11:3436. doi: 10.1038/s41467-020-17317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ochoa S.A., Franco O.H., Rojas L.Z., Raguindin P.F., Roa-Díaz Z.M., Wyssmann B.M., Guevara S.L.R., Echeverría L.E., Glisic M., Muka T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2020 doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl S., Rabenau H., Berger A., Kortenbusch M., Cinatl J., Bojkova D., Behrens P., Boddinghaus B., Gotsch U., Naujoks F., Neumann P., Schork J., Tiarks-Jungk P., Walczok A., Eickmann M., Vehreschild M., Kann G., Wolf T., Gottschalk R., Ciesek S. Evidence of SARS-CoV-2 Infection in Returning Travelers from Wuhan, China. New Engl. J. Med. 2020;382:1278–1280. doi: 10.1056/NEJMc2001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter B.R., Dbeibo L., Weaver C.S., Beeler C., Saysana M., Zimmerman M.K., Weaver L. Seroprevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) antibodies among healthcare workers with differing levels of coronavirus disease 2019 (COVID-19) patient exposure. Infect. Control Hosp. Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses. 2020;12 doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.-H., Joo E.-J., Park S.-J., Baek J.Y., Kim W.D., Jee J., Kim C.J., Jeong C., Kim Y.-J., Shon H.J., Kang E.-S., Choi Y.K., Peck K.R. Neutralizing Antibody Production in Asymptomatic and Mild COVID-19 Patients, in Comparison with Pneumonic COVID-19 Patients. J. Clin. Med. 2020;9:2268. doi: 10.3390/jcm9072268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmer, N., Westhaus, S., Rühl, C., Ciesek, S., Rabenau, H.F., Clinical performance of different SARS-CoV-2 IgG antibody tests. J. Med. Virol. n/a. [DOI] [PMC free article] [PubMed]
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M., Cordes S., Ross B., Esser S., Lindemann M., Kribben A., Dittmer U., Witzke O., Herrmann A. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. Off. Publicat. Pan Am. Soc. Clin. Virol. 2020;128 doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.-T.-Y., Shum M.-H.-H., Zhu H.-C., Tong Y.-G., Ni X.-B., Liao Y.-S., Wei W., Cheung W.-Y.-M., Li W.-J., Li L.-F., Leung G.M., Holmes E.C., Hu Y.-L., Guan Y. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020 doi: 10.1038/s41586-020-2169-0. [DOI] [PubMed] [Google Scholar]
- Lisboa Bastos M., Tavaziva G., Abidi S.K., Campbell J.R., Haraoui L.-P., Johnston J.C., Lan Z., Law S., MacLean E., Trajman A., Menzies D., Benedetti A., Ahmad Khan F. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ (Clin. Res. ed.) 2020;370 doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgaço, J.G., Azamor, T., Ano Bom, A.P.D., 2020. Protective immunity after COVID-19 has been questioned: What can we do without SARS-CoV-2-IgG detection? Cell Immunol. 353, 104114-104114. [DOI] [PMC free article] [PubMed]
- MOH-KSA, 2020. https://twitter.com/SaudiMOH/status/1264992536453660673?s=20 [accessed 27 May 2020].
- Nikolai L.A., Meyer C.G., Kremsner P.G., Velavan T.P. Asymptomatic SARS Coronavirus 2 infection: Invisible yet invincible. Int. J. Infect. Dis. IJID : Off. Publicat Int. Soc. Infect. Diseas. 2020;100:112–116. doi: 10.1016/j.ijid.2020.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto J. Covid-19 mass testing facilities could end the epidemic rapidly. BMJ (Clin. Res. ed.) 2020;368 doi: 10.1136/bmj.m1163. [DOI] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., Hägglöf T., Oliveira T.Y., Viant C., Hurley A., Hoffmann H.-H., Millard K.G., Kost R.G., Cipolla M., Gordon K., Bianchini F., Chen S.T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A.W., Waltari E., Pak J.E., Huey-Tubman K.E., Koranda N., Hoffman P.R., West A.P., Rice C.M., Hatziioannou T., Bjorkman P.J., Bieniasz P.D., Caskey M., Nussenzweig M.C. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunjaya A.F., Sunjaya A.P. Pooled Testing for Expanding COVID-19 Mass Surveillance. Disaster Med. Public Health Preparedn. 2020:1–5. doi: 10.1017/dmp.2020.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., Wang, B., Xiang, H., Cheng, Z., Xiong, Y., Zhao, Y., Li, Y., Wang, X., Peng, Z., 2020. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama. [DOI] [PMC free article] [PubMed]
- WHO, Keep health workers safe to keep patients safe: WHO. https://www.who.int/news/item/17-09-2020-keep-health-workers-safe-to-keep-patients-safe-who [Last accessed on 8 November 2020].
- WHO, World Health Organization (WHO). COVID-19 dashboard. Available from: https://who.sprinklr.com [Last accessed on 8 November 2020].
- WHO, World Health Organization (WHO). Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Availabe from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Last accessed on 8 November 2020].
- WHO, World Health Organization (WHO). Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. Availabe from: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 [Last accessed on 8 November 2020].
- Wu, F., Zhao, S., Yu, B., Chen, Y.-M., Wang, W., Hu, Y., Song, Z.-G., Tao, Z.-W., Tian, J.-H., Pei, Y.-Y., Yuan, M.-L., Zhang, Y.-L., Dai, F.-H., Liu, Y., Wang, Q.-M., Zheng, J.-J., Xu, L., Holmes, E., Zhang, Y.-Z., 2020a. Complete genome characterisation of a novel coronavirus associated with severe human respiratory disease in Wuhan, China. bioRxiv.
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E.C., Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. Respirat. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezli S., Alotaibi B., Al-Abdely H., Balkhy H.H., Yassin Y., Mushi A., Maashi F., Pezzi L., Benkouiten S., Charrel R., Raoult D., Gautret P. Acquisition of respiratory and gastrointestinal pathogens among health care workers during the 2015 Hajj season. Am. J. Infect. Control. 2019;47:1071–1076. doi: 10.1016/j.ajic.2019.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]