Abstract
Bilirubin has been proven to possess significant anti-inflammatory, antioxidant and antiviral activities. Recently, it has been postulated as a metabolic hormone. Further, moderately higher levels of bilirubin are positively associated with reduced risk of cardiovascular diseases, diabetes, metabolic syndrome and obesity. However, due to poor solubility the therapeutic delivery of bilirubin remains a challenge. Nanotechnology offers unique advantages which may be exploited for improved delivery of bilirubin to the target organ with reduced risk of systemic toxicity. Herein, we postulate the use of intravenous administration or inhalational delivery of bilirubin nanomedicine (BNM) to combat systemic dysfunctions associated with COVID-19, owing to the remarkable preclinical efficacy and optimistic results of various clinical studies of bilirubin in non-communicable disorders. BNM may be used to harness the proven preclinical pharmacological efficacy of bilirubin against COVID-19 related systemic complications.
Keywords: COVID-19, Bilirubin, Biliverdin, Nanomedicine, Anti-inflammatory, Antioxidant
Introduction
SARS-CoV-2 pandemic is a global health challenge with no proven treatment regimens available. Current therapeutic strategies concentrate on the alleviation of symptoms, though to a lesser extent, emergency approved antiviral drugs and vaccines do provide relief [1]. However, treatment of this multifaceted disease, which poses severe complications in multiple organs, requires strategic attention to control viral load and simultaneously address other systemic complexities [2]. To this end, as a life-saving drug, corticosteroids like dexamethasone, which modulate the inflammatory microenvironment and reduce oxidative stress, have shown promising efficacy [3], [4], [5]. In such a scenario, it is imperative to demonstrate the clinical effectiveness of therapeutically active substances individually or in combination with other drugs having proven preclinical efficacy. Bilirubin possesses unique pharmacological effects, and its moderately higher levels have been shown to potentially reduce the risk of cardiovascular diseases (CVDs), diabetes, metabolic syndrome and obesity [6], [7], [8], [9], [10]. However, some controversial reports suggest that bilirubin plays a negative role in COVID-19, as bilirubin levels were found higher in a pool of such patients. However, this preliminary observation was due to systemic sepsis or viremia, further, no conclusive findings indicated any detrimental role of bilirubin in the clinical course of COVID-19 [11]. In previous studies, bilirubin as an endogenous antioxidant compound or its exogenous administration has demonstrated immense antioxidant, anti-inflammatory and immunomodulatory potential in various chronic diseases [12], [13]. Further, recently it was postulated as a metabolic hormone [14]. At the cellular and molecular levels, bilirubin reduces M1 macrophage polarization, expression of proinflammatory cytokines, improves gut barrier integrity, increases MHC-II+, CD11c+, CD11– dendritic cells (DCs), β-defensin-3, superoxide dismutase 1 and modulates molecular mechanistic cascades relevant to pathogenesis of COVID-19 [15]. As the increased incidence and high mortality associated with COVID-19 has become a global burden and a grave challenge, there is still a great need for novel treatment options that can cure and ultimately eradicate this disease. This encourages us to propose the nanocarrier-mediated delivery of bilirubin that can be used as a potential alternative strategy to manage SARS-CoV-2 infection.
Hypothesis
We hypothesize that bilirubin nanomedicine (BNM) can be used as an alternative strategy for the management of COVID-19 by its property to halt the development of systemic inflammatory complications and cytokine storm by modulating the expression of transforming growth factor-beta (TGF-β), mitogen-activated protein kinase (MAPKs) and nuclear factor kappa light chain enhancer of activated B cells (NFκB) signaling pathways.
Justification of the hypothesis
Mechanism of pharmacological effects of bilirubin
Bilirubin has gained wide attention in biomedical research owing to its strong antioxidant, immunomodulatory and anti-inflammatory responses [16]. Moderately high serum bilirubin concentration protects against multiple clinical pathologies. In various preclinical studies, bilirubin at nanomolar concentrations showed potential pharmacological effects. Bilirubin elicits its anti-inflammatory and immunomodulatory activities by modulating the expression of NFκB, nuclear factor erythroid 2-related factor 2 (Nrf2), MAPKinase, inducible nitric oxide synthase (iNOS) enzyme and reducing toll-like receptor 4 (TLR4) mediated inflammatory signaling pathways. Bilirubin possesses reactive oxygen species (ROS) scavenging activity, inflammatory cytokines inhibitory potential and the ability to restore physiological redox balance [17], [18], [19].
In the early 1970s, Nejedla reported that hyperbilirubinemia exerted a suppressive effect on antibody formation in newborn infants [20]. Later, it has been shown that intraperitoneal administration of bilirubin influences the expression of Fc receptors for immunoglobulin (Ig) M, IgG2B, IgA and IgE in immature mouse peritoneal macrophages via activating the tubulin system and altering the lipid environment of the plasma membrane [21]. Haga et al., reported that in human B lymphocytes, unconjugated bilirubin dose-dependently reduces the activity of class I antigens which activate cytotoxic T lymphocytes [22]. Similarly, in extended studies, bilirubin showed a protective effect in several autoimmune diseases, including encephalomyelitis through its broad spectrum suppressive effect on T cells reactivity and interference with NFκB [23], [24]. Liu et al., reported that bilirubin inhibits the antigen-specific-polyclonal T-cell response, suppresses CD4+ T-cell response and causes apoptosis in reactive CD4(+) T cells at high doses. However, at non-apoptotic concentration, bilirubin suppressed CD4(+) T-cell reactivity by inhibiting co-stimulatory activity, suppressing immune transcription factor activation, and downregulation of inducible MHC class II expression. Further, bilirubin treatment suppressed experimental autoimmune encephalomyelitis in SJL/J mice [24]. In addition, bilirubin showed an immunosuppressive effect by activating apoptosis via caspases 9 in lymphocytes, downregulation of protein kinase, and inhibiting MHC II class cell surface expression in molecules containing antigen presenting cells. Moreover, unconjugated bilirubin impairs the expression of Fc subsets, modulates the activity of macrophage and antigen-presenting cells through the upregulation of Fcγ1, Fcµ, Fcε, Fcα, and the downregulation of Fcγ2B receptors [25]. Altogether, bilirubin could have great potential to modulate the immune system.
Introduction to nanoformulations and potential of bilirubin as a nanomedicine against immune-mediated inflammation
Nanotechnology-based drug delivery systems are currently being used as a smart strategy to treat pathogenic infections due to their penetration ability across cell barriers owing to their smaller size and permeation into cellular compartments for site-specific drug delivery [21], [26]. Among the different types of novel strategies, such as controlled release, sustained release or pH triggered/responsive release drug delivery systems, nanoparticles have attracted wide attention for intracellular delivery of drugs [27]. Nanoparticles offer distinctive physicochemical properties, such as high biocompatibility and negligible cellular toxicity in physiological systems [28]. Tailoring nanoparticles with bilirubin or other drugs may improve their therapeutic effectiveness by rapidly internalizing conjugated/encapsulated drugs into microbial cells through interactions with the negatively charged cell membrane [29]. These nanosystems offer excellent drug loading capacity, improved pharmacokinetics and minimal dose-related side effects. In addition, biocompatible polycationic polymers such as chitosan with proven antimicrobial properties while polyesters and polyurethanes as bio-based polymers with aided antimicrobial properties offer a highly positive surface charge, which helps to transport drugs across the cell membrane [30].
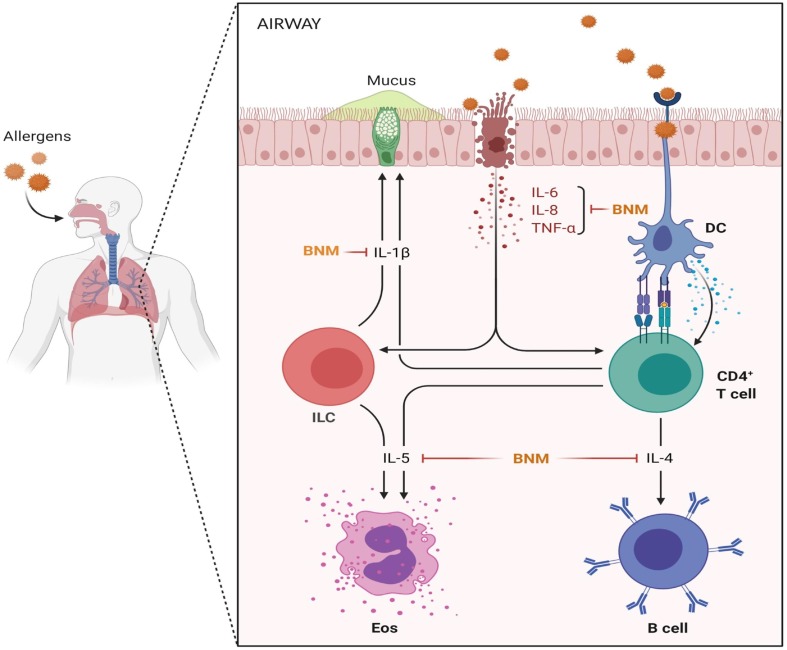
The primary concern of COVID-19 is the diffuse alveolar epithelium damage resulting in lung injury and other cellular responses. Although the clinical course of molecular events and pathobiology of COVID-19 associated respiratory tract damage remains unknown. The primary pathophysiological mechanism for this is the triggered host innate immune response and subsequent accelerated inflammation caused by the cytokine constellation. Further, the condition may worsen when elevated cytokine levels promote lung fibrosis, making the disease almost incurable. DCs act as antigen-presenting cells (APCs) for coronavirus and display the virus on their surface on MHCII [31]. DCs migrate to the lymph nodes and activate allergen-specific T-cells that induce clonal selection and Th2 cells polarization. Th2 cells, in turn, produce cytokines (IL-1β, IL-4 and IL-5) responsible for inflammatory and fibrotic reactions (Fig. 1 ). Bilirubin, a product of the enzyme heme oxygenase (HO-1) has strong antioxidant, anti-inflammatory and cytoprotective effects [32]. Over the last decades, several efforts were made to unravel the salient therapeutic features of bilirubin using a nanocarrier-mediated delivery system and overcome the limitations of its hydrophobicity and toxicity. Therefore, targeting the respiratory system with nano-based bilirubin delivery as an alternative therapeutic strategy to protect against coronavirus would be a plausible approach. BNMs have been reported to possess enhanced therapeutic effect as the volume-to-surface ratio significantly increases, which imparts bilirubin the improved catalytic properties [33]. Treatment of C57BL/6 mice with bilirubin-based nanoparticles suppressed experimental allergic asthma complications by modulating the expression of Th2 cytokines compared to untreated controls. Observed effect may be due to decreased T-cells population and their associated proinflammatory cytokines (IL-4, IL-5 and IL-13), via NFκB activation and co-stimulatory molecules of T cells in an antigen-presenting cell (APC)-independent way significantly attenuated Th2-related allergic lungs inflammation. In addition, PEGylation of bilirubin into nanoparticles improved its pharmacokinetics and dispersibility compared to free bilirubin. This study showed bilirubin as an alternative nanomedicine to treat allergic pulmonary inflammatory diseases compared to currently available corticosteroids with potential side effects [34]. Fig. 2 depicts our postulated hypothesis for the probable mechanistic benefits of BNM. The high potency of bilirubin as an endogenous anti-inflammatory molecule makes it an attractive option for clinical implications. In a mouse model of dextran sodium sulfate-induced ulcerative colitis, unconjugated bilirubin treated animals showed reduced clinical severity of colitis and colonic tissue injury marked by restored intestinal mucosal barrier function and intestinal microbiota homeostasis. Furthermore, reduced immune inflammation was also characterized by significantly reduced levels of proinflammatory cytokines (IL-1β, IL-6, IFN-γ and TNF-α) through the alteration of different molecular signaling pathways (TLR4, TRAF6 and NFκB) [18]. Bilirubin encapsulated silk fibroin-based nanoparticles (BRSNPs) were found to be highly biocompatible and protected pancreatic acinar cells from free radicals mediated oxidative damage and reduced the disease severity in rat acute pancreatitis model, evident by suppressed NFκB and activated Nrf2/HO-1 pathway. This study showed the site-specific targeting capability of the synthesized BRSNPs demonstrated by the accumulation and internalization of BRSNPs into inflammatory pancreas. This study concluded the immense therapeutic potential of BNM with advantage of increased vascular permeability and retention of bilirubin in cellular and subcellular compartments through enhanced permeability and retention (EPR) effects. Such a bilirubin-functionalized nanocarrier-mediated delivery strategy can improve high payload of drugs, achieve controlled release of encapsulated drugs, and reduce the dose associated side effects [13], [35]. Collectively, previous findings indicate that BNMs are highly effective against the prevention and management of various inflammatory diseases by harmonizing the inflammatory immune cells and levels of their related proinflammatory cytokines making them an interesting arsenal in the fight against COVID-19.
Fig. 1.
Respiratory airways exposure to coronavirus. Dendritic cells (DCs) in the respiratory airways capture, internalize, and display coronavirus on the cell surface of MHCII molecules. DCs migrate to the lymph node (LN) and encounter coronavirus-specific T cells. The DCs induce the differentiation of the T cells into Th2 cells. Th2 cells produce cytokines (IL-4, IL-5 and IL-13) that induce mucus production and smooth muscle contraction in the airways. This forms the basis of allergic airway sensitization and cytokine mediated lungs insufficiency. The figure was created with BioRender.com.
Fig. 2.
Coronavirus get inhaled and travel to the respiratory airways. Once there, their stimulus damages the epithelium, which causes the release of different alarmins: IL-33, IL-25 and TSLP. ILC2, innate immune cells, are activated and release proinflammatory type 2 cytokines, such as IL-5 and IL-13 for the recruitment of eosinophils and mucus production, respectively. T helper cells, adaptive immune cells, are activated in an antigen-dependent manner via dendritic cells and cause major respiratory problems, including fibrotic lungs and inflammation. The figure was created with BioRender.com.
More recently, hyaluronic acid-bilirubin nanoparticles for inflammatory bowel diseases and doxorubicin-loaded-biotinylated bilirubin-nanoparticles, folate-gold-bilirubin nanoconjugate for cancer have been developed as BNM and found to be significantly effective [36], [37], [38]. The potential strategy to treat associated systemic infections and management of COVID-19 may be a combination drug delivery system that facilitates the easy transport of drugs to the target site. BNMs functionalized with biocompatible polymers would enhance cellular internalization of bilirubin due to interaction between nanoformulation and cell membrane resulting in membrane disruption and enhanced penetration, thus, providing a smart strategy to combat the threatening effects of COVID-19.
Potential of bilirubin nanomedicine (BNM) against cytokine storm
Cytokine storm is the most prominent cause, making COVID-19 pathology very complex and life-threatening [39], [40]. Currently available medications have limited efficacy or elicit significant adverse effects, hampering their clinical use in COVID-19 management [41]. In this context, BNMs could be considered for their ability to restrain the cytokine signaling pathways like NFκB, Janus kinases and signal transducer and activator of transcription proteins (JAK-STAT) etc. and halt the triggered expression and release of proinflammatory cytokines [42]. Administration of BNM to murine pancreatic islet allograft recipients extensively enhanced graft viability by alleviating cell apoptosis and reducing the expression of inflammatory cytokines such as IL-1β, TNF-α, monocyte chemoattractant protein-1 (MCP-1) and soluble intercellular adhesion molecule-1 (sICAM-1) [43]. Similarly, BNM abrogated the severity of acute pancreatitis by dampening NF-κB and TNF-α expression and stimulating the Nrf2 activity [35]. Hyaluronic acid decorated BNM protected the experimental animals from dextran sulfate sodium-induced colitis. Developed nanoformulation found accumulated in the inflamed colonic epithelium and restored the epithelium barriers integrity in the in vivo murine model of acute colitis by decreasing the expression of IL-1β, IL-6, IL-10, TNF-α and TGF-β [36].
Potential of BNM to fight against multiple organ dysfunctions
The clinical spectrum of COVID-19 is not limited to lung pneumonia; it also represents the multiple organ dysfunction and potential risk of other systemic complications [44]. Therapeutic intervention with bilirubin loaded nanoparticles has been shown to alleviate imiquimod (IMQ)-induced psoriasis in mice. Topical treatment of IMQ-induced psoriasis mice with BNM showed reduced clinical severity of psoriasis, downregulated ROS levels and decreased expression of proinflammatory chemical mediators. This study demonstrated BNM with high biocompatibility and reduced cellular toxicity for psoriasis management [45]. The anti-fibrotic effect of bilirubin conjugated chitosan nanoparticles loaded with losartan was investigated in a thioacetamide/ethanol-induced liver fibrosis mice model. Findings of the in vitro and in vivo experiments suggested that the ROS stimuli-responsive nanoparticles having losartan might be an appealing therapeutic choice to manage hepatic dysfunction and fibrosis [46], [47]. Under strong antioxidant and anti-inflammatory potential, BNM reversed the typical characteristics of pancreatitis by inhibiting proinflammatory cytokines and restoring the altered levels of lipase and amylase. Moreover, BNM attenuated the expression of NFκB pathway, resulting in an overall improvement of the pancreas in L-arginine induced acute pancreatitis in mice [13], [15]. Similarly, PEGylated BNM has been shown beneficial against allergen-induced allergic asthma by ameliorating Th2 mediated allergic lung inflammation confirmed by reduced Th2 cell population and related cytokines level [34].
Santangelo et al., demonstrated the virucidal effect of bilirubin against the enterovirus EV71 and human herpes simplex virus type 1 (HSV-1). Bilirubin-IX-α, at concentrations of 1–10 μM, close to those found in blood and tissues, significantly reduced the EV71 and HSV-1 replication in Vero and Hep-2 cell lines, respectively. Bilirubin-IX-α inhibited the viral infection of Vero cells and Hep-2 when given 2 h before and concomitantly after 2 h of the viral infection [6]. Similarly, biliverdin (BV) has been reported as a potent inhibitor of hepatitis C virus (HCV) NS3/4A protease, which exerts antiviral action against HCV by inducing dose-dependent activity of heme oxygenase-1. BV showed remarkable antiviral activity at concentrations as low as 20 μM. BV also augmented the antiviral activity of α-interferon in replicons. All these findings suggested that bilirubin and BV or its derivatives have potential antiviral activities which can also be investigated against the SARS-CoV-2.
Possible novel treatment strategies of bilirubin for COVID-19 therapeutics
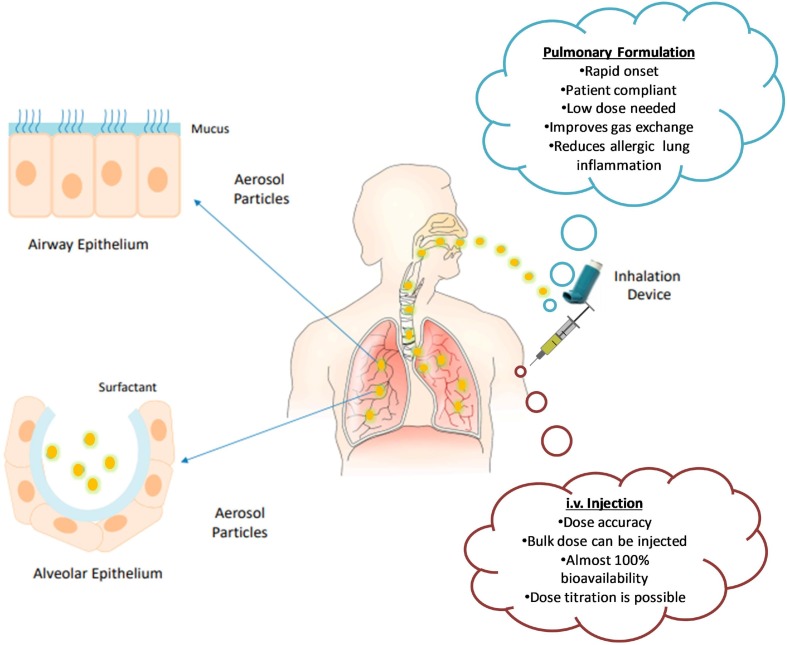
Dosage form and route of drug administration to the patients remain key factors for an effective treatment strategy when using conventional or novel treatment options. The critical assessment of physicochemical and pre-formulation parameters is essential to develop a robust formulation that can deliver maximum therapeutic outcomes in patients [48]. To this end, nanotechnology and nanobiotechnology are providing innovative solutions [49]. Principally, COVID-19 causes significant damage to the lungs and renders them inefficient for routine function; therefore, pulmonary drug delivery must be incorporated as a therapeutic strategy for prominent action [50]. In this context, aerosol-based inhalation formulations might be a better approach which is not only patient compliant but can also exhibit the rapid relief over a defined period. In its free form, bilirubin has problems with its solubility; hence, its administration in nanoparticulate form offers enhanced solubility and improved efficacy. BNM as aerosol-based drug delivery system can deliver cargo directly to the lungs and reduce the complications of COVID-19 resulting in improved patient status. As the dose is required to be taken at short time intervals, it poses compliance issues. In such cases, BNM sustained release formulation can be administered directly through the intravenous route, which can provide 100% bioavailability and enhanced clinical response.
Bilirubin has unveiled encouraging effects in eliminating overproduced ROS and maintaining the cellular redox homeostasis; therefore, it is considered an efficacious therapeutic agent. Exogenous bilirubin also serves as an alternative for the shielding of heme oxygenase in various organs of the body. However, ample information regarding the toxic traits of bilirubin is present, such as neonatal hyperbilirubinemia and kernicterus [51], [52]. In particular, the low solubility of bilirubin at physiological pH due to internal hydrogen bonds between the different polar groups is a hindrance to its intravenous administration. Several attempts have been made to overcome this limitation, such as binding bilirubin with glutathione-S-transferase, an intracellular protein or with albumin [53]. Kristin et al., demonstrated a protocol for intravenous administration of the exogenous bilirubin for its protective effects on ischemia-reperfusion induced injury in rats' kidneys [54], where, bilirubin was added to a solution of pH 8 with the exception of albumin [55].
Moreover, it was suggested that upon intravenous administration, the protective effects of bilirubin have been observed to be model specific and organ specific. These effects may arise as a result of the variance in regional oxygen tension and microcirculatory hemodynamics of that particular organ, which plays climacteric role in the reperfusion phase that occurs instantly after a cardiac ischemic injury. Therefore, intravenous infusion of bilirubin was effective in prompt elevations of serum bilirubin concentrations, for a very short period of time. Besides, Nakao et al. have also observed the identical effects but were unable to produce these results in the kidney and heart in animal models [56]. Similarly, Clark et al., demonstrated similar effects in the isolated perfused rat heart model [57]. Hence, it delineates that various efforts have been made for intravenous administration of bilirubin to explore its usefulness as a therapeutic tool. Further, adding other treatment regimens in combination with BNM would serve as a dual synergistic approach. Fig. 3 shows the proposed routes for BNM administration.
Fig. 3.
Administration of BNM by various routes and their advantages. Part of the figure were adapted and reproduced from reference [70] under the Creative Commons Attribution License (CCBY).
Experimental strategy to test the hypothesis of bilirubin nanomedicine (BNM) as a potential candidate against COVID-19
The airway epithelial cells are located at the interface between the external and internal milieu and demonstrate a broad spectrum of activities associated with pulmonary inflammation, immune response, host defense and tissue remodeling. The airway epithelium plays a crucial role in the defense against various inhaled substances by modulating innate and adaptive immunity [58]. Moreover, airway epithelial cells show highly-expressed angiotensin-converting enzyme 2 (ACE2) and transmembrane serine proteinase 2 (TMPRSS2), as essential receptors for SARS-CoV-2 entry [59], [60]. Further, experiments with SARS-CoV-2 infection using primary human airway epithelial cells have been found to demonstrate cytopathic effects 96 h after the infection [61]. Therefore, primary human airway epithelial cells can be utilized to test the potential anti-COVID-19 effects of BNM. Herein, we propose the following experimental design to investigate the effectiveness of BNM against COVID-19- group-I: control-bronchial primary human airway epithelial cells, group-II: bronchial primary human airway epithelial cells infected with SARS-CoV-2, group III: treatment of SARS-CoV-2 infected primary airway epithelial cells with various doses of BNM. Bronchial primary human airway epithelial cells can be maintained and cultured as earlier described by Vanderheiden et al. [62]. Infection in the bronchial primary human airway epithelial cells would trigger strong immune-mediated inflammatory cytokine response, while BNM treatment can reduce the cytokine storm and other proinflammatory chemical mediators.
In the case of COVID-19, where disease pathogenesis is complex and progressive, combination therapy is needed to cure or prevent further disease severity through a multidimensional mechanism of action. While proposing bilirubin as nanomedicine for the clinical management of COVID-19, which itself holds the therapeutic effects, the question arises how to design a safe and effective BNM rationally and effectively. We sincerely believe that this is a part at the end of both, i.e., clinical and formulation scientists. Therefore, when developing BNM, one should choose site-specific targeting to avoid the accumulation of free drug in healthy tissues and to enhance transportation across various biological barriers for improved drug bioactivity [63]. Further developed BNM should be characterized using state-of-the-art biophysical techniques for physicochemical analysis such as physical and surface properties, architectural composition and stability using standardized protocols [64]. Biocompatibility and comprehensive toxicity analysis of developed BNM choosing standard protocols and appropriate cell line based in vitro assay and the in vivo animal models following the guidelines of Organisation for Economic Co-operation and Development (OECD) for testing of chemicals are necessary, before its preclinical or clinical evaluation [65], [66].
Until now, we know that the most prominent pathophysiological features of COVID-19 are upper respiratory tract infections that gradually lead to alveolar epithelial damage and chronic inflammation. In severe cases, when the virus severely attacks the immune system and modulates cellular and molecular homeostasis, it can cause systemic infection and life-threatening complications. Furthermore, there is a lack of understanding of the precise pathobiology and mechanistic molecular cascades involved in the pathogenesis of COVID-19. Now if we specifically focus on assessing the therapeutic effectiveness of BNM developed for COVID-19, this can be done using clinically relevant cell lines based in vitro and in vivo animal models. In order to evaluate the altered expression of major cytokines relevant to COVID-19, an enzyme-linked immunosorbent assay (ELISA) may be performed on physiological fluid samples, or tissue homogenate of a particular organ, such as the lungs of the BNM treated experimental animals infected with SARS-CoV-2 [34]. In addition, intracellular cytokines staining with the aid of flow cytometry may also be performed. The tissue histopathological analysis can be performed to observe the major pathological changes in vital organs, such as lungs, kidneys and heart of the experimental animals. It is known that COVID-19 cause the upper respiratory tract collapse and bronchial inflammation, ROS generation assay using 5-(and-6)-chloromethyl-2′,7′-dichlorofluorescein diacetate (DCF-DA) staining and levels of proinflammatory cytokines and chemokines can also be analyzed using ELISA [34]. Further experiments such as quantitative polymerase chain reaction (qPCR) assay and indirect immunofluorescence assay (IFA) may be performed to analyze the inhibition of viral replication, its entry into the host cells and virucidal effect of BNMs [67], [68].
Implications of the hypothesis
In conclusion, BNM may provide a novel strategy for the pharmacotherapy of COVID-19 as it possesses not only antiviral efficacy but also anti-inflammatory and antioxidant effects. Further, it can be administered either systemically by intravenous route or locally to the lungs as an aerosol. Given that moderately higher serum levels of bilirubin have positive health benefits, the use of BNMs for COVID-19 management sounds appealing. Owing to the physiological relevance of bilirubin, BNMs may be excellent choice for effective management of this deadly viral infection.
Limitations and practical challenges in the implementation of proposed hypothesis
There is always the possibility of viral mutations and resistance development against the currently available medications, which may lose their effectiveness due to their specific response under different pathophysiological conditions. Therefore, evaluating effectiveness and safety and repurposing existing drugs are of utmost importance and should be considered a continuous process. For any new chemical entity or a drug molecule in the preclinical or clinical phase; it is crucial to determine its appropriate dosage form and route of administration to achieve maximum therapeutic response in patients. Since bilirubin is a hydrophilic molecule, it is susceptible to first-pass hepatic metabolism. This results in low systemic bioavailability of bilirubin and limits its clinical application as a drug candidate. Therefore, we propose the clinical use of bilirubin as a nanomedicine using appropriate drug delivery systems to overcome the limitations of poor solubility, sustained or controlled drug release and improved pharmacokinetics of bilirubin. Concurrently, nano-based drug delivery systems offer the advantage of high drug payload, smaller size and reduced dose associated side effects. Besides, it is necessary to identify a suitable route of administration for BNM and a comprehensive toxicity assessment using clinically relevant animal models. There are significantly less data available on direct inhalation toxicological studies of different types of nanoparticles. The determination of the actual inhalation dose requirement is a daunting process which has restricted the specific designs of direct BNM inhalation formulations. Also, we are still a long way from deducing the nanotoxicological assessments in the relevant disease model [69]. Therefore, a comprehensive evaluation is needed before implementing the proposed hypothesis in the therapeutics of COVID-19.
Author contributions
IK, PA, AK, AKK, USN, AKB, BKK- Conceptualization, Methodology, Writing-original draft. PA, AK, BKK- Writing - review and editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the Dean, Faculty of Veterinary Science, PV Narasimha Rao Telangana Veterinary University (PVNRTVU), Hyderabad, India.
References
- 1.Oroojalian F., Haghbin A., Baradaran B., Hemat N., Shahbazi M.-A., Baghi H.B., et al. Novel insights into the treatment of SARS-CoV-2 infection: an overview of current clinical trials. Int J Biol Macromol. 2020 doi: 10.1016/j.ijbiomac.2020.09.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Contini C., Caselli E., Martini F., Maritati M., Torreggiani E., Seraceni S., et al. Covid-19 is a multifaceted challenging pandemic which needs urgent public health interventions. Microorganisms. 2020;8(8):1228. doi: 10.3390/microorganisms8081228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cain D.W., Cidlowski J.A. After 62 years of regulating immunity, dexamethasone meets COVID-19. Nat Rev Immunol. 2020;20(10):587–588. doi: 10.1038/s41577-020-00421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group R.C. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammers T., Sofias A.M., van der Meel R., Schiffelers R., Storm G., Tacke F., et al. Dexamethasone nanomedicines for COVID-19. Nat Nanotechnol. 2020;15(8):622–624. doi: 10.1038/s41565-020-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santangelo R., Mancuso C., Marchetti S., Di Stasio E., Pani G., Fadda G. Bilirubin: an endogenous molecule with antiviral activity in vitro. Front Pharmacol. 2012;3:36. doi: 10.3389/fphar.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara R., Haag M., Schaeffeler E., Nies A.T., Zanger U.M., Schwab M. Systemic regulation of bilirubin homeostasis: potential benefits of hyperbilirubinemia. Hepatology. 2018;67(4):1609–1619. doi: 10.1002/hep.29599. [DOI] [PubMed] [Google Scholar]
- 8.Ziberna L., Martelanc M., Franko M., Passamonti S. Bilirubin is an endogenous antioxidant in human vascular endothelial cells. Sci Rep. 2016;6:29240. doi: 10.1038/srep29240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Q., Sun R., Bao S., Chen R., Kou L. Bilirubin protects transplanted islets by targeting ferroptosis. Front Pharmacol. 2020;11:907. doi: 10.3389/fphar.2020.00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vítek L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol. 2012;3:55. doi: 10.3389/fphar.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takei R., Inoue T., Sonoda N., Kohjima M., Okamoto M., Sakamoto R., et al. Bilirubin reduces visceral obesity and insulin resistance by suppression of inflammatory cytokines. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0223302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Q., Jiang X., Zhai Y.-Y., Luo L.-Z., Xu H.-L., Xiao J., et al. Protective effects and mechanisms of bilirubin nanomedicine against acute pancreatitis. J Control Release. 2020;322:312–325. doi: 10.1016/j.jconrel.2020.03.034. [DOI] [PubMed] [Google Scholar]
- 14.Creeden J.F., Gordon D.M., Stec D.E., Hinds T.D., Jr. Bilirubin as a metabolic hormone: the physiological relevance of low levels. Am J Physiol Endocrinol Metab. 2020 doi: 10.1152/ajpendo.00405.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vítek L., Tiribelli C. Bilirubin, intestinal integrity, the microbiome, and inflammation. N Engl J Med. 2020;383(7):684–686. doi: 10.1056/NEJMcibr2013250. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y., Kim H., Kang S., Lee J., Park J., Jon S. Bilirubin nanoparticles as a nanomedicine for anti-inflammation therapy. Angew Chem Int Ed Engl. 2016;55(26):7460–7463. doi: 10.1002/anie.201602525. [DOI] [PubMed] [Google Scholar]
- 17.Zahir F., Rabbani G., Khan R.H., Rizvi S.J., Jamal M.S., Abuzenadah A.M. The pharmacological features of bilirubin: the question of the century. Cell Mol Biol Lett. 2015;20(3):418–447. doi: 10.1515/cmble-2015-0012. [DOI] [PubMed] [Google Scholar]
- 18.Zheng J.D., He Y., Yu H.Y., Liu Y.L., Ge Y.X., Li X.T., et al. Unconjugated bilirubin alleviates experimental ulcerative colitis by regulating intestinal barrier function and immune inflammation. World J Gastroenterol. 2019;25(15):1865–1878. doi: 10.3748/wjg.v25.i15.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S.Y., Park S.C. Physiological antioxidative network of the bilirubin system in aging and age-related diseases. Front Pharmacol. 2012;3(45) doi: 10.3389/fphar.2012.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nejedlá Z. The development of immunological factors in infants with hyperbilirubinemia. Pediatrics. 1970;45(1):102–104. [PubMed] [Google Scholar]
- 21.Větvička V., Miler I., Šíma P., Táborský L., Fornůsbk L. The effect of bilirubin on the Fc receptor expression and phagocytic activity of mouse peritoneal macrophages. Folia Microbiol. 1985;30(4):373–380. doi: 10.1007/BF02927593. [DOI] [PubMed] [Google Scholar]
- 22.Haga Y., Tempero M.A., Kay D., Zetterman R.K. Intracellular accumulation of unconjugated bilirubin inhibits phytohemagglutin-induced proliferation and interleukin-2 production of human lymphocytes. Dig Dis Sci. 1996;41(7):1468–1474. doi: 10.1007/BF02088574. [DOI] [PubMed] [Google Scholar]
- 23.Kapitulnik J. Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol Pharmacol. 2004;66(4):773–779. doi: 10.1124/mol.104.002832. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y., Li P., Lu J., Xiong W., Oger J., Tetzlaff W., et al. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J Immunol (Baltimore, Md.: 1950) 2008;181(3):1887–1897. doi: 10.4049/jimmunol.181.3.1887. [DOI] [PubMed] [Google Scholar]
- 25.Jangi S., Otterbein L., Robson S. The molecular basis for the immunomodulatory activities of unconjugated bilirubin. Int J Biochem Cell Biol. 2013;45(12):2843–2851. doi: 10.1016/j.biocel.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Panwar R., Raghuwanshi N., Srivastava A.K., Sharma A.K., Pruthi V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mater Sci Eng C. 2018;92:381–392. doi: 10.1016/j.msec.2018.06.055. [DOI] [PubMed] [Google Scholar]
- 27.Raghuwanshi N., Srivastava A.K., Yadav T.C., Gupta S., Khatri K., Pruthi V., et al. Biogenic nanoparticles as theranostic agents: prospects and challenges. Integr Green Chem Sustain Eng. 2019:647–684. [Google Scholar]
- 28.Khurana A., Tekula S., Saifi M.A., Venkatesh P., Godugu C. Therapeutic applications of selenium nanoparticles. Biomed Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 29.Mishra P., Gupta P., Pruthi V. Cinnamaldehyde incorporated gellan/PVA electrospun nanofibers for eradicating Candida biofilm. Mater Sci Eng C. 2020;119 doi: 10.1016/j.msec.2020.111450. [DOI] [PubMed] [Google Scholar]
- 30.Yadav T.C., Saxena P., Srivastava A.K., Singh A.K., Yadav R.K., Prasad R., et al. Potential applications of chitosan nanocomposites: recent trends and challenges. Adv Funct Text Polym Fabric Process Appl. 2019:365–403. [Google Scholar]
- 31.Khurana A., Sayed N., Allawadhi P., Weiskirchen R. It’s all about the spaces between cells: role of extracellular matrix in liver fibrosis. Ann Transl Med. 2020 doi: 10.21037/atm-20-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araujo J., Zhang M., Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol. 2012;3(119) doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar S., Makhal A., Baruah S., Mahmood M.A., Dutta J., Pal S.K. Nanoparticle-sensitized photodegradation of bilirubin and potential therapeutic application. J Phys Chem C. 2012;116(17):9608–9615. [Google Scholar]
- 34.Kim D.E., Lee Y., Kim M., Lee S., Jon S., Lee S.-H. Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials. 2017;140:37–44. doi: 10.1016/j.biomaterials.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Kim M.J., Lee Y., Jon S., Lee D.Y. PEGylated bilirubin nanoparticle as an anti-oxidative and anti-inflammatory demulcent in pancreatic islet xenotransplantation. Biomaterials. 2017;133:242–252. doi: 10.1016/j.biomaterials.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y., Sugihara K., Gillilland M.G., Jon S., Kamada N., Moon J.J. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater. 2019:1–9. doi: 10.1038/s41563-019-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rathinaraj P., Muthusamy G., Prasad N.R., Gunaseelan S., Kim B., Zhu S. Folate–gold–bilirubin nanoconjugate induces apoptotic death in multidrug-resistant oral carcinoma cells. Eur J Drug Metab Pharmacokinet. 2019:1–12. doi: 10.1007/s13318-019-00600-9. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y., Lee S., Jon S. Biotinylated bilirubin nanoparticles as a tumor microenvironment-responsive drug delivery system for targeted cancer therapy. Adv Sci. 2018;5(6):1800017. doi: 10.1002/advs.201800017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allawadhi P., Khurana A., Allwadhi S., Joshi K., Packirisamy G., Bharani K.K. Nanoceria as a possible agent for the management of COVID-19. Nano Today. 2020;35 doi: 10.1016/j.nantod.2020.100982. 100982–100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allawadhi P., Khurana A., Allwadhi S., Navik U.S., Joshi K., Banothu A.K., Bharani K.K. Potential of electric stimulation for the management of COVID-19. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110259. 110259–110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Wang Y., Agostinis P., Rabson A., Melino G., Carafoli E., et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11(7):512. doi: 10.1038/s41419-020-2721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Q., Chen R., Ganapathy V., Kou L. Therapeutic application and construction of bilirubin incorporated nanoparticles. J Control Release. 2020;328:407–424. doi: 10.1016/j.jconrel.2020.08.054. [DOI] [PubMed] [Google Scholar]
- 43.Fullagar B., Rao W., Gilor C., Xu F., He X., Adin C.A. Nano-encapsulation of bilirubin in pluronic F127-chitosan improves uptake in β cells and increases islet viability and function after hypoxic stress. Cell Transpl. 2017;26(10):1703–1715. doi: 10.1177/0963689717735112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robba C., Battaglini D., Pelosi P., Rocco P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Exp Rev Respir Med. 2020:1–4. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keum H., Kim T.W., Kim Y., Seo C., Son Y., Kim J., et al. Bilirubin nanomedicine alleviates psoriatic skin inflammation by reducing oxidative stress and suppressing pathogenic signaling. J Control Release. 2020;325:359–369. doi: 10.1016/j.jconrel.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Kim J.Y., Lee D.Y., Kang S., Miao W., Kim H., Lee Y., et al. Bilirubin nanoparticle preconditioning protects against hepatic ischemia-reperfusion injury. Biomaterials. 2017;133:1–10. doi: 10.1016/j.biomaterials.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Surendran S.P., Thomas R.G., Moon M.J., Park R., Lee J.H., Jeong Y.Y. A bilirubin-conjugated chitosan nanotheranostics system as a platform for reactive oxygen species stimuli-responsive hepatic fibrosis therapy. Acta Biomater. 2020 doi: 10.1016/j.actbio.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 48.6 – Drug delivery systems. In Bruschi ML, ed., Strategies to modify the drug release from pharmaceutical systems. Woodhead Publishing; 2015, pp. 87–194.
- 49.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tse G.M., To K.F., Chan P.K., Lo A.W., Ng K.C., Wu A., et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J Clin Pathol. 2004;57(3):260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bratlid D. The effect of pH on bilirubin binding by human erythrocytes. Scand J Clin Lab Invest. 1972;29(4):453–459. doi: 10.3109/00365517209080265. [DOI] [PubMed] [Google Scholar]
- 52.Hahm J.S., Ostrow J.D., Mukerjee P., Celic L. Ionization and self-association of unconjugated bilirubin, determined by rapid solvent partition from chloroform, with further studies of bilirubin solubility. J Lipid Res. 1992;33(8):1123–1137. [PubMed] [Google Scholar]
- 53.Ostrow J.D., Mukerjee P., Tiribelli C. Structure and binding of unconjugated bilirubin: relevance for physiological and pathophysiological function. J Lipid Res. 1994;35(10):1715–1737. [PubMed] [Google Scholar]
- 54.Kirkby K., Baylis C., Agarwal A., Croker B., Archer L., Adin C. Intravenous bilirubin provides incomplete protection against renal ischemia-reperfusion injury in vivo. Am J Physiol Renal Physiol. 2007;292(2):F888–F894. doi: 10.1152/ajprenal.00064.2006. [DOI] [PubMed] [Google Scholar]
- 55.Adin C.A., Croker B.P., Agarwal A. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am J Physiol Renal Physiol. 2005;288(4):F778–F784. doi: 10.1152/ajprenal.00215.2004. [DOI] [PubMed] [Google Scholar]
- 56.Nakao A., Choi A.M.K., Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. 2006;10(3):650–671. doi: 10.1111/j.1582-4934.2006.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clark J.E., Foresti R., Sarathchandra P., Kaur H., Green C.J., Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278(2):H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 58.Hiemstra P.S., McCray P.B., Jr., Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J. 2015;45(4):1150–1162. doi: 10.1183/09031936.00141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H., Rostami M.R., Leopold P.L., Mezey J.G., O'Beirne S.L., Strulovici-Barel Y., et al. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am J Respir Crit Care Med. 2020;202(2):219–229. doi: 10.1164/rccm.202003-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Djomkam A.L.Z., Olwal C.O., Sala T.B., Paemka L. Commentary: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Front Oncol. 2020;10:1448. doi: 10.3389/fonc.2020.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takayama K. In vitro and animal models for SARS-CoV-2 research. Trends Pharmacol Sci. 2020;41(8):513–517. doi: 10.1016/j.tips.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanderheiden A., Ralfs P., Chirkova T., Upadhyay A.A., Zimmerman M.G., Bedoya S., et al. Type I and Type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J Virol. 2020;94(19) doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao Q., Chen R., Ganapathy V., Kou L. Therapeutic application and construction of bilirubin incorporated nanoparticles. J Control Release. 2020 doi: 10.1016/j.jconrel.2020.08.054. [DOI] [PubMed] [Google Scholar]
- 64.Lin P.-C., Lin S., Wang P.C., Sridhar R. Techniques for physicochemical characterization of nanomaterials. Biotechnol Adv. 2014;32(4):711–726. doi: 10.1016/j.biotechadv.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel P., Shah J. Safety and toxicological considerations of nanomedicines: the future directions. Curr Clin Pharmacol. 2017;12(2):73–82. doi: 10.2174/1574884712666170509161252. [DOI] [PubMed] [Google Scholar]
- 66.Soares S., Sousa J., Pais A., Vitorino C. Nanomedicine: principles, properties, and regulatory issues. Front Chem. 2018;6:360. doi: 10.3389/fchem.2018.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci. 2019;26(1):1–10. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Medhi R., Srinoi P., Ngo N., Tran H.-V., Lee T.R. Nanoparticle-based strategies to combat COVID-19. ACS Appl Nano Mater. 2020;3(9):8557–8580. doi: 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- 69.Saifi M.A., Khurana A., Godugu C. Nanomaterials in chromatography. Elsevier; 2018. Nanotoxicology: toxicity and risk assessment of nanomaterials; pp. 437–465. [Google Scholar]
- 70.Orienti I., Gentilomi G.A., Farruggia G. Pulmonary delivery of fenretinide: a possible adjuvant treatment in COVID-19. Int J Mol Sci. 2020;21(11):3812. doi: 10.3390/ijms21113812. [DOI] [PMC free article] [PubMed] [Google Scholar]