Abstract
Synechococcus are picocyanobacteria with a cosmopolitan distribution. They are capable of surviving in a wide variety of environmental conditions. Synechococcus have been isolated from the Chesapeake Bay during winter months, and they show an impressive tolerance to cold temperatures. Cold-adapted Synechococcus are unique and diverse, as they have complex phylogenetic lineages closely related to subalpine cluster II, Bornholm Sea cluster, CB7 cluster, and some novel lineages which are independent from summer estuarine strains in subcluster 5.2. CBW1002 and CBW1006 are the first complete genomes to represent Bornholm Sea cluster Synechococcus strains. They have some of the largest genomes among the Synechococcus (3.8 Mb) and share many unique and cryptic homologs which could give insight into their ability to tolerate such cold and dynamic conditions in the Chesapeake Bay estuary.
Keywords: Bornholm Sea cluster, genome, cold adaptation, Synechococcus, Cyanobium
Significance
These two Synechococcus strains CBW1002 and CBW1006 were isolated from the estuarine environment during the winter, and they are able to grow in low temperature (4–10 °C). The analysis of these two genomes gives some insight into the mechanisms that these organisms may have in order to survive traditionally adverse conditions, such as cold temperatures.
Introduction
The Chesapeake Bay (CB) is the largest estuary in the United States. As a temperate ecosystem, CB exhibits dynamic seasonal and spatial patterns of microbial community (Kan et al. 2006; Wang et al. 2020). Picocyanobacteria contribute significantly to phytoplankton biomass and primary production in the Bay in summer (Wang et al. 2011), and their abundance can exceed more than 1 million cells per milliliter during the bloom season. A large portion of picocyanobacterial strains that have been isolated belong to marine subcluster 5.2 Synechococcus (Chen et al. 2004, 2006) and two genomes of CB Synechococcus strains (CB0101 and CB0205) have been sequenced, representing two different clades CB4 and CB5 of subcluster 5.2 (Marsan et al. 2014, 2017; Fucich et al. 2019).
During the winter, picocyanobacterial abundance in CB decreases dramatically (<1,000 cells/ml). The community structure of CB picocyanobacteria in winter is distinct from that in summer (Cai et al. 2010). In order to understand their cold adaptation, we isolated 17 strains of CB picocyanobacteria from the Baltimore Inner Harbor in winter (Xu et al. 2015). The water temperature varied between 2 and 8 °C during the sampling period and salinity ranged between 5 and 22 ppt. These winter isolates are able to grow in low temperatures and tolerate a wide range of salinities. All the CB winter isolates are not affiliated with marine subcluster 5.2 but instead related to subalpine cluster II, Bornholm Sea cluster (named after the 50-m halocline Bornholm Basin located in the Baltic Sea; Jakobsen 1996), CB7 cluster, and some novel lineages. Interestingly, more than half of these CB winter isolates (9 of 17) are closely related to the Bornholm Sea cluster picocyanobacteria, and CBW1002 and CBW1006 are the two members in this cluster. CBW1002 and CBW1006 were isolated from water with temperature at 6.5 and 6.2 °C, and salinity at 17 and 19 PSU, respectively. Many members of the Bornholm Sea cluster were isolated from the Baltic Sea, a large brackish estuarine ecosystem (Ernst et al. 2003). The close phyletic relationship between CB winter isolates and the Baltic Sea isolates suggests that this group of picocyanobacteria could be important in the estuarine environment, especially under cold climate conditions. It is noteworthy that many CB winter strains in the Bornholm Sea are able to elongate their cells during the cultivation, an interesting feature not commonly seen in other picocyanobacterial lineages (Xu et al. 2015). To the best of our knowledge, no genome sequences have been reported for picocyanobacteria in the Bornholm Sea cluster prior to this study.
Materials and Methods
CBW1002 and CBW1006 were grown in SN15 media (Xu et al. 2015). For DNA extraction, Synechococcus cells of each strain were collected from 20 ml of exponential culture by centrifugation at 10,000 × g for 10 min. Cell pellets were then ground with liquid nitrogen, transferred into 2-ml tubes with CTAB lysis buffer, and then incubated at 65 °C in a water bath for 60 min. Genomic DNA was then extracted by using the phenol–chloroform method described by Kan et al. (2006). The DNA samples were sent to the Beijing Genome Institute (BGI) for sequencing. CBW complete genome sequences were obtained using a combination of Illumina HiSeq and PacBio Sequel platforms. For the raw reads from Illumina sequencing, low quality (≤20), high N nucleotide percentage (>10%), adapter, and duplication reads were removed to obtain clean reads. For PacBio raw sequences, hairpin-shaped adapters were concatenated to DNA templates and formed into a dumbbell shape called SMRTbell, which is deposited in the SMRT cell with 1 million Zero-Mode Waveguides (ZMV) wells. ZMV wells with only one template (P1) were selected to generate polymerase reads. Adapters and poor-quality reads were cut from polymerase reads to generate multiple subreads. Subreads with less than 1,000 nucleotides were filtered out, and remaining subreads were integrated into one circular consensus sequencing read of insert. Subreads were corrected with multiple programs (Pbdagocon, FalconConsensus, and Proovread) to generate corrected reads, which were further constructed using Celera and Falcon to yield optimal assemblies. The yielded assemblies were checked with second-generation Illumina seq for single-nucleotide correction (Quiver, GATK, SOAPsnp/SOAPindel) for the final assemblies. CBW1002 and CBW1006 contain one circular chromosome with no plasmids.
Both genomes were annotated using the BGI pipeline and were confirmed with the Rapid Annotations using Subsystems Technology (RAST) server (Aziz et al. 2008; Overbeek et al. 2014). Homologs were determined in silico using reciprocal best hits were using the BlastP (e-value < 1 × 10−10) of amino acid sequences from open reading frames. Homologs were visualized using the online Circos table viewer (http://mkweb.bcgsc.ca/tableviewer/visualize/) (Krzywinski et al. 2009).
Results and Discussion
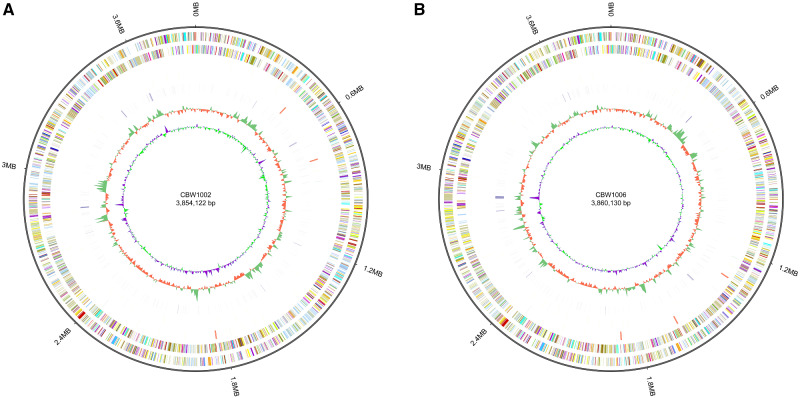
The genome size of CBW1002 and CBW1006 is similar, 3.85 and 3.86 Mb, respectively (fig. 1, table 1). These genome sizes are among the largest genomes for picocyanobacteria. According to the publicly available JGI and NCBI databases, the largest complete Synechococcus genome was the freshwater Synechococcus PCC6312 (3.72 Mb) in current databases. CBW1002 and CBW1006 contain high GC content (>65%). This high GC content is not normally seen in Synechococcus.
Fig. 1.
Circular genome representation of (A) CBW1002 and (B) CBW1006. From the outer to inner, circles represent: 1) genome size; 2) forward strand genes, colored according to cluster of orthologous groups (COG) classification; 3) reverse strand genes, colored according to COG classification; 4) forward strand ncRNA; 5) reverse strand ncRNA; 6) repeat regions; 7) percent GC content; 8) percent GC-SKEW.
Table 1.
Genomic Statistics of CBW1002 and CBW1006.
The large genome size and high GC content make the classification of CBW1002 and CBW1006 uncertain. We named them Synechococcus primarily based on their Synechococcus-like morphology. Given the current revolutionary classification restructuring among Synechococcus and adjacent picocyanobacteria (Coutinho et al. 2016; Salazar et al. 2020), it is probably more appropriate to name them Cyanobium after considering their genomic features and phylogenetic position. Cyanobium spp. in Cyanobiaceae tend to have higher GC content (>65%) and larger genome size compared with the rest members in the order Synechococcales (Salazar et al. 2020). With CBW1002 and CBW1006 genome sequences available, we are now trying to understand their genomic function and evolution via comparative genomics and phylogenomic analysis.
In their large genomes, CBW1002 and CBW1006 have an expansive capacity of coding sequences. CBW1002 contains 3,854,122 bp, a 65.15% GC content, 3,994 coding sequences, and 61 ncRNAs. CBW1006 has a slightly larger genome with 3,860,130 bp, 65.08% GC content, 4,047 coding sequences, and 62 ncRNAs (table 1). Both genomes encode many genes induced by cold conditions (Barria et al. 2013), such as fatty acid synthesis and desaturation related to membrane fluidity modulation, and chaperone proteins that support proper protein folding (supplementary table S1, Supplementary Material online). CBW1002 and CBW1006 contain eight and nine desaturase-related genes, and 29 and 33 chaperone-related genes, respectively. In addition, CBW1002 and CBW1006 have 59 and 35 transposase genes, respectively, suggesting that the ability of acquiring new genes via horizontal gene transfer is important to these two winter strains. Coastal and open ocean picocyanobacteria have no or very low number of transposase genes. Surprisingly, neither strain contained any bacterial cold shock proteins (cspA, cspB, cspC, or cspG). This suggests that CB winter strains have an alternate capacity to handle cold weather conditions and have developed mechanisms to survive in the CB. Detailed comparative genomics between CBW1002 and CBW1006 and other picocyanobacteria is underway.
CBW strains share unique homologs among themselves. In congruence with the phylogeny described previously (Xu et al. 2015), all CBW strains share more homologs with each other than with CB0101 (subcluster 5.2), marine Synechococcus WH8102, or freshwater Synechocystis PCC6803 (fig. 2). The proportion of a strains shared homologs can be seen on the outer most ring, whereas the number of homologs shared between any two strains can be read on the inner ring. CBW1002 and CBW1006 share the most homologs among the CBW strains (n = 3,023), have the largest genomes, and are the most closely related with regard to the partial 16S rRNA gene marker (Xu et al. 2015). Unfortunately, many of these shared homologs do not have well-annotated functions and may be cryptic in nature. Further research should be completed on genes of interest unique to CBW1002 and CBW1006 genomes, as they could play an integral role into how Bornholm Sea cluster Synechococcus are able to survive the harsh winters of the CB estuary.
Fig. 2.
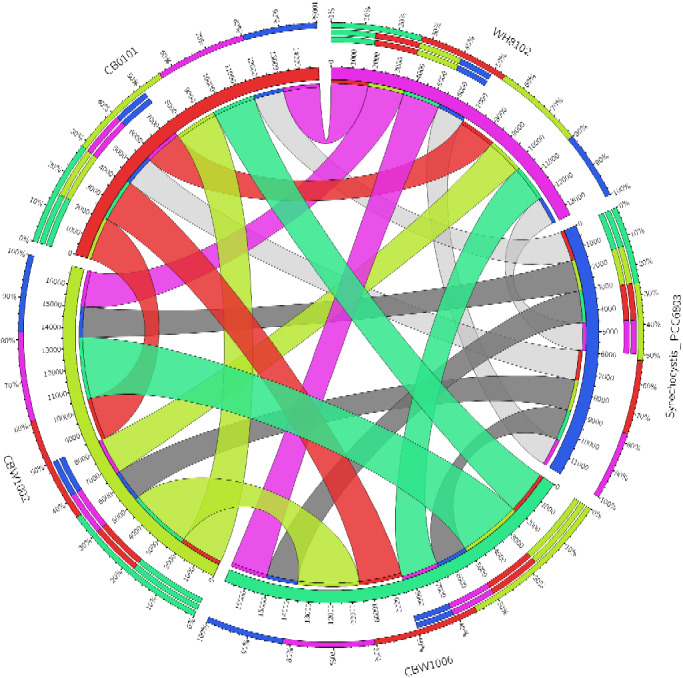
Homologs between selected picocyanobacteria genomes. Chesapeake Bay strains include two winter (CBW) and one summer (CB0101) strain. Model marine strain WH8102, and model freshwater strain Synechocystis PCC6803 were included as outgroups for comparison. Highly shared homologs are in color (top 50th percentile), whereas the fewest shared homologs are in gray. Homologs were determined in silico using reciprocal best hits were using the BlastP (e-value < 1 × 10−10) of amino acid sequences from open reading frames.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2018YFA0605800), the National Natural Science Foundation of China (41861144018, 41706161), the Ratcliffe Environmental Entrepreneurship Fellowship, and the Senior User Project of RV KEXUE (KEXUE2020G10).
Data Availability
Full genome sequences can be found on the National Center for Biotechnology Information (NCBI) website under the accession numbers CP060398 for CBW1002 and CP060396 for CBW1006.
Literature Cited
- Aziz RK, et al. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria C, Malecki M, Arraiano CM. 2013. Bacterial adaptation to cold. Microbiology (United Kingdom) 159(Pt_12):2437–2443. [DOI] [PubMed] [Google Scholar]
- Cai H, Wang K, Huang S, Jiao N, Chen F. 2010. Distinct patterns of picocyanobacterial communities in winter and summer in the Chesapeake Bay. Appl Environ Microbiol. 76(9):2955–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Wang K, Kan J, Suzuki MT, Wommack KE. 2006. Diverse and unique picocyanobacteria in Chesapeake Bay, revealed by 16S-23S rRNA internal transcribed spacer sequences. Appl Environ Microbiol. 72(3):2239–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, et al. 2004. Phylogenetic diversity of Synechococcus in the Chesapeake Bay revealed by Ribulose-1,5-bisphosphate carboxylase-oxygenase (RuBisCO) large subunit gene (rbcL) sequences. Aquat Microb Ecol. 36:153–164. [Google Scholar]
- Coutinho F, Tschoeke DA, Thompson F, Thompson C. 2016. Comparative genomics of Synechococcus and proposal of the new genus Parasynechococcus. PeerJ. 4:e1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst A, Becker S, Wollenzien UIA, Postius C. 2003. Ecosystem-dependent adaptive radiations of picocyanobacteria inferred from 16S rRNA and ITS-1 sequence analysis. Microbiology 149(1):217–228. [DOI] [PubMed] [Google Scholar]
- Fucich D, Marsan D, Sosa A, Chen F. 2019. Complete genome sequence of subcluster 5.2 Synechococcus sp. strain CB0101, isolated from the Chesapeake Bay. Microbiol Resour Announc. 8(35):e00484-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen F. 1996. The dense water exchange of the Bornholm basin in the Baltic Sea. Dtsch Hydrogr Zeitschrift. 48(2):133–145. [Google Scholar]
- Kan J, Crump BC, Wang K, Chen F. 2006. Bacterioplankton community in Chesapeake Bay: predictable or random assemblages. Limnol Oceanogr. 51(5):2157–2169. [Google Scholar]
- Krzywinski M, et al. 2009. Circos: an information aesthetic for comparative genomics. Genome Res. 19(9):1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsan D, Place A, Fucich D, Chen F. 2017. Toxin-antitoxin systems in estuarine Synechococcus strain CB0101 and their transcriptomic responses to environmental stressors. Front Microbiol. 8:1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsan D, Wommack KEK, Ravel J, Chen F. 2014. Draft genome sequence of Synechococcus sp. strain CB0101, isolated from the Chesapeake Bay Estuary. Genome Announc. 2(1):e01111–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek R, et al. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 42:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar VW, et al. 2020. A new genomic taxonomy system for the Synechococcus collective. Environ Microbiol. 22(11):4557–4570. [DOI] [PubMed] [Google Scholar]
- Wang K, Wommack KE, Chen F. 2011. Abundance and distribution of Synechococcus spp. and cyanophages in the Chesapeake Bay. Appl Environ Microbiol. 77(21):7459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang C, Chen F, Kan J. 2020. Spatial and temporal variations of bacterioplankton in the Chesapeake Bay: A re-examination with high-throughput sequencing analysis. Limnol Oceanogr. 65(12):3032-3045.
- Xu Y, Jiao N, Chen F. 2015. Novel psychrotolerant picocyanobacteria isolated from Chesapeake Bay in the winter. J Phycol. 51(4):782–790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full genome sequences can be found on the National Center for Biotechnology Information (NCBI) website under the accession numbers CP060398 for CBW1002 and CP060396 for CBW1006.