Abstract
Background
Although adipose tissue has been proposed to harbor part of the human immunodeficiency virus 1 (HIV-1) reservoir, the influence of host characteristics, including sex and body mass index (BMI), on measures of HIV-1 persistence during antiretroviral therapy (ART) are incompletely understood.
Methods
We evaluated age, sex, BMI, waist circumference, years on ART, pre-ART HIV-1 RNA, pre-ART CD4+ T-cell count, and initial ART regimen with measures of HIV-1 persistence in blood (residual viremia, cellular HIV-1 DNA and RNA) in a cohort of 295 individuals with well-documented long-term virologic suppression (HIV-1 RNA <50 copies/mL) on ART (AIDS Clinical Trials Group study A5321).
Results
Men were more likely than women to have detectable plasma HIV-1 RNA by single-copy assay (52% vs 29%; P = .003), and the proportion of participants with detectable residual viremia increased in a stepwise fashion by BMI category (normal weight or underweight, 38%; overweight, 50%; and obese, 55%). ART regimen type was not associated with measures of HIV-1 persistence after controlling for ART duration.
Conclusions
Sex and obesity are independently associated with residual viremia in people on long-term ART. Additional studies to confirm these relationships and to define the mechanisms by which sex and obesity affect HIV-1 persistence are needed to inform HIV-1 cure strategies.
Keywords: HIV-1, sex, obesity
In 295 individuals with human immunodeficiency virus suppressed by antiretroviral therapy for a median of 7.1 years, detectability of plasma viremia was significantly associated with male sex and high body mass index.
People living with human immunodeficiency virus 1 (HIV-1) and taking antiretroviral therapy (ART) often have low levels of residual viremia when this is measured using research assays, even when the plasma HIV-1 RNA level is below the lower limits of detection of Food and Drug Administration–cleared clinical assays (<20–40 copies/mL) [1–8]. Low-level viremia is often dominated by a relatively small number of clonal sequences [9, 10] and is associated with a shorter time to HIV-1 rebound after treatment interruption [11], implying that infected cell clones with identical proviruses are an important component of the HIV-1 reservoir [12]. Determining the host and viral factors associated with residual viremia is critical to understanding how HIV-1 persists on ART and to informing curative strategies. To date, the level of plasma viremia before ART initiation and the CD8+ T-cell count and CD4/CD8 T-cell ratio during ART have been the only factors identified to be associated with residual viremia [7, 8].
The individuals with male sex have been shown to influence levels of HIV-1 viremia before ART initiation [13–15]. In several cross-sectional and longitudinal studies in people with untreated HIV-1 infection, women had lower levels of plasma viremia than men [16–19]. In a meta-analysis, women had 41% lower plasma HIV-1 RNA levels before starting ART than men, even after controlling for CD4+ T-cell counts [13]. In addition, there is accumulating evidence for sex-based differences in HIV-1 latency and transcription [20] and cellular susceptibility to HIV-1 infection [21]. Other studies demonstrate differences in innate immunity between men and women: women have higher levels of Toll-like receptor-induced interferon-stimulated genes in plasmacytoid dendritic cells during HIV-1 infection [22–24], and macrophages isolated from blood of female donors can be less susceptible to HIV-1 infection due to increased SAMHD1 activity [21]. Other sex-based differences in HIV-1 infection have been documented [14, 15], but more insight is needed into the relationship between sex and measures of HIV-1 persistence during ART.
Another factor that may affect HIV-1 persistence is body fat composition [25]. Recent evidence has demonstrated that CD4+ T cells sorted from human adipose tissue harbor replication-competent HIV-1 at levels similar to those in peripheral blood, suggesting that adipose tissue is an important and overlooked viral reservoir [26, 27]. Both CD8+ and CD4+ T cells are readily isolated from adipose tissue, with a large percentage of T cells expressing memory or T-helper 17 phenotypic markers [28]. In addition, HIV-1 infection and specific antiretroviral agents can affect the amount and distribution of adipose tissue and significantly alter the function of adipocytes and stromal-vascular cells [29–32].
In the current article, we report on the evaluation of participants in a large cohort study of individuals with well-documented HIV-1 suppression on ART, to assess whether sex, adiposity, and ART regimen components are associated with markers of HIV-1 persistence including residual viremia. We also assessed whether persistent inflammation is associated with sex or adiposity in persons on ART.
METHODS
Study Population
We conducted a cross-sectional evaluation of 295 adults who had initiated ART during chronic HIV-1 infection and had sustained virologic suppression (AIDS Clinical Trials Group study [ACTG] studies A5001 and A5321) [33]. The duration of ART was ≥4 years, but in some participants was as long as 16 years. Participants had plasma HIV-1 RNA levels <50 copies/mL by commercial assays by week 48 of ART and at all subsequent time points (isolated measurements <200 copies/mL were allowed), with no reported ART interruptions >21 days. Measurements of HIV-1 persistence, inflammation, and T-cell activation were performed on blood samples obtained during ART and with plasma HIV-1 RNA <40 copies/mL. Participant characteristics were assessed before ART and at the time point when virologic and inflammatory/activation markers were measured, except that waist circumference was assessed at the last available study A5001 time point (which was before the time point when blood measurements were performed),and adherence was assessed over multiple study visits. Adherence was based on self-report of any missed ART doses over the 4 days before an A5001 study visit, using the ACTG adherence questionnaire [34].
Ethics Statement
The institutional review boards at the participating site institutions approved the study. All participants provided written informed consent for their participation in the study.
Measures of HIV-1 Persistence
All assays were performed in batch on frozen samples that had been processed from peripheral blood. HIV-1 RNA in ethylenediaminetetraacetic acid–anticoagulated plasma by single-copy assay (SCA) and HIV-1 DNA and cell-associated HIV-1 RNA (CA-RNA) in peripheral blood mononuclear cells (PBMC) were measured by means of quantitative polymerase chain reaction (qPCR) targeting the integrase region of pol, using previously published methods, primers, and probes [2, 3, 35]. During blood processing, plasma was obtained and spun at 400g for 10 minutes, followed by a second spin at 1350g for 15 minutes to reduce cellular contamination. In addition, each participant plasma sample was spun at 2700g for 15 minutes at 4°C to pellet and further remove debris, fibrinogen, lipids, albumin, or other insoluble complexes that may differ between men and women [36]. Nucleic acid was extracted from plasma or PBMCs using guanidium hydrochloride and proteinase K, followed by guanidium isothiocyantate and glycogen. Glycogen pellets were precipitated with isopropanol, washed with 70% ethanol, dried and resuspended in 5 mmol/L Tris-hydrochloride, and then quantified by UV specrotroscopy.
Plasma HIV-1 RNA extracts were reverse-transcribed and then analyzed with reverse-transcription PCR. Half of the PBMC extract was used for HIV-1 DNA detection, the other half was DNAsed, reextracted, and reverse-transcribed for CA-RNA detection by reverse-transcription PCR. The limit of detection for HIV-1 SCA was 0.4 copies/mL with testing of a 5-mL plasma sample. Every sample tested by SCA included an internal extraction control of replication-competent avian sarcoma leukosis virus long terminal repeat with a splice acceptor virion that has properties similar to those of HIV-1, which must be recovered above a minimum threshold for the assay to pass quality control. Additional SCA controls for each run included plasma samples with HIV-1 levels of 0, 5, or 20-copies/mL HIV-1 generated by the National Institutes of Health–funded Virology Quality Assurance Laboratory at Rush University, Chicago, IL. Cellular qPCR assays included HIV-1–negative PBMCs and validated low copy HIV-infected PBMCs. All HIV-1 control sequences have been validated as a match with our qPCR primer system and must be measured within a predetermined range to pass quality control.
Immunologic Assays
Soluble inflammatory biomarkers were evaluated from frozen plasma samples. Concentrations of interleukin 6 (IL-6), soluble CD14 (sCD14), soluble CD163 (sCD163), neopterin, CXCL10, and tumor necrosis factor (TNF) were quantified using enzyme-linked immunosorbent assay kits, according to the manufacturer’s instructions (R&D). Levels of T-cell activation and cell cycling in cryopreserved PBMCs were determined in batch using multicolor flow cytometry performed with a BD FACSCanto II cytometer and with Live/Dead Aqua cell stain (Invitrogen), CD3 allophycocyanin-H7, CD4 Alexa 488, CD8 V450, PD-1 (clone M1H4) A488, HLA-DR phycoerythrin, and CD38 allophycocyanin (all BD Biosciences). Daily laser calibration and instrument upkeep was performed according to the manufacturer’s recommendation.
Statistical Analyses
Rank-based correlations (Spearman) were performed for analyses; partial correlations (also rank based) generated adjusted correlations. Groups were compared using the Wilcoxon rank sum or Kruskal-Wallis tests. Adjustment for other factors, including pre-ART log10 HIV-1 RNA level, pre-ART CD4+ T-cell count, years on ART, age, and sex at birth (as reported by the participant at study enrollment) were undertaken using rank-based regression models, with the regression outcome variable being the rank of each of the 3 HIV-1 persistence measures [37]. Body mass index (BMI) categories were defined as follows: underweight, BMI <18.5, normal weight (18.5 to <25), overweight (25 to <30), or obese (≥30) (BMI calculated as weight in kilograms divided by height in meters squared). Supplemental analyses examined plasma HIV-1 RNA level as a binary outcome (above vs below assay limit; ie, detectable vs undetectable).
RESULTS
Study Population
A total of 295 participants with HIV-1 on long-term ART who had ≥1 measure of HIV-1 persistence (plasma HIV-1 RNA by SCA, HIV-1 DNA, or CA-RNA) were included in the analysis. The median duration of ART at the time of evaluation was 7 years (interquartile range, 6–10 years). Over the prior 3 years (median, 4 assessments), 80% of participants reported 100% adherence at each visit (79% for men, 87% for women) and 92% reported 100% adherence at all but 1 visit (92% for men, 94% for women). All participants had well-documented virologic suppression during long-term ART. Their median age was 48 years; 18% of the cohort (n = 53) was female. Median weight, hip circumference, and waist circumference were 81.6 kg, 99.5 cm, and 94.4 cm, respectively. The median BMI was 27.0; 31% of participants (n = 91) were normal weight or underweight (31%), 42% (n = 124) were overweight, and 27% (n = 80) were obese. Table 1 shows participant characteristics before ART and at the time of the current study evaluation.
Table 1.
Characteristics of 295 Study Participants Before Antiretroviral Therapy or at Entry into the Studya
| Characteristic | Study Participants | ||
|---|---|---|---|
| Male (n = 242) | Female (n = 53) | Total (N =295) | |
| General | |||
| Age, median (IQR), y | 48 (39–53) | 51 (44–57) | 48 (41–54) |
| Race/ethnicity, no. (%) | |||
| White non-Hispanic | 145 (60) | 20 (38) | 165 (56) |
| Black non-Hispanic | 43 (18) | 15 (28) | 58 (20) |
| Hispanic | 47 (19) | 17 (32) | 64 (22) |
| Time on ART, median (IQR), y | 7 (6–10) | 8 (6–10) | 7 (6–10) |
| BMI related | |||
| Weight, median (IQR), kg | |||
| Pre-ART | 77 (70–88) | 69 (61–82) | 76 (68–87) |
| At study entry | 83 (73–94) | 73 (66–92) | 82 (71–94) |
| Change from pre-ART to study entry | 4.3 (−1.1 to 10.8) | 4.5 (−1.8 to 12.7) | 4.3 (−1.2 to 11.8) |
| Pre-ART BMI, median (IQR)b | 25.3 (23.0–27.9) | 26.7 (23.3–31.0) | 25.7 (23.1–28.1) |
| Pre-ART BMI category, no. (%) | |||
| Underweight | 4 (2) | 1 (2) | 5 (2) |
| Normal weight | 108 (45) | 15 (28) | 123 (42) |
| Overweight | 96 (40) | 22 (42) | 118 (40) |
| Obese | 33 (14) | 15 (28) | 48 (16) |
| BMI at study entry, median (IQR)b | 26.9 (24.0–29.6) | 29.1 (25.2–33.4) | 27.0 (24.3–31.0) |
| BMI category at study entry, no. (%) | |||
| Underweight | 1 (0) | 1 (2) | 2 (1) |
| Normal weight | 77 (32) | 12 (23) | 89 (30) |
| Overweight | 108 (45) | 16 (30) | 124 (42) |
| Obese | 56 (23) | 24 (45) | 80 (27) |
| BMI change from pre-ART to study entry, median (IQR)b | 1.4 (−0.3 to 3.4) | 1.5 (−0.6 to 4.9) | 1.4 (−0.4 to 3.6) |
| Waist and hip measurements, median (IQR) | |||
| Waist circumference, cm | 94 (87–101) | 95 (86–109) | 94 (87–102) |
| Hip circumference, cm | 99 (93–105) | 107 (98–120) | 100 (94–107) |
| Waist-hip ratio | 1.0 (0.9–1.0) | 0.9 (0.9–0.9) | 0.9 (0.9–1.0) |
| HIV-1-related | |||
| CD4+ T-cell count, median (IQR), cells/μL | |||
| Pre-ART | 276 (118–380) | 232 (101–353) | 261 (113–375) |
| At study entry | 669 (512–849) | 718 (563–920) | 681 (515–863) |
| CD4/CD8 ratio, median (IQR) | |||
| Pre-ART | 0.3 (0.2–0.5) | 0.3 (0.2–0.5) | 0.3 (0.2–0.5) |
| At study entry | 1.0 (0.7–1.3) | 1.1 (0.8–1.5) | 1.0 (0.7–1.3) |
| Pre-ART plasma HIV-1 RNA, median (IQR), log10 copies/mL | 4.6 (4.3–5.1) | 4.5 (4.1–4.9) | 4.6 (4.2–5.0) |
| HIV-1 RNA, <40 copies/mL | 242 (100) | 53 (100) | 295 (100) |
| Initial ART regimen | |||
| NNRTI + NRTI | 111 (46) | 27 (51) | 138 (47) |
| PI + NRTI | 87 (36) | 17 (32) | 104 (35) |
| INSTI + NRTI | 34 (14) | 7 (13) | 41 (14) |
| Other | 10 (4) | 2 (4) | 12 (4) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV-1, human immunodeficiency virus 1; INSTI, integrate strand transfer inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
aAt entry into the study, participants had been on ART for a median of 7.1 years.
bBMI calculated as weight in kilograms divided by height in meters squared.
Associations Between Pre-ART Characteristics and on-ART Measures of HIV-1 Persistence
Plasma HIV-1 RNA by SCA (n = 285), HIV-1 DNA (n = 285) and CA-RNA (n = 276) on ART were all positively correlated with pre-ART plasma HIV-1 RNA (r = 0.20, 0.35, and 0.29, respectively; P < .001), and negatively correlated with pre-ART CD4+ T-cell count (r = −0.12, −0.35, and −0.21, respectively; all P < .05). On-ART plasma HIV-1 RNA by SCA and HIV-1 DNA were negatively correlated with years on ART (r = −0.15 and −0.12, respectively; P = .01 and .05, adjusting for pre-ART plasma HIV-1 RNA). CA-RNA was not correlated with years on ART. Neither the CD8+ T-cell count nor the CD4/CD8 ratio during-ART was correlated with plasma HIV-1 RNA by SCA (all P > .2).
Sex and Measures of HIV-1 Persistence
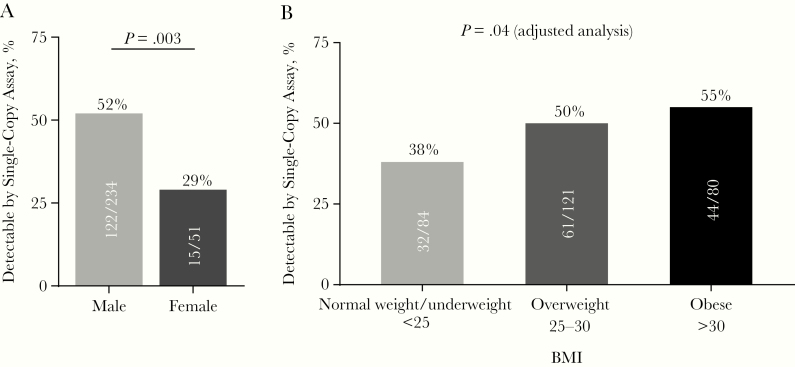
Plasma HIV-1 RNA was detectable using SCA in 137 of 285 plasma samples (48%). In participants with detectable plasma viremia, the median HIV-1 RNA level was 1.5 copies/mL with a range of 0.4–24.9 copies/mL. Men were more likely women to have detectable plasma HIV-1 RNA (≥0.4 copies/mL) (52% vs 29%, respectively; P = .003) (Figure 1A). This relationship between sex and residual viremia persisted after adjustment for participant age, pre-ART plasma HIV-1 RNA, and CD4+ T-cell count, years on ART, and BMI (P = .004). The association between sex and residual viremia remained after further adjustment for race (white vs nonwhite, P = .009) and ethnicity (Hispanic vs non-Hispanic, P = .006). Sex was not associated with median levels of HIV-1 DNA (534 and 629 copies/million CD4+ T cells in men and women, respectively; P = .43) or CA-RNA (47.3 and 25.9 copies/million CD4+ T cells, respectively; P = .61). We did not have information on the menopausal status of the women in the study but did not see an association between age in women (<50 vs ≥50 years) and frequency of detectable residual viremia (detectable in 26% of the 23 women aged <50 years vs 32% of the 28 aged ≥50 years; P = .76).
Figure 1.
Plasma human immunodeficiency virus 1 (HIV-1) RNA detectability by sex or body mass index (BMI) category. Plasma HIV-1 RNA single-copy assay results for 285 of the 295 participants in AIDS Clinical Trials Group (ACTG) study A5321 were grouped according to participant sex (A) or BMI (B). Illustrated are the proportions of HIV-1 RNA levels >0.4 copies per mL by single-copy assay of approximately 5 mL of plasma. The normal weight/underweight group in B includes 82 normal weight (BMI, 18.5–25) and 2 underweight (BMI <18.5) individuals (BMI calculated as weight in kilograms divided by height in meters squared).
Adiposity and Measures of HIV-1 Persistence
Higher BMI (Figure 1B) and larger waist circumference were each associated with more frequent detection of residual viremia (HIV-1 RNA ≥0.4 copies/mL). These associations (r = 0.12 and 0.13, respectively; P < .04) persisted even after adjustment for age, sex, pre-ART HIV-1 RNA and CD4+ T-cell count, and years on ART. Associations remained when correlations were further adjusted for race (white vs nonwhite) and ethnicity (Hispanic vs non-Hispanic). The proportion of participants with detectable viremia increased in a stepwise fashion by BMI category: normal weight or underweight, 38%; overweight, 50%; obese, 55% (Figure 1B). There was no evidence that change in BMI from pre-ART was associated with residual viremia (adjusted r = 0.07; P = .26). BMI and waist circumference (not shown) were not associated with CA-DNA (adjusted r = 0.04; P = .46) or CA-RNA (adjusted r = 0.00; P = .94).
When we examined the relationship between residual viremia and BMI separately in men and women (adjusted for age, pre-ART HIV-1 RNA, CD4+ T-cell count, years on ART, and race [white vs nonwhite]), the correlation estimates were similar for men (r = 0.11; P = .08) and women (r = 0.13; P = .38). The sample sizes when the sexes are analyzed separately are smaller, and the P values are higher. As noted above, the significant relationship between residual viremia and higher BMI in the overall study population was independent of sex.
Sex and Inflammation/Immune Activation
We compared levels of the soluble inflammatory markers (IL-6, CXCL10, neopterin, sCD163, sCD14, and TNF) between men and women. Women had higher levels than men of IL-6 (2.03 vs 1.38 pg/mL, respectively; P = .002), sCD163 (580 vs 522 pg/mL; P = .05), and sCD14 (2.2 vs 1.9 µg/mL; P = .02) (Table 2). None of the soluble inflammatory markers assayed were associated with any of the measures of HIV-1 persistence. CD4+ and CD8+ T-cell activation (CD38/HLA-DR coexpression) was measured in a subset of 98 participants and did not differ significantly between men and women, nor did expression of the checkpoint inhibitor, PD-1 (Table 2). Additional data from analyses that differentiated PD-1hi versus PD-1low expression on CD4+ or CD8+ T cells did not show significant differences between men and women [38], nor did expression of the cell cycling marker, Ki-67, in CD4+ or CD8+ T cells.
Table 2.
Associations of Inflammatory Markers and Cellular Activation Markers With Sex
| Markers and Activation | Median Value (IQR) | P Value | Body Mass Index | ||
|---|---|---|---|---|---|
| Male Participants | Female Participants | Spearman r | P Value | ||
| Soluble inflammatory markers (N = 295) | |||||
| IL-6, pg/mL | 1.4 (0.9–2.0) | 2.0 (1.1–3.0) | .002a | 0.3 | <.001a |
| CXCL10, pg/mL | 120 (84–164) | 128 (93–157) | .7 | 0.09 | .13 |
| Neopterin, pg/mL | 9.3 (7.3–11.0) | 8.7 (7.0–11.5) | .49 | 0.01 | .84 |
| sCD14, µg/mL | 1.9 (1.4–2.4) | 2.2 (1.7–2.5) | .02a | −0.02 | .74 |
| sCD163, pg/mL | 522 (381–732) | 580 (437–916) | .05a | 0.11 | .07a |
| TNF, pg/mL | 2.0 (1.2–3.4) | 1.7 (1.1–2.9) | .32 | 0.05 | .40 |
| Activation of CD4+ T cells, % (n = 98)b | |||||
| CD38+ HLA-DR+ | 3.9 (3.0–5.5) | 3.3 (2.2–4.5) | .09 | −0.18 | .08 |
| PD-1+ | 39.1 (18.2–57.3) | 49.8 (22.1–69.4) | .22 | −0.08 | .45 |
| PD-1hi | 0.3 (0.1–0.9) | 0.4 (0.2–0.7) | .78 | −0.01 | .93 |
| Ki-67+ | 0.6 (0.5–1.0) | 0.5 (0.4–0.7) | .26 | −0.12 | .26 |
| Activation of CD8+ T cells, % (n = 98)b | |||||
| CD38+ HLA-DR+ | 8.5 (4.6–14.7) | 10.2 (6.8–13.7) | .56 | −0.17 | .10 |
| PD-1+ | 33.3 (16.9–62.0) | 57.0 (25.9–71.0) | .14 | −0.1 | .33 |
| PD-1hi | 0.4 (0.2–0.9) | 0.4 (0.2–0.6) | .91 | 0.02 | .88 |
| Ki-67+ | 0.5 (0.3–0.9) | 0.4 (0.4–0.9) | .85 | −0.07 | .47 |
Abbreviations: IL-6, interleukin 6; TNF, tumor necrosis factor; sCD14, soluble CD14, sCD163, soluble CD163.
aSignificant at P < .05.
bFor PD-1 expression, n = 92.
Adiposity and Inflammation/Immune Activation
Of the soluble inflammatory markers measured (CXCL10, neopterin, sCD163, sCD14, and TNF), only IL-6 was positively associated with BMI (r = 0.30; P < .001) (Table 2). BMI was not associated with the CD38/HLA-DR or PD-1 expression on CD4+ (r = −0.18 and −0.08, respectively; P = .08 and .45) or CD8+ T cells (r = −0.17 and −0.10, respectively; P = .10 and .33). Additional analyses showed that BMI was also not associated with PD-1hi or Ki-67+ expression on CD4+ (r = −0.01 and −0.12, respectively; P = .93 and .26) or CD8+ (r = 0.02 and −0.07; P = .88 and .47) T cells.
ART Regimen and HIV-1 Persistence
We assessed whether the initial type of ART regimen (nonnucleoside reverse-transcriptase inhibitor [NNRTI] plus nucleoside reverse-transcriptase inhibitor [NRTI], protease inhibitor (PI) plus NRTI, or integrase strand transfer inhibitor [INSTI] plus NRTI) was associated with on-ART measures of HIV-1 persistence and BMI. Initial ART regimen was not significantly associated with on-ART HIV-1 DNA or CA-RNA (P = .36 and .31, respectively). On-ART residual viremia by SCA was correlated with initial ART regimens containing INSTI (P = .04), but this association was lost after adjustment for duration of ART (P = .16). There was no association of on-ART BMI with initial ART regimen (P = .6; median BMI, 26.9 for NNRTI, 27.8 for PI, and 26.9 for INSTI).
DISCUSSION
In this study of individuals with HIV-1 on long-term suppressive ART, we find that male sex and higher BMI were independently associated with residual plasma viremia measured by HIV-1 RNA SCA. Men were substantially more likely than women to have detectable levels of residual viremia (52% vs 29%, respectively). Although the correlation between BMI and residual viremia was modest, the plausibility of the relationship is supported by the finding that there is a stepwise association between BMI category and the proportion with detectable residual viremia, as follows: normal weight or underweight, 38%; overweight, 50%; and obese, 55%. Adjusting for other factors, such as race or ethnicity, did not reduce the association between BMI and SCA. These findings imply that the host factors of sex [39] and adiposity have an impact on the steady-state levels of virion production and/or clearance in people on ART. Additional studies to confirm these relationships are warranted.
In addition, we found that women had higher peripheral levels of IL-6, sCD14, and sCD163 than men, consistent with other studies [40], but we found no significant association of T-cell activation or proliferation with sex or BMI. IL-6 was positively correlated with BMI [41]. None of the soluble inflammatory markers assayed was associated with any of the measures of HIV-1 persistence. These current findings also support our prior report of no significant correlations between measures of HIV-1 persistence and soluble or cell surface markers of inflammation on ART [42]. Participants who initiated ART with an INSTI-containing regimen had residual viremia detected more frequently than those receiving an NNRTI- or protease inhibitor–containing regimens, but these individuals were generally on ART for a shorter time, and the association between residual viremia and INSTI-based ART was no longer significant after controlling for the duration of ART [8].
Both obesity and sex can have significant effects on hormonal pathways [14, 31] that in turn may affect HIV-1 susceptibilty and persistence. Some studies have shown that the sex hormones estrogen and progesterone affect vaginal HIV-1 and simian immunodeficiency virus transmission and reservoir dynamics [43–46]. However, recent results from the Evidence for Contraceptive Options and HIV-1 Outcomes (ECHO) Trial Consortium did not show increased HIV-1 risk in women using an intramuscular medroxyprogesterone acetate depot or a levonorgestrel implant for contraception [47]. Other recent work has shown that estrogen receptor 1 is a key factor in maintaining HIV-1 latency by inhibiting HIV-1 transcription in infected cells [20]. No transgender individuals participated in this study, but it may be valuable to include individuals taking estrogen in future studies to further examine the impact of sex hormones on residual viremia and other measures of HIV-1 persistence.
Fat also has important hormonal effects, such that adipose tissue is referred to as “an endocrine organ” [48] with profound impact on metabolism and inflammation. We found that higher BMI was associated with more frequent detection of residual viremia, which is consistent with studies that describe adipose tissue as an important HIV-1 reservoir [29, 49]. However, BMI was not associated with levels of HIV-1 DNA or CA-RNA in blood. The association of BMI with residual viremia but not with HIV-1 DNA or CA-RNA in blood could be due to virion production from adipose tissue that equilibrates with plasma and retention of infected cells in fat. This hypothesis could be addressed by measuring HIV-1–infected cell numbers and virions in samples of adipose tissue. BMI was generally higher in women and was associated with higher levels of residual plasma viremia and IL-6, although residual plasma viremia was significantly lower in women even after adjustment for BMI. These findings, combined with the lack of association between inflammation and measures of HIV-1 persistence, indicates that the relationship between adiposity, inflammation, and HIV-1 persistence is complex and may involve other factors that were not measured in the current study.
There have been a number of recent insights into how adipose tissue may serve as an HIV-1 reservoir. Immune cells, such as T cells and macrophages, are found in adipose tissue [27]. CD8+ T cells isolated from adipose tissue usually have higher levels of activation markers than those found in the periphery and are more terminally differentiated [49]. Migration of HIV-1–infected cells into adipose tissue can occur early in HIV-1 infection and persist thereafter, but human adipocytes and preadipocytes lack the HIV-1 entry receptors CD4, CCR5, and CXCR4 and cannot be productively infected. Although HIV-1 DNA is often detectable in adipose tissue, HIV-1 transcription and cell-free HIV-1 RNA appear to be rare [26, 50].
Penetrance of antiretrovirals into adipose tissue has not been extensively studied, but some evidence suggests that NNRTI and INSTI are able to enter adipose tissue, whereas PI and NRTI are more restricted [27, 51]. The recently reported connection between INSTI-based regimens and weight gain [52] may be related to enhanced adipose tissue penetration by these drugs, although the exact metabolic processes involved and the impact of INSTI-based weight gain on residual viremia are not clear. It is conceivable that higher HIV-1 RNA in obese individuals could result from lower penetration of some antiretrovirals into adipose tissue, although previous work in this same cohort demonstrated no significant correlation between cumulative drug concentrations (as reflected by levels in hair) and measures of HIV-1 persistence or soluble markers of inflammation [53].
Our work has revealed associations between residual viremia, sex, and BMI but not markers of inflammation. Further study is needed to determine whether the origin of residual viremia is adipose tissue or whether there are indirect effects of fat on virus production and/or clearance at other sites. These investigations will require sampling and analysis of adipose tissue, which were not part of the current study. Despite this limitation, our findings contribute new insights into host factors that may influence the persistence and expression of HIV-1 on ART. Additional studies to confirm these relationships and to define the mechanisms by which sex and obesity affect HIV-1 persistence are needed to inform HIV-1 cure strategies.
Notes
Acknowledgments. We thank the participants of AIDS Clinical Trials Group study A5321 for their generous involvement in the study, as well as all clinical and research site staff for their efforts. We also thank former statistical team member Christina Lalama for her dedication to this study.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) (grants UM1AI068636, UM1AI106701, UM1AI068634, and UM1AI069481).
Potential conflicts of interest. C. G. wrote this manuscript in her capacity as an NIH employee, but the views expressed herein do not necessarily represent those of the NIH. J. W. M. is a consultant for Gilead and Merck and owns share options in Co-Crystal Pharmaceuticals. R. T. G. has served on a scientific advisory board for Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cillo AR, Sobolewski MD, Bosch RJ, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A 2014; 111:7078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cillo AR, Vagratian D, Bedison MA, et al. Improved single-copy assays for quantification of persistent HIV-1 viremia in patients on suppressive antiretroviral therapy. J Clin Microbiol 2014; 52:3944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hong F, Aga E, Cillo AR, et al. Novel assays for measurement of total cell-associated HIV-1 DNA and RNA. J Clin Microbiol 2016; 54:902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong JK, Hezareh M, Günthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 1997; 278:1291–5. [DOI] [PubMed] [Google Scholar]
- 5. Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 6. Chun TW, Stuyver L, Mizell SB, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 1997; 94:13193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog 2007; 3:e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Riddler SA, Aga E, Bosch RJ, et al. ; ACTG A5276s Protocol Team Continued slow decay of the residual plasma viremia level in HIV-1-infected adults receiving long-term antiretroviral therapy. J Infect Dis 2016; 213:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tobin NH, Learn GH, Holte SE, et al. Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol 2005; 79:9625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bailey JR, Sedaghat AR, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 2006; 80:6441–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Scheerder MA, Vrancken B, Dellicour S, et al. HIV rebound is predominantly fueled by genetically identical viral expansions from diverse reservoirs. Cell Host Microbe 2019; 26:347–58.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Napravnik S, Poole C, Thomas JC, Eron JJ Jr. Gender difference in HIV RNA levels: a meta-analysis of published studies. J Acquir Immune Defic Syndr 2002; 31:11–9. [DOI] [PubMed] [Google Scholar]
- 14. Scully EP Sex differences in HIV infection. Curr HIV/AIDS Rep 2018; 15:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 2014; 209(suppl 3):S86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meditz AL, MaWhinney S, Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis 2011; 203:442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis 1999; 180:666–72. [DOI] [PubMed] [Google Scholar]
- 18. Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med 2001; 344:720–5. [DOI] [PubMed] [Google Scholar]
- 19. Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis 2002; 35:313–22. [DOI] [PubMed] [Google Scholar]
- 20. Das B, Dobrowolski C, Luttge B, et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc Natl Acad Sci U S A 2018; 115:E7795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szaniawski MA, Spivak AM, Bosque A, Planelles V. Sex influences SAMHD1 activity and susceptibility to human immunodeficiency virus-1 in primary human macrophages. J Infect Dis 2019; 219:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol 2006; 177:2088–96. [DOI] [PubMed] [Google Scholar]
- 23. Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med 2009; 15:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seillet C, Laffont S, Trémollières F, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 2012; 119:454–64. [DOI] [PubMed] [Google Scholar]
- 25. Godfrey C, Bremer A, Alba D, et al. Obesity and fat metabolism in human immunodeficiency virus-infected individuals: immunopathogenic mechanisms and clinical implications. J Infect Dis 2019; 220:420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Damouche A, Lazure T, Avettand-Fènoël V, et al. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog 2015; 11:e1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Couturier J, Lewis DE. HIV persistence in adipose tissue reservoirs. Curr HIV/AIDS Rep 2018; 15:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fabbrini E, Cella M, McCartney SA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 2013; 145:366–74.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koethe JR, Hulgan T, Niswender K. Adipose tissue and immune function: a review of evidence relevant to HIV infection. J Infect Dis 2013; 208:1194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giralt M, Domingo P, Villarroya F. Adipose tissue biology and HIV-infection. Best Pract Res Clin Endocrinol Metab 2011; 25:487–99. [DOI] [PubMed] [Google Scholar]
- 31. Erlandson KM, Lake JE. Fat matters: understanding the role of adipose tissue in health in HIV infection. Curr HIV/AIDS Rep 2016; 13:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caron M, Auclair M, Lagathu C, et al. The HIV-1 nucleoside reverse transcriptase inhibitors stavudine and zidovudine alter adipocyte functions in vitro. AIDS 2004; 18:2127–36. [DOI] [PubMed] [Google Scholar]
- 33. Smurzynski M, Collier AC, Koletar SL, et al. AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials 2008; 9:269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reynolds NR, Sun J, Nagaraja HN, Gifford AL, Wu AW, Chesney MA. Optimizing measurement of self-reported adherence with the ACTG adherence questionnaire: a cross-protocol analysis. J Acquir Immune Defic Syndr 2007; 46:402–9. [DOI] [PubMed] [Google Scholar]
- 35. Besson GJ, Lalama CM, Bosch RJ, et al. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis 2014; 59:1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Silliman CC, Dzieciatkowska M, Moore EE, et al. Proteomic analyses of human plasma: Venus versus Mars. Transfusion 2012; 52:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics 1982; 38:715–24. [PubMed] [Google Scholar]
- 38. Macatangay BJC, Gandhi RT, Jones RB, et al. ; ACTG A5321 Team T cells with high PD-1 expression are associated with lower HIV-specific immune responses despite long-term antiretroviral therapy. AIDS 2020; 34:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scully EP, Gandhi M, Johnston R, et al. Sex-based differences in human immunodeficiency virus type 1 reservoir activity and residual immune activation. J Infect Dis 2019; 219:1084–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208:1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koethe JR, Jenkins CA, Furch BD, et al. Brief report: circulating markers of immunologic activity reflect adiposity in persons with HIV on antiretroviral therapy. J Acquir Immune Defic Syndr 2018; 79:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith SM, Baskin GB, Marx PA. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J Infect Dis 2000; 182:708–15. [DOI] [PubMed] [Google Scholar]
- 44. Smith SM, Mefford M, Sodora D, et al. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS 2004; 18:1637–43. [DOI] [PubMed] [Google Scholar]
- 45. Mugo NR, Heffron R, Donnell D, et al. ; Partners in Prevention HSV/HIV Transmission Study Team Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS 2011; 25:1887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fiscus SA, Cu-Uvin S, Eshete AT, et al. ; A5185s Team Changes in HIV-1 subtypes B and C genital tract RNA in women and men after initiation of antiretroviral therapy. Clin Infect Dis 2013; 57:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet 2019; 394:303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 2013; 9:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koethe JR, McDonnell W, Kennedy A, et al. Adipose tissue is enriched for activated and late-differentiated CD8+ T cells and shows distinct CD8+ receptor usage, compared with blood in HIV-infected persons. J Acquir Immune Defic Syndr 2018; 77:e14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hsu DC, Wegner MD, Sunyakumthorn P, et al. CD4+ cell infiltration into subcutaneous adipose tissue is not indicative of productively infected cells during acute SHIV infection. J Med Primatol 2017; 46:154–7. [DOI] [PubMed] [Google Scholar]
- 51. Dupin N, Buffet M, Marcelin AG, et al. HIV and antiretroviral drug distribution in plasma and fat tissue of HIV-infected patients with lipodystrophy. AIDS 2002; 16:2419–24. [DOI] [PubMed] [Google Scholar]
- 52. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 53. Gandhi M, Gandhi RT, Stefanescu A, et al. ; A5321 Team Cumulative antiretroviral exposure measured in hair is not associated with measures of HIV persistence or inflammation among individuals on suppressive ART. J Infect Dis 2018; 218:234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]