Abstract
This case series analyzes brains from autopsies of patients who died of coronavirus disease 2019 as confirmed by nucleic acid test and with severe pulmonary pathology.
Evidence suggests brain involvement in coronavirus disease 2019 (COVID-19). Manifestations in acutely ill individuals often include confusion and alteration of consciousness. After this phase, many patients experience continued neurologic symptoms such as dysexecutive syndrome1 or “brain fog.”2 However, in autopsies from patients with COVID-19 who had neurologic abnormalities (reviewed in the study by Mukerji and Solomon3), investigations have largely not identified the chronic inflammation or marked neural changes typically associated with viral infection, and viral genetic material has been minimal or absent. It has been difficult to reconcile the experience of patients and clinicians that COVID-19 is altering cognition with tissue studies that show no evidence of encephalitis involving higher brain centers. We hypothesized that histopathology might provide insight. We report here a finding that may contribute in some cases, identified by analysis of brain tissue from patients who died of COVID-19.
Methods
The institutional review boards of Johns Hopkins University and Mass General Brigham approved this study, and the next of kin of each patient consented for use of tissues for research. We evaluated brain tissue from autopsies of patients with nucleic acid–proven severe acute respiratory syndrome coronavirus 2 infection and confirmed pulmonary pathology. We assessed the brains from the first 5 such cases at Johns Hopkins University; for 2 of these, only fragments of brain were available. Cases from Mass General Brigham were randomly selected from autopsies of individuals with COVID-19 performed between April 14 and May 15, 2020, and free of infarcts. Detailed information on these 15 patients as well as the 2 control patients without COVID-19 is provided in the eTable in the Supplement. COVID-19–negative cases were chosen because of comparable patient age and the presence of hypoxic-ischemic changes in brain. At autopsy, the cranium was opened with hand tools or a saw with a vacuum shroud to prevent aerosolization. Brains were fixed in neutral buffered formalin, 10%, for 2 weeks, then grossly examined and sectioned for microscopic assessment.
Results
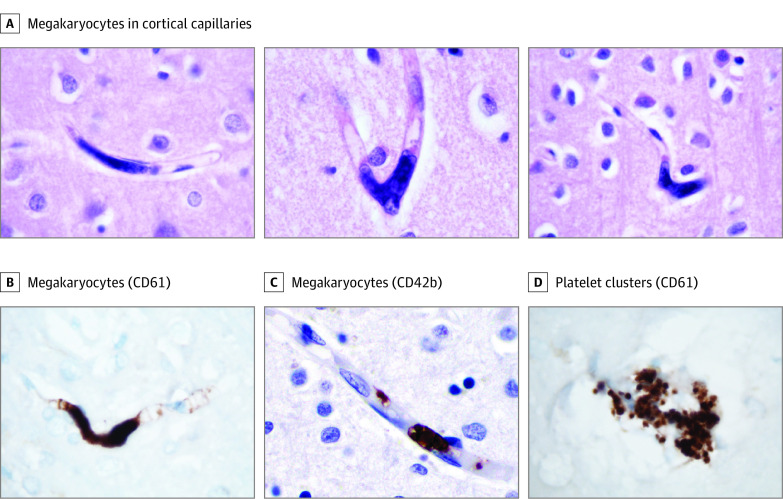
In 5 cases (Table) in cortical capillaries, we identified large cell nuclei morphologically consistent with megakaryocytes (Figure, A). To further characterize these cells, we performed immunohistochemistry for CD61 and CD42b, markers of platelets and megakaryocytes. CD61 labels these cells (Figure, B), as does CD42b (Figure, C), confirming their megakaryocyte identity. The cells were distinct from platelet clusters, which were found in postmortem intravascular precipitates (Figure, D). Evaluation of the cortex of 2 patients who tested negative for COVID-19 who had hypoxic brain changes demonstrated no megakaryocytes on CD61.
Table. Clinical Information for Affected Patientsa.
| Age, y/sex | Key medical history | Platelet count, ×103/µL | Hematocrit, % | White blood cell count, /µL | |||
|---|---|---|---|---|---|---|---|
| Range | Laboratory reference range | Range | Laboratory reference range | Range | Laboratory reference range | ||
| 50s/Male | Myasthenia gravis | 143-494 | 150-350 | 22.5-43.8 | 41.0-53.0 | 8500-45 260 | 4500-11 000 |
| 60s/Female | Coronary artery disease, chronic kidney disease, monoclonal gammopathy of undetermined significance | 137-219 | 150-450 | 18.2-27.9 | 36.0-48.0 | 3210-6700 | 4000-10 000 |
| 70s/Male | End-stage kidney disease, vascular dementia | 170-212 | 150-450 | 34.0-38.4 | 40.0-54.0 | 10 170-27 630 | 4000-10 000 |
| 40s/Male | Diabetes | No values measured; the patient died shortly after arrival to emergency department | |||||
| 70s/Male | Rheumatoid arthritis, hypertension, Parkinson disease | 187-642 | 150-450 | 33.0-38.2 | 40.0-54.0 | 2500-26 860 | 4000-10 000 |
SI conversion factors: To convert hematocrit to proportion of 1.0, multiply by 0.01; platelet count to ×109/L, multiply by 1; white blood cell count to ×109/L, multiply by 0.001.
No patients underwent extracorporeal membrane oxygenation.
Figure. Megakaryocytes in Cortical Capillaries of Patients With Coronavirus Disease 2019.
A, Examples of megakaryocytes in cortical capillaries from affected cases (hematoxylin-eosin). B, CD61 antibody confirms megakaryocyte identity (cellmarque 161M-18; clone Ms/2f2). C, CD42b antibody further confirms megakaryocyte identity (abcam Ab183345; clone SP219). D, Platelet clusters also stained with CD61 antibody and are readily distinguished from megakaryocytes morphologically. The section thickness was 10 μm. Original magnification was ×1000 except for panel C (original magnification ×600).
Discussion
Prior to this pandemic, the study neuropathologists (D.W.N. and I.H.S.) had not seen megakaryocytes in brain vessels, and we find no reference to this in the literature. A recent report showed these cells in an infarcted brain in COVID-19,4 suggesting they could have been present in the brain circulation and entered the parenchyma during hemorrhage.
Multiple lines of evidence indicate endothelial dysfunction may contribute to severe COVID-19 illness. Lung examination demonstrates megakaryocytes,5 and the cells have now been reported in other organs.6 One possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs. Although this initial study does not investigate mechanism, it is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable. By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment.
eTable. Clinical Information for All Study Patients
References
- 1.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268-2270. doi: 10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couzin-Frankel J. From ‘brain fog’ to heart damage, COVID-19’s lingering problems alarm scientists. Science. Published July 31, 2020. Accessed January 28, 2021. doi: 10.1126/science.abe1147 [DOI]
- 3.Mukerji SS, Solomon IH. What can we learn from brain autopsies in COVID-19? Neurosci Lett. 2021;742:135528. doi: 10.1016/j.neulet.2020.135528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen MP, Le Quesne J, Officer-Jones L, et al. Neuropathological findings in two patients with fatal COVID-19. Neuropathol Appl Neurobiol. Published online September 8, 2020. doi: 10.1111/nan.12662 [DOI] [PubMed] [Google Scholar]
- 5.Duarte-Neto AN, Monteiro RAA, da Silva LFF, et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77(2):186-197. doi: 10.1111/his.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapkiewicz AV, Mai X, Carsons SE, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Clinical Information for All Study Patients