Abstract
Objective:
To study the effectiveness of various concentrations of citric acid (CA) added to 2% chlorhexidine (CHX) on smear layer removal from the root canal wall and antimicrobial efficacy against Enterococcus faecalis (E. faecalis) and Candida albicans (C. albicans).
Methods:
Fifty-three single-rooted mandibular premolars were decoronate and the root canals underwent mechanical instrumentation using MTwo rotary files to size 40/0.06. The samples were then randomly divided into 5 groups according to the root canal irrigants to be used: 17% ethylenediaminetetraacetic acid (EDTA), 2% CHX, 1%, 6%, and 10% citric acid-modified 2% chlorhexidine (CAmCHX). Three teeth irrigated with phosphate-buffered saline (PBS) were used as a negative control. The smear layer removal effectiveness was evaluated under scanning electron microscopy (SEM). Images were randomly taken at the apical, middle, and coronal third level. Statistical analysis was performed using Kruskal-Wallis and Mann-Whitney U tests. Disc diffusion and direct exposure tests were performed along with three additional control groups consisting of 1%, 6%, and 10% CA groups to assess and compare the antimicrobial efficacy of irrigants against E. faecalis and C. albicans. Statistical analysis was conducted using one-way ANOVA and Dunnett’s T3 tests.
Results:
Smear layer removal effectiveness in 17% EDTA group and 6% and 10% CAmCHX groups were not significantly different in the coronal and apical third of the root canal (P>0.05), however at the middle third of the root canal, the 10% CAmCHX group had significantly less remaining smear layer than all of the other experimental groups (P<0.05). There was significantly more smear layer remnant in the CHX group (P<0.01). For antimicrobial efficacy, the largest growth inhibition zone against E. faecalis was recorded in the 10% CAmCHX group (P<0.05). For planktonic E. faecalis, 1%, 6%, and 10% CAmCHX demonstrated an insignificant difference in antimicrobial efficacy compared to CHX (P>0.05). CA demonstrated no antifungal effect against C. albicans. Whereas, 6% and 10% CAmCHX resulted in the largest growth inhibition zone. Also, adding CA to CHX resulted in an insignificant difference in antifungal effect against planktonic C. albicans compared to CHX (P>0.05).
Conclusion:
When CA was added into CHX, the mixed irrigant demonstrated smear layer removal ability. Additionally, its antimicrobial effect remained the same.
Keywords: Citric acid, chlorhexidine, root canal irrigants, smear layer
HIGHLIGHTS.
CA concentration of at least 6% added into CHX provided equal smear layer removal efficacy to 17% EDTA
CHX antimicrobial efficacy against planktonic E. faecalis and C. albicans remains unchanged following the addition of CA
The mixed irrigant can help reduce clinical procedure and time compared to the current method without losing efficiency
INTRODUCTION
In root canal treatment, biomechanical instrumentation utilizes a series of instruments to scrape off infected dentine (1). This process creates the smear layer which consists of dentine debris, remnants of odontoblastic processes, pulp tissue, and bacteria. Removal of the smear layer, in addition to eliminating bacteria, also increases the penetration efficiency of irrigants and medicaments into the complex anatomy of the root canal (2). Furthermore, the removal of the loosely adhered smear layer promotes good adaptation of the root filling material to the root canal wall (3).
Effective irrigants play an important role in the elimination of the smear layer. One method to debride the smear layer is the use of sodium hypochlorite (NaOCl) together with an inorganic substance-altering solution, such as chelating agents (ethylenediaminetetraacetic acid; EDTA) or acids (polyacrylic, maleic, or citric acid; CA) (4, 5). Some studies have reported that EDTA is an inefficient root canal disinfectant and should not be used as a stand-alone irrigant (6, 7). A proposed irrigation protocol for smear layer removal suggests using 10 ml of 17% EDTA followed by 1 ml of 5% NaOCl (5, 7). The complexity of this procedure has led to many attempts to develop a new and convenient single irrigant that combines both smear layer removal and disinfection ability. BioPure MTAD (Dentsply, Tulsa, OK, USA) which contains CA, a smear layer remover, and doxycycline, a disinfectant, and QMiX (Dentsply) which contains EDTA and chlorhexidine (CHX), are some example of such new irrigants.
CA is used as a root canal irrigant to remove the smear layer because it is a weak organic acid (8). Studies have evaluated concentrations of CA from 1-50% in their smear layer removal and antimicrobial efficacy (9-11) with a report indicating that the root canal wall can be debrided with 10% CA (12). Also, 10% CA has an equal smear layer removal efficiency to that of 17% EDTA (3, 13). Yamaguchi et al. (10) have demonstrated that CA is able to kill many bacterial strains isolated from infected root dentine. Many in vitro studies have also shown that CA has greater biocompatibility, and less tissue irritation, than does EDTA (14, 15). Therefore, CA may be better suited than EDTA as a root canal irrigant.
CHX, a bis-biguanide synthetic compound, is a broad-spectrum disinfectant. A study shows that 2% CHX can eliminate Enterococcus faecalis (E. faecalis) and Candida albicans (C. albicans) (16, 17). These two microorganisms can be found in root-filled teeth with apical periodontitis (18). In addition, CHX has no effect on the adhesion of resin composite filling materials to the dentine of the pulp chamber (19) or the adhesion of root canal sealers (20). A study has demonstrated that CHX has a substantivity effect, or the long-lasting antimicrobial effect, in the root canal (21). Therefore, CHX seems to be highly effective as a final irrigating solution.
CA has the ability to remove the smear layer, while CHX can eliminate microorganisms. To simplify the irrigation procedure performed using EDTA followed by NaOCl, the authors propose citric acid-modified chlorhexidine (CAmCHX) as a final rinse irrigant. A study detected no formation of a precipitate when CA was added to CHX (22) while another study also detected no change in the demineralization effect of CA when added to CHX (23). Therefore, the aim of this study was to determine the efficacy of various concentrations (1%, 6%, and 10%) of CA added to 2% CHX on smear layer removal, their antibacterial efficacy against E. faecalis, and their antifungal efficacy against C. albicans. The null hypothesis was that there is no difference in smear layer removal and antimicrobial efficacy of the irrigants tested.
MATERIALS AND METHODS
Assessment of smear layer removal
Specimen preparation
This study protocol was approved by the Human Experimentation Committee of the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand (Clearance No.13/2018). Fifty-three single-rooted human mandibular premolars with complete root formation, no dental caries, were extracted for orthodontic reasons and stored in 0.1% thymol solution. Two radiographic images (mesiodistal and buccolingual views) of the teeth were taken to exclude teeth with moderate to severe root curvature and multiple root canals. A diamond disc (3M ESPE, St. Paul, MN, USA) was used to decoronate and flattened the occlusal table to the length of 16 mm. After endodontic access was achieved, a K-file is inserted to a working length of 15 mm, and teeth with an initial apical file size larger than 25 were also excluded from this study to standardize the initial canal size. Two longitudinal grooves, 0.5 mm in depth, were created on the buccal and lingual surfaces using a diamond disc, to facilitate the splitting of the tooth. The roots were then coated with two layers of nail polish and embedded in putty silicone (3M ESPE) to simulate a closed system. The root canals were then mechanically prepared using rotary instruments (MTwo, VDW GmbH, Munich, Germany) up to size 40/0.06 at a working length of 15 mm. During root canal preparation, 20 ml of 5.25% NaOCl (Sigma-Aldrich, St. Louis, MO, USA) was used to irrigate the canal with a side-vented 27-gauge needle placed 2 mm short of the working length. All specimens were then incubated at 37°C.
Experimental groups and SEM examination
The specimens were randomly divided into 5 groups (n=10 for each group) to be irrigated with different freshly-prepared solutions. Side-vented 27-gauge needles placed 2 mm short of the working length were used to irrigate the root canals. The total volume of irrigants (13 ml) and irrigation time were equally measured in every group and the irrigants were administered with a constant 2 mm vertical movement of the needle. The irrigants were aspirated and canals dried with paper points between the use of each irrigant. The experimental groups were classified as follow:
Control group: 13 ml of phosphate-buffered saline (PBS)
Group 1 (17% EDTA): 5 ml of 2.5% NaOCl followed by 3 ml of 17% EDTA (Sigma-Aldrich) and left for 1 minute. Then, rinse with 5 ml of 2.5% NaOCl.
Group 2 (2% CHX): 5 ml of 2.5% NaOCl before flushing out by 5 ml of normal saline solution (NSS). Then, rinse with 3 ml of 2% CHX for 1 minute.
Group 3 (1% CAmCHX): 5 ml of 2.5% NaOCl before flushing out by 5 ml of NSS. Then, rinse with 3 ml of 1% CAmCHX for 1 minute.
Group 4 (6% CAmCHX): 5 ml of 2.5% NaOCl before flushing out by 5 ml of NSS. Then, rinse with 3 ml of 6% CAmCHX for 1 minute.
Group 5 (10% CAmCHX): 5 ml of 2.5% NaOCl before flushing out by 5 ml of NSS. Then rinse with 3 ml of 10% CAmCHX for 1 minute.
After irrigation, all specimens were split longitudinally using chisel and mallet and then kept in 1% osmium tetroxide in 0.1% PBS, pH 7.1 at 4°C for two hours. All teeth were sequentially dehydrated using a graded ethyl alcohol series: 30%, 50%, 70%, 90%, and 100%, then critical-point dried with liquid carbon dioxide, and sputter-coated with gold. Each specimen was examined under a scanning electron microscope (JSM 6610LV, JEOL, Akishima, Japan) at a magnification of 2,000x to assess the efficacy of smear layer removal. For every specimen, three images were randomly recorded at each of three levels: the coronal, middle, and apical third of the root canal. Two blinded and calibrated examiners assessed the amount of remaining smear layer according to the scoring method described by Gutmann et al. (24).
Assessment of antimicrobial properties
Disc diffusion
E. faecalis (ATCC 29212) and C. albicans (ATCC 10231) were used for the assessment of the antimicrobial property of CHX modified with three concentrations of CA (1%, 6%, and 10%) compared to plain 2% CHX, 1% CA, 6% CA, 10% CA, and PBS. The experiment was carried out using disc diffusion. Each test was performed three times. Disc diffusion was performed by incubating E. faecalis and C. albicans overnight at 37ºC on Brain Heart Infusion (BHI) agar and Sabouraud Dextrose Agar (SDA), respectively. The microbes were subcultured in BHI broth (E. faecalis) and Sabouraud Dextrose Broth (SDB) (C. albicans) to a concentration of 0.5 McFarland standard (E. faecalis=1x108 CFU/ml and C. albicans=1x106 CFU/ml). An aseptic cotton swab was used to gather the microbes by placing it in the broth and gently pressed against the tube wall to remove the excess liquid. Then the swab was streaked throughout the agar and left to dry for 5 minutes. 20 ml of each root canal irrigating solution was pipetted and dropped on aseptic filter papers with a diameter of 6 mm before placing the papers on the agar. The agar plates were then incubated at 37ºC for 24 hours and the inhibition zones were measured in millimeters.
Direct exposure test
E. faecalis and C. albicans were incubated overnight at 37ºC on BHI agar and SDA, respectively, as performed in the disc diffusion test. Then, the microbes were subcultured in BHI broth (E. faecalis) and SDB (C. albicans) to a concentration of 0.5 McFarland standard. Following that, 100 µl of the cultured solution was centrifuged at 10.000 rpm for 5 minutes. The supernatants were removed and 100 µl of the root canal irrigating solution was added and incubated for 30, 60, 90, and 120 seconds. After incubation, 900 µl of PBS was added to perform single plate-serial dilution spotting (SP-SDS) (25) on the agar. The agar was then incubated at 37ºC for 24 hours and the number of colonies was counted.
Statistical analysis
The difference in the remaining smear layer and size of the inhibition zone was analyzed using Kruskal-Wallis and Mann-Whitney U tests with a significance level of P<0.05. The difference in the number of bacterial colonies was analyzed using one-way ANOVA and Dunnett’s T3 tests with a significance level of P<0.05. SPSS Statistics 17.0 (IBM Corporation) was used for statistical analysis.
RESULTS
Evaluation of smear layer removal
SEM images representing the coronal, middle, and apical third of the root canal wall are shown in Figure 1. The dentinal tubules in the control group (Fig. 1a-c) and the 2% CHX group (Fig. 1g-i) were completely covered with a smear layer in all three levels. In the EDTA group and every concentration of CAmCHX groups, the tubules were mostly visible at the coronal and middle third of the root canal wall (Fig. 1d-e, 1j-k, 1m-n, 1p-q). However, fairly clean root canal walls with little smear layer covering the dentinal tubules were observed at the apical third. (Fig. 1f, 1l, 1o, 1r).
Figure 1.
SEM images (2.000x) demonstrating the results of each irrigant. The dentinal tubules in the control group and CHX group were completely covered with a smear layer at every level of the root canal (a, b, c, g, h, i). The dentinal tubules in the EDTA group were mostly free of smear layer at the coronal and middle third of the root canal (d,e) but were partially covered with smear layer at the apical third of the root canal (f), similar to the 6% CAmCHX group (m, n, o) and 10% CAmCHX group (p, q, r). The dentinal tubules in the 1% CAmCHX group were heavily covered with smear layer at the apical third but were partially covered at the middle and coronal third of the root canal (j, k, l)
CA: Citric acid, CHX: Chlorhexidine, CAmCHX: Citric acid-modified chlorhexidine, EDTA: Ethylenediaminetetraacetic acid
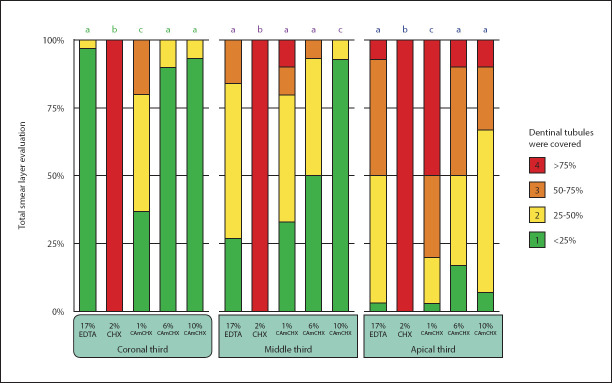
The statistical analysis revealed that the CHX group had significantly more remaining smear layer than all the other experimental groups in all three tested locations (P<0.05). At the coronal and apical third of the root canal, the 6% CAmCHX and 10% CAmCHX groups had comparable remaining smear layer to the EDTA group (P>0.05) while the 1% CAmCHX group had significantly more remaining smear layer than the EDTA group (P<0.05) (Fig. 2). However, at the middle third of the root canal, the 10% CAmCHX group had significantly less remaining smear layer than all of the other experimental groups (P<0.05).
Figure 2.
Stacked bar chart showing the percentage scores for the remaining smear layer coronal, middle, and apical third of the root canal after irrigating with each irrigant. Different superscript letters indicate a statistically significant difference between groups (P<0.05)
CA: Citric acid, CHX: Chlorhexidine, CAmCHX: Citric acid-modified chlorhexidine, EDTA: Ethylenediaminetetraacetic acid
Evaluation of antimicrobial properties
Disc diffusion
For E. faecalis (Table 1), the diameter of the inhibition zone provided by 2% CHX was significantly larger than every concentration of CA (1%, 6%, and 10%) (P<0.05). Statistical analysis showed that the growth inhibition zone provided by 1% CAmCHX was not different from 2% CHX (P>0.05) while 10% CAmCHX had a significantly larger diameter of inhibition zone than 6% CAmCHX and 1% CAmCHX (P<0.05).
TABLE 1.
Mean and standard deviation of the diameter of the growth inhibition zones (mm) provided by different irrigants against E. faecalis and C. albicans
| Irrigant | pH | Diameter of the growth inhibition zones (mm) | |
|---|---|---|---|
| E. faecalis | C. albicans | ||
| PBS | 7.31 | 0 | 0 |
| 1% CA | 2.51 | 7.72±2.97a | 0 |
| 6% CA | 2.03 | 8.67±2.52b | 0 |
| 10% CA | 1.77 | 10.67±1.15c | 0 |
| 2% CHX | 5.51 | 16.67±0.29d | 10.50±1.80A |
| 1% CAmCHX | 3.04 | 16.67±0.29d | 11.33±1.53B |
| 6% CAmCHX | 2.44 | 17.17±0.76e | 11.67±0.58C |
| 10% CAmCHX | 2.38 | 17.50±0.50f | 11.67±0.58D |
CA: Citric acid, CHX: Chlorhexidine, CAmCHX: Citric acid-modified chlorhexidine, PBS: Phosphate-buffered saline. Different superscript letters indicate a statistically significant difference between groups
For C. albicans (Table 1), the inhibition zone was not observed with PBS and all concentrations of CA (1%, 6%, and 10%). However, in the mixed solutions (all concentrations of CAmCHX), a significantly larger diameter of the inhibition zone was observed than with only 2% CHX (P<0.05). There was no significant difference between the inhibition zones of 6% CAmCHX and 10% CAmCHX (P>0.05).
Direct exposure test
As depicted in Table 2, 10% CA can eliminate E. faecalis after 60 seconds, while 2% CHX and the mixture of all concentrations of CAmCHX were able to eliminate the microorganism after only 30 seconds. Every concentration of CA was not capable of eliminating C. albicans (Table 3), opposite to 2% CHX, and the mixture of all concentrations of CAmCHX groups displayed the ability to eliminate the fungus.
TABLE 2.
Number of E. faecalis colonies (LogCFU/ml±SD) after tested with various irrigants for 30, 60, 90 and 120 seconds
| Irrigant | E. faecalis(LogCFU/ml±SD) | |||
|---|---|---|---|---|
| 30 sec | 60 sec | 90 sec | 120 sec | |
| PBS | 7.99±0.01ab | 7.58±0.02ace | 7.42±0.05cde | 8.05±0.10b |
| 1% CA | 7.98±0.09ab | 7.48±0.09cde | 7.36±0.06de | 8.00±0.07ab |
| 6% CA | 7.83±0.06abc | 7.40±0.00cde | 7.10±0.04d | 7.48±0.01cde |
| 10% CA | 7.25±0.54de | 4.91±0.02f | 4.70±0.04f | 3.05±0.10g |
| 2% CHX | 0 | 0 | 0 | 0 |
| 1% CAmCHX | 0 | 0 | 0 | 0 |
| 6% CAmCHX | 0 | 0 | 0 | 0 |
| 10% CAmCHX | 0 | 0 | 0 | 0 |
CA: Citric acid, CHX: Chlorhexidine, CAmCHX: Citric acid-modified chlorhexidine, PBS: Phosphate-buffered saline. Different superscript letters indicate a statistically significant difference between groups
TABLE 3.
Number of C. albicans colonies (LogCFU/ml±SD) after tested with various irrigants for 30, 60, 90 and 120 seconds
| Irrigant | C. albicans (LogCFU/ml±SD) | |||
|---|---|---|---|---|
| 30 sec | 60 sec | 90 sec | 120 sec | |
| PBS | 5.99±0.01a | 5.85±0.05ab | 5.78±0.02ab | 5.88±0.09ab |
| 1% CA | 5.91±0.05ab | 5.77±0.12ab | 5.69±0.02ab | 5.77±0.11b |
| 6% CA | 5.86±0.06ab | 5.71±0.17ab | 5.66±0.07ab | 5.75±0.17b |
| 10% CA | 5.86±0.69ab | 5.73±0.05ab | 5.63±0.08ab | 5.78±0.12b |
| 2% CHX | 0 | 0 | 0 | 0 |
| 1% CAmCHX | 0 | 0 | 0 | 0 |
| 6% CAmCHX | 0 | 0 | 0 | 0 |
| 10% CAmCHX | 0 | 0 | 0 | 0 |
CA: Citric acid, CHX: Chlorhexidine, CAmCHX: Citric acid-modified chlorhexidine, PBS: Phosphate-buffered saline. Different superscript letters indicate a statistically significant difference between groups
DISCUSSION
The results demonstrated that every concentration of CA equally added into 2% CHX could effectively remove the smear layer from the root canal wall comparable to the current standard method of using 17% EDTA. CHX displays a high antimicrobial activity. Also, due to dentine absorption and slow release of CHX, it helps maintain the antimicrobial effect for an extended period of time (26). Despite its antimicrobial efficacy, CHX cannot remove the smear layer which covers the root canal wall following biomechanical debridement, as demonstrated by the CHX group which was used as an internal control. Therefore, in order to remove the smear layer, further procedures involving the use of chelating agents, such as EDTA or CA, are necessary. A study reported that mixing CHX into CA does not change the decalcifying properties of the solution (23). The combination of CA and CHX provides a broad antimicrobial activity (17) and smear layer removal capability. As a result, this mixture may simplify irrigation procedures and decrease tissue toxicity.
The present study found that there was no significant difference between the effectiveness in smear layer removal between 6% and 10% CAmCHX solutions, but both were more effective than the 1% CAmCHX solution. The increasing pH of 2.38, 2.44, and 3.04 after adding 10%, 6%, and 1% CA into CHX, respectively, may have influenced the decreasing smear layer removal ability of the irrigants. Haznedaroglu (9) also reported that at the same concentration, the lower pH solution showed greater smear layer removal ability.
In the current study, no significant difference in smear layer removal ability between 6% and 10% CAmCHX solutions and EDTA was found, which corresponds with other in vitro studies (23, 27). In contrast, Yamada et al. (7) suggested that CA showed less smear layer removal when compared to EDTA. However, irrigating with EDTA for a long period of time can cause peritubular and intertubular dentin erosion, dentin softening, and degradation of collagen fibers, which may affect the bonding of root canal fillings (28).
The second part of this study aimed at assessing the antimicrobial efficiency of the mixed irrigants. The antimicrobial efficiency of various irrigants was tested with E. faecalis and C. albicans because they are the representative bacteria and fungus found in persistent endodontic infection, and have been reported with a high prevalence in failed root canal treated teeth (29, 30). Disc diffusion and direct exposure tests have been selected for this study to determine the antimicrobial efficiency of all experimental groups. Antibacterial and antifungal tests with disc diffusion method demonstrated that 2% CHX is more effective than all concentrations of CA (1%, 6%, and 10%). The results were similar to those of a study by Prado et al. (31), who tested the antimicrobial effect of root canal irrigants on E. faecalis and C. albicans using the disc diffusion method. That study found that 2% CHX had a larger diameter of microbial growth inhibition zone than 10% CA. Interestingly, in that study, all concentrations of CA had no antimicrobial effect on C. albicans. The findings of Prado et al. corresponds with that of Fidalgo et al. (32), who tested the inhibitory activity of 3 root canal irrigants using disc diffusion test and reported that CA did not present any antimicrobial activity on C. albicans. The current study demonstrated that CAmCHX has the ability to kill both microorganisms, and the solution of 6% CAmCHX and 10% CAmCHX had a significantly broader inhibition zone than other groups. These results indicated that the addition of CA into CHX does not decrease the disinfecting ability of the irrigant.
The diameter of the inhibition zone depends on many factors, such as contact time, temperature, the polarization of solutions, dissolution, and diffusion properties (33). To confirm the result, this study tested further with the direct exposure method and found that the results were consistent. 2% CHX and all concentrations of CAmCHX have excellent sterilization effect in every tested duration (30, 60, 90, and 120 seconds). While CA does not affect C. albicans, 10% CA used for more than 60 seconds is required to sterilize E. faecalis. On the contrary, Arias-moliz et al. (34) reported the disinfecting capability of endodontic irrigants using the dilution-neutralization method. Those researchers found that 10% CA required more than 5 minutes to disinfect the tested solutions and 1 minute contact time is not enough to reduce the number of microorganisms. Nevertheless, these discrepancies may have been caused by the differences in methodology, inoculum size, or incubation time.
The authors attempted to develop a new protocol and root canal irrigant to eliminate the smear layer and disinfect the root canal simultaneously. At present, there is no solution that can eliminate both the inorganic structure and the organic structure of the smear layer. Therefore, the current protocol recommends the use of NaOCl to debride the organic component of the smear layer, followed by 17% EDTA to demineralize the inorganic substances of the smear layer. And lastly, rinse with a final irrigant with an antiseptic effect such as NaOCl or CHX to kill any remaining bacteria in the root canal (6, 7). CHX is effective in disinfection, especially against E. faecalis and C. albicans, which can be found with a high prevalence in root-filled teeth with apical periodontitis (18). CA has not only the ability to eliminate the smear layer, but also the antimicrobial ability (10). It is less cytotoxic than EDTA (14), easily attainable, and affordable. The combination of these two compounds is, therefore, an effective enhancement of antimicrobial and smear layer removal irrigant. According to this study, mixed solutions can maintain the efficiency of both irrigants.
Since the combination of the two solutions in this study was freshly mixed, a further examination of the stability of the mixed irrigant should be examined. The cell toxicity and biofilm disinfection efficacy of the irrigant should also be studied.
The limitation of the current study lies in the use of disc diffusion and direct exposure test to assess the antimicrobial efficacy of the mixed irrigant. Root canal infection is polymicrobial, presenting in the form of biofilm (29). Therefore, an antimicrobial study in the form of multispecies and antibiofilm effect may better represent the true working condition of root canal disinfection. This is due to the multiple virulence factors or mechanisms between microorganisms which could affect the efficacy of irrigants. Nevertheless, the aim of the current study of assessing the smear layer removal and antimicrobial effect of the mixed irrigant resulted in initial pilot data of the working irrigant that can be developed further.
CONCLUSION
The addition of 6% or 10% CA to CHX had equal efficacy in removing the smear layer from the root canal wall to 17% EDTA which is the current standard, while CHX remains viable and effective in killing planktonic microorganisms of E. faecalis and C. albicans.
Footnotes
Conflict of Interest: Authors declared no conflict of interest.
Ethics Committee Approval: This study protocol was approved by the Human Experimentation Committee of the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand (Clearance No.13/2018).
Peer-review: Externally peer-reviewed.
Financial Disclosure: No grant or other financial support was received for this study.
Authorship contributions: Concept – A.D., P.L.; Design – A.D., P.L.; Supervision – A.D., P.L.; Funding - A.D.; Materials - A.D.; Data collection &/or processing – A.D., C.U., D.C.; Analysis and/or interpretation – A.D., C.U., D.C.; Literature search – A.D.; Writing – A.D., C.U., D.C.; Critical Review – A.D., C.U., D.C., P.L.
REFERENCES
- 1.McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975;1(7):238–42. doi: 10.1016/S0099-2399(75)80226-3. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Shen Y, Haapasalo M. Effect of smear layer against disinfection protocols on Enterococcus faecalis-infected dentin. J Endod. 2013;39(11):1395–400. doi: 10.1016/j.joen.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Machado R, Garcia LDFR, da Silva Neto UX, Cruz Filho AMD, Silva RG, Vansan LP. Evaluation of 17% EDTA and 10% citric acid in smear layer removal and tubular dentin sealer penetration. Microsc Res Tech. 2018;81(3):275–82. doi: 10.1002/jemt.22976. [DOI] [PubMed] [Google Scholar]
- 4.Di Lenarda R, Cadenaro M, Sbaizero O. Effectiveness of 1 mol L-1 citric acid and 15% EDTA irrigation on smear layer removal. Int Endod J. 2000;33(1):46–52. doi: 10.1046/j.1365-2591.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- 5.American Association of Endodontists. Winter 2011 Endodontics:collegues for excellence newsletter:root canal irrigants and disinfectants. [Accessed Apr 21 2020]. Available at: https://f3f142zs0k2w1kg84k5p9i1o-wpengine.netdna-ssl.com/specialty/wp-content/uploads/sites/2/2017/07/rootcanalirrigantsdisinfectants.pdf .
- 6.Goldman LB, Goldman M, Kronman JH, Lin PS. The efficacy of several irrigating solutions for endodontics:a scanning electron microscopic study. Oral Surg Oral Med Oral Pathol. 1981;52(2):197–204. doi: 10.1016/0030-4220(81)90319-4. [DOI] [PubMed] [Google Scholar]
- 7.Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions:Part 3. J Endod. 1983;9(4):137–42. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- 8.Herrera DR, Santos ZT, Tay LY, Silva EJ, Loguercio AD, Gomes BP. Efficacy of different final irrigant activation protocols on smear layer removal by EDTA and citric acid. Microsc Res Tech. 2013;76(4):364–9. doi: 10.1002/jemt.22175. [DOI] [PubMed] [Google Scholar]
- 9.Haznedaroğlu F. Efficacy of various concentrations of citric acid at different pH values for smear layer removal. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(3):340–4. doi: 10.1016/s1079-2104(03)00164-1. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi M, Yoshida K, Suzuki R, Nakamura H. Root canal irrigation with citric acid solution. J Endod. 1996;22(1):27–9. doi: 10.1016/S0099-2399(96)80232-9. [DOI] [PubMed] [Google Scholar]
- 11.Machado-Silveiro LF, González-López S, González-Rodríguez MP. Decalcification of root canal dentine by citric acid. EDTA and sodium citrate. Int Endod J. 2004;37(6):365–9. doi: 10.1111/j.1365-2591.2004.00813.x. [DOI] [PubMed] [Google Scholar]
- 12.Wayman BE, Kopp WM, Pinero GJ, Lazzari EP. Citric and lactic acids as root canal irrigants in vitro. J Endod. 1979;5(9):258–65. doi: 10.1016/S0099-2399(79)80171-5. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Guo LY, Fang HZ, Zou WL, Yang YM, Gao Y, et al. An in vitro study on the efficacy of removing calcium hydroxide from curved root canal systems in root canal therapy. Int J Oral Sci. 2017;9(2):110–6. doi: 10.1038/ijos.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malheiros CF, Marques MM, Gavini G. In vitro evaluation of the cytotoxic effects of acid solutions used as canal irrigants. J Endod. 2005;31(10):746–8. doi: 10.1097/01.don.0000157994.49432.67. [DOI] [PubMed] [Google Scholar]
- 15.Sousa SM, Bramante CM, Taga EM. Biocompatibility of EDTA. EGTA and citric acid. Braz Dent J. 2005;16(1):3–8. doi: 10.1590/s0103-64402005000100001. [DOI] [PubMed] [Google Scholar]
- 16.Onçağ O, Hoşgör M, Hilmioğlu S, Zekioğlu O, Eronat C, Burhanoğlu D. Comparison of antibacterial and toxic effects of various root canal irrigants. Int Endod J. 2003;36(6):423–32. doi: 10.1046/j.1365-2591.2003.00673.x. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi Z, Jafarzadeh H, Shalavi S. Antimicrobial efficacy of chlorhexidine as a root canal irrigant:a literature review. J Oral Sci. 2014;56(2):99–103. doi: 10.2334/josnusd.56.99. [DOI] [PubMed] [Google Scholar]
- 18.Molander A, Reit C, Dahlén G, Kvist T. Microbiological status of root-filled teeth with apical periodontitis. Int Endod J. 1998;31(1):1–7. [PubMed] [Google Scholar]
- 19.Santos JN, Carrilho MR, De Goes MF, Zaia AA, Gomes BP, Souza-Filho FJ, et al. Effect of chemical irrigants on the bond strength of a self-etching adhesive to pulp chamber dentin. J Endod. 2006;32(11):1088–90. doi: 10.1016/j.joen.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Nassar M, Awawdeh L, Jamleh A, Sadr A, Tagami J. Adhesion of Epiphany self-etch sealer to dentin treated with intracanal irrigating solutions. J Endod. 2011;37(2):228–30. doi: 10.1016/j.joen.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Carrilho MR, Carvalho RM, Sousa EN, Nicolau J, Breschi L, Mazzoni A, et al. Substantivity of chlorhexidine to human dentin. Dent Mater. 2010;26(8):779–85. doi: 10.1016/j.dental.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi-Fedele G, Doğramaci EJ, Guastalli AR, Steier L, de Figueiredo JA. Antagonistic interactions between sodium hypochlorite chlorhexidine, EDTA and citric acid. J Endod. 2012;38(4):426–31. doi: 10.1016/j.joen.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 23.González-López S, Camejo-Aguilar D, Sanchez-Sanchez P, Bolaños-Carmona V. Effect of CHX on the decalcifying effect of 10% citric acid 20% citric acid or 17% EDTA. J Endod. 2006;32(8):781–4. doi: 10.1016/j.joen.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Gutmann JL, Saunders WP, Nguyen L, Guo IY, Saunders EM. Ultrasonic root-end preparation Part 1. SEM analysis. Int Endod J. 1994;27(6):318–24. doi: 10.1111/j.1365-2591.1994.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 25.Thomas P, Sekhar AC, Upreti R, Mujawar MM, Pasha SS. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnol Rep (Amst) 2015;8:45–55. doi: 10.1016/j.btre.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souza MA, Menon CZ, Nery LF, Bertol CD, Rossato-Grando LG, Cecchin D. Effect of root canal preparation techniques on chlorhexidine substantivity on human dentin:a chemical analysis. Clin Oral Investig. 2018;22(2):859–65. doi: 10.1007/s00784-017-2162-7. [DOI] [PubMed] [Google Scholar]
- 27.Prado M, Gusman H, Gomes BP, Simão RA. Scanning electron microscopic investigation of the effectiveness of phosphoric acid in smear layer removal when compared with EDTA and citric acid. J Endod. 2011;37(2):255–8. doi: 10.1016/j.joen.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002;28(1):17–9. doi: 10.1097/00004770-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Du J, Peng Z. Correlation between Enterococcus faecalis and Persistent Intraradicular Infection Compared with Primary Intraradicular Infection:A Systematic Review. J Endod. 2015;41(8):1207–13. doi: 10.1016/j.joen.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Bernal-Treviño A, González-Amaro AM, Méndez González V, Pozos-Guillen A. Frequency of Candida in root canals of teeth with primary and persistent endodontic infections. Rev Iberoam Micol. 2018;35(2):78–82. doi: 10.1016/j.riam.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Prado M, Silva EJ, Duque TM, Zaia AA, Ferraz CC, Almeida JF, et al. Antimicrobial and cytotoxic effects of phosphoric acid solution compared to other root canal irrigants. J Appl Oral Sci. 2015;23(2):158–63. doi: 10.1590/1678-775720130691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fidalgo TK, Barcelos R, Portela MB, Soares RM, Gleiser R, Silva-Filho FC. Inhibitory activity of root canal irrigants against Candida albicans. Enterococcus faecalis and Staphylococcus aureus. Braz Oral Res. 2010;24(4):406–12. doi: 10.1590/s1806-83242010000400006. [DOI] [PubMed] [Google Scholar]
- 33.Dickert H, Machka K, Braveny I. The uses and limitations of disc diffusion in the antibiotic sensitivity testing of bacteria. Infection. 1981;9(1):18–24. [Google Scholar]
- 34.Arias-Moliz MT, Ferrer-Luque CM, Espigares-Rodríguez E, Liébana-Ureña J, Espigares-García M. Bactericidal activity of phosphoric acid citric acid and EDTA solutions against Enterococcus faecalis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(2):e84–9. doi: 10.1016/j.tripleo.2008.04.002. [DOI] [PubMed] [Google Scholar]