Abstract
Aim:
this study was conducted to investigate expression of the genes associated with CD in the target tissue in order to estimate contribution of each single gene to development of immune response. Then, the same set of genes was evaluated in peripheral blood mononuclear cells (PBMCs).
Background:
Celiac disease (CD) is a chronic systemic autoimmune disease of the small intestine occurring in genetically-susceptible individuals. There are several genes related to immune response.
Methods:
For this purpose, the genes related to CD were extracted from public databases (documents of proteomics and microarray-based techniques) and were organized in a protein-protein interaction network using the search tool for retrieval of interacting genes/proteins (STRING) database as a plugin of Cytoscape software version 3.6.0. The main genes were introduced and enriched via ClueGO to find the related biochemical pathways. The network was analyzed, and the most important genes were introduced based on central indices.
Results:
Among 20 CD genes as hub and bottleneck nodes, there were 7 genes with common expression in blood and intestinal tissue (C-X-C motif chemokine 11(CXCL11), granzyme B (GZMB), interleukin 15(IL-15), interleukin 17(IL-17A), interleukin 23(IL-23A), t-box transcription factor 21(TBX21), and tumor necrosis factor alpha-induced protein 3(TNFAIP3)).
Conclusion:
The enriched biological process related to the central nodes of celiac network indicated that most of hub-bottleneck genes are the well-known ones involved in different types of autoimmune and inflammatory diseases.
Key Words: Celiac Disease, PBMC, Intestinal Tissue, Autoimmune, Inflammatory
Introduction
Celiac disease (CD) is a chronic systemic autoimmune disease of the small intestine occurring in genetically-susceptible individuals (1). CD as a multifactorial disease involves genetic elements (human leukocyte antigen (HLA)-DQ2 and HLA-DQ8) and environmental trigger (gluten) (2).
The primary mechanism involved in development of CD is related to an inappropriate adaptive immune response to gliadin, a prolamin found in wheat and related cereals. Prolamins contain critical epitopes presented by either HLA-DQ2 or HLA-DQ8, leading to induction of a cluster of differentiation 4(CD4)+ T-lymphocytes response(3).
High levels of pro-inflammatory cytokines are produced by the activated CD4+ T-lymphocytes (4). Cytokines can prime the innate immune response by polarizing dendritic cells and intraepithelial lymphocyte function (5). Following innate immune response, intestinal cells can be directly exposed to intestinal damage. Intestinal damage is developed gradually from completely normal mucosa, to mucosal inflammation, followed by crypt hyperplasia, and culminating in villous atrophy (6).
Gene expression profiling (GEP) as a robust test is potentially used for classification purposes, with a high inter laboratory reproducibility (7, 8).Gene expression studies are a useful approach to provide evidence regarding implication of several functional pathways in complex diseases, such as CD (9). Genes displaying differential expression in CD intestine and PBMC compared to normal and common expression of both CD intestine and PBMC can be useful in developing a gene expression-based diagnostic test. Moreover, histological and functional alterations associated with CD may be revealed by analyzing the genes differentially expressed in CD (10, 11) , or by monitoring the genes potentially involved in intestinal immune response in CD (12).
There are several genes related to immune response. Therefore, this study was performed to investigate expression of the genes associated with CD in the target tissue in order to estimate contribution of each single gene to development of immune response. Then, the same set of genes was evaluated in PBMC or whole blood.
Methods
Microarray experiments were used in CD intestine tissues and PBMC or whole blood was measured in the microarray-based techniques. Whole genome profile with differential expression in CD small intestine compared to control (533 genes), and in PBMC or whole blood compared to control (252 genes) was collected from public databases and gene expression omnibus(GEO) dataset published by March 2019. Also, all the genes with differential expression were collected. Fold change (FC)≥2 was considered to screen the differentially expressed genes. The protein-protein interaction (PPI) network was constructed using the search tool for retrieval of interacting genes/proteins (STRING) database as a plugin of Cytoscape software version 3.6.0 (13). Such that, genes (proteins) were nodes, and edges between gene nodes were formed when a gene was found to be significantly differentially expressed in the disease state. The main connected component of PPI network was analyzed by the network analyzer plugin of Cytoscape software. The most important topological properties of the PPI network nodes including degree and betweenness of nodes were considered to rank nodes of the network. The number of top 20% of the genes based on degree values was selected as hub genes and 20% of genes based on betweenness were identified as bottleneck nodes. The top 20 nodes based on degree value and betweenness centrality were selected as hub and bottleneck nodes, respectively. Interactions between hub-bottleneck nodes were identified by a related sun-network. Hub-bottleneck nodes of the celiac network were enriched by Kyoto encyclopedia of genes and genomes(KEGG) via ClueGO (14, 15). The resulting biochemical pathways were clustered. A p-value less than 0.05 was considered as statistically significant.
Results
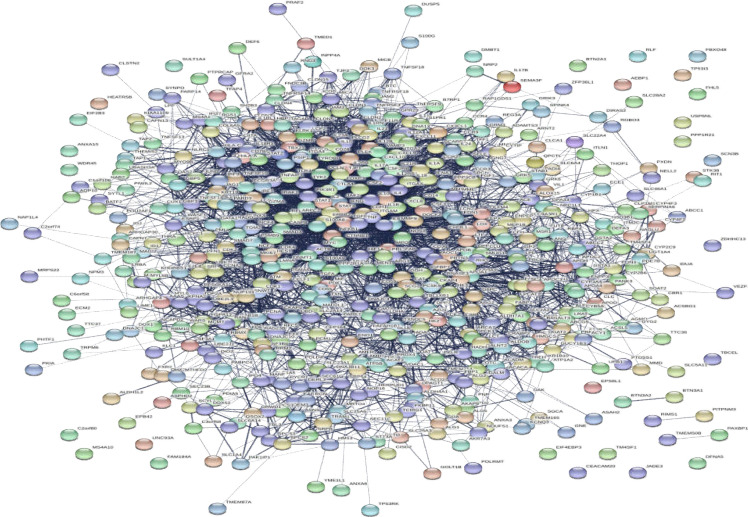
For integrating the data provided through an experimental study, literature survey, or database, differential expressions of the genes present in the patients with CD were combined compared to those of healthy controls. There are 533 genes with differential expression in CD small intestine compared to control and 259 genes with differential expression in CD PBMC or whole blood vs. control (16-29). The genes related to CD were extracted from the STRING database. As shown in Figures 1, 2 a total of 533 genes were included in the main connected component. The network was analyzed, and the nodes were ranked on the basis of centrality parameters. Top 20% of nodes based on the degree value and betweenness centrality were selected and organized in 2 groups. As described in the Methodology Section, the top 20 nodes based on degree value and betweenness centrality were selected as hub and bottleneck nodes, respectively as presented in Table1.
Figure 1.
PPI network of CD constructed by 533 genes extracted from STRING database
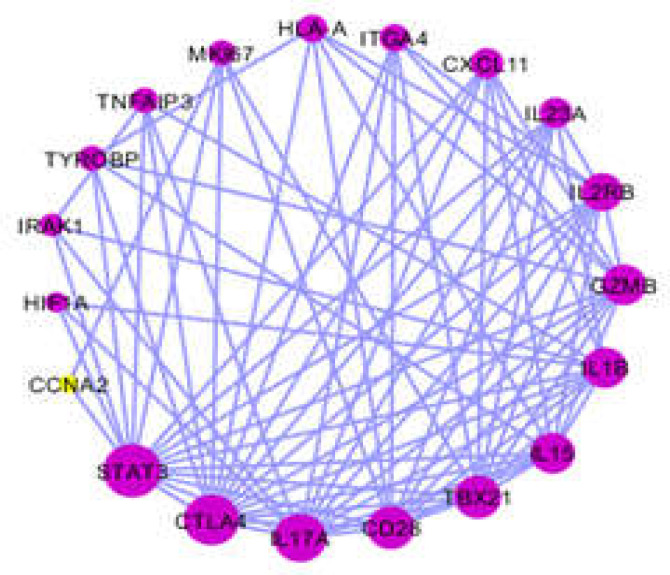
Figure 2.
The 20 central nodes of CD network organized in a sub-network
Table 1.
The top 20 nodes based on degree value and betweenness centrality were selected as hub and bottleneck nodes with differential expression in CD blood and intestine compared to control. CXCL11, GZMB, IL-15, IL-17A, IL-23A, TBX21, and TNFAIP3 had common expression in CD blood and intestinal tissue
| Hub& bottleneck | Degree | Betweenness centrality | Expression | ||
|---|---|---|---|---|---|
| Intestine | Blood | ||||
| 1 | APOA1 | 40 | 0.00634588 | Down (20, 21) | - |
| 2 | CCNA2 | 30 | 0.00841483 | Down (30) | - |
| 3 | CD28 | 63 | 0.00839965 | Up (23) | - |
| 4 | CTLA4 | 88 | 0.02961893 | Up (19) | - |
| 5 | CXCL11 | 45 | 0.02059243 | Up (19(31)) | Up (32) |
| 6 | GZMB | 57 | 0.01230656 | Up (19) | Up |
| 7 | HIF1A | 35 | 0.00923055 | Up (27) | - |
| 8 | HLA-A | 40 | 0.00915393 | Up (30) | - |
| 9 | IL-15 | 67 | 0.01475644 | Up (19) | Up (33) |
| 10 | IL-17A | 86 | 0.01610106 | Up (19) | Up (28, 34) |
| 11 | IL-1B | 95 | 0.01405327 | Up (19) | - |
| 12 | IL-23A | 35 | 0.0535164 | Up (21) | Up (35) |
| 13 | IL-2RB | 53 | 0.00721186 | Up (14) | - |
| 14 | IRAK1 | 32 | 0.00518941 | Down (18) | - |
| 15 | ITGA4 | 32 | 0.03352843 | Down (18) | - |
| 16 | MKI67 | 32 | 0.01219633 | Up (19) | - |
| 17 | STAT3 | 115 | 0.00616552 | Up (21) | - |
| 18 | TBX21 | 55 | 0.02993936 | Up (19) | Up (28, 34) |
| 19 | TNFAIP3 | 35 | 0.00532498 | Up (22) | Up (23) |
| 20 | TYROBP | 40 | 0.0078105 | Up (19) | - |
Also, change in expression of the genes in CD small intestine and PBMC or whole blood vs. control was investigated via the literature survey, and the findings are presented in Table1. Among 20 CD genes as hub and bottleneck nodes, there were 7 genes with common expression in blood and intestinal tissue (CXCL11 (C-X-C motif chemokine ligand 11), GZMB (granzyme B), IL-15 (interleukin 15), IL-17A (interleukin 17A), IL-23A (interleukin 23A), TBX21 (T-box 21), and TNFAIP3 (tumor necrosis factor alpha-induced protein 3)).
Since, the gene attribution in biological processes is an important role of gene in the investigated disease, 20 genes were enriched through gene ontology (GO) and significant processes were determined as shown in Table2.
Table 2.
Biological process of the genes as hub-bottleneck nodes
| Associated genes | GOTerm |
|---|---|
| [CTLA4, TNFAIP3, TYROBP] | Negative regulation of B cell activation |
| [APOA1, IL-15, IL-1B, IL-2RB] | Positive regulation of phagocytosis |
| [IL-15, IL-2RB, STAT3] | Response to IL-15 IL-15-mediated signaling pathway Cellular response to IL-15 |
| [CD28, IL-23A, TYROBP] | Production of IL-10 Regulation of IL-10 production Production of IL-10 Regulation of IL-10 production |
| [CD28, IL-1B, STAT3, TYROBP] | Positive regulation of cytokine biosynthetic process |
| [IL-1B, STAT3, TYROBP] | IL-6 biosynthetic process Regulation of IL-6 biosynthetic process Positive regulation of IL-6 biosynthetic process |
| [IL-23A, STAT3, TBX21] | CD4-positive, alpha-beta T cell differentiation involved in immune response T-helper cell lineage commitment T-helper 17 cell differentiation T-helper 17 type immune response T-helper 17 cell lineage commitment Activation of alpha-beta T cell involved in immune response |
| [CD28, IL-1B, TBX21, TNFAIP3] | Production of IL-2 |
| [HLA-A, IL-23A, TYROBP] | Positive regulation of leukocyte-mediated cytotoxicity |
| [CD28, IL-23A, TYROBP] | |
| [CD28, IL-1B, TBX21, TNFAIP3] | Regulation of IL-2 production |
| [HLA-A, IL-1B, TBX21] | T cell cytokine production Regulation of T cell cytokine production |
| [CD28, HLA-A, IL-1B, IL-23A, TBX21] | |
| [IL-15, IL-1B, IL-23A, TNFAIP3] | Regulation of defense response to virus Regulation of defense response to virus by host |
| [CD28, HLA-A, IL-1B, IL-23A, TBX21] | Positive regulation of adaptive immune response Positive regulation of lymphocyte-mediated immunity Positive regulation of adaptive immune response based on somatic recombination of immune receptors built from immunoglobulin superfamily domains |
| [IL-15, IL-23A, TYROBP] | Regulation of natural killer cell activation Positive regulation of natural killer cell activation |
| [CD28, IL-1B, STAT3, TYROBP] | Positive regulation of cytokine biosynthetic process |
| [HLA-A, IL-1B, IL-23A, TBX21] | Regulation of T cell-mediated immunity |
| [CD28, HLA-A, IL-15, IL-23A] | Proliferation of alpha-beta T cell |
| [HLA-A, IL-1B, IL-23A] | Positive regulation of T cell-mediated immunity |
| [CD28, IL-15, IL-23A, STAT3, TBX21] | T cell selection |
| [IL-23A, STAT3, TBX21] | T cell lineage commitment |
| [CD28, HLA-A, IL-23A] | Regulation of alpha-beta T cell proliferation |
| [IL-23A, STAT3, TBX21] | Positive T cell selection Lineage commitment of alpha-beta T cell Lineage commitment of CD4-positive or CD8-positive, and alpha-beta T cell |
| [CD28, HLA-A, IL-23A] | Positive regulation of alpha-beta T cell proliferation |
| [IL-17A, IL-23A, TYROBP] | Positive regulation of osteoclast differentiation |
| [IL-23A, STAT3, TBX21] | Differentiation of alpha-beta T cell involved in immune response of CD4-positive, alpha-beta T cell lineage commitment |
The enriched pathways from KEGG related to 20 hub and bottleneck nodes of CD network are also shown. Ten terms related to 20 hub- bottleneck nodes were identified and clustered (Table 3). Also, biological process and enriched pathways from KEGG related to the genes as hub and bottleneck nodes with common expression in both CD small intestine biopsy and blood were identified and clustered (Table 4). Important roles of these biological processes in relation to CD are discussed in detail in the following section.
Table 3.
The enriched pathways from KEGG related to 20 central nodes of CD network
| Associated genes | GOTerm |
|---|---|
| [CD28, IL-15, ITGA4] | Intestinal immune network for IgA production |
| [IL-1B, IRAK1, ITGA4] | Leishmaniasis |
| [CD28, IL-1B, IL-2RB, IRAK1, STAT3, TNFAIP3] | Measles |
| [CD28, CTLA4, IL-15, IL-17A, IL-1B, IL-23A] | Rheumatoid arthritis |
| [HIF1A, IL-17A, IL-1B, IL-23A, IL-2RB, STAT3, TBX21] | Differentiation of Th17 cell |
| [IL-17A, IL-1B, IL-23A, STAT3, TBX21] | Inflammatory bowel disease (IBD) |
| [CD28, GZMB, HLA-A, IL-1B] | Type I diabetes mellitus Graft-versus-host disease |
| [CD28, CTLA4, GZMB, HLA-A] | Autoimmune thyroid disease |
| [CD28, GZMB, HLA-A] | Allograft rejection |
Table 4.
Biological process and enriched pathways from KEGG related to the genes as hub and bottleneck nodes with common expression in both CD small intestine biopsy and blood
| Associated genes | GOTerm |
|---|---|
| [IL-15, IL-23A, TBX21] | T cell selection |
| [IL-15, IL-23A, TNFAIP3] | Regulation of defense response to virus by host |
| [IL-17A, IL-23A, TBX21] | Inflammatory bowel disease (IBD) |
Discussion
There are large amount of data in genomics and proteomics (high throughput methods) applied as suitable screening tools (36). In this research, the reported data related to CD were screened by PPI network analysis to find the crucial genes among them. Differentially expressed genes present in the patients with CD compared to healthy controls were combined. There are 533 genes with differential expression in CD small intestine compared to control and 259 genes with differential expression in CD PBMC or whole blood vs. control. Totally, 533 genes were analyzed using the PPI network and 20 central genes were introduced as hub-bottleneck nodes. The roles of hub-bottleneck genes are introduced in Table 2.
The GO can be useful for obtaining the information about roles of a gene set (37). The enriched biological process related to the central nodes of CD network (Table 3) indicated that most of hub-bottleneck genes are the well-known ones involved in different types of autoimmune and inflammatory diseases (38, 39). Among the mechanisms involved in innate immune system, chemokine signaling pathway is involved in development of CD. CXCL11 is a chemokine that binds to its receptor CXCR3 as a member of family G-protein. Expression of CXCR3 and its ligands is strongly associated with autoimmune diseases, such as CD (38). High expression levels of CXCR3, CXCL10, and CXCL11 in small intestinal biopsies of the untreated patients with CD suggest that these chemokines are able to induce an innate immune response and are important factors in development of CD (29, 38).
Immune reaction in CD involves an adaptive innate immune response and is characterized by the presence of anti-gluten and anti-transglutaminase 2 antibodies, and expression of multiple cytokines and other signaling proteins in the intestinal epithelial membrane. Interleukins, such as IL-15 are a group of cytokines, which can prime innate immune response by polarizing dendritic cells and intraepithelial lymphocyte. They have been reported to be associated with numerous inflammatory conditions including CD, Crohns҆ disease, psoriasis, Rheumatoid arthritis, and type 1 diabetes (40-42). IL-15 contributes to pathogenesis of autoimmune diseases, such as CD through activation of immune responses. Therefore, genetic or environmental factors controlling expression of IL-15 and responsiveness in the intestine are likely involved in pathogenesis of CD (43). The roles of IL-15 and IL-23 in generation, activation ,and expansion of CD4+ T-lymphocytes still remain unknown (44). IL-15 provides activating and survival signals to indirectly modulate CD4+ T cell responses. Activated CD4+ T-lymphocytes are thought to perpetuate inflammation by secreting pro-inflammatory cytokines, such as interferon γ (IFNγ), IL-17, and interleukin 21(IL-21) that act on immune and non-immune cells. Regulation of T cell differentiation and cytokine pathway seems to be provided by cytoplasmic and nuclear transcription factors. TBX21 has been identified as a key transcription factor for development of T helper lymphocytes. The signal transducer and activator of transcription (STAT) proteins are cytoplasmic transcription factors activated via receptors from cytokines of signal transducer and activator of transcription 3(STAT3) that provides multiple functions in cytokine -mediated signaling in many cell types including differentiation, survival, apoptosis ,and mobility. It is involved in generation of cells and acute inflammatory response. STAT3 is activated by several cytokines(45, 46).
TNFAIP3 is involved in regulation of the nuclear factor kappa B (NF-kB) inflammatory signaling pathway in pathology of coeliac disease and is one of the key mediators in this nuclear activating complex (47). TNFAIP3 is an attractive candidate for both inflammatory and autoimmune pathogenesis. TNFAIP3 is required for mediation of the NF-kB signal by innate immune receptors via de-ubiquitination of several NF-kB signaling factors. NF-kB as a transcription complex has a key role in regulation of the cellular immune response to stimuli. In various inflammatory disorders including CD, arthritis ,and inflammatory bowel disease, NF-kB is activated and NF-kB pathway plays an independent role in innate mechanisms of disease development (48).
These cytokines lead to histopathological changes like villous atrophy and crypt hyperplasia (49, 50). Upon cell death, mRNAs are released into the surrounding environment and then, reaching peripheral blood circulation or body fluids; hence detection of tissue-specific mRNA in bio fluids might be used as a biomarker for specific tissue damage and specific tissue event. In addition, a biomarker panel for CD introduced through analyzing and screening significantly differentially expressed genes should be considered as an important player in pathology of CD.
Acknowledgment
This study supported by Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Zali MR, Rostami Nejad M, Rostami K, Alavian SM. Liver complications in celiac disease. Hepat Mon. 2011;11:333–41. [PMC free article] [PubMed] [Google Scholar]
- 2.Liu E, Lee HS, Aronsson CA, Hagopian WA, Koletzko S, Rewers MJ, et al. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med. 2014;371:42–9. doi: 10.1056/NEJMoa1313977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiao SW, Sollid LM, Blumberg R. Antigen presentation in celiac disease. Curr Opin Immunol. 2009;21:111–7. doi: 10.1016/j.coi.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björck S, Lindehammer S, Fex M, Agardh D. Serum cytokine pattern in young children with screening detected coeliac disease. Clin Exp Immunol. 2015;179:230–5. doi: 10.1111/cei.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Ménard S, Candalh C, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143–54. doi: 10.1084/jem.20071204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurppa K, Koskinen O, Collin P, Mäki M, Reunala T, Kaukinen K, et al. Changing phenotype of celiac disease after long-term gluten exposure. J Pediatr Gastroenterol Nutr. 2008;47:500–3. doi: 10.1097/MPG.0b013e31817d8120. [DOI] [PubMed] [Google Scholar]
- 7.Kohlmann A, Haschke-Becher E, Wimmer B, Huber-Wechselberger A, Meyer-Monard S, Huxol H, et al. Intraplatform reproducibility and technical precision of gene expression profiling in 4 laboratories investigating 160 leukemia samples: the DACH study. Clin Chem. 2008;54:1705–15. doi: 10.1373/clinchem.2008.108506. [DOI] [PubMed] [Google Scholar]
- 8.Kohlmann A, Kipps TJ, Rassenti LZ, Downing JR, Shurtleff SA, Mills KI, et al. An international standardization programme towards the application of gene expression profiling in routine leukaemia diagnostics: the Microarray Innovations in LEukemia study prephase. Br J Haematol. 2008;142:802–7. doi: 10.1111/j.1365-2141.2008.07261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Jimenez N, Castellanos-Rubio A, Plaza-Izurieta L, Irastorza I, Elcoroaristizabal X, Jauregi-Miguel A, et al. Coregulation and modulation of NFκB-related genes in celiac disease: uncovered aspects of gut mucosal inflammation. Hum Mol Genet. 2013;23:1298–310. doi: 10.1093/hmg/ddt520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107:686–96. doi: 10.17235/reed.2015.3846/2015. [DOI] [PubMed] [Google Scholar]
- 11.Turner J. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 12.Diosdado B, Van Bakel H, Strengman E, Franke L, Van Oort E, Mulder CJ, et al. Neutrophil recruitment and barrier impairment in celiac disease: a genomic study. Clin Gastroenterol Hepatol. 2007;5:574–81. doi: 10.1016/j.cgh.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Ge Q, Chen L, Tang M, Zhang S, Liu L, Gao L, et al. Analysis of mulberry leaf components in the treatment of diabetes using network pharmacology. Eur J Pharmacol. 2018;833:50–62. doi: 10.1016/j.ejphar.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Karbalaei R, Allahyari M, Rezaei-Tavirani M, Asadzadeh-Aghdaei H, Zali MR. Protein-protein interaction analysis of Alzheimers disease and NAFLD based on systems biology methods unhide common ancestor pathways. Gastroenterol Hepatol Bed Bench. 2018;11:27. [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Z, Kong Q, Han J, Deng J, Wu M, Deng H. Circular RNAs are differentially expressed in liver ischemia/reperfusion injury model. J Cell Biochem. 2018;119:7397–405. doi: 10.1002/jcb.27047. [DOI] [PubMed] [Google Scholar]
- 16.Diosdado B, Wapenaar M, Franke L, Duran K, Goerres M, Hadithi Ma, et al. A microarray screen for novel candidate genes in coeliac disease pathogenesis. Gut. 2004;53:944–51. doi: 10.1136/gut.2003.018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortazavi S, Zali M, Raoufi M, Nadji M, Kowsarian P, Nowroozi A. The Prevalence of Human Papillomavirus in Cervical Cancer in Iran. Asian Pac J Cancer Prev. 2002;3:69–72. [PubMed] [Google Scholar]
- 18.Simula MP, Cannizzaro R, Canzonieri V, Pavan A, Maiero S, Toffoli G, et al. PPAR signaling pathway and cancer-related proteins are involved in celiac disease-associated tissue damage. Mol Med. 2010;16:199–209. doi: 10.2119/molmed.2009.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavirani MR, Bashash D, Rostami FT, Tavirani SR, Nikzamir A, Tavirani MR, et al. Celiac disease microarray analysis based on system biology approach. Gastroenterol Hepatol Bed Bench. 2018;11:216. [PMC free article] [PubMed] [Google Scholar]
- 20.Bragde H, Jansson U, Fredrikson M, Grodzinsky E, Söderman JJC. Celiac disease biomarkers identified by transcriptome analysis of small intestinal biopsies. Cell Mol Life Sci. 2018;75:4385–401. doi: 10.1007/s00018-018-2898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bragde H, Jansson U, Jarlsfelt I, Söderman J. Gene expression profiling of duodenal biopsies discriminates celiac disease mucosa from normal mucosa. Pediatr Res. 2011;69:530. doi: 10.1203/PDR.0b013e318217ecec. [DOI] [PubMed] [Google Scholar]
- 22.Sangineto M, Graziano G, D’Amore S, Salvia R, Palasciano G, Sabbà C, et al. Identification of peculiar gene expression profile in peripheral blood mononuclear cells (PBMC) of celiac patients on gluten free diet. PLoS ONE. 2018;13:e0197915. doi: 10.1371/journal.pone.0197915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascual V, Medrano L, López-Palacios N, Bodas A, Dema B, Fernández-Arquero M, et al. Different gene expression signatures in children and adults with celiac disease. PLoS One. 2016;11:e0146276. doi: 10.1371/journal.pone.0146276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cielo D, Galatola M, Fernandez-Jimenez N, De Leo L, Garcia-Etxebarria K, Loganes C, et al. Combined Analysis of Methylation and Gene Expression Profiles in separate Compartments of small Bowel Mucosa Identified Celiac Disease patients’ signatures. Sci Rep. 2019;9:10020. doi: 10.1038/s41598-019-46468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galatola M, Izzo V, Cielo D, Morelli M, Gambino G, Zanzi D, et al. Gene expression profile of peripheral blood monocytes: a step towards the molecular diagnosis of celiac disease? PLoS One. 2013;8:e74747. doi: 10.1371/journal.pone.0074747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Jimenez N, Santin I, Irastorza I, Plaza-Izurieta L, Castellanos-Rubio A, Vitoria JC, et al. Upregulation of KIR3DL1 gene expression in intestinal mucosa in active celiac disease. Hum Immunol. 2011;72:617–20. doi: 10.1016/j.humimm.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Castellanos-Rubio A, Caja S, Irastorza I, Fernandez-Jimenez N, Plaza-Izurieta L, Vitoria JC, et al. Angiogenesis-related gene expression analysis in celiac disease. Autoimmunity. 2012;45:264–70. doi: 10.3109/08916934.2011.637531. [DOI] [PubMed] [Google Scholar]
- 28.Frisullo G, Nociti V, Iorio R, Patanella AK, Plantone D, Bianco A, et al. T‐bet and pSTAT‐1 expression in PBMC from coeliac disease patients: new markers of disease activity. Clin Exp Immunol. 2009;158:106–14. doi: 10.1111/j.1365-2249.2009.03999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvati V, Bajaj-Elliott M, Poulsom R, Mazzarella G, Lundin K, Nilsen E, et al. Keratinocyte growth factor and coeliac disease. Gut. 2001;49:176–81. doi: 10.1136/gut.49.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juuti-Uusitalo K, Mäki M, Kaukinen K, Collin P, Visakorpi T, Vihinen M, et al. cDNA microarray analysis of gene expression in coeliac disease jejunal biopsy samples. Autoimmun. 2004;22:249–65. doi: 10.1016/j.jaut.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Haghbin M, Rostami-Nejad M, Forouzesh F, Sadeghi A, Rostami K, Aghamohammadi E, et al. The role of CXCR3 and its ligands CXCL10 and CXCL11 in the pathogenesis of celiac disease. Medicine. 2019;98:e15949. doi: 10.1097/MD.0000000000015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bragde H, Jansson U, Fredrikson M, Grodzinsky E, Söderman J. Potential blood-based markers of celiac disease. BMC Gastroenterol. 2014;14:176. doi: 10.1186/1471-230X-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Sabatino A, Ciccocioppo R, Cupelli F, Cinque B, Millimaggi D, Clarkson MM, et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut. 2006;55:469–77. doi: 10.1136/gut.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myhr CB, Hulme MA, Wasserfall CH, Hong PJ, Lakshmi PS, Schatz DA, et al. The autoimmune disease-associated SNP rs917997 of IL18RAP controls IFNγ production by PBMC. J Autoimmun. 2013;44:8–12. doi: 10.1016/j.jaut.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahdenperä A, Ludvigsson J, Fälth-Magnusson K, Högberg L, Vaarala O. The effect of gluten-free diet on Th1–Th2–Th3-associated intestinal immune responses in celiac disease. Scand J Gastroenterol. 2011;46:538–49. doi: 10.3109/00365521.2011.551888. [DOI] [PubMed] [Google Scholar]
- 36.Jin BJ, Lee S, Verkman A. Hollow Micropillar Array Method for High-Capacity Drug Screening on Filter-Grown Epithelial Cells. Anal Chem. 2018;90:7675–81. doi: 10.1021/acs.analchem.8b01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safari-Alighiarloo N, Rezaei-Tavirani M, Taghizadeh M, Tabatabaei SM, Namaki SJP. Network-based analysis of differentially expressed genes in cerebrospinal fluid (CSF) and blood reveals new candidate genes for multiple sclerosis. PreeJ. 2016;4:e2775. doi: 10.7717/peerj.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama S, Watanabe N, Sato N, Perera PY, Filkoski L, Tanaka T, et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci USA. 2009;106:15849–54. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DePaolo R, Abadie V, Tang F, Fehlner-Peach H, Hall J, Wang W, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Geboes K, Colpaert S, D’Haens GR, Rutgeerts P, Ceuppens J. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol. 2000;164:3608–15. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 41.Kuczyński S, Winiarska H, Abramczyk M, Szczawińska K, Wierusz-Wysocka B, Dworacka M, et al. IL-15 is elevated in serum patients with type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005;69:231–6. doi: 10.1016/j.diabres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Bernardo D, Garrote JA, Allegretti Y, León A, Gómez E, Bermejo‐Martin J, et al. Higher constitutive IL15Rα expression and lower IL‐15 response threshold in coeliac disease patients. Clin Exp Immunol. 2008;154:64–73. doi: 10.1111/j.1365-2249.2008.03743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris KM, Fasano A, Mann D. Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: implications for celiac disease. Clin Immunol. 2010;135:430–9. doi: 10.1016/j.clim.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jafari F, Hamidian M, Rezadehbashi M, Doyle M, Salmanzadeh-Ahrabi S, Derakhshan F, et al. Prevalence and antimicrobial resistance of diarrheagenic Escherichia coli and Shigella species associated with acute diarrhea in Tehran, Iran. Can J Infect Dis Med Microbiol. 2009;20:e56–62. doi: 10.1155/2009/341275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stepkowski SM, Chen W, Ross JA, Nagy ZS, Kirken R. STAT3: an important regulator of multiple cytokine functions. Transplantation. 2008;85:1372–7. doi: 10.1097/TP.0b013e3181739d25. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz J, Dahmen H, Grimm C, Gendo C, Müller-Newen G, Heinrich PC, et al. The cytoplasmic tyrosine motifs in full-length glycoprotein 130 have different roles in IL-6 signal transduction. J Immunol. 2000;164:848–54. doi: 10.4049/jimmunol.164.2.848. [DOI] [PubMed] [Google Scholar]
- 47.Trynka G, Zhernakova A, Romanos J, Franke L, Hunt K, Turner G, et al. Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-κB signalling. Gut. 2009;58:1078–83. doi: 10.1136/gut.2008.169052. [DOI] [PubMed] [Google Scholar]
- 48.Perkins N. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 49.Alaedini A, Green P. Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann Intern Med. 2005;142:289–98. doi: 10.7326/0003-4819-142-4-200502150-00011. [DOI] [PubMed] [Google Scholar]
- 50.Faghih M, Barartabar Z, Nasiri Z. The role of Th1 and Th17 in the pathogenesis of celiac disease. Gastroenterol Hepatol Open Access. 2018;9:83–7. [Google Scholar]