Abstract
Introduction:
A body of literature reported associations between late-life general adiposity measures (eg, body mass index) and dementia. Little is known about the association of late-life body composition with dementia risk.
Methods:
We determined this association among cognitively normal participants from the Cardiovascular Health Study- Cognition Study. Body composition was assessed by dual-energy x-ray absorptiometry in 1994–1995. Dementia was ascertained at annual follow-up from 1998–1999 to 2013. Associations of body composition with incident dementia were assessed by the Fine-Gray model.
Result:
Among 344 participants (mean age 78, 62.2% women), body composition was significantly different between men and women, despite similar body mass indexes (BMIs). Increased dementia risk was significantly associated with lower lean mass in men and marginally with low appendicular lean mass in women.
Discussion:
Decreased lean mass was an indicator of increased dementia risk in older adults. Studies should test whether preventing lean mass loss in older adults reduces dementia risk.
Keywords: body composition, cohort study, dementia, older adults
1 |. INTRODUCTION
Higher mid-life BMI (body mass index) is associated with increased dementia risk, but in late life, lower BMI is associated with increased dementia risk.1,2 One hypothesis to explain this observation is reverse causality such that subclinical dementia causes the decrease in late-life BMI.3 However, although BMI is often used as a marker of obesity, it cannot fully capture body composition which is important for the health of older adults.4,5
In older adults, low lean mass6–8 and high fat mass9 were associated with poor cognitive function in cross-sectional and longitudinal studies. Cross-sectional studies further showed that individuals with dementia, compared to those with normal cognition, tended to have lower lean mass and higher fat mass.10,11 Cross-sectional studies cannot clarify the temporality of body composition and dementia; this question will be better informed by prospective longitudinal studies. In addition, the association between body composition and dementia risk may differ by sex, given that the distribution of body composition is significantly different between men and women.12–14 Specifically, men have significantly higher lean mass and lower fat mass than women.
The aim of the present study was to determine the association of lean and fat mass with dementia risk by sex among older adults with normal cognition at baseline (mean age 78) in the Cardiovascular Health Study-Cognition Study (CHS-CS) over 15 years of follow-up. We hypothesized that lower lean mass and higher fat mass would be associated with increased risk of dementia in older adults.
2 |. METHODS
The data that support the findings of this study are available at the National Heart, Lung, and Blood Institute-CHS website (https://chsnhlbi.org/) and will be made available by the corresponding author on request.
2.1 |. Participants
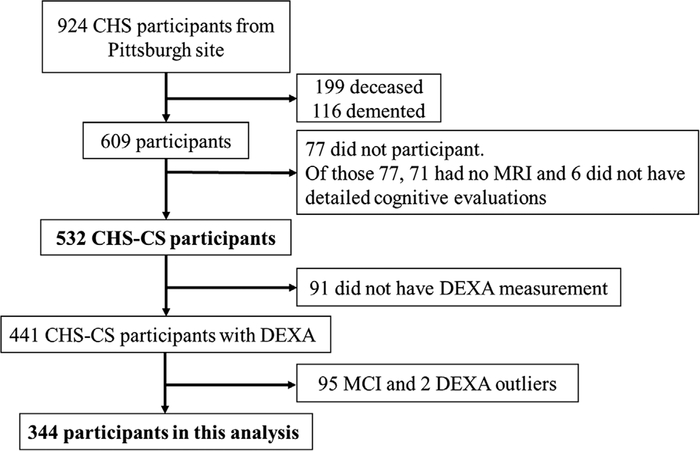
The present analysis included 344 participants from CHS-CS who were cognitively normal at baseline and had dual-energy x-ray absorptiometry (DEXA) measurement in 1994–1995 (Figure 1 and Supplementary Figure 1). The CHS-CS has been described previously.15–18 Briefly, starting in 1998–1999, CHS-CS performed annual cognitive evaluations on 532 participants, who were not demented at baseline, in the longitudinal Cardiovascular Health Study (CHS) in Pittsburgh. As described previously, from 924 participants who had brain magnetic resonance imaging (MRI) in 1992–1994, 199 (22%) were deceased, 116 (16%) were demented by 1998–1999, and 77 (13%) did not have detailed cognitive evaluation in 1998–1999, leaving 532 Pittsburgh CHS-CS participants free from dementia at baseline (1998–1999). During 1994–1995, an ancillary study of CHS had conducted DEXA scans on 441 CHS-CS participants (346 normal cognition and 95 mild cognitive impairment).19 After excluding two outliers who had normal weight but extremely low body mass based on DEXA, we included 344 cognitively normal participants. This study was approved by the University of Pittsburgh Institutional Review Board, and informed consent was obtained from all participants in the study.
FIGURE 1.
Flowchart of study participation
2.2 |. Assessment of dementia
Dementia was ascertained at annual follow-up. The annual cognitive assessments of CHS-CS participants have been described.16,17 Briefly, dementia diagnosis among CHS-CS participants was based on a progressive or static cognitive deficit of sufficient severity to affect the participant’s activities of daily living, and a history of normal intellectual function before the onset of cognitive abnormalities. Cognitive tests evaluated overall cognitive function (modified Mini-Mental State Examination (3MSE)), IQ (The American National Adult Reading Test (AMNART) and Raven’s), memory (California Verbal Test and REY figure delayed recall), construction (REY figure copy and, block design), language (naming and word generation), psychomotor speed (Trials A, Digit symbol substitution test), and executive function (Trials B/A and Stroop color word). Dementia diagnosis required impairments in two cognitive domains (eg, language, constructional, executive functions), which did not necessarily include memory.16 The classification of dementia was made by an experienced neurologist or psychiatrist with extensive experience in dementia diagnosis. For CHS-CS participants who died between annual evaluations during follow-up, the status of dementia prior to death was carefully evaluated.17
2.3 |. Assessment of body composition and other risk factors
Body composition was measured once in 1994–1995. The measurement of lean and fat mass in CHS has been described previously.20 Briefly, the DEXA measurements used QDR-2000 bone densitometers (Hologic, Inc., Bedford, Massachusetts).19 The lean and fat mass was calculated from whole-body DEXA data. Scans were read blinded to participant’s characteristics and were monitored for quality control by the University of California, San Francisco reading center. Lean mass did not include bone mineral content. Appendicular lean mass was defined as the sum of arm and leg lean mass at both sides. Grip strength was assessed with an isometric dynamometer for the dominant hand.21
Sex was assessed by asking participants to choose between male and female. Cardiovascular risk factors were assessed by standardized protocols during the 1998–1999 examination.18 Lipid and glucose assays were performed on 12-hour fasting blood samples.22 Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg or antihypertensive medication use. Mean arterial pressure (MAP) was calculated as 2/3 DBP 1/3 SBP. Diabetes was defined as fasting blood glucose ≥7.0 mmol/L or self-reported use of oral hypoglycemic agents or insulin. Physical activity was assessed by a validated Minnesota Leisure-Time Activities questionnaire.23 The questionnaire evaluated the type, frequency, and duration of physical activity during the prior 2 weeks. The weekly energy expenditure (kcal/week) was calculated according to the pre-defined metabolic equivalent task units and participant responses. Apolipoprotein E genotype (APOE) was determined with the whole-blood samples.24 APOE ε4 carrier was defined as those with ε2/ε4, ε3/ε4, or ε4/ε4 genotypes, and noncarrier was defined as ε2/ε2, ε2/ε3, or ε3/ε3 genotypes. A 10-item version of the Center for Epidemiological Studies Depression (CES-D) scale was used annually to assess self-reported depressive symptoms experienced in the past week.25,26
Brain MRI scanning was performed in 1998–1999 using a 1.5-T scanner.27 White matter lesions and ventricular size were graded on a scale of 0 to 9.28 As in prior reports, subclinical brain MRI abnormalities were defined as ≥5 for ventricular grade, ≥3 for white matter grade, or presence of ≥1 infarct (>3 mm).17,28
2.4 |. Statistical analysis
Depending on distribution, continuous variables were summarized as mean ± SD or median (interquartile range) and categorical variables as number (%). T-test and Mann-Whitney U test were used for continuous variables with normal and non-normal distributions, respectively. For categorical variables, chi-square test was used.
Time-to-event (person-years) was defined from the baseline year of CHS-CS (1998–1999) to onset of dementia, or end of follow-up (ie, end of study or death). To account for body size and be comparable with BMI, we adjusted the waist circumference and body composition measures by height.2 Multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs) for BMI, waist circumference, lean and fat mass, and grip strength (quartiles) predicting incident dementia were calculated using the Fine-Gray sub distribution hazard model in the presence of competing risk of death. We used sex-specific quartiles to account for the significant difference in the distribution of body composition and grip strength between men and women. For quartiles (reference = lowest), P-for-trend was determined using ordinal values of the quartiles (0, 1, 2, 3). BMI was also categorized into four groups: underweight (<20), normal weight (20–25), overweight (>25–30), and obese (>30) based on Fitzpatrick et al.2 In addition, we categorized grip strength and appendicular lean mass using recommended cut-points (see Cruz-Jentoft et al.). 29 to categorize the grip strength and appendicular lean mass. Models were sequentially adjusted using selected covariates based on literature review and their association with body composition and dementia risk. We sequentially adjusted for age, race, education, APOE ε4 carrier status, diabetes, hypertension, physical activity, triglycerides, smoking status, alcohol consumption, depression scale, history of transient ischemic attack (TIA) or stroke, cancer, or chronic obstructive pulmonary disease (COPD). The results of age- race-adjusted and fully adjusted models are presented. In sensitivity analyses, we further adjusted for baseline 3MSE score, subclinical brain diseases, and BMI. In addition, we conducted the analysis without dementia cases occurred within 5 years of DEXA measurement.
All analyses analysis was performed using SAS version 9.4 (Cary, NC, USA). For statistical testing, significance level was set at P < .05.
3 |. RESULTS
Among the 344 participants, the mean (SD) age was 77.9 (3.8); 37.8% were men; 19.5% were APOE ε4 carriers, 64.2% had hypertension, and 9.3% had diabetes at baseline (Table 1). During the follow-up period (median 6 years), 199 cases (57.8%) of dementia occurred, and 87 deaths (25.3%) occurred. Body composition measures were higher among those who did not develop dementia than those who did during the follow-up, but the difference was significantly attenuated when standardized by height (Supplementary Table S1). Body composition measures and grip strength significantly differed by sex (Supplementary Table S2). Men had significantly higher grip strength and more lean mass, and women had more fat mass. Body composition measures and grip strength were not associated with baseline cognitive function or subclinical brain diseases (data not shown).
TABLE 1.
Baseline characteristics of participants with normal cognition in the CHS-CS (1998–1999), by dementia status at end of follow-up
| Demented (n = 199) | Nondemented(n = 145) | P-value* | |
|---|---|---|---|
| Age, years | 78.3 ± 3.7 | 77.4 ± 4.0 | .02 |
| Women (n, %) | 134 (67.3) | 80(55.2) | .02 |
| Black (n, %) | 36(18.1) | 25 (17.2) | .84 |
| Education, years median (IQR)a | 17.0 (12.0,20.0) | 18.0(12.0, 20.0) | .22 |
| Hypertension (n,%) | 131 (66.5) | 90 (62.9) | .50 |
| Anti-hypertensive medication (n, %) | 113(56.8) | 74(51.0) | .29 |
| SBP, mm Hg | 130.7 ± 20.5 | 129.0 ± 18.8 | .43 |
| DBP, mm Hg | 66.8 ± 10.8 | 67.0 ± 9.2 | .85 |
| Mean arterial pressure, mm Hg | 88.1 ± 12.4 | 87.6 ± 10.3 | .69 |
| Diabetes mellitus (n, %) | 23(11.9) | 9(6.3) | .09 |
| HDLc, mmol/L | 1.4 ± 0.4 | 1.4 ± 0.4 | .61 |
| LDLc, mmol/L | 3.3 ± 0.7 | 3.0 ± 0.7 | .02 |
| Triglycerides, mmol/L median (IQR)b | 1.2 (0.9,1.6) | 1.4 (1.0,1.7) | .02 |
| Lipid-lowering medication (n, %) | 46 (23.1) | 25(17.2) | .18 |
| APOE ε4 carriers (n, %) | 42 (23.1) | 25 (18.5) | .33 |
| 3MSE, median (IQR)b | 97.0 (94.0,99.0) | 98.0 (96.0, 99.0) | <.01 |
| >3 mm infarct (n, %) | 46 (25.7) | 29 (23.4) | .65 |
| ≥5 Ventricular grade (n, %) | 43 (24.0) | 14(11.3) | <.01 |
| ≥3 White matter grade (n, %) | 60 (33.5) | 40 (32.3) | .82 |
3MSE,modified Mini-Mental State Examination; BMI, body mass index;CHS-CS, Cardiovascular Health Study-Cognition Study;DBP, diastolic blood pressure; HDLc, high-density lipoprotein cholesterol; IQR, interquartile range; LDLc, low-density lipoprotein cholesterol; SBP, systolic BP.
Median (IQR) and Mann-Whitney U test were used for skewed distribution.
T-test and Mann-Whitney U test were used for continuous variables with normal and non-normal distributions, respectively. For categorical variables, chi- square test was used.
The association between body composition measures and dementia risk differed significantly between men and women for BMI, total and appendicular lean mass. P-values for sex interaction were <.05 for BMI and <.01 for total and appendicular lean mass, respectively. P-values for sex interaction were.09 and.12 for waist circumference and trunk lean mass, respectively. For total, trunk and appendicular fat mass, P-values for sex interaction were .42,.51, and.25. When stratified by sex, lower BMI, total lean mass, and appendicular lean mass were significantly associated with increased dementia risk in men but not in women (Tables 2 and 3). In contrast, waist circumference, fat mass, and trunk lean mass had no association with dementia risk in men or women.
TABLE 2.
Dementia risk (HR (95% CI)) for BMI, waist circumference, and fat mass (men and women)
| Men (HR (95%CI)) |
Women (HR(95%CI)) |
|||||
|---|---|---|---|---|---|---|
| Range | Model 1 | Model 2 | Range | Model 1 | Model 2 | |
| BMI (kg/m2) | ||||||
| 1st Quartilea | 18.72–23.69 | 1 | 1 | 17.52–23.40 | 1 | 1 |
| 2nd Quartile | 23.79–25.99 | 0.78 (0.44,1.40) | 0.56(0.27,1.14) | 23.45–26.38 | 0.88 (0.55,1.39) | 0.91 (0.54,1.52) |
| 3rd Quartile | 26.04–28.29 | 0.38 (0.19,0.76) | 0.28 (0.11,0.71) | 26.44–29.64 | 0.94 (0.59,1.50) | 0.87 (0.46,1.63) |
| 4th Quartile | 28.62–34.27 | 0.59(0.29,1.19) | 0.35 (0.12,1.02) | 29.64–41.15 | 1.04 (0.65,1.67) | 0.92 (0.48,1.76) |
| P-trend | .03 | .03 | .81 | .77 | ||
| Waist circumference/height2 (m/m2) | ||||||
| 1st Quartile | 0.23–0.30 | 1 | 1 | 0.24–0.34 | 1 | 1 |
| 2nd Quartile | 0.30–0.32 | 1.25 (0.68, 2.29) | 1.27 (0.60, 2.70) | 0.34–0.37 | 1.16 (0.73,1.83) | 1.12 (0.69,1.85) |
| 3rd Quartile | 0.32–0.35 | 0.79 (0.38,1.62) | 0.85 (0.36, 2.00) | 0.38–0.42 | 1.03 (0.65,1.62) | 0.93 (0.54,1.60) |
| 4th Quartile | 0.35–0.42 | 0.79 (0.38,1.65) | 0.70(0.22, 2.22) | 0.42–0.57 | 0.90 (0.56,1.44) | 0.67 (0.38,1.20) |
| P-trend | .29 | .42 | .55 | .14 | ||
| Total fat mass/height2 (kg/m2) | ||||||
| 1st Quartile | 2.24–5.93 | 1 | 1 | 2.37–8.82 | 1 | 1 |
| 2nd Quartile | 5.97–7.32 | 1.05 (0.56,1.96) | 0.77(0.28, 2.10) | 8.83–11.50 | 0.97 (0.61,1.54) | 1.00 (0.59,1.67) |
| 3rd Quartile | 7.32–8.82 | 0.73 (0.39,1.36) | 1.11(0.48, 2.58) | 11.50–13.81 | 0.85 (0.53,1.39) | 0.68 (0.37,1.27) |
| 4th Quartile | 8.85–14.00 | 0.61 (0.29,1.29) | 0.66(0.21, 2.12) | 13.87–24.17 | 0.84 (0.52,1.36) | 0.67 (0.37,1.22) |
| P-trend | .11 | .73 | .41 | .12 | ||
| Trunkfat mass/height2 (kg/m2) | ||||||
| 1st Quartile | 0.61–2.81 | 1 | 1 | 0.59–3.89 | 1 | 1 |
| 2nd Quartile | 2.82–3.79 | 0.82 (0.43,1.55) | 0.85 (0.38,1.94) | 3.91–5.24 | 1.19 (0.76,1.89) | 1.46 (0.89, 2.38) |
| 3rd Quartile | 3.80–4.58 | 0.57 (0.30,1.09) | 0.71 (0.32,1.58) | 5.25–6.41 | 0.99 (0.60,1.61) | 0.94 (0.50,1.78) |
| 4th Quartile | 4.68–8.97 | 0.52 (0.25,1.08) | 0.69 (0.24, 2.01) | 6.42–10.68 | 0.92 (0.57,1.49) | 0.72 (0.37,1.39) |
| P-trend | .04 | .37 | .55 | .26 | ||
| Appendicularfat mass/height2 (kg/m2) | ||||||
| 1st Quartile | 1.37–2.63 | 1 | 1 | 1.43–4.65 | 1 | 1 |
| 2nd Quartile | 2.64–3.20 | 0.67(0.35,1.27) | 0.46 (0.18,1.20) | 4.68–5.86 | 0.88 (0.56,1.38) | 0.68 (0.41,1.13) |
| 3rd Quartile | 3.21–4.00 | 0.87 (0.48,1.57) | 0.94 (0.44, 2.03) | 5.86–7.10 | 0.87 (0.54,1.39) | 0.64 (0.36,1.15) |
| 4th Quartile | 4.01–6.44 | 0.55(0.27,1.12) | 0.72 (0.27,1.89) | 7.10–13.23 | 0.83(0.51,1.36) | 0.66(0.37,1.15) |
| P-trend | .17 | .85 | .48 | .17 | ||
BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Quartiles were defined using all the participants in this analysis. From the lowest to the highest, there are four quartiles (1st quartile: 0–25th percentile, 2nd quartile: 25th–50th percentile, 3rd quartile: 50th–75th percentile, 4th quartile: 75th–100th percentile).
Model 1: age (year) + race (black vs white).
Model 2: age (year) + race (black vs white) + education (≥ 12 years vs <12 years) + APOE ε4 (carrier vs noncarrier) + diabetes (yes vs no) + hypertension (yes vs no) + physical activity (kcal/week) + triglycerides (mmol/L) + current smoking status (yes vs no) + alcohol consumption (drinks/week) + depression scale (point) + TIA/stroke history (yes vs no) + cancer history (yes vs no) + diagnosis of COPD (yes vs no).
TABLE 3.
Dementia risk (HR (95% CI)) for lean mass and grip strength (men and women)
| Men (HR (95%CI)) |
Women (HR(95%CI)) |
|||||
|---|---|---|---|---|---|---|
| Range | Model 1 | Model 2 | Range | Model 1 | Model 2 | |
| Total lean mass/height2 (kg/m2) | ||||||
| 1st Quartile | 13.99–16.97 | 1 | 1 | 11.09–13.70 | 1 | 1 |
| 2nd Quartile | 16.99–18.34 | 0.76 (0.41,1.41) | 0.48 (0.19,1.17) | 13.71–14.71 | 1.21 (0.75,1.94) | 1.10 (0.63,1.93) |
| 3rd Quartile | 18.35–19.53 | 0.67 (0.34,1.29) | 0.75 (0.33,1.73) | 14.71–15.79 | 0.98 (0.60,1.61) | 0.88 (0.48,1.61) |
| 4th Quartile | 19.62–22.84 | 0.52 (0.26,1.06) | 0.32 (0.13,0.76) | 15.82–19.44 | 1.32 (0.81,2.15) | 1.20 (0.66, 2.17) |
| P-trend | .06 | .03 | .42 | .75 | ||
| Trunk lean mass/height (kg/m2) | ||||||
| 1st Quartile | 6.90–8.47 | 1 | 1 | 6.01–7.08 | 1 | 1 |
| 2nd Quartile | 8.49–9.08 | 1.60 (0.85,3.00) | 1.00 (0.46, 2.20) | 7.08–7.54 | 0.98 (0.60,1.60) | 0.68 (0.38,1.19) |
| 3rd Quartile | 9.10–9.73 | 1.04 (0.48,2.26) | 1.15(0.47,2.81) | 7.54–8.01 | 1.22 (0.79,1.88) | 0.98 (0.59,1.62) |
| 4th quartile | 9.73–11.21 | 0.97 (0.47, 2.01) | 0.66 (0.27,1.58) | 8.01–9.93 | 1.18 (0.74,1.90) | 0.77 (0.42,1.41) |
| P-trend | .62 | .43 | .35 | .66 | ||
| Appendicular lean mass/height2 (kg/m2) | ||||||
| 1st Quartile | 5.43–7.03 | 1 | 1 | 3.60–5.27 | 1 | 1 |
| 2nd Quartile | 7.06–7.74 | 0.60 (0.33,1.10) | 0.49 (0.23,1.05) | 5.27–5.78 | 1.10 (0.67,1.81) | 0.91 (0.52,1.59) |
| 3rd Quartile | 7.78–8.51 | 0.49 (0.25,0.96) | 0.46 (0.17,1.27) | 5.79–6.40 | 1.11 (0.68,1.81) | 1.03 (0.58,1.82) |
| 4th Quartile | 8.55–10.76 | 0.45 (0.22,0.89) | 0.23(0.10,0.53) | 6.40–8.09 | 1.37 (0.84,2.22) | 1.28 (0.72, 2.26) |
| P-trend | .02 | <.01 | .23 | .45 | ||
| Grip strength (kg) | ||||||
| 1st Quartile | 20.00–32.67 | 1 | 1 | 7.67–19.33 | 1 | 1 |
| 2nd Quartile | 33.00–38.67 | 1.50 (0.77,2.91) | 1.19(0.50, 2.83) | 19.33–22.67 | 1.15 (0.71,1.87) | 1.54 (0.89, 2.65) |
| 3rd Quartile | 39.00–44.33 | 0.84 (0.39,1.79) | 0.60 (0.20,1.83) | 23.00–27.33 | 0.88 (0.52,1.47) | 0.71 (0.40,1.28) |
| 4th quartile | 44.67–56.33 | 0.93 (0.45,1.91) | 0.94 (0.36, 2.51) | 27.33–38.67 | 1.24 (0.76,2.02) | 1.44 (0.83, 2.49) |
| P-trend | .48 | .56 | .66 | .85 | ||
CI, confidence interval; HR, hazard ratio.
Model l:age (year) + race (black vs white)
Model 2: age (year) + race (black vs white) + education (≥12 years vs <12 years) +APOE ε4 (carrier vs noncarrier) + diabetes (yes vs no) + hypertension (yes vs no) + physical activity (kcal/week) + triglycerides (mmol/L) + current smoking status (yes vs no) + alcohol consumption (drinks/week) + depression scale (point) + TIA/stroke history (yes vs no) + cancer history (yes vs no) + diagnosis of COPD (yes vs no).
Table 4 presents the results for BMI and sarcopenia cut-points. As compared to normal weight, overweight was associated with lower dementia risk in men but not in women (Table 4). Underweight was marginally associated with increased dementia risk in women. Low appendicular lean mass was associated with increased dementia risk in men (HR 3.38, 95% CI 1.85, 6.18) and modestly in women (HR 1.45, 95% CI 0.92, 2.30). After adjusting for height, the association between low appendicular lean mass and dementia remained significant in men but not women.
TABLE 4.
Dementia risk (HR (95%CI)) for sex-specific cut-points of BMI, grip strength, and appendicular lean mass
| Men (HR (95%CI)) |
Women (HR(95%CI)) |
|||||
|---|---|---|---|---|---|---|
| Range | Model 1 | Model 2 | Range | Model 1 | Model 2 | |
| BMI (kg/m2)a | ||||||
| Underweightb | <20 | –a | –a | <20 | 1.99(1.10,3.62) | 1.56(0.75, 3.21) |
| Normal | 20–25 | 1 (ref) | 1 (ref) | 20–25 | 1 (ref) | 1 (ref) |
| Overweight | >25–30 | 0.50 (0.30,0.81) | 0.38 (0.18,0.77) | >25–30 | 1.27 (0.84,1.91) | 1.07 (0.66,1.73) |
| Obese | >30 | 0.32 (0.11,0.90) | 0.14 (0.02,0.80) | >30 | 1.35 (0.82,2.22) | 1.19(0.64, 2.19) |
| Sarcopenia-related cut-pointsc | ||||||
| Low grip strength (kg) | <27 | 0.74 (0.32,1.74) | 0.47 (0.14,1.62) | <16 | 0.57(0.29,1.13) | 0.50 (0.22,1.13) |
| ≥27 | 1 (ref) | 1 (ref) | ≥16 | 1 (ref) | 1 (ref) | |
| Low appendicular lean mass (kg) | <20 | 1.92(1.14,3.22) | 3.38 (1.85, 6.18) | <15 | 1.29 (0.88,1.88) | 1.45 (0.92, 2.30) |
| ≥20 | 1 (ref) | 1 (ref) | ≥15 | 1 (ref) | 1 (ref) | |
| Low appendicular lean mass/height2 (kg/m2) | <7 | 1.85(1.12,3.05) | 2.28(1.23,4.23) | <5.5 | 0.75 (0.52,1.08) | 0.81 (0.52,1.28) |
| ≥7 | 1 (ref) | 1 (ref) | ≥5.5 | 1 (ref) | 1 (ref) | |
BMI, body mass index; CI, confidence interval; HR, hazard ratio.
The BMI was categorized according to was defined according to Fitzpatrick et al.2
Four men were categorized as underweight, three of whom died during the follow-up.
The sarcopenia was defined according to Cruz-Jentoft et al.30
Model l:age (year) + race (black vs white).
Model 2: age (year) + race (black vs white) + education (≥12 years vs <12 years) +APOE ε4 (carrier vs noncarrier) + diabetes (yes vs no) + hypertension (yes vs no) + physical activity (kcal/week) + triglycerides (mmol/L) + current smoking status (yes vs no) + alcohol consumption (drinks/week) + depression scale (point) + TIA/stroke history (yes vs no) + cancer history (yes vs no) + diagnosis of COPD (yes vs no).
In sensitivity analyses, associations of lean mass with dementia from Cox models were similar to those from our primary Fine-Gray models, both for men and women (Supplementary Table S3). By dementia type, results were similar for Alzheimer’s disease (AD) or possible AD cases (n=141). Results for other types of dementia were not statistically significant due to the very small number. Results were also similar when further adjusted for 3MSE score, hippocampal volume, and subclinical brain diseases, including brain infarcts, white matter hyperintensities, and ventricular enlargement at baseline (Supplemental Table S4) or after excluding dementia cases that occurred within 5 years from DEXA measurements (Supplementary Table S5). Associations of lean mass with dementia were similar for APOE ε4 carriers and noncarriers (P-values for interaction >.50). Both total lean and total fat mass are highly correlated with BMI. When adjusted for BMI in the model, BMI was not associated with dementia risk, whereas lean mass was significantly associated with dementia risk for men (Supplementary Table S6).
4 |. DISCUSSION
Among 344 older adults with normal cognition and mean age of 78, we found that lower lean mass, especially appendicular lean mass, was associated with increased dementia risk in men over 15 years of follow-up. In women, low appendicular lean mass was marginally associated with increased dementia risk. In contrast, fat mass was not associated with dementia risk in fully adjusted model in men or women.
To our knowledge, this is the first study to evaluate the longitudinal association between body composition and dementia risk. A cross-sectional study in 70 older adults (mean ag 74) showed that those with early AD had significantly lower lean mass but similar BMI and fat mass compared to healthy controls.10 They also found that higher lean mass was related to greater whole brain volume, especially white matter volume. However, a nested case-control study of 647 women (age ≥75) failed to show a significant difference of lean mass between dementia cases and controls.30 This latter study only measured cognitive function twice, baseline and 7-year follow-up, and only 43.3% of the participants returned to the 7-year follow-up. In contrast, the current study measured cognition annually and had 100% follow-up, and found that lower late-life lean mass is associated with higher dementia risk over 15-year follow-up. Our study results suggest that the association of higher late-life BMI with decreased risk of dementia may be mediated by high lean mass preservation among individuals with high BMI, instead of merely due to reverse causality. Our results also suggest that preservation of lean mass during aging may reduce dementia risk. Additional studies are needed to test this hypothesis.
There are several mechanisms that may contribute to the association between body composition and dementia risk. First, the association between lean mass and dementia might be partially through cardiovascular risk. It has been shown an inverse association between lean mass and white matter hyperintensities among middle-age individuals.31 Our study found that adjusting for multiple cardiovascular risk factors and subclinical brain disease had little effect on our results. However, it should be noted that our participants were older adults with a high prevalence of cardiovascular risk factors and chronic disease. Second, appropriate size of lean mass might support the production of some proteins, for example plasma-derived transthyretin, that are involved in tau and amyloid pathways.32 It is also possible that the association of body composition and dementia is not causal but due to shared risk factors or shared mechanisms, such as hormones and chronic inflammation. Myostatin is an endogenous protein, which is related to loss of muscle mass,33 especially in men.34,35 A recent study found that myostatin can induce cognitive deficits in male mice.36 Therefore, myostatin might directly decrease both lean mass and cognitive function, resulting in an association between them that might be stronger in men than women. Previous studies of chronic inflammation showed that it is associated with loss in lean mass and decline in white matter integrity.37–39 Further studies are needed to understand the underlying mechanisms for the association of lean mass and dementia risk.
In our study, the inverse association between lean mass and dementia is significant in men but only marginally significant in women. Our results agree with previous studies showing sex-specific association between body composition and cognitive function. A British cohort of 1570 older men (mean age 78) reported that lower lean mass was associated with a higher risk of cognitive impairment at the 30-year follow-up.9 A cross-sectional study reported an inverse association between lean mass and cognitive impairment in men but not women.40 At a similar BMI, men and women have a significantly different distribution of body composition with men having substantially higher % lean mass and lower % fat mass. For example, in our study, the third highest quartile of lean mass in women overlaps with the first (lowest) quartile in men. Therefore, the different association between lean mass and dementia risk may relate to the significant difference in the amount of lean mass between men and women. The results of our study and others highlight the importance of sex-specific analysis in future studies of body composition and dementia risk. In addition, we did not observe an association between grip strength and dementia in the sex-specific analysis. An inverse association between grip strength and dementia has been reported by some,41,42 but not all,43 longitudinal studies. Both studies that found an association between grip strength and dementia had a higher percentage of women who developed dementia, especially in Boyle et al.42 Without sex-specific analysis, the results were driven significantly by the higher grip strength in men. Our analysis suggested that the grip strength was not strongly associated with dementia risk among older adults.
An increasing number of studies emphasize the importance of body composition as a risk factor for various disease outcomes. Low lean mass is associated with increased risk of mortality20,44 and cardiovascular events.45 Body composition is more informative than general adiposity indicators, for example, BMI and waist circumference, given the fact that change in BMI and waist circumference lags behind changes in body composition with aging and disease progression. In the progression of AD, a decrease in muscle mass happens prior to the change in BMI.46 Participants with early stage of AD can have a BMI similar to that of healthy controls but lower lean mass.10 Previous studies showed that body composition was significantly associated with health outcomes even after adjusting for BMI.44,45,47 Similarly, in the current study, the inverse association of dementia with total lean mass in men was attenuated but still showed significant trend after adjusting for BMI. In contrast, the association of BMI with dementia risk was diminished by adjustment for total lean mass. This suggests that the association between general adiposity measures and dementia risk is attributed to body composition. In addition, the attenuation by BMI might imply that other variables related to BMI might account for the association between lean mass and dementia risk. Overall, our results suggest the importance of assessing body composition to identify older adults with higher risk of dementia and other diseases.
Our study has several strengths and limitations. It is the first prospective cohort to investigate the association between body composition and dementia in older adults. In addition, we considered the competing risk of death, which is an emerging statistical technique of great importance in dementia research. The consistent results from both Fine-Gray and Cox models show the robustness of the association of higher lean mass with lower dementia risk in our study. Moreover, CHS-CS has annual highly detailed assessment of cognitive status over 15 years, and measurement of many potential confounders including subclinical MRI brain disease measures. Study limitations include a relatively small sample size and a select sample of individuals who were well educated and had survived dementia free to a mean age of 78. Potential survival bias must be carefully considered in interpreting our results, although the results were similar when we conducted various sensitivity analyses. Our study cannot exclude the possibility of reverse causality due to the long prodromal phase of dementia pathophysiology, although the analysis excluded individuals with mild cognitive impairment at baseline. Preclinical cognitive impairment could reduce physical activity and lead to loss of body mass. In addition, we did not observe a significant change in the association between body composition and dementia by excluding dementia cases that occurred within 5 years of DEXA measurement. Finally, our study measured body composition only once, so we cannot assess the association between the change in body composition and dementia risk.
In this cohort of older adults, we found that higher late-life lean mass, especially appendicular lean mass, was significantly associated with decreased dementia risk over 15 years of follow-up, particularly among men. Our results suggest that the association of higher late-life BMI with lower dementia risk is due to higher lean mass, not higher fat mass. These findings should be confirmed by other studies, especially those that follow younger participants for a long period. Future research should determine the underlying mechanisms between body composition and dementia risk, and investigate if interventions on lean mass could delay the onset of dementia in older adults.
Supplementary Material
Supplementary Figure 1. Timeline of the study
HIGHLIGHTS.
Lower lean mass at mean age of 78 was associated with increased dementia risk in older adults over a 15-year follow-up.
With similar body mass index (BMI), men and women had significantly different body composition. The association between lean mass and dementia risk was more prominent in men than in women.
This study clarified prior studies by showing in late life, lower risk of dementia with higher BMI appears to be related to higher lean mass, not higher fat mass.
RESEARCH IN CONTEXT.
Systematic review: The authors searched and screened titles and abstracts on PubMed. Higher late-life body mass index (BMI) is associated with decreased dementia risk. Cross-sectional study reports higher lean mass among cognitively normal individuals than in individuals with dementia. Few longitudinal studies have examined the association between late-life body composition and dementia risk.
Interpretation: This longitudinal study shows that higher late-life lean mass but not fat mass is associated with decreased dementia risk with 15 years of follow-up. It suggests that previous findings of inverse association between late-life BMI and dementia risk is due to lean mass not fat mass.
Future directions: Large longitudinal studies, especially those that follow younger participants for a long period, are warranted to confirm the findings of this study. In addition, future research should investigate the underlying mechanisms between body composition and dementia risk, and investigate if interventions on lean mass could delay the onset of dementia.
ACKNOWLEDGMENTS
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Sources of Funding: This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, R01HL64587, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG15928, R01AG20098, R01AG023629 and RF1AG051615 from the National Institute on Aging (NIA).
Footnotes
DECLARATION OF INTEREST
None.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Pedditzi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45: 14–21. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller S, Preische O, Sohrabi HR, et al. Decreased body mass index in the preclinical stage of autosomal dominant Alzheimer’s disease. Sci Rep. 2017;7:1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond). 2016;40:761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luchsinger JA, Biggs ML, Kizer JR, et al. Adiposity and cognitive decline in the cardiovascular health study. Neuroepidemiology. 2013;40:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nourhashemi F, Andrieu S, Gillette-Guyonnet S, et al. Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. J Am Geriatr Soc. 2002;50:1796–1801. [DOI] [PubMed] [Google Scholar]
- 8.Tolea MI, Chrisphonte S, Galvin JE. Sarcopenic obesity and cognitive performance. Clin Interv Aging. 2018;13:1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papachristou E, Ramsay SE, Lennon LT, et al. The relationships between body composition characteristics and cognitive functioning in a population-based sample of older British men. BMC Geriatr. 2015;15:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010;67:428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong HL, Chang SH, Abdin E, et al. Association of grip strength, upper arm circumference, and waist circumference with dementia in older adults of the WiSE study: A Cross-Sectional Analysis. J Nutr Health Aging 2016;20:996–1001. [DOI] [PubMed] [Google Scholar]
- 12.Bredella MA. Sex Differences in Body Composition. Adv Exp Med Biol. 2017;1043:9–27. [DOI] [PubMed] [Google Scholar]
- 13.Bishop P, Cureton K, Collins M. Sex difference in muscular strength in equally-trained men and women. Ergonomics. 1987;30:675–687. [DOI] [PubMed] [Google Scholar]
- 14.Hinton BJ, Fan B, Ng BK, Shepherd JA. Dual energy X-ray absorptiometry body composition reference values of limbs and trunk from NHANES 1999–2004 with additional visualization methods. PLoS One. 2017;12:e0174180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuller LH, Lopez OL, Mackey RH, et al. Subclinical cardiovascular disease and death, dementia, and coronary heart disease in patients 80+ years. J Am Coll Cardiol. 2016;67:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. [DOI] [PubMed] [Google Scholar]
- 17.Kuller LH, Lopez OL, Becker JT, Chang Y, Newman AB. Risk of dementia and death in the long-term follow-up of the Pittsburgh Cardiovascular Health Study-Cognition Study. Alzheimers Dement. 2016;12:170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. [DOI] [PubMed] [Google Scholar]
- 19.Kern LM, Powe NR, Levine MA, et al. Association between screening for osteoporosis and the incidence of hip fracture. Ann Intern Med. 2005;142:173–181. [DOI] [PubMed] [Google Scholar]
- 20.Spahillari A, Mukamal KJ, DeFilippi C, et al. The association of lean and fat mass with all-cause mortality in older adults: The Cardiovascular Health Study. Nutr Metab Cardiovasc Dis 2016;26:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 22.Odden MC, Shlipak MG, Whitson HE, et al. Risk factors for cardiovascular disease across the spectrum of older age: the Cardiovascular Health Study. Atherosclerosis. 2014;237:336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares-Miranda L, Siscovick DS, Psaty BM, Longstreth WT Jr., Mozaffarian D. Physical activity and risk of coronary heart disease and stroke in older adults: The Cardiovascular Health Study. Circulation. 2016;133:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29:388–398. [DOI] [PubMed] [Google Scholar]
- 25.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 26.Win S, Parakh K, Eze-Nliam CM, Gottdiener JS, Kop WJ, Ziegelstein RC. Depressive symptoms, physical inactivity and risk of cardiovascular mortality in older adults: the Cardiovascular Health Study. Heart. 2011;97:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue NC, Arnold AM, Longstreth WT Jr., et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997;202:33–39. [DOI] [PubMed] [Google Scholar]
- 28.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr., Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. [DOI] [PubMed] [Google Scholar]
- 29.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abellan van Kan G, Rolland Y, Gillette-Guyonnet S, et al. Gait speed, body composition, and dementia. The EPIDOS-Toulouse cohort. J Gerontol A Biol Sci Med Sci. 2012;67:425–432. [DOI] [PubMed] [Google Scholar]
- 31.Kohara K, Okada Y, Ochi M, et al. Muscle mass decline, arterial stiffness, white matter hyperintensity, and cognitive impairment: Japan Shimanami Health Promoting Program study. J Cachexia Sarcopenia Muscle. 2017;8:557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingenbleek Y, Bernstein LH. Downsizing of lean body mass is a key determinant of Alzheimer’s disease. J Alzheimers Dis. 2015;44:745–754. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. [DOI] [PubMed] [Google Scholar]
- 34.Tay L, Ding YY, Leung BP, et al. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordr). 2015;37:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng LN, Lee WJ, Liu LK, Lin MH, Chen LK. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J Cachexia Sarcopenia Muscle 2018;9:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin YS, Lin FY, Hsiao YH. Myostatin is associated with cognitive decline in an animal model of Alzheimer’s disease. Mol Neurobiol. 2019;56(3):1984–1991. [DOI] [PubMed] [Google Scholar]
- 37.Westbury LD, Fuggle NR, Syddall HE, et al. Relationships between markers of inflammation and muscle mass, strength and function: findings from the hertfordshire Cohort Study. Calcif Tissue Int. 2018;102:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker KA, Windham BG, Power MC, et al. The association of mid-to late-life systemic inflammation with white matter structure in older adults: The Atherosclerosis Risk in Communities Study. Neurobiol Aging. 2018;68:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wåhlin-Larsson B, Wilkinson DJ, Strandberg E, Hosford-Donovan A, Atherton PJ, Kadi F. Mechanistic links underlying the impact of c-reactive protein on muscle mass in elderly. Cell Physiol Biochem. 2017;44:267–278. [DOI] [PubMed] [Google Scholar]
- 40.Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29:66–73. [DOI] [PubMed] [Google Scholar]
- 42.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66:1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milne JS, Maule MM. A longitudinal study of handgrip and dementia in older people. Age Ageing. 1984;13:42–48. [DOI] [PubMed] [Google Scholar]
- 44.Padwal R, Leslie WD, Lix LM, Majumdar SR. Relationship among body fat percentage, body mass index, and all-cause mortality: A Cohort Study. Ann Intern Med. 2016;164:532–541. [DOI] [PubMed] [Google Scholar]
- 45.Hioki H, Miura T, Motoki H, et al. Lean body mass index prognostic value for cardiovascular events in patients with coronary artery disease. Heart Asia. 2015;7:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa Y, Kaneko Y, Sato T, Shimizu S, Kanetaka H, Hanyu H. Sarcopenia and muscle functions at various stages of Alzheimer disease. Front Neurol. 2018;9:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azarbal F, Stefanick ML, Assimes TL, et al. Lean body mass and risk of incident atrial fibrillation in post-menopausal women. Eur Heart J. 2016;37:1606–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Timeline of the study