Abstract
OBJECTIVE:
This study aimed to investigate the effects on postoperative pain of ketamine and dexmedetomidine addition to bupivacaine in a transversus abdominis plane (TAP) block in laparoscopic cholecystectomy.
METHODS:
A retrospective study was conducted patients who underwent ultrasound-guided TAP block in laparoscopic cholecystectomy. The patients were divided into three groups: Group BD (Bupivacaine+Dexmedetomidine), Group BK (Bupivacaine+Ketamine), and Group B (Bupivacaine). Our primary outcomes were pain scores with Visual Analogue Scale (VAS), postoperative first analgesic time and tramadol consumption in 24 hours postoperatively. Secondary outcomes were intraoperative hemodynamic changes, rescue analgesic requirement and side effects.
RESULTS:
The first analgesic administration time was significantly shorter in Group B and significantly longer in Group BD than the other two groups. Pain score at rest in Group B at 0th hours was significantly higher than that of Group BD and VAS pain score Group BD at 2nd hours was significantly lower than the other two groups. There was no significant difference between the groups regarding tramadol consumption and the requirement of rescue analgesics.
CONCLUSION:
Dexmedetomidine and ketamine can be added to the bupivacaine for the TAP block without major side-effects. The combination of dexmedetomidine and bupivacaine provides better analgesia in the first postoperative 2nd hour than other groups and hence extends the time to the first analgesic demand.
Keywords: Bupivacaine, dexmedetomidine, nerve block, ultrasonography ketamine
Highlight key points.
TAP block is an effective, safe, and technique with no significant side effects.
Ketamine and dexmedetomidine as an adjuvant agent can be added in the TAP block.
The first analgesia requirement was significantly longer in the dexmedetomidine group.
Many methods, such as patient-controlled thoracic epidural analgesia, intravenous patient-controlled analgesia, the intraperitoneal injection of local anesthetics, non-steroidal anti-inflammatory drugs, opioids, and multimodal analgesia, have been used for postoperative pain after laparoscopic cholecystectomies [1, 2]. Rafiin was first described transversus abdominis plane (TAP) block in 2001, and the TAP block provides up to 24 hours of analgesia [3]. Local anesthetics administration between the T6-L1 spinal nerve roots alleviates pain in abdominal procedures [4]. Improvements have been made to TAP with the addition of ultrasound guidance to confirm the proper region and avoid complications [4]. Ultrasonography (USG) guided techniques may have the advantage of being effective and safe through direct needle visualization [5]. Bupivacaine, ropivacaine, and levobupivacaine are generally preferred local anesthetic agents for TAP block [6]. Only few studies have studied the additions to the TAP block of local anesthetics, such as dexamethasone [7], epinephrine [8], fentanyl [9], and dexmedetomidine [10, 11].
We studied the effects on postoperative pain of ketamine and dexmedetomidine addition to bupivacaine in a TAP block applied under ultrasonography in laparoscopic cholecystectomy.
MATERIALS AND METHODS
The study protocol was approved by the Local Ethics Committee (Uludag University Ethics Committee Decision Number: 28 March 2017/2017-4/22). Our study was a single-center, retrospective, and observational trial and performed in accordance with the principles of the Declaration of Helsinki. The patients included were those aged 18–65 years who were applied with TAP block using ultrasonography in a laparoscopic cholecystectomy under general anesthesia between September 2016 and March 2017. The archive records of the patients were examined. Any patients with incomplete records were excluded from this study.
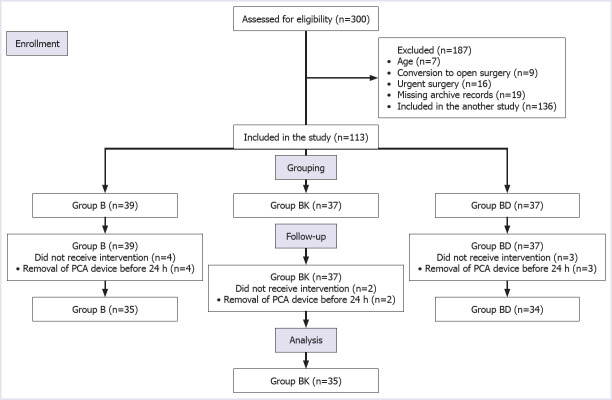
A total of 187 of 300 patients were excluded from this study. The remaining 113 patients were grouped according to the drugs used for TAP block, and 104 of them were analyzed retrospectively (Fig. 1). The patients were grouped according to the drugs used for TAP block.
FIGURE 1.
Flow chart of this study.
Bupivacaine Group (B) received 40 ml solution containing 1 mg/kg bupivacaine (Bustesin®, Vem, Ankara, Turkey) + saline (n=39).
Bupivacaine Ketamine Group (BK) received 40 ml solution containing 1 mg/kg bupivacaine+0.5 mg/kg ketamine (Ketalar®, Pfizer, Istanbul, Turkey)+saline (n=37).
Bupivacaine Dexmedetomidine Group (BD) received 40 ml solution containing 1 mg/kg bupivacaine+1 mcg/kg dexmedetomidine (Precedex®, Meditera, Izmir, Turkey)+saline (n=37).
Outcomes
The primary outcomes evaluated the pain scores with a visual analogue scale (VAS) (0=no pain, 10=worst imaginable pain), time of the first analgesic, and the amount of tramadol consumption at the 24th postoperative hour (time to admission to recovery room was defined as 0th). The secondary outcomes evaluated the side effects (e.g., nausea, vomiting, respiratory depression, nystagmus, hallucinations, bradycardia and hypotension) requirement for rescue analgesia, intraoperative bradycardia, tachycardia, hypotension and hypertension during 24 h follow-up.
Statistical Analyses
All statistical analyses were performed using IBM SPSS ver. 23.0 (Statistical Package for the Social Sciences, NY, USA). Continuous variables were described as means and standard deviations, or median, minimum and maximum as necessary. Categorical variables were defined as frequency and percentage. Continuous variables were compared using ANOVA and Kruskal Wallis tests when the data were not normally distributed. A Bonferroni test was used for multiple pairwise comparisons. For responses at different time points, percent changes and differences were calculated according to the baseline measurement. These percent changes and differences were compared between groups. Categorical variables were compared using Pearson’s chi-squared test and the Fisher-Freeman-Halton test. A p-value <0.05 was considered significant. According to a pilot study, using a pooled standard deviation of VAS score (2.3), a power analysis indicated that a sample of 34 patients would be needed for each group to detect an effect size (d=0.31) with 80% power with alpha at 0.05, 2-sided significance level.
RESULTS
Among 300 patients who had been undergoing laparoscopic cholecystectomy, 113 were included in this study and 104 of them analyzed statistically (Fig. 1). No statistically significant differences were found between the groups regarding the demographic data (Table 1).
TABLE 1.
Demographic characteristics of the patients
| Group B (n=35) | Group BK (n=35) | Group BD (n=34) | p* | |
|---|---|---|---|---|
| Age (year), Mean±SD | 46.43±12.65 | 43.71±10.86 | 46.12±14 | 0.613 |
| Sex (Female %) | 82.9 | 80 | 73.5 | 0.624 |
| BMI (kg/m2), Mean±SD | 27.27±2.81 | 27.48±3.57 | 29.11±4.46 | 0.102 |
| ASA, % | ||||
| I | 57.1 | 51.4 | 32.4 | 0.098 |
| II | 42.9 | 48.6 | 67.6 | |
| Operation time (minute), Mean±SD | 44±12.11 | 40.85±13.36 | 42.20±12.44 | 0.561 |
SD: Standard deviation; BMI: Body mass index; ASA: American Society of Anesthesiology;
ANOVA and Kruskal Wallis tes.
Pain scores at rest were significantly lower in Group BD than in Group B (p=0.030) at 0th hours. VAS pain scores in Group BD at 2nd hours were significantly lower than the other groups (p=0.004, Table 2). No significant difference was found between the three groups regarding the pain scores examined at the other time points (p>0.05, Table 2). A significant difference was observed between the groups regarding the time of requirement for the first analgesic (p<0.05). In Group B, the time of requirement for the first analgesic was significantly shorter than in the other two groups and in Group BD, the time was significantly longer than in the other two groups (Table 3). No significant difference was observed between the groups regarding the amount of tramadol consumption in the first 24 hours (p>0.05, Table 3).
TABLE 2.
Comparison of Visual Analogue Scale scores between groups
| Group B (n=35) | Group BK (n=35) | Group BD (n=34) | p* | Binary comparisons** | |
|---|---|---|---|---|---|
| 0 | 3 (0–8) | 1 (0–8) | 1 (0–6) | 0.035 | Group BD-BK: 0.772 |
| Group BD-B: 0.030 | |||||
| Group BK-B:0.436 | |||||
| 2nd h | 0 (-3–5) | 0 (-8–3) | 0.5 (-3–4) | 0.004 | Group BD-BK: 0.009 |
| Group BD-B: 0.016 | |||||
| Group BK-B: 1.000 | |||||
| 4th h | -1 (-3–5) | 0 (-8–3) | 0 (-6–4) | 0.410 | – |
| 6th h | -1 (-4–6) | 0 (-8–5) | 0 (-6–4) | 0.143 | – |
| 12th h | -2 (-5–3) | 0 (-8–3) | 0 (-5–3) | 0.184 | – |
| 24th h | -2 (-5–3) | 0 (-8–2) | 0 (-6–5) | 0.180 | – |
VAS: Visual Analogue Scale;
Kruskal Wallis test;
Bonferroni. For the comparison of VAS scores (at 2nd, 4th, 6th, 12th and 24th) changes were calculated as the difference of VAS according to baseline VAS.
TABLE 3.
Analgesic usage profile of the groups
| Group B (n=35) | Group BK (n=35) | Group BD (n=34) | p* | |
|---|---|---|---|---|
| Time of first analgesia (minute), Median (min.–max.) | 20 (5–240) | 90 (5–240) | 120 (10–600) | <0.001† |
| Total tramadol consumption during 24h (mg), Median (min.–max.) | 200 (50–550) | 200 (50–500) | 150 (0–400) | 0.064 |
| Requirement of rescue analge-sics, % | 25.7 | 11.4 | 14.7 | 0.255 |
min.: Minimum; max.: Maximum;
Kruskal Wallis test;
Bonferroni (Group B-BK: 0.016, Group B-BD: <0.001, Group BK-BD: 0.009).
No significant difference was observed between the groups regarding the heart rate values at baseline, before intubation, after intubation and at 5, 10, 15, 20, 25 and 30 minutes intraoperatively (p>0.05). When a comparison was made regarding the mean arterial pressure (MAP), the values measured at 25 minutes were significantly higher in Group B than in the other two groups (p=0.006). No significant difference was observed between the groups regarding the other MAP values (Fig. 2). No significant difference was observed between the groups regarding the intraoperative development of hypotension, hypertension, bradycardia, and tachycardia (p>0.05). No significant difference was observed between the groups regarding the requirement for rescue analgesia (p>0.05, Table 3). Rescue analgesia, diclofenac 75 mg was applied intramuscularly. In the postoperative period, nausea was observed in one patient in Group BK. In Group BD, hypotension was seen in one patient and bradycardia in one patient. No complications were observed in Group B. No significant difference was observed between the groups with respect to patient satisfaction (p>0.05).
FIGURE 2.
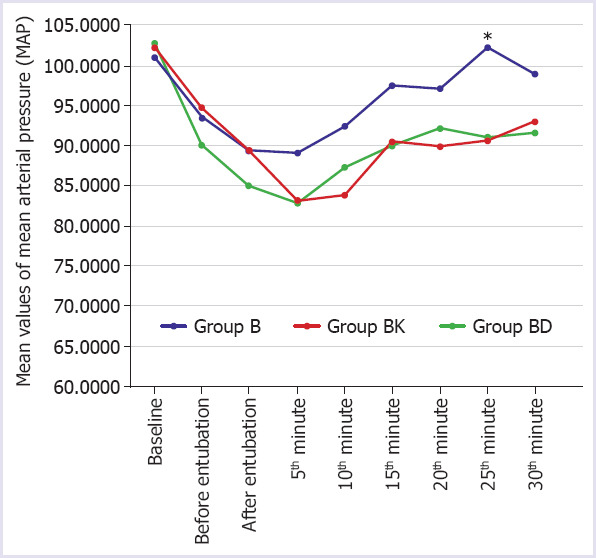
Mean values of Mean Arterial Pressure (MAP) according to groups (ANOVA).
Group B: Bupivacaine group; Group BK: Bupivacaine+Ketamine group; Group BD: Bupivacaine+Deskmetedomidine group; *: p Group B-BK: 0.016, Group B-BD: 0.015, Group BD-BK: 1.000.
Our Clinical Anesthesia Management
All patients were premedicated with iv midazolam (0.01–0.02 mg/kg). After standard monitoring, patients were intubated using fentanyl (1–2 mcg/kg), propofol (2–3 mg/kg) and rocuronium (0.6 mg/kg). Anesthesia maintenance was provided with sevoflurane in an air+ O2 mixture administered. Ondansetron 4 mg IV was administered intraoperatively. All TAP blocks were performed after induction of anesthesia with ultrasound guidance by the anesthesiologist. A linear ultrasound (Esaote®, MyLab30Gold Cardiovascular, Florence, Italy) probe was placed on the wall of the upper abdomen, obliquely along the subcostal margin close to the midline after intubation of the patient. After identifying the rectus abdominis muscle, the ultrasound probe was then moved in a lateral and oblique direction along the subcostal margin until the transversus abdominis muscle was identified posterior to the rectus muscle. Bilateral application of the TAP block was performed with an 80 mm 22 G needle (Stimuplex® Ultra, B. BraunMelsungen AG, Germany) into the plane between the rectus abdominis and transversus abdominis muscles. All patients received patient-controlled intravenous analgesia (PCA) with 90 ml saline and 10 ml tramadol for postoperative pain management (bolus dose 5 ml, and no continuous infusion) for 24 hours.
DISCUSSION
This study was evaluated the postoperative pain of ketamine or dexmedetomidine addition to bupivacaine for TAP block applied under ultrasonography guidance in laparoscopic cholecystectomy. The main result of this study was that the time to a requirement for analgesia was significantly longer in the dexmedetomidine group compared to the other groups. Pain scores in Group B at 0th hours were significantly higher than Group BD and pain scores in Group BD at 2nd hours were significantly lower than that of the other two groups. There was no difference in VAS values after the second hour. No significant difference was observed between the groups regarding tramadol consumption and the need for rescue analgesia in the first 24 hours.
In recent years, the use of ultrasound-guided TAP block has become more widespread in laparoscopic cholecystectomy. TAP block can provide efficient postoperative analgesia [12, 13]. In the reports in the literature, different volumes (15–30 ml), different agents (bupivacaine, ropivacaine, and levobupivacaine) and different concentrations have been used in TAP blocks [12, 13]. Bupivacaine concentration varies from 0.125% to 0.5% [12, 13]. Thus, the risk of local anesthetic toxicity was kept low by administering a low dose of bupivacaine in the fixed total volume.
Several adjuvant agents are added to local anesthetic in the application of peripheral or regional blocks to provide effective, long-lasting and safe analgesia in a single administration [14]. Dexmedetomidine is a selective α2-adrenoreceptor agonist and when administered IV, provides analgesia and sedation without causing respiratory depression [15]. Studies have shown that the addition of dexmedetomidine to local anesthetics in central and peripheral blocks is effective in potentiating the local anesthetic effect and decreasing the need for analgesia [16–18]. In a study conducted by McDonnell et al. [19], complete regression of the sensory deficit caused by a TAP block was observed after 24 hrs. Various adjuvants have been described to prolong the sensory block and these are used in daily clinical practice. In previous studies in the literature, dexmedetomidine has been used at a dose of bilateral 1 mcg/kg as appropriate for TAP block [15, 17, 18]. The dose used in the current study was the same as that in the literature. Several studies have shown that prolonged analgesia with the addition of dexmedetomidine to bupivacaine in the TAP block has provided a lower need for morphine and lower VAS pain scores [10, 11]. However, some studies have shown that the addition of dexmedetomidine had no benefit on the pain scores [15, 17, 18]. In our study, we found that the time to the first analgesic requirement was significantly shorter in the bupivacaine group and significantly longer in the dexmedetomidine group. However, no statistically significant difference was determined between the bupivacaine, dexmedetomidine and ketamine groups regarding pain scores, amount of tramadol consumption and a requirement for rescue analgesia throughout the first 24 hours postoperatively. It has been suggested that there could be an association between dexmedetomidine and some side-effects, such as hypotension, bradycardia, and sedation, especially with the use of higher doses [16]. In the current study, these side-effects were not observed as a low dose of dexmedetomidine was used. Only one patient had bradycardia.
The unique property of ketamine is its mechanism of action at the NMDA receptors. Although it has been defined as a general anesthetic, nowadays, it is commonly used in sub-anesthetic doses for cases of severe depression and acute and chronic pain management. It has been believed that, for chronic pain, ketamine reverses central sensitization and enhances descending modulatory pathways [20]. Ketamine is also used in both inpatient and outpatient settings for acute pain management. Ketamine is a good choice in emergency departments for patients with refractory pain in the perioperative period, to alleviate procedure-related discomfort, and in opioid-tolerant patients [21]. As an agent with anesthetic and analgesic properties, the efficacy of ketamine in regional anesthesia has been reported in several studies [22–25]. These studies are limited to the application site of the peripheral block with ketamine. In stellate ganglion blockade and infiltration to the peritonsillar region, ketamine has been applied at a dose of 0.5 mg/kg [26, 27]. In the groups applied with ketamine in both studies, effective analgesia was determined to have been provided without any evident side-effects in the postoperative period [26, 27]. To our knowledge, there has been no previous study where ketamine has been added to bupivacaine in the TAP block. Ricciardelli et al. [28] showed that the addition of ketamine (0.5 mg/kg bolus and 0.2 mg/kg/h infusion) to the standard pain control regimen for spinal fusion procedures for the correction of idiopathic scoliosis successfully reduced the total morphine consumption. Kasputyte et al. [29] found that pre-incisional single dose ketamine (0.15 mg/kg iv bolus) reduces postoperative opioids consumption but does not have an effect on postoperative pain intensity and side effects after remifentanil infusions in patients undergoing bariatric surgery. In our study, ketamine, one of the multimodal analgesia methods, was administered at a dose of 0.5 mg/kg, similar to the literature.
There are some limitations to this study. The retrospective design of this study was the first limitation. Secondly, it was not possible to evaluate parameters at the onset of anesthesia because the TAP block was performed following the induction of general anesthesia. Thirdly, clinical signs or symptoms of neurotoxicity were not assessed. Nevertheless, there were no significant changes in hemodynamics intraoperatively or postoperatively. The other limitation was that dexmedetomidine and ketamine plasma concentrations could not be compared between patients to determine whether their actions were related to systemic absorption or a purely local effect. Furthermore, that the doses of adjuvant ketamine in TAP block have not been specified in previous studies can be considered a limitation.
Conclusion
Ketamine or dexmedetomidine can be added to bupivacaine without major side effects for the TAP block. The combination of dexmedetomidine and bupivacaine compare to ketamine to bupivacaine prolongs the time to first analgesic requirement. This study can be considered to contribute to the literature as a study which has investigated the effects of ketamine or dexmedetomidine as adjuvant agents added to bupivacaine for TAP block. However, there is a need for further prospective studies on this subject.
Footnotes
Ethics Committee Approval: The Uludag University Clinical Research Ethics Committee granted approval for this study (date: 28.03.2017, number: 2017-4/22).
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
Authorship Contributions: Concept – DK, SEO, CY, DY; Design – DK, CY, SEO, DY; Supervision – DK, CY, SEO, DY; Fundings – DY; Materials – DK, CY, SEO- DY; Data collection and/or processing – DK, DY; Analysis and/or interpretation – GO; Literature review – CY, DY; Writing – DK, SEO; Critical review – DK, GO.
REFERENCES
- 1.Michaloliakou C, Chung F, Sharma S. Preoperative multimodal analgesia facilitates recovery after ambulatory laparoscopic cholecystectomy. Anesth Analg. 1996;82:44–51. doi: 10.1097/00000539-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Wills VL, Hunt DR. Pain after laparoscopic cholecystectomy. Br J Surg. 2000;87:273–84. doi: 10.1046/j.1365-2168.2000.01374.x. [DOI] [PubMed] [Google Scholar]
- 3.Rafi AN. Abdominal field block:a new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–6. doi: 10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- 4.Hebbard P, Fujiwara Y, Shibata Y, Royse C. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care. 2007;35:616–7. [PubMed] [Google Scholar]
- 5.Sandeman DJ, Bennett M, Dilley AV, Perczuk A, Lim S, Kelly KJ. Ultrasound-guided transversus abdominis plane blocks for laparoscopic appendicectomy in children:a prospective randomized trial. Br J Anaesth. 2011;106:882–6. doi: 10.1093/bja/aer069. [DOI] [PubMed] [Google Scholar]
- 6.Ma N, Duncan JK, Scarfe AJ, Schuhmann S, Cameron AL. Clinical safety and effectiveness of transversus abdominis plane (TAP) block in post-operative analgesia:a systematic review and meta-analysis. J Anesth. 2017;31:432–52. doi: 10.1007/s00540-017-2323-5. [DOI] [PubMed] [Google Scholar]
- 7.Gulhas N, Kayhan G, Sanlı M, Kıtlık A, Durmus M. Effect of adding dexamethasone to bupivacaine on transversus abdominis plane block. Med-Science. 2015;4:2732–42. [Google Scholar]
- 8.Soltani Mohammadi S, Dabir A, Shoeibi G. Efficacy of transversus abdominis plane block for acute postoperative pain relief in kidney recipients:a double-blinded clinical trial. Pain Med. 2014;15:460–4. doi: 10.1111/pme.12311. [DOI] [PubMed] [Google Scholar]
- 9.John R, Ranjan RV, Ramachandran TR, George SK. Analgesic Efficacy of Transverse Abdominal Plane Block after Elective Cesarean Delivery - Bupivacaine with Fentanyl versus Bupivacaine Alone:A Randomized. Double-blind Controlled Clinical Trial. Anesth Essays Res. 2017;11:181–4. doi: 10.4103/0259-1162.186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aksu R, Patmano G, Biçer C, Emek E, Çoruh AE. Efficiency of bupivacaine and association with dexmedetomidine in transversus abdominis plane block ultrasound guided in postoperative pain of abdominal surgery. Rev Bras Anestesiol. 2018;68:49–56. doi: 10.1016/j.bjane.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luan H, Zhang X, Feng J, Zhu P, Li J, Zhao Z. Effect of dexmedetomidine added to ropivacaine on ultrasound-guided transversus abdominis plane block for postoperative analgesia after abdominal hysterectomy surgery:a prospective randomized controlled trial. Minerva Anestesiol. 2016;82:981–8. [PubMed] [Google Scholar]
- 12.Abdallah FW, Chan VW, Brull R. Transversus abdominis plane block:a systematic review. Reg Anesth Pain Med. 2012;37:193–209. doi: 10.1097/AAP.0b013e3182429531. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui MR, Sajid MS, Uncles DR, Cheek L, Baig MK. A meta-analysis on the clinical effectiveness of transversus abdominis plane block. J Clin Anesth. 2011;23:7–14. doi: 10.1016/j.jclinane.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Patacsil JA, McAuliffe MS, Feyh LS, Sigmon LL. Local Anesthetic Adjuvants Providing the Longest Duration of Analgesia for Single- Injection Peripheral Nerve Blocks in Orthopedic Surgery:A Literature Review. AANA J. 2016;84:95–103. [PubMed] [Google Scholar]
- 15.Almarakbi WA, Kaki AM. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients:A prospective randomized controlled trial. Saudi J Anaesth. 2014;8:161–6. doi: 10.4103/1658-354X.130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 17.Mishra M, Mishra SP, Singh SP. Ultrasound-guided transversus abdominis plane block:What are the benefits of adding dexmedetomidine to ropivacaine? Saudi J Anaesth. 2017;11:58–61. doi: 10.4103/1658-354X.197348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding W, Li W, Zeng X, Li J, Jiang J, Guo C, et al. Effect of Adding Dexmedetomidine to Ropivacaine on Ultrasound-Guided Dual Transversus Abdominis Plane Block after Gastrectomy. J Gastrointest Surg. 2017;21:936–46. doi: 10.1007/s11605-017-3402-5. [DOI] [PubMed] [Google Scholar]
- 19.McDonnell JG, O'Donnell BD, Farrell T, Gough N, Tuite D, Power C, et al. Transversus abdominis plane block:a cadaveric and radiological evaluation. Reg Anesth Pain Med. 2007;32:399–404. doi: 10.1016/j.rapm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Niesters M, Martini C, Dahan A. Ketamine for chronic pain:risks and benefits. Br J Clin Pharmacol. 2014;77:357–67. doi: 10.1111/bcp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S, et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43:456–66. doi: 10.1097/AAP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang J, Jeon Y, Park HP, Lim YJ, Oh YS. Comparison of alfetanil and ketamine in combination with propofol for patient-controlled sedation during fiberoptic bronchoscopy. Acta Anaesthesiol Scand. 2005;49:1334–8. doi: 10.1111/j.1399-6576.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- 23.Hirota K, Lambert DG. Ketamine:new uses for an old drug? Br J Anaesth. 2011;107:123–6. doi: 10.1093/bja/aer221. [DOI] [PubMed] [Google Scholar]
- 24.Cook B, Grubb DJ, Aldridge LA, Doyle E. Comparison of the effects of adrenaline clonidine and ketamine on the duration of caudal analgesia produced by bupivacaine in children. Br J Anaesth. 1995;75:698–701. doi: 10.1093/bja/75.6.698. [DOI] [PubMed] [Google Scholar]
- 25.Semple D, Findlow D, Aldridge LM, Doyle E. The optimal dose of ketamine for caudal epidural blockade in children. Anaesthesia. 1996;51:1170–2. doi: 10.1111/j.1365-2044.1996.tb15063.x. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni KR, Kadam AI, Namazi IJ. Efficacy of stellate ganglion block with an adjuvant ketamine for peripheral vascular disease of the upper limbs. Indian J Anaesth. 2010;54:546–51. doi: 10.4103/0019-5049.72645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiqui AS, Raees US, Siddiqui SZ, Raza SA. Efficacy of pre-incisional peritonsillar infiltration of ketamine for post-tonsillectomy analgesia in children. J Coll Physicians Surg Pak. 2013;23:533–7. [PubMed] [Google Scholar]
- 28.Ricciardelli RM, Walters NM, Pomerantz M, Metcalfe B, Afroze F, Ehlers M, et al. The efficacy of ketamine for postoperative pain control in adolescent patients undergoing spinal fusion surgery for idiopathic scoliosis. Spine Deform. 2020;8:433–40. doi: 10.1007/s43390-020-00073-w. [DOI] [PubMed] [Google Scholar]
- 29.Kasputytė G, Karbonskienė A, Macas A, Maleckas A. Role of Ketamine in Multimodal Analgesia Protocol for Bariatric Surgery. Medicina (Kaunas) 2020;56:96. doi: 10.3390/medicina56030096. [DOI] [PMC free article] [PubMed] [Google Scholar]