Abstract
Delayed post-hypoxic leukoencephalopathy (DPHL) is a syndrome that may occur as a result of the hypoxic event, including opiate overdose. The pathophysiology of this entity is not fully known. Within a neuropsychiatric context, the diagnosis of this rare disease is important. A 39-year-old man with a history of methadone overdose presented with loss of consciousness and fever. After clinical evaluations, laboratory analysis, including various tests on blood and cerebrospinal fluid and magnetic resonance imaging, the patient was diagnosed with methadone-induced DPHL. Treatment with antioxidants, including vitamins E, C and B complex, produced a favorable outcome. In rare cases, methadone overdose may lead to DPHL. Antioxidants therapy should be considered in the treatment of this rare disorder.
Keywords: Antioxidants, anoxia, delayed post-hypoxic leukoencephalopathy, hypoxia, leukoencephalopathy, methadone, overdose, vitamin
Delayed post-hypoxic leukoencephalopathy (DPHL) syndrome is a rare disorder that may occur following any event that induces cerebral hypoxia and presents with acute neuropsychiatric symptoms [1]. Carbon monoxide poisoning, overdose with opioids, benzodiazepines, barbiturates and other conditions are associated with the oxygen deprivation of the brain. With the exception of carbon monoxide poisoning, DPHL patients always go through a period of unconsciousness [2, 3]. In the absence of widely acceptable diagnostic criteria, the diagnosis is generally possible using a series of assessments, including clinical history, analysis of cerebrospinal fluid (CSF) and myelin basic protein, electroencephalography (EEG), and magnetic resonance imaging (MRI). These are necessary for differentiating DPHL from other diseases with similar neurological characteristics [4]. Treatment is not well established. Here, we introduced a case with methadone-induced DPHL with a favorable outcome after antioxidants therapy. Written informed consent was obtained from the patient.
CASE REPORT
A 39-year-old man with a history of opium and amphetamine addiction for a few years presented with loss of consciousness and fever. Based on the patient’s history, he was admitted to the intensive care unit in another hospital a month before with a diagnosis of methadone overdose, rhabdomyolysis and acute renal failure, and was discharged with a normal level of consciousness. A week after discharge, he developed symptoms, such as delusion and loss of consciousness. CSF analysis, together with other tests showed normal range results. Also, brain MRI was performed (Fig. 1). Then, the patient was referred to our hospital. Upon admission, he was febrile and was in an aphasic and unconscious status and did not respond to painful stimuli. The laboratory study showed white blood cells: 9800/mm3, hemoglobin: 10.4 g/dl, platelets: 239000/mm3, creatinine: 1.1 mg/dl, sodium: 142 mEq/L, potassium: 4.3 mEq/L, erythrocyte sedimentation rate: 16 mm/hr and C reactive protein: 1. Thyroid and liver function tests, vasculitis tests, antibodies for human immunodeficiency virus (HIV), hepatitis C virus with hepatitis B surface antigen all yielded normal results. The results of the CSF smear, culture, herpes simplex polymerase chain reaction (PCR) and autoimmune encephalitis panel were reported as negative with normal analysis. Serum HIV RNA PCR was negative. Serum vitamin B12 and folate levels were normal. Three days after admission to our hospital, brain MRI with and without contrast was repeated (Fig. 2). The EEG result revealed a generalized slowing pattern. Given the previous hypoxic event identified from history, imaging findings and clinical picture of akinetic-mutism, the patient was diagnosed with DPHL. Treatment was initiated with the daily intake of vitamin E 400 mg/day, vitamin C 1 gr/day, vitamin B complex and intravenous infusion of magnesium sulfate 1 gr/hr, which at the beginning of the treatment, it did not bring about a considerable favorable effect. We continued intravenous magnesium sulfate infusions for 10 days but the neurological status of the patient, his ability to speak and movement was not improved. Hence, it was discontinued, and we continued antioxidant therapy with vitamin E, C and B complex. The neurocognitive status improved gradually. Neurologic follow-up for two months after hospital discharge demonstrated dramatically improvement in the patient’s condition. He could speak; he was alert and communicative. His gait also returned to baseline.
FIGURE 1.
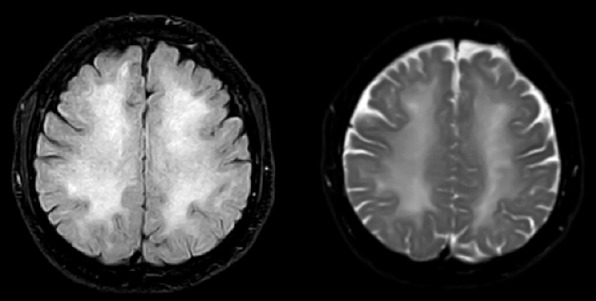
An axial T2-weighted MR image and FLAIR MR- image showed bilateral symmetric subcortical hyperintensities.
FIGURE 2.

Diffusion-weighted image (DWI) showed leukoencephalopathy with high-intensity apparent diffusion coefficient (ADC) mapping confirmed restricted diffusion of the cerebral white matter.
DISCUSSION
In 1962, Plum et al. [5] reported, for the first time, that a delayed neurological deficit may be observed following anoxia. The review of reports showed that this state might occur on average 19 days after the initial hypoxic event. The pathophysiology of this disease is not yet clear. Studies conducted on these patients suggest a disorder in the white matter with subsequent demyelination. The mechanisms to cause demyelination are different, such as impairing the myelin turnover pathway enzymes and this amount of delay is consistent with the time needed in clinical manifestations for myelin lipids’ and proteins’ replacement half-life [3–6]. Methadone is a synthesized opioid that has been in use since 1960 as an analgesic and for the treatment of heroin addiction. Methadone mitigates the effects of a heroin overdose but it may have adverse effects if overdosed [7]. Methadone overdose-induced DPHL is a rare and complex condition that is accompanied by various neuropsychiatric characteristics, including parkinsonism or akinetic-mutism [4, 8]. The abnormal symmetrical bilateral signals in MRI, especially in the supratentorial white matter with no gyral edema, are highly certain to indicate DPHL and can be used for differentiating this syndrome from other damages to the cerebral white matter, such as acute poisoning or metabolic problems. Performing MRI is therefore necessary. Measuring the myelin basic protein in the CSF as a marker of acute demyelination can be used for the diagnosis in conjunction with MRI. Since recovery is likely, early diagnosis is important [2, 9, 10]. In most cases, recovery signs emerge after three to six months, although abnormal manifestations in MRI may persist for several years. The chance of resuming the normal state is vastly dependent on the patient’s age [2, 6, 11]. Treatments are not well established and are mostly supportive. Recovery time varies depending on the severity of the disorder. Treatments based on steroids and amantadine [12], magnesium sulfate [13], levodopa [6], baclofen [8] have produced different outcomes. Given that the production of free radicals and oxidative damages are highly likely in the process of hypoxia, Mittal et al. [14] examined a treatment based on steroid plus antioxidants. King et al. [15] proposed antioxidant therapy using vitamins C, E and B complex as a treatment measure for this disease. In this case, given the presence of akinetic-mutism symptoms, in addition to treatment with antioxidants, intravenous magnesium sulfate was also used to abate neurological symptoms. In contrast to the Rozen TD study [13], our case did not respond to intravenous magnesium sulfate rapidly. We continued intravenous magnesium sulfate for 10 days, but the patient’s neurological status, ability to speak and movement was not improved. Hence, we discontinued it. Vitamin E, C and B complex therapy were continued. The neurocognitive status improved gradually. Our result consistent with the study by King et al. [15] that showed that antioxidant therapy might play a role in the treatment of DPHL.
Conclusion
DPHL may occur following any hypoxic event, such as methadone overdose. Given that the production of free radicals and oxidative damages are highly likely to have a role in the process of hypoxia, the antioxidants should be considered in treatment. This therapeutic effect needs to be investigated in future cases.
Footnotes
Authorship Contributions: Concept – AH; Design – AH; Supervision – AH, SH; Materials – AH, SH; Data collection and/or processing – AH, SH, SA; Analysis and/or interpretation – AH, SH, FK; Literature review – AH, SH, FK; Writing – AH, SH; Critical review – AH, SH, FK, SA.
Informed Consent: Written informed consent was obtained from the patient for the publication of the case report and the accompanying images.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Lee HB, Lyketsos CG. Delayed post-hypoxic leukoencephalopathy. Psychosomatics. 2001;42:530–3. doi: 10.1176/appi.psy.42.6.530. [DOI] [PubMed] [Google Scholar]
- 2.Zamora CA, Nauen D, Hynecek R, Ilica AT, Izbudak I, Sair HI, et al. Delayed posthypoxic leukoencephalopathy:a case series and review of the literature. Brain Behav. 2015;5:e00364. doi: 10.1002/brb3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer MA. Delayed post-hypoxic leukoencephalopathy:case report with a review of disease pathophysiology. Neurol Int. 2013;5:e13. doi: 10.4081/ni.2013.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shprecher D, Mehta L. The syndrome of delayed post-hypoxic leukoencephalopathy. NeuroRehabilitation. 2010;26:65–72. [PMC free article] [PubMed] [Google Scholar]
- 5.Plum F, Posner JB, Hain RF. Delayed neurological deterioration after anoxia. Arch Intern Med. 1962;110:18–25. doi: 10.1001/archinte.1962.03620190020003. [DOI] [PubMed] [Google Scholar]
- 6.Salazar R, Dubow J. Delayed posthypoxic leukoencephalopathy following a morphine overdose. J Clin Neurosci. 2012;19:1060–2. doi: 10.1016/j.jocn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Paulozzi LJ, Logan JE, Hall AJ, McKinstry E, Kaplan JA, Crosby AE. A comparison of drug overdose deaths involving methadone and other opioid analgesics in West Virginia. Addiction. 2009;104:1541–8. doi: 10.1111/j.1360-0443.2009.02650.x. [DOI] [PubMed] [Google Scholar]
- 8.Bileviciute-Ljungar I, Häglund V, Carlsson J, von Heijne A. Clinical and radiological findings in methadone-induced delayed leukoencephalopathy. J Rehabil Med. 2014;46:828–30. doi: 10.2340/16501977-1820. [DOI] [PubMed] [Google Scholar]
- 9.Nzwalo H, SáF, Cordeiro I, Ferreira F, Basílio C. Delayed hypoxic-ischemic leukoencephalopathy. BMJ Case Rep. 2011;2011:bcr0620114344. doi: 10.1136/bcr.06.2011.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katyal N, Narula N, George P, Nattanamai P, Newey CR, Beary JM. Delayed Post-hypoxic Leukoencephalopathy:A Case Series and Review of the Literature. Cureus. 2018;10:e2481. doi: 10.7759/cureus.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shprecher DR, Flanigan KM, Smith AG, Smith SM, Schenkenberg T, Steffens J. Clinical and diagnostic features of delayed hypoxic leukoencephalopathy. J Neuropsychiatry Clin Neurosci. 2008;20:473–7. doi: 10.1176/jnp.2008.20.4.473. [DOI] [PubMed] [Google Scholar]
- 12.Carroll I, Heritier Barras AC, Dirren E, Burkhard PR, Horvath J. Delayed leukoencephalopathy after alprazolam and methadone overdose:a case report and review of the literature. Clin Neurol Neurosurg. 2012;114:816–9. doi: 10.1016/j.clineuro.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 13.Rozen TD. Rapid resolution of akinetic mutism in delayed post-hypoxic leukoencephalopathy with intravenous magnesium sulfate. NeuroRehabilitation. 2012;30:329–32. doi: 10.3233/NRE-2012-0763. [DOI] [PubMed] [Google Scholar]
- 14.Mittal M, Wang Y, Reeves A, Newell K. Methadone-induced delayed posthypoxic encephalopathy:clinical radiological, and pathological findings. Case Rep Med. 2010;2010:716494. doi: 10.1155/2010/716494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King F, Morris NA, Schmahmann JD. Delayed Posthypoxic Leukoencephalopathy:Improvement with Antioxidant Therapy. Case Rep Neurol. 2015;7:242–6. doi: 10.1159/000441892. [DOI] [PMC free article] [PubMed] [Google Scholar]


