Summary
The oropharyngeal mucosa serves as a perpetual pathogen entry point and a critical site for viral replication and spread. Here, we demonstrate that type 1 innate lymphoid cells (ILC1s) were the major immune force providing early protection during acute oral mucosal viral infection. Using intravital microscopy, we show that ILC1s populated and patrolled the uninfected labial mucosa. ILC1s produced interferon-γ (IFN-γ) in the absence of infection, leading to the upregulation of key antiviral genes which were downregulated in uninfected animals upon genetic ablation of ILC1s or antibody-based neutralization of IFN-γ. Thus, tonic IFN-γ production generates increased oral mucosal viral resistance even before infection. Our results demonstrate barrier-tissue protection through tissue surveillance in the absence of rearranged-antigen receptors and the induction of an antiviral state during homeostasis. This aspect of ILC1 biology raises the possibility that these cells do not share true functional redundancy with other tissue-resident lymphocytes.
eToc
ILC1s provide antiviral protection at initial sites of viral encounter, but how these cells accomplish this spatially in the tissue remains unexplored. Shannon et al. show that ILC1s patrol the uninfected epithelium of the oral mucosa and provide protection even before infection through the production of IFN-γ.
Graphical Abstract
Introduction
Innate lymphoid cells (ILCs) are a heterogenous group of tissue-resident lymphocytes that bridge innate and adaptive immunity by responding rapidly to invading pathogens without the antigen-specific receptors found on adaptive lymphocytes. ILCs are divided into three groups based upon their cell-surface phenotype, expression of transcription factors, and production of specific cytokines. Group I ILCs include natural killer cells (NK cells) and ILC1s and are thought to mirror the phenotypes and functions of T cells, with NK cells being the innate counterparts of CD8+ T cells and ILC1s of Th1 cells (Eberl et al., 2015). In line with this, NK cells are profoundly cytolytic, whereas ILC1s present in the tissue respond to infection through the production of IFN-γ but do not directly kill infected cells (Weizman et al., 2017). Whether ILC1s perform functions truly unique from T cells or rather serve as a “spare tire” when the adaptive immune response is lacking or absent is currently unclear (Adams and Sun, 2018).
The separation of NK cells and ILC1s has proven challenging owing to their shared expression of many cell surface markers, including NK1.1 and NKp46 (O’Sullivan, 2019), along with ILC1 heterogeneity in the tissue. Adding to the complexity, NK cells can convert to ILC1-like cells in both the tissue and circulation during infection (Cortez et al., 2017; Park et al., 2019). Nonetheless, parabiosis studies have demonstrated that while conventional NK cells circulate through the tissues, ILC1s are primarily permanent tissue residents and thus often express markers of intraepithelial residency, including CD49a, CD103, and CD69 (Cortez et al., 2016; Cortez et al., 2017; Gasteiger et al., 2015; Sojka et al., 2014). ILC1s have been characterized within most tissues thus far examined, including the liver (the primary site used to examine ILC1 function), skin, lungs, adipose tissue, intestinal mucosa, and salivary glands (Vivier et al., 2018).
There is a rich and diverse literature detailing direct NK cell-mediated control of viral infection (Vivier et al., 2008). Despite this, only a handful of studies have unequivocally demonstrated that ILC1s can protect against tissue-replicating virus with most of our understanding of the role of ILC1s in viral infection drawn from their proposed functionality akin to CD8+ T cells. Recently, in an elegant study, Weizman et al. have shown that ILC1s limit viral titers and protect mice against lethal challenge with murine cytomegalovirus (MCMV) (Weizman et al., 2017). In this hydrodynamic infection model, high doses of MCMV injected intravenously result in rapid lethality in hobit-deficient mice or in mice depleted of NK1.1+ cells. In a separate study, Vashist et al. have shown that the transfer of in vitro-generated ILC1s into mice genetically lacking these cells could reduce influenza virus titers at 3 days post infection (but not other timepoints) with no effect on morbidity (Vashist et al., 2018). Thus, even with these groundbreaking studies, much remains to be learned regarding the precise nature and timing of the response of ILC1s to naturally replicating virus. Currently, ILC1s are thought to serve as a “second wave” of antiviral cells, functioning after the arrival of myeloid cells and before NK cells and T cells (Adams and Sun, 2018), but this has not been systematically assessed.
ILC1s have been shown to respond to viral infection within the first 24 hours of infection through IFN-γ production (Adams and Sun, 2018). As their precise tissue locations are unknown, it is unclear how ILC1s mobilize to restrict early viral replication. The decrease in MCMV or Sendai virus titers observed 36 hours after infection suggests that ILC1s are present in sufficient numbers at the precise area of infection to exert early control (Weizman et al., 2017). As tissue-resident lymphocytes are often located in proximity to the basement membrane of the epithelium, ILC1s might need to rapidly move to respond to more superficial epithelial infection that initially results from many viral infections (Gebhardt et al., 2011; Khairallah et al., 2018; Zaid et al., 2014). Recently, γδ intraepithelial lymphocytes (IELs) have been shown to constantly move within the intestine during homeostasis, surveying a large number of epithelial cells (Edelblum et al., 2012; Hoytema van Konijnenburg et al., 2017). After infection, γδ IELs preferentially accumulate in pathogen-rich areas and increase movement between epithelial cells (termed “flossing”). Whether ILC1s also “floss” pre- or post-infection has not been determined.
In the oropharyngeal cavity, murine ILC1s reside in salivary glands (Cortez et al., 2016) and gingiva (Brown et al., 2018), although ILC1 function in oral health and oral mucosal defense remains to be determined. To investigate the mechanisms underlying the resilience of the oral mucosa against viral infection, we established a murine model of viral oral mucosal infection using the poxvirus vaccinia virus (VACV). Here, we show that ILC1s present in the basal epithelium prior to infection serve as epithelial guardians, limiting viral replication and dissemination. Further, tonic production of IFN-γ by ILC1s was critical for the induction of an antiviral state even prior to viral encounter and within the first few hours of infection, indicating these cells serve an omnipresent, initial line of antiviral defense.
Results
Group 1 ILCs restrict early VACV replication in the oral mucosa
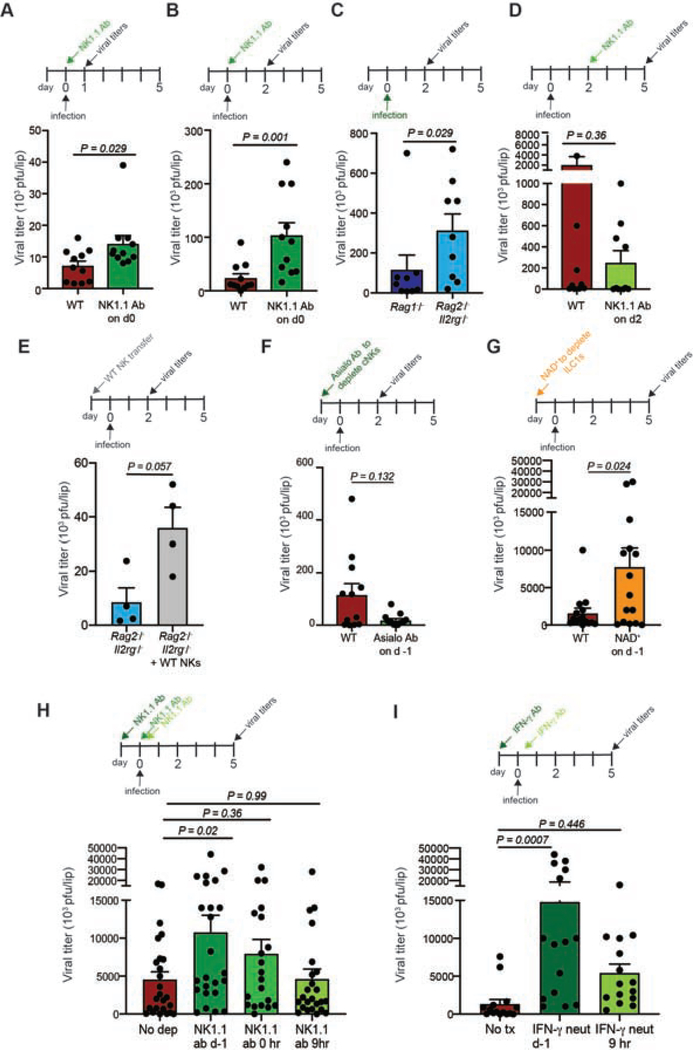
Little is known about the immune mechanisms that restrict viral infection of the oral mucosa and limited mouse models exist of viral infection in this important barrier tissue (Chapman et al., 2010; Kollias et al., 2015). Because many poxviruses, including smallpox and monkeypox, can invade through and produce lesions in the oral cavity (Baron, 2003; Chapman et al., 2010; Fenner, 1988; Slifka and Hanifin, 2004), we developed a mouse model of labial mucosal (inner lip) viral infection using vaccinia virus (VACV). Although VACV replicated well in multiple areas of the mouth, we focused on the labial mucosa due to ease of intravital imaging compared to other areas such as the buccal mucosa (Figure 1A and Figure S1). Following labial mucosal infection via the same bifurcated needle used for human smallpox vaccination, VACV replicated to titers >107 plaque forming units (pfu) per lip by day (d) 7 in WT mice, after which titers declined (Figure 1B and Figure S1F). Peak oral mucosal titers were higher than those we previously observed after similar infection of the ear skin (Hickman et al., 2013). We did not detect viral dissemination post infection, suggesting that infection remains local at these timepoints (Figure S1E). At d10, we no longer detected virus by either plaque assay or using intravital multiphoton imaging of the lips of WT animals infected with VACV expressing GFP (Figure S1D–1E). For the first 5 days of infection, Rag1−/− animals (lacking B and T cells with rearranged antigen receptors), controlled virus equally or better than WT animals (Figure 1B). After d7, however, the adaptive immune response is clearly needed to control viral replication as titers increased in Rag1−/− animals (Figure S1F).
Figure 1. Group 1 ILCs restrict viral replication in the oral mucosa.
(A) Diagram of the location of VACV infection of the oral mucosa.
(B-E) VACV titers in the oral mucosa as determined by plaque assay.
(B) Titers in the labial mucosa at the indicated d post infection in either WT (red bars) or Rag1−/− (blue bars) mice. n=6 mice. The complete timecourse was performed twice.
(C) Titers in the labial mucosa of the WT or Rag1−/− mice on d5 post infection.
(D) Titers in WT or Rag2−/−Il2rg−/− animals on d5 post infection.
(E) Titers in WT mice on d5 post infection with NK1.1 Ab administration at d-1 and every 48 hrs throughout infection. Ab depletion efficiencies are shown in Fig. S2.
All data except b are representative of at least 3 individual experiments with 3–10 mice/group, which were pooled. Dots represent individual animals. Statistics = Mann-Whitney unpaired t test. Error bars = SEM.
To further investigate early immune control of viral infection in the labial mucosa, we infected mice devoid of different lymphocyte populations and analyzed viral titers on d5 post infection (which consistently yielded high viral titers in the mucosa of WT animals). As seen in the timecourse analysis, Rag1−/− animals had lower viral titers than WT controls on d5 (Figure 1C). As Rag1−/− mice retain both NK cells and ILC1s, we next examined Rag2−/−Il2rg−/− mice, which lack both, finding higher viral titers than WT animals on d5 (Figure 1D). Likewise, antibody-based depletion of NK1.1+ ILCs prior to labial mucosal infection enhanced viral replication to similar titers (Figure 1E (depletion efficiencies are shown in Figure S2)). Taken together, these data show that VACV can be used as a model to study viral infection of the oral mucosa and that early viral replication is restrained by mucosal group 1 ILCs.
ILC1s reside in the basal layers of the mucosal epithelium
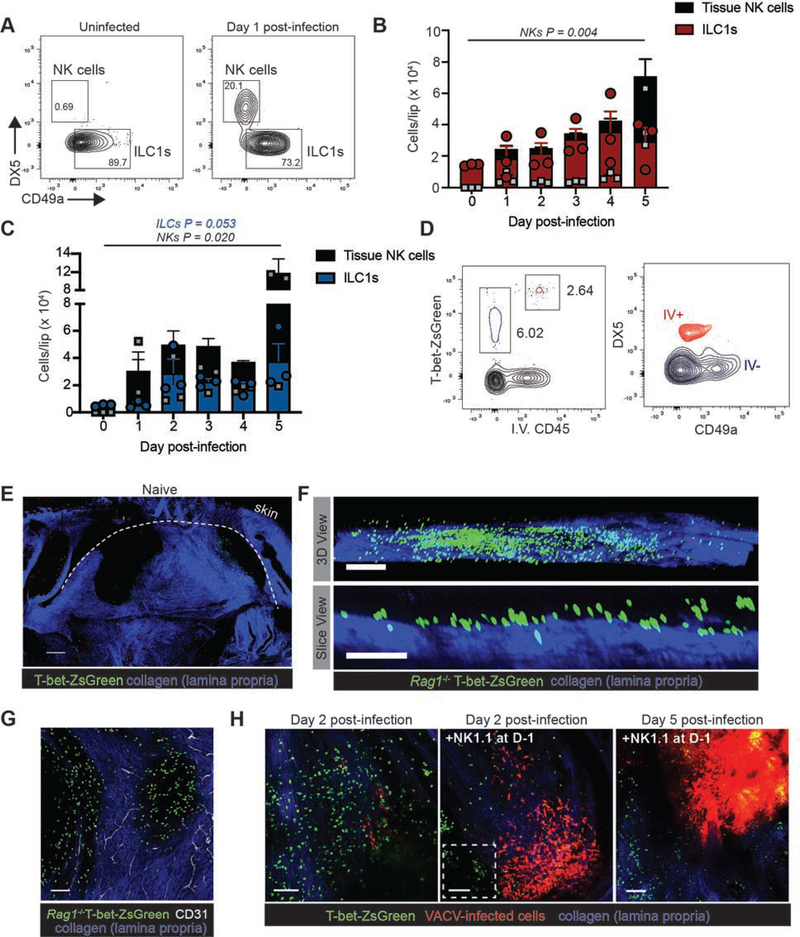
To further characterize group I ILCs in the labial mucosa, we generated single cell suspensions from both uninfected and infected tissue and analyzed cellular phenotypes in WT and Rag1−/− animals via flow cytometry (Figure 2A–C). We distinguished ILC1s from NK cells based on tissue residency (determined by injection of a labeled Ab intravenously prior to tissue harvest (Figure 2D (Adams and Sun, 2018; Anderson et al., 2014)) and a combination of DX5 expression on NK cells and CD49a on ILC1s. We also confirmed that these cells were not NKp46+ ILC3s (Figure S3). In the uninfected tissue, we did not typically detect NK cells (CD45+ iv CD45+ CD3− NK1.1+ T-bet+ DX5+ CD49a−) using this staining panel. In contrast, both the labial mucosa of uninfected WT and Rag1−/− mice was populated by ILC1s. Most ILC1s expressed CD69 and CD103, the latter of which is thought to promote tissue residency (Cortez et al., 2017) (Figure S3). The number of both ILC1s and NK cells in the labial mucosa gradually increased over the first 5 days of infection in WT and Rag1−/− mice (Figure 2B–C). By 24 h post-infection, we noted an influx of NK cells into the tissue which was more pronounced in Rag1−/− compared to WT animals. By d5, both sets of animals had a statistically significant population of NK cells in the tissue.
Figure 2. Imaging group 1 ILCs in the oral mucosa.
(A) Flow cytometric analysis of group I ILCs in the uninfected labial mucosa cells. Cells were gated on live, non-vascular CD45+CD3−NK1.1+ cells. Anti-CD45 was injected i.v. prior to tissue harvest in order to exclude vascular cells. ILC1s and NKs were distinguished on the basis of CD49a (ILC1) and CD49b (DX5, NK) expression. See also Fig. S3.
(B) Number of ILC1s (red portion of bar and red circles) and NKs (black portion of bar and grey squares) at the indicated day post infection in WT animals.
(C) Number of ILC1s (blue portion of bar and blue circles) and NKs (black portion of bar and grey squares) at the indicated day post infection in Rag1−/− animals.
(D) Flow cytometric analysis of labial mucosal cells from T-bet-ZsGreen reporter mice. Left panel shows T-bet-ZsGreen+ cells separated based on intravenous injection of CD45. Red outline = intravascular cells. Blue outline = cells present in the tissue. Right panel shows CD49a and CD49b expression of vascular and tissue-populating cells.
(E) Maximum intensity projections (MIP) of multiphoton microscopy (MPM) images of an entire, excised lip from a naïve T-bet-ZsGreen reporter mouse. A dotted line shows the floor of the label mucosa. ZsGreen+ ILCs can be seen primarily in areas lacking collagen (blue signal). Scalebar = 500 μms.
(F) (top) MIP view of the side of an uninfected Rag1−/− T-bet-ZsGreen reporter mouse lip. (bottom) Slice-view image of the top panel taken from the side. Collagen of the lamina propria is visualized in blue. Flow cytometric analyses of uninfected tissue (B and C) reveals that T-bet-ZsGreen+ cells are ILC1s. Scalebar = 200 μms (top panel) and 100 μms bottom panel.
(G) MIP MPM image looking down on a Rag1−/− T-bet-ZsGreen mouse lip. Blood vessels in the lamina propria are shown in white (visualized by iv injection of CD31 prior to imaging). Scalebar = 100 μms.
(H) MIP MPM images of the infected labial mucosa on d2 in T-bet ZsGreen animals (left panel), NK1.1-depleted animals (middle panel), or on d5 in NK1.1-depleted animals (right panel). All mice were also depleted of CD4 prior to imaging. Boxed area denotes an area of viral replication in green. Scalebars = 100 μms.
All data are representative of at least 3 individual experiments with 3–4 mice/group, which were pooled. Dots represent individual animals. Statistics = one-way ANOVA. Error bars = SEM.
See also Figure S3.
We next sought to analyze the movement of tissue-resident ILC1s in response to viral infection in the oral mucosa. ILC1s require the transcription factor T-bet for their development and both cNK cells and ILC1s constitutively express this protein (Lazarevic et al., 2013). Thus, we used MPM to image T-bet-expressing group I ILCs in T-bet-ZsGreen reporter mice (expressing ZsGreen after the Tbx21 promoter in a BAC while retaining endogenous Tbx21 expression (Zhu et al., 2012)) (Figure 2E–G). As steady-state memory phenotype CD4+ T cells expressing T-bet-ZsGreen have been reported in the spleen (Kawabe et al., 2017), we administered CD4-depleting Ab prior to imaging WT T-bet-ZsGreen mice, reasoning that these cells were dispensable for the early control of viral replication (prior to day 6 (Figure 1 and Figure S1)). Confocal and MPM imaging revealed a population of T-bet-ZsGreen+ ILC1s in the uninfected labial mucosa (Figure 2E–G). In the uninfected tissue, ILC1s formed a layer above the lamina propria. Consistent with the increase in tissue ILC1s and NK cells, confocal analyses of frozen tissue sections revealed that some of the T-bet-ZsGreen+ cells also expressed Ki67, a marker of cellular division, even in uninfected tissues (Figure S3).
We next imaged the labial mucosa of T-bet-ZsGreen mice infected with VACV expressing the red fluorescent protein TurboRFP (Figure 2H). On d2 post infection, we visualized numerous T-bet-ZsGreen+ group 1 ILCs in the mucosa; flow data indicated that approximately 8.5 ± 2.0 % of these are NK cells rather than ILC1s (Figure 2H, left panel). Consistent with viral titer data, depletion of NK1.1+ cells prior to infection resulted in increased viral-promoter driven RFP expression on both d2 and d5 post-infection (Figure 2H, middle and right panels). We observed similar populations of Ncr1-gfp+ cells (Sheppard et al., 2013) in both infected an uninfected tissue (Figure S4). As Ncr1-gfp+/+ mice lack NKp46 (the human ortholog of which specifically recognizes the hemagglutinin protein of VACV (Jarahian et al., 2011)), we focused imaging studies primarily on T-bet ZsGreen mice, although we detected no loss of viral control in Ncr1-gfp+/+ mice (Figure S4B). These data indicate that primarily tissue-resident ILC1s are present in the uninfected labial mucosal epithelium and that NK cells are recruited into the tissue after infection.
ILC1s are mobile prior to infection
As the precise spatial locations and interstitial movements of ILC1s remain an open question (Adams and Sun, 2018), we first examined frozen cross-sections of uninfected labial mucosa of T-bet ZsGreen mice using confocal microscopy to determine ILC1 location at steady state (Figure 3A). In uninfected tissue, T-bet-ZsGreen+ ILC1s predominated in the basal layers of the epithelium, both contacting and just above the basement membrane overlying the lamina propria (LP) with few ILC1s present in the LP. ILC1s were particularly concentrated in the downward undulations of the rete ridges of the epithelium, which we observed both in the frozen cross-sections as well as via MPM imaging (Figure 3B).
Figure 3. Group 1 ILCs patrol the uninfected oral epithelium.
(A) Confocal images of frozen tissue cross-sections from the uninfected lip showing both the labial mucosal side as well as the skin side of T-bet-ZsGreen mice. Boxed areas are magnified in panels to the right. Scalebars = 200 μms (left panel); 500 μms (middle panel); 10 μms (right panel).
(B) MPM images of the uninfected labial mucosa of T-bet-ZsGreen reporter mice. Panels decrease in depth by 7 μms moving from left to right. Tracks of individual cells are shown over the course of a 1 hr imaging prior. Tracks are color-coded to show displacement over the imaging period from low displacement (blue) to high (red). Scalebars = 50 μms.
(C) MPM images taken over 1 hr imaging period showing movement of group 1 ILCs in uninfected T-bet-ZsGreen reporter mice. Times shown in upper left = minutes. Scalebars = 50 μms.
(D) Speeds of T-bet-ZsGreen+ cells in the uninfected labial mucosa.
(E) MPM images taken over time (minutes, upper left) in mice injected with Hoescht to label epithelial nuclei. An arrow indicates a cell moving between epithelial cells. Dashed red line = junction of lamina propria and epithelium. Scalebars = 15 μms.
All experiments were performed at least 3 times with 1–2 mice/group. Error bars = SEM.
We next use MPM imaging to investigate whether ILC1s in the uninfected epithelium were motile or remained sessile (Figure 3B–E and Movie S1). Over the course of a 1 h imaging period, we plotted the length of the movement (track) of ILC1s either in the epithelium or LP (distinguished by a combination of imaging depth and the presence of LP collagen and blood vessels). Although we observed a few LP T-bet-ZsGreen+ cells, they were primarily sessile. In contrast, non-LP T-bet-ZsGreen+ cells slowly moved through the epithelium over time (mean speed = 2.4 ± 0.04 SEM μm/min) (Figure 3D). To understand how tissue-resident ILC1s navigated the closely packed basal epithelial cells, we labeled nuclei in vivo in the oral mucosa using i.v. Hoechst injection (shown in white) (Figure 3E). MPM imaging under these conditions revealed ILC1s squeezed between the nuclei of epithelial cells. Collectively, these data show that ILC1s slowly patrol the uninfected epithelium, contacting and squeezing between epithelial cells.
ILC1s do not accumulate around virus-infected cells
We have previously reported that VACV-specific CD8+ T cells rapidly accumulate around infected cells in the skin, facilitating their elimination (Hickman et al., 2015). We therefore hypothesized that ILC1s would also rapidly accumulate in close proximity to infected cells. Thus, we next examined the spatial distribution of ILC1s relative to virus-infected cells in the infected labial mucosa on d2 post-infection. MPM imaging of the entire, excised labial mucosa of T-bet-ZsGreen mice (~91% ILC1s on d2 (Figure 2B)) revealed increased concentrations of tissue-resident T-bet-ZsGreen+ ILCs compared to uninfected controls (Figure 4A and Figure 2E). Although we observed ILCs in areas of viral infection, T-bet-ZsGreen+ cells were distributed throughout the tissue and not consistently enriched near infected epithelial cells.
Figure 4. ILC1s remain distributed in infected tissue and produce IFN-γ that restricts viral replication.
(A) MIPs of MPM images of entire, excised lips from a T-bet-ZsGreen mouse on day 2 post infection with VACV expressing RFP (red). A dotted line shows the floor of the label mucosa. Scalebars = 500 μms.
(B) MIPs of MPM images taken over a 6 hr imaging period. Time in hr:min:sec shown in the lower right. Top panels = naïve, Bottom panels = d2 post infection. Scalebars = 100 μms.
(C) MPM images showing a side-view of either a slice (MIP, top panel) or a 3D view (blend view tilted, bottom panel) of the labial mucosa of a T-bet-ZsGreen mouse 2 days post infection illustrating the location of ILC1s in the epithelium. VACV-infected cells = red; ILCs = green; lamina propria collagen = blue. Scalebars = 100 μms.
(D) Confocal image of frozen tissue sections of the labial mucosa taken on d2 post infection. The floor of the epithelium is indicated by staining with laminin (white). Scalebar = 50 μms.
(E) Confocal image of frozen tissue sections taken from uninfected animals (left panels) or d2 post infection (middle, right panels) staining for Eomes expression (white). The green channel is omitted in the bottom panels to more clearly show intracellular Eomes staining. Higher magnification image of the middle panel is shown on the right. Scalebars = 100 μms (left and middle panels) and 20 μms (right panels).
(F) Color-coded 3D view of the image in the middle panel of (E) showing ILC1s (green) or NK cells (purple, Eomes+) in infected labial mucosal tissue. Scalebar = 200 μms.
(G) Confocal image of frozen tissue sections of the labial mucosa taken on d2 post infection. Circles indicate ILCs in the epithelium producing IFN-γ. Scalebar = 50 μms.
(H) Viral titers in the labial mucosa on d5 post infection. Mice were treated with a single dose of IFN-γ neutralizing Ab just prior to infection (+Ab d0 group) or on d3 and d4 (+Ab d3&4 group).
(I) MIPs of MPM images of the labial mucosa taken on d5 post infection. Control animal (left) or animal given a single dose of neutralizing Ab on d0 (right). Scalebars = 400 μms.
All data are representative of at least 3 individual experiments with 2–4 mice/group. Dots in (H) represent individual animals which were pooled from 3 experiments. Statistics = ordinary one-way ANOVA. Error bars = SEM.
To better understand the spatial distribution and accumulation of group 1 ILCs post infection, we imaged the mucosa continuously for 6 h in either naïve or d2 post-infection animals (Figure 4B and Movie S2). Over the imaging period, we observed stable numbers of slowly motile tissue-resident T-bet ZsGreen+ ILC1s in naïve mice (Figure 4B, top panel). In contrast, in infected animals, group 1 ILCs accumulated in the mucosa, though they did not concentrate around virus-infected cells and remained primarily distributed in the epithelium rather than the lamina propria (Figure 4B, bottom panel). We quantified the speed and total displacement over time of the smaller number of cells that contacted VACV-infected cells compared to those that moved through the tissue but did not encounter infected cells (Figure S5). Interacting ILCs moved at slower speeds and exhibited less displacement than their non-interacting counterparts; however, they were not completely immobilized by contact with infected cells (Figure S5 and Movie S3). At any time, more ILCs were not in contact with infected cells than were (Figure S5 A–C). This behavior contrasted with previous reports of the long-lasting, relatively immobile contacts characteristic of Ag-engaged CD8+ T cells (Hickman et al., 2015; Mempel et al., 2004). Further, we did not observe lysis of VACV-infected cells in the oral mucosa. Consistent with a lack of stable contacts leading to viral cell killing, VACV titers were unchanged in the oral mucosa of perforin-deficient mice (necessary for NK-induced cytotoxic pore formation) (Figure S5G). This suggested that the major role for ILC1s during oral mucosal VACV infection was not direct cytolysis.
Although our flow cytometric data revealed >90% of the group I ILCs in the d2 infected oral mucosa were ILC1s, we wished to further examine the spatial distribution of ILC1s versus NK cells. After infection, we noted that T-bet-ZsGreen+ cells remained in the epithelium while T-bet-ZsGreen+ cells increased in numbers in the lamina propria (Figure 4C–D). To distinguish ILC1s from NK cells on D2, we stained frozen cross-sections from d2 post infection T-bet-ZsGreen mice with anti-Eomesodermin Ab (Figure 4E–F). Consistent with flow data, we did not detect Eomes+ T-bet-ZsGreen+ cells in uninfected tissue (Figure 4E, left panel). In contrast, also consistent with flow, we detected a small proportion of Eomes+ T-bet-ZsGreen+ cells in the mucosal epithelium (Figure 4E, middle and right panels). Classifying these cells based on Eomes positivity revealed that ILC1s that were Eomes− remained distributed throughout the epithelium on d2 post infection (Figure 4F).
Because of the lack of contact of most ILC1s with virus-infected cells (as well as no change in viral titers in perforin-deficient mice (although other modes of killing certainly exist), we hypothesized that group I ILCs might control infection through non-cytolytic methods. ILC1s secrete the potent antiviral cytokine interferon-gamma (IFN-γ) which induces antiviral gene expression (Farlik et al., 2012; Groom and Luster, 2011; Spits et al., 2016). Accordingly, we detected IFN-γ production by epithelial ILCs on d2 post infection (Figure 4G). To examine the possible effect of IFN-γ in the oral mucosa, we neutralized IFN-γ with Ab either 1) at the time of infection (Ab administered once on day 0) or after viral replication had begun (Ab given on days 3 and 4 post-infection) and analyzed viral titers on d5 (Figure 4H). Viral titers were significantly increased only when IFN-γ was neutralized at the time of infection. Consistent with this, MPM imaging revealed a greatly enhanced virus-driven fluorescent signal on d5 after anti-IFN-γ treatment on d0 (Figure 4I). Neutralizing IFN-γ did not obviously alter the distribution of T-bet-ZsGreen+ ILCs on d2 post-infection, which remained largely distributed in the tissue and did not accumulate around infected cells (Movie S4).
Group I ILCs invoke an antiviral state in the infected mucosa
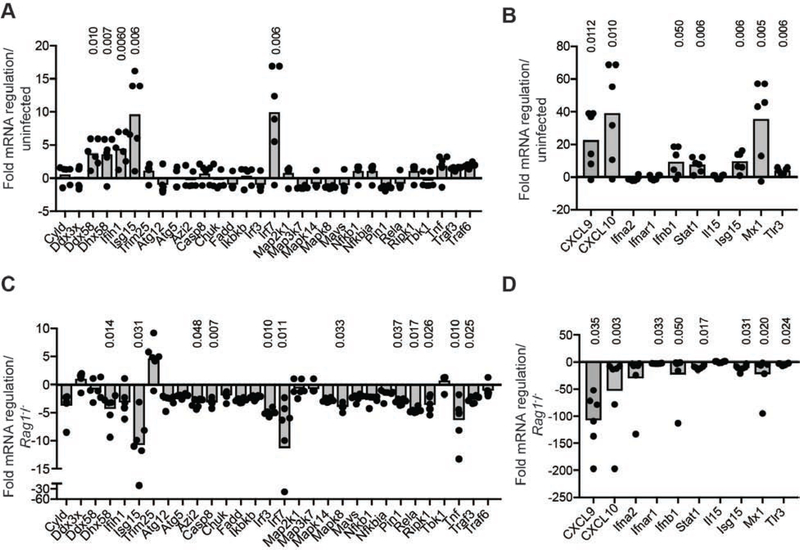
Because of the widespread distribution of group I ILCs in the infected tissue compared to our previous studies of effector T cell accumulation around infected cells, we questioned whether the spread of T-bet-ZsGreen+ group I ILCs throughout the epithelium could maximize the area of tissue coverage and potentially induce epithelial antiviral immunity in areas lacking infection. This could be particularly important after infection with VACV, which encodes a potent secreted type I IFN-binding protein that subverts signaling (Hernaez and Alcami, 2018). To examine this, we isolated RNA from the labial mucosa on d2 post infection and performed qPCR arrays focused on genes involved in antiviral signaling pathways (Figure 5A–B). After infection, the expression of a number of IFN-regulated genes increased, including Cxcl10 (~32 x increase), Ifnb1 (~10 x), Stat1 (~7.9 x), Isg15 (~8.7 x) and Irf7 (~9.7 x). Thus, an antiviral transcriptional signature was present in the tissue by d2 post infection.
Figure 5. Group 1 ILCs invoke an antiviral state in the labial mucosal after infection.
(A-B) mRNA regulation in the labial mucosa of mice on d2 post infection compared to naïve animals as determined by qPCR arrays for antiviral genes.
(C-D) mRNA regulation in the labial mucosa of Rag2−/−Il2rg−/− mice on d2 post infection compared to d2 Rag1−/− animals as determined by qPCR arrays for antiviral genes.
Statistically significant differences in mRNA determined using an unpaired t-test on the replicate ΔΔCT values for each gene in the control and treatment group are shown above gene where applicable. Dots show individual mice. Results were pooled from 2 separate experiments with 3 mice/group.
To determine if group I ILCs contributed to the induction of antiviral genes, we isolated RNA from the labial mucosa on d2 post-infection, comparing Rag2−/−Il2rg−/− to Rag1−/− mice (Figure 5C–D). In contrast to wild-type animals or Rag1−/− animals, mice lacking group I ILCs failed to upregulate a majority of antiviral signaling genes after infection, with the exception of Trim25. Together, these data show that ILCs are required for the upregulation of antiviral genes in the oral mucosa within 48 hours of tissue-replicating VACV infection.
ILC1s invoke an antiviral state in the uninfected epithelium
The very rapid restriction of viral replication, along with the findings that resting ILC1s are continuously producing IFN-γ mRNA (Bezman et al., 2012; Weizman et al., 2017), led us to question whether ILC1s could induce an antiviral state even in the uninfected epithelium to pre-empt viral infection. To examine this, we stained frozen tissue cross-(Figure 6A–D and Figure S6) from the uninfected labial mucosa of T-bet-ZsGreen reporter mice for IFN-γ protein. Even in the uninfected epithelium, T-bet-ZsGreen+ ILCs expressed IFN-γ. In contrast, memory CD8+T cells present in the uninfected oral mucosa did not express IFN-γ when stained with identical methods (Figure S6B). We detected IFN-γ in Ncr1-gfp+ cells in the labial mucosa of uninfected Ncr1-gfp+/+ mice (Figure 6B), indicating that murine NKp46 is not necessary for IFN-γ production. Additionally, we stained with the anti-IFN-γ Ab XMG1.2, which works well for flow cytometry but we have found difficult to use for IHC (Figure 6C).
Figure 6. ILC1s induce an antiviral state in the uninfected epithelium.
(A) Confocal images of a frozen tissue cross-section of uninfected labial mucosa of T-bet ZsGreen mice. Panel on the right lacks T-bet-ZsGreen to more easily visualize the IFN-γ staining (white). Scalebars = 20 μms. Results were repeated at least 3 times with 2–4 mice/group.
(B) As in (A) except in the uninfected labial mucosa of Ncr1-gfp+/+ mice. Scalebars = 10 μms.
(C) As in (B) except IFN-γ staining was performed using the XMG1.2 Ab. Scalebars = 10 μms.
(D) Confocal images of two different fields from a cross-section of the uninfected labial mucosa of a GREAT+/+ mouse. Scalebars = 50 μms (left) and 20 μms (right).
(E) Flow histograms of all ILCs from the labial mucosa of uninfected WT (red) or GREAT+/+ (light green) mice. Dark green histogram is gated on the small population of GREAT-YFPHI ILC1s.
(F) Geometric mean fluorescent intensity of GREAT-YFP in the populations shown in (E) (left panel). Right panel shows the percentage of GREAT-YFPHI ILC1s in B6 versus WT. Data are pooled from 2 experiments with 3 mice/group.
(G) qPCR array data of antiviral genes in the uninfected labial mucosa showing fold mRNA regulation of Rag2−/−Il2rg−/− mice compared to Rag1−/− mice. Results were pooled from 2 separate experiments with 3 mice/group.
(H) As in (F) but in uninfected Rag1−/− mice after treatment with IFN-γ-neutralizing Ab compared to untreated, uninfected Rag1−/− mice. Results were pooled from 2 separate experiments with 3 mice/group.
(I) Confocal images of a frozen tissue cross-section of the uninfected labial mucosa of a T-bet-ZsGreen mouse. Right panels omit DAPI signal to more easily visualize IRF7 staining (white). Boxed area is magnified in the image to the right. Scalebars = 100 μms (left and middle) and 20 μms (right).
Statistically significant differences in mRNA were determined using a Student’s t-test on the replicate ΔΔCT values for each gene in the control and treatment groups are shown above gene where applicable. Dots show individual mice. Confocal images were repeated at least 2 times with 2–4 mice/group, except for (D).
See also Figure S6.
To complement antibody-based detection of IFN-γ, we analyzed tissue sections and single cells suspensions from the labial mucosa of uninfected “interferon-gamma reporter with endogenous polyA transcript” or GREAT mice (Reinhardt et al., 2009) for eYFP production as a commonly used surrogate of IFN-γ expression (Weizman et al., 2017). Analysis of frozen sections revealed numerous eYFP+ cells in the basal epithelium of the labial mucosa (Figure 6D), although we were not successful in co-staining for ILC1s in these section to conclusively demonstrate eYFP+ cells were ILC1s. Instead, we isolated cells from the labial mucosa of GREAT mice and analyzed ILC1s by flow cytometry (Figure 6E–F). As a whole ILC1s, from GREAT mice exhibited a shift in YFP fluorescent compared to WT controls (seen in histograms and geometric mean intensities). A small population of isolated ILC1s, ranging from 0–7%, exhibited bright YFP fluorescent indicative of IFN-γ transcription.
We next analyzed whether tissue-resident ILC1s in the oral mucosa could induce localized antiviral immunity prior to infection (Figure 6G). Comparison of antiviral gene signatures in the labial mucosa of Rag2−/−Il2rg−/− compared to Rag1−/− mice revealed downregulation of the mRNA for IFN-γ-regulated chemokine Cxcl10 as well as many of the antiviral genes upregulated on d2 post-VACV infection in WT mice (Figure 5A–B). Similarly, IFN-γ neutralization in Rag1−/− animals ablated a majority of the antiviral gene signature, including the transcription factor Irf7 (Figure 6H).
We next stained frozen labial mucosal cross-sections from uninfected WT mice for IRF7, an antiviral signaling transcription factor with a short half-life that can be directly activated through IFN-γ signaling (Farlik et al., 2012; Ning et al., 2011). Although IRF7 was expressed throughout the uninfected epithelium of the labial mucosa, it was most concentrated in areas above and surrounding T-bet-ZsGreen+ ILC1s (Figure 6I) and was reduced in the absence of IFN-γ (Figure S6C).
Taken together, these data demonstrate that tissue-resident ILC1s produce IFN-γ under homeostatic conditions that primes the epithelium to restrict viral infection through the induction of antiviral genes.
ILC1s act before widespread viral replication to restrict infection
The data thus far suggested a very early role for group 1 ILCs in restricting viral replication in the oral mucosal epithelium and were consistent with previous reports of early control of viral infection of the liver by resident ILC1s (Weizman et al., 2017). While we and others had previously analyzed mice depleted of group I ILCs or ILC1s prior to infection (Figure 1C) (Vashist et al., 2018; Weizman et al., 2017), we now administered depleting antibody at the same time as infection, thus allowing ILC1s to be present for only the first few hours post-infection (Figure 7A–B, S2). Under these conditions we recovered twice as much infectious virus from ILC-depleted animals as controls by 24 hours post infection; by 48 hours over 4.5 times more virus. Comparison of viral titers between Rag1−/− and Rag2−/−Il2rg−/− mice revealed significantly more infectious virus in ILC-deficient mice on d2 (Figure 7C).
Figure 7. Oral mucosal ILC1s protect at the earliest stages of infection.
(A-B) Viral titers in WT (red bar) or NK1.1 Ab-treated WT (green bar) mice on d1 (A) or d2 (B) post infection as determined by plaque assay.
(C) Viral titers in the labial mucosa of Rag1−/− (dark blue bar) or Rag2−/−Il2rg−/− (light blue bar) mice on d2 post infection.
(D) Viral titers on d5 in WT animals (red bar) or in WT mice that received NK1.1 Ab on d2 post infection (light green bar).
(E) Viral titers on d2 post infection in the labial mucosa of Rag2−/−Il2rg−/− mice with (grey bar) or without (blue bar) adoptive transfer of WT NK prior to infection.
(F) Viral titers on d2 post infection in WT (red bar) or titrated asialo-GM1-Ab-treated (green bar) mice.
(G) Viral titers on d5 post-infection in WT animals (red bar) or in animals given NAD+ (orange bar) prior to infection.
(H) Viral titers on d5 post infection in WT mice administered NK1.1 Ab before infection (darkest green), at the same time as infection (medium green bar), or 9 hours after infection (light green bar).
(I) Viral titers on d5 post infection in WT mice given IFN-γ-neutralizing Ab before infection (dark green bar) or 9 hours after infection (light green bar).
All data are representative of at least 3 individual experiments with 3–4 mice/group, which were pooled. Dots represent individual animals. Statistics = unpaired t test (for two groups) or one-way ANOVA for 3 or more groups (panels H-I). Error bars = SEM.
To more formally address the role of NK cells, which we found entered the tissue in sizeable numbers by 24 hours post-infection (Figure 2), we first depleted WT mice of both NK cells and ILC1s 48 hours after infection using NK1.1 Ab administration and examined viral titers on d5 post-infection (Figure 7D). With this late depletion of NK1.1+ cells, viral titers decreased upon ILC depletion, indicating ILC1s exert their control well before day 2 of infection (in line with our IFN-γ neutralization data (Figure 4H)). We next transferred WT NK cells into Rag2−/−Il2rg−/− mice (Figure 7E). Consistent with a limited role in protection, NK transfer prior to infection did not improve viral titers and trended toward enhanced replication.
To more conclusively address the role ILC1s during oral epithelial virus replication, we depleted circulating NK cells through delivery of a titrated dose of asialo-GM1 as previously reported (Victorino et al., 2015). Asialo-GM1 treatment was effective at depleting most NK cells in the blood, while maintaining most of the tissue-resident ILC1s (Figure S2). Confocal imaging of frozen tissue sections confirmed that T-bet-ZsGreen+ ILC1s were maintained in the basal epithelium after asialo-GM1 treatment in similar locales as those in untreated mice (Figure S2). Decreasing the number of NK cells did not alter viral titers on day 2 post-infection (which trended even lower in treated mice), suggesting that ILC1s present in the tissue controlled viral infection rather than recently recruited NK cells (Figure 7F). We also did the converse experiment, depleting tissue-resident ILC1s while preserving circulating NK cells using i.v. NAD+ injection as previously described for tissue-resident memory CD8+ T cells (Borges da Silva et al., 2018; Stark et al., 2018) (Figure S2). NAD+ administration significantly increased viral titers in depleted mice (Figure 7G). Together, these data strongly suggest that ILC1s present in the tissue control infection rather than NK cells.
As only ILC1s were present in uninfected tissue, and our depletion studies suggested that ILC1s must act very early in infection, we next performed time depletion studies to determine the timing of ILC1-restriction of viral replication (Figure 7H). Depletion prior to infection yielded the largest increase in viral titers, with depletion at 0 hours also showing enhanced titers. By 9 hours post infection, however, depletion of all ILCs had no impact on viral titers. Flow cytometric data showed limited entry of NK cells into the labial mucosa by 9 hours post infection, further indicating that ILC1s mediate the protective effect (Figure S7). To corroborate these data, we also neutralized IFN-γ either before or 9 hours after infection (Figure 7I). Neutralizing either before or at (Figure 4H) the time of infection dramatically increased titers; this effect was largely limited by 9 hours post infection.
Collectively, these data show that tissue-resident ILC1s function even prior to viral exposure to prevent future viral replication.
Discussion
The oral mucosal epithelium forms an important defensive barrier against incoming pathogens and is constantly challenged by new microbes, incoming food antigens, and masticatory damage. Several viruses establish infection in the oral mucosa, including human papillomavirus (HPV) and herpes simplex virus (HSV), with medical and economical importance--the former is responsible for HPV-associated oropharyngeal carcinomas that continue to increase in incidence in the US (Berman and Schiller, 2017). Recently, a role for oral mucosal viral shedding has been implicated in the transmission of severe acute respiratory syndrome virus 2 (SARS-CoV-2) (Huang et al., 2020). Thus, a much better understanding of the innate and adaptive cellular effectors responsible for providing protection against viral infection in the oral mucosa is warranted.
Using our poxviral model of infection of the labial mucosa and a combination of ex vivo approaches as well as static and dynamic imaging, we demonstrate that ILC1s are the major early restrictive factor limiting viral replication in the labial mucosa. ILC1s populate the tissue at steady-state, while NKs are recruited from the blood rapidly post-infection. The increase in ILC1 numbers over time suggests that, as described for other infections, NK cells convert to ILC1s or ILC1-like cells after VACV infection (Cortez et al., 2017; Park et al., 2019), or that infection induces local proliferation of ILC1s, which remains to be elucidated.
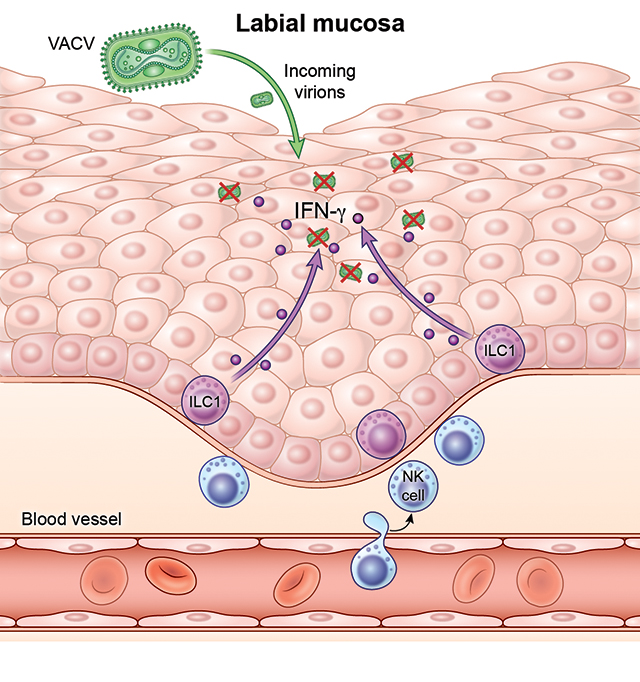
Static and dynamic visualization of T-bet ZsGreen+ ILC1s revealed that these cells reside near the basement membrane of the mucosal epithelium overlying the LP. As HPV and HSV both establish infection in basal keratinocytes after epithelial microabrasions (McBride, 2017; Roberts et al., 2007; Thier et al., 2017), this likely represents a critical oral mucosal area for protection. T-bet ZsGreen+ ILC1s slowly patrolled the uninfected oral epithelium, squeezing between epithelial cells. In a similar manner, γδ intestinal IELs migrate almost exclusively in the space between the epithelium and the basement membrane (Hoytema van Konijnenburg et al., 2017), and tissue-resident CD8+ T cells also patrol this locale near the basement membrane separating the dermis from the epidermis (Masopust and Soerens, 2019). Thus, ILC1s occupy the same tissue-niches as other tissue-resident sentinel lymphocytes. Despite similar locations, the production of IFN-γ in uninfected tissues and ILC1-dependent upregulation of antiviral genes in the uninfected epithelium suggests that ILC1s serve a unique function in host defense compared to other tissue-resident cells that use their rearranged receptors to detect antigen.
There are many possible stimuli for ILC-IFN-γ production in the uninfected oral mucosa. Like intestinal IELs, the microbiota and/or their potential metabolites could promote ILC1-production of IFN-γ directly or indirectly after DC sensing. Consistent with this hypothesis, Schaupp et al. recently demonstrated that microbiota-induced type I IFNs instruct epigenetic and metabolic changes in conventional DCs (Schaupp et al., 2020). Alternatively, the neuronal system has recently been shown to regulate ILC function, particularly at barrier surfaces, although this remains to be explored for ILC1s in the oral mucosa (reviewed in (Klose and Artis, 2019) and (Veiga-Fernandes and Mucida, 2016)). Another possible mediator of ILC1 activation is mechanical damage in the oral mucosa; ongoing mastication has been shown to elicit tissue-destructive Th17 cells in the gingiva (Dutzan et al., 2017). Similarly, ILC1s could sense damage and upregulate IFN-γ production. By flow cytometry, we failed to detect IFN-γ protein present in ILC1s isolated from the oral mucosa after enzymatic digestion (which we speculate results in degranulation); this technical limitation prevented a large-scale screening of ILC1 stimuli.
Unlike naïve T cells, memory T cells and NK cells constitutively transcribe Ifng mRNA (Stetson et al., 2003), and liver ILC1s expressed Ifng transcripts as determined by IFN-γ-YFP expression in reporter mice (Weizman et al., 2017). Likewise, ILC1 IFN-γ-protein production is IL-18-independent, suggesting ILC1s may more easily produce IFN-γ-than NK cells which require both IL-12 and IL-18 for optimal cytokine production (Adams and Sun, 2018). Previous studies of ILC1 function during viral infection did not identify homeostatic production of IFN-γ protein in the liver, but this was not directly examined in situ and may reflect the consequences of the harsh procedures needed to release ILC1s from the tissue. Alternatively, a lack of homeostatic IFN-γ production may reflect differences in commensal access or mechanical damage present in the oral mucosa but not the liver, an organ that rapidly clears bacteria that breach the epithelium (Balmer et al., 2014). Additional studies will be needed to assess the regulation of ILC1-IFN-γ production in different organs and tissues.
The distribution of IFN-γ throughout the labial epithelium by slowly motile ILC1s is likely a key feature of ILC1 function, facilitating a widespread induction of antiviral genes prior to infection (Hoytema van Konijnenburg et al., 2017). Thus, as mobile ILC1s move throughout the epithelium and produce IFN-γ before and early during infection, the resultant upregulation of antiviral genes forms a firewall against the spread of viral infection. In contrast to the IELs in the intestinal epithelium which slowed in response to bacterial challenge, T-bet ZsGreen+ ILCs increased motility on day 2 in the oral mucosa. At this timepoint, however, group I ILCs are comprised of a heterogenous mixture of cells with approximately 91% representing ILC1s; thus, we cannot determine whether ILC1s slow relative to recruited NK cells and potential ILC1-like cells.
Inducing protection even prior to and at the earliest stages of infection may be particularly important for protection against viruses that encode immunomodulatory proteins. VACV blocks IFN-γ signal transduction in infected cells, preventing and even reversing STAT1 activation (Koksal and Cingolani, 2011; Mann et al., 2008; Najarro et al., 2001). Additionally, the VACV protein C6 binds to TBK-1 and inhibits IRF7 activation (Unterholzner et al., 2011). IFN-γ directed at VACV-infected cells therefore would likely fail to completely restrict VACV infection. Thus, our data suggest that ILC1s represent a pre-formed line of defense guarding the epithelium through the induction of antiviral genes in uninfected epithelial cells, thus maintaining epithelial integrity.
In summary, we have uncovered a role for ILC1s in protecting oral mucosal epithelium against viral infection through tonic production of IFN-γ. Homeostatic IFN-γ-priming of the mucosal epithelium presents a mechanism to protect this oft-exposed barrier tissue regardless of the presence of immunological memory, suggesting a unique function for ILC1s during antiviral immune responses.
Limitations of the study
Although we used multiple methods to separate and understand immune protection afforded by NK cells versus ILC1s, we did not have the use of Ncr1-icre Eomesfl/fl mice (Narni-Mancinelli et al., 2011; Pikovskaya et al., 2016) for this study, which would have allowed genetic discrimination of NKs versus ILC1s. It will be important to confirm and extend our findings in these mice moving forward. Further, while we predict that the upregulation of antiviral genes in the epithelium will afford broad antiviral protection, we did not directly test this in the current study. As age and sex are also associated with differences in antiviral responses, the impact of these factors on viral control will also need to be addressed. On a technical note, we failed to detect IFN-γ protein in ILC1s using flow cytometry (though we confirmed this extensively by microscopy). We speculate that the enzymatic digestion we used to release ILC1s from the oral mucosa results in ILC1 degranulation but have not found a method to remove the cells that would prevent this.
STAR METHODS
Resource Availability
Lead Contact
Information and requests for resources and reagents should be directed to the Lead Contact (hhickman@mail.nih.gov).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The published article includes all of the datasets generated during this study.
Experimental Model and Subject Details
Animals
Specific pathogen-free C57Bl/6N mice were obtained from Taconic Farms. Ncr1-gfp+/+ (stock number 22739), GREAT (stock number 17581), and Stock Tg(CAG-DsRed*MST)1Nagy/J mice (stock number 5441) were obtained from Jackson Laboratories. T-bet-ZsGreen (stock number 8419), Rag1−/− (stock number 146), Rag2−/Il2rg−/− (stock number 111), Perf −/− (stock number 2407), OT-I TCR transgenic mice (stock number 175) and Ifng−/− (stock number 248) mice were obtained from the NIAID Intramural Research Repository. Rag1−/− (stock number 146) were crossed with T-bet-ZsGreen (stock number 8419) and bread to homozygosity to create Rag1−/− T-bet-ZsGreen mice. Stock Tg(CAG-DsRed*MST)1Nagy/J mice were crossed with OT-I TCR transgenic mice and bread to homozygosity to create DsRed OT-I mice. 6- to 12-week-old female mice were used in all experiments except for the GREAT flow cytometry, where male mice were used due to availability. There was variation in viral titers in the oral mucosa depending on the age of the mice (with higher titers in younger mice). Mice were housed under specific pathogen-free conditions (including surveillance and negativity for murine norovirus, mouse parvovirus, and mouse hepatitis virus) and maintained on standard rodent chow and water supplied ad libitum. All animal studies were approved by and performed in accordance with the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases.
Microbe strains
Viruses used include VACV NP-S-eGFP (a fusion protein consisting of influenza nucleoprotein (NP), the SIINFEKL T-cell determinate (S), and green fluorescence protein (eGFP); fluorescence is nuclear); VACV NP-S-TurboRFP (with red fluorescent protein); and VACV CR19 (wild type TK+ VACV) and have been previously described (Cush et al., 2016; Hickman et al., 2015; Hickman et al., 2013). All recombinant viruses were generated using the Western Reserve strain of VACV.
METHOD DETAILS
Viral infections and titers
Mice were anesthetized and infected in the cheek (buccal mucosa), tongue (masticatory mucosa), or lower lip (labial mucosa) by five pokes with a bifurcated needle dipped in VACV (stock titer ~ 1 × 108 pfu/ml). To determine viral titers, lips were collected at various d post-infection and single-cell suspensions prepared by collagenase digestion for 40 mins at 37°C. Cells were disrupted by vigorous pipetting, suspension were freeze-thawed three times, serially diluted and plated on TK- cells. Cells were incubated at 37°C for 2 days, then stained with crystal violet and plaques counted.
Antibody and drug treatment
For Ab depletions, mice received either 0.5 mg of anti-CD8 Ab (clone 2.43), 0.25 mg of anti-NK1.1 (clone PK136), 0.4 mg of anti-CD4 (clone GK1.5), or 0.2 mg of isotype control (clone 2A3). For IFN-γ neutralization mice were treated with 0.2 mg of anti-IFN-γ (clone XMG1.2). Unless otherwise indicated, Ab were given intraperitoneally (i.p.) at day −1 and every other day thereafter. One dose of Anti-Asialo-GM1 Ab (Clone Poly21460) was administered at 0.5 μl i.p. 12 hrs prior to infection. NAD+ was dissolved in normal saline and pH adjusted to 5–6, and 60mg was injected intravenously (i.v.) 24 hrs prior to infection. We found batch-to-batch variability in the NAD+ which must be controlled.
Flow cytometric analyses after enzymatic tissue dissociation
Single-cell suspensions of lips were generated by chopping dissected lips with scissors in collagenase P (1mg/ml, Sigma Aldrich) for 45 mins – 1 hour at 37°C followed by filtering through a 70 μm nylon cell strainer. For phenotyping and IV staining shown in Figure S3 (A–C), prior to collagenase digestion, lips were incubated for 45 mins in dispase (1U/ML, Stemcell). Cells were stained with a combination of CD45 (clone 30-F11), CD8 (clone 53–6.7), CD3 (clone 17A2), NK1.1 (clone PK136), CD4 (clone GK1.5), CD49b (clone DX5), CD69 (clone H12.F3), CD103 (clone 2F7), CD49a (clone Ha31/8) and fixable viability dye (efluor780) from eBioscience, BD Biosciences or Biolegend. Tbet+,Ncr1+, and Great+ cells were identified based on fluorescent protein expression. For transcription factor staining an intracellular fixation & permeabilization buffer set (eBioscience) was used and stained for T-bet (clone 4B10, Biolegend) and Eomes (clone Dan11mag, eBioscience). For intravascular staining, 3 ug of CD45 (clone 30-F11) was injected i.v. in a volume of 200 μl 3 mins prior to tissue isolation. Cells were analyzed either on an LSR II or LSRFortessa flow cytometer (BD Bioscience), and data analyzed with Flowjo software (TreeStar).
Adoptive Transfers
NK cells were purified from spleens of WT mice using an EasySep Mouse Negative NK Cell Isolation Kit. 2.5 × 106 NK cells were transferred i.v. into Rag2−/−Il2rg−/− mice prior to infection.
Confocal microscopy of frozen LN sections
Lips were removed on indicated d. post infection, fixed in periodate-lysine-paraformaldehyde (PLP) buffer for two days, and moved to 30% sucrose/PBS for 24 hrs. before embedding. Tissues were embedded in optimal-cutting-temperature (OCT) medium (Electron Microscopy Sciences) in longitudinal or cross section orientation and frozen in dry-ice-cooled isopentane. 17-μm sections were cut on a Lecia cryostat (Leica Microsystems), blocked with 10% bovine and donkey serum then stained with one of the following anti-IFN-γ (clone EPR1108, Abcam or clone XMG1.2, Bioxcell), IRF7 (rabbit polyclonal, cat#PA520281, Lot#UG2815528), anti-TBR2 (clone EPR19012, Abcam) or laminin (rabbit polyclonal, ab11575, lot# GR233309–2, Abcam). Spectral DAPI (Perkin Elmer) was used for nucleic acid stain. Sections were incubated with secondary antibodies as needed and as controls. In some instances, Huygens Essentials was used to deconvolve confocal images. Images were acquired on a Leica SP8 confocal microscope equipped with acousto-optical tunable filters and hybrid detectors.
MPM imaging
MPM imaging was performed as previously described with minor modifications (Hickman et al., 2015; Shannon et al., 2019). Briefly, images were acquired on a Leica DIVE (Deep In Vivo Explorer) inverted microscope (Leica Microsystems) equipped with MaiTai and InSight DeepSee lasers (Spectra Physics). To visualize blood vessels in the lamina propria of the mucosa, 0.25 mg of Alexa Fluor-647 conjugated anti-CD31 (clone MEC13.3) was injected i.v. Epithelial nuclei of the mucosal tissue were labeled using 0.5 mg of Hoeschst 33258 (ThermoFisher) diluted in 100 μl of PBS and injected i.v. Lips were immobilized and immersed in lubricating jelly. Images were acquired with a 25x inverted objective (numerical aperture = 0.95). Images were obtained at 910 nm for ZsGreen, Hoechst, and collagen; at at 1150 for dsRed and Alexa 647. Emitted fluorescence was collected with 4 tunable non-descanned hybrid detectors (HyD) of the DIVE detection system. For time-lapse recording, images were acquired with 1x zoom, a 2.5 – 5 μm z step for a depth of 50–70 μm every 30–90 s. Most videos and large static images were tiled, and a merged mosaic was constructed using Leica tile scanning software. To visualize blood vessels in the mucosal tissue, 50 μl of Alexa Fluor 647-conjugated anti-CD31 (clone MEC13.3) was injected i.v. Epithelial nuclei of the mucosal tissue were labeled using 0.5 mg of Hoeschst 33258 (ThermoFisher) diluted in 100 μl of PBS and injected i.v.
MPM and confocal images were analyzed using Imaris (Bitplane). Cellular speeds were calculated using the spot function of Imaris. Cells were classified as sessile if their speed was under 1.5 μm/min and displacement was equal to or less than 5 μm during each imaging period. To calculate speeds of interacting and non-interacting cells, cells were manually assigned to groups and then cellular speeds and displacements calculated automatically using the spot function.
Real-time PCR arrays
Lips were removed and immediately placed in buffer RLT (Qiagen). Lips were homogenized and RNA isolated using Lysing Matrix S (1/8″) metal beads (MPBio) and a FastPrep®−24 Instrument (MPBio). RNA was then purified using a Qiagen RNAEasy Mini Kit (Qiagen). An on column DNAse digestion was performed prior to RNA elution. CDNA were made using the RT2 First Strand Kit (Qiagen) with ~ 1.5 g of RNA/sample. CDNA were loaded onto an Inflammatory Cytokines and Receptors qPCR array (PAMM-011Z, Qiagen) and Antiviral Response qPCR array (PAMM-122Z, Qiagen), and RT-PCR data acquired on a Real Time-PCR machine (ThermoFisher). Rox was used as a loading control. Fold-changes of genes were calculated against 5 standard housekeeping genes included on the same plate using the manufacturer’s data analysis software based upon ΔΔCT (Qiagen).
Statistical analyses
Significances were calculated using Prism V 8.3.0 (Graphpad Software) using unpaired two-tailed Mann-Whitney t tests (when only two groups were present) or using a one-way ANOVA as indicated in the figure legends. For real-time PCR arrays, significances were calculated using an unpaired t-test on the replicate ΔΔCT values for each gene in the control and treatment group. Values of p < 0.05 was considered to be stastically significant and were reported. Exact p values are shown throughout the manuscript.
Supplementary Material
Movie S1 T-bet-Zsgreen+ group I ILCs slowly move through the epithelium, related to Figure 3
Maximum intensity projections (MIPs) of time-lapse MPM images taken of the labial mucosa in naïve animals. Mice were treated with CD4-depleting antibody 12 hours before imaging; Alexa Fluor 647-conjugated anti-CD31 Ab was administered immediately before imaging to visualize blood vessels (purple). Lamina propria collagen = blue. Primarily the epithelium is visualized at a shallow tissue depth (left), while the right image contains a larger area of the lamina propria. Scale bar = μm. Time = hr:min:sec.
Movie S2. T-bet-Zsgreen+ group I ILCs patrol the naïve and inflamed tissue, related to Figure 4
MIPs (showing both the epithelium and lamina propria) of time-lapse MPM images taken of the labial mucosa in naïve animals (left) or day 2 post-infection with VACV-NP-S-TurboRFP (red, right). Collagen = blue. Scale bar = μm. Time = hr:min:sec.
Movie S3. T-bet Zsgreen+ group I ILCs transiently interact with VACV-infected cells, related to Figure 4
MIPs (showing both the epithelium and lamina propria) of time-lapse MPM images taken of the labial mucosa on day 2 post-infection with VACV-NP-S-TurboRFP (red). Collagen = blue. A track of an individual ILC over the 1 hr imaging perior is shown in gray. Scale bar = μm. Time = hr:min:sec:ms.
Movie S4. T-bet Zsgreen+ group I ILCs do not accumulate around VACV-infected cells, related to Figure 4
MIPs (showing both the epithelium and lamina propria) of time-lapse MPM images taken of the labial mucosa on day 2 post-infection with VACV-NP-S-TurboRFP (red). Collagen = blue, vasculature = purple (CD31). Scale bar = μm. Time = hr:min:sec.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD45 (30-F11) | eBioscience | Catalog # MCD4528 RRID:AB_10373710 |
| Anti-mouse CD45 (30-F11) | Biolegend | Catalog #103132 RRID: AB_893340 |
| Anti-mouse CD45 (30-F11) | Biolegend | Catalog #103112 RRID: AB_312977 |
| Anti-mouse CD45 (30-F11) | Biolegend | Catalog #103128 RRID: AB_493715 |
| Anti-mouse CD45 (30-F11) | Biolegend | Catalog #103116 RRID: AB_312981 |
| Anti-mouse CD8 (53-6.7) | Biolegend | Catalog #100712 RRID: AB_312751 |
| Anti-mouse CD3 (17A2) | Biolegend | Catalog # 100204 RRID: AB_312661 |
| Anti-mouse CD3 (17A2) | Biolegend | Catalog # 100220 RRID: AB_1732057 |
| Anti-mouse NK1.1 (PK136) | Biolegend | Catalog #108708 RRID: AB_313395 |
| Anti-mouse NK1.1 (PK136) | Biolegend | Catalog #108720 RRID: AB_2132713 |
| Anti-mouse CD4 (GK1.5) | eBioscience | Catalog #100406 RRID: AB_312691 |
| Anti-mouse CD49b (DX5) | Biolegend | Catalog #108908 RRID: AB_313415 |
| Anti-mouse CD49b (DX5) | Biolegend | Catalog #108910 RRID: AB_313417 |
| Anti-mouse CD49a (Ha31/8) | BD Biosciences | Catalog #564862 RRID: AB_2734135 |
| Anti-mouse Eomes (Dan11mag) | eBioscience | Catalog #25-4875-82 RRID:AB_2573454 |
| Anti-mouse T-bet (4B10) | Biolegend | Catalog # 644810 RRID:AB_2200542 |
| Anti-mouse CD31 (MEC13.3) | Biolegend | Catalog #102516 RRID:AB_2161029 |
| Anti-mouse CD69 (H12.F3) | Biolegend | Catalog #104513 RRID: AB_492844 |
| Anti-mouse CD103 (2F7) | Biolegend | Catalog #121432 RRID:AB_2566552 |
| Anti-mouse CD103 (2F7) | Biolegend | Catalog #121422 RRID: AB_2562901 |
| Normal bovine serum | Jackson Immunoresearch lab | Catalog #001-000-121 RRID:AB_2336944 |
| Normal donkey serum | Jackson Immunoresearch lab | Catalog #017-000-121 RRID:AB_2337258 |
| Anti-interferon gamma (EPR1108) | Abcam | Catalog #Ab133566 RRID: N/A |
| Anti-IRF7 (rabbit polyclonal) | Invitrogen | Catalog #PA520281 RRID:AB_11157833 |
| Anti-Laminin (rabbit polyclonal) | Abcam | Catalog #Ab11575 RRID: AB_384243 |
| Anti-Eomes TBR2/Eomes (EPR19012) | Abcam | Catalog #Ab183991 RRID: AB_2721040 |
| Anti-Ki67 (rabbit polyclonal) | Abcam | Catalog #Ab66155 RRID: AB_1140752 |
| Donkey anti-rabbit IgG | Jackson Immunoresearch lab | Catalog #711-605-152 RRID:AB_2492288 |
| Purified anti-mouse CD8 (2.43) | Bioxcell | Catalog #BE0061 RRID:AB_1125541 |
| Purified anti-mouse NK1.1 (PK136) | Bioxcell | Catalog #BE0036 RRID:AB_1107737 |
| Purified anti-mouse CD4 (GK1.5) | Bioxcell | Catalog #BE0003-1 RRID:AB_1107636 |
| Purified rat IgG2a isotype control (2A3) | Bioxcell | Catalog #BE0089 RRID:AB_1107769 |
| Purified anti-mouse IFN gamma (XMG1.2) | Bioxcell | Catalog #BE0055 RRID:AB_1107694 |
| Purified anti-Asialo-GM1 (Poly21460) | Biolegend | Catalog # 146002 RRID:AB_2562206 |
| Bacterial and Virus Strains | ||
| Vaccinia virus NP-S-eGFP (with influenza nucleoprotein (NP), SIINFEKEL T cell determinate (S), and green fluorescence protein). | Laboratory of H.D.H, | N/A |
| Vaccinia virus NP-S-TurboRFP (with red fluorescent protein). | Laboratory of H.D.H, | N/A |
| Vaccinia Virus wild type (CR19) | Laboratory of H.D.H, | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Hoeschst 33258 | ThermoFisher | H3569 |
| Spectral DAPI | Perkin Elmer | FP1490 |
| BSA | Sigma-Aldrich | A3059-500G |
| DNase I | Sigma-Aldrich | DN25-5G |
| FBS | Hyclone | SH30070.03 |
| Triton X | Sigma-Aldrich | T9284 |
| Paraformaldehyde | Electron Microscopy Sciences | 15714-S |
| Sodium (meta)periodate | Sigma-Aldrich | S1878-500G |
| Lysine | Sigma-Aldrich | L5501-100G |
| Saline | Quality Biological Inc | 114055101 |
| Optimal-cutting-temperature (OCT) compound | Electron Microscopy Sciences | 62550-01 |
| 2-Methylbutane (Isopentane) | Sigma-Aldrich | M32631-500ML |
| Collagenase (type I) | Worthington Biochemicals | LS004216 |
| Collagenase P | Sigma-Aldrich | 11249002001 |
| NAD | Sigma-Aldrich | 10127973001 |
| Dispase (1U/mL) | Stemcell | 07923 |
| Crystal Violet | Sigma-Aldrich | C0775-100G |
| Formaldehyde | Sigma-Aldrich | 252549-500ML |
| Gentamicin (50mg/mL) | Sigma-Aldrich | G1397-10ML |
| Critical Commercial Assays | ||
| Fixable viability dye (eflour780) | eBioscience | 65-0865-14 |
| Mouse T cell Isolation kit | Stemcell | 19851 |
| Foxp3/Transcription Factor Staining Buffer Set | eBioscience | 00-5523-00 |
| BD Perm/Wash | BD Bioscience | 554723 |
| Lysing Matric S (1/8”) metal beads | MPBio | 116925100 |
| Qiagen RNAEasy Mini Kit | Qiagen | 74104 |
| RT2 First Stand Kit | Qiagen | 330404 |
| Inflammatory Cytokines and Receptor qPCR array | Qiagen | PAMM-011Z-24 |
| Antiviral Response qPCR array | Qiagen | PAMM-122Z-24 |
| RT2 SYBR Grenn ROX qPCR Mastermix | Qiagen | 330523 |
| EasySep™ Mouse NK Cell Isolation Kit | Stemcell | 19855 |
| Experimental Models: Cell Lines | ||
| 143B | ATCC | CRL-8303 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57Bl/6 | Taconic Farms | Mouse strain: C57Bl/6NTac |
| Mouse: NCR1gfp/gfp | Jackson Laboratories | Mouse strain: Jax, 22739 |
| Mouse: Great | Jackson Laboratories | Mouse strain: Jax 017580 |
| Mouse: T-bet-ZsGreen | Taconic Farms – NIAID exchange | Mouse strain: Tac 8419 |
| Mouse: rag−/− | Taconic Farms – NIAID exchange | Mouse strain: Tac 146 |
| Mouse: rag−/−IL-2rg−/− | Taconic Farms – NIAID exchange | Mouse strain: Tac 111 |
| Mouse: perf−/− | Taconic Farms – NIAID exchange | Mouse strain: Tac 2407 |
| Mouse: ifng−/− | Taconic Farms – NIAID exchange | Mouse strain: Tac 248 |
| Mouse: rag−/− × T-bet-ZsGreen | Laboratory of H.D.H. | N/A |
| Mouse: rag−/− × Great | Laboratory of H.D.H. | N/A |
| Software and Algorithms | ||
| Flowjo software | Treestar | RRID:SCR_008520 |
| Imaris software | Bitplane | RRID:SCR_007370 |
| Prism software | Graphpad | RRID:SCR_002798 |
Highlights.
Oral mucosa ILC1s restrict viral replication in the epithelium.
ILC1s patrol the basal layers of the oral epithelium at steady state.
ILC1s produce tonic IFN-γ, upregulating antiviral genes in the epithelium.
ILC1s prime uninfected tissue to restrict viral replication.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the NIAID, NIH. Graphical abstract was drawn by Ethan Tyler, NIH Medical Arts Branch. We thank Dr. Jinfang (Jeff) Zhu for kindly sharing T-bet-ZsGreen reporter mice, Dr. KPJM van Gisbergen for helpful comments regarding NAD+ depletion of tissue-resident cells, and Drs. Eric Vivier and Ron Germain for helpful discussions on this study.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams NM, and Sun JC (2018). Spatial and temporal coordination of antiviral responses by group 1 ILCs. Immunol Rev 286, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, and Masopust D (2014). Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9, 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S, Miele L, Grieco A, Van Vlierberghe H, Fahrner R, Patuto N, et al. (2014). The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med 6, 237ra266. [DOI] [PubMed] [Google Scholar]
- Baron S (2003). Smallpox: the main site of transmission is the oropharynx. J Dent Res 82, 252. [DOI] [PubMed] [Google Scholar]
- Berman TA, and Schiller JT (2017). Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer 123, 2219–2229. [DOI] [PubMed] [Google Scholar]
- Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, Best JA, Goldrath AW, Lanier LL, and Immunological Genome Project C (2012). Molecular definition of the identity and activation of natural killer cells. Nat Immunol 13, 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, et al. (2018). The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8(+) T cells. Nature 559, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, Campbell L, Malcolm J, Adrados Planell A, Butcher JP, and Culshaw S (2018). Enrichment of Innate Lymphoid Cell Populations in Gingival Tissue. J Dent Res 97, 1399–1405. [DOI] [PubMed] [Google Scholar]
- Chapman JL, Nichols DK, Martinez MJ, and Raymond JW (2010). Animal models of orthopoxvirus infection. Vet Pathol 47, 852–870. [DOI] [PubMed] [Google Scholar]
- Cortez VS, Cervantes-Barragan L, Robinette ML, Bando JK, Wang Y, Geiger TL, Gilfillan S, Fuchs A, Vivier E, Sun JC, et al. (2016). Transforming Growth Factor-beta Signaling Guides the Differentiation of Innate Lymphoid Cells in Salivary Glands. Immunity 44, 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez VS, Ulland TK, Cervantes-Barragan L, Bando JK, Robinette ML, Wang Q, White AJ, Gilfillan S, Cella M, and Colonna M (2017). SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat Immunol 18, 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cush SS, Reynoso GV, Kamenyeva O, Bennink JR, Yewdell JW, and Hickman HD (2016). Locally Produced IL-10 Limits Cutaneous Vaccinia Virus Spread. PLoS Pathog 12, e1005493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, et al. (2017). On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity 46, 133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Colonna M, Di Santo JP, and McKenzie AN (2015). Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelblum KL, Shen L, Weber CR, Marchiando AM, Clay BS, Wang Y, Prinz I, Malissen B, Sperling AI, and Turner JR (2012). Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A 109, 7097–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlik M, Rapp B, Marie I, Levy DE, Jamieson AM, and Decker T (2012). Contribution of a TANK-binding kinase 1-interferon (IFN) regulatory factor 7 pathway to IFN-gamma-induced gene expression. Mol Cell Biol 32, 1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner FHD; Arita I; Jezek Z; Ladnyi I (1988). Smallpox and Its Eradicaiton. World Health Organization, Geneva, 1460. [Google Scholar]
- Gasteiger G, Fan X, Dikiy S, Lee SY, and Rudensky AY (2015). Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, and Mueller SN (2011). Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219. [DOI] [PubMed] [Google Scholar]
- Groom JR, and Luster AD (2011). CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunology and cell biology 89, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernaez B, and Alcami A (2018). New insights into the immunomodulatory properties of poxvirus cytokine decoy receptors at the cell surface. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman HD, Reynoso GV, Ngudiankama BF, Cush SS, Gibbs J, Bennink JR, and Yewdell JW (2015). CXCR3 chemokine receptor enables local CD8(+) T cell migration for the destruction of virus-infected cells. Immunity 42, 524–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman HD, Reynoso GV, Ngudiankama BF, Rubin EJ, Magadan JG, Cush SS, Gibbs J, Molon B, Bronte V, Bennink JR, and Yewdell JW (2013). Anatomically restricted synergistic antiviral activities of innate and adaptive immune cells in the skin. Cell host & microbe 13, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, and Mucida D (2017). Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell 171, 783–794 e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Perez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Dominguez Conde C, Gasmi B, Stein S, Beach M, et al. (2020). Integrated Single-Cell Atlases Reveal an Oral SARS-CoV-2 Infection and Transmission Axis. medRxiv. [Google Scholar]
- Jarahian M, Fiedler M, Cohnen A, Djandji D, Hammerling GJ, Gati C, Cerwenka A, Turner PC, Moyer RW, Watzl C, et al. (2011). Modulation of NKp30- and NKp46-mediated natural killer cell responses by poxviral hemagglutinin. PLoS Pathog 7, e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T, Jankovic D, Kawabe S, Huang Y, Lee PH, Yamane H, Zhu J, Sher A, Germain RN, and Paul WE (2017). Memory-phenotype CD4(+) T cells spontaneously generated under steady-state conditions exert innate TH1-like effector function. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairallah C, Chu TH, and Sheridan BS (2018). Tissue Adaptations of Memory and Tissue-Resident Gamma Delta T Cells. Front Immunol 9, 2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CS, and Artis D (2019). Neuronal regulation of innate lymphoid cells. Curr Opin Immunol 56, 94–99. [DOI] [PubMed] [Google Scholar]
- Koksal AC, and Cingolani G (2011). Dimerization of Vaccinia virus VH1 is essential for dephosphorylation of STAT1 at tyrosine 701. J Biol Chem 286, 14373–14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias CM, Huneke RB, Wigdahl B, and Jennings SR (2015). Animal models of herpes simplex virus immunity and pathogenesis. J Neurovirol 21, 8–23. [DOI] [PubMed] [Google Scholar]
- Lazarevic V, Glimcher LH, and Lord GM (2013). T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol 13, 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann BA, Huang JH, Li P, Chang HC, Slee RB, O’Sullivan A, Anita M, Yeh N, Klemsz MJ, Brutkiewicz RR, et al. (2008). Vaccinia virus blocks Stat1-dependent and Stat1-independent gene expression induced by type I and type II interferons. J Interferon Cytokine Res 28, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, and Soerens AG (2019). Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol 37, 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AA (2017). Mechanisms and strategies of papillomavirus replication. Biol Chem 398, 919–927. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, and Von Andrian UH (2004). T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159. [DOI] [PubMed] [Google Scholar]
- Najarro P, Traktman P, and Lewis JA (2001). Vaccinia virus blocks gamma interferon signal transduction: viral VH1 phosphatase reverses Stat1 activation. J Virol 75, 3185–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narni-Mancinelli E, Chaix J, Fenis A, Kerdiles YM, Yessaad N, Reynders A, Gregoire C, Luche H, Ugolini S, Tomasello E, et al. (2011). Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc Natl Acad Sci U S A 108, 18324–18329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning S, Pagano JS, and Barber GN (2011). IRF7: activation, regulation, modification and function. Genes Immun 12, 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan TE (2019). Dazed and Confused: NK Cells. Front Immunol 10, 2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Patel S, Wang Q, Andhey P, Zaitsev K, Porter S, Hershey M, Bern M, Plougastel-Douglas B, Collins P, et al. (2019). Toxoplasma gondii infection drives conversion of NK cells into ILC1-like cells. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikovskaya O, Chaix J, Rothman NJ, Collins A, Chen YH, Scipioni AM, Vivier E, and Reiner SL (2016). Cutting Edge: Eomesodermin Is Sufficient To Direct Type 1 Innate Lymphocyte Development into the Conventional NK Lineage. J Immunol 196, 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Liang HE, and Locksley RM (2009). Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol 10, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, and Schiller JT (2007). Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 13, 857–861. [DOI] [PubMed] [Google Scholar]
- Schaupp L, Muth S, Rogell L, Kofoed-Branzk M, Melchior F, Lienenklaus S, Ganal-Vonarburg SC, Klein M, Guendel F, Hain T, et al. (2020). Microbiota-Induced Type I Interferons Instruct a Poised Basal State of Dendritic Cells. Cell 181, 1080–1096 e1019. [DOI] [PubMed] [Google Scholar]
- Shannon JP, Kamenyeva O, Reynoso GV, and Hickman HD (2019). Intravital Imaging of Vaccinia Virus-Infected Mice. Methods Mol Biol 2023, 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard S, Triulzi C, Ardolino M, Serna D, Zhang L, Raulet DH, and Guerra N (2013). Characterization of a novel NKG2D and NKp46 double-mutant mouse reveals subtle variations in the NK cell repertoire. Blood 121, 5025–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, and Hanifin JM (2004). Smallpox: the basics. Dermatol Clin 22, 263–274, vi. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, et al. (2014). Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. Elife 3, e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Bernink JH, and Lanier L (2016). NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol 17, 758–764. [DOI] [PubMed] [Google Scholar]
- Stark R, Wesselink TH, Behr FM, Kragten NAM, Arens R, Koch-Nolte F, van Gisbergen K, and van Lier RAW (2018). T RM maintenance is regulated by tissue damage via P2RX7. Sci Immunol 3. [DOI] [PubMed] [Google Scholar]
- Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, and Locksley RM (2003). Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med 198, 1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier K, Petermann P, Rahn E, Rothamel D, Bloch W, and Knebel-Morsdorf D (2017). Mechanical Barriers Restrict Invasion of Herpes Simplex Virus 1 into Human Oral Mucosa. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner L, Sumner RP, Baran M, Ren H, Mansur DS, Bourke NM, Randow F, Smith GL, and Bowie AG (2011). Vaccinia virus protein C6 is a virulence factor that binds TBK-1 adaptor proteins and inhibits activation of IRF3 and IRF7. PLoS Pathog 7, e1002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist N, Trittel S, Ebensen T, Chambers BJ, Guzman CA, and Riese P (2018). Influenza-Activated ILC1s Contribute to Antiviral Immunity Partially Influenced by Differential GITR Expression. Front Immunol 9, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Fernandes H, and Mucida D (2016). Neuro-Immune Interactions at Barrier Surfaces. Cell 165, 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victorino F, Sojka DK, Brodsky KS, McNamee EN, Masterson JC, Homann D, Yokoyama WM, Eltzschig HK, and Clambey ET (2015). Tissue-Resident NK Cells Mediate Ischemic Kidney Injury and Are Not Depleted by Anti-Asialo-GM1 Antibody. J Immunol 195, 4973–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. (2018). Innate Lymphoid Cells: 10 Years On. Cell 174, 1054–1066. [DOI] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, and Ugolini S (2008). Functions of natural killer cells. Nat Immunol 9, 503–510. [DOI] [PubMed] [Google Scholar]
- Weizman OE, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, Degli-Esposti MA, Leslie CS, Sun JC, and O’Sullivan TE (2017). ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 171, 795–808 e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, Manton JH, Heath WR, and Mueller SN (2014). Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci U S A 111, 5307–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, Guo L, Yagi R, Yamane H, Punkosdy G, et al. (2012). The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 37, 660–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1 T-bet-Zsgreen+ group I ILCs slowly move through the epithelium, related to Figure 3
Maximum intensity projections (MIPs) of time-lapse MPM images taken of the labial mucosa in naïve animals. Mice were treated with CD4-depleting antibody 12 hours before imaging; Alexa Fluor 647-conjugated anti-CD31 Ab was administered immediately before imaging to visualize blood vessels (purple). Lamina propria collagen = blue. Primarily the epithelium is visualized at a shallow tissue depth (left), while the right image contains a larger area of the lamina propria. Scale bar = μm. Time = hr:min:sec.
Movie S2. T-bet-Zsgreen+ group I ILCs patrol the naïve and inflamed tissue, related to Figure 4
MIPs (showing both the epithelium and lamina propria) of time-lapse MPM images taken of the labial mucosa in naïve animals (left) or day 2 post-infection with VACV-NP-S-TurboRFP (red, right). Collagen = blue. Scale bar = μm. Time = hr:min:sec.
Movie S3. T-bet Zsgreen+ group I ILCs transiently interact with VACV-infected cells, related to Figure 4
MIPs (showing both the epithelium and lamina propria) of time-lapse MPM images taken of the labial mucosa on day 2 post-infection with VACV-NP-S-TurboRFP (red). Collagen = blue. A track of an individual ILC over the 1 hr imaging perior is shown in gray. Scale bar = μm. Time = hr:min:sec:ms.
Movie S4. T-bet Zsgreen+ group I ILCs do not accumulate around VACV-infected cells, related to Figure 4
MIPs (showing both the epithelium and lamina propria) of time-lapse MPM images taken of the labial mucosa on day 2 post-infection with VACV-NP-S-TurboRFP (red). Collagen = blue, vasculature = purple (CD31). Scale bar = μm. Time = hr:min:sec.
Data Availability Statement
The published article includes all of the datasets generated during this study.