Abstract
Objective:
Mineralocorticoid receptor antagonists (MRAs) are effective in patients with resistant hypertension and/or primary aldosteronism (PA). Screening for PA should ideally be conducted after stopping medications that might interfere with the renin-angiotensin-aldosterone system, but this is challenging in patients with recalcitrant hypertension or hypokalemia. Herein, we aimed to evaluate the impact of MRAs on PA screening in clinical practice.
Methods:
We conducted a retrospective cohort study of patients with hypertension who had plasma aldosterone and renin measurements before and after MRA use in a tertiary referral center, over 19 years.
Results:
A total of 146 patients, 91 with PA, were included and followed for up to 18 months. Overall, both plasma renin and aldosterone increased after MRA initiation (from median, interquartile range: 0.5 [0.1, 0.8] to 1.2 [0.6, 4.8] ng/mL/hour and from 19.1 [12.9, 27.7] to 26.4 [17.1, 42.3] ng/dL, respectively; P<.0001 for both), while the aldosterone/renin ratio (ARR) decreased from 40.3 (18.5, 102.7) to 23.1 (8.6, 58.7) ng/dL per ng/mL/hour (P<.0001). Similar changes occurred irrespective of the MRA treatment duration and other antihypertensives used. Positive PA screening abrogation after MRA initiation was found in 45/94 (48%) patients. Conversely, 17% of patients had positive PA screening only after MRA treatment, mostly due to correction of hypokalemia. An initially positive screening test was more likely altered by high MRA doses and more likely persistent in patients with confirmed PA or taking beta-blockers.
Conclusion:
MRAs commonly reduce ARR and the proportion of positive PA screening results. When PA is suspected, screening should be repeated off MRAs.
INTRODUCTION
Primary aldosteronism (PA) is a common form of secondary hypertension, and it is characterized by renin-independent aldosterone excess (1,2). Compared to patients with essential hypertension of similar severity, patients with PA have enhanced cardiovascular and renal morbidity and mortality (3–5). Early detection and targeted therapy of PA are crucial for abrogating the higher cardiovascular risk attributed to inappropriate mineralocorticoid receptor activation (6,7).
PA screening consists of concomitant measurement of peripheral renin and aldosterone and evaluation of their ratio (ARR) (8). Several classes of antihypertensive medications can alter the renin-angiotensin-aldosterone system (RAAS) and lead to inaccuracies in PA screening (9). Consequently, expert guidelines recommend avoiding such antihypertensive agents, and in particular mineralocorticoid receptor antagonists (MRAs), for up to 4 weeks prior to PA screening (8,10). Many RAAS-interfering agents are, however, widely used in the treatment of hypertension (11,12). Additionally, practical aspects, such as resistant hypertension with or without recalcitrant hypokalemia in patients at high risk for cardiovascular complications, preclude even a temporary interruption of all antihypertensive agents with RAAS-interfering potential. RAAS inhibitors and MRAs are commonly added to thiazide diuretics in patients with hypertension, both to ensure eukalemia and for their cardio-renal protective effects (13–15). Very few previous reports have investigated the impact of MRA use on plasma aldosterone concentration (PAC) and renin activity (PRA) alterations, and existing evidence has focused on patients with confirmed PA (16–18). To expand our understanding of MRA impact on RAAS, including in cases with equivocal PA screening results, we conducted a comprehensive study of patients with all forms of hypertension treated with these agents, with the hypothesis that MRAs reduce the number of positive PA screening results.
METHODS
Study Subjects
We conducted a retrospective review of all patients with hypertension treated with MRAs seen within the University of Michigan system between January 1, 2000, to September 30, 2019, who had plasma renin and aldosterone measurements both before and after initiation of MRAs. We employed an internal database search engine, DataDirect (19), to identify patients of interest based on the following entry criteria: age 18 years old and above; with a documented diagnosis of hypertension; who were prescribed MRAs; and had laboratory data on plasma renin and aldosterone both at baseline and after MRA initiation (Fig. 1). To eliminate major confounders, we imposed the following exclusion criteria: Cushing syndrome; glucocorticoid use; adrenal cortical carcinoma; congenital adrenal hyperplasia; renovascular hypertension; end-stage renal disease; and critically ill patients. In addition, we excluded patients with no follow-up data for longer than 18 months after MRA initiation. This study was conducted with the University of Michigan Internal Review Boards approval (HUM00055821). A waiver of consent was granted for the retrospective review of medical records.
Fig. 1.
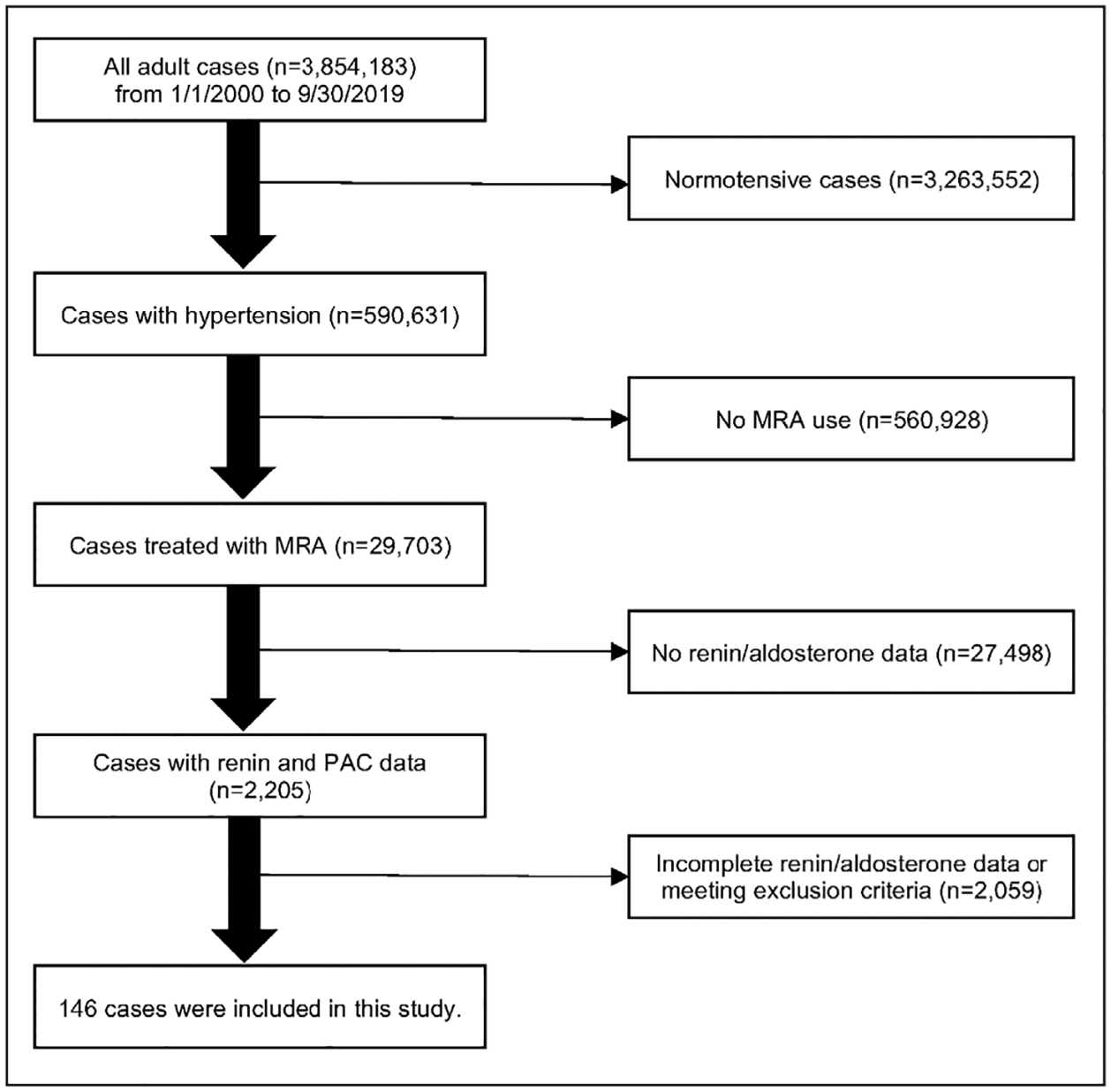
Selection of study participants. MRA = mineralocorticoid receptor antagonist; PAC = plasma aldosterone concentration.
Clinical Data Collection
Clinical data extracted from the medical records of study participants included: demographics, body mass index (BMI), blood pressure, medical diagnoses, medications, renal function, serum and urinary electrolytes and aldosterone, and plasma renin results. Hypokalemia was defined as serum potassium <3.5 mM or use of potassium supplements. PAC and PRA were measured as previously described (20). Since February 2018, direct renin concentration (DRC) has substituted PRA, and DRC was measured in 32 of 146 patients (21.9%) using a DiaSorin Liaison competitive chemiluminescent immunoassay with a coefficient of variability <10%. To enable within- and between-patient comparisons in all cases, we used a conversion factor of DRC/PRA of 8, based on rigorous in-house studies conducted over 9 months during the transition between the two assays, when both PRA and DRC were measured. Also, to standardize the MRA doses used, eplerenone doses were divided by a factor of 2 to convert them to spironolactone equivalent doses (4,16).
PA Screening and Diagnosis Criteria
Screening for PA was considered positive based on: a PAC >10 ng/dL and a suppressed renin value (PRA <1.0 ng/mL/hour or DRC <8.0 pg/mL); and an ARR >20 ng/dL per ng/mL/hour (8). The diagnosis of PA was established based on any of the following: an oral salt loading test (followed by a 24-hour urinary aldosterone >12 μg); an intravenous saline infusion test (PAC >6.8 ng/dL at 4 hours); or PAC ≥20 ng/dL along with suppressed renin.
Statistical Analysis
Two-group comparisons for continuous variables were assessed using the Student’s t-test (for normally distributed data) or Mann-Whitney U test (for variables lacking normal distribution). The Wilcoxon signed rank test was used for paired data analysis. Chi-squared and Fisher’s exact tests were used to compare proportions between groups. Two-way repeated measures analyses of variance and multiple logistic regression analyses were also employed where appropriate. All statistical analyses were performed with StatFlex software (version 7.0; Artech Co, Ltd, Osaka, Japan) or Prism (version 8.0; GraphPad Software, La Jolla, CA), and P values <.05 were considered significant.
RESULTS
Study Flow and Characteristics of Participants
Of 590,631 patients with hypertension seen in our institution over 19 years, 29,703 (5%) were prescribed MRAs. Of 2,205 patients who had at least one measurement of PAC and renin available to us, 146 patients met all inclusion criteria (Fig. 1). The baseline characteristics of the study participants are summarized in Table 1. The median age of study participants was 56 years (range, 19 to 88 years), and 76 (52%) were men. The median number of antihypertensive agents used was 3.0, and calcium channel blockers were the most commonly prescribed class (62% of patients). Of all patients, 71 (49%) had a history of hypokalemia.
Table 1.
Baseline Characteristics of Study Patients
| Variable | All participants | PA | No PA | P value |
|---|---|---|---|---|
| n | 146 | 91 | 55 | |
| Age (years) | 56 [47, 64] | 55 [48, 64] | 57 [46, 66] | .85 |
| Sex (n men, %) | 76 (52.1) | 55 (60.4) | 21 (38.2) | .009 |
| BMI (kg/m2) | 32.6 [28.5, 36.9] | 32.6 [28.8, 36.7] | 32.5 [27.7, 37.2] | .86 |
| SBP (mm Hg) | 150 [134, 171] | 148 [130, 161] | 160 [141, 179] | .02 |
| DBP (mm Hg) | 81 [74, 90] | 82 [74, 90] | 81 [71, 98] | .15 |
| eGFR (mL/min/1.73 m2) | 83 [61, 97] | 83 [60, 94] | 82 [69, 100] | .91 |
| Serum potassium (mM) | 3.8 [3.5, 4.2] | 3.7 [3.4, 4.1] | 4.0 [3.6, 4.3] | .009 |
| PAC (ng/dL) | 19.1 [12.9, 27.7] | 23.3 [16.9, 31.9] | 12.2 [8.3, 20.0] | <.0001 |
| PRA (ng/mL/h) [n = 114] | 0.5 [0.1, 0.8] | 0.3 [0.1, 0.6] | 0.8 [0.3, 3.1] | <.0001 |
| DRC (pg/mL) [n = 32] | 3.6 [2.1, 8.2] | 2.1 [2.1, 6.8] | 5.6 [2.1, 9.4] | .20 |
| Suppressed renin cases (n, %) | 121 (82.3) | 87 (94.5) | 34 (61.8) | <.0001 |
| ARR (ng/dL per ng/mL/h) | 40.3 [18.5, 102.7] | 73.3 [35.5, 152.5] | 14.0 [5.7, 30.3] | <.0001 |
| Diabetes mellitus (n, %) | 52 (35.6) | 28 (30.8) | 24 (43.6) | .12 |
| Cardiovascular disease (n, %) | 41 (28.1) | 21 (23.1) | 20 (36.4) | .08 |
| Stroke (n, %) | 17 (11.6) | 7 (7.7) | 10 (18.2) | .06 |
| Antihypertensive agents (n) | 3.0 [2.0, 4.0] | 3.0 [2.0, 4.0] | 3.0 [1.3, 4.0] | .64 |
| Alpha-blocker (n, %) | 15 (10.3) | 10 (11.0) | 5 (9.1) | .71 |
| Beta-blocker (n, %) | 88 (61.0) | 56 (61.5) | 33 (60.0) | .85 |
| Central agonists (n, %) | 38 (26.0) | 23 (25.3) | 15 (27.3) | .79 |
| Potassium-wasting diuretics (n, %) | 67 (45.9) | 41 (45.1) | 26 (47.3) | .79 |
| Potassium-sparing diuretics (n, %) | 9 (6.2) | 6 (6.6) | 3 (5.5) | .78 |
| RAAS inhibitors (n, %) | 81 (55.5) | 43 (47.3) | 38 (69.1) | .01 |
| Ca blocker (n, %) | 91 (62.3) | 66 (72.5) | 25 (45.5) | .001 |
| Other antihypertensives (n, %) | 9 (6.2) | 7 (7.7) | 2 (3.6) | .32 |
| Potassium replacement (n, %) | 52 (35.6) | 44 (48.4) | 8 (14.5) | <.0001 |
Abbreviations: ARR = aldosterone/renin ratio; BMI = body mass index; DBP = diastolic blood pressure; DRC = direct renin concentration; eGFR = estimated glomerular filtration rate; PA = primary aldosteronism; PAC = plasma aldosterone concentration; PRA = plasma renin activity; SBP = systolic blood pressure.
Data are shown as median [interquartile range] or number (n) and %. Comparisons between groups were performed using the Mann Whitney U test for continuous variables or Chi-squared test for categorical variables.
In total, 91 (62%) patients were diagnosed with PA. At study entry, patients with PA and those with other forms of hypertension had similar ages, BMI, kidney function, and prevalence of diabetes, cardiovascular disease, and stroke (Table 1). Compared to PA patients, those without PA were more commonly women (62% vs. 40%; P = .009) and more frequently treated with RAAS inhibitors (69% vs. 47%; P = .01). Conversely, PA patients had higher rates of hypokalemia (62% vs. 27%; P<.0001) and suppressed renin (95% vs. 62%; P<.0001), and they were treated more often with calcium channel blockers (73% vs. 46%; P = .001).
Effects of MRA Therapy on the RAAS
The median duration of follow-up from the time of MRA initiation was 103 days, interquartile range (IQR): 48 to 208 days. The median dose of MRA used by the last follow-up visit was 25 (IQR: 25, 50) mg/day. Serum potassium levels increased from 3.8 (3.5, 4.2) to 4.3 (4.0, 4.7) mM (P<.0001), while the estimated glomerular filtration rate decreased from 83 (61, 97) to 70 (55, 90) mL/min/1.73 m2 (P<.0001). Overall, PAC, PRA, and DRC increased significantly after MRA initiation (PAC, from 19.1 [12.9, 27.7] to 26.4 [17.1, 42.3] ng/dL, P<.0001; PRA, from 0.5 [0.1, 0.8] to 1.2 [0.6, 4.8] ng/mL/hour, P<.0001; and DRC, from 3.6 [2.1, 8.2] to 7.5 [2.9, 29.1] pg/mL, P = .0001), while ARR decreased from 40.3 (18.5, 102.7) to 23.1 (8.6, 58.7) ng/dL per ng/mL/hour (P<.0001). Nevertheless, 52% of patients continued to have a suppressed renin by the end of follow-up after MRA initiation. Patients with hypokalemia at baseline (n = 41) had a significantly larger increase in PAC compared to eukalemic patients (11.3 [1.5, 28.4] vs. 4.5 [−0.95, 11.3] ng/dL; P = .03), while the changes of PRA and DRC were similar between groups.
Expectedly, PAC was higher and renin was lower in patients with PA than in those with essential hypertension (Table 1). These differences persisted during MRA treatment (PAC, 32.8 [21.3, 46.5] vs. 17.1 [11.7, 26.3] ng/dL; PRA, 0.8 [0.4, 1.8] vs. 4.8 [1.3, 7.3] ng/mL/hour; and ARR, 34.8 [16.6, 77.8] vs. 6.7 [2.1, 20.3] ng/dL per ng/mL/hour; P≤.01 for all, Fig. 2). Compared to baseline, however, the PRA increase was larger in patients with essential hypertension as compared to the PA group (P = .02, Fig. 2).
Fig. 2.

Comparison of plasma renin and aldosterone changes after mineralocorticoid receptor antagonist (MRA) initiation between patients with and without primary aldosteronism (PA). Changes of: (A) plasma aldosterone concentration (PAC); (B) plasma renin activity (PRA); (C) direct renin concentration (DRC); and (D) aldosterone-to-renin ratio (ARR) in patients with PA (white boxes) and without PA (gray boxes) from baseline to the last follow-up visit after initiation of MRA therapy. Number of patients: total PA patients, n = 91 (panels A and D); with PRA, n = 76; with DRC, n = 15: patients without PA, n = 55 (panels A and D); with PRA, n = 38; with DRC, n = 17. To calculate ARR in all cases, DRC was converted to PRA by dividing by 8. Comparisons are based on two-way analysis of variance tests. Significant interactions were found between the changes of PRA and ARR and the two groups (P = .02 and .006, respectively). a, P<.05; b, P<.01; c, P<.001; d, P<.0001. *, ng/dL per ng/mL/h.
Of all patients treated with MRA, 107 (73%) had additional changes in their antihypertensive regimen during the study period. In total, 63 patients were treated with beta-blockers, and 61 patients were treated with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs). The changes in PAC, PRA, and ARR were overall similar, regardless of other antihypertensive agents used (Table 2).
Table 2.
Impact of MRA Treatment on Plasma Renin and Aldosterone Concentrations Relative to Other Antihypertensive Agents
| A | AHT changes (n = 107) | No AHT changes (n = 39) | P value | |||
|---|---|---|---|---|---|---|
| Baseline | After MRA | Baseline | After MRA | a | b | |
| PAC | 19.8 [13.9, 27.9] | 27.2 [17.9, 45.5] | 16.5 [10.2, 26.8] | 21.2 [14.2, 39.0] | .0003 | .11 |
| PRA | 0.5 [0.1, 0.8] | 1.2 [0.6, 5.0] | 0.6 [0.1, 0.8] | 1.2 [0.6, 2.3] | .003 | .20 |
| ARR | 41.0 [19.5, 103.7] | 27.2 [7.4, 61.3] | 34.8 [16.1, 98.2] | 17.9 [8.7, 38.3] | .0009 | .27 |
| B | Beta-blocker use (n = 26) | No beta-blocker use (n = 13) | P value | |||
| Baseline | After MRA | Baseline | After MRA | a | b | |
| PAC | 22.2 [13.0, 29.0] | 23.9 [20.2, 40.1] | 15.2 [7.7, 18.7] | 14.4 [10.3, 27.4] | .004 | .34 |
| PRA | 0.6 [0.1, 1.0] | 0.9 [0.6, 2.1] | 0.5 [0.2, 0.7] | 1.2 [0.6, 3.0] | .003 | .54 |
| ARR | 45.7 [16.6, 125.0] | 24.0 [8.6, 70.5] | 31.2 [10.4, 52.4] | 15.8 [10.4, 20.1] | .03 | .11 |
| C | ACEI and ARB use (n = 20) | No ACEI and ARB use (n = 19) | P value | |||
| Baseline | After MRA | Baseline | After MRA | a | b | |
| PAC | 18.2 [9.7, 29.2] | 23.3 [12.7, 41.6] | 16.5 [10.4, 23.8] | 20.3 [15.1, 32.6] | .003 | .88 |
| PRA | 0.6 [0.2, 1.7] | 1.3 [0.6, 2.7] | 0.2 [0.1, 0.7] | 0.9 [0.3, 2.2] | .003 | .31 |
| ARR | 21.7 [10.8, 83.1] | 14.3 [2.4, 33.4] | 50.5 [27.1, 102.4] | 24.0 [14.5, 51.5] | .02 | .99 |
Abbreviations: ACEI = angiotensin-converting enzyme inhibitor; AHT = antihypertensive agent; ARB = angiotensin receptor blocker; ARR = aldosterone/renin ratio; MRA = mineralocorticoid receptor antagonist; PAC = plasma aldosterone concentration; PRA = plasma renin activity.
PAC (ng/dL), PRA (ng/mL/h), and ARR (ng/dL per ng/mL/h) are shown as median [interquartile range]. The number of cases with available PRA in patients with vs. no additional medication changes were: 84 and 30 in the panel A; 19 and 11 in the panel B; 15 and 15 in the panel C, respectively. Direct renin concentration (DRC) is not shown due to small number of patients per subgroup. In cases with DRC measurements, ARRs are calculated using a conversion factor of DRC/PRA of 8. Two-way repeated measures analyses of variance were used to assess the effect of MRA use (a) and subgroup (b) on PAC, PRA, and ARR. The interactions between MRA use and subgroup were not significant in all cases.
Temporal and Dose-Dependent Changes in Renin and Aldosterone Levels Following MRA Initiation
Following initiation of MRAs, renin and aldosterone were assessed at 1 month in 60 (41%) patients, at 3 to 6 months in 82 (56%) patients, and at 1 year in 30 (21%) patients. On average, PAC and PRA increased significantly by 1 month after MRA initiation (PAC, from 17.8 [12.6, 25.8] to 25.8 [18.1, 39.5] ng/dL, P<.0001; PRA, from 0.6 [0.2, 1.0] to 1.0 [0.6, 3.1] ng/mL/hour, P<.0001), while ARR decreased (from 38.0 [16.7, 83.9] to 30.2 [11.4, 77.1] ng/dL per ng/mL/hour, P<.05). Similar changes of renin, aldosterone, and ARR were also observed in patients evaluated at 3 to 6 and 12 months, respectively.
Based on the daily MRA dose used, patients were grouped into three tiers: <50 mg/day (80 patients); 50 to 100 mg/day (49 patients); and >100 mg/day (17 patients). PAC, PRA, and DRC increased, and ARR declined in all groups after MRA initiation (Fig. 3). The absolute change in PAC was significantly larger in patients taking >100 mg daily as compared to those taking <50 mg daily (11.3 [2.6, 57.8] vs. 4.5 [−2.4, 11.4] ng/dL; P=0.006). Similar, albeit nonsignificant, trends were observed for renin and ARR.
Fig. 3.

Dose-dependent changes of plasma renin and aldosterone during treatment with mineralocorticoid receptor antagonist (MRAs). Comparisons of: (A) plasma aldosterone concentration (PAC); (B) plasma renin activity (PRA); (C) direct renin concentration (DRC); and (D) aldosterone-to-renin ratio (ARR) between baseline and the last follow-up visit, divided into three groups based on the daily dose of MRAs: Group 1, <50 mg/day (total n = 80: PRA, n = 62; DRC, n = 18); Group 2, 50–100 mg/day (total n = 49: PRA, n = 37; DRC, n = 12); Group 3, >100 mg/day (total n = 17: PRA, n = 15; DRC, n = 2). To calculate ARR in all cases, DRC was converted to PRA by dividing by 8. Statistical significance based on the Wilcoxon signed rank test are shown as follows: a, P<.01; b, P<.001; c, P<.0001. * ng/dL per ng/mL/h.
MRA Therapy Impact on PA Screening
Of 94 patients with positive PA screening at baseline, positive screening criteria were no longer met at the last visit after MRA initiation in 45 patients (48%). Of these, PA was confirmed in 32 patients and excluded in 13 patients. Patients in whom MRA initiation altered the initial positive screening for PA had higher blood pressure and were treated with higher doses of MRA (Table 3A). Conversely, patients in whom PA screening remained positive despite MRA treatment were more often cases with confirmed PA and treated with beta-blockers than those in whom MRA treatment altered an initially positive PA screening result (Table 3A). In multiple logistic regression analysis, the use of beta-blockers and confirmed PA were associated with higher odds of persistent positive PA screening results following MRA initiation, while higher MRA doses were more likely to abrogate an initially positive PA screening test (Table 4).
Table 3.
Comparison of Baseline Characteristics Between Patients With and Without Changes in PA Screening After MRA Initiation
| A. Patients with positive PA screening at baseline | |||
|---|---|---|---|
| PA screening after MRA use | |||
| Variable | Negative | Persistent positive | P value |
| n | 45 | 49 | |
| Age (years) | 56 [47, 66] | 57 [47, 64] | .97 |
| Sex (n men, %) | 30 (66.7) | 29 (59.2) | .45 |
| BMI (kg/m2) | 34.7 [29.7, 38.8] | 32.6 [28.8, 36.2] | .12 |
| SBP (mm Hg) | 158 [143, 175] | 146 [122, 159] | .01 |
| DBP (mm Hg) | 88 [80, 98] | 81 [70, 88] | .003 |
| eGFR (mL/min/1.73 m2) | 88 [67, 101] | 81 [56, 90] | .44 |
| Serum potassium (mM) | 3.7 [3.5, 4.3] | 3.7 [3.5, 4.0] | .09 |
| PAC (ng/dL) | 18.8 [15.6, 27.3] | 24.1 [19.2, 32.4] | .03 |
| PRA (ng/mL/h) | 0.3 [0.1, 0.6] | 0.2 [0.1, 0.6] | .25 |
| DRC (pg/mL) | 2.1 [2.1, 5.1] | 2.1 [2.1, 3.2] | .69 |
| ARR (ng/dL per ng/mL/h) | 57.3 [35.8, 110.4] | 100.3 [42.5, 216.2] | .04 |
| PA cases (n, %) | 32 (71.1) | 45 (91.8) | .009 |
| Antihypertensive agents (n) | 3.0 [2.0, 4.0] | 3.0 [2.0, 4.0] | .25 |
| Beta-blocker (n, %) | 24 (53.3) | 39 (79.6) | .007 |
| MRA dose (mg/day) | 25.0 [25.0, 75.0] | 25.0 [25.0, 50.0] | .03 |
| MRA dose per body weight (mg/kg) | 0.37 [0.24, 0.67] | 0.29 [0.21, 0.48] | .06 |
| Duration of MRA treatment (days) | 113 [54, 330] | 101 [42, 204] | .26 |
| B. Patients with negative PA screening at baseline | |||
| Variable | Positive | Persistent negative | P value |
| n | 9 | 43 | |
| Age (years) | 51 [38, 67] | 55 [48, 64] | .75 |
| Sex (n men, %) | 3 (33.3) | 14 (32.6) | .96 |
| BMI (kg/m2) | 30.7 [28.9, 32.7] | 31.5 [27.2, 36.7] | .61 |
| SBP (mm Hg) | 164 [139, 174] | 150 [135, 171] | .59 |
| DBP (mm Hg) | 76 [75, 98] | 78 [70, 88] | .61 |
| eGFR (mL/min/1.73 m2) | 94 [58, 97] | 82 [73, 100] | .77 |
| Serum potassium (mM) | 3.4 [2.9, 4.0] | 4.1 [3.8, 4.4] | .009 |
| PAC (ng/dL) | 15.0 [9.7, 17.0] | 12.2 [6.7, 21.7] | .65 |
| PRA (ng/mL/h) | 1.0 [0.8, 1.1] | 1.0 [0.6, 3.6] | .57 |
| DRC (pg/mL) | 6.3 [3.0, 10.2] | 9.8 [6.6, 22.2] | .32 |
| ARR (ng/dL per ng/mL/h) | 16.0 [13.8, 23.1] | 11.4 [5.2, 19.4] | .12 |
| PA cases (n, %) | 5 (55.6) | 9 (20.9) | .03 |
| Antihypertensive agents (n) | 3.0 [1.8, 3.3] | 2.0 [1.0, 3.8] | .97 |
| Beta-blocker (n, %) | 6 (66.7) | 20 (46.5) | .27 |
| MRA dose (mg/day) | 50.0 [25.0, 50.0] | 25.0 [25.0, 50.0] | .49 |
| MRA dose per body weight (mg/kg) | 0.48 [0.36, 0.75] | 0.40 [0.23, 0.65] | .39 |
| Duration of MRA treatment (days) | 111 [91, 205] | 79 [46, 150] | .054 |
Abbreviations: ARR = aldosterone/renin ratio; BMI = body mass index; DBP = diastolic blood pressure; DRC = direct renin concentration; eGFR = estimated glomerular filtration rate; MRA = mineralocorticoid receptor antagonist; PA = primary aldosteronism; PAC = plasma aldosterone concentration; PRA = plasma renin activity; SBP = systolic blood pressure.
Data are shown as median [interquartile range] or number (n) and %. Comparisons between groups were performed using the Mann Whitney U test for continuous variables or Chi-squared or Fisher’s exact test for categorical variables.
Table 4.
Factors Associated With Alterations of an Initially Positive PA Screening Test by MRA Therapy
| Variable | β | SE (β) | P | Odds ratio | 95% CI |
|---|---|---|---|---|---|
| Beta-blocker use | −1.76 | 0.55 | .001 | 0.17 | 0.06–0.50 |
| PA | −2.35 | 0.72 | .001 | 0.10 | 0.02–0.39 |
| MRA dose (mg/day) | 0.03 | 0.01 | .01 | 1.03 | 1.01–1.06 |
Abbreviations: CI = confidence interval; MRA = mineralocorticoid receptor antagonist; PA = primary aldosteronism; SE = standard error.
Notably, of 52 patients with negative PA screening at baseline, 9 patients (17%) had subsequent positive PA screening after MRA initiation (Table 3B). Of these, 5 patients had hypokalemia at baseline, which normalized during MRA treatment. Overall, PAC increased during MRA therapy in these 9 patients (from 15.0 [9.7, 17.0] to 21.0 [17.9, 30.6] ng/dL; P = .004), while renin remained relatively stable. Compared to patients who continued to have negative PA screening results after MRA initiation, patients with subsequent positive screening were more often confirmed PA cases (Table 3B).
DISCUSSION
Although PA is the most common form of secondary hypertension, this curable disorder remains grossly under-diagnosed (21–23). Practical aspects that discourage PA screening include the concern for inaccurate results while taking several classes of antihypertensive agents that can interfere with the RAAS, and the high risk of hypertension-related complications and hypokalemia when such medications are discontinued. MRAs are the most effective medical treatment for PA (4,8,24). Moreover, MRAs are the most effective add-on agents in patients with resistant hypertension (14), and they are recommended by the guidelines from European Society of Cardiology-European Society of Hypertension and American Heart Association in these patients (11,12). Several lines of evidence suggest that MRAs attenuate inflammation, fibrosis, and endothelial dysfunction mediated by mineralocorticoid receptors activation, preventing end-organ damage (25,26). We and others have shown that in patients with unequivocal PA, MRAs do not typically interfere with diagnostic and subtyping tests (18,27,28). Because early detection and treatment of PA is critical for mitigating the associated cardio-renal morbidity (4,5), we herein aimed to assess the impact of MRA use on PA screening when including all patients with hypertension treated with these agents. We found an overall elevation of both aldosterone and renin and a consequential decrease in the ARR. As a result, a large proportion (48%) of positive screening tests became negative following MRA initiation. Conversely, ARR increased during treatment with MRAs in a smaller group of patients, most often due to resolution of baseline hypokalemia.
Renin secretion is regulated by renal artery perfusion and sodium delivery. By reducing renal sodium reabsorption and causing mild diuresis, MRAs indirectly stimulate renin secretion and activate the RAAS cascade, including aldosterone elevation. Indeed, we found that, on average, both plasma renin and aldosterone increased following MRA initiation, and this effect was visible as early as within 1 month of therapy, remaining relatively stable up to 1 year into MRA treatment. Moreover, such effects were observed both in patients with and without PA. PRA, however, demonstrated a more subtle elevation in patients with PA than in those with other forms of hypertension, suggestive of residual renin suppression by autonomous aldosterone synthesis in the former group. In a recent prospective study of 42 patients with confirmed PA, aldosterone and renin remained overall unaltered by canrenone, which was administered at doses between 50 and 100 mg daily (18). Conversely, in our study, elevations of aldosterone and renin were observed even at modest doses of spironolactone or eplerenone, and they were more pronounced when high MRA doses were used. Canrenone is one of the major active metabolites of spironolactone, but it has only a third of the pharmacologic potency of spironolactone (29,30). The renin elevations observed in our study could be partially attributed to 7α-thiomethylspironolactone, a second active metabolite of spironolactone (31). In addition, we found that, expectedly, renin suppression was more resistant to MRA treatment in patients with robust PA, as were those recruited in the EMIRA study. Patients with severe PA often require large doses of MRA (2,32). Indeed, PA diagnosis and lateralization have been shown to often remain positive in patients with persistent renin suppression during MRA use, indicative of incomplete MR blockade (27,28). Nevertheless, our results indicate that in many patients with modest ARR, treatment with MRA can abolish an initially positive PA screening test. Thus, in patients with borderline screening results while taking MRAs, repeating the test after MRA discontinuation should be considered in order to capture patients with milder forms of PA.
MRAs are rarely used as first-line therapy for hypertension. In our study, only 5% of all hypertensive patients were prescribed an MRA, which is comparable to other populational studies (33,34). Thus, the vast majority of patients in this study were also treated with other medications with RAAS-interfering potential, such as beta-blockers, ACEIs/ARBs, and diuretics. While beta-blockers are known to inhibit renin release, variability has been reported regarding their impact on aldosterone (8,35,36). We found that the use of beta-blockers was associated with lower odds of abrogating an initially positive PA screening result by MRAs. Conversely, ACEIs, ARBs, and diuretics commonly lead to renin elevations (8,9,36). Nevertheless, the effects of MRAs on aldosterone and renin were overall similar in patients who received other antihypertensive agents, and regardless of other changes in the antihypertensive regimens during the follow-up period.
In addition to the many cases in which positive PA screening was annulled by MRA use, we have also identified a small number of patients in whom PA screening was positive only after initiation of MRAs. Over half of these patients eventually had confirmed PA, and their initial negative screening occurred in the context of hypokalemia. Serum potassium is a direct regulator of aldosterone secretion, and elevations or falls in serum potassium concentrations enhance or suppress aldosterone production, respectively (8,37,38). Maintenance of eukalemia is, thus, essential for accurate interpretation of renin and aldosterone during PA diagnosis and subtyping. In addition, MRAs could lead to aldosterone elevations via paracrine mechanisms. Mineralocorticoid receptor blockade has been reported to directly regulate the activity of aldosterone synthase in the zona glomerulosa and enhance both basal and angiotensin II–stimulated aldosterone production in rodents (39).
The main limitations of our study are its retrospective design, variability in follow-up and concomitant medications, and lack of confirmatory PA tests in a subgroup of patients. In addition, most patients were treated with relatively low doses of MRAs. Despite these limitations reflective of the real-world practice, we observed that MRA initiation was generally followed by disproportionate elevations of renin, leading to lower ARR and obliteration of positive PA screening in almost half of the patients. We also identified a small number of PA cases who became apparent only after hypokalemia was corrected by MRAs. Our findings support previous reports suggesting that in patients with difficult to control hypertension and/or hypokalemia, initial PA screening can be performed while taking MRAs (2). Nevertheless, to ensure accurate PA screening results, particularly so in patients who might be at the mild end of the PA spectrum (40), temporary discontinuation of MRAs should be attempted whenever possible.
ACKNOWLEDGMENT
We thank the University of Michigan Medical School Research Data Warehouse and DataDirect for providing data aggregation and management services in support of the research reported in this publication.
A.F.T. was supported by grants 1K08DK109116 from the NIDDK, DDCF_2019087 from the Doris Duke Charitable Foundation, and U070002 from the Michigan Institute for Clinical & Health Research.
Abbreviations:
- ACEI
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- ARR
aldosterone/renin ratio
- DRC
direct renin concentration
- MRA
mineralocorticoid receptor antagonist
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- PRA
plasma renin activity
- RAAS
renin-angiotensin-aldosterone system
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39:1057–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrd JB, Turcu AF, Auchus RJ. Primary aldosteronism: practical approach to diagnosis and management. Circulation. 2018;138:823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monticone S, D’Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50. [DOI] [PubMed] [Google Scholar]
- 4.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638–2645. [DOI] [PubMed] [Google Scholar]
- 7.Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. [DOI] [PubMed] [Google Scholar]
- 8.Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. [DOI] [PubMed] [Google Scholar]
- 9.Stowasser M, Ahmed AH, Pimenta E, Taylor PJ, Gordon RD. Factors affecting the aldosterone/renin ratio. Horm Metab Res. 2012;44:170–176. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism--the Japan Endocrine Society 2009. Endocr J. 2011;58:711–721. [DOI] [PubMed] [Google Scholar]
- 11.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 12.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 13.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 14.Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386: 2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger MH, Roniker B, Krause SL, Weiss RJ. Eplerenone, a selective aldosterone blocker, in mild-to-moderate hypertension. Am J Hypertens. 2002;15:709–716. [DOI] [PubMed] [Google Scholar]
- 17.Pilz S, Trummer C, Verheyen N, et al. Mineralocorticoid receptor blockers and aldosterone to renin ratio: a randomized controlled trial and observational data. Horm Metab Res. 2018;50:375–382. [DOI] [PubMed] [Google Scholar]
- 18.Rossi GP, Ceolotto G, Rossitto G, Maiolino G, Cesari M, Seccia TM. Effects of mineralocorticoid and AT1 receptor antagonism on the aldosterone-renin ratio in primary aldosteronism-the EMIRA Study. J Clin Endocrinol Metab. 2020;105.dgaa080. [DOI] [PubMed] [Google Scholar]
- 19.Kheterpal S RDW/DataDirect: a self-serve tool for data retrieval. 2015; University of Michigan, Ann Arbor, MI. [Google Scholar]
- 20.Nanba AT, Nanba K, Byrd JB, et al. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf). 2017;87:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funder JW. Primary aldosteronism. Hypertension. 2019;74: 458–466. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe G, Gray Z, Krishnan G, et al. Screening rates for primary aldosteronism in resistant hypertension: a cohort study. Hypertension. 2020;75:650–659. [DOI] [PubMed] [Google Scholar]
- 23.Heinrich DA, Adolf C, Rump LC, et al. Primary aldosteronism: key characteristics at diagnosis: a trend toward milder forms. Eur J Endocrinol. 2018;178:605–611. [DOI] [PubMed] [Google Scholar]
- 24.Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126–148. [DOI] [PubMed] [Google Scholar]
- 25.Chrissobolis S Vascular consequences of aldosterone excess and mineralocorticoid receptor antagonism. Curr Hypertens Rev. 2017;13:46–56. [DOI] [PubMed] [Google Scholar]
- 26.Parviz Y, Iqbal J, Pitt B, Adlam D, Al-Mohammad A, Zannad F. Emerging cardiovascular indications of mineralocorticoid receptor antagonists. Trends Endocrinol Metab. 2015;26:201–211. [DOI] [PubMed] [Google Scholar]
- 27.Nanba AT, Wannachalee T, Shields JJ, et al. Adrenal vein sampling lateralization despite mineralocorticoid receptor antagonists exposure in primary aldosteronism. J Clin Endocrinol Metab. 2019;104:487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haase M, Riester A, Kröpil P, et al. Outcome of adrenal vein sampling performed during concurrent mineralocorticoid receptor antagonist therapy. J Clin Endocrinol Metab. 2014;99:4397–4402. [DOI] [PubMed] [Google Scholar]
- 29.Ramsay LE, Shelton JR, Wilkinson D, Tidd MJ. Canrenone-the principal active metabolite of spironolactone? Br J Clin Pharmacol. 1976;3:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsay L, Shelton J, Harrison I, Tidd M, Asbury M. Spironolactone and potassium canrenoate in normal man. Clin Pharmacol Ther. 1976;20:167–177. [DOI] [PubMed] [Google Scholar]
- 31.Kolkhof P, Borden SA. Molecular pharmacology of the mineralocorticoid receptor: prospects for novel therapeutics. Mol Cell Endocrinol. 2012;350:310–317. [DOI] [PubMed] [Google Scholar]
- 32.Parthasarathy HK, Ménard J, White WB, et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29: 980–990. [DOI] [PubMed] [Google Scholar]
- 33.Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of apparent treatment-resistant hypertension in the United States. Hypertension. 2019;73:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang AY, Dave C, Smith SM. Trends in antihypertensive medication use among US patients with resistant hypertension, 2008 to 2014. Hypertension. 2016;68:1349–1354. [DOI] [PubMed] [Google Scholar]
- 35.Browne GA, Griffin TP, O’Shea PM, Dennedy MC. b-Blocker withdrawal is preferable for accurate interpretation of the aldosterone-renin ratio in chronically treated hypertension. Clin Endocrinol (Oxf). 2016;84:325–331. [DOI] [PubMed] [Google Scholar]
- 36.Mulatero P, Rabbia F, Milan A, et al. Drug effects on aldosterone/plasma renin activity ratio in primary aldosteronism. Hypertension. 2002;40:897–902. [DOI] [PubMed] [Google Scholar]
- 37.Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Himathongkam T, Dluhy RG, Williams GH. Potassim-aldosterone-renin interrelationships. J Clin Endocrinol Metab. 1975;41:153–159. [DOI] [PubMed] [Google Scholar]
- 39.Chong C, Hamid A, Yao T, et al. Regulation of aldosterone secretion by mineralocorticoid receptor-mediated signaling. J Endocrinol. 2017;232:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JM, Robinson-Cohen C, Luque-Fernandez MA, et al. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med. 2017;167:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]


