Abstract
Background
Marigold (Tagetes erecta L) accounts for over half of the world’s loose flower production, and marigold crop residue (MCR) are abundantly available and should be used as a forage. In this study, MCR from the last commercial flower pickings was ensilaged with lactic acid bacteria (LAB) and the shift in their volatile organic compounds (VOCs) profiles was monitored. Samples were collected at 6 different times during ensilage (3, 6, 9, 12, 15, 30 days) to determine and quantify the VOCs changes using a solid-phase microextraction (SPME) technique and gas chromatography – mass spectrometry (GC-MS).
Results
After 30 days, the caryophyllene and piperitone, which account for 14.7 and 12.1% of total VOCs, decreased by 32.9 and 9.6% respectively, alcohols increased from 2.8 to 8.1%, and the acetic acid content increased by 560%.
Conclusion
We have confirmed LAB can degrade the content of terpenes and enhance the content of alcohols and acids in MCR, which was for the first time on terpene degradation in fodder by ensilage. These results have shed light on our understanding of how to improve fodder odor and to enhance terpene degradation by lactic acid bacteria fermentation.
Keywords: Biodegradation, Marigold, Terpenes, Volatile organic compounds
Background
Marigold (Tagetes erecta L) is one of the most widely cultivated commercial flower crops in the world and accounts for over half of the world’s loose flower production [1]. Since the harvest takes only the flower (used to extract lutein), a large number of marigold residues were randomly discarded. In fact, the crude protein content in the stem of marigold can reach 26.53%, and the content in the leaf is 6.97%, the crude fat content in the stem is nearly double that in the leaf, which can reach 5%. The crude fiber content is 35.09%, while the crude fiber content in the stem is less than 10%, and the stems and leaves are rich in a variety of amino acids [2]. Therefore, marigold crop residue (MCR) should be used as a forage for its high nutritional value and abundantly available. However, studies on the volatile substances in the flowers and leaves of marigolds have indicated that there is a large proportion of various terpenoids, which produces the terpenes of volatile organic compounds (VOCs) would rejected by cattle [3–5].
All domestic mammals have an acute sense of smell, and aroma is one of the most important factors that influencing feed acceptance and intake in cattle [6]. The influence of a specific volatile compound on the final aroma depends on its concentration in fodder and its perception threshold [7–9]. How to reduce the concentrations of main terpenes in total VOC is therefore the key to using MCR as a forage.
Although the VOC terpene level can be reduced through physical and chemical treatments [10, 11], those may cause other issues such as loss of nutritional value, palatability, and safety of such feeds. Another approach to reduce terpene levels is to use biodegradation and biotransformation by microorganisms. Fungi, yeasts, bacteria, cyanobacteria, microalgae, enzymes, plants, and animal cells have all been used in the biodegradation or biotransformation of terpenes [12, 13], however, despite the relative safety of microorganisms and enzymes, only a limited number have been used as feed additives.
In forage processing, ensilage can improve forage palatability and preservation. Ensilage relies mainly on lactic acid bacteria (LAB) fermentation to convert water-soluble carbohydrates into organic acids, and LAB have been widely used as feed additives [14, 15]. The combination of an acidic environment and the microbial fermentation process may synergistically degrade and/or produce new volatiles, and will often produce alcohols and acids which can make the fodder aroma acidic, fragrant, and alcoholic [14, 15] . LAB can also be used in biodegrading and biotransforming terpenes in food fermentation and brewing [16–19].
To the best of our knowledge, there is limited research about the VOC of fodder, and no studies to date on terpene degradation in fodder by ensilage. In order to address this, this study took samples of MCR silage with LAB and used solid-phase microextraction (SPME) and gas chromatography – mass spectrometry (GC-MS) methods to determine and quantify the changes of VOCs over time. The objective of this study was to relate the VOCs changes to ensilage times, and investigate the suitable length of ensilage needed to reduce terpenes while enhancing alcohols and acids to ensure good silage quality.
Results
Analysis of fresh MCR (CK) showed that the main VOCs were terpenes, which accounted for 63.5% of the 60 VOCs found. Fresh MCR also contained aldehydes (11.35%), ketones (4.61%), esters (3.81%), alcohols (2.8%), alkenes (2.12%), benzenes (1.33%), acids (0.57%), furans (0.56%) phenols (0.48%), and alkanes (0.26%), with other VOCs accounting for the remaining 0.83% (Table. 1).
Table 1.
Changes in the VOCs (%) of terpenes of MCR ensilage with Lactic acid over time
| No | Compounds | CAS | 0 day (cK) | 3 day | 6 day | 9 day | 12 day | 15 day | 30 day |
|---|---|---|---|---|---|---|---|---|---|
| Terpenes | |||||||||
| 1 | (+)-α-pinene | 7785-70-8 | 0.19 ± 0.02A | 0.13 ± 0.01AB | 0.09 ± 0.05AB | 0.09 ± 0.05AB | 0.05 ± 0.05AB | ND | 0.03 ± 0.03B |
| 2 | tricyclo[2.2.1.0(2,6)]heptane, 1,7,7-trimethyl- | 508–32-7 | 0.17 ± 0a | 0.09 ± 0.05abc | 0.05 ± 0.05bc | 0.04 ± 0.04bc | 0.13 ± 0.01ab | 0.1 ± 0.05abc | ND |
| 3 | Linalool | 78–70-6 | 0.57 ± 0.01C | 0.81 ± 0.08 BC | 0.97 ± 0.11B | 0.89 ± 0.04 BC | 0.92 ± 0.05 BC | 1.05 ± 0.04B | 1.42 ± 0.15A |
| 4 | terpinen-4-ol | 562–74-3 | 0.47 ± 0.11ab | 0.65 ± 0.18a | 0.5 ± 0.26ab | 0.84 ± 0.18a | 0.31 ± 0.31ab | ND | 0.01 ± 0.01b |
| 5 | Copaene | 3856-25-5 | 0.08 ± 0.04b | 0.12 ± 0.06ab | 0.1 ± 0.05ab | 0.12 ± 0.06ab | 0.17 ± 0ab | 0.05 ± 0.05b | 0.24 ± 0.01a |
| 6 | beta-elemene | 515–13-9 | 0.18 ± 0.11a | 0.21 ± 0.01a | 0.15 ± 0.08a | 0.11 ± 0.11a | 0.12 ± 0.06a | 0.11 ± 0.05a | 0.08 ± 0.08a |
| 7 | (−)-alpha-gurjunene | 489–40-7 | 0.79 ± 0.04AB | 0.84 ± 0.07AB | 0.8 ± 0.06AB | 0.65 ± 0.04B | 0.69 ± 0.1B | 0.86 ± 0.08AB | 1.13 ± 0.1A |
| 8 | Caryophyllene | 87–44-5 | 14.67 ± 0.45A | 15.7 ± 0.2A | 14.19 ± 0.53A | 15.24 ± 0.09A | 15.21 ± 0.08A | 14.62 ± 0.45A | 9.85 ± 0.26B |
| 9 | cis-a-bergamotene | 18,252–46-5 | 0.47 ± 0.02a | 0.38 ± 0.19a | 0.2 ± 0.17a | 0.21 ± 0.21a | 0.26 ± 0.15a | 0.56 ± 0.28a | 0.42 ± 0.22a |
| 10 | cis-β-farnesene | 28,973–97-9 | 9.46 ± 0.18AB | 13.46 ± 1.66A | 14.03 ± 1.44A | 11.54 ± 0.95AB | 11.11 ± 0.96AB | 14.04 ± 1.42A | 7.97 ± 0.32B |
| 11 | 2-epi-trans-β-caryophyllene | 68,832–35-9 | 0.24 ± 0.03b | 0.69 ± 0.11a | 0.6 ± 0.22ab | 0.45 ± 0.04ab | 0.44 ± 0.04ab | 0.53 ± 0.18ab | 0.24 ± 0.12b |
| 12 | (1e,4e)-germacrene b | 15,423–57-1 | 0.9 ± 0.03 BC | 1.93 ± 0.12A | 1.49 ± 0.13AB | 1.48 ± 0.08AB | 1.6 ± 0.15AB | 0.93 ± 0.23 BC | 0.26 ± 0.26C |
| 13 | (1 s,2e,6e,10r)-3,7,11,11-tetramethylbicyclo[8.1.0]undeca-2,6-diene | 24,703–35-3 | 0.6 ± 0.06A | 0.32 ± 0.07B | 0.11 ± 0.02C | ND | ND | ND | ND |
| 14 | ß-longipinene | 41,432–70-6 | 0.24 ± 0.09b | 0.46 ± 0.06ab | 0.39 ± 0.1ab | 0.56 ± 0.19ab | 0.6 ± 0.12a | 0.25 ± 0.05ab | 0.29 ± 0.06ab |
| 15 | 2-carene | 554–61-0 | 0.17 ± 0.02A | ND | ND | ND | ND | ND | ND |
| 16 | naphthalene, 1,2,3,5,6,7,8,8a-octahydro-1,8a-dimethyl-7-(1-methylethenyl)-, [1 s-(1a,7a,8aa)]- | 10,219–75-7 | 0.09 ± 0.09b | 0.33 ± 0.02a | 0.18 ± 0.09ab | 0.23 ± 0.04av | 0.22 ± 0.06ab | 0.19 ± 0.05ab | 0.18 ± 0.05ab |
| 17 | (−)-germacrene-d | 317,819–80-0 | ND | 1.95 ± 0.18A | 0.97 ± 0.31ABC | 1.43 ± 0.49AB | 1.58 ± 0.36AB | 0.35 ± 0.14 BC | 0.8 ± 0.04ABC |
| 18 | (−)-spathulenol | 77,171–55-2 | ND | 0.13 ± .0.01a | 0.15 ± .0.02a | 0.16 ± .0.01a | 0.15 ± 0.02a | 0.13 ± .0.03a | 0.15 ± 0.02a |
| 19 | Junenol | 472–07-1 | ND | 0.2 ± 0.03CD | 0.21 ± 0.01CD | 0.42 ± 0.03 BC | 0.45 ± 0.01B | 0.48 ± 0.01B | 1.1 ± 0.13A |
| 20 | Carveol | 99–48-9 | ND | ND | ND | ND | 0.37 ± 0.19ab | 0.17 ± 0.17ab | 0.07 ± 0.07b |
| 21 | Isothujol | 7712-79-0 | ND | ND | ND | ND | ND | 0.05 ± 0.05B | 0.34 ± 0.01A |
| 23 | (−)-α-thujone | 33,766–30-2 | ND | ND | ND | ND | ND | ND | 0.07 ± 0.01A |
| 24 | (−)-.β.-bourbonene | 5208-59-3 | ND | ND | ND | ND | ND | ND | 0.14 ± 0.01A |
| 25 | (s)-β-bisabolene | 495–61-4 | ND | ND | ND | ND | ND | ND | 0.14 ± 0.02A |
| 26 | Nerolidol | 7212-44-4 | ND | ND | ND | ND | ND | ND | 0.89 ± 0.11A |
| 27 | 3,7,11,15-tetramethyl-2-hexadecen-1-ol | 102,608–53-7 | ND | ND | ND | ND | ND | ND | 0.24 ± 0.07A |
| 28 | Isoneral | 72,203–97-5 | ND | ND | ND | ND | ND | 0.15 ± 0.01B | 0.27 ± 0.03A |
| 29 | (±)-trans-nerolidol | 40,716–66-3 | ND | ND | ND | ND | ND | ND | 0.88 ± 0.07A |
| 30 | bicyclo[3.1.1] heptane, 6,6-dimethyl-2-methylene-, (1 s)- | 18,172–67-3 | 3.01 ± 0.27A | 0.96 ± 0.15 BC | 1.18 ± 0.07 BC | 1 ± 0.2 BC | 1.24 ± 0.08 BC | 1.21 ± 0.04 BC | 0.56 ± 0.05 BC |
| 31 | terpinolene | 586–62-9 | 8.24 ± 0.25A | 7.72 ± 0.2AB | 7.15 ± 0.18ABC | 6.8 ± 0.15 BC | 7.18 ± 0.59ABC | 6.27 ± 0.05C | 4.62 ± 0.15D |
| 32 | d-limonene | 5989-27-5 | 5.56 ± 0.14A | 3.55 ± 0.13B | 3.49 ± 0.24 BC | 3.32 ± 0.12 BC | 3.44 ± 0.22 BC | 3.3 ± 0.05 BC | 2.57 ± 0.36C |
| 33 | ß-ocimene | 13,877–91-3 | 4.92 ± 0.31A | 4.2 ± 0.23A | 4.07 ± 0.24A | 3.98 ± 0.27A | 4.43 ± 0.32A | 4.21 ± 0.22A | 2.38 ± 0.27B |
| 34 | Piperitone | 89–81-6 | 12.17 ± 0.11a | 9.93 ± 0.47b | 10.08 ± 0.68b | 10.76 ± 0.32ab | 10.16 ± 0.31b | 10.51 ± 0.24b | 10.1 ± 0.7b |
| 35 | Sabinene | 3387-41-5 | 0.31 ± 0.06A | ND | ND | ND | ND | ND | ND |
CAS Chemical Abstract Service, ND Not detected, experiments were performed in triplicate and shown are the means ± S.D., different uppercase letters in the same column correspond to significance at the 0.01 level, and different lowercase letters correspond to a significant difference at the 0.05 level (Duncan’s new multiple range test). The same as Table 2
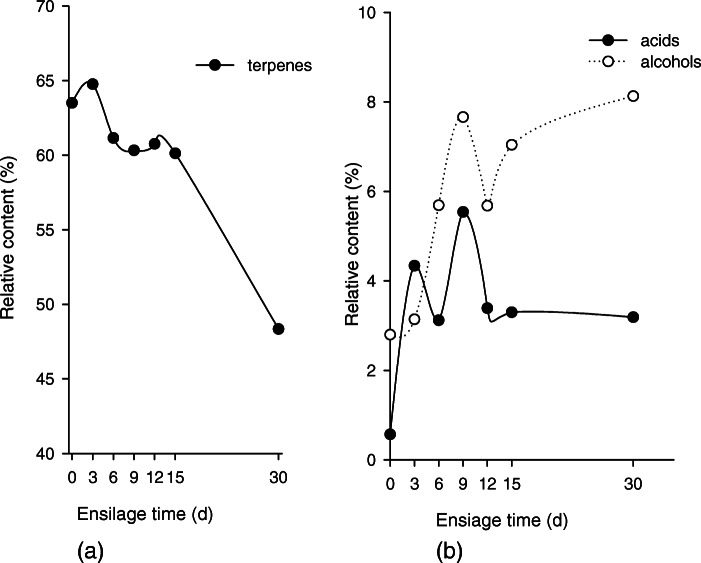
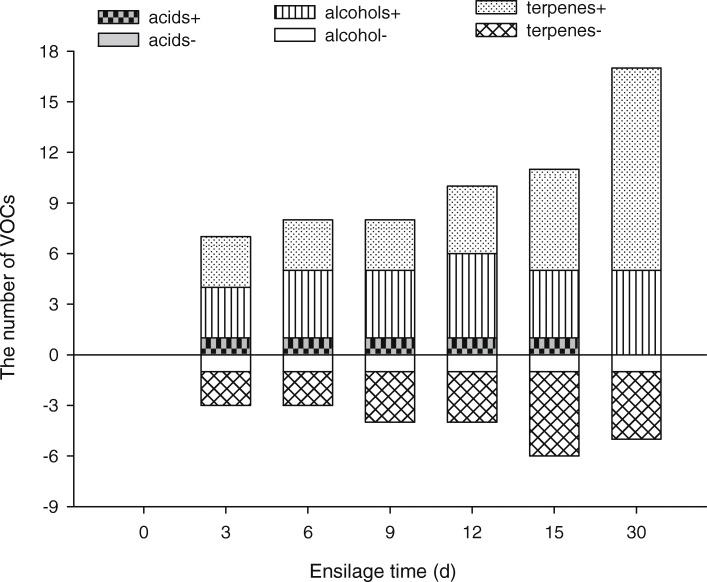
The differences in terpene levels between fresh MCR and MCR at different silage treatment times are shown in Table. 1. The levels of caryophyllene, the main VOC which accounted for 14.67% of total VOCs, were relatively stable on days 3, 6, 9, 12, 15 (P > 0.01), but on day 30 had decreased by 32.86% (P < 0.01). Another main VOC, piperitone, which accounted for 12.17% of total VOCs, declined on days 3, 6, 9, 12, 15, and 30 by 18.41% (P < 0.05), 17.17% (P < 0.05), 11.59% (P < 0.05) and 16.52% (P < 0.05), 13.64% (P < 0.05), and 17.01% (P < 0.05) respectively. Compared with the fresh MCR group, levels of caryophyllene, the most abundant constituent of total VOCs, decreased noticeably on day 30 by 32.86% (P < 0.01), but not at any other time during ensilage. While the levels of some terpenes that accounted for a small amount of total VOCs increased with silage time, overall the total amount of terpene VOCs decreased by 33.87% after 30 days (Fig. 1a). As ensilage progressed, not only did some terpenes disappear, new terpenes were produced, among those, 12 of which are exclusively found after ensilage. Many of the newly generated VOCs were produced on the 30th day of ensilage (Fig. 2).
Fig. 1.
Changes in terpene (a) and alcohols and acids (b) content in MCR during ensilage
Fig. 2.
Changes in number of main VOCs in MCR during ensilage. + represents the VOCs that appeared, − represents the VOCs that disappeared
The alcohol levels of fresh MCR and MCR at different ensilage times are shown in Table 2. After 30 days of ensilage, the content of the original alcohols did not significantly change, and only one of the alcohols disappeared. This study found that 6 alcohols appeared after ensilage (Fig. 2). Compared with other alcohols, (3-Methyl-oxiran-2-yl)-methanol was the most abundant alcohol found in silage, accounting for 4.14% of total VOCs. After 30 days’ ensilage the total amount of alcohols in VOCs increased from 2.8 to 8.13% (Fig. 1 b).
Table 2.
Changes in the VOCs (%) of alcohols and acids of MCR ensilage with Lactic acid over time
| No | Compounds | CAS | 0 day (cK) | 3 day | 6 day | 9 day | 12 day | 15 day | 30 day |
|---|---|---|---|---|---|---|---|---|---|
| Alcohols | |||||||||
| 36 | (2r,3r)-(−)-2,3-butanediol | 24,347–58-8 | 0.04 ± 0.04a | 0.38 ± 0.26a | 0.21 ± 0.13a | 0.56 ± 0.34a | 0.8 ± 0.11a | 0.25 ± 0.05a | 0.21 ± 0.02a |
| 37 | leaf alcohol | 928–96-1 | 1.42 ± 0.19A | 0.46 ± 0.16B | 0.27 ± 0.04B | 0.32 ± 0.06B | 0.33 ± 0.07B | 0.13 ± 0.07B | 0.09 ± 0.09B |
| 38 | 1-hexanol | 111–27-3 | 0.42 ± 0.07A | 0.21 ± 0.05B | 0.18 ± 0.02B | 0.18 ± 0.01B | 0.17 ± 0.02B | 0.15 ± 0.01B | 0.04 ± 0.04B |
| 39 | 9-oxabicyclo[6.1.0]nonan-4-ol | 69,853–85-6 | 0.03 ± 0.02 | ND | ND | ND | ND | ND | ND |
| 40 | phenylethyl alcohol | 60–12-8 | 0.49 ± 0.27a | 0.49 ± 0.39a | 0.33 ± 0.33a | 0.58 ± 0.19a | 0.66 ± 0.14a | 1.02 ± 0.15a | 0.36 ± 0.19a |
| 41 | trans-chrysanthenol | 38,043–83-3 | 0.15 ± 0.01C | 0.25 ± 0.02B | 0.27 ± 0.02B | 0.27 ± 0.01B | 0.27 ± 0.01B | 0.31 ± 0.01B | 0.39 ± 0.02A |
| 42 | 2-(4-methylphenyl)propan-2-ol | 1197-01-9 | 0.25 ± 0.02 a | 0.3 ± 0.03a | 0.37 ± 0.05a | 0.34 ± 0.05a | 0.35 ± 0.15a | 0.34 ± 0.21a | 0.62 ± 0.08b |
| 43 | (3-methyl-oxiran-2-yl)-methanol | 872–38-8 | ND | 0.48 ± 0.22CD | 2.82 ± 0.56AB | 2.18 ± 0.59ABC | 1.7 ± 0.68BCD | 3.25 ± 0.1AB | 4.14 ± 0.43A |
| 44 | 3-methylcyclopentane-1,2-diol | 27,583–37-5 | ND | ND | ND | ND | 0.11 ± 0.06A | ND | ND |
| 45 | benzyl alcohol | 100–51-6 | ND | 0.38 ± 0.15a | 0.44 ± 0.2a | 0.27 ± 0.02ab | 0.23 ± 0.03ab | 0.26 ± 0.05ab | 0.38 ± 0.07a |
| 46 | (+)-maaliol | 527–90-2 | ND | 0.19 ± 0.02 BC | 0.22 ± 0.05 BC | 0.32 ± 0.04B | 0.39 ± 0.04B | 0.33 ± 0.03B | 0.65 ± 0.11A |
| 47 | 2-butanol | 15,892–23-6 | ND | ND | 0.58 ± 0.04a | 2.64 ± 2.26a | 0.56 ± 0.06a | 1 ± 0.17a | 1.02 ± 0.26a |
| 48 | 3-methylcyclopentane-1,2-diol | 27,583–37-5 | ND | ND | ND | ND | 0.11 ± 0.06A | ND | ND |
| 49 | decan-1-ol | 112–30-1 | ND | ND | ND | ND | ND | ND | 0.23 ± 0.01A |
| Acids | |||||||||
| 50 | acetic acid | 64–19-7 | 0.57 ± 0.01b | 4.18 ± 1.40a | 2.98 ± 1.03ab | 5.37 ± 1.50a | 3.17 ± 0.33ab | 3.27 ± 0.43a | 3.19 ± 0.37ab |
| 51 | cyclohexanebutanoic acid | 4441-63-8 | ND | 0.16 ± 0.09a | 0.14 ± 0.09a | 0.17 ± 0.09a | 0.22 ± 0.08a | 0.03 ± 0.01a | ND |
Only two acids, cyclohexanebutanoic acid and acetic acid, were present during the whole fermentation process (Table. 2). The cyclohexanebutanoic acid content was very low, and was detected on only the third to fifteenth day of ensilage, while the acetic acid accounted for 0.57% of total VOCs in fresh MCR, but increased by 560% (P < 0.05) after ensilage.
Ensilage not only changed the levels of terpenes, alcohols, and acids in the MCR, but also changed the volatile profile of other quantitative and qualitative compounds (Table 3). On day 30, declines in the total aldehyde (14.45%) and benzene (53.38%) VOCs, and increases in the total VOCs of esters (60.63%), phenols (454.17%), alkanes (434.62%), ketones (47.36%), alkenes (74.06%), furans (103.57%), and miscellaneous compounds (174.70%) were observed.
Table 3.
Changes in the VOCs (%) of others of MCR ensilage with Lactic acid over time
| No | Compounds | 0 day (cK) | 3 day | 6 day | 9 day | 12 day | 15 day | 30 day |
|---|---|---|---|---|---|---|---|---|
| 1 | Aldehydes | 11.35 | 6.23 | 6.61 | 7.05 | 6.42 | 7.48 | 9.71 |
| 2 | Phenols | 0.48 | 0.88 | 1.16 | 1.31 | 1.6 | 1.81 | 2.66 |
| 3 | ketones | 4.61 | 5.43 | 5.57 | 6.02 | 5.87 | 5.84 | 6.13 |
| 4 | Esters | 3.81 | 6.8 | 7.01 | 7.65 | 5.77 | 6.11 | 6.12 |
| 5 | alkanes | 0.26 | 0.47 | 0.64 | 0.41 | 2.05 | 0.7 | 1.39 |
| 6 | alkenes | 2.12 | 1.1 | 0.99 | 1.03 | 0.7 | 0.44 | 3.71 |
| 7 | benzenes | 1.33 | 1.2 | 1.02 | 0.94 | 0.95 | 0.78 | 0.62 |
| 8 | Furans | 0.56 | 0.68 | 0.62 | 0.64 | 0.65 | 0.65 | 1.14 |
| 9 | miscellaneous | 0.83 | 0.77 | 0.76 | 0.86 | 1.69 | 2.07 | 2.05 |
Discussion
Caryophyllene, piperitone, cis-β-farnesene, and terpinolene found in this study represented the major components of the essential oil of marigold leaves and flowers as well [4, 20]. Terpene was the major component of VOCs in marigold flowers which consistent with a previous report and may suggest that high terpene content of VOCs could be the main reason for MCRs’ pungent taste [21].
This study describes the effects of LAB on the biotransformation of VOCs from MCR. These results showed LAB mediated degradation of some terpenes, which agreed with those of a previous study conducted by Figueiredo et al. [22] who found that terpenes in red clover forages decreased greatly after ensilage. Park et al. [19] also found that LAB significantly reduced the terpene content of blueberry juice, including a 92% reduction in vitispirane. The causes of terpene reduction were not fully known, but could involve oxidation to secondary products, glycoside hydrolysis, or ester conversion, as well as isomerization and / or interconversion of some monoterpenols [13, 23–25].
However, not all terpene levels were changed. This study has shown that some of the main occurring terpenes were not degraded by LAB, similar to the study of Belviso et al. [26] which showed that while alpha-campholenal can be completely degraded in LAB cultures, alpha-pinene, alpha-terpineol, beta-myrcene, and myrtenal did not degrade at all. Liu et al. [18] reported that some terpenes might be difficult to hydrolyze because their precursors were in the bound form. This could mean that some of these terpenoids present may be in their bound form at the end of the ensilage, or this might be due to enzymatic hydrolysis by glycosidases from microorganisms being limited under the specific conditions found during fermentation.
An explanation for this late silage degradation of terpenes may be that glycoside precursors were mainly released by acid hydrolysis, a process that occurred slowly [8]. Therefore, terpene levels changing at different times during ensilage could be a result of the different levels of glycoside resistance to acid hydrolysis.
According to the current literature, total content of terpene in forage can be reduced by ensilage, but there were still a small amount of terpenes increased [22], which is consistent with our results. Similarly, total terpene content declined when LAB was used to ferment berry juice [19], but the total terpene content increased when pomegranate juice was fermented [17]. There is a paucity of information regarding terpene biodegradation by LAB, and studies have shown that terpene biodegradation varies across different species and strains of microorganisms, including LAB. The results from this study have provided preliminary information for future studies on terpene biodegradation in MCR fermentation.
Belviso et al. [26] found that LAB cultures can completely degrade alpha-campholenal and form a new monoterpenoid in 48 h. Although terpenes are formally composed of one biosynthetic unit, the fact that they can be biotransformed by mechanisms including hydration, isomerization, dehydrogenation, conjugation, oxidation, reduction, decarboxylation, and β-oxidation, means that multiple structures can be produced [2, 13]. Microorganisms that promote the biological transformation of terpenes include bacteria, fungi, and yeast. These microorganisms can transform the original terpenes into new ones and other substances via various biotransformation reactions [2, 13]. Thus, LAB is responsible for both the degradation of terpenes and the production of the new terpenoidic metabolites. The terpene biotransformation mechanisms of LAB are not well established. Although there have been some reports about the biotransformation activities of LAB during juice and pickle fermentation [16, 19], it is difficult to infer the complex relationships between them based on the changes in either the final amount of terpenes or in the kinetics, since there are many other compounds that could interact with terpenes or influence the metabolic behavior of LAB.
These results are consistent with those of Figueiredo et al. [22] who also found that the levels of original alcohols in red clover did not significantly change after ensilage and that some alcohols disappeared.
Wide variations in alcohol levels have been observed for different forage, with comparable or lower concentrations seen in corn, alfalfa, cereal and red clover silages [3, 22, 27, 28]. Current research suggests that a large amount of alcohol is produced during ensilage and that the volatile content of ethanol in corn silage is up to 70% of total VOCs [3, 27, 28], however, in this study, no ethanol was detected at any stage. Except for ethanol, there is a lack of data on alcohols in silage which are probably generated by amino acid catabolism or by the reduction of aldehydes and ketones [16].
High ethanol contents have been observed in high-dry-matter grass silages due to their high content of fermentable carbohydrates. Low carbohydrate legume forages do not produce more ethanol during the ensiling process [22, 28]. Low fermentable carbohydrate may be the main reason for the absence of ethanol during the MCR fermentation process, while silage quality is not measured by the production of large quantities of ethanol, which can adversely affect both the environment and the animals themselves [3, 27, 28].
Acetic acid is the most important organic acid in silage, affects its quality, and is known to possess a sour odor [29]. Acetic acid accumulation depends on substrate supply and the sugar metabolism of the starter culture [30]. In fat metabolism during ensilage, LAB could degrade fatty acids to produce short-chain fatty acids such as butyric acid, acetic acid, butyric acid, and caprylic acid. Goswami et al. [31] found that acetic acid and butyric acid concentrations were significantly increased during the fermentation of horse gram by Lactobacillus plantarum (NRRL-B 4496) and Lactobacillus plantarum (NCDO 1133), indicating that these two strains can effectively metabolize fatty acids to produce short chain fatty acids.
As more acid could be produced in other silage and food fermentation processes, the detection of only two acids in this experiment make this study differ from the rest of the current literature. Since the acids produced by LAB species are strain-dependent [32], further research is needed on the importance of organic acids to silage quality.
Other VOCs, even at lower concentrations, might considerably influence animal acceptance of forage [33]. In this study, it was not possible to elucidate a clear and definite relationship between MCR ensilage with LAB and VOC biotransformation or to distinguish between the effects of the various VOCs observed. Hence, more research on this specific relationship should be conducted.
Conclusions
This work presents the first investigation of the biotransformation of VOCs in MCR by LAB ensilage. The results reported in this study show that during ensilage, LAB influences type and levels of VOCs. Compared with the fresh MCR group, the main VOCs caryophyllene and piperitone were decreased by 32.9 and 17.0%, respectively after 30 days of ensilage, while the content of alcohols increased from 2.8 to 8.1%, and the acetic acid content increased by 560%. The findings of this study should form the base foundation for future studies leading to elucidate more suitable LAB strains and their optimal environmental conditions, including concentration, pH and temperature which would allow for operations to be scaled-up. Meanwhile, these results have shed light on our understanding of how to improve fodder odor and to enhance terpene degradation by lactic acid bacteria fermentation.
Methods
Plant materials and bacterial strains
MCR was obtained from Tengchong city, Yunnan Province, at the end of September 2019 after the last commercial flowers had been picked while the stems and leaves were still green and fresh. The MCR was manually mowed leaving 2–3 cm of stubble and air-dried away from light until moisture levels had dropped to about 75%. Lactobacillus plantarum LP-115 (Danisco USA Inc., Madison, WI, USA) was used in the fermentation of MCR.
Silage preparation
The MCR was chopped into pieces approximately 3 cm in length using a forage cutter (Lingong Machinery, Shandong, China), thoroughly mixed and either treated with LAB or left untreated (control). A total of 18 silage and 3 control replicates were set up. On the first day of the experiment, 5 mg/kg of Lactobacillus plantarum, containing lactic acid bacteria (LAB) at (1.0 × 105) colony forming unit (cfu)/g, was added to the fresh MCR as per manufacturer’s instructions. To produce silage, the MCR was compressed into a 1 L polyethylene bag silo (Beijing meat processing company, Beijing) and in order to ensure an anaerobic fermentation environment, all bags were sealed with a vacuum packer (Beijing Keyoujia, Beijing) and stored indoors in the dark for 30 days at 25 °C. Three samples were taken from the control group and silage treatments at 3, 6, 9, 12, 15 and 30 days and frozen at − 20 °C prior to analysis of VOCs.
SPME experimental conditions
After the sample was melted, 3 g of MCR sample was put into a 20 mL Agilent crimp-top headspace vial, and heated in a 60 °C water bath to allow the aroma substances in the extraction bottle to reach equilibrium. After 5 min, the aged extraction head was inserted and extraction at 60 °C for 30 min was performed before GC-MS analysis.
GC-MS analysis
GC-MS was used to analyze the VOCs from MCR. A TRACE1310/ISQ7000 mass selective detector (ThermoFisher) was used in conjunction with a TG-5MS column (30 m*0.25 mm*0.25um; ThermoFisher). Operation conditions were as follows: Set injection to splitless mode for 5 min at 250 °C. Helium flow rate, 1.0 mL/min. Temperature programing: 40 °C for 2 min then 4 °C/min to 160 °C for 4 min and finally ramped to 250 °C at 15 °C/min and maintained for 2 min. The temperature of ion source was set to 230 °C and the inlet line temperature was set at 250 °C. The MS detector operated in positive electron ionization (EI+) mode at 70 eV under a mass scan range of 35–450 amu (m/z). VOCs were initially identified by comparison with the mass spectra data registered in the National Institute of Standards and Technology database (NIST 11) (Avila-Sosaet al., 2010), and identity was further ascertained based on the probable percentage of the three candidate components provided by GC-MS. The relative percentage of each component was calculated using the total percentage of peak area which was expressed as a percentage of the sum peak area of all identified compounds.
Statistical analysis
SPSS 19. 0 Statistical software was used to perform analysis of variance, and multiple comparisons using Duncan’s method (P = 0.01 and P = 0.05). Mapping was performed using Sigmaplot 10.0.
Acknowledgements
We thank Meiyan Zhang for helpful data analysis.
Abbreviations
- CAS
Chemical Abstract Service
- GC-MS
Gas chromatography – mass spectrometry
- LAB
Lactic acid bacteria
- MCR
Marigold crop residue
- SPME
Solid-phase microextraction technique
- VOCs
Volatile organic compounds
Authors’ contributions
ZH, JL, BH and MW conceived and designed the experiments. MC and YL performed the experiments. ZH and JL wrote the manuscript and analyzed the data. LM and YG performed data curation. BH and MW finalized the manuscript. All authors read and approved the manuscript.
Funding
Authors are grateful acknowledged for financial support by Special Fund for China Agriculture Research System (grant number CARS-37), Yunling Scholar and Yunling Super-Talent Initiative-Yunling High-end Foreign Experts Program, Academician workstation of Zhibiao Nan (grant number 2018IC074), High-tech Talents Introduction Program of Yunnan Province (grant number 2012HA012), “Light of West China” Program(2020), Yunnan Province Candidate talents Project for Academic and Technical leaders of Middle-aged and Young talents (grant number 2019HB035), Yunnan Fundamental Research Projects (Grant number 202001 AU070094), Demonstration Project of Exploitation and Utilization of High-Quality Green and Rough Feed Resources (grant number 16190051). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Sampling was conducted on private land and the land owner gave permission for this.
Consent for publication
Not applicable.
Competing interests
The authors have declared no competing financial or non-financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhijiang Hou and Jianyong Liu contributed equally to this work.
References
- 1.Baskaran V, Abirami K. Effect of pinching on yield of African marigold (Tagetes erecta L.) cv. Pusa Narangi Gainda under Andaman conditions. Agric Sci Digest. 2017;37:148–50.
- 2.Wu R, Kan C, Zhao W, Pan D. Nutrition analysis of marigold in Inner Mongolia. Guangzhou Chem. 2010;38:184–185. [Google Scholar]
- 3.Malkina IL, Kumar A, Green PG, Mitloehner FM. Identification and quantitation of volatile organic compounds emitted from dairy silages and other feedstuffs. J Environ Qual. 2011;40:28–36. doi: 10.2134/jeq2010.0302. [DOI] [PubMed] [Google Scholar]
- 4.Ogunwande IA, Olawore NO. The Essential Oil from the Leaves and Flowers of “African Marigold,” Tagetes erecta L. J Essent Oil Res. 2006;18:366–368. doi: 10.1080/10412905.2006.9699115. [DOI] [Google Scholar]
- 5.Singh G, Prakash Singh O, de Lampasona MP, Catalán CAN. Studies on essential oils. Part 35: chemical and biocidal investigations on Tagetes erecta leaf volatile oil. Flavour Frag J. 2003;18:62–65. doi: 10.1002/ffj.1158. [DOI] [Google Scholar]
- 6.Cannas A, Mereu A, Decandia M, Molle G. Role of sensorial perceptions in feed selection and intake by domestic herbivores. Ital J Anim Sci. 2009;8(SUPPL. 2):243–251. doi: 10.4081/ijas.2009.s2.243. [DOI] [Google Scholar]
- 7.Estell RE, Fredrickson EL, Tellez MR, Havstad KM, Shupe WL, Anderson DM, et al. Effects of volatile compounds on consumption of alfalfa pellets by sheep. J Anim Sci. 1998;76:228–233. doi: 10.2527/1998.761228x. [DOI] [PubMed] [Google Scholar]
- 8.Dziadas M, Jeleń HH. Comparison of enzymatic and acid hydrolysis of bound flavor compounds in model system and grapes. Food Chem. 2016;190:412–418. doi: 10.1016/j.foodchem.2015.05.089. [DOI] [PubMed] [Google Scholar]
- 9.Rapisarda T, Mereu A, Cannas A, Belvedere G, Licitra G, Carpino S. Volatile organic compounds and palatability of concentrates fed to lambs and ewes. Small Ruminant Res. 2012;103:120–132. doi: 10.1016/j.smallrumres.2011.08.011. [DOI] [Google Scholar]
- 10.Gabriel M, Paczkowski S, Nicke S, Schütz S, Behn C, Kraft R, et al. Effect of some treatments on emission of volatile organic compounds (VOC) from chips used in pellets making processes. Int Wood Pro J. 2015;6:60–68. doi: 10.1179/2042645314Y.0000000091. [DOI] [Google Scholar]
- 11.Roffael E, Schneider T, Dix B. Effect of oxidising and reducing agents on the release of volatile organic compounds (VOCs) from strands made of scots pine (Pinus sylvestris L.) Wood Sci Technol. 2015;49:957–967. doi: 10.1007/s00226-015-0744-6. [DOI] [Google Scholar]
- 12.Zhang B, Huo Y, Zhang J, Zhang X, Zhu C. Agrobacterium rhizogenes-mediated RNAi of Tripterygium wilfordii and application for functional study of terpenoid biosynthesis pathway genes. Ind Crop Prod. 2019;139:111509. doi: 10.1016/j.indcrop.2019.111509. [DOI] [Google Scholar]
- 13.Krings U, Berger RG. Terpene bioconversion - how does its future look? Nat Prod Commun. 2010;5:1507–1522. [PubMed] [Google Scholar]
- 14.Zhang YC, Li DX, Wang XK, Lin YL, Zhang Q, Chen XY, et al. Fermentation quality and aerobic stability of mulberry silage prepared with lactic acid bacteria and propionic acid. Anim Sci J. 2019;90:513–522. doi: 10.1111/asj.13181. [DOI] [PubMed] [Google Scholar]
- 15.Alhan R, Can A. Determining effect of straw and inoculant addition on silage quality of sugar beet leaves silage. Bulg J Agric Sci. 2017;23:639–643. [Google Scholar]
- 16.Choi YJ, Yong S, Lee MJ, Park SJ, Yun YR, Park SH, et al. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. Lwt. 2019;105:118–26.
- 17.di Cagno R, Filannino P, Gobbetti M. Lactic acid fermentation drives the optimal volatile flavor-aroma profile of pomegranate juice. Int J Food Microbiol. 2017;248:56–62. doi: 10.1016/j.ijfoodmicro.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Liu F, Li S, Gao J, Cheng K, Yuan F. Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. Lwt. 2019;109:233–40.
- 19.Park JB, Lim SH, Sim HS, Park JH, Kwon HJ, Nam HS, et al. Changes in antioxidant activities and volatile compounds of mixed berry juice through fermentation by lactic acid bacteria. Food Sci and Biotechnol. 2017;26:441–446. doi: 10.1007/s10068-017-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérez Gutiérrez RM, Luna HH, Garrido SH. Antioxidant activity of Tagetes erecta essential oil. J Chil Chem Soc. 2006;51:883–886. doi: 10.4067/S0717-97072006000200010. [DOI] [Google Scholar]
- 21.Gao QY, Gao Y, Zhang RM, Du ML, Li G. Aromatic composition in three plant species using TDS-GC/MS. J Zhejiang A&F Univ. 2011;28:326–332. [Google Scholar]
- 22.Figueiredo R, Rodrigues AI, do Céu Costa M. Volatile composition of red clover (Trifolium pratense L.) forages in Portugal: The influence of ripening stage and ensilage. Food Chem. 2007;104:1445–1453. doi: 10.1016/j.foodchem.2007.02.022. [DOI] [Google Scholar]
- 23.Takoi K, Itoga Y, Koie K, Kosugi T, Shimase M, Katayama Y, et al. The contribution of geraniol metabolism to the citrus flavour of beer: synergy of geraniol and β-citronellol under coexistence with excess linalool. J I Brewing. 2010;116:251–260. doi: 10.1002/j.2050-0416.2010.tb00428.x. [DOI] [Google Scholar]
- 24.Gamero A, Manzanares P, Querol A, Belloch C. Monoterpene alcohols release and bioconversion by Saccharomyces species and hybrids. Int J Food Microb. 2011;145:92–97. doi: 10.1016/j.ijfoodmicro.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 25.King AJ, Dickinson JR. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003;3:53–62. doi: 10.1016/S1567-1356(02)00141-1. [DOI] [PubMed] [Google Scholar]
- 26.Belviso S, Giordano M, Dolci P, Zeppa G. Degradation and biosynthesis of terpenoids by lactic acid bacteria isolated from cheese: first evidence. Dairy Sci Technol. 2011;91:227–236. doi: 10.1007/s13594-011-0003-z. [DOI] [Google Scholar]
- 27.Hafner SD, Howard C, Muck RE, Franco RB, Montes F, Green PG, et al. Emission of volatile organic compounds from silage: compounds, sources, and implications. Atmos Environ. 2013;77:827–839. doi: 10.1016/j.atmosenv.2013.04.076. [DOI] [Google Scholar]
- 28.Hafner SD, Windle M, Merrill C, Smith ML, Franco RB, Kung L. Effects of potassium sorbate and lactobacillus plantarum MTD1 on production of ethanol and other volatile organic compounds in corn silage. Anim Feed Sci Tech. 2015;208:79–85. doi: 10.1016/j.anifeedsci.2015.07.007. [DOI] [Google Scholar]
- 29.Nsogning DS, Procopio S, Sacher B, Becker T. Flavor of lactic acid fermented malt based beverage: current status and perspective. Trends Food Sci Tech. 2016;54:37–51. doi: 10.1016/j.tifs.2016.05.017. [DOI] [Google Scholar]
- 30.Peyer LC, Zannini E, Arendt EK. Lactic acid bacteria as sensory biomodulators for fermented cereal based beverage. Trends Food Sci Tech. 2016;54:17–25.
- 31.Goswami RP, Jayaprakasha GK, Shetty K, Patil BS. Lactobacillus plantarum and natural fermentation-mediated biotransformation of flavor and aromatic compounds in horse gram sprouts. Process Biochem. 2018;66:7–18.
- 32. Lau, ASY, Liong MT. Lactic acid bacteria and bifidobacteria-inhibited Staphylococcus epidermidis. Wounds. 2014;26:121–31. [PubMed]
- 33.Mereu A. Palatability of concentrates fed to sheep. Ph.D. dissertation. University of Sassari, Sassari, Italy 2009.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.