Abstract
Objectives
A subgroup of patients with SARS-CoV-2 infection was thought to have developed cytokine release syndrome and were treated with tocilizumab; however, a significant percentage of patients evolved. This study aimed to determine the usefulness of anakinra as a rescue treatment for patients with tocilizumab-refractory COVID-19 disease.
Methods
A prospective cohort of patients with COVID-19 pneumonia who received anakinra as salvage therapy after failure of tocilizumab were compared (1:1) with selected controls in a historical cohort of patients treated with tocilizumab. Cases and controls were matched by age, comorbidities, pulse oximetry oxygen saturation to fraction of inspired oxygen (SpO2/FiO2) ratio at baseline, and time elapsed since the initiation of treatment with tocilizumab. The primary outcome was the improvement in clinical status measured by a 6-point ordinal scale, from baseline to day 21.
Results
The study included 20 cases and 20 controls (mean age 65.3 ± 12.8 years, 65% males). No differences were found in the clinical improvement rates at 7, 14 and 21 days of follow-up. The in-hospital mortality rate for patients receiving anakinra was 55% vs. 45% in the control group (P = 0.527).
Conclusions
Treatment with anakinra was not useful in improving the prognosis of patients with tocilizumab-refractory severe COVID-19.
Keywords: COVID-19, Anakinra, Tocilizumab, Therapy, Immunomodulation, Outcome
Introduction
As of January 2021, the worldwide outbreak of the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), responsible of the viral pneumonia known as COVID-19, has caused more than 94 million infections and 2 million deaths (Johns Hopkins University of Medicine, 2021). SARS-CoV-2 infection produces mild symptoms of respiratory tract infection in most individuals; however, a subgroup of patients with COVID-19 develop severe disease, with acute respiratory distress syndrome (ARDS) and/or multiple organ dysfunction especially affecting the heart, liver and kidneys. Increased systemic levels of cytokines and chemokines are observed in these patients, resulting in harmful effects as hypothesized by similarities with other cytokine release syndromes (CRS) (Huang et al., 2020). CRS may occur as part of the clinical picture of haemophagocytic lymphohistiocytosis or macrophage activation syndrome observed after triggering by viral infections, systemic diseases such as adult-onset Still’s disease or systemic juvenile idiopathic arthritis, or after therapeutic immune-activation by CAR T-cells. In these conditions, treatment with IL-1 or IL-6 cytokine antagonists has been found effective (Bruck et al., 2011, Grom et al., 2016).
Previous studies have postulated that treating patients with severe COVID-19 and hyperinflammation with immunomodulatory drugs could improve prognosis. Tocilizumab, an IL-6 antagonist, has become the most commonly used drug and several clinical trials have been published on patients with moderate-severe COVID-19 to date (Salama et al., 2021, Stone et al., 2020, Hermine et al., 2021, Salvarani et al., 2021, Rosas et al., 2021). Other anti-cytokine strategies have been proposed, including the use of the IL-1 receptor antagonist anakinra, with conflicting results in studies published to date (Aouba et al., 2020; Balkhair et al., 2020; Cavalli et al., 2020; Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1: a randomised controlled trial, 2021). Nevertheless, the prognosis remains ominous for a subgroup of patients and evidence from randomized controlled trials is still limited. Therefore, the decision to target one or another cytokine is still difficult. Anakinra was prescribed at the current institution as a rescue therapy for those patients with ARDS in the context of Covid-19 infection who remained in respiratory failure despite previous treatment with tocilizumab, which was the first choice based upon preliminary reports of potential efficacy.
It is believed that this is the first series to analyse the potential effectiveness of anakinra after tocilizumab failure for improving prognosis in COVID-19, in comparison with a historical series of patients in the same hospital who did not receive anakinra in this context.
Methods
Study design and patients
This study was conducted at the Hospital Universitario “12 de Octubre” in Madrid (Spain). The hospital has 1,300 beds, and it is one of the largest hospitals in the region of Madrid. It serves a population of 370,000 inhabitants of the south of Madrid. The hospital admitted more than 3,000 patients with COVID-19 between March and May 2020. The study was approved by the Clinical Research Ethics Committee and they granted a waiver of informed consent due to its observational design.
This study included a prospective cohort of hospitalised patients with COVID-19 severe pneumonia, aged ≥18 years and who received anakinra as rescue therapy after not achieving clinical respiratory improvement despite treatment with tocilizumab, from April 6 to May 8. They were compared with selected controls in a historical cohort of patients treated with tocilizumab from March 18 to May 14. Four of the patients included in this series were included in a previously published series of haematological patients at the current centre who had received anakinra (Villegas et al., 2020).
Patients with confirmed (by reverse transcription-PCR on nasopharyngeal swab) SARS-CoV-2 infection or with high clinical suspicion, who presented chest X-rays compatible with COVID-19 pneumonia, oxygen saturations while breathing ambient air of ≤90% and hyperinflammation analytical data (C-reactive protein (CPR) ≥10 mg/dL, ferritin ≥500 mg/dL or D-dimer ≥1500 ng/mL) were candidates to receive tocilizumab. Doses of tocilizumab were 600 mg in a single dose for patients whose weight was ≥75 kg or 400 mg in a single dose in patients weighing <75 kg. The administration of a second dose within the following 24 h was indicated according to the criteria of the attending physician. Exclusion criteria were evidence of concomitant bacterial infection, history of diverticular disease, neutropenia <1.5 × 103 cells/μL or baseline elevation of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels >5-fold the upper limit of the normal range.
After administration of tocilizumab, the clinical status of patients was evaluated daily by a 6-point ordinal scale, according to World Health Organization (WHO) recommendations and those used in previous studies of patients admitted to hospital with COVID-19 (Cao et al., 2020) on the basis of the following categories: 1, not hospitalised; 2, hospitalised, not requiring supplemental oxygen but requiring ongoing medical care; 3, hospitalised, requiring low-flow rate oxygen ≤6 L/min; 4, hospitalised, high-flow rate oxygen ≥8 L/min delivered through a Venturi or non-rebreather mask; 5, invasive mechanical ventilation or extracorporeal membrane oxygenation; and 6, death. If the patient did not experience an improvement on the clinical scale in the days following the administration of tocilizumab, the physicians could choose to start treatment with anakinra. Anakinra was administrated subcutaneously at 100 mg/12 h on day 0, then at 100 mg/24 h from day 1 to day 5. On the sixth day, the indication for treatment with anakinra was re-evaluated according to the clinical status on that day, maintaining the treatment or stopping it if it was not considered necessary based on clinical improvement. Exclusion criteria for treatment with anakinra were evidence of concomitant bacterial infection and presence of neutropenia <1.5 × 103 cells/μL.
An ad hoc steering committee was created in the hospital to establish standardised therapeutic decisions concerning the use of immunomodulating drugs in patients with infections caused by SARS-CoV-2. This committee was developed due to the uncertainties in tocilizumab and anakinra effectiveness and safety and due to potential shortages in drug supply. It included all the involved clinical specialties and the Department of Pharmacy. The decisions to initiate treatment with tocilizumab and anakinra in the patients included in this study were adopted in a scholarly manner by this committee.
Control selection
Controls were consecutively selected from the historical cohort of patients admitted to the centre who had received treatment with tocilizumab. For each case, the first chronologically matching control fulfilling the following criteria was selected: age ±5 years, Charlson comorbidity index score ±2 points, same clinical status according the 6-point ordinal scale, and the same number of days without improvement in oxygen requirements since the first day of treatment with tocilizumab as the corresponding case.
Outcomes
Patients and controls were followed up to discharge from hospital or death. Epidemiological data and baseline comorbidities, time between debut of symptoms and inclusion, previous therapies administered and clinical characteristics, laboratory values, respiratory status, and radiological features on day 0 were collected from electronic medical records using a standardised case report form, as well as on days 7, 14 and 21 after inclusion. Adverse events potentially attributable to anakinra were also collected. All laboratory and imaging investigations were performed as part of standard of care. Respiratory function was assessed by means of the pulse oximetry oxygen saturation/fraction of inspired oxygen (SpO2/FiO2) ratio, which shows good correlation with the partial pressure of arterial oxygen (PaO2)/FiO2 ratio (SpO2/FiO2 = 64 + 0.84 × PaO2/FiO2) (Rice et al., 2007).
The primary outcome was the change in clinical status using the 6-point ordinal scale, from baseline [day 0] to day 7 and to day 21. This change was defined by discharge from the hospital and/or by a decrease of ≥1 points from baseline on the previously mentioned ordinal scale. In the case group, the date of administration of the first dose of anakinra was considered as day 0. In the control group, day 0 corresponded to the day on which the anakinra would have been started if the same interval between the administration of tocilizumab and anakinra had been maintained, as in the corresponding case (pseudo date of treatment). Secondary outcomes included the difference in 6-point ordinal scale scores between day 0 and days 7, 14 and 21.
Concomitant treatment
Patients and controls were initially treated with supportive care with or without a combination of oral lopinavir-ritonavir (400 mg twice daily for 5 days), hydroxychloroquine ± oral azithromycin (400 mg twice daily for two doses on day 1, followed by 200 mg twice daily on days 2–5 ± 500 mg of azithromycin for 3–5 days) subcutaneous interferon-β (0.25 mg SC every 48 h for 14 days), corticosteroids (iv methylprednisolone 0.5–1 mg/kg daily for 3 days or as boluses of 100–250 mg daily for 3 days), and antibiotics at the criterion of their physicians in charge and in accordance with the institutional protocol. No patient in the present study received concomitant treatment of remdesivir with tocilizumab or anakinra, as it was one exclusion criterion for the clinical trial with which this drug was used in the hospital at that time.
Microbiological methods
The diagnosis of COVID-19 was made by means of SARS-CoV-2 real-time reverse transcription polymerase chain reaction (rRT-PCR) in nasopharyngeal or oropharyngeal swabs or sputum samples. In detail, nucleic acid extraction was performed with the Microlab® STARlet IVD platform using the STARMag 96 × 4 Universal Cartridge Kit (Seegene, Seoul, South Korea) or the NucliSENS® easyMAG® instrument (bioMerieux, Marcy l’Etoile, France). Amplification was carried out by using the TaqManTM 2019-nCoV assay kit v1 (Thermo Fisher Scientific, Waltham, MA) in the LightCycler® 480 instrument II (Roche Life Science, Indianapolis, IN). Appropriate (positive and negative) controls were tested in each run, as well as an internal control to rule out the presence of PCR inhibitors. The diagnosis of COVID-19 was also assumed in patients with a highly suggestive clinical and radiological presentation and compatible epidemiological history but repeatedly negative RT-PCR.
Statistical analysis
Quantitative data were reported as mean ± standard deviation (SD) or median with interquartile range (IQR), whereas qualitative variables were expressed as absolute and relative frequencies. Categorical variables were compared using the χ2 test or Fisher's exact test, when applicable. Student’s t-test or Mann–Whitney U test were applied for continuous variables. The threshold for significance was set at a P-value < 0.05. Statistical analysis was performed with SPSS version 25.0 (IBM Corp., Armonk, NY) and graphs were generated with Prism version 6.0 (GraphPad Software Inc., La Jolla, CA).
Results
Characteristics of patients and controls
Twenty patients diagnosed with SARS-CoV-2 infection who had been treated with tocilizumab without respiratory improvement were selected to receive treatment with anakinra. The mean age of patients was 65.3 ± 12.7 years, and 70% were males. The median time elapsed between tocilizumab treatment and anakinra was 6 days (IQR 4.0–8.75). Subsequently, 20 controls with the previously described characteristics were selected. Demographic data, baseline comorbidities, laboratory severity markers, as well as treatments received during admission are shown in Table 1 . Both groups were comparable in all variables, except the lymphocyte nadir value recorded prior to inclusion: median (IQR) 0.25 (0.1–0.7) in the anakinra group vs. 0.5 (0.4–0.7) in the control group (P = 0.035). Comorbidity was high in both groups, with a median (IQR) Charlson index score of 3.5 (2–5); cardiovascular disease was the most frequent previous disease. The median time from symptom onset to inclusion was 16 days (IQR 12–24), with no between-group differences.
Table 1.
Demographics, clinical and laboratory characteristics in both groups.
| Variable | Anakinra group (n = 20) | Control group (n = 20) | P-value |
|---|---|---|---|
| Age, years [mean ± SD] | 65.3 ± 12.7 | 65.3 ± 13.3 | 0.990 |
| Male sex [n (%)] | 14 (70) | 12 (60) | 0.507 |
| Ethnicity [n (%)] | |||
| Hispanic | 4 (20) | 5 (25) | 1.000 |
| Caucasian | 16 (80) | 15 (75) | 1.000 |
| Charlson index score [median (IQR)] | 4 (3–5) | 3 (2–5) | 0.495 |
| Comorbidities [n (%)] | |||
| Cardiovascular diseasea | 9 (45) | 13 (65) | 0.204 |
| Diabetes mellitus | 7 (35) | 7 (35) | 1.000 |
| Obesityb | 7 (38.9) | 11 (57.9) | 0.248 |
| Chronic lung diseasec | 2 (10) | 3 (15) | 1.000 |
| Immunosuppressiond | 9 (45) | 7 (35) | 0.519 |
| Chronic kidney disease | 3 (15) | 1 (5) | 0.605 |
| Chronic liver disease | 1 (5) | 0 | 1.000 |
| Current or former smoker | 6 (30) | 10 (50) | 0.197 |
| Duration of symptoms, days [median (IQR)]e | 16 (13–25) | 16 (12–22.5) | 0.771 |
| Laboratory values | |||
| Lymphocytes nadir x 109 cells/L [median (IQR)] | 0.25 (0.1–0.7) | 0.5 (0.4–0.7) | 0.035 |
| Ferritin peak, ng/mL [median (IQR)]f | 1,237 (691–2,595) | 1,811 (934–2,233) | 0.372 |
| CRP peak, mg/dL [median (IQR)] | 17.5 (11.2–30.7) | 19.9 (15.8–24.9) | 0.495 |
| LDH peak, U/L [median (IQR)] | 606 (518–772) | 705 (550–943) | 0.102 |
| D–dimer, ng/mL [median (IQR)]g | 2,266 (949–33,083) | 7,172 (1,467–18,449) | 0.762 |
| Prior or concomitant therapies | |||
| Hydroxychloroquine [n (%)] | 20 (100) | 19 (95) | 0.311 |
| Lopinavir/ritonavir [n (%)] | 5 (25) | 11 (55) | 0.053 |
| Azithromycin [n (%)] | 13 (65) | 18 (90) | 0.058 |
| Interferon-β [n (%)] | 1 (5) | 4 (20) | 0.342 |
| Corticosteroids [n (%)] | 17 (85) | 13 (65) | 0.273 |
| Interval, days [median (IQR)]h | 8 (4.5–10) | 8 (7–18) | 0.229 |
| Interval TCZ to day 0, days [median (IQR)]i | 6 (4–8.75) | 6 (4–8) | 0.799 |
SD: standard deviation; IQR: interquartile range; CRP: C-reactive protein; LDH: lactate dehydrogenase.
Cardiovascular disease included coronary heart disease, heart failure, stroke and hypertension.
Obesity was established when BMI was ≥30; data available for 37 patients.
Chronic lung disease included chronic obstructive pulmonary disease, asthma or severe obstructive sleep apnoea.
Inmunossupresion included active malignant neoplasia, autoimmune disease, solid organ transplantation, HIV infection, use of steroids or chemotherapy. Use of steroids was defined as: 1) >20 mg/day of oral prednisone for ≥7 days or 2) <20 mg/day for a minimum of 3 months.
From the onset of symptoms to day 0 (i.e. day of administration of first dose of anakinra or the corresponding pseudo date in the control group).
Ferritin levels available for 18 patients in the anakinra group and 18 patients in the control group.
D-dimer levels available for 20 patients in the anakinra group and 18 in the control group.
From the initiation of corticosteroids to day 0 (i.e. day of administration of first dose of anakinra or the corresponding pseudo date in the control group).
From the initiation of tocilizumab to day 0 (i.e. day of administration of first dose of anakinra or the corresponding pseudo date in the control group).
The clinical and respiratory status of patients and controls on day 0, as well as their status according to the 6-point ordinal scale is shown in Table 2 . Most patients in both groups (n = 17, 85%) were on high-flow supplemental oxygen using non-invasive ventilation or high-flow masks; two cases (10%) and two controls (10%) were on mechanical ventilation and one patient from each group (5%) was on low-flow supplemental oxygen using nasal cannula. Notably, 11 patients (55%) in the anakinra group and eight patients (40%) in the control group presented severe ARDS (PaO2:FiO2 ≤ 100 mmHg) on day 0.
Table 2.
Vital signs, respiratory status and laboratory values at day 0.
| Variable | Anakinra group (n = 20) | Control group (n = 20) | P-value |
|---|---|---|---|
| Vital signs at day 0 | |||
| Axillary temperature, ºC [mean ± SD] | 36.7 ± 0.7 | 36.9 ± 0.8 | 0.418 |
| Respiratory rate, rpm [median (IQR)] | 24.6 ± 5.2 | 23.6 ± 4.6 | 0.537 |
| Heart rate, bpm [mean ± SD] | 87.1 ± 16.6 | 90.2 ± 11.8 | 0.500 |
| PaO2:FiO2, ratio [mean ± SD] | 108.6 ± 48.9 | 132.9 ± 68.7 | 0.206 |
| PaO2:FiO2 ≥ 300 mmHg [n (%)] | 0 | 1 (5) | 1.000 |
| PaO2:FiO2 200–300 mmHg [n (%)] | 2 (10) | 1 (5) | 1.000 |
| PaO2:FiO2 100–200 mmHg [n (%)] | 7 (35) | 10 (50) | 0.337 |
| PaO2:FiO2 < 100 mmHg [n (%)] | 11 (55) | 8 (40) | 0.342 |
| Laboratory values at day 0 | |||
| Lymphocytes, x 109 cells/L [median, IQR] | 0.7 (0.2–0.8) | 0.9 (0.6–1.3) | 0.040 |
| CRP, mg/dL [median, (IQR)]a | 1.44 (0.9–2.9) | 1.27 (0.5–1.3) | 0.681 |
| LDH, U/L [median, (IQR)]b | 598 (330–716) | 567 (414–697) | 1.000 |
| Ferritin, ng/mL [median (IQR)]c | 1,110 (738–3,579) | 2,088 (861–10,275) | 0.441 |
| D-dimer, ng/mL [median (IQR)]d | 3,045 (1,165–17,278) | 6,585 (1,241–18,449) | 0.904 |
| SOFA score [median, IQR] | 4 (3–4) | 3.5 (3–6) | 0.799 |
| NEWS score [median, IQR] | 6.5 (5–7.7) | 6 (5–8) | 0.550 |
| Six-point ordinal scale | |||
| 1: not hospitalised | 0 | 0 | |
| 2: hospitalised, not requiring supplemental oxygen | 0 | 0 | |
| 3: hospitalised, low-flow rate oxygen [n (%)] | 1 (5) | 1 (5) | |
| 4: hospitalised, high-flow rate oxygen [n (%)] | 17 (85) | 17 (85) | |
| 5: IMV or ECMO [n (%)] | 2 (10) | 2 (10) | |
| 6: Death | 0 | 0 |
SD: standard deviation; IQR: interquartile range; bpm: beats per minute; CRP: C-reactive protein; LDH: lactate dehydrogenase; rpm: respirations per minute; SpO2/FiO2: pulse oximetry oxygen saturation/fraction of inspired oxygen; ECMO: extracorporeal membrane oxygenation; IMV: invasive mechanical ventilation.
CRP levels available for 20 patients in anakinra group and 15 patients in control group.
LDH levels available for 19 patients in anakinra group and 15 patients in control group.
Ferritin levels available for 11 patients in anakinra group and 5 patients in control group.
D-dimer levels available for 16 patients in anakinra group and 15 patients in control. group.
Response to treatment
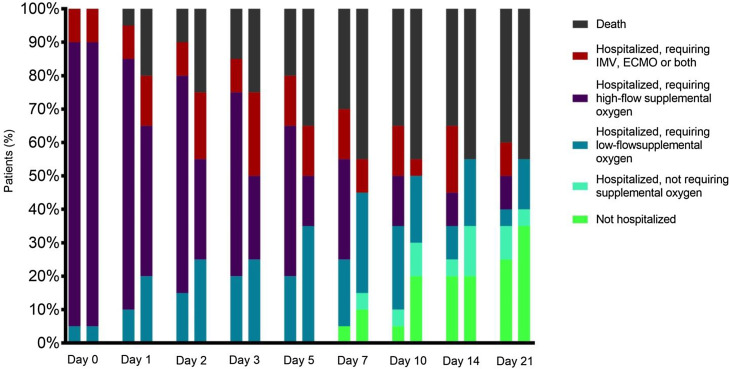
As shown in Table 3, five (25%) patients in the anakinra group had improved their clinical status according to the 6-point ordinal scale vs. nine patients in the control group (45%, P = 0.185) after 7 days of follow-up. No differences were found in the clinical improvement rates at 14 and 21 days after inclusion. Similar scores in the 6-point ordinal scale were obtained by days 7, 14 and 21 between groups (, Figure 1 ). Six (30%) and seven (35%) patients had been discharged on day 21 in the anakinra group and control group, respectively. The in-hospital mortality rate in patients receiving anakinra was 55% vs. 45% in the control group (P = 0.527).
Table 3.
Comparison of study outcomes.
| Variable | Anakinra group (n = 20) | Control group (n = 20) | P-value | Absolute risk difference (95% CI) |
|---|---|---|---|---|
| Clinical improvement at day 7 [n (%)] | 5 (25) | 9 (45) | 0.185 | 0.642–9.391 |
| 6-point ordinal scale score at day 7 [median (IQR)] | 3 (1–3.75) | 2 (1–4) | 0.752 | |
| Difference in 6-point ordinal scale scores between day 0 and day 7 [median (IQR)] | 0 (-2–0) | −1 (-2–1) | 0.177 | |
| Clinical improvement at day 14 [n (%)] | 7 (35) | 11 (55) | 0.204 | 0.636–8.106 |
| 6-point ordinal scale score at day 14 [median (IQR)] | 2 (1–5.5) | 4 (1–6) | 0.752 | |
| Difference in 6-point ordinal scale scores between day 0 and day 14 [median (IQR)] | 0 (-2–2.5) | 1 (-2–3) | 0.340 | |
| Clinical improvement at day 21 [n (%)] | 8 (40) | 11 (55) | 0.342 | 0.522–6.434 |
| 6-point ordinal scale score at day 21 [median (IQR)] | 2.5 (1–6.75) | 4 (1–7) | 0.527 | |
| Difference in 6-point ordinal scale scores between day 0 and day 21 [median (IQR)] | 0 (-2–3) | 1 (-2–4) | 0.527 | |
| All-cause mortality at day 14 [n (%)] | 7 (35) | 9 (45) | 0.519 | 0.425–5.426 |
| All-cause mortality at day 21 [n (%)] | 8 (40) | 9 (45) | 0.749 | 0.350–4.307 |
| Hospital discharge at day 21 | 6 (30) | 7(35) | 0.736 | 0.334–4.733 |
| In-hospital mortality | 11 (55) | 9 (45) | 0.527 | 0.193–2.327 |
Figure 1.
Patient status according to the 6-point ordinal scale during the follow-up.
The first bar of each day indicates the clinical situation of the cases and the second bar that of the controls.
Adverse events
Four patients (20%) in the anakinra group and six (30%) in the control group developed a thromboembolic event during their hospital stay. Four patients (20%) in the control group presented an increase in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels >3-fold the upper limit of the normal range; there were none in the anakinra group. Three patients (15%) in the anakinra group and two patients (10%) in the control group presented some microbiologically documented bacterial infection during the hospital stay: a nosocomial-acquired pneumonia due to Klebsiella pneumonia was diagnosed in two of them, and all of them presented catheter-related bacteraemia, two due to Enterococcus faecalis and the other due to coagulase-negative staphylococci.
Discussion
There is scarce information available about the best approach for patients with COVID-19 with persistent severe disease after tocilizumab therapy. The steering committee for immunomodulatory treatment at the current hospital proposed the use of anakinra as a rescue treatment for patients who do not improve their respiratory status after treatment with tocilizumab. IL-1 antagonists, including anakinra, are the first therapeutic choice in CRS associated with many autoinflammatory disorders, where inflammasome activation seems a key pathogenic element (Mehta et al., 2020). The complementary or hierarchical roles of IL-1 and IL-6 are unclear in the COVID-19 setting, but an upstream role for IL-1 in the cytokine cascade can be postulated based upon the direct activation effects of conserved SARS coronaviruses protein E on the NLPR3 inflammasome (Shah, 2020). The addition of anakinra to the standard treatment of COVID-19 at the current hospital, including tocilizumab for patients with severe inflammatory response, did not provide any benefit in terms of respiratory improvement or mortality reduction. Previous studies have reported an improvement in the prognosis of severe COVID-19 patients treated with anakinra (Cavalli et al., 2020, Aouba et al., 2020, Balkhair et al., 2020, Pontali et al., 2020), although the time elapsed between symptom onset and anakinra treatment initiation was shorter, and the patients had not previously been treated with immunomodulating drugs. Also, anecdotal isolated case reports have communicated the beneficial effect of immunomodulators for severe COVID-19 refractory to treatment with tocilizumab, such as ruxolitinib (a Janus kinase inhibitor) (Innes et al., 2020) and anakinra itself (Figuero-Pérez et al., 2020).
There are some explanations to justify the negative results of the present study. One possibility is that the therapeutic effect of IL-1 inhibition by anakinra could depend on downstream suppression of IL-6 synthesis and its systemic proinflammatory effects. In this case, no additional effects would be expected in patients previously treated with tocilizumab, which efficiently blocks the IL-6 pathway in COVID-19, as inferred by its potent effect on CRP synthesis in these patients (Xu et al., 2020). However, additional markers for macrophage activation in tocilizumab-treated patients, such as persistent ferritin elevation, are consistent with additional pathogenic roles for IL-1b or other macrophagic cytokines (Giamarellos-Bourboulis et al., 2020). On the other hand, CRS suppression is likely to be more effective in earlier phases before severe lung damage is established. It has to be highlighted that the median days since symptom debut to anakinra treatment was 16 days (IQR 13–25) and 90% of patients in each group presented a PAFI <200 on day 0, which reflected the advanced stage of the disease. In addition to this, the high comorbidity of the patients was responsible for many of them not being potential candidates for ICU admission or mechanical ventilation. This fact definitely influenced the extremely high mortality of the current sample compared with other studies (Zangrillo et al., 2020) and could have influenced the lack of response to treatments. A potential benefit of anakinra as a rescue therapy after failure of therapy with tocilizumab when administered to younger patients or at earlier stages of the disease cannot be excluded.
Another explanation may be the relatively low dose of anakinra selected by the local committee, which was based on previous recommendations from experience in patients with non-infections systemic inflammatory rheumatological diseases (Divithotawela et al., 2015). Since most recommendations are based upon consensus and no randomised trials have been performed, there is remarkable disparity in the recommended dosing (Rice et al., 2007). A study (Cavalli et al., 2020) involving a moderately severe COVID-19 cohort compared a low-dose anakinra (100 mg subcutaneous twice daily) cohort with a high-dose anakinra cohort (10 mg/kg per day intravenously) and a retrospective control cohort; treatment with anakinra was only beneficial for those patients who received the higher dose. Nevertheless, other studies have obtained favourable results with low doses similar to those used at the current centre (Aouba et al., 2020).
This study had several limitations. First, it had a limited number of cases and controls. Second, although great effort was made to select comparable controls for baseline characteristics and disease severity, bias due to unmeasured confounding cannot be excluded. The single-centre design hampered the generalisability of the results to other institutions that might have applied different criteria for the sequential use of tocilizumab and anakinra. Also, a potential beneficial effect of higher doses of anakinra cannot be ruled out. Finally, this study was carried out with patients treated in the first weeks of the pandemic, with high rates of hospital occupancy and limited access to the ICU; therefore, it cannot be ruled out that the prognosis of patients would have been different under other epidemiological context.
This is the first study to assess the effectiveness of anakinra as rescue therapy in patients with tocilizumab-refractory severe COVID-19, using a control group for comparison. It could not demonstrate a beneficial effect of anakinra over standard of care in patients not responding positively to tocilizumab treatment. Nevertheless, the blockade of both cytokine pathways, IL-1 and IL-6, is being evaluated in larger series and ongoing clinical trials, which will help to clarify its clinical usefulness (Maes et al., 2020) and the adequate temporal sequence for its use.
Ethical approval
The study protocol was approved by the University Hospital 12 de Octubre Clinical Research Ethics Committee in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Author contributions
All authors meet the requirements for authorship. C.C, F.L-M, J.L-T, C.L and J.M.A designed the study. C.C, F.L-M, JL.P, M.S-F, MA.P-J, JM.C-T, R.G-G, M.C, J.M.L, A.S, J.O, M.R, R.S.J, A.L, H.B, B.D-M, F.A, C.G and J.M.A screened the patients. C.C. and M.S-F performed acquisition of data. C.C, G.M-D and M.F-R. performed the statistical analysis. C.C, F.L-M, J-L-T, J.L.P, G.M-D, M.S-F, E.P-A, C.L and J.M.A participated in data interpretation and edited the article. C.C. and F.L-M. created the first draft of the article. J.L.P, J.L-T, JM.C-T, J.M.L, A.S, R.G-G, M.R, H.B, C.L, O.C. and JM.A participated in project administration and supervision. C.C. and F.L-M wrote the final draft of the article and made all the changes suggested by the co-authors. All authors had access to the clinical study report, reviewed the article and approved the submission of the article.
Competing interests
All the authors declare no potential conflict of interest regarding this study.
Funding sources
This work was supported by Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation (COV20/00181), co‐financed by European Development Regional Fund “A way to achieve Europe”.
M.F.R. holds a research contract “Miguel Servet” (CP18/00073) from the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III.
Acknowledgements
The authors would like to acknowledge all the healthcare workers involved in the response to the current pandemic in our hospital and, singularly, those who suffered COVID-19.
Appendix
Other members of the H12O Immunomodulation Therapy for COVID-19 Group:
Department of Internal Medicine: Mikel Mancheño-Losa, Ana García-Reyne, Raquel Díaz-Simón; Unit of Infectious Diseases: Manuel Lizasoain, Tamara Ruiz-Merlo, Patricia Parra; Department of Pharmacy: José Miguel Ferrari; Department of Pneumology: Javier Sayas Catalán, Eva Arias Arias; Department of Nephrology: Fernando Caravaca, Amado Andrés, Manuel Praga; Department of Rheumatology: María Martín-López; Department of Haematology: Denis Zafra, Cristina García Sánchez; Department of Medical Oncology: Carmen Díaz-Pedroche, Flora López, Luis Paz-Ares; Department of Intensive Medicine: Jesús Abelardo Barea Mendoza, Paula Burgueño Laguía, Helena Domínguez Aguado, Amanda Lesmes González de Aledo, Juan Carlos Montejo; Department of Emergency Medicine: Antonio Blanco Portillo, Laura Castro Reyes, Manuel Gil Mosquera, José Luis Montesinos Díaz, Isabel Fernández Marín; Department of Immunology: Óscar Cabrera-Marante, Antonio Serrano-Hernández, Daniel Pleguezuelo, Édgar Rodríguez de Frías, Paloma Talayero, Laura Naranjo-Rondán, Ángel Ramírez-Fernández, María Lasa-Lázaro, Daniel Arroyo-Sánchez; Department of Microbiology: Rafael Delgado, María Dolores Folgueira.
References
- Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N. Targeting the inflammatory cascade with Anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- Balkhair A., Al-Zakwani I., Al Busaidi M., Al Mubaihsi S., BaTaher H., Al Aghbari J. Anakinra in hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy: results of a prospective, open-label, interventional study. Int J Infect Dis. 2020;103:288–296. doi: 10.1016/j.ijid.2020.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck N., Suttorp M., Kabus M., Heubner G., Gahr M., Pessler F. Rapid and sustained remission of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome through treatment with anakinra and corticosteroids. J Clin Rheumatol. 2011;17(1):23–27. doi: 10.1097/RHU.0b013e318205092d. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divithotawela C., Garrett P., Westall G., Bhaskar B., Tol M., Chambers D.C. Successful treatment of cytomegalovirus associated hemophagocytic lymphohistiocytosis with the interleukin 1 inhibitor - Anakinra. Respirol Case Rep. 2015;4(1):4–6. doi: 10.1002/rcr2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figuero-Pérez L., Olivares-Hernández A., Escala-Cornejo R.A., Terán-Brage E., López-Gutiérrez A., Cruz-Hernández J.J. Anakinra as a potential alternative in the treatment of severe acute respiratory infection associated with SARS-CoV-2 refractory to tocilizumab. Reumatol Clin. 2020;(June) doi: 10.1016/j.reumae.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grom A.A., Horne A., De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12(5):259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes A.J., Cook B.L., Marks S., Bataillard E., Crossette-Thambiah C., Sivasubramaniam G. Ruxolitinib for tocilizumab-refractory severe COVID-19 infection. Br J Haematol. 2020;10 doi: 10.1111/bjh.16979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University of Medicine . 2021. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)https://coronavirus.jhu.edu/map.html (Accessed January 16 2021) [Google Scholar]
- Maes B., Bosteels C., De Leeuw E., Declercq J., Van Damme K., Delporte A. Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):468. doi: 10.1186/s13063-020-04453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., Cron Q.R., Hartwell J., Manson J., Tattersall S.R. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2(6):e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020;146(1):213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice T.W., Wheeler A.P., Bernard G.R., Hayden D.L., Schoenfeld D.A., Ware L.B. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- Rosas I., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S. Tocilizumab in hospitalized patients with COVID-19 pneumonia. MedRxiv. 2021 doi: 10.1101/2020.08.27.20183442. (pre-print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;384(1):20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah Adnan. Novel Coronavirus-induced nlrp3 inflammasome activation: a potential drug target in the treatment of COVID-19. Front Immunol. 2020;11:1021. doi: 10.3389/fimmu.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas C., Poza M., Talayero P., Caro-Teller J.M., Zafra D., García C. IL-1R blockade is not effective in patients with hematological malignancies and severe SARS-CoV-2 infection. Ann Hematol. 2020:1–4. doi: 10.1007/s00277-020-04160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangrillo A., Beretta L., Scandroglio A., Monti G., Fominskiy E., Colombo S. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;(April) doi: 10.1016/S1441-2772(23)00387-3. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORIMUNO-Collaborative group Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;S2213–2600(20):30556–30557. doi: 10.1016/S2213-2600(20)30556-7. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]