Abstract
Antiviral agents with different mechanisms of action could induce synergistic effects against SARS-CoV-2 infection. Some reports suggest the therapeutic potential of the heme oxygenase-1 (HO-1) enzyme against virus infection. Given that hemin is a natural inducer of the HO-1 gene, the aim of this study was to develop an in vitro assay to analyze the antiviral potency of hemin against SARS-CoV-2 infection. A SARS-CoV-2 infectivity assay was conducted in Vero-E6 and Calu-3 epithelial cell lines. The antiviral effect of hemin, and chloroquine as a control, against SARS-CoV-2 virus infection was quantified by RT-qPCR using specific oligonucleotides for the N gene. Chloroquine induced a marked reduction of viral genome copies in kidney epithelial Vero-E6 cells but not in lung cancer Calu-3 cells. Hemin administration to the culture medium induced a high induction in the expression of the HO-1 gene that was stronger in Vero-E6 macaque-derived cells than in the human Calu-3 cell line. However, hemin treatment did not modify SARS-CoV-2 replication, as measured by viral genome quantification 48 h post-infection for Vero-E6 and 72 h post-infection for the Calu-3 lineages. In conclusion, although exposure to hemin induced strong HO-1 up-regulation, this effect was unable to inhibit or delay the progression of SARS-CoV-2 infection in two epithelial cell lines susceptible to infection.
Keywords: SARS-CoV-2 infection, Antiviral effect, Heme oxygenase-1 induction, Hemin
Graphical Abstract
1. Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has spread globally causing a pandemic of respiratory illness, the so-called coronavirus disease 2019 (COVID-19). Drugs against COVID-19 are urgently needed and promising antiviral agents that delay the progression of SARS-CoV-2 infection are currently being tested in clinical trials [1]. Antivirals could have different mechanisms of action and combined administration of different agents could induce synergistic effects. Some reports have shown an inverse correlation between the induction of the catabolic enzyme heme oxygenase-1 (HO-1, EC: 1.14.14.18) and pathogen infections [2], [3], [4]. A dose response anti-viral effect to hemin treatment has been reported in Ebola-infected HeLa and HFF1 cells [5], MARC-145 cells infected with porcine reproductive and respiratory syndrome virus [6], as well as in A301 and Jurkat cells [7] or human monocytes [8] infected with human immunodeficiency virus (HIV). Indeed, it has been reported that cobalt protoporphyrin (CoPP), a potent HO-1 inducer similar to hemin, inhibits influenza A virus replication through HO-1 interaction with IRF3 and subsequent induction of the expression of IFNα/β [9]. Of note, the antiviral effect of HO-1, mediated by CoPP, was able to control numerous other viral infections, such as those produced by human respiratory syncytial virus, Ebola virus, and HIV by inhibiting virus replication [10], [11], [12].
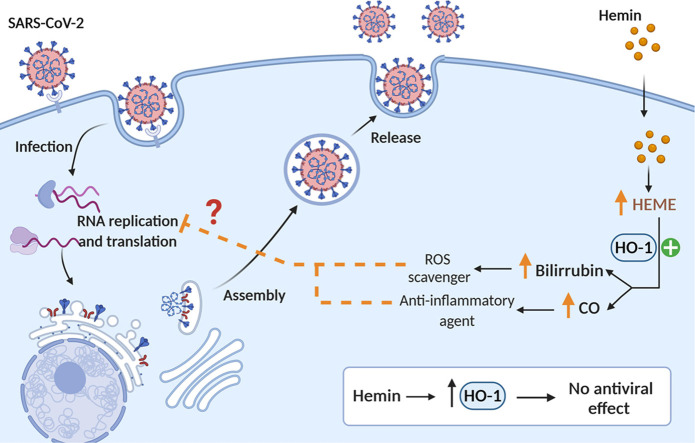
Given that hemin is a natural inducer of the HO-1 gene, and an approved drug for the treatment of acute intermittent porphyria [13] the aim of this study was to develop an in vitro assay to analyze the antiviral potency of hemin treatment against SARS-CoV-2 (Graphical abstract and Fig. 1A).
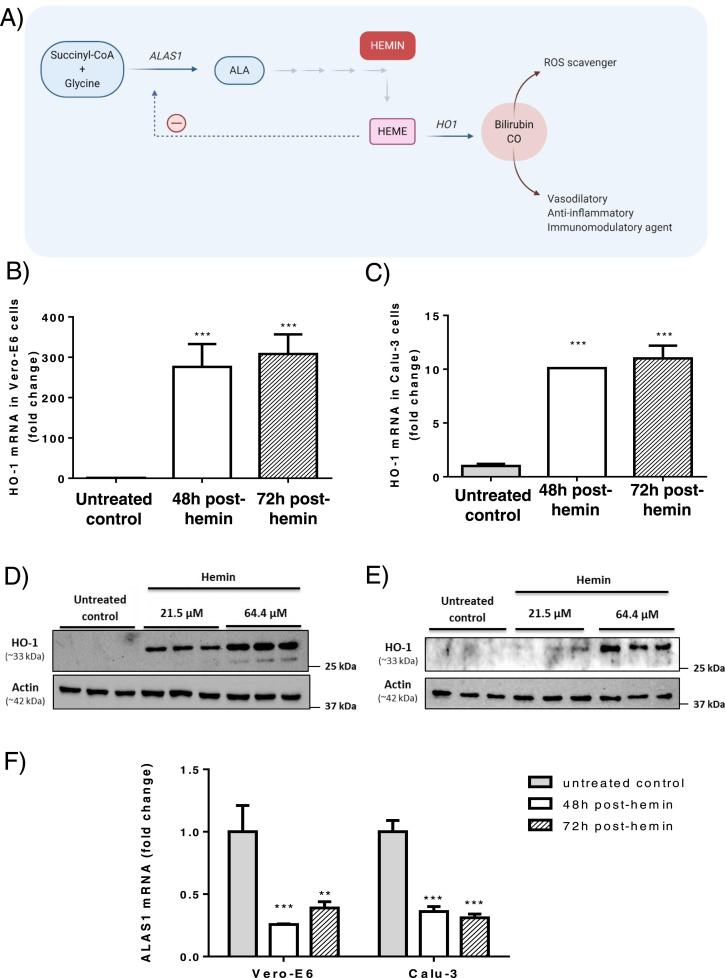
Fig. 1.
Effects of hemin administration on rate limiting enzymes of heme synthesis and catabolism. A) Regulation of heme synthesis and potential antiviral effect of hemin administration. The first and rate limiting step in heme synthesis is the conversion of glycine and succinyl-coenzyme A to form δ-aminolevulinic acid (ALA), catalyzed by ALA synthase-1 (ALAS1). Heme oxygenase-1 (HO-1) is the inducible isoform of HO responsible for the oxidative cleavage of heme groups to yield carbon monoxide (CO) and biliverdin, which is subsequently reduced to bilirubin by the cytosolic biliverdin reductase enzyme. CO is a strong vasodilatory, anti-inflammatory and immunomodulatory agent and both biliverdin reductase B and bilirubin are efficient scavengers of Reactive Oxygen Species (ROS) and reduce the formation of peroxidation products. Fold-change expression of HO-1 in B) Vero-E6 and C) Calu-3 cell lines was measured after exposure to 64.4 µM of hemin. Immunoblot analyses of HO-1 protein levels in D) Vero-E6 and E) Calu-3 cell lines treated with two hemin concentrations (21.5 μM and 64.4 μM) for 48 h. F) Fold change of ALAS1 expression after hemin exposure. Results were plotted as mean ± SD from three experiments. *** p < 0.001 vs untreated control condition.
2. Material and methods
SARS-CoV-2 infectivity assay in cultured cells. SARS-CoV-2 susceptible Vero-E6 (isolated from kidney epithelial cells extracted from an African green monkey, Chlorocebus sp., ATCC® CRL-1586™) and Calu-3 (human lung cancer epithelial cell, ATCC® HTB-55™) cell lines were used. Vero subtype E6 cells have shown to be infected better by SARS-CoV-2 than regular Vero cells [14]. Calu-3 cells have been used to analyze the effect of several antiviral compounds against SARS-CoV-2 [15], [16]. The fact that Calu-3 cells derive from human lung epithelium, a tissue preferentially infected by SARS-CoV-2, as well as the fact that these cells maintain a normal type I interferon response, make them a very good model for testing antivirals in vitro. SARS-CoV-2 virus (isolate NAVARRA-2473) was obtained from the nasal sample of a COVID-19 patient admitted to the University of Navarra Clinic (Pamplona, Spain). Informed consent and the necessary Regional Government permits were obtained. As a first step, the virus was grown in Vero-E6 cells; supernatants were collected at 48 h post-inoculation and titrated by means of a lysis plate assay using Vero-E6 cell monolayers, resulting in a titer of 4.3 × 107 plaque forming units/ml. For inhibition assays, confluent monolayers of Vero-E6 and Calu-3 cells were infected with a low multiplicity of infection (MOIVeroE6: 0.05; MOICALU-3:0.1) in the presence or absence of hemin (64.4 µM) (heme arginate, Normosang®, Recordati) or chloroquine diphosphate salt (10 µM, C6628 Sigma-Aldrich). In a pre-treatment approach, drugs were administered 24 h before infection with SARS-CoV-2. The medium was refreshed every 24 h and supernatants were collected for reverse transcription PCR (RT-qPCR) assays 48 h post-infection for Vero-E6 or 72 h post-infection for Calu-3 lineages using the “iCycler IQ real-time PCR” (ref#170–8740, Bio-Rad, CA, USA) thermal cycler using “iQ SYBR Green Supermix” (ref#1708880, Bio-Rad, CA, USA). Collection times were based on previous studies in which maximum viral titers were obtained between 24 and 72 h and at 72 h post-infection in Vero-E6 and Calu-3 cells, respectively [16].
For HO-1 protein detection, cells were lysed 48 h post-hemin administration in RIPA lysis buffer (50 mM Tris-HCl pH 7.4; 150 mM KCl; 0.1% SDS; 2% NP40 and 10 mM sodium deoxycholate) containing a protease inhibitor cocktail (Roche) and phosphatase inhibitors (1 mM sodium orthovanadate and 1 mM sodium fluoride). Lysates were centrifuged at 250,000 x g at 4⁰C for 20 min and protein concentrations were determined using the Bio-Rad Protein Assay Dye Reagent kit. 20 μg of each sample were resolved by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked for 1 h at RT with 5% milk in Tris-buffered saline with 0.1%Tween 20. Specific primary anti-body were applied overnight at 4ºC and then, blots were probed with HRP-conjugated secondary anti-body for 1 h at RT. Signals were detected using a western lightning Plus ECL kit (Perkin Elmer, Waltham, MA). The primary antibodies used were: rabbit anti-HO1 (Cat. Nº ADI-SPA-895; Enzo Life Sciences) and rabbit anti-Actin (Cat. Nº A2066; Sigma-Aldrich).
Viral RNA was extracted from cell supernatants using Dynabeads™ MyOne™ Silane beads (ThermoFisher) and quantified by RT-qPCR using specific oligonucleotides for the SARS-CoV-2 N gene (sense 5′-GACCCCAAAATCAGCGAAAT-3′, antisense 5′-TCTGGTTACTGCCAGTTGAATCTG-3′), using as a control a standard curve of the NAVARRA-2473 isolate, which was titrated as described above. For the analysis of δ-Aminolevulinate Synthase-1 (ALAS1) and HO-1 gene expression, mRNA was extracted from Vero-E6 and Calu-3 cells using TRIzol (Invitrogen, Thermo Fisher Scientific Inc. Madrid, Spain) and then measured by RT-qPCR with specific primers designed to amplify the genes of both human and non-human primate origin: ALAS1 (sense 5′-GATGATGGAAGTTGGGGCCA-3′, antisense 5′-AAGGGCATTTGCTTGCAGTG-3′, product length: 224 bp); HO-1 (sense: 5′-ACTTCCCAGAAGAGCTGCAC-3′, antisense 5′-GCTTGAACTTGGTGGCACTG-3′, product length: 306 bp); β-actin was used as a control housekeeping gene (sense 5′-GAGCGGGAAATCGTGCGTGACATT-3′, antisense 5′-GAAGGTAGTTTCGTGGATGCC-3′; product length: 225 bp). Results are expressed according to the formula 2ΔCt(Actin) − ΔCt(gene). To test toxicity, Vero-E6 and Calu-3 cells were incubated with hemin at 64.4 µM or chloroquine at 10 µM during 72 h and 96 h, respectively, and cell viability was analyzed using a commercial kit (CellTiter-Gloassay, Promega).
Procedures involving SARS-CoV-2 were performed in a BSL3 (P3 Security) laboratory at CIMA-Universidad de Navarra with the authorization and permissions from the Regional Government of Navarra, Spain.
Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software). Before statistical analysis, data were transformed using the formula log (1 + x) to normalize the variances. Data were analyzed using one-way ANOVA, and pairwise comparisons were made using Bonferroni’s multiple comparison tests. P < 0.05 was used to indicate statistically significant differences.
3. Results
As expected, administration of hemin (64.4 µM) to the culture medium induced a marked increase in the expression of the HO-1 gene (Fig. 1B-C), the inducible isoform of the HO that mediates the regulatory step in heme catabolism. HO-1 up-regulation was stronger in Vero-E6 than in Calu-3 cells and the induction was maintained throughout the exposure period in both cell lines. HO-1 protein levels, as detected by immunoblot, were also strongly induced by hemin (Fig. 1D-E). In fact, HO-1 expression increased with hemin concentrations from 21.5 to 64.4 µM, which was the dose chosen for the anti-viral studies.
The expression of the ALAS1 gene, the rate-limiting enzyme in the heme synthesis pathway was significantly reduced by the presence of the final product of this pathway, i.e. hemin (Fig. 1F). Given that down-regulation of the human ALAS1 gene has been previously documented in the human hepatoma Huh-7 cell line after hemin exposure [17], reduced ALAS1 mRNA levels confirmed efficient hemin uptake in the two cell lines used in the in vitro study. Overall, these data suggest that the induction of HO-1 is the consequence of a regulatory mechanism activated to eliminate the excess of hemin accumulating inside the cells (Fig. 1A).
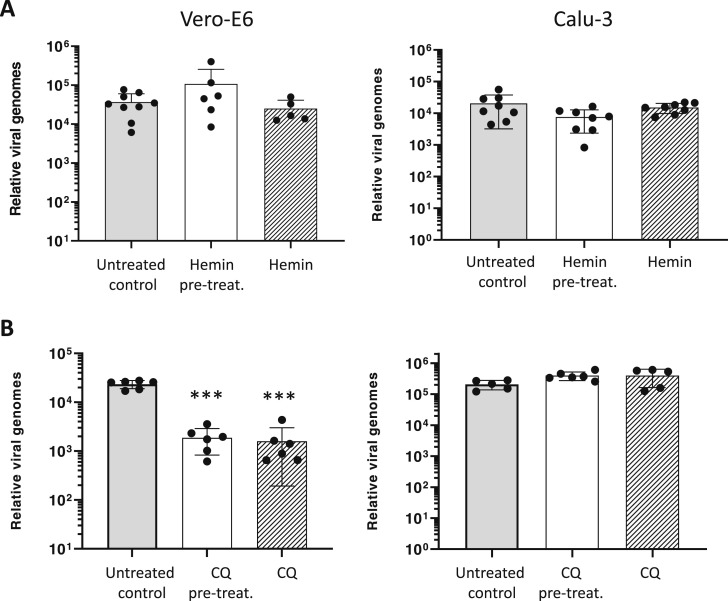
Although hemin administration induced a strong up-regulation of the HO-1 gene in both cell lines, it was not able to inhibit SARS-CoV-2 infection and replication, as measured by viral genome quantification 48 h post-infection for Vero-E6 and 72 h post-infection for Calu-3 cells ( Fig. 2A). These data rule out any potential protective effect of hemin catabolism products against the infection and the replication of the SARS-CoV-2 genome. No toxicity was observed in Vero-E6 and Calu-3 cells incubated with 64.4 µM of hemin, which was measured as described in Methods (data not shown).
Fig. 2.
Hemin did not inhibit SARS-CoV-2 infection in Vero-E6 and Calu-3 cell lines. A) Quantification of SARS-CoV-2 genomes determined by RT-qPCR of supernatants collected at 48 h for Vero-E6 cells or at 72 h for Calu-3 cells in the absence (untreated control) or presence of hemin (64.4 µM) added 24 h before infection (hemin pre-treatment) or at the same time as infection (hemin). B) A similar study was performed using chloroquine (10 µM). In all conditions, the drug was maintained during the incubation period and the medium changed every 24 h. Pre-treat: pre-treatment. CQ: chloroquine. Results were plotted as mean ± SD (one representative experiment is shown of at least two with similar results). *** p < 0.001 vs untreated control condition.
As a control in our study, we verified that the administration of 10 µM of chloroquine significantly inhibited viral replication in Vero-E6 cells but not in Calu-3 cells, as previously reported [18]. These data confirm that the study design was valid to quantify viral infectivity of the SARS-CoV-2 (Fig. 2B).
4. Discussion
The effect of human hemin (heme arginate, Normosang® in Europe and crystallized hemin, Panhematin® in the US, both from Recordati) against SARS-CoV-2 infection was assayed in in vitro assays and compared to chloroquine, whose clinical efficacy is being studied [19]. Various reports showed anti-inflammatory and anti-thrombotic effects of chloroquine therapy that could improve the treatment of COVID-19 patients. Furthermore, it has been described that hydroxychloroquine efficiently blocked viral entry and reduced SARS-CoV-2 genomes in Vero-E6 cells [20], [21]. Thus, chloroquine was used as a positive control to evaluate changes of SARS-CoV-2 genome.
Different reports showed that increased levels of HO-1 serve as a cellular defensive response, because the biliverdin and carbon monoxide (CO), products released in the HO-1 reaction, have immunomodulatory, anti-inflammatory and antiapoptotic properties [2], [3], [4], [5], [6], [7], [8]. Huang and collaborators also suggested an important role for HO-1 induction in primary human monocyte-derived macrophages as an intracellular mediator in restricting Ebola [5] and Zika virus [22] replication. Since most studies testing anti-viral effects of hemin have reported an EC50 ≈ 30 μM, we reasoned that a concentration of 64.4 μM should be adequate to test SARS-CoV-2 inhibition [5]. In addition, hemin is nephrotoxic at high doses and its continued use can generate iron overload and chronic inflammatory hepatic disease [23]. Thus, based on current knowledge of hemin pharmacology in humans, we used a dose of 64.4 µM, which is similar to the concentration that the intravenous administration of 3 mg/kg/day (a total of 210 mg of hemin circulating in 5 L of blood in an individual of 70 kg) would reach, as recommended for the treatment of acute intermittent porphyria attacks [13].
However, in the present study, no direct anti-SARS-CoV-2 effect was observed after the exposure of epithelial cells to hemin. The induction of HO-1 gene was stronger in Vero-E6 macaque-derived cells than in the human Calu-3 cell line, but this strong effect did not modify SARS-CoV-2 infection and replication. Although the number of tested cell lines is limited, we believe that similar results would be expected in other cell lines.
Nevertheless, we cannot rule out that hemin and/or CO could have an effect in vivo associated to the suppression/reduction of the inflammatory signaling pathways and production of pro-inflammatory cytokines, such as TNF-α, IL-6, and IFN-γ, or the inhibition of antigen presentation and subsequent immune activation, as reported for chloroquine.
5. Conclusion
Although exposure to hemin induced a strong expression of the HO-1 gene in two epithelial cell lines susceptible to SARS-CoV-2 infection, this response was not able to inhibit or delay SARS-CoV-2 infection and replication.
Funding
This work was supported in part by grants from Institute of Health Carlos III (PI18/00860) co-funded by European Union (ERDF/ESF, "A way to make Europe"/"Investing in your future")(Spain), Fundación Mutua Madrileña de Investigación Médica (Spain), Fundación Eugenio Rodríguez Pascual (Spain), Fundación Federación Española de Enfermedades Raras para la investigación de enfermedades raras (Spain) and Fundación Mario Losantos del Campo (FMLC)(Spain). Hemin was kindly provided for this study by M. Miñano and C. Salim form Recordati Rare Diseases.
CRediT authorship contribution statement
The conception and design of the study, JA, MAA, GG-A, CS, AF; Methodology, SM, KMC, CO, JA, CS, AF; Acquisition of data, SM, KMC, CO; Formal analysis and interpretation of data, SM, KMC, CS, AF.; Investigation, SM, KMC, CO, CS, AF; Resources, JA, MAA, GG-A, CS, AF; Writing - original draft Preparation and revising it critically for important intellectual content, JA, MAA, GG-A, CS, AF; Funding acquisition, JA, MAA, GG-A, CS, AF; Final approval of the version to be submitted: SM, KMC, CO, JA, MAA, GG-A, CS, AF.
Ethical approval
This study was approved by the Ethics Committee of the University of Navarra (protocol 2020.083).
Conflict of interest statement
Authors declare no conflicts of interest.
References
- 1.Humeniuk R., Mathias A., Cao H., Osinusi A., Shen G., Chng E., Ling J., Vu A., German P. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin. Transl. Sci. 2020;13:896–906. doi: 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svensson L., Mohlin C., Persson K. Upregulation of heme oxygenase-1 as a host mechanism for protection against nitric oxide–induced damage in human renal epithelial cells. Urology. 2009;73:1150–1155. doi: 10.1016/j.urology.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Wang C., Zhang Y., Han L., Guo L., Zhong H., Wang J. Hemin ameliorates influenza pneumonia by attenuating lung injury and regulating the immune response. Int. J. Antimicrob. Agents. 2017;49:45–52. doi: 10.1016/j.ijantimicag.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Park J.-S., Choi H.-S., Yim S.-Y., Lee S.-M. Heme oxygenase-1 protects the liver from septic injury by modulating TLR4-mediated mitochondrial quality control in mice. Shock. 2018;50:209–218. doi: 10.1097/SHK.0000000000001020. 〈https://journals.lww.com/shockjournal/Fulltext/2018/08000/Heme_Oxygenase_1_Protects_the_Liver_from_Septic.12.aspx〉 [DOI] [PubMed] [Google Scholar]
- 5.Huang H., Konduru K., Solovena V., Zhou Z.-H., Kumari N., Takeda K., Nekhai S., Bavari S., Kaplan G.G., Yamada K.M., Dhawan S. Therapeutic potential of the heme oxygenase-1 inducer hemin against Ebola virus infection. Curr. Trends Immunol. 2016;17:117–123. 〈https://pubmed.ncbi.nlm.nih.gov/28133423〉 [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao S., Zhang A., Zhang C., Ni H., Gao J., Wang C., Zhao Q., Wang X., Wang X., Ma C., Liu H., Li N., Mu Y., Sun Y., Zhang G., Hiscox J.A., Hsu W.H., Zhou E.-M. Heme oxygenase-1 acts as an antiviral factor for porcine reproductive and respiratory syndrome virus infection and over-expression inhibits virus replication in vitro. Antivir. Res. 2014;110:60–69. doi: 10.1016/j.antiviral.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Shankaran P., Vlkova L., Liskova J., Melkova Z. Heme arginate potentiates latent HIV-1 reactivation while inhibiting the acute infection. Antivir. Res. 2011;92:434–446. doi: 10.1016/j.antiviral.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Devadas K., Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J. Immunol. 2006;176:4252–4257. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- 9.Ma L.-L., Zhang P., Wang H.-Q., Li Y.-F., Hu J., Jiang J.-D., Li Y.-H. heme oxygenase-1 agonist CoPP suppresses influenza virus replication through IRF3-mediated generation of IFN-α/β. Virology. 2019;528:80–88. doi: 10.1016/j.virol.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Espinoza J.A., León M.A., Céspedes P.F., Gómez R.S., Canedo-Marroquín G., Riquelme S.A., Salazar-Echegarai F.J., Blancou P., Simon T., Anegon I., Lay M.K., González P.A., Riedel C.A., Bueno S.M., Kalergis A.M. Heme oxygenase-1 modulates human respiratory syncytial virus replication and lung pathogenesis during infection. J. Immunol. 2017;199:212–223. doi: 10.4049/jimmunol.1601414. [DOI] [PubMed] [Google Scholar]
- 11.Espinoza J.A., González P.A., Kalergis A.M. Modulation of antiviral immunity by heme oxygenase-1. Am. J. Pathol. 2017;187:487–493. doi: 10.1016/j.ajpath.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Hill-Batorski L., Halfmann P., Neumann G., Kawaoka Y. The cytoprotective enzyme heme oxygenase-1 suppresses Ebola virus replication. J. Virol. 2013;87:13795–13802. doi: 10.1128/JVI.02422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson K.E., Bloomer J.R., Bonkovsky H.L., Kushner J.P., Pierach C.A., Pimstone N.R., Desnick R.J. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann. Intern. Med. 2005;142:439–450. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- 14.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U. S. A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schäfer A., Dinnon K.H., 3rd, Stevens L.J., Chappell J.D., Lu X., Hughes T.M., George A.S., Hill C.S., Montgomery S.A., Brown A.J., Bluemling G.R., Natchus M.G., Saindane M., Kolykhalov A.A., Painter G., Harcourt J., Tamin A., Thornburg N.J., Swanstrom R., Denison M.R., Baric R.S. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu H., Chan J.F.-W., Yuen T.T.-T., Shuai H., Yuan S., Wang Y., Hu B., Yip C.C.-Y., Tsang J.O.-L., Huang X., Chai Y., Yang D., Hou Y., Chik K.K.-H., Zhang X., Fung A.Y.-F., Tsoi H.-W., Cai J.-P., Chan W.-M., Ip J.D., Chu A.W.-H., Zhou J., Lung D.C., Kok K.-H., To K.K.-W., Tsang O.T.-Y., Chan K.-H., Yuen K.-Y. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng J., Shan Y., Lambrecht R.W., Donohue S.E., Bonkovsky H.L. Differential regulation of human ALAS1 mRNA and protein levels by heme and cobalt protoporphyrin. Mol. Cell. Biochem. 2008;319:153–161. doi: 10.1007/s11010-008-9888-0. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann M., Mösbauer K., Hofmann-Winkler H., Kaul A., Kleine-Weber H., Krüger N., Gassen N.C., Müller M.A., Drosten C., Pöhlmann S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020;585:588–590. doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Wang Y., Agostinis P., Rabson A., Melino G., Carafoli E., Shi Y., Sun E. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11:512. doi: 10.1038/s41419-020-2721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou T., Mou H., Zhang L., Ojha A., Choe H., Farzan M. Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. PLOS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R., Naninck T., Pizzorno A., Lemaitre J., Gonçalves A., Kahlaoui N., Terrier O., Fang R.H.T., Enouf V., Dereuddre-Bosquet N., Brisebarre A., Touret F., Chapon C., Hoen B., Lina B., Calatrava M.R., van der Werf S., de Lamballerie X., Le Grand R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:584–587. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 22.Huang H., Falgout B., Takeda K., Yamada K.M., Dhawan S. Nrf2-dependent induction of innate host defense via heme oxygenase-1 inhibits Zika virus replication. Virology. 2017;503:1–5. doi: 10.1016/j.virol.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt C., Lenglet H., Yu A., Delaby C., Benecke A., Lefebvre T., Letteron P., Paradis V., Wahlin S., Sandberg S., Harper P., Sardh E., Sandvik A.K., Hov J.R., Aarsand A.K., Chiche L., Bazille C., Scoazec J.-Y., To-Figueras J., Carrascal M., Abian J., Mirmiran A., Karim Z., Deybach J.-C., Puy H., Peoc’h K., Manceau H., Gouya L. Recurrent attacks of acute hepatic porphyria: major role of the chronic inflammatory response in the liver. J. Intern. Med. 2018;284:78–91. doi: 10.1111/joim.12750. [DOI] [PubMed] [Google Scholar]