Abstract
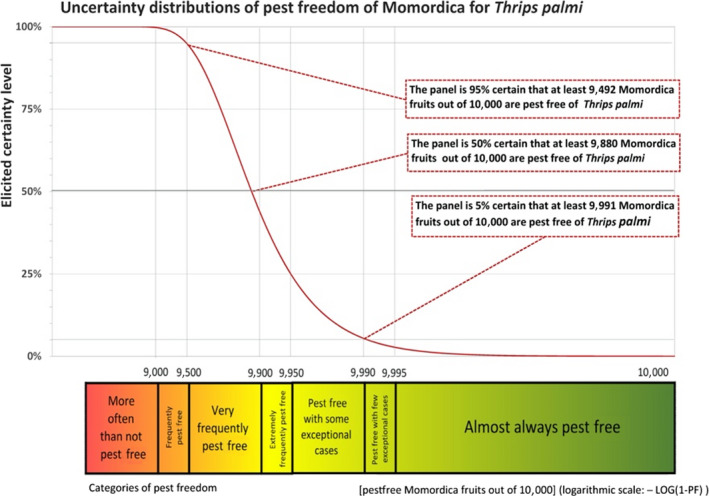
The European Commission requested the EFSA Panel on Plant Health to prepare and deliver risk assessments for commodities listed in Commission Implementing Regulation (EU) 2018/2019 as ‘High risk plants, plant products and other objects’. Momordica fruits originating from countries where Thrips palmi is known to occur qualify as high risk plants. This Scientific Opinion covers the introduction risk for T. palmi posed by fruits of Momordica charantia L. imported from Mexico, taking into account the available scientific information, including the technical information provided by the National Service of Health, Safety and Agrifood Quality (Senasica) of Mexico. The risk mitigation measures proposed in the technical dossier from Mexico were evaluated taking into account the possible limiting factors. An expert judgement is given on the likelihood of pest freedom taking into consideration the potential pest pressure in the field, the risk mitigation measures acting on the pest in the field and in the packinghouse, including uncertainties associated with the assessment. For T. palmi on M. charantia fruits from Mexico, an expert judgement is given on the likelihood of pest freedom following the evaluation of the risk mitigation measures acting on T. palmi, including any uncertainties. The Expert Knowledge Elicitation indicated, with 95% certainty that between 9,492 and 10,000 M. charantia fruits per 10,000 will be free from T. palmi.
Keywords: European Union, plant health, plant pest, quarantine, Thrips palmi, Momordica charantia, bitter gourd, bitter melon, melon thrips
1. Introduction
1.1. Background and Terms of Reference as provided by European Commission
1.1.1. Background
The new Plant Health Regulation (EU) 2016/20311, on the protective measures against pests of plants, has been applied from December 2019. Provisions within the above Regulation are in place for the listing of ‘high risk plants, plant products and other objects’ (Article 42) on the basis of a preliminary assessment, and to be followed by a commodity risk assessment. A list of ‘high risk plants, plant products and other objects’ has been published in Regulation (EU) 2018/20192. Scientific opinions are therefore needed to support the European Commission and the Member States in the work connected to Article 42 of Regulation (EU) 2016/2031, as stipulated in the terms of reference.
1.1.2. Terms of reference
EFSA is expected to prepare and deliver risk assessments for commodities listed in the relevant Implementing Act as “High risk plants, plant products and other objects”. Article 42, paragraphs 4 and 5, establishes that a risk assessment is needed as a follow‐up to evaluate whether the commodities will remain prohibited, removed from the list and additional measures will be applied or removed from the list without any additional measures. This task is expected to be on‐going, with a regular flow of dossiers being sent by the applicant required for the risk assessment.
In view of the above and in accordance with Article 29 of Regulation (EC) No. 178/2002, the Commission asks EFSA to provide a scientific opinion in the field of plant health for Momordica charantia fruits from Mexico taking into account the available scientific information, including the technical dossier provided by Mexico.
1.2. Interpretation of the Terms of Reference
The EFSA Panel on Plant Health (hereafter referred to as ‘the Panel’) was requested to conduct a commodity risk assessment of Momordica charantia fruits from Mexico following the Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019).
As stated in the EU implementing regulation 2018/2019, fruits of Momordica L. are known to host and provide a significant pathway for the introduction and establishment of the pest Thrips palmi Karny, which is known to have the potential to have a major impact on plant species which are of a major economic, social or environmental importance to the Union territory. However, this pest does not occur in all third countries nor in all areas within a third country where it is known to occur. Certain third countries also have effective mitigation measures in place for that pest. In view of this, fruits of Momordica L. that originate in third countries or parts thereof where T. palmi is known to occur and which lack effective mitigation measures for T. palmi, qualify as high‐risk plants, within the meaning of Article 42(1) of Regulation (EU) 2016/2031, and therefore, the introduction into the Union of those plants should be provisionally prohibited. Where demand for the importation of these plant products is identified, a risk assessment will be carried out in accordance with an implementing act to be adopted pursuant to Article 42(6) of Regulation (EU) 2016/2031.
Based on the information provided in the dossier, the panel will make an assessment to evaluate if the mitigation measures against T. palmi on M. charantia fruits from Mexico are effective to substantiate pest freedom. When necessary, additional information was requested to the applicant.
Risk management decisions are not within EFSA's remit. Therefore, the Panel provided a rating based on expert judgement regarding the likelihood of pest freedom for T. palmi given the risk mitigation measures proposed by the applicant.
2. Data and methodologies
2.1. Data provided by the National Service of Health, Safety and Agrifood Quality (Senasica) of Mexico
The Panel considered all the data and information (hereafter called ‘the Dossier’) provided by the National Service for Health, Safety and Agrifood Quality (Senasica) of Mexico on 01 November 2019, including the additional information provided by Senasica of Mexico on 09 July 2020 after EFSA's request. The Dossier is managed by EFSA.
The structure and overview of the Dossier is shown in Table 1. The number of the relevant section is indicated in the opinion when referring to a specific part of the Dossier.
Table 1.
Structure and overview of the Dossier and the additional material submitted by Senasica of Mexico
| Dossier section | Overview of contents | Filename |
|---|---|---|
| 1 | Main document‐dossier | ANEXO 10256.pdf |
| 2 | Official Letter to the EU with additional information | 10256‐ INFORMACION TECNICA DE MELON AMARGO A LA UNION EUROPEA.pdf |
| 3 | Point by point reply to requested additional information by EFSA |
OFICIO 5134 EXPEDIENTE MOMORDICA CHARANTIA UE09‐07‐2020‐182615.pdf (in Spanish) Translation_ID 95_OFICIO 5134 EXPEDIENTE MOMORDICA CHARANTIA UE09‐07‐2020‐182615_EN.docx |
2.2. Literature searches performed by EFSA
A literature search was undertaken by EFSA to assess the state of the art regarding 1) the pest pressure in the applicant country; 2) efficacy of pre‐ and post‐harvest measures applied to control T. palmi; 3) efficacy of insecticides to control T. palmi. The searches were run on 29/6/2020 (Appendix B). No language, date or document type restrictions were applied in the search strategy. Additional searches, limited to retrieve documents, were run when developing the opinion. The available scientific information, including previous EFSA opinions on the relevant pest (see pest data sheets in Appendix A) and the relevant literature and legislation (e.g. Regulation (EU) 2016/2031; Commission Implementing Regulations (EU) 2018/2019; (EU) 2018/2018 and (EU) 2019/2072) were taken into account.
2.3. Methodology
When developing the opinion, the Panel followed the EFSA Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019). Therefore, the proposed risk mitigation measures for T. palmi were evaluated in terms of efficacy or compliance with EU requirements as explained in Section 1.2. A conclusion on the likelihood of the commodity being free from T. palmi was determined and uncertainties identified using expert judgements. Pest freedom was assessed by estimating the number of infested fruits out of 10,000 exported fruits.
2.3.1. Listing and evaluation of risk mitigation measures
All currently used risk mitigation measures in the country of export were listed and evaluated.
The risk mitigation measures adopted in the production places and packinghouses as communicated by Senasica were evaluated with Expert Knowledge Elicitation (EKE) according to the Guidance on uncertainty analysis in scientific assessment (EFSA Scientific Committee, 2018).
Estimates of the pest pressure of T. palmi in the production places and the effect of the mitigation measures taken in the field during production and the post‐harvest mitigation measures taken in the packinghouse were summarised in a pest data sheet (see Appendix A).
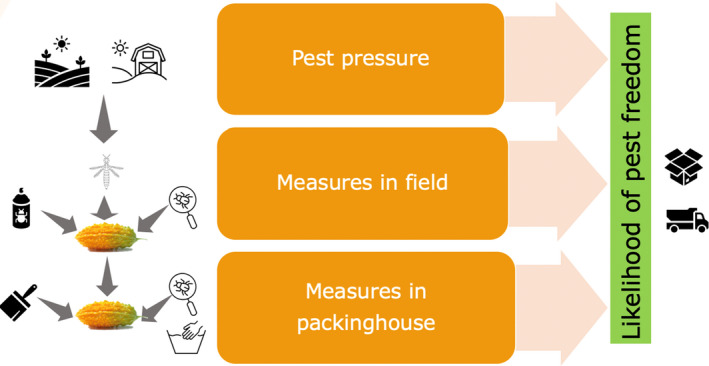
To estimate the pest freedom of the commodity, a three‐step approach was adopted following EFSA guidance (Annex B.8 of EFSA Scientific Committee, 2018). Therefore, three independent elicitations were conducted i.e. one to estimate the pest pressure in the field; one to estimate the efficacy of mitigation measures applied in the field; and a final one to estimate the efficacy of post‐harvest mitigation measures applied in the packinghouse. Combining these three estimations, the level of pest freedom for T. palmi on M. charantia fruits from Mexico was determined (see Section 2.3.2). The final result indicates how many fruits out of 10,000 will be infested with T. palmi when arriving in the EU.
The uncertainties associated with the EKE were taken into account and quantified in the probability distribution applying the semi‐formal method described in Section 3.5.2 of the EFSA‐PLH Guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Finally, the results were reported in terms of the likelihood of pest freedom. The lower 5% percentile of the uncertainty distribution reflects the opinion that pest freedom is with 95% certainty above this limit.
2.3.2. Conceptual model for risk of entry
The risk of entry of Thrips palmi via import of M. charantia fruits from Mexico was estimated in three steps using a formal conceptual model. In this model, the estimated pest pressure is used as starting point and corrected by the independent effects of measures in the field and in the packing house. The result of this model is the level of infestation at import calculated as follows:
All values are expressed in numbers of fruits out of 10,000.
The input parameters ppressure, pfield and ppacking are determined by separate Expert Knowledge Elicitations (EKE). The uncertainties associated with the EKE were taken into account and quantified in the probability distribution applying the semi‐formal method described in Section 3.5.2 of the EFSA‐PLH Guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018).
The model results rimport and PFimport were calculated using Monte Carlo simulation. A final distribution is fitted to the simulation results.
Finally, the results were reported in terms of the likelihood of pest freedom. The lower 5% percentile of the uncertainty distribution reflects the opinion that pest freedom is with 95% certainty above this limit.
3. Thrips palmi
3.1. Biology of the pest
Thrips palmi Karny (Thysanoptera: Thripidae), commonly known as melon thrips, oriental thrips and southern yellow thrips, was first described in 1925 from Sumatra and Java (Indonesia) (Karny, 1925). The species previously had the common name ‘palm thrips’; however, no palm species are known to host this pest and the origin of this name is in honour of Dr B.T. Palm, a well‐known specialist of this group.
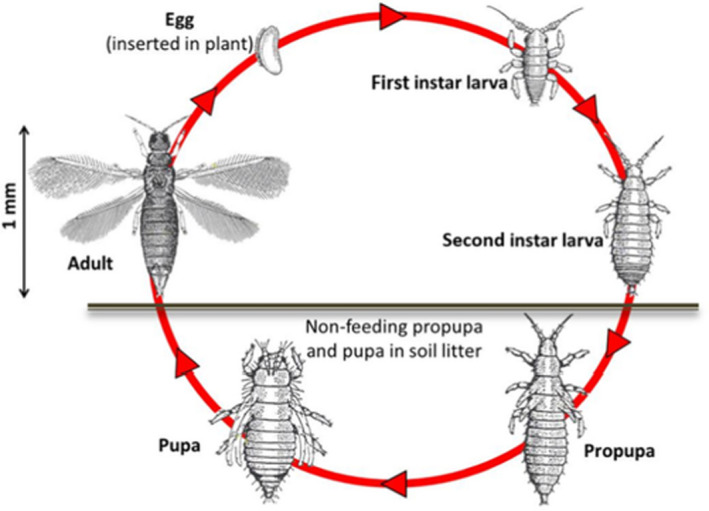
At 25°C, the life cycle from egg to egg lasts 17.5 days (OEPP/EPPO, 1989). The life cycle differs little from that of most phytophagous Thripidae (Figure 1). The adults emerge from the pupa in the soil and consequently, move to the leaves, flowers and fruits of the plant, where they lay their eggs in an incision made with the ovipositor. They preferably lay their eggs in young growing tissue of leaves, and also the flowers and fruit of a wide range of host plants, especially Cucurbitaceae, Solanaceae and Leguminosae. The two larval stages (LI and LII) and male and female adults feed on the maturing leaves, stems, flowers and flower petals and surfaces of fruits. They suck the contents of tissue cells with their specialised mouthparts, leaving them empty, causing silvery scars or leaf bronzing. The second‐stage larva drops from the plant to the soil (or packing cases or growing medium) and completes its cycle by pupating (pupa I and pupa II) in the substrate (EPPO, 2018a,b).
Figure 1.
Conceptual framework to assess the likelihood of pest freedom for Thrips palmi on Momordica charantia fruits
The life cycle and population dynamics of T. palmi in Japan have been reviewed by Kawai (1990).
Figure 2.
Life cycle of Thripidae (e.g. Thrips palmi)
Thrips palmi is primarily a subtropical and tropical species. Tsumuki et al. (1987) analysed the cold hardiness of T. palmi in Japan and concluded that it could not survive outdoor winter conditions in southern Honshu island.
Sakimura et al. (1986) set the outdoor northern limit to 34°N, which corresponds to the very south of Honshu. However, Nagai and Tsumuki (1990) reported no reduction of adult populations at temperatures as low as from −3 to −7°C on weeds in an unheated glasshouse between mid‐January and mid‐February in Japan. Developmental time decreased with increasing temperature up to 32.5°C in all stages. The total developmental time was longest at 12.5°C (64.2 days) and shortest at 32.5°C (9.2 days), 12.7 days at 25°C (Park et al., 2010). The mean developmental time for the egg stage varied between 24 days at 12.5°C, 6–7 days at 25°C, 4–5 days at 30°C and 3.3 days at 32.5°C (Park et al., 2010). Developmental times varied, however, between different lab assays, host plants, photoperiod etc. by a few days between different experiments in particular larval development at lower temperatures. The lower developmental threshold was 10.6, 10.6, 9.1, and 10.7°C for egg, larva, prepupa and pupa, respectively. The thermal constant required to complete the respective stage was 71.7, 59.2, 18.1 and 36.8 degree‐days (DD). The lower threshold temperature and thermal requirements varied a bit between different studies ranging from 10.1°C and 194 DD (McDonald et al., 1999) and 10.6°C and 183.3 DD for egg to adult development (Park et al., 2010) to 11.3°C and 196 DD (Yadav and Chang, 2014) and 11.6°C and 189.1 DD (Kawai, 1985).
Parthenogenesis (arrhenotoky) in T. palmi has been reported by Yoshihara and Kawai (1982). The oviposition behaviour of the species was observed in Taiwan (Wang et al., 1989); a preoviposition period of 1–3 days for virgin females and 1–5 days for mated ones was recorded. Virgin females laid 3–164 eggs (1.0–7.9 eggs per day) during their lifespan, while mated females laid 3–204 eggs (0.8–7.3 eggs per day). At 25°C, the net reproductive rate (28.0), female fecundity (59.6 eggs/female) and daily oviposition rate (3.8 eggs/day) reached the maximum level (Kawai, 1985). At the optimum temperature for population growth (25–30°C), the number of generations was estimated in 25–26/year (Huang and Chen, 2004). Significant differences in population growth among crops were highlighted (Kawai, 1986). The survival rates of the larval and pupal stages fed on cucumber, kidney bean, eggplant and balsam pear were high, whereas the survival rates of those fed on okra and chrysanthemum were low. The larvae fed on tomato and strawberry were unable to pupate. Duration of the larval and pupal stages fed on chrysanthemum and okra was longer than the duration of those fed on other crops. The longevity of the adults fed on cucumber, pumpkin, eggplant and kidney bean was increased, whereas the longevity of those fed on chrysanthemum, tomato and strawberry was decreased. The fecundity of adult females (no eggs/female) fed on cucumber was maximum (60), while the fecundity of those fed on melon, eggplant and pumpkin amounted to 20. The differences in the generation time were not significant among crops, unlike the differences in the net reproductive rate. The intrinsic rate of natural increase of T. palmi fed on cucumber was maximum and the value was 0.134, while that of T. palmi fed on melon, eggplant and pumpkin ranged from 0.08 to 0.11 (Kawai, 1986).
3.2. Symptoms
3.2.1. General symptoms
On plant material, at inspection, silvery feeding scars on the leaf surface, especially alongside the midrib and veins, can be seen (Cannon et al., 2007). Heavily infested plants are characterised by a silvered or bronzed appearance of the leaves, stunted leaves and terminal shoots. At high densities, feeding by T. palmi may cause damage to fruits (Kawai, 1986) as well, such as scarring, discoloration and deformation in developed fruits or fruit abortion in an early stage. Cucumber, eggplant and pepper fruit are damaged when thrips feed in the blossoms. Symptoms may be found on all parts of a wide range of plant species (Sakimura et al., 1986). Although T. palmi feeds on Momordica sp., no specific information of symptoms and damage caused to fruits of M. charantia is available.
3.2.2. Pest density of Thrips palmi in fruits
Despite its wide host range, including fruits and vegetables, the information about the actual pest density levels of T. palmi itself in various crops is limited. Most relevant papers measure the economic injury level (EIL) and the economic threshold (ET), which are calculated by the damage caused by the pest correlated with pest density. Yet, no information has been found of EILs and ETs calculated for T. palmi infestations in M. charantia under greenhouse or semi‐field conditions in particular.
Rosenheim et al. (1990) recorded that in cucumber, densities of T. palmi (number per unit area of plant substrate) were greatest on foliage, and lowest on fruits, with an average ratio of 0.55 per female flower and 0.19 per fruit compared to foliage. During the early stage of development, fruits physically support the female flowers, but as the densities of T. palmi in flowers are low, the opportunities for them to incidentally feed upon and scar young fruit are low as well, this in contrast to Frankliniella occidentalis.
At high densities, T. palmi feeding may cause damage to fruits (Kawai, 1986; Welter et al., 1990). No records, however, are available specifically for M. charantia, and data available in literature for cucumber likely better reflect the incidence on M. charantia than those on Solanaceous crops like eggplant or sweet pepper. Kawai (1985) estimated EILs for cucumber the tolerable density of adults – at a constant high density – at 4.4 per leaf for uninjured fruit yield and at 5.3 adults per leaf for the total fruit yield (at a level of yield loss of 5%) and 8.8 adults per leaf (at a level of yield loss of 10%). In addition, Kawai (1990) reported EILs of 0.08 adults per leaf for eggplant and 0.11 adults per flower for sweet pepper. In other studies, in Japan, EILs were estimated at densities of 1–10 adults per cucumber leaf or 2–3 adults or larvae per pepper flower in south Florida, USA (Capinera, 2020). In case of high infestations in eggplant, less fruits are produced and of smaller size (Yadav and Chang, 2013). They recommended as an action threshold 1.05–1.50 thrips per flower or 4.91–10.17 adults per sticky trap over a 4‐day period. Welter et al. (1990) calculated an action threshold of 94 thrips/cucumber leaf early in the growing season, showing that an EIL for fruits is relatively high for T. palmi. EILs are quite variable and differ per crop, per country and timing in the season and ETs depend on variable and dynamic economic factors such as costs for control, labour, yield, market price etc. (Pedigo et al., 1986). Yadav and Chang (2013) indicated that the percentage of fruit damage correlates with the population dynamics of the thrips. Besides, thrips‐related fruit damage in eggplants can best be evaluated in terms of the damaged fruit percentage, not in terms of yield loss.
3.3. Confusion with other pests
Thrips palmi identification is hampered by its small size and great similarity with other yellow species of thrips. Indeed, T. palmi can be mistaken for common thrips species with similar characteristics, e.g. T. flavus Schrank and T. tabaci Lindeman distributed worldwide, T. alatus Bhatti and T. pallidulus Bagnall in the Oriental region, T. nigropilosus Uzel and T. alni Uzel in the Palaearctic region and T. urticae Fabricius in Europe. For the distinction between look‐alike species, microscopic examination by a seasoned expert of the morphological characteristics is required, or by molecular analysis (EPPO, 2018a,b).
3.4. Effectiveness of control options worldwide
A variety of chemical, cultural, biological and physical measures is used by growers across the world to manage T. palmi (Morse and Hoddle, 2006; Cannon et al., 2007), to prevent or maintain populations at a very low‐density level. Management measures include the use of systemic and contact insecticides, insecticidal soaps, essential oils/plants extracts, soil treatments, the use of resident or introduced natural enemies, exclusion of the crops by physical barriers such as windbreaks, screenhouses, row covers, bagging of fruits, covering the soil with organic or plastic mulch or film, the removal of alternative weed hosts, trap crops (Salas, 2004), alternation of susceptible crops (Young and Zhang, 1998; Maltby and Walsh, 2005) and the use of less susceptible cultivars. Each of them separately has an effect, to restrict the entry and colonisation of the crop, to limit or suppress population growth (Kawai, 1990; Matsui et al., 1995; Shibao, 2016).
Other techniques are used to monitor the number of thrips in order to establish the level and distribution of thrips infestation in a crop, such as the use of sticky traps, alone or with lures or pheromones, water pan traps, sampling of leaves and leaf beating. Monitoring results can be used to establish the distribution in a crop, to establish economic threshold levels and to facilitate the decision‐making for which and when measures need to be taken to manage T. palmi infestations (Sánchez et al., 2011; Nakamura et al., 2014; Shibao and Tanaka, 2014; Thongjua et al., 2015; Shibao, 2016; Dong and Hsiu, 2019).
3.5. Detection and monitoring
3.5.1. Sampling
Thrips palmi adults and larvae generally are found on the foliage: adults aggregate on the young vegetative parts, sometimes in the flowers, larvae on the underside of maturing leaves, concentrated in the upper third part of the crop (Kawai, 1990; Bacci et al., 2008; Zhang et al., 2014). Which parts of the plant best reflect the relationship between the density of thrips and the resulting damage depends on the crop type: flowers in orchids (Maketon et al., 2014) and eggplant (Yadav and Chang, 2013), leaves in cucumber (Bacci et al., 2008) and bean (Osorio and Cardona, 2003). The number of leaves or flowers sampled depends on the crop, stage of infestation, the experimental set‐up etc. For cucumber, reflecting best a bitter gourd crop, the best sampling size consisted of 35 leaflets per field or 40 leaflets per ha (Osorio and Cardona, 2003), taken at random from the uppermost part of plants to establish the action threshold.
3.5.2. Monitoring with traps
Adults can be sampled with water pan traps, sticky traps and LED light traps. The use of sticky traps is common practice around the world for monitoring thrips, whereas water pan traps are uncommon and LED light traps not yet implemented at a commercial level. Blue and white have shown to be attractive colours for monitoring T. palmi in cucumber, eggplant and sweet pepper (Kawai, 1983; Kawai and Kitamura, 1987, 1990; Kawai, 1990; Yadav and Chang, 2013; Zhang et al., 2014) or wax gourd respectively (Huang, 1989); for some crops, e.g. in orchids, yellow is more attractive (Culliney, 1990; Thongjua et al., 2015; Maketon et al., 2014). Besides trap colour and relation to the background colour of the crop and the environment, its efficacy in a crop also depends on placement height in the crop (upper third). In recent years a combination of LED lights covered by transparent plates show that T. palmi is attracted to light at wavelengths from 500 to 525 nm (Hajime et al., 2014; Shibao and Tanaka, 2014).
3.6. Management options
3.6.1. Chemical control
Contact and systemic insecticides combined with insecticidal soaps, essential oils/plant extracts, are frequently applied for suppression of T. palmi, in particular during the first years after invading a new area or when the pest needs to be eradicated (MacLeod et al., 2004; Cannon et al., 2007). Then, efficacy of control can be very high (90–95%) when timely and regularly applied. However, application of insecticides alone is not an adequate tool to control T. palmi because the eggs (in the foliar tissue) and the pupae (in the soil) are relatively insensitive to insecticide application. Given the polyphagous nature of T. palmi and the short life cycle, the population density in the surrounding environment of a crop may be very high and this may require repeated insecticide applications.
In addition, T. palmi is able to develop insecticide resistance already after a few years requiring alternation of different active ingredients which most often do not match with integration of biological or integrated control methods. Insecticide resistance in T. palmi was recorded as early as 1994 (Nozawa et al., 1994). In recent years, resistance has been recorded in Asia for insecticides such as cypermethrin (Kim et al., 2019; Ghosh et al., 2020), imidacloprid (Bao et al., 2015; Kim et al., 2019; Ghosh et al., 2020), and in particular spinosad (Kim et al., 2019) and spinetoram (Gao et al., 2019; Shi et al., 2020). Field populations in Korea also showed reduced mortality to emamectin benzoate, chlorfenapyr, cyantraniliprole and dinotefuran (Kim et al., 2019). Resistance varies geographically and locally (Kim et al., 2019). To slow down insecticide resistance, it is important to apply insecticides that are effective in a rotation programme.
3.6.2. Mass trapping
Mass trapping with sticky traps/ribbons can reduce the numbers of T. palmi in some crops, such as sweet pepper and eggplant (Kawai, 1990, 2001; Murai, 2002). When these ribbons were set every 2–3 m2 in a greenhouse, the density of T. palmi was reduced 10–20% compared to that in greenhouses without ribbons (Nonaka and Nagai, 1984). In strawberry, it could reduce adult thrips (F. occidentalis) numbers per flower by 61% and fruit bronzing by 55% (Sampson and Kirk, 2013). However, in these and other studies on thrips (see Sampson and Kirk, 2013), either no assessment of crop damage was made, or it failed to prevent damage (Trdan et al., 2005 for T. tabaci in onion crops), and therefore, no evidence is available of its economic viability. Nevertheless, mass trapping could be cost‐effective at an early stage of invasion (Kawai and Kitamura, 1987, 1990), in high‐value crops (Sampson and Kirk, 2013) and when part of an overall IPM programme. As a part of a combination of measures, it could maintain thrips numbers below the damage threshold during specific periods of preharvest, when pesticides cannot be used because of residue levels.
3.6.3. Cultural control
Several cultural practices can effectively reduce the level of infestation by T. palmi. Physical barriers hampering the access to the host plants can protect a crop from infestation, such as windbreaks, growing the crop in glasshouses or fine meshed screenhouses, crop covers and or row covers, bagging of fruits, covering the soil with organic or plastic mulch or silver plastic or spraying kaolin. Additionally, intercropping, the use of trap plants and the removal of alternative weed hosts (Salas, 2004; Cannon et al., 2007) (Kawai, 2001; Salas, 2004; Ingrid et al., 2012; Shibao, 2016; Shirotsuka et al., 2016; Razzak and Seal, 2017; Razzak et al., 2018) also contributes to a better crop hygiene and thus a lower infestation level. Population build‐up is often hampered by periods of heavy rains in the open field (Huang, 1989; Etienne et al., 1990), but overhead irrigation of the crop does not. Cultural control measures can be part of a systems approach for the control of T. palmi.
3.6.3.1. Fruit bagging
Preharvest fruit bagging is an extensively used practice in many countries around the world (Faci et al., 2014; Sharma et al., 2014; Shen et al., 2014). The use is twofold, it ensures homogeneity, aesthetics and quality of the product and it protects against diseases and pests, such as fruit flies (Tephritidae) and fruit borers (Lepidoptera). In the literature, there is not so much information for the effect on the prevention of damage by thrips, indicating it is primarily for other insect pests.
Few studies have been performed on the use of fruit bagging in reducing the incidence of thrips pests: Affandi et al. (2008) found a reduction in scarring of mango fruits (caused by an unspecified species of thrips) of 32–42% in Indonesia using double‐layered bags of plastic and paper. Karar et al. (2019) found that harvested fruits of mango in closed paper bags (brown paper inner black and butter – wet resistant/greaseproof – paper) were 100% free of (unspecified) thrips in Pakistan. Martins (2018) noticed a 30–50% reduction in lesions caused by F. brevicaulis in Brazil, and according to de López et al. (2020) bagging alone of bananas reduced losses by 90–100% by the red rust thrips (Chaetanaphothrips signipennis) compared to bunches with no bags. In banana plantings, covering bunches with polyethylene bags during fruit development provides a physical barrier to insect infestations, but bags cannot fully protect the fruit when a thrips infestation is heavy (Hara et al., 2002). No records have been found in literature on the effect of preharvest fruit bagging of M. charantia fruits.
3.6.4. Biological control
Macroorganisms
Augmentative biological control by seasonal or inundative releases of natural enemies such as predatory mites, (e.g. Neoseoiulus spp. or Amblyseius spp.) or predatory bugs (e.g. Orius spp.) can be very effective in greenhouses or in an outdoor Mediterranean climate when other crop pests are carefully managed and applications are timely made. Other generalist predators such as lacewings (Chrysoperla spp.), mirid bugs (Macrolophus spp.) or lady bugs (Coccinellidae) can prey on T. palmi, but will predominantly target preys which are prevalent, and thus only partly contribute to thrips control (Van Lenteren and Loomans, 1999). Conservation biological control, relying on the natural colonisation of a crop by natural enemies already present in the environment, is often too late and too less, and therefore, much less effective in an early and timely control of T. palmi. Control of thrips pests heavily relies on chemical applications; however, the use of insecticides may have detrimental effects on biological control agents (Cuthbertson, 2014).
Microorganisms
Application of entomopathogens, such as the fungi Akanthomyces lecanii (previously named as Lecanicillium lecanii and Verticillium lecani), Metarhizium anisopliae, M. rileyi (synonym Nomuraea rileyi), Beauveria bassiana and Paecilomyces fumosoroseus can have a certain control effect on thrips whereas others like Bacillus thuringiensis have a limited effect (Saito, 1991, 1992; Vestergaard et al., 1995; Castineiras et al., 1996; Parker et al., 1996; Ekesi et al., 2000; Ekesi and Maniania, 2002; Trujillo et al., 2003; Visalakshy et al., 2004; Cuthbertson et al., 2005; Smith et al., 2005; North et al., 2006; Silva et al., 2011; Shao et al., 2015; Hadiya et al., 2016). Others, such as Purpureocillium lilacinum (Hotaka et al., 2015) and Isaria javanica (Park et al., 2018), are still in a developmental phase.
Biotechnical control and semiochemicals
The effect of semiochemicals (Kirk, 2017; Qin et al., 2004) – either as a repellent or attractant ‐ on the behaviour and trapping efficiency is still in an experimental phase. An aggregation pheromone for T. palmi has been identified (Akella et al., 2014), it can be used for monitoring, but implementation is still in an experimental phase (Kirk, 2017). In experimental set‐ups, methyl salicylate (MeSA) has shown to attract natural enemies and to reduce populations in cucumber plants (Dong and Hsiu, 2019), but has not been developed to a commercial scale.
3.6.5. Host plant resistance
A few research reports mention differences in susceptibility to foliar injury among cultivars of pepper (Nuessly and Nagata, 1995), sweet pepper (Matsui et al., 1995; Visschers et al., 2019) and bean (Cardona et al., 2002; Frei et al., 2004), but host plant resistance has shown a low or no effectiveness in the management of T. palmi. No records have been found which specifically refer to breeding resistance genes into M. charantia or other Momordica species.
3.6.6. Post‐harvest treatments
Potassium salts of fatty acids also known as insecticidal soaps are used as insecticides, herbicides, fungicides and algaecides. Mixtures of potassium salts of fatty acids and essential oils may be used as selective acaricides (Tsolakis and Ragusa, 2008), and insecticides (Wafula et al., 2017) as an alternative to synthetic chemical pesticides enabling farmers to produce with acceptable residue levels that meet market requirements. In snap bean in Kenya (Wafula et al., 2017) potassium salts of fatty acids reduced thrips (Frankliniella spp. and Megalurothrips sjostedti) populations up to 54%, comparable with synthetic pesticides.
Washing produce – fruits and vegetables – with chlorinated or ozonated water is used to sanitise water systems and to disinfect the surface of produce to prevent decay caused by microorganisms such as bacteria, fungi and yeasts and other pathogens at concentrations between 100 and 200 ppm active ingredient, at pH around 7 (Bornhorst et al., 2018; Ilic et al., 2018). It is not designed to kill insects, and little or no scientific evidence is available that it works as such.
4. Commodity data
4.1. Description of the commodity
The commodity to be imported are fruits of Momordica charantia also known as bitter gourd or bitter melons or cundeamor, balsamina, bálsamo, pepinillo amargo, ampalayá and catajera in Spanish. Fruits of M. charantia, of the Chinese and Indian varieties, exported to EU markets are intended for human consumption and are exported in boxes of either 4 or 13.6 kg each. The trade volume for 2020 targeting the European Union market is expected at a minimum of 4,000 tons of M. charantia fruits (Table 3).
Table 3.
Overview of estimated export volumes (in tons) for Momordica charantia fruits designated for export to the EU from Mexico in 2020 (volumes as provided by Mexico in the dossier 7). On 1 November 2019, no data of expected shipments were available for Santiago Ixcuintla, Nayarit
| Location | Variety | J | F | M | A | M | J | J | A | S | O | N | D | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacalar ‐Quintana Roo | Not specified | 59.5 | 59.5 | 31.5 | 31.5 | 31.5 | 31.5 | 31.5 | 31.5 | 31.5 | 59.5 | 59.5 | 59.4 | 518 |
| Navolato‐ Sinaloa | Indian | 228 | 228 | 228 | 228 | 228 | 228 | 0 | 0 | 0 | 0 | 228 | 228 | 1,824 |
| Chinese | 12 | 12 | 12 | 12 | 12 | 12 | 0 | 0 | 0 | 0 | 12 | 12 | 96 | |
| Temozon‐Yucatan | Indian (Palee) | 22 | 19 | 27 | 29 | 24 | 25 | 0 | 0 | 10 | 10 | 19 | 20 | 205 |
| Indian (Preeti) | 87 | 78 | 489.5 | 72 | 64 | 56 | 50 | 20 | 0 | 50 | 67 | 80 | 1,113.5 | |
| Chinese | 24 | 16 | 24 | 20 | 22 | 32 | 35 | 36 | 18 | 21 | 24 | 27 | 299 | |
| Total | 432.5 | 412.5 | 812 | 392.5 | 381.5 | 384.5 | 116.5 | 87.5 | 59.5 | 140.5 | 409.5 | 426.5 | 4,055.5 |
4.2. Description of the production areas
Momordica charantia production is concentrated in four areas in Mexico: in Bacalar (Quintana Roo) and Temozón (Yucatán) in the east, and Santiago Ixcuintla (Nayarit) and Navolato (Sinaloa) in the west (Figure 3). All 4 production areas of M. charantia in Mexico are destined for exportation. Currently, all the production of M. charantia is carried out in open fields. Thrips palmi has only been reported from eastern Mexico including the production areas in Bacalar (Quintana Roo) and Temozón (Yucatan) (see Figure 3) but not from the production areas in the west, Santiago Ixcuintla (Nayarit) and Navolato (Sinaloa). However, there are areas where T. palmi has not been recorded, but Mexico does not recognise any of these as officially pest‐free areas.
Figure 3.
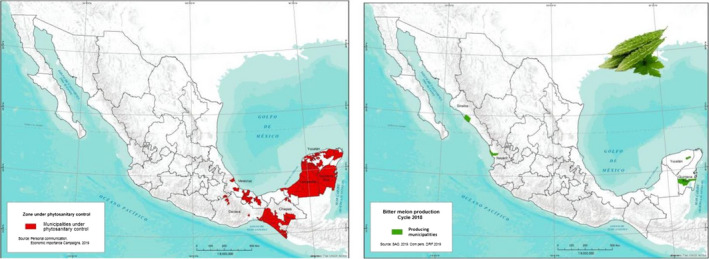
Areas in Mexico under Thrips palmi phytosanitary control (left, red colour) and producing areas of bitter melon (Momordica charantia L.) for export (right, green colour). Mexico own elaboration, Department of Risk Analysis – DCNRF, National Service for Health, Safety and Agrifood Quality (Senasica, 2019: Dossier 1)
4.2.1. Source of planting material
According to the information provided in the dossier seeds of bitter melon are used as propagating material, but do not have any official certification by Senasica.
4.2.2. Production cycle
The general phenology of bitter melon and densities of plants/ha in Mexico varies between the different growing areas. The growing cycle of M. charantia as described in the dossier starts with an initial period of up to 13 days, during which seeds are sown, germinated and young plants prepared. In Yucatan plants are prepared in greenhouses and also directly in the field. Twelve to 15 days after germination, greenhouse grown seedlings are transplanted into the field, and up to 25 after transplantation, plants are brought to a flowering stage, which usually starts between 45 and 55 days after sowing, and plants keep flowering for 6–8 months. The harvest starts 60–70 days (week 8–10) after planting, depending on environmental conditions (temperature and precipitation) reaching a peak between weeks 12 and 14.
The production of bitter melon in Mexico is carried out throughout the year. However, in each production area, there is a 2‐ to 3‐month gap in the general phenology of Momordica (tables 2, 3 and 4 of Dossier section 1: August–October in Nayarit and Sinaloa; April–June in Quintana Roo; July–August in Yucatan).
5. Overview of interceptions
According to Europhyt/TRACES‐NT accessed on 14 July 2020 and covering all interceptions since 1995 there were 10 interceptions of T. palmi from Mexico destined for the EU: 5x on M. charantia fruits in 2018, 3x on M. charantia in 2019 and 2x on Momordica sp. In 2020, one interception of T. palmi on M. charantia from Mexico has been notified from Switzerland.
6. Pest pressure and risk mitigation measures
The evaluation of the efficacy of the risk mitigation measures against T. palmi was done in a three‐step approach. First, an estimate was made for the pest pressure of T. palmi in the production environment. Secondly, the control effect of the pest management measures in the field was estimated. Thirdly, the control effect of the post‐harvest measures (packing house) was estimated.
The information used in the evaluation of the effectiveness of the risk mitigation measures is summarised in a pest data sheet (see Appendix A).
6.1. Pest pressure in production places
Based on monitoring data in production fields available in the dossier (see Table 2 in the reply to EFSA), the Panel estimated the pest pressure in the production places under a no‐intervention scenario (i.e. no mitigation measures). For details on the evaluation of pest pressure, see Appendix A. Moreover, the climatic conditions in Mexico (based on monthly average temperatures) are very favourable for the development of this pest.
Table 2.
Parameters for three‐step conceptual model to estimate the likelihood of pest freedom from Thrips palmi in Momordica charantia fruits
| Parameter | Unit | Description |
|---|---|---|
| rimport | [No out of 10,000 fruits] | The number (out of 10,000) M. charantia fruits imported to the European Union (EU) from Mexico, which will be infested with T. palmi when arriving the EU |
| ppressure | [No out of 10,000 fruits] | The number (out of 10,000) M. charantia fruits harvested on production sites in Mexico, which will be infested with T. palmi without application of specific measures against the pest (pest pressure under general agricultural practise) |
| pfield | [No out of 10,000 fruits] | The number of M. charantia fruits (out of 10,000 infested fruits) that remain infested after applying measures on production sites |
| ppacking | [No out of 10,000 fruits] | The number of M. charantia fruits (out of 10,000 infested fruits) that remain infested after applying measures at the packing house |
| PFimport | [No out of 10,000 fruits] | The number (out of 10,000) M. charantia fruits imported to the EU from Mexico, which will be pest free of T. palmi when arriving the EU |
6.2. Risk mitigation measures applied in production fields
With the information provided by Senasica (Dossier), the Panel summarised the risk mitigation measures that are currently applied in the production places (Table 4).
Table 4.
Overview of currently applied risk mitigation measures for Momordica charantia fruits designated for export to the EU from Mexico, based on the information provided in sections the dossier
| Risk mitigation measure | Description of applied measures |
|---|---|
| Production of seedlings in greenhouse | Seedlings develop in protected environments free of pests, ensuring plants which are not suitable are discarded and after 2 weeks only healthy plants are transplanted into the field (in some areas in Yucatan seeds develop directly in the field). |
| Protected cultivation | Not applied, only seedlings are raised in greenhouse conditions in most areas. |
| Pest specific monitoring | Mexico adopted a specific ‘National Campaign Against Eastern Thrips’ including surveillance and monitoring systems for T. palmi. Sampling data are recorded by field staff (project professionals/field assistants). This consists of several measures:
|
| Chemical control | Various insecticides are frequently applied (see details in Appendix A). |
| Weed control | Elimination of alternative hosts through hand weeding or by applications of glyphosate. |
| Biological control | Biological control agents are applied (dossier section 6) during pre‐harvest and harvest. Application to foliage. In addition to naturally occurring control agents in the area of M. charantia production, at the moment of detecting T. palmi at low levels (1–3.9 ind/organ sampled) biological control is applied:
|
| Cultural control |
Padding: the crop rows are covered with silver padding that prevents the emergence of weeds next to the plants, which can be alternative hosts of T. palmi; in addition, the silver padding acts as pest repellent. Cleaning and disinfection of tools: the scissors and boxes used to cut the fruits are cleaned and of polluting materials where they could accommodate T. palmi. |
| Inspection | Fruits are visually inspected in the field during harvesting by the technical field staff, and should be free from visible defects. |
| Pest‐free area | Not applied; there are no official pest‐free areas in Mexico |
6.3. Risk mitigation measures applied in the packinghouse
With the information provided by the Senasica (Dossier 1, section 3), the Panel summarised the risk mitigation measures that are currently applied in the packinghouse (Table 5).
Table 5.
Overview of currently applied risk mitigation measures applied in the packinghouse on Momordica charantia fruits designated for export to the EU from Mexico, based on the information provided in sections of the dossier 1, Chapter 3, Section 3.1 (Table 12) and Section 3.5
| Risk mitigation measure | Description of applied measure |
|---|---|
| Transport from field to packing house | The field collected fruits are transported in chlorine cleaned, plastic boxes to field collection points by pickup vehicles with a mesh shade roof and transported by non‐refrigerated trailers to the packing house. |
| Inspection upon arrival to the packing house | Fruit sampling upon reception at the packing house, searching for fruits with symptoms of damage and/or presence of pests. |
| Product washing |
|
| Sorting/Classification/Packing | Once the fruit enters, they proceed to the selection and classification process. At this stage, possible physical damages are detected. The selection parameters consist of separating all those fruits that are damaged or malformed, inappropriate colour or any type of damage that detracts value and quality. |
| Fruit brushing and cleaning | Brushing between cut‐outs on the M. charantia fruit (Indian variety) or cleaning with cloth dampened in a solution of peracetic acid of 80–100 ppm (Chinese variety). |
| Storage | Boxes with fruits are stored in cooled rooms at 10–12°C with 85–90% for 2 weeks within an anti‐aphid mesh, which prevents cross‐contamination during the journey of the product up to its final destination. |
| Inspection of the export consignment | Inspection of the shipment prior to export and issuance of an International Phytosanitary Certificate by personnel authorised by Senasica, which corroborates that the shipment meets the phytosanitary requirements established by the European Union (Phytosanitary requirements for fruits of M. charantia established by the EU): pest free and free of plant residues and soil (dossier section 1). |
6.4. Overview of the evaluation of Thrips palmi
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free fruits * | 9,492 out of 10,000 fruits | 9,762 out of 10,000 fruits | 9,880 out of 10,000 fruits | 9,950 out of 10,000 fruits | 9,991 out of 10,000 fruits |
| Proportion of infested fruits * | 9 out of 10,000 fruits | 50 out of 10,000 fruits | 120 out of 10,000 fruits | 238 out of 10,000 fruits | 508 out of 10,000 fruits |
| Summary of the information used for the evaluation |
Possibility that the pest could become associate with the commodity Environmental conditions in Mexico are optimal for T. palmi development. Although weather conditions (rainfall, low temperatures) may affect pest pressure, T. palmi is recorded on Momordica plants throughout the growing cycle. T. palmi is widespread in the east, including two areas of production (Yucatan and Quintana Roo) and is considered a national phytosanitary problem. In two other areas of production (Nayarit, Sinaloa), no records of T. palmi are known. The frequency and the number of sprays are very high probably underlying high infestations in the field. Measures taken against the pest and their efficacy The main control measures applied in the field until harvest are official inspections, monitoring, conservation biological control, applications of natural enemies (macro‐organisms, microorganisms), chemical control and inspection during harvesting. Although effectiveness of measures is estimated as high (80–100%) by the applicant, based on reports in international literature, the efficacy of the applied biological organisms (pathogens, predators) against T. palmi is expected to be low (20–30%); whereas the efficacy of insecticides applied during the development and flowering period of the crop and during the production stage of the crop is expected to be intermediate to high. |
||||
|
Measures in the packing house include inspection before processing, washing, brushing and air drying and pest and product inspections before packing. Measures in the packing house mainly target adults and larvae and have minimal effect on eggs. Interception records From 2018 to 2019, there are 10 interceptions reported in Europhyt/TRACES‐NT of T. palmi on M. charantia (eight times) and Momordica sp. (twice) fruits originating from Mexico. In 2020, there is a single notification from M. charantia from Switzerland. Shortcomings of current measures/procedures Application of insecticides is mainly performed on a calendar‐like basis. Continuous use of insecticides is likely to cause development of resistant populations of T. palmi. Most measures applied in the packing house are not likely to have an effect on eggs that may be present in fruits. Main uncertainties There are limited data on population dynamics of T. palmi on M. charantia in Mexico. Since identification of thrips at species level may be difficult in the field, it cannot be excluded that field observations of thrips refer to mixtures of T. palmi and other species. Data of the efficacy for field applied measures are not specific for the field situation in different regions, and if so, either limited or not available. Data on efficacy of the methods applied in the packing house in removing T. palmi from fruits are not available. The level of insecticide resistance against the insecticides applied in Mexico is uncertain. |
|||||
Numbers rounded off to the nearest whole number.
6.5. Outcome of Expert Knowledge Elicitation
Table 6 and Figure 4 show the outcome of the EKE regarding pest freedom after the evaluation of the currently proposed risk mitigation measures for T. palmi.
Table 6.
Assessment of the likelihood of pest freedom following evaluation of current risk mitigation measures against Thrips palmi on Momordica charantia fruits from Mexico designated for export to the EU. In panel A, the median value for the assessed level of pest freedom for each pest is indicated by ‘M’, the 5% percentile is indicated by L and the 95% percentile is indicated by U. The percentiles together span the 90% uncertainty range regarding pest freedom. The pest freedom categories are defined in panel B of the table
| Pest species | Sometimes pest free | More often than not pest free | Frequently pest free | Very frequently pest free | Extremely frequently pest free | Pest free with some exceptional cases | Pest free with few exceptional cases | Almost always pest free |
|---|---|---|---|---|---|---|---|---|
| Thrips palmi | L | M | U |
| PANEL A | |||||
|---|---|---|---|---|---|
| Pest freedom category | Pest‐free fruits out of 10,000 | Legend of pest freedom categories | |||
| Sometimes pest free | ≤ 5,000 | L | Pest freedom category includes the elicited lower bound of the 90% uncertainty range | ||
| More often than not pest free | 5,000–≤ 9000 | M | Pest freedom category includes the elicited median | ||
| Frequently pest free | 9,000–≤ 9500 | U | Pest freedom category includes the elicited upper bound of the 90% uncertainty range | ||
| Very frequently pest free | 9,500–≤ 9900 | ||||
| Extremely frequently pest free | 9,900–≤ 9950 | ||||
| Pest free with some exceptional cases | 9,950–≤ 9990 | ||||
| Pest free with few exceptional cases | 9,990–≤ 9995 | ||||
| Almost always pest free | 9,995–≤ 10000 | ||||
| PANEL B | |||||
Figure 4.
Explanation of the descending distribution function describing the likelihood of pest freedom from Thrips palmi after the evaluation of the currently proposed risk mitigation measures for fruits of Momordica charantia from Mexico designated for export to the EU
Figure 4 provides an explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the currently proposed risk mitigation measures for M. charantia fruits designated for export to the EU for T. palmi.
7. Conclusions
For Thrips palmi on Momordica charantia fruits from Mexico, the likelihood of pest freedom following evaluation of current risk mitigation measures was estimated as ‘very frequently pest free’ with the 90% uncertainty range reaching from ‘frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,492 and 10,000 fruits per 10,000 will be free from T. palmi.
Glossary
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017)
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017)
- Measures
Control (of a pest) is defined in ISPM 5 (FAO, 2017) as ‘Suppression, containment or eradication of a pest population’ (FAO, 1995). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk mitigation measures that do not directly affect pest abundance.
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017)
- Pest pressure
Local population density of a pest (often used in economic threshold levels in IPM)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017)
- Protected zone
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017)
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017)
- Risk mitigation measure
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A risk mitigation measure may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017)
Abbreviations
- CABI
Centre for Agriculture and Bioscience International
- EKE
Expert Knowledge Elicitation
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- INS
Insect
- ISPM
International Standards for Phytosanitary Measures
- PLH
Plant Health
- PRA
Pest Risk Assessment
- RNQPs
Regulated Non‐Quarantine Pests
Appendix A – Data sheets of pests selected for further evaluation via Expert Knowledge Elicitation
A.1. Thrips palmi
A.1.1. Organism information
| Taxonomic information |
Current valid scientific name: Thrips palmi Karny * Synonyms: Thrips clarus Moulton, 1928; Thrips leucadophilus Priesner, 1936; Thrips gossypicola Ramakrishna & Margabandhu,1939; Chloethrips aureus Ananthakrishnan & Jagadish, 1967 Thrips gracilis Ananthakrishnan & Jagadish, 1968. Name used in the EU legislation: Thrips palmi Karny [THRIPL] Order: Thysanoptera Family: Thripidae Common name: oriental thrips, palm thrips, southern yellow thrips Name used in the Dossier: Thrips palmi * see Symptoms: confusion with other pests |
| Group | Insects |
| EPPO code | THRIPL |
| Regulated status |
Thrips palmi is regulated in the European Union, and it is listed in the Union Quarantine pests: Annex II Part A – Pests not known to occur in the European Union. Commission Implementing Regulation (EU) 2019/2072. A1 list: East Africa (2001), Egypt (2018), Southern Africa (2001), Argentina (2019), Chile (2019), Paraguay (1993), Uruguay (1993), Bahrain (2003), Jordan (2013), Kazakhstan (2017), Azerbaijan (2007), Georgia (2018), Moldova (2006), Russia (2014), Turkey (2016), Ukraine (2019), EAEU (2016), EPPO (1988) A2 list: CAHFSA (1990), COSAVE (2018) Quarantine: Morocco (2018), Tunisia (2012), Mexico (2018), Israel (2009), Norway (2012), New Zealand (2000) |
| Pest status in Mexico | Present: few occurrences (EPPO, Online; CABI CPC, Online) |
| Pest status in the EU | Absent (EPPO, Online; CABI CPC, Online) |
| Host status on Momordica charantia L. | According to the Pest categorisation of Thrips palmi (EFSA, 2019), Momordica charantia is one of the main host plants of Thrips palmi. |
| PRA information | Pest Risk Assessments currently available:
|
| Interceptions (Europhyt – TRACES‐NT) | There are 10 interceptions of T. palmi on M. charantia fruits (eight times) and Momordica fruits (twice) originating from Mexico between 2018 and 2019 in the EU. A single interception has been notified form Switzerland. |
| Surveillance information |
The National Service for Health, Safety and Agrifood Quality (Senasica) of Mexico implements ‘National Campaign Against Eastern Thrips’ since 2011 (Senasica, 2011; 2018ab). For T. palmi, there is a specific sampling methodology in the field as well as instalment of five blue sticky traps per field, which are checked every 3 weeks in areas under phytosanitary control. Sampling is carried out twice a week, starting from the establishment of the crop and until the beginning of the harvest. Field measures are carried out by the technicians of the producer, based on the pests under surveillance, their operational actions, symptom and damage guides, fact sheets, technical reports and consultation of operating manuals operated by Senasica. |
Country specific information
A.1.1.1. Pest pressure in the production area
Temperature ranges between 20 and 30 °C which is an optimal range for multiplication of T. palmi.
In Mexico, M. charantia is a little‐known crop, production is only carried out for export purposes by some producing companies and therefore, the presence of pests and diseases in the crop is not documented. However, based on the information provided by the different exporting companies, the pests that have been associated with the crop are: Bemisia tabaci, Helicoverpa zea, Spodoptera frugiperda and T. palmi (Table 7, Dossier). Interceptions of T. palmi have been reported on Momordica spp. (M. charantia, M. dioica) and on Solanum melongena (Europhyt/TRACES ‐NT, Accessed on June 2020).
T. palmi was first detected on watermelon (Citrullus lanatus [Thunb.] Matsum. & Nakai) in March 2004, in the state of Campeche. Even though surveillance and sampling measures were put in place T. palmi is now reportedly present in the Yucatan of Mexico (Canon et al., 2006) and in the states Chiapas, Campeche and Quintana Roo (Williams et al., 2013).
According to the information provided by the Mexican NPPO in the dossier (section 1–3), T. palmi is currently under phytosanitary control in several states in Mexico i.e. Oaxaca, Puebla, Quintana Roo Veracruz, Yucatan. T. palmi is not present in the M. charantia producing areas of Nayarit and Sinaloa.
Prevalence of the pest during the season: In the producing areas of Temozon, Yucatan, it has been observed through monitoring that the presence of T. palmi is correlated with climatic conditions, being the months with higher temperatures and lower relative humidity (February, March, April and May) in which the greater percentage of individuals is detected; they are mainly observed in the aerial parts of the plant (leaves, flowers and fruits), the parts of the plant where T. palmi is present, may differ according to the above‐mentioned variables as well as the phenological stage of the plant. During the months of June, July, August and September, its presence is considered low due to the increase in relative humidity caused by rains. The following months (October, November, December and January) show a very low presence, resulting from the change in temperature, where temperatures stay below 20°C and this period is considered as winter. For the producing areas in Bacalar, Quintana Roo, the presence of T. palmi peaks in the months of June and July.
In February 2018, the (average) population densities of T. palmi in the capital states of Campeche, Chiapas, Oaxaca, Puebla, Quintana Roo, Tabasco, Veracruz and Yucatán (Figure 5) were 0.04, 0.04, 0.14, 0.16, 0.17, 0.24, 0.09 and 0.23 individuals/organ sampled, respectively. The national population density was 0.13 individuals/organ, although the presence of the insect has not been significant and it has not exceeded the national economic threshold (seven individuals/sampled organ). This was attributed to that phytosanitary actions were carried out in a timely manner, in accordance with the campaign operational strategy (Senasica, 2011, 2018ab) (Reply, Comment B.2).
The number of interceptions in relation to the number of export consignments is low.
Uncertainties:
There are limited data on population dynamics of T. palmi on M. charantia.
Since identification of juvenile stages of thrips at the species level is difficult in the field, it is possible that field observations of thrips refer to other species than T. palmi (e.g. mixtures of F. occidentalis and T. palmi).
A.1.1.2. Evaluation of measures applied in the field
The main control measures applied in the field until harvest are summarised in Tables A.1 and A.2.
Table A.1.
Overview, evaluation and uncertainties of measures applied in the field against Thrips palmi on Momordica charantia fruits from Mexico designated for export to the EU, based on the information provided in the dossier
| Risk mitigation measure | Description of applied measures | Evaluation and uncertainties by the Panel |
|---|---|---|
| Protected cultivation | Not applied, only seedlings are raised in greenhouse conditions in most areas. | Only applied for seedlings |
| Pest specific monitoring | Mexico adopted a specific ‘National Campaign Against Eastern Thrips’ including surveillance and monitoring systems for T. palmi. Sampling data are recorded by field staff (project professionals/field assistants). This consists of several measures:
|
A pest‐specific monitoring is in place in Mexico. Monitoring and sampling data are used to determine the population density. When population levels are low, biological products are used, when levels are high (4 individuals/organ) insecticides are used. Uncertainties Number of thrips recorded during field inspections, in particular larvae, may also include other species like F. occidentalis. |
| Chemical control |
|
Insecticides applied are effective against T. palmi. Insecticides are applied when the population level of 100 organs per field on sampling and monitoring data, exceeds an average of 4 individuals/organ. The repeated use of insecticides is likely to cause development of resistant populations of T. palmi. Uncertainties Number of thrips recorded after insecticide application, may also include other species like F. occidentalis. There are uncertainties on the level of resistance of T. palmi to chemical insecticides |
| Weed control | Elimination of alternative hosts through hand weeding or by applications of glyphosate. |
The availability of alternative host plants for T. palmi is reduced in and around production fields. Uncertainties The intensity and frequency of weed control is uncertain |
| Biological control |
Biological control agents are applied (dossier section 6) during preharvest and harvest. In addition to naturally occurring control agents in the area of M. charantia production, at the moment of detecting T. palmi at low levels (1–3.9 ind/organ sampled) biological control is applied: – lacewings (Chrysoperla carnea) are curatively applied onto infested leaves at a dose of 2 ml/ha or 10–50/m2. – Cc Beauveria bassiana (1x107 CFU/mL): 20%; Cc Nomurea Rileyi (1 × 107 CFU/mL): 10%; Cc Metarhizium anisopliae (1 × 10 CFU/mL): 15%; Cc Verticillium lecanii (1 × 10 CFU/mL): 10%; Cc Paecilomyces fumosoroseus (1 × 10 CFU/mL): 15%; multiple oleic‐active concentrate: 15%. |
Biological control is applied at low population levels. The chemical control applied is not compatible with biological control. The agents used are not specifically targeting T. palmi, and have a low overall efficacy on thrips. Uncertainties: No data are available on biological control efficacy |
| Cultural control |
Padding: the crop rows are covered with silver padding that prevents the emergence of weeds next to the plants, which can be alternative hosts of T. palmi; in addition, the silver padding acts as pest repellent. Cleaning and disinfection of tools: the scissors and boxes used to cut the fruits are cleaned and of polluting materials where they could accommodate T. palmi. |
Low effect |
| Inspection | Fruits are visually inspected in the field during harvesting by the technical field staff, and should be free from visible defects. |
Detection of early larval stages of T. palmi in the field is difficult. Eggs are not detected. |
| Pest‐free area | Not applied; there are no official pest‐free areas in Mexico | Not applied |
Table A.2.
Overview of insecticides and other phytosanitary products used for the control of Thrips palmi in Momordica charantia fields in Mexico, based on the information provided in dossier
| Insecticides and other phytosanitary products used to control Thrips palmi | |||
|---|---|---|---|
| Product | Type of product | Efficacy as reported (see Table 11 in dossier #1) | Efficacy evaluation by the Panel on T. palmi |
| Azadirachta indica | Insect growth regulator | 100% | Medium effect on thrips |
| Imidacloprid + Betacyflutrin | Systemic insecticides | 85% | High on thrips |
| Lambda cyhalothrin | Synthetic pyrethroid contact | 85% | Medium effect on thrips |
| Z‐cypermethrin | Synthetic pyrethroid contact | 91% | Medium effect on thrips |
| Spinosad | Contact insecticide | 90% | High on thrips |
| Spinetoram | Contact insecticide | 100% | High on thrips |
|
Beauveria bassiana (1 × 107 CFU/mL) Nomurea Rileyi (1 × 107 CFU/mL) Metarhizium anisopliae (1 × 10 CFU/mL) Verticillium lecanii (1 × 10 CFU/mL) Paecilomyces fumosoroseus (1 × 10 CFU/mL) |
Entomopathogenic fungi | 95% | Low‐medium on thrips |
Uncertainties:
Data on own efficacy of the various control methods for T. palmi in the field were not made available.
It is not clear what % of volume is originating from the two areas where T. palmi is not recorded so far.
A.1.1.3. Evaluation of measures applied in the packing house
The main control measures applied in the packing house are: (a) inspection before processing, (b), washing, (c) brushing and air blowing, (d) pest inspections before packing and certification of the shipment.
When M. charantia fruits are delivered to packing houses, packing house personnel will take samples after weighing, during classification and packing to inspect the quality and pest infestation on fruits. If the quality of M. charantia fruits is lower than standard or any pest infestation notice over standard, the fruits will be refused to process in the packing house. However, data on frequency of rejections at packing houses were not made available. Fruits are air dried after the washing procedures and individually brushed (Indian variety) or cleaned (Chinese variety) with sanitising products such as peroxyacetic acid. However, these products are not indented to remove pests such as thrips but mainly for disinfecting fruits. Finally, samples of fruits will be inspected by packing house personnel for signs of insect infestation.
Uncertainties:
Specific data on efficacy of the above methods in removing T. palmi from fruits post‐harvest were not made available upon request.
Table A.3.
Overview of currently applied risk mitigation measures applied in the packinghouse on M. charantia fruits designated for export to the EU from Mexico, based on the information provided in the dossier
| Risk mitigation measure | Description of applied measure | Evaluation and uncertainties by the Panel |
|---|---|---|
| Protected transport | The field collected fruits are transported in chlorine cleaned, plastic boxes to field collection points by pickup vehicles with a mesh shade roof, and transported by non‐refrigerated trailers to the packing house. | |
| Inspection upon arrival to the packing house | Fruit sampling after weighing at the packing house, searching for fruits with the symptoms of damage and/or presence of pests. | Inspection is intended mainly as a first filter to discard fruits infested by pests or which do not fulfil quality (visual) requirements. As such, is not aimed to detect T. palmi. This method will only detect heavily infested fruits showing clear symptoms of infestation. |
| Product washing |
Pre‐washing of boxes with fruits with pressurised water in 360° in a tunnel to cool fruits and remove dirt; water may contain peracetic acid at 100 ppm or sodium hypochlorite at 80–100 ppm. Washing: washing in water (12–15°C) in stainless steel tanks during 5 min with detergent solution with food grade alkaline inorganic matter, to remove any solid matter. Sanitisation: Immersion of fruits in tanks during 5–10 min in water with 15% peracetic acid using concentrations between 80 and 150 ppm. Fans to remove excess water. |
Washing is predominantly used to remove any solid matter, but also removes larvae and adults of T. palmi. Immersion of fruits in a detergent solution is a sanitising solution only. Eggs are not affected. Uncertainties: Unclear how often washing solvents are renewed. |
| Sorting/Classification/Packing | Once the fruit enters, they proceed to the selection and classification process. At this stage, possible physical damages are detected. The selection parameters consist of separating all those fruits that are damaged or malformed, inappropriate colour or any type of damage that detracts value and quality. |
Sorting is intended mainly as second filter to discard fruits infested by pests or do not fulfil quality (visual) requirements. This method will only detect heavily infested fruits showing clear symptoms of infestation. |
| Fruit brushing and cleaning | Brushing between cut‐outs on the M. charantia fruit (Indian variety) or cleaning with cloth dampened in a solution of peracetic acid of 80–100 ppm (Chinese variety). |
Fruit brushing has a low to intermediate effect on larvae and adults. Brushed adults may not be killed and therefore re‐infest other fruits in the packing station. Brushing has no effect on eggs as eggs are laid inside the fruit tissue, especially when using soft brushing. Uncertainties: Efficacy data are not provided. |
| Storage | Boxes with fruits are stored in cooled rooms at 10–12°C with 85–90% for 2 weeks within an anti‐aphid mesh, which prevents cross‐contamination during the journey of the product up to its final destination. |
Cooling at 10–12°C will stop development of thrips. Peracetic acid/sodium hypochlorite is used for sanitisation of pallets and containers. Only 1 company – Agricola Alejandra del Valle in Navolato Sinaloa – exerts anti‐aphid mesh boxes. No effect on T. palmi. |
| Inspection of the export consignment | Inspection of the shipment prior to export and issuance of an International Phytosanitary Certificate by personnel authorised by Senasica, which corroborates that the shipment meets the phytosanitary requirements established by the European Union (Phytosanitary requirements for fruits of M. charantia established by the EU): pest free and free of plant residues and soil. | Inspection is intended a final filter to discard fruits infested by pests or which do not fulfil quality (visual) requirements. |
A.1.2. Information from interceptions
According to Europhyt/TRACES‐NT accessed on 14 July 2020 and covering all interceptions since 2018 there were 10 interceptions of T. palmi from Mexico destined for the EU: 5x on M. charantia fruits in 2018, three times on M. charantia in 2019 and twice on Momordica sp. In 2020, one interception of T. palmi on M. charantia from Mexico has been notified from Switzerland.
A.1.3. Overall likelihood of pest freedom
| Rating of the likelihood of pest freedom | Very frequently pest free (based on the median) | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free fruits * | 9,492 out of 10,000 fruits | 9,762 out of 10,000 fruits | 9,880 out of 10,000 fruits | 9,950 out of 10,000 fruits | 9,991 out of 10,000 fruits |
| Proportion of infested fruits * | 9 out of 10,000 fruits | 50 out of 10,000 fruits | 120 out of 10,000 fruits | 238 out of 10,000 fruits | 508 out of 10,000 fruits |
| Summary of the information used for the evaluation |
Possibility that the pest could become associate with the commodity Environmental conditions in Mexico are optimal for T. palmi development. Although weather conditions (rainfall, low temperatures) may affect pest pressure, T. palmi is recorded on Momordica plants throughout the growing cycle. T. palmi is widespread in the east, including two areas of production (Yucatan and Quintana Roo) and is considered a national phytosanitary problem. In two other areas of production (Nayarit, Sinaloa), no records of T. palmi are known. The frequency and the number of sprays are very high probably underlying high infestations in the field. Measures taken against the pest and their efficacy The main control measures applied in the field until harvest are official inspections, monitoring, conservation biological control, applications of natural enemies (macro‐organisms, microorganisms), chemical control and inspection during harvesting. Although effectiveness of measures is estimated as high (80–100%) by the applicant, based on reports in international literature, the efficacy of the applied biological organisms (pathogens, predators) against T. palmi is expected to be low (20–30%); whereas the efficacy of insecticides applied during the development and flowering period of the crop and during the production stage of the crop is expected to be intermediate to high. Measures in the packing house include inspection before processing, washing, brushing and air drying and pest and product inspections before packing. Measures in the packing house mainly target adults and larvae and have minimal effect on eggs. Interception records From 2018 to 2019, there are 10 interceptions reported in Europhyt/TRACES‐NT of T. palmi on M. charantia (eight times) and Momordica sp. (twice) fruits originating from Mexico. In 2020, there is a single notification from M. charantia from Switzerland. Shortcomings of current measures/procedures Application of insecticides is mainly performed on a calendar‐like basis. Continuous use of insecticides is likely to cause development of resistant populations of T. palmi. Most measures applied in the packing house are not likely to have an effect on eggs that may be present in fruits. Main uncertainties There are limited data on population dynamics of T. palmi on M. charantia in Mexico. Since identification of thrips at species level may be difficult in the field, it cannot be excluded that field observations of thrips refer to mixtures of T. palmi and other species. Data of the efficacy for field applied measures are not specific for the field situation in different regions, and if so, either limited or not available. Data on efficacy of the methods applied in the packing house in removing T. palmi from fruits are not available. The level of insecticide resistance against the insecticides applied in Mexico is uncertain. |
||||
Numbers rounded off to the nearest whole number.
A.1.3.1. Reasoning for a scenario which would lead to a reasonably low number of infested consignments
Pest pressure
Exported volumes come largely from the pest‐free production areas.
The surrounding environment provides few hosts for T. palmi (i.e. population sources).
There is general pest management programme in place for thrips in agricultural areas where M. charantia is cultivated.
Natural biological control agents are very active and preserved and keep T. palmi controlled.
Thrips monitored are not always T. palmi. Other species of thrips (e.g. F. occidentalis) may be present.
Field measures
Regular and frequent inspection/monitoring targeted to T. palmi.
Exports match harvest periods where pest pressure is low.
Sampling and monitoring allow appropriate timing and use of proper control measures and active ingredients to control T. palmi.
Measures in the packing house
Low number of T. palmi flying inside the packing house; packed products are cooled.
Inspections at packing house and initial sorting of fruits are conducted properly and are effective in detecting and discarding infested fruits.
Cleaning measures (with water and other products, manually or submerged) are effective against T. palmi and render pest‐free fruits.
Proper replacement of water and other products in the washing area.
Additives and other products used have an effect on the mortality of T. palmi.
Large proportion of infestation is in adult stage and/or juveniles (mobile stages).
A.1.3.2. Reasoning for a scenario which would lead to a reasonably high number of infested consignments
Pest pressure
Exported volumes come largely from infested areas.
Density/plant in examples provided seem to be high and recover very high after pesticide treatments which indicate high background infestation.
The surrounding environment provides many hosts for T. palmi.
There are uncontrolled sites where the pest occurs (e.g. and eggplant plantation without efficient control), natural or roadside vegetation.
Environment contains natural biological control agents which are not very active and preserved to control T. palmi due to poor management in other crops.
Most monitored thrips are T. palmi.
Measures in the field
Inspection/monitoring is based on averages, focal points are overlooked.
Exports do not match harvest periods where pest pressure is low.
There is an inadequate timing or calendar spraying when exceeding the threshold levels and use of active ingredients that are not efficient against T. palmi.
Measures in the packing house
High number of T. palmi flying inside the packing house.
Inspections at packing house and initial sorting of fruits are not conducted properly and are not effective in detecting and discarding infested fruits.
Cleaning measures (with water and other products, manually or using machines) are not effective against T. palmi and do not render pest‐free fruits.
Poor replacement of water and other products in the washing area.
Additives and other products used do not have an effect on the mortality of T. palmi.
Large proportion of infestation are eggs.
A.1.3.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested consignments (Median)
An unknown part of M. charantia shipments originates from western pest‐free areas.
The surrounding environment provides sufficient hosts for T. palmi.
Most monitored thrips are likely to be T. palmi.
Insecticides are applied on a regular basis.
Procedures in the packinghouse are effective in removing larvae and adult stages of T. palmi and detecting infested fruits.
A.1.3.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
Identification of thrips at species level is difficult in the field and observations of thrips may refer to T. palmi, but also to mixtures of T. palmi and other species) and leading to either over‐ or underestimations of T. palmi pressure in the field.
Specific efficacy data for field applied measures are either limited or not available.
Data on efficacy of the methods applied in the packing house in removing T. palmi from fruits are not available.
It is uncertain to what extent infestation reported in the field on vegetative plant parts (e.g. leaves) is related to infestation numbers on the fruits.
The level of insecticide resistance against the insecticides applied in Mexico is uncertain.
The clarification is given by the level of uncertainty which is higher for the values below the median.
A.1.3.5. Elicitation outcomes of the assessment of the pest freedom for Thrips palmi
The following tables show the elicited values for pest freedom in Momordica charantia fruits according to a three‐step approach (i.e. estimating pest pressure, effectiveness of the measures applied in the field and in the packing house) (Table A.4) to come to a final estimation of likelihood of pest freedom (Table A.5) (Figures A.1 and A.2).
Table A.4.
Elicited values to estimate the likelihood of pest freedom (i.e. no. of pest free fruits out of 10,000, elicited as 10,000 minus no. of infested fruits) and the fitted distributions in a three‐step approach (i.e. Import risk: Rimport = ppressure × pfield/10,000 × ppacking/10000; Pest freedom: PFimport = 10,000 Rimport)
| Percentile | Parameter | 1% | 25% | 50% | 75% | 99% | Fitted distribution |
|---|---|---|---|---|---|---|---|
| Elicited values for pest pressure | ppressure | 500 | 1,500 | 2,500 | 3,500 | 5,000 | BetaGeneral (1.21, 1.5169, 430, 5200) |
| Elicited values for measures in the field | pfield | 1,000 | 2,300 | 3,500 | 5,000 | 6,700 | BetaGeneral (1.0721, 1.2735, 940, 6850) |
| Elicited values for measures in the packing house | Ppacking | 100 | 900 | 1,700 | 2,800 | 4,000 | Beta general (0.95631, 1.2207, 75, 4100) |
| Resulting model values for the import risk after Monte Carlo simulation | rimport | 4.2 | 48 | 123 | 235 | 800 | Calculated with @Risk version 7.6 |
| As pest‐free fruits | 9,200 | 9,765 | 9,887 | 9,952 | 9,995.8 |
Table A.5.
The uncertainty distribution of fruits free of Thrips palmi per 10,000 fruits calculated by taking into account a three‐step procedure and according to elicited values in Table A.4
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Three‐step approach for pest freedom | 9,200 | 9,345 | 9,465 | 9,595 | 9,691 | 9,765 | 9,817 | 9,887 | 9,934 | 9,952 | 9,968 | 9,980 | 9,988 | 9,992.9 | 9,995.8 |
| EKE results | 9,223 | 9,376 | 9,492 | 9,608 | 9,694 | 9,762 | 9,811 | 9,880 | 9,930 | 9,950 | 9,968 | 9,981 | 9,991 | 9,995.4 | 9,998.2 |
The EKE results are the fitted values for a Weibull distribution (1.0128, 172.03) fitted with @Risk version 7.6.
Figure A.1.
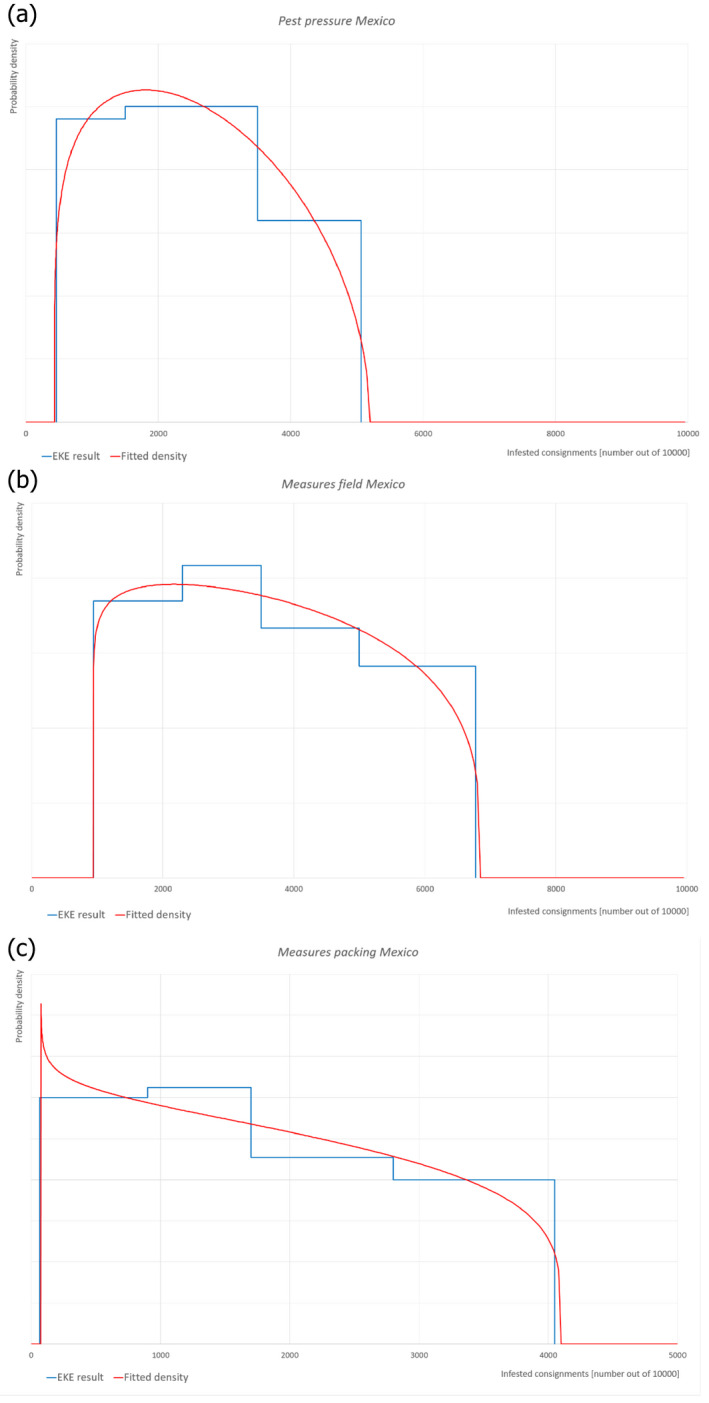
Probability densities for the number of pest‐free Momordica charantia fruits (x‐axis) out of 10,000 designated for export to the EU introduced according to (a) estimated pest pressure in the field; (b) measures applied in the field; and (c) measures applied in the packing house for Thrips palmi
Figure A.2.
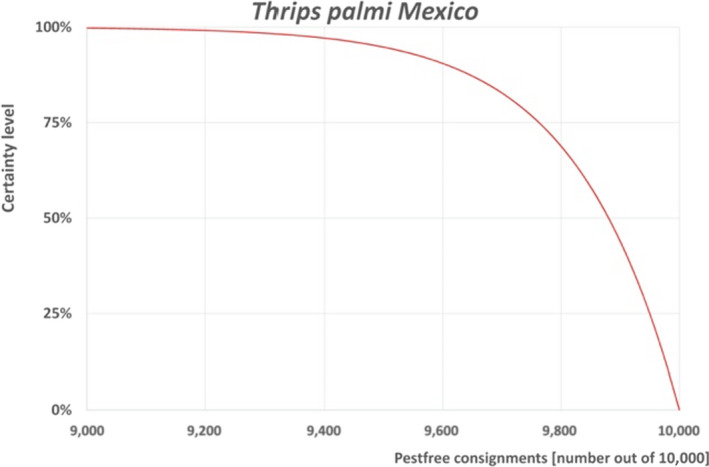
Elicited certainty (y‐axis) of the number of pest‐free Momordica charantia fruits (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU introduced from Mexico for Thrips palmi visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%). The Panel is 95% sure that 9492 or more fruits per 10,000 will be free from T. palmi
A.1.4. Reference list
Australian Government Department of Agriculture and Water Resources, 2017. Final group pest risk analysis for thrips and orthotospoviruses on fresh fruit, vegetable, cut‐flower and foliage imports.
CABI CPC (Centre for Agriculture and Bioscience International), online. Datasheet Thrips palmi. Available online: https://www.cabi.org/cpc/datasheet/5374 [Accessed: 22 July 2020].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Fejer Justesen A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappala L, Malumphy C, Czwienczek E and MacLeod A, 2019. Scientific Opinion on the pest categorisation of Thrips palmi. EFSA Journal 2019;17(2):5620, 39 pp. https://doi.org/10.2903/j.efsa.2019.5620
EPPO (European and Mediterranean Plant Protection Organizatio), online. Thrips palmi. Available online: https://gd.eppo.int/taxon/THRIPL [Accessed: 20 July 2020].
EUROPHYT, online. European Union Notification System for Plant Health Interceptions – EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm [Accessed: 22 April 2020].
Senasica, 2011. Manual operativo de la campagña contra el trips oriental (Thrips palmi Karny). National Agri‐food Health, Safety and Quality Service (Senasica). Circular 065. Available online: http://www.cesvo.org.mx/downloads/Manual_trips_oriental.pdf. [Accessed: 26 August 2020].
Senasica, 2018a. Operating strategy of the campaign against oriental thrips National Agri‐food Health, Safety and Quality Service (Senasica). Available online: https://www.qob.mx/cms/uploads/attachment/file/29Q259/ESTRATEGIA OPERAT IVA DETRIPS ORIENTAL 2018.pdf [Accessed: 26 August 2020].
Senasica, 2018b. 2018 reports and evaluations on oriental thrips (Thrips palmi Karny). National Agri‐food Health, Safety and Quality Service (Senasica). Available online: https://www.qob.mx/senasica/documentos/informes-v-evaluaciones‐ 2018‐to [Accessed: 26 August 2020].
Appendix B – Web of Science All Databases Search String
1.
In the table below, the search string used in Web of Science is reported.
| Web of Science |
TOPIC: (“Momordica” OR “Momordica charantia” OR “M. charantia” OR “Momordica anthelmintica Guin.” OR “Momordica elegans Salisb.” OR “Momordica muricata Willd.” OR “Momordica operculata Vell.” OR “Momordica senegalensis Lam.” OR “bitter gourd” OR “bitter melon” OR “Cucurbitaceae” OR “balsam apple” OR “balsam pear” OR “bitter balsam apple” OR “bitter cucumber” OR “bitter melon” OR “carilla gourd” OR “paria” OR “wild balsam‐apple” OR “cucumber” OR “melon”) AND TOPIC: (“Thrips palmi” OR “melon thrips” OR “Thrips palmi Karny, 1925” OR “Chloethrips aureus Ananthrakrishnan & Jagadish, 1967” OR “Thrips clarus Moulton, 1928” OR “Thrips gossypicola (Priesner, 1939)” OR “Thrips gracilis Ananthrakrishnan & Jagadish, 1968” OR “Thrips leucadophilus Priesner, 1936” OR “Thrips nilgiriensis Ramakrishna 1928” OR “Oriental thrips” OR ”southern yellow thrips”) AND TOPIC: (“pest pressure” OR “population build‐up” OR “pesticide application$” OR “pesticide$” OR “risk reduction option$” OR “mitigation measure$” OR “efficac*” OR “resistance” OR “population dynamic$” OR “phytosanitary product$” OR “registered pesticide$” OR “high pressure water*” OR ”air pressur*” OR “population dynamic$” OR ”field densit*” OR “occurrence” OR “monitor*” OR ”sticky trap$” OR ”sticky trap$ efficac*”) AND TOPIC: (“Mexico”) |
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Lucchi A, Loomans A, Mosbach‐Schulz O, de la Peña E and Milonas P, 2021. Scientific Opinion on the Commodity risk assessment of Momordica charantia fruits from Mexico. EFSA Journal 2021;19(2):6398, 37 pp. 10.2903/j.efsa.2021.6398
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00792
Panel members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to acknowledge the important contribution of Oresteia Sfyra to the literature search, the preparation of the data sheets and her support in drafting and reviewing this opinion.
Adopted: 31 December 2020
Notes
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) 228/2013, (EU) 652/2014 and (EU) 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. OJ L 317, 23.11.2016, pp. 4–104.
Commission Implementing Regulation (EU) 2018/2019 of 18 December 2018 establishing a provisional list of high risk plants, plant products or other objects, within the meaning of Article 42 of Regulation (EU) 2016/2031 and a list of plants for which phytosanitary certificates are not required for introduction into the Union, within the meaning of Article 73 of that Regulation C/2018/8877. OJ L 323, 19.12.2018, pp. 10–15.
References
- Affandi A, Emilda D and Jawal ASM, 2008. Application of fruit bagging, sanitation, and yellow sticky trap to control thrips on mangosteen. Indonesian Journal of Agricultural Science, 9, 19–23. [Google Scholar]
- Akella SV, Kirk WD, Lu YB, Murai T, Walters KF and Hamilton JG, 2014. Identification of the aggregation pheromone of the melon thrips, Thrips palmi. PLoS ONE, 9, e103315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci L, Picanço MC, Moura MF, Semeão AA, Fernandes FL and Morais EG, 2008. Sampling plan for thrips (Thysanoptera: Thripidae) on cucumber. Neotropical Entomology, 37, 582–590. [DOI] [PubMed] [Google Scholar]
- Bao WX, Kataoka Y, Fukada K and Sonoda S, 2015. Imidacloprid resistance of melon thrips, Thrips palmi, is conferred by CYP450‐mediated detoxification. Journal of Pesticide Science, D15‐004. [Google Scholar]
- Bornhorst ER, Luo YG, Millner PD, Nou XW, Park EH, Turner E, Vinyard BT and Zhou B, 2018. Immersion‐free, single‐pass, commercial fresh‐cut produce washing system: an alternative to flume processing. Postharvest Biology and Technology, 146, 124–133. [Google Scholar]
- Cannon RJC, Matthews L and Collins DW, 2007. A review of the pest status and control options for Thrips palmi . Crop Protection, 26, 1089–1098. [Google Scholar]
- Capinera JL, 2020. Melon thrips, Thrips palmi Karny (Insecta: Thysanoptera: Thripidae). Electronic Data Information Source (EDIS). Publication EENY135. University of Florida, Gainesville, Florida, USA. [Google Scholar]
- Cardona C, Frei A, Bueno JM, Diaz J, Gu H and Dorn S, 2002. Resistance to Thrips palmi (Thysanoptera: Thripidae) in beans. Journal of Economic Entomology, 95, 1066–1073. [DOI] [PubMed] [Google Scholar]
- Castineiras A, Pena JE, Duncan R and Osborne L, 1996. Potential of Beauveria bassiana and Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) as biological control agents of Thrips palmi (Thysanoptera: Thripidae). Florida Entomologist, 458. [Google Scholar]
- Culliney TW, 1990. Population performance of Thrips palmi (Thysanoptera: Thripidae) on cucumber infected with a mosaic virus.
- Cuthbertson AGS, 2014. Compatibility of predatory mites with pesticides for the control of Thrips palmi Karny. 103, 17–21. [Google Scholar]
- Cuthbertson AGS, North JP and Walters KFA, 2005. Effect of temperature and host plant leaf morphology on the efficacy of two entomopathogenic biocontrol agents of Thrips palmi (Thysanoptera: Thripidae). Bulletin of Entomological Research, 95, 321. [DOI] [PubMed] [Google Scholar]
- Dong YJ and Hsiu BC, 2019. Methyl salicylate attracts predators and reduces melon thrips population (Thrips palmi Karny) (Thysanoptera: Thripidae) in cucumber plants. J. Taiwan Agric. Res., 68, 128–136. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), 2019. Guidance on commodity risk assessment for the evaluation of high‐risk plants dossiers. EFSA Journal 2019;17(4):5668, 20 pp. 10.2903/j.efsa.2019.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekesi S and Maniania NK, 2002. Metarhizium anisopliae: an effective biological control agent for the management of thrips in horti‐and floriculture in Africa. In Advances in Microbial Control of Insect Pests. Springer, Boston, MA: pp. 165–180. [Google Scholar]
- Ekesi S, Maniania NK, Akpa AD, Onu I and Dike MC, 2000. Entomopathogenicity of Beauveria bassiana and Metarhizium anisopliae (Hyphomycetes) to the onion thrips, Thrips tabaci Lindeman (Thysanoptera: Thripidae) Nig. J. Ent, 17, 21–30. [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2018a, online. EPPO Global Database. Available online: https://gd.eppo.int
- EPPO (European and Mediterranean Plant Protection Organization), 2018b. PM 7/3 (3) Thrips palmi . EPPO Bulletin, 48, 446–460. [Google Scholar]
- Etienne J, Guyot J and van Waetermeulen X, 1990. Effect of insecticides, predation, and precipitation on populations of Thrips palmi on aubergine (eggplant) in Guadeloupe. The Florida Entomologist, 73, 339–342. [Google Scholar]
- EUROPHYT , online. European Union Notification System for Plant Health Interceptions ‐ EUROPHYT. Available online: http://ec.europa.eu/food/plant/plant_health_biosecurity/europhyt/index_en.htm
- Faci JM, Medina ET, Martínez‐Cob A and Alonso JM, 2014. Fruit yield and quality response of a late season peach orchard to different irrigation regimes in a semi‐arid environment. Agricultural Water Management, 143, 102–112. [Google Scholar]
- Frei A, Bueno JM, Diaz‐Montano J, Gu H, Cardona C and Dorn S, 2004. Tolerance as a mechanism of resistance to Thrips palmi in common beans. Entomologia experimentalis et applicata, 112, 73–80. [Google Scholar]
- Gao YF, Gong YJ, Cao LJ, Chen JC, Gao YL, Mirab‐balou M, Chen ML, Hoffmann AA and Wei SJ, 2019. Geographical and interspecific variation in susceptibility of three common thrips species to the insecticide, spinetoram. Journal of Pest Science, 1–7. 10.1007/s10340-019-01128-2 [DOI] [Google Scholar]
- Ghosh A, Jagdale SS, Basavaraj YB, Dietzgen RG and Jain RK, 2020. Genetics of Thrips palmi (Thysanoptera: Thripidae). Journal of Pest Science, 93, 27–39. 10.1007/s10340-019-01160-2 [DOI] [Google Scholar]
- Hadiya GD, Kalariya GB and Kalola NA, 2016. Efficacy of different entomopathogenic fungus on chilli thrips. Adv Life Sci, 5, 1658–1660. [Google Scholar]
- Hajime H, Katai Y, Mannen J and Masui S, 2014. Attraction of Melon Thrips, Thrips palmi (Karny), to color sheets and LED Lights. Japanese Journal of Applied Entomology & Zoology, 58, 17–22. 10.1303/jjaez.2014.17 [DOI] [Google Scholar]
- Hara AH, Mau RFL, Heu R, Jacobsen C and Niino‐DuPonte R, 2002. Banana rust thrips. Damage to banana and brnamentals in Hawaii. CTAHR — Insect Pests, IP‐10. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/IP-10.pdf
- Hotaka D, Amnuaykanjanasin A, Maketon C, Siritutsoontorn S and Maketon M, 2015. Efficacy of Purpureocillium lilacinum CKPL‐053 in controlling Thrips palmi (Thysanoptera: Thripidae) in orchid farms in Thailand. Applied Entomology and Zoology, 50, 317–329. [Google Scholar]
- Huang KC, 1989. The population fluctuation and trapping of Thrips palmi in waxgourd. Bulletin of the Taichung District Agricultural Improvement Station, 25, 35–41. [Google Scholar]
- Huang LH and Chen CN, 2004. Temperature effect on the life history traits of Thrips palmi Karny (Thysanoptera:Thripidae) on eggplant leaf. Plant Protection Bulletin Taipei, 46, 99–111. [Google Scholar]
- Ilic ZS, Fallik E, Manojlovic M, Kevresan Z and Mastilovic J, 2018. Postharvest practices for organically grown products. Contemporary Agriculture, 67, 71–80. 10.2478/contagri-2018-0011 [DOI] [Google Scholar]
- Ingrid A, Marcano C, Contreras J, Jiménez O, Escalona A and Pérez P, 2012. Characterization of agronomic crop management of cucumber (Cucumis sativus L.) at Humocaro Bajo, Lara state, Venezuela. Revista Unellez de Ciencia y Tecnología, Producción Agrícola, 30, 36–42. [Google Scholar]
- Karar H, Ahmad M, Ullah H, Wajid M, Zubair M and Raza H, 2019. Effectiveness of fruit bagging for the control of insect‐pests complex and its impact on quality of mango fruits. Journal of Horticultural Science and Technology, 2, 45–48. [Google Scholar]
- Karny HH, 1925. Thrips found on tobacco in Java and Sumatra. Bulletin Deli Proefstation, 23, 3–55. [Google Scholar]
- Kawai A, 1983. Studies on population ecology of Thrips palmi Karny. I. Population growth and distribution pattern on cucumber in the greenhouse. Japanese Journal of Applied Entomology and Zoology, 27, 261–264. [Google Scholar]
- Kawai A, 1985. Studies on population ecology of Thrips palmi Karny. VII. Effect of temperature on population growth. Japanese Journal of Applied Entomology and Zoology, 29, 140–143. Available online: https://www.jstage.jst.go.jp/article/jjaez1957/29/2/29_2_140/_pdf/-char/ja [Google Scholar]
- Kawai A, 1986. Studies on population ecology of Thrips palmi Karny. XI. Analysis of damage to cucumber. Japanese Journal of Applied Entomology and Zoology, 30, 12–16. 10.1303/jjaez.30.12 [DOI] [Google Scholar]
- Kawai AKIRA, 1990. Life cycle and population dynamics of Thrips palmi Karny. Japan Agricultural Research Quarterly, 23, 282–288. [Google Scholar]
- Kawai A, 2001. Population Management of Thrips palmi Karny. Japanese Journal of Applied Entomology and Zoology, 45, 39–59. [Google Scholar]
- Kawai A and Kitamura C, 1987. Studies on Population Ecology of Thrips palmi KARNY: XV. Evaluation of Effectiveness of Control Methods Using a Simulation Model. Applied Entomology and Zoology, 22, 292–302. [Google Scholar]
- Kawai A and Kitamura C, 1990. Studies on population ecology of Thrips palmi Karny 18. evaluation of effectiveness of control methods of thrips on eggplant and sweet pepper using a simulation model. Applied Entomology and Zoology, 25, 161–175. [Google Scholar]
- Kim K, Kim MJ, Han SH, Kim SH, Kim JH and Lee SH, 2019. Amount and time course of ingestion of plant subcellular fractions by two thrips and one reference mite species. Journal of Asia‐Pacific Entomology, 22, 733–736. [Google Scholar]
- Kirk WD, 2017. The aggregation pheromones of thrips (Thysanoptera) and their potential for pest management. International Journal of Tropical Insect Science, 37, 41–49. [Google Scholar]
- de López MA, Corozo‐Ayovi RE, Delgado R, Osorio B, Moyón D, Rengifo D, Suárez P, Paulino A, Medrano S, Sanchez L, Rojas JC, Vegas U, Alburqueque D, Staver C, van Tol R and Clercx L, 2020. Red rust thrips in smallholder organic export banana in Latin America and the Caribbean: Pathways for control, compatible with organic certification. Acta Horticulturae, 1272, 153–161. [Google Scholar]
- MacLeod A, Head J and Gaunt A, 2004. An assessment of the potential economic impact of Thrips palmi on horticulture in England and the significance of a successful eradication campaign. Crop Protection, 23, 601–610. [Google Scholar]
- Mahato S and Misra HP, 2018. Field efficacy of some new insecticides against thrips Thrips palmi karny (Thysanoptera: Thripidae) on cucumber. Journal of Plant Protection and Environment, 15, 1–4. [Google Scholar]
- Maketon M, Amnuaykanjanasin A, Hotaka D and Maketon C, 2014. Population ecology of Thrips palmi (Thysanoptera: Thripidae) in orchid farms in Thailand. Applied Entomology and Zoology, 49, 273–282. [Google Scholar]
- Maltby J and Walsh B, 2005. Melon thrips in potatoes. The State of Queensland, DPI&F (Department of Primary Industries and Fisheries) note. File No: H0299. http://www.dpi.qld.gov.au/horticulture/14155.htmlS.
- Martins RC, 2018. Produção, qualidade e sanidade de frutos de bananeira ‘BRS Conquista’ ensacados com polipropileno de diferentes cores. Universidade Estadual Paulista (UNESP), Dissertação de mestrado: Available online: http://hdl.handle.net/11449/153588 [Google Scholar]
- Matsui M, Monma S and Koyama K, 1995. Screening of resistant plants in the genus Solanum to Thrips palmi Karny (Thysanoptera: Thripidae) and factors related to their resistance. Bulletin of the National Research Institute of Vegetables, Ornamental Plants and Tea. Series A: Vegetables and Ornamental Plants, 10, 13–24. [Google Scholar]
- McDonald JR, Bale JS and Walters KFA, 1999. Temperature, development and establishment potential of Thrips palmi in the UK. European Journal of Entomology, 96, 169–173. [Google Scholar]
- Morse JG and Hoddle MS, 2006. Invasion biology of thrips. Annual Review of Entomology, 51, 67–89. [DOI] [PubMed] [Google Scholar]
- Murai T, 2002. The pest and vector from the East: Thrips palmi In: Marullo R and Mound LA (eds.). pp. 19–32. [Google Scholar]
- Nagai H and Tsumuki H, 1990. Search for winter host plants of T. palmi in winter [in Japanese]. Japanese Journal of Applied Entomology and Zoology, 34, 105–108. [Google Scholar]
- Nakamura Y, Shibao M, Tanaka H and Yano E, 2014. Timing of the Attraction of Melon Thrips, Thrips palmi (Thysanoptera: Thripidae), to Reflective‐type Traps Combined with Blue Sticky Board and a Blue LED Array. Japanese Journal of Applied Entomology & Zoology, 58. [Google Scholar]
- Nonaka K and Nagai K, 1984. Ecology and control of the thrips infesting fruit vegetables. 8. Control of Thrips palmi using blue coloured sticky ribbons. Kyushu Agric. Res., 44, 119. [Google Scholar]
- North JP, Cuthbertson AG and Walters KF, 2006. The efficacy of two entomopathogenic biocontrol agents against adult Thrips palmi (Thysanoptera: Thripidae). Journal of Invertebrate Pathology, 92, 89–92. [DOI] [PubMed] [Google Scholar]
- Nozawa H, Matsui M and Koyama K, 1994. An examination on susceptibility of Thrips palmi Karny to insecticides collected from various locations in Japan. Proceedings of the Kanto‐Tosan Plant Protection Society, 41, 205–207 (In Japanese). [Google Scholar]
- Nuessly GS and Nagata RT, 1995. Pepper varietal response to thrips feeding Thrips biology and management. Springer, Boston, MA: pp. 115–118. [Google Scholar]
- OEPP/EPPO , 1989. Data sheets on quarantine organisms No. 175, Thrips palmi. Bulletin OEPP/EPPO Bulletin, 19, 717–720. [Google Scholar]
- Osorio J and Cardona C, 2003. Phenology, population dynamics and sampling methods for Thrips palmi (Thysanoptera: Thripidae) on snap beans and beans. Revista Colombiana de Entomología, 29, 43–49. [Google Scholar]
- Park CG, Kim HY and Lee JH, 2010. Parameter estimation for a temperature‐dependent development model of Thrips palmi Karny (Thysanoptera: Thripidae). Journal of Asia‐Pacific Entomology, 13, 145–149. [Google Scholar]
- Park SE, Kim JC, Lee SJ, Lee MR, Kim S, Li D and Shin TY, 2018. Solid cultures of thrips‐pathogenic fungi Isaria javanica strains for enhanced conidial productivity and thermotolerance. Journal of Asia‐Pacific Entomology, 21, 1102–1109. [Google Scholar]
- Pedigo LP, Hutchins SH and Higley LG, 1986. Economic injury levels in theory and practice. Annual Review of Entomology, 31, 341–368. [Google Scholar]
- Qin Y, Wu W and Liang G, 2004. Natural predators of Thrips palmi (Kamy) and their role in natural control. Chinese Agricultural Science Bulletin, 20, 250–264. [Google Scholar]
- Razzak MA and Seal DR, 2017. Effect of plastic mulch on the abundance of Thrips palmi Karny (Thysanoptera: Thripidae) and yield of jalapeno pepper in South Florida. Vol 130 Florida State Horticultural Society; pp. 124–128. [Google Scholar]
- Razzak MA, Seal DR and Schaffer B, 2018. Vegetable Section. In Proceedings of the Florida State Horticultural Society (Vol. 131, pp. 126–131). Florida State Horticultural Society.
- Rosenheim JA, Welter SC, Johnson MW, Mau RF and Gusukuma‐Minuto LR, 1990. Direct feeding damage on cucumber by mixed‐species infestations of Thrips palmi and Frankliniella occidentalis (Thysanoptera: Thripidae). Journal of Economic Entomology, 83, 1519–1525. [Google Scholar]
- Saito T, 1991. A field trial of an entomopathogenic fungus, Beauveria bassiana (Bals.) Vuill., for the control of Thrips palmi Karny (Thysanoptera: Thripidae). Japanese Journal of Applied Entomology and Zoology, 35, 80–81. [Google Scholar]
- Saito T, 1992. Control of Thrips palmi and Bemisia tabaci by a mycoinsecticidal preparation of Verticillium lecanii . Proceedings of the Kanto‐Tosan Plant Protection Society, 39, 209–210. [Google Scholar]
- Sakimura K, Nakahara LM and Denmark HA, 1986. A thrips, Thrips palmi Karny (Thysanoptera: Thripidae). Entomology Circular, Division of Plant Industry, Florida Department of Agriculture and Consumer Services, 280, 1–4. [Google Scholar]
- Salas J, 2004. Evaluation of cultural practices to control Thrips palmi (Thysanoptera: Thripidae) on green pepper. Entomotropica, 19, 39–46. [Google Scholar]
- Sampson C and Kirk WDJ, 2013. Can mass trapping reduce thrips damage and is it economically viable? Management of the western flower thrips in strawberry. PLoS ONE, 8 e80787 10.1371/journal.pone.0080787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez MDC, Figueroa R, Campos A and Romero R, 2011. Evaluación del color y de la orientación de trampas adhesivas en la atracción de trips en siembras comerciales de vainita. Agronomía Tropical, 61, 141–148. [Google Scholar]
- Shao F, Yang D and Ren L, 2015. Field experiment on control effects of 14 biopesticides on Thrips palmi Karny. Journal of Southern Agriculture, 46, 1237–1242. [Google Scholar]
- Sharma RR, Reddy SVR and Jhalegar MJ, 2014. Pre‐harvest fruit bagging: a useful approach for plant protection and improved post‐harvest fruit quality–a review. The Journal of Horticultural Science and Biotechnology, 89, 101–113. [Google Scholar]
- Shen JY, Wu L, Liu HR, Zhang B, Yin XR, Ge YQ and Chen KS, 2014. Bagging treatment influences production of C6 aldehydes and biosynthesis‐related gene expression in peach fruit skin. Molecules, 19, 13461–13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Guo SK, Gao YF, Cao LJ, Gong YJ, Chen JC, Yue L, Li H, Hoffmann AA and Wei SJ, 2020. Variable resistance to spinetoram in populations of Thrips palmi across a small area unconnected to genetic similarity. Evolutionary Applications, 13, 2234–2245. 10.1111/eva.12996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibao M, 2016. The efficacy of red LED (Light Emitting Diode) irradiation for controlling the density of melon thrips, Thrips palmi Karny on eggplants. Japanese Journal of Applied Entomology & Zoology, 59, 1–6. 10.1303/jjaez.2014.29 [DOI] [Google Scholar]
- Shibao M and Tanaka H, 2014. Attraction of the Melon Thrips, Thrips palmi Karny (Thysanoptera: Thripidae), to Traps Combined with a Colored Sticky Board and an LED (Light Emitting Diode). Japanese Journal of Applied Entomology & Zoology, 58, 29–32. 10.1303/jjaez.2014.29 [DOI] [Google Scholar]
- Shirotsuka K, Hamasaki K, Shibao M and Okada K, 2016. Control of melon thrips, Thrips palmi Karny, on greenhouse cucumber with the combined use of a red insect‐proof net, Amblyseius swirskii, and Metarhizium anisopliae. Report of The Kansai Plant Protection Society, 58, 45–49. 10.4165/kapps.58.45. [DOI] [Google Scholar]
- Silva AIE, Morales CAM and Torres MM, 2011. Patogenicidad De Los Hongos Metarhizium anisopliae (METSCHN.), Lecanicillium lecanii (ZIMM.) ZARE & GAMS Y Beauveria bassiana (BALS.‐CRIV.) VUILL. sobre Thrips palmi karny en el cultivo de la papa (Solanum tuberosum L.). Fitosanidad, 15, 147–151. [Google Scholar]
- Smith RM, Cuthbertson AG and Walters KF, 2005. Note: extrapolating the use of an entomopathogenic nematode and fungus as control agents for Frankliniella occidentalis to Thrips palmi . Phytoparasitica, 33, 436. [Google Scholar]
- Thongjua T, Thongjua J, Sriwareen J and Khumpairun J, 2015. Attraction effect of thrips (Thysanoptera: Thripidae) to sticky trap color on orchid greenhouse condition. Journal of Agricultural Technology, 11, 2451–2455. [Google Scholar]
- Trdan S, Valic N, Zezlina I, Bergant K and Znidarcic D, 2005. Light blue sticky boards for mass trapping of onion thrips, Thrips tabaci Lindeman (Thysanoptera: Thripidae), in onion crops: fact or fantasy? Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 112, 173–180. [Google Scholar]
- Trujillo Z, Pérez R, Borroto D and Concepción E, 2003. Efectividad de hongos entomopatógenos y Bacillus thuringiensis sobre Thrips palmi Karny en el cultivo del pepino. Fitosanidad, 7, 13–18. [Google Scholar]
- Tsolakis H and Ragusa S, 2008. Effects of a mixture of vegetable and essential oils and fatty acid potassium salts on Tetranychus urticae and Phytoseiulus persimilis . Ecotoxicology and Environmental Safety, 70, 276–282. [DOI] [PubMed] [Google Scholar]
- Tsumuki H, Nagai K and Kanehisa K, 1987. Cold hardiness of Thrips palmi. I. Survival period of winter and summer populations at low temperatures [in Japanese]. Japanese Journal of Applied Entomology and Zoology, 31, 328–332. [Google Scholar]
- Van Lenteren JC and Loomans AJM, 1999. Biological control of thrips: how far are we. Bulletin IOBC, 22, 141–144. [Google Scholar]
- Vestergaard S, Gillespie AT, Butt TM, Schreiter G and Eilenberg J, 1995. Pathogenicity of the hyphomycete fungi Verticillium lecanii and Metarhizium anisopliae to the western flower thrips. Frankliniella occidentalis. Biocontrol Science and Technology, 5, 185–192. [Google Scholar]
- Visalakshy PG, Kumar AM and Krishnamoorthy A, 2004. Epizootics of a fungal pathogen, Verticillium lecanii Zimmermann on Thrips palmi Karny. Insect Environment, 10, 134–135. [Google Scholar]
- Visschers IG, Peters JL, van de Vondervoort JA, Hoogveld RH and van Dam NM, 2019. Thrips resistance screening is coming of age: leaf position and ontogeny are important determinants of leaf‐based resistance in pepper. Frontiers in Plant Science, 10, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafula GO, Muthomi JW, Nderitu JH and Chemining'wa GN, 2017. Efficacy of potassium salts of fatty acids in the management of thrips and whitefly on snap beans. Sustainable Agriculture Research, 6, 45 10.5539/sar.v6n4p45 [DOI] [Google Scholar]
- Wang CL, Chu YI and Lo KC, 1989. The reproductive mechanism of Thrips palmi Karny. 1. The female oviposition behaviour. Chinese Journal of Entomology, 9, 251–261. [Google Scholar]
- Welter SC, Rosenheim JA, Johnson MW, Mau RFL and Gusukuma‐Minuto LR, 1990. Effects of Thrips palmi and western flower thrips (Thysanoptera: Thripidae) on the yield, growth, and carbon allocation pattern in cucumbers. Journal of Economic Entomology, 83, 2092–2101. [Google Scholar]
- Yadav R and Chang NT, 2014. Effects of temperature on the development and population growth of the melon thrips, Thrips palmi, on eggplant, Solanum melongena . Journal of Insect Science, 14, 78 10.1093/jis/14.1.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R and Chang NT, 2013. Economic thresholds of Thrips palmi (Thysanoptera: Thripidae) for eggplants in a greenhouse. Appl Entomol Zool, 48, 195–204. 10.1007/s13355-013-0172-8. [DOI] [Google Scholar]
- Yoshihara T and Kawai A, 1982. 28, 130–131. [Google Scholar]
- Young GR and Zhang L, 1998. Control of the melon thrips, Thrips palmi. Primary Industry and Fisheries Northern Territory, Darwin, Australia: Available online: https://dpir.nt.gov.au/__data/assets/pdf_file/0020/233606/753.pdf [Google Scholar]
- Zhang J, Idowu OJ, Wedegaertner T and Hughs SE, 2014. Genetic variation and comparative analysis of thrips resistance in glandless and glanded cotton under field conditions. Euphytica, 199, 373–383. [Google Scholar]


