Abstract
Purpose
This study aimed to assess the frequency of sleep and mood disturbances, and their association with COVID-like symptoms in healthcare workers (HCWs) with and without positive Coronavirus RT-PCR in a corona referral center.
Methods
This study was a cross-sectional, anonymous survey of adult HCWs. Data collection was performed in May and June 2020, while governmental restrictions were in place. The participants completed the forms including six separate parts: personal and occupational information, Insomnia Severity Index (ISI), Generalized Anxiety Disorder-7 (GAD-7), Patient’s Health Questionnaire (PHQ-9), Pittsburgh Sleep Quality Index (PSQI), and COVID-like symptoms and Coronavirus RT-PCR status.
Results
Among the 372 HCW participants, 245 (66%) were women and mean age was 34.5 ± 7.1 years (age range 23 to 58). The mean scores of all questionnaires except ISI were significantly higher in the HCWs with positive Coronavirus RT-PCR than another group (PSQI, 9 ± 3.4 vs. 6.9 ± 3.1; GAD-7, 9.8 ± 3.6 vs. 7.9 ± 5.3; PHQ-9, 12.8 ± 6.1 vs. 9.5 ± 6.4, P < 0.05; and ISI, 13.8 ± 5.3 vs. 12.3 ± 6 P = 0.163). Positive association between COVID-like symptoms and sleep and mood disturbances was found in the group without a positive test result. Analysis of questionnaires showed higher scores in the group directly involved except for ISI (P < 0.001 and P = 0.053 respectively).
Conclusions
During the COVID-19 pandemic, the HCWs in this sample experienced a high rate of sleep and mood disturbances. There was also a strong association between sleep and mood disturbances and COVID-like symptoms in the group without a positive RT-PCR result. With all this considered, effective psychological support for HCWs during crisis seems to be necessary.
Keywords: COVID-19, Insomnia, Sleep, Anxiety, Depression, Healthcare worker
Introduction
COVID-19, a disease caused by severe acute respiratory syndrome Coronavirus (SARS-CoV-2) was first identified in December 2019 in China. In a short time, COVID-19 became a worldwide health problem [1]. Due to the high infectivity of the virus and the large number of patients in several countries, quarantine of the general population has been a major governmental strategy [2]. Although isolation has had benefits such as decreasing the number of patients and providing time for the healthcare system to cope with the burden of the pandemic, it also had some adverse effects on the mental health of the population [3]. In fact, COVID-19 pandemic has had diverse effects on all aspects of peoples’ lives all around the globe other than the disease itself. These effects include social, mental, psychological, and economic problems. Healthcare workers (HCWs) are subject to a higher degree of stress than the general population [4]. The high prevalence of the disease and the large number of patients requiring intensive respiratory care overwhelmed the healthcare system and HCWs in different countries. HCWs faced different issues and stresses including increased workload and night shifts, fear of becoming sick and transmission to their family, and demoralization due to observed mortality. Despite paying attention to the prevention and treatment of patients, the psychological consequences on the general population and especially the vulnerable medical personnel were ignored [5]. On the other hand, the occurrence of the psychosomatic symptoms among HCWs related to the emotional distress of the current pandemics has been reported in Italy [6].
Iran was one of the first countries to be affected with COVID-19 outbreak. The international preventive and control-of-disease strategies such as isolation were implemented. Isfahan as the third largest city of Iran with 2,243,249 inhabitants was overwhelmed by a large number of patients, a large number of whom were critically ill. The physicians and the other personnel faced high workload, insomnia, depression, and anxiety.
Additionally, in several hospitals identified as corona referral center (CRC) in Isfahan, physicians and nurses with no experience in the care of respiratory patients were involved in the treatment of COVID-19 patients after a brief course of training programs. They encountered significantly higher mental strain and various other consequences. Considering these, the sleep quality and social support were important contributing factors in the personnel’s anxiety and depression [7, 8].
During this period, some of the personnel experienced COVID-like symptoms which are common in respiratory infections, which in turn caused further stress among them. However, many of these HCWs who experienced such symptoms showed no evidence of COVID-19 in diagnostic tests.
Therefore, we aimed to assess the prevalence of sleep and mood disturbances among HCWs and also to investigate whether insomnia, poor sleep quality, anxiety, and depression have an association with the occurrence of COVID-like symptoms among them, both in those infected with Coronavirus and those who did not show any evidence of infection.
Methods
Participants
A total of 580 HCWs were invited, and finally 372 HCWs from different parts of the CRC were enrolled (Fig. 1). A video clip about this research, and its aim was prepared and was presented to all HCWs. All participants signed informed consent forms before taking part in this study. Inclusion criteria were age > 18 and a hospital work experience of more than 3 months. Participants included HCWs such as physicians, nurses, paramedics, office and service workers, and security personnel. Participants with previous history of severe psychiatric disorders and severe insomnia with need for drug consumption were excluded. Also, forms with more than 10% of data missing were excluded. Demographic and clinical characteristics including age, gender, marital status, and psychological and medical disease were statistically analyzed, and only gender was found to have a significant effect on the outcome (P value = 0.000).
Fig. 1.
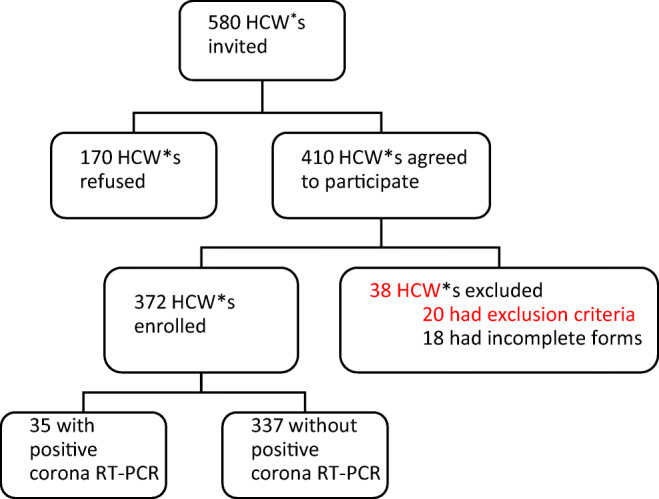
Fellow diagram of participants. *healthcare worker
Procedures
Data collection of this cross sectional study was performed in May and June, 2020. That timespan began about 3 months after the beginning of the outbreak in Iran and the peak prevalence of the disease. At that time, some restrictions such as the closure of high risk jobs, but not the general population lockdown, were applied by the government. The forms were distributed among HCWs who gave consent forms, and they were supported for the completion of the forms if needed. Each form included 6 separate sections: personal and occupational information, Insomnia Severity Index (ISI) questionnaire, Pittsburgh’s Sleep Quality Index (PSQI) questionnaire, Generalized Anxiety Disorder-7 (GAD-7) questionnaire, Patient’s Health Questionnaire-9 (PHQ-9), and some questions about COVID-like symptoms and history of positive Coronavirus RT-PCR (reverse transcription-polymerase chain reaction) of nasopharyngeal and oropharyngeal swabs during the previous three months. Mentioned symptoms included fever, chills, dyspnea, cough, rhinorrhea, diarrhea, fatigue, myalgia, and anosmia.
The ISI is a self-report questionnaire that evaluates subjective perception of insomnia. This measurement tool includes seven items evaluating difficulties falling asleep, difficulties maintaining sleep, waking up too early in the morning, satisfaction with sleep pattern, interference of sleep problems with daily functions, noticeability of sleep problems for others, and distress about sleep problems during the last month. Each item is scored on a 5-point Likert scale (0–4), and the total score ranges from 0 to 28. Scoring included four categories: no clinically significant insomnia (0–7), subthreshold insomnia (8–14), moderately severe clinical insomnia (15–21), and severe clinical insomnia (22–28) [9, 10]. Validity of the Persian version of ISI has been studied [11].
The PSQI is a valid self-reported instrument for assessing subjective sleep quality. It consists of seven domains about sleep quality, latency, duration, efficiency, disturbances, use of sleep medication, and daytime dysfunction over the past 1 month. Items are scored 0–3 based, and a score of 5 or higher is indicative of poor sleep quality [12]. We used the valid Persian version of PSQI in our study [13].
The GAD-7 is a 7-item instrument for evaluating subjective anxiety symptoms during the past 2 weeks. Each item is rated on a 4-point Likert scale from 0 (not at all) to 3 (nearly every day). The severity of anxiety is categorized to minimal (0–4), mild (5–9), moderate (10–14), and severe (15–21) based on GAD-7 score [14].
The PHQ-9 is a 9-item valid instrument for assessing depression symptoms. It is consisted of questions about interest in doing things, feeling down, sleep problems, tiredness, appetite abnormality, bad feeling about himself/herself, poor concentration, slowness of movement and speech, and self-hurt thoughts during the last 2 weeks. Items are scored on a 4-point Likert scale from 0 (not at all) to 3 (nearly every day). The severity of depression is categorized to minimal (0–4), mild (5–9), moderate (10–14), moderately severe (15–19), and severe (20–27) based on the PHQ-9 score [15]. The valid Persian translation of this tool was applied in the current research [16].
Statistical analysis
For the purposes of the current study, the Chi-square and independent T-test were used to perform descriptive statistical analysis for categorical and quantitative data, respectively. Results are reported in number with percentage for categorical data and the mean with standard deviation for quantitative data. To assess the associations between COVID-like symptoms and the scores of the questionnaires, appropriate cutoff values were presented for the insomnia, anxiety, and depression questionnaires. Then the Cochran-Mantel-Haenszel test was applied in order to adjust the P values by sex and age variables. The results were considered statistically significant with a P value of < 0.05. STATA (V.12.0) software was used to carry out all the statistical analysis.
Results
Of the 372 participants, 245(65.8%) were female, and the mean age of all participants were 34.5 (± 7.1) (Table 1). The mean age for men and women (33.4 ± 7.5 and 35.1 ± 6.9 respectively; P = 0.040) was different. In this population, 35 individuals had a history of COVID-19 disease (positive Coronavirus RT-PCR) from the beginning of the outbreak. As shown in Table 1, there is no difference between the demographic and occupational characteristics of two groups.
Table 1.
Demographic and occupational characteristics of participants
| Items | All personnel (N = 372) | Personnel with positive PCR test (N = 35) | Personnel without positive PCR test (N = 337) | P value |
|---|---|---|---|---|
| Age (mean, ± SD) | 34.5 (± 7.1) | 34.4 (± 5.7) | 34.5 (± 7.3) | 0.521 |
| Gender (female)% | 245 (65.8%) | 25 (71.4%) | 220 (65.2%) | 0.493 |
| Marriage (married)% | 280 (75.2%) | 27 (77.1%) | 253 (75.1%) | 0.787 |
| Occupation % | ||||
| Physician | 13 (3.5%) | 1 (2.9%) | 12 (3.6%) | 0.84148 |
| Nurse | 242 (65.1%) | 26 (74.3%) | 216 (64.1%) | 0.23014 |
| Paramedic | 28 (7.5%) | 1 (2.9%) | 27 (8.0%) | 0.27133 |
| Office personnel | 35 (9.4%) | 3 (8.5%) | 32 (9.5%) | 0.92034 |
| Service personnel | 37 (9.9%) | 3 (8.5%) | 34 (10.1%) | 0.76418 |
| Guard personnel | 17 (4.6%) | 1 (2.9%) | 16 (4.7%) | 0.61708 |
| Education % | ||||
| High school | 19 (5.1%) | 1 (2.9%) | 18 (5.3%) | 0.54851 |
| Diploma | 66 (17.7%) | 4 (11.4%) | 62 (18.4%) | 0.36812 |
| Bachelor | 245 (65.9%) | 21 (60%) | 224 (66.5%) | 0.61708 |
| Master | 29 (7.8%) | 8 (22.8%) | 21 (6.2%) | 0.00373 |
| PhD | 4 (1.1%) | 0 (0%) | 4 (1.2%) | 0.54851 |
| Specialty | 9 (2.4%) | 1 (2.9%) | 8 (2.4%) | 0.84148 |
| Night shifts % | ||||
| None | 118 (31.8%) | 9 (25.7%) | 109 (32.4%) | 0.42371 |
| 1–3 | 43 (11.6%) | 5 (14.3%) | 38 (11.3%) | 0.61708 |
| 4–6 | 66 (17.7%) | 7 (20%) | 59 (17.5%) | 0.68916 |
| 6–9 | 60 (16.1%) | 8 (22.9%) | 52 (15.4%) | 0.23014 |
| > 10 | 85 (22.8%) | 6 (17.1%) | 79 (23.4%) | 0.42371 |
P values are derived from the chi square tests and independent sample T-tests. The Bonferroni corrections were conducted for multiple comparisons
Also, demographic and clinical characteristics including age, gender, marital status, parental status, psychological and medical disease of physicians, nurses, paramedics, and office, service, and guard personnel were statistically analyzed, and only gender was found to be different (P = 0.000).
The frequency of all COVID-like symptoms except diarrhea (28.6% vs. 20.8%; P = 0.29) and rhinorrhea (31.4% vs. 25.2%; P = 0.45) was significantly higher in the group with positive Coronavirus RT-PCR (P < 0.05).
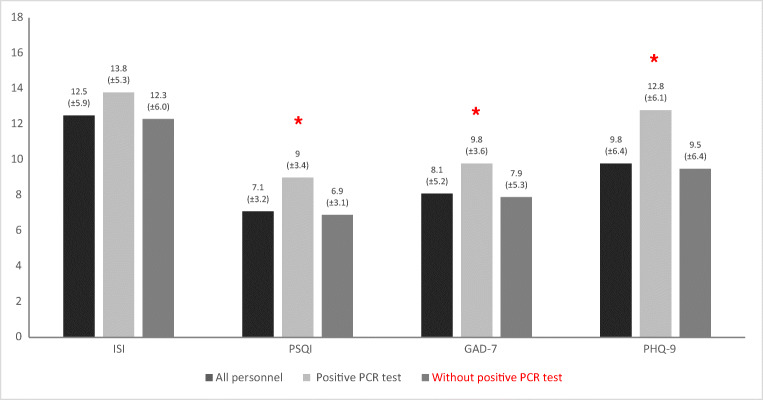
In analysis of the mean of the four questionnaires’ scores, the total scores of all questionnaires except ISI were significantly higher in participants with positive Coronavirus RT-PCR (Fig. 2). The mean scores of ISI for groups with and without positive Coronavirus RT-PCR (12.5 and 12.3 respectively) were both in the category of subthreshold insomnia (Fig. 2).
Fig. 2.
Descriptive analysis of questionnaire score separately for participants with and without PCR positive. *P < 0.05. P values are derived by the independent sample T-tests. ISI Insomnia Severity Index, PSQI Pittsburgh’s Sleep Quality Index, GAD-7 Generalized Anxiety Disorder-7, PHQ-9 Patient’s Health Questionnaire-9
Analysis by independent sample T-tests showed that there was no association between the score of the four questionnaires and COVID-like symptoms, except for PHQ9 with fever (P = 0.002) in HCWs with positive Coronavirus RT-PCR.
There was, however, a positive association between the severity of sleep and mood disturbances based on the four questionnaires and all COVID-like symptoms, in HCWs without positive Coronavirus RT-PCR (P < 0.05).
To assess the associations between symptoms and the scores of the questionnaires, appropriate cutoff values were presented for each questionnaires, and the Cochran-Mantel-Haenszel test was applied in order to adjust the P values by gender and age variables. These cutoff values indicate moderate severity of insomnia, depression, anxiety, and poor sleep quality (15, 10, 10, and 5 for ISI, PHQ-9, GAD-7, and PSQI respectively) (Table 2). As shown in Table 2, a strong association between positive Coronavirus RT-PCR with anxiety was observed.
Table 2.
Association between symptoms and four questionnaire score in all participants
| Symptoms | ISI (cutoff = 15) | PHQ-9 (cutoff = 10) | GAD-7 (cutoff = 10) | PSQI (cutoff = 5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 15 N = 241 |
≥ 15 N = 131 |
P value | < 10 N = 204 |
≥ 10 N = 168 |
P value | < 10 N = 239 |
≥ 10 N = 133 |
P value | < 5 N = 80 |
≥ 5 N = 292 |
P value | |
| Fever | 32 (13.3%) | 25 (19.1%) | 0.185 | 20 (9.8%) | 37 (22%) | 0.004 | 30 (12.6%) | 27 (20.3%) | 0.087 | 10 (12.5%) | 47 (16.1%) | 0.297 |
| Chills | 63 (26.1%) | 58 (44.3%) | 0.002 | 44 (21.6%) | 77 (45.8%) | < 0.001 | 61 (25.5%) | 60 (45.1%) | 0.004 | 15 (18.8%) | 106 (36.3%) | 0.006 |
| Cough | 78 (32.4%) | 64 (48.9%) | 0.007 | 60 (29.4%) | 82 (48.8%) | 0.003 | 77 (32.2%) | 65 (48.9%) | 0.013 | 25 (31.3%) | 117 (40.1%) | 0.258 |
| Dyspnea | 73 (30.3%) | 62 (47.3%) | 0.004 | 53 (26%) | 82 (48.8%) | < 0.001 | 69 (28.9%) | 66 (49.6%) | 0.001 | 20 (25%) | 115 (39.4%) | 0.010 |
| Nausea | 36 (14.9%) | 41 (31.3%) | 0.001 | 23 (11.3%) | 54 (32.1%) | < 0.001 | 31 (13%) | 46 (34.6%) | 0.002 | 10 (12.5%) | 67 (22.9%) | 0.121 |
| Diarrhea | 30 (12.4%) | 50 (38.2%) | < 0.001 | 19 (9.3%) | 61 (36.3%) | < 0.001 | 32 (13.4%) | 48 (36.1%) | < 0.001 | 9 (11.3%) | 71 (24.3%) | 0.043 |
| Anorexia | 52 (21.6%) | 48 (36.6%) | 0.003 | 35 (17.2%) | 65 (38.7%) | < 0.001 | 53 (22.2%) | 47 (35.3%) | 0.026 | 11 (13.8%) | 89 (30.5%) | 0.004 |
| Abdominal pain | 36 (14.9%) | 49 (37.4%) | < 0.001 | 31 (15.2%) | 54 (32.1%) | 0.001 | 39 (16.3%) | 46 (34.6%) | 0.001 | 9 (11.3%) | 76 (26%) | 0.044 |
| Rhinorrhea | 55 (22.8%) | 41 (31.3%) | 0.225 | 39 (19.1%) | 57 (33.9%) | 0.014 | 51 (21.3%) | 45 (33.8%) | 0.074 | 13 (16.3%) | 83 (28.4%) | 0.039 |
| Nasal congestion | 46 (19.1%) | 40 (30.5%) | 0.032 | 34 (16.7%) | 52 (31%) | 0.003 | 44 (18.4%) | 42 (31.6%) | 0.003 | 9 (11.3%) | 77 (26.4%) | 0.006 |
| Anosmia | 21 (8.7%) | 23 (17.6%) | 0.006 | 14 (6.9%) | 30 (17.9%) | 0.001 | 19 (7.9%) | 25 (18.8%) | 0.007 | 6 (7.5%) | 38 (13%) | 0.190 |
| Weight loss | 22 (9.1%) | 21 (16%) | 0.143 | 12 (5.9%) | 31 (18.5%) | < 0.001 | 18 (7.5%) | 25 (18.8%) | 0.007 | 6 (7.5%) | 37 (12.7%) | 0.201 |
| Chest pain | 54 (22.4%) | 46 (35.1%) | 0.026 | 38 (18.6%) | 62 (36.9%) | < 0.001 | 55 (23%) | 45 (33.8%) | 0.065 | 8 (10%) | 92 (31.5%) | < 0.001 |
| Myalgia | 63 (26.1%) | 72 (55%) | < 0.001 | 46 (22.5%) | 89 (53%) | < 0.001 | 64 (26.8%) | 71 (53.4%) | < 0.001 | 17 (21.3%) | 118 (40.4%) | 0.001 |
| Fatigue | 103 (42.7%) | 96 (73.3%) | < 0.001 | 77 (37.7%) | 122 (72.6%) | < 0.001 | 98 (41%) | 101 (75.9%) | < 0.001 | 27 (33.8%) | 172 (58.9%) | 0.001 |
| positive Corona-virus RT-PCR | 21 (8.7%) | 14 (10.7%) | 0.869 | 13 (6.4%) | 22 (13.1%) | 0.134 | 15 (6.27%) | 20 (15.0%) | 0.027 | 4 (5%) | 31 (10.6%) | 0.178 |
P values < 0.05 are statistically significant
P values are based on the Cochran-Mantel-Haenszel test were confounded by age and sex
ISI Insomnia Severity Index, PHQ-9 Patient’s Health Questionnaire-9, GAD-7 Generalized Anxiety Disorder-7, PSQI Pittsburgh’s Sleep Quality Index
Sleep and mood disturbances were compared among the participants not only based on Coronavirus RT-PCR test results, but also considering whether they were directly or indirectly caring for the patients. Those participants who were directly caring for the patients including physicians and nurses had significantly higher PHQ-9, GAD-7, and PSQI, but not for ISI questionnaire scores relative to other participants. The ISI mean score for directly and indirectly caring groups were 12.92 ± 5.89 vs. 11.64 ± 6.09 respectively (P = 0.053). These scores were 10.98 ± 6.32 vs. 7.5 ± 6.23 (P < 0.001) for PHQ-9, 9.09 ± 5.26 vs. 6.01 ± 4.45 (P < 0.001) for GAD-7, and 7.56 ± 3.24 vs. 6.08 ± 2.9 (P < 0.001) for PSQI questionnaire.
Discussion
COVID-19 disease has rapidly spread throughout the world after beginning in China on December 2019, and it has become a full blown pandemic [17]. Due to the high risk of transmission and fatality, medical staff are at risk for stress and mental health problems in addition to infection. The infection and loss of colleagues and friends are also important causes of mental stress and tension. Our country, Iran, was one of the first sites of Coronavirus outbreak, and a great number of people were infected.
In our study, the prevalence of insomnia, anxiety, depression, and poor sleep quality in HCWs were high. All disturbances except insomnia were more severe in group with COVID-19 disease. There were positive association between the severity of sleep and mood disturbances and COVID-like symptoms in HCWs without COVID-19 disease, but not in group with COVID-19 disease. The reason could be the small sample size in the group with COVID-19 disease. Additionally, there were positive associations between moderately severe insomnia, anxiety, depression, poor sleep quality, and COVID-like symptoms in all participants. Actual explanation of this association may be one directional or bidirectional. The sleep and mood disturbances increase the prevalence of somatic symptoms without physical illness. On the other hand, this potentially fatal infection per se could induce or worsen sleep and mood disorders.
In our study, the severity of poor sleep quality, anxiety, and depression, but not insomnia, were higher in HCWs with positive Coronavirus RT-PCR. The psychological impact of COVID-19 disease has been reported in a large retrospective cohort in the USA conducted by Taquet et al. In their study, patients diagnosed with COVID-19 were more frequently diagnosed with their first psychiatric disorder, including anxiety and insomnia, in the following 14–90 days after being infected, compared to six other health-related events [18]. The different result regarding insomnia might be due to the differences of the population studied.
Similar findings were reported from other countries involved with the outbreak. In the study of Huang in China, the mental health burden of the pandemic was shown by a high prevalence of anxiety, depression, and poor sleep quality among the general population. In their research, being a healthcare worker was a risk factor for mental illness that explained the difference in prevalence with our research [19]. In another study in China, anxiety, depression, insomnia, and acute stress disorder were prevalent during the outbreak. Also, the threat degree that is highest for medical staff was an important factor in mental illness [20]. In study of Zhang et al, 36.1% of frontline HCWs had insomnia. The severity of insomnia was associated with factors including low education, being a doctor, working in an isolated unit, depression, and anxiety [21].
In a meta-analysis that included thirteen studies on the psychological impacts of COVID-19 outbreak on HCWs, the authors reported that a significant proportion of HCWs experienced mood and sleep disturbances [22]. Their results are completely in agreement with ours, but not necessarily with the same prevalence rates of insomnia, depression, and anxiety.
HCWs of India and Singapore involved in the care of COVID-19 patients had high rates of anxiety, depression, and stress. There was a significant association between physical symptoms and the mean score of questionnaires [23]. The similarities of their results with ours are the high prevalence of anxiety and depression and their association with physical symptoms. Although the prevalence of anxiety and depression were different, that could be explained by the unequal exposure and prevalence of the disease and different cultural and social characteristics. The outbreak and the overwhelming of the healthcare system were more severe in Iran compared to India and Singapore at least until the time of data collection.
The relationship between anxiety and depression with somatic symptoms has found in the study of a large population in the HUNT-II Study [24]. Some of evaluated symptoms were the same symptoms we assessed in our study including chest pain, breathlessness, nausea, and diarrhea. Explanation of no association between the sleep and mood disturbances with symptoms in group with positive RT-PCR could be that the main etiology of the symptoms in this group was the infection. In the same way, Huang and colleagues excluded the participants with organic diseases [24].
Regarding the association between the level of exposure and the sleep and mood disturbances, relatively similar to our findings were reported from China [25]. In their study, the severity of anxiety and depression were high in HCWs, but significantly higher among frontline HCWs compared to other HCWs [25]. The different findings about insomnia may be due to our small sample size. Another explanation is the basic sleep characteristics of participants.
To our best knowledge, this is the first study of the association between sleep and mood disorders with COVID-like symptoms and in HCWs in Iran. Nevertheless, there are some limitations to our research. One drawback of this study is the lack of baseline sleep and psychological characteristics of participants for comparison with the new results. Additionally, the number of participants was relatively low in our study.
In conclusion, our results showed a high prevalence of anxiety, depression, and sleep disturbances during the outbreak in HCWs. The severity of sleep and mood disorders was higher in HCWs who suffered from COVID-19 compared to their colleagues. Also, there was a significant association between sleep and mood disturbances with COVID-like symptoms without any disease. Therefore, an organized scientific team for reduction of mental and emotional effects of the outbreak seems necessary.
Acknowledgment
We appreciate the collaboration of staff of Khorshid Hospital, Isfahan, Iran.
Authors’ contributions
BA and CM contributed to the concept and design of the work. FS, MS, RS, and MN contributed to data collection and interpretation. MM and KG analyzed the data. The first draft was written by FS. All authors commented and approved the final manuscript.
Declarations
Ethics approval
The project was approved by the ethical committee of the Institutional Board Review of Isfahan University of Medical Sciences, Isfahan, Iran (IR.MUI.REC.1399.008).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Babak Amra, Email: amrababak@gmail.com.
Mehrzad Salmasi, Email: mehrzad_salmasi@yahoo.com.
Forogh Soltaninejad, Email: soltaninejad.fg@gmail.com, Email: f.soltaninejad@med.mui.ac.ir.
Ramin Sami, Email: R.sami@med.mui.ac.ir.
Mina Nickpour, Email: minanickpour@yahoo.com.
Marjan Mansourian, Email: jmansourian@gmail.com.
Khojasteh Ghasemi, Email: Khojasteghasemi1372@gmail.com.
Charles M. Morin, Email: cmorin@psy.ulaval.ca
References
- 1.Organization WHO (2020) Coronavirus disease ( COVID-19): situation report, 182
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Bansal P, Bingemann TA, Greenhawt M, et al. Clinician wellness during the COVID-19 pandemic: extraordinary times and unusual challenges for the allergist/immunologist. J Allergy Clin Immunol Pract. 2020;8(6):1781–1790.e3. doi: 10.1016/j.jaip.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Wang K, Yin L, Zhao W, Xue Q, Peng M, Min BQ, Tian Q, Leng HX, du JL, Chang H, Yang Y, Li W, Shangguan FF, Yan TY, Dong HQ, Han Y, Wang YP, Cosci F, Wang HX. Mental health and psychosocial problems of medical health workers during the COVID-19 epidemic in China. Psychother Psychosom. 2020;89:242–250. doi: 10.1159/000507639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kar SK, Arafat SY, Kabir R, Sharma P, Saxena SK. Coping with mental health challenges during COVID-19. In: Saxena SK, editor. Coronavirus Disease 2019 (COVID-19), Medical Virology: from Pathogenesis to Disease Control. Singapore: Springer; 2020. pp. 199–213. [Google Scholar]
- 6.Marinaci T, Carpinelli L, Venuleo C, Savarese G, Cavallo P. Emotional distress, psychosomatic symptoms and their relationship with institutional responses: a survey of Italian frontline medical staff during the Covid-19 pandemic. Heliyon. 2020;6:e05766. doi: 10.1016/j.heliyon.2020.e05766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Q, Luo D, Haase JE, Guo Q, Wang XQ, Liu S, et al. The experiences of health-care providers during the COVID-19 crisis in China: a qualitative study. Lancet Glob Health. 2020;8(6):e790–e798. doi: 10.1016/S2214-109X(20)30204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao H, Zhang Y, Kong D, Li S, Yang N. The effects of social support on sleep quality of medical staff treating patients with coronavirus disease 2019 (COVID-19) in January and February 2020 in China. Med Sci Monit. 2020;26:e923549–e923541. doi: 10.12659/MSM.923549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morin CM (1993) Insomnia Severity Index
- 10.Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadeghniiat-Haghighi K, Montazeri A, Khajeh-Mehrizi A, Nedjat S, Aminian O. The insomnia severity index: cross-cultural adaptation and psychometric evaluation of a Persian version. Qual Life Res. 2014;23(2):533–537. doi: 10.1007/s11136-013-0489-3. [DOI] [PubMed] [Google Scholar]
- 12.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Moghaddam JF, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P) Sleep Breath. 2012;16(1):79–82. doi: 10.1007/s11325-010-0478-5. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardestani MS, Ashtiani RD, Rezaei Z, Vasegh S, Gudarzi SS. Validation of Persian version of PHQ-9 for diagnosis of major depressive episode in psychiatric wards in IRAN. IJABS. 2019;5(2):1–8. [Google Scholar]
- 17.Zhou P, Yang X, Wang X, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):27 0-3. [DOI] [PMC free article] [PubMed]
- 18.Taquet M, Luciano S, Geddes JR, Harrison PJ (2020) Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. Published Online November 9, 2020. 10.1016/S2215-0366(20)30462-4 [DOI] [PMC free article] [PubMed]
- 19.Huang Y, Zhao N (2020) Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res 112954 [DOI] [PMC free article] [PubMed]
- 20.Lin L, Wang J, Ou-yang X, Miao Q, Chen R, Liang F, Zhang YP, Tang Q, Wang T (2020) The immediate impact of the 2019 novel coronavirus (COVID-19) outbreak on subjective sleep status. Sleep Med [DOI] [PMC free article] [PubMed]
- 21.Zhang C, Yang L, Liu S, Ma S, Wang Y, Cai Z, du H, Li R, Kang L, Su M, Zhang J, Liu Z, Zhang B. Survey of insomnia and related social psychological factors among medical staff involved in the 2019 novel coronavirus disease outbreak. Front Psychiatry. 2020;11:306. doi: 10.3389/fpsyt.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pappa S, Ntella V, Giannakas T, Giannakoulis VG, Papoutsi E, Katsaounou P (2020) Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun [DOI] [PMC free article] [PubMed]
- 23.Chew NWS, Lee GKH, Tan BYQ, Jing M, Goh Y, Ngiam NJH, Yeo LLL, Ahmad A, Ahmed Khan F, Napolean Shanmugam G, Sharma AK, Komalkumar RN, Meenakshi PV, Shah K, Patel B, Chan BPL, Sunny S, Chandra B, Ong JJY, Paliwal PR, Wong LYH, Sagayanathan R, Chen JT, Ying Ng AY, Teoh HL, Tsivgoulis G, Ho CS, Ho RC, Sharma VK. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–565. doi: 10.1016/j.bbi.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haug TT, Mykletun A, Dahl AA. The Association Between Anxiety, Depression, and Somatic Symptoms in a Large Population: The HUNT-II Study. Psychosom Med. 2004;66:845–851. doi: 10.1097/01.psy.0000145823.85658.0c. [DOI] [PubMed] [Google Scholar]
- 25.Lai J, Ma S, Wang Y, Cai Z, Hu J, Wei N, et al (2020) Factors associated with mental health outcomes among health careworkers exposed to coronavirus disease 2019. 3(3):e203976. 10.1001/jamanetworkopen.2020.3976 [DOI] [PMC free article] [PubMed]