Abstract
Background
Outpatient coronavirus disease 2019 (COVID-19) has been insufficiently characterized. To determine the progression of disease and determinants of hospitalization, we conducted a prospective cohort study.
Methods
Outpatient adults with positive reverse transcription polymerase chain reaction results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were recruited by phone between April 21 and July 23, 2020, after receiving outpatient or emergency department testing within a large health network in Maryland, United States. Symptoms were collected by participants on days 0, 3, 7, 14, 21, and 28, and portable pulse oximeter oxygen saturation (SaO2), heart rate, and temperature were collected for 15 consecutive days. Baseline demographics, comorbid conditions, and vital signs were evaluated for risk of subsequent hospitalization using negative binomial and logistic regression.
Results
Among 118 SARS-CoV-2-infected outpatients, the median age (interquartile range [IQR]) was 56.0 (50.0–63.0) years, and 50 (42.4%) were male. Among individuals in the first week of illness (n = 61), the most common symptoms included weakness/fatigue (65.7%), cough (58.8%), headache (45.6%), chills (38.2%), and anosmia (27.9%). Participants returned to their usual health a median (IQR) of 20 (13–38) days from symptom onset, and 66.0% of respondents were at their usual health during the fourth week of illness. Over 28 days, 10.9% presented to the emergency department and 7.6% required hospitalization. The area under the receiver operating characteristics curve for the initial home SaO2 for predicting subsequent hospitalization was 0.86 (95% CI, 0.73–0.99).
Conclusions
Symptoms often persisted but uncommonly progressed to hospitalization among outpatients with COVID-19. Home SaO2 may be a helpful tool to stratify risk of hospitalization.
Keywords: ambulatory care, coronavirus infections/epidemiology, middle aged, recovery of function, treatment outcome
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the coronavirus disease 2019 (COVID-19) pandemic that has affected nearly every region of the world and by October 30, 2020, is responsible for the deaths of more than 1 180 000 people [1]. In persons who are hospitalized, the clinical features of COVID-19 and disease course are well described [2–4]. However, most SARS-CoV-2-infected persons are not hospitalized, and relatively little is known about the progression of symptoms, clinical outcomes, and severity predictors among outpatients [5–7]. The prevalence and time course of unique clinical features of COVID-19 such as the occurrence of low oxygen saturations with a delayed patient sense of dyspnea, or “silent hypoxemia,” have yet to be fully characterized [8–10]. Additionally, seroprevalence studies suggest that the number of outpatient cases is much greater than has been reported [11].
To investigate COVID-19 in the home setting, a prospective outpatient observational cohort was recruited and studied using structured measurements to characterize the course of disease. To better study risk factors for severe disease, the cohort recruitment efforts enriched for older individuals [12]. Given the dominance of pulmonary syndromes in those hospitalized and to investigate the incidence of asymptomatic hypoxemia, we supplemented home monitoring with daily pulse oximetry [9].
METHODS
Study Design
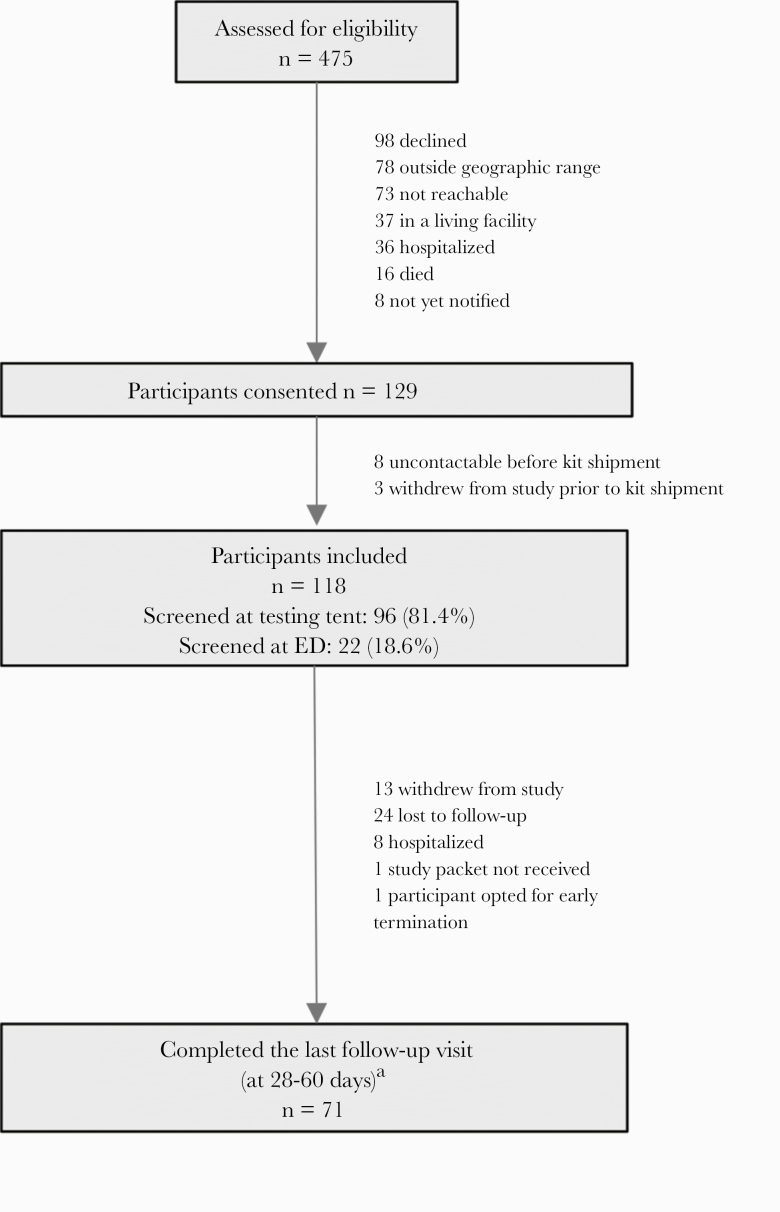
In an Institutional Review Board–approved study, persons age ≥18 who attended 1 of the Johns Hopkins Health System COVID-19 testing sites (5 hospital-based tent testing facilities and emergency rooms) and tested positive for SARS-CoV-2 were offered enrollment in the study via a telephone call between April 21 and July 23, 2020. Research personnel received a daily report of all individuals who had a positive test within the health system, which was 3991 patients in total during this period. To maximize recruitment of older persons, we limited recruitment to the 2453 individuals who were midlife adults or older (≥40 years) using convenience sampling [12]. Of these, 1978 people including 727 non-English-speakers were not contacted due to research personnel capacity limits for a remote longitudinal study. There were 475 individuals assessed for eligibility (Figure 1), and there were 118 individuals who provided informed consent and received a study kit [13]. Spanish speakers were included between June 27, 2020, and July 23, 2020. Participants had to be notified of their SARS-CoV-2 test result, living independently not in a congregate setting, and living in the Baltimore–Washington corridor. Additionally, hospitalized individuals and people in a living facility were not included. After providing verbal consent, a study coordinator contacted the participant to confirm their willingness to participate and verify the shipping address, to which a study self-testing kit was shipped and received by the participant within 24–48 hours. This kit contained a thermometer (CVS Health, Woonsocket, RI, USA), a pulse oximeter (Zewa, Fort Myers, FL, USA), and supplies for self-testing [14]. A study coordinator scheduled a video or phone study visit (day 0) to occur upon receipt of the study kit to instruct participants on self-testing procedures and appropriate use of the pulse oximeter. Study visits occurred via phone for days 0, 3, 7, 14, and 21. These follow-up calls and the health system electronic medical records were used to determine if participants were subsequently hospitalized. Participants attended an in-person follow-up visit between study days 28 and 60 if they were asymptomatic at the time, consistent with local hospital infection control procedures.
Figure 1. .
Screening, enrollment, and follow-up. aOne participant completed follow-up after hospitalization, but others were not able to complete an in-person follow-up visit. Abbreviation: ED, emergency department.
Vital signs (heart rate, oxygen saturation, and temperature) were prospectively collected and recorded by participants for the first 15 study days and reviewed with the study coordinator at each study visit. Participants were instructed to use the pulse oximeter at least once a day at rest after sitting for about 10 minutes. If participants were able to ambulate safely, they were asked to ambulate for 30 to 60 seconds before measuring the ambulatory oxygen saturation. Participants were requested to call the study team and their primary care physician for oxygen saturation values ≤92%. At study days 0, 3, 7, 14, 21, and 28, participants completed a 32-item influenza Patient–Reported Outcome (FLU-PRO) [15] questionnaire verbally over the phone with the study coordinator. This questionnaire assessed participants’ sense of physical well-being including symptoms, a return to “usual activities,” and a return to “usual health.” Symptoms were reported using a Likert scale. This instrument has been previously validated for patient-reported outcomes for influenza and other respiratory viruses [15–18]. The FLU-PRO was modified to allow participants to report symptoms they perceived to be related to COVID-19 that were not already listed on the questionnaire (eg, ageusia and anosmia). These additional items were not included in the mean FLU-PRO score. Study data were collected and managed using the REDCap electronic data capture tool hosted at Johns Hopkins University [19].
Preceding symptoms were recorded at enrollment, and present symptoms were recorded on study days. Summary statistics including prevalence, incidence, and duration were calculated for baseline demographics, baseline comorbidities, and for time-varying parameters such as vital signs and modified FLU-PRO symptoms. FLU-PRO total score means and symptom domain (eg, respiratory) means were calculated [17]. The Likert scale was dichotomized to presence or absence of symptoms. To identify common symptom patterns at the onset of illness, we evaluated the frequency of combinations of CDC COVID-19 case definition symptoms in addition to diarrhea and weakness using an UpSet plot [20]. After excluding nonrespondents and 5 asymptomatic individuals, date of symptom onset was used to divide longitudinal symptom prevalence into the following categories: days 0–7 (n = 68), days 8–14 (n = 67), days 15–21 (n = 64), days 22–28 (n = 52), and days >28 (n = 84). A single result per week per individual from participants with repeated measures was chosen with randomization to reduce confounding from dropout. Date of symptom onset was used to center the data, and therefore descriptive symptom and physiologic parameter results were restricted to those that had a date of symptom onset. Participants who did not initially have symptoms but subsequently developed symptoms were considered symptomatic, and the time of onset of symptoms was incorporated into summary results. To reduce the effect of recall bias, prior symptoms were included in the determination of symptom prevalence in the first week of illness only if symptoms had started within a week of enrollment. To evaluate the correlation between oxygen saturation at rest and with ambulation, a Pearson’s correlation was performed, and a Bland-Altman plot was created.
Baseline demographics were compared between those who completed the study and those who dropped out using a Mann-Whitney U test (age and Charlson Comorbidity Index), Fisher’s exact test (sex and ethnicity), and chi-square test (race). While there was appreciable dropout, with 71 of 118 participants completing the study, there was no significant difference in age (P = .66), sex (P = .57), race (P = .08), or Charlson comorbidity index (P = .62) between those who completed the study and those who did not. However, those who did not complete follow-up were more likely to be Hispanic/Latinx participants (27.7%, compared with 7% of those who completed; P = .003). Kaplan-Meier plots were created to describe the time from symptom onset to a return to usual health and the time to a return to usual activities. After checking the proportional hazards assumption, Cox regression was performed to evaluate for baseline demographics and duration of illness affecting activities. Univariate negative log binomial regression was performed to evaluate the association between age, sex, baseline comorbid conditions (dichotomous), heart rate (continuous), temperature (continuous), and oxygen saturation (continuous) and the occurrence of a subsequent hospitalization. Logistic regression was performed for significant values, and an area under the receiver operating characteristic (AUROC) curve was determined. Logistic and negative binomial regression was performed using baseline data from all participants regardless of a history of symptoms. Persons who withdrew or were lost to follow-up were excluded from regression analyses. Sample size calculations were performed before study initiation with a target accrual of 500 participants to determine the duration of viral shedding (clinicaltrials.gov NCT04496466). The results described here include those that were achievable during the described study period, which included the region’s first peak of the pandemic. Analyses were performed using Stata, version 16.0 (StataCorp LLC, College Station, TX, USA), and figures were created using Stata or R, version 4.0.1 (R Foundation).
RESULTS
From April 21 to June 23, 2020, 118 participants enrolled a median (interquartile range [IQR]) of 5.0 (3.0–10.0) days from symptom onset (Figure 1). Their positive SARS-CoV-2 results were obtained from tent testing (81.4%) or from an emergency department (18.6%). Participants were a median (IQR) of 56.0 (50.0–63.0) years of age, 42.4% male (n = 50), and the median Charlson Comorbidity Index (IQR) was 2 (1–3) (Table 1). In the prior 2 weeks before developing symptoms, 40.2% of respondents (39 of 97) had contact with someone with confirmed COVID-19, and an additional 21.6% (n = 21) had contact with someone with symptoms concerning for unconfirmed COVID-19.
Table 1. .
Baseline Demographics and Clinical Characteristics of the Cohort, Stratified by Subsequent Hospitalization
| Characteristic | Total | Subsequently Hospitalized | |
|---|---|---|---|
| Male sex, No./total No. (%) | 50/118 (42.4) | 4/9 (44.4) | |
| Age, median (IQR), y | 56.0 (50.0–63.0) | 65.0 (54.0–69.0) | |
| Race, No. (%) | |||
| White | 56 (47.5) | 7 (77.8) | |
| Black | 46 (39.0) | 1 (11.1) | |
| Other | 8 (6.8) | 0 (0.0) | |
| Asian | 6 (5.1) | 1 (11.1) | |
| Native American | 1 (0.8) | 0 (0.0) | |
| Native Hawaiian or other Pacific Islander | 1 (0.8) | 0 (0.0) | |
| Ethnicity, No. (%) | Hispanic | 18 (15.3) | 3 (33.3) |
| Charlson Comorbidity Index, median (IQR) | 2 (1–3) | 2 (1–3) | |
| Coexisting disorder, No. (%) | |||
| Hypertension | 46 (39.0) | 6 (66.7) | |
| Current or history of tobacco use | 27 (26.0) | 3 (33.3) | |
| Diabetes | 19 (16.1) | 3 (33.3) | |
| Asthma or COPD | 18 (15.3) | 2 (22.2) | |
| Solid tumor malignancy | 18 (15.3) | 1 (11.1) | |
| HIV | 7 (6.6) | 0 (0.0) | |
| Hematologic malignancy | 6 (5.1) | 0 (0.0) | |
| CKD stage 3 or 4 | 4 (3.4) | 0 (0.0) | |
| History of myocardial infarction | 2 (1.7) | 1 (11.1) | |
| Employment, No. (%) | |||
| Health care setting | |||
| Hospital | 15 (12.7) | 0 (0.0) | |
| Skilled nursing facilities, long-term care facility, or nursing home | 9 (7.6) | 0 (0.0) | |
| Clinic | 3 (2.5) | 0 (0.0) | |
| Other | 3 (2.5) | 0 (0.0) | |
| Home health | 2 (1.7) | 0 (0.0) | |
| Prison or jail | 1 (0.8) | 1 (11.1) | |
| Essential workera, no. (%) | 21 (20.6) | 1 (14.3) | |
| Body mass index, median (IQR), kg/m2 | 30.0 (26.0–36.0) | 31.0 (29.0–36.0) | |
| Duration of symptoms at enrollment among participants with symptoms (nb = 95), median (IQR), d | 5.0 (3.0–10.0) | 3.0 (2.0–7.0) | |
| Duration of symptoms until initial vital sign among participants with symptoms (nb = 89), median (IQR), d | 8.0 (3.0–13.0) | 5.0 (3.0–11.0) | |
| Initial vital sign parameters (nb = 96), | |||
| Resting oxygen saturation (IQR), % | 98.0 (96.0–98.5) | 95.0 (90.0–97.0) | |
| Ambulatory oxygen saturation (IQR), % | 97.0 (95.0–98.0) | 95.0 (88.0–96.0) | |
| Heart rate (IQR), beats per minutes | 85.0 (74.0–91.0) | 85.0 (73.0–94.0) | |
| Highest temperature (IQR), °Fahrenheit | 98.3 (97.6–98.8) | 98.4 (97.9–100.2) |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
aEssential workers outside of a health care setting.
bParticipants with data available out of the cohort of 118 participants. Missingness for duration of symptoms was due to lack of symptoms (n = 5) or incomplete/unknown dates (n = 18).
Symptoms and Physiologic Parameters at the Onset of Illness
The most common initial symptoms reported at enrollment were suspected fever (28.0%), dry cough (23.7%), body aches (21.2 %), weakness or fatigue (20.3 %), and headache (17.0 %) (Supplementary Figure 1). The median duration of symptoms at enrollment (IQR, range) was 5.0 (3.0–10.0, –4 to 75) days (Table 1). There were 5 (4.2%) asymptomatic participants and 4 patients (3.4%) who were pauci-symptomatic (1 symptom before or at enrollment). Asymptomatic patients were tested because they had a positive contact (n = 3) or during screening for medical encounters (n = 2).
Symptoms and Physiologic Parameters During the Clinical Course of Disease
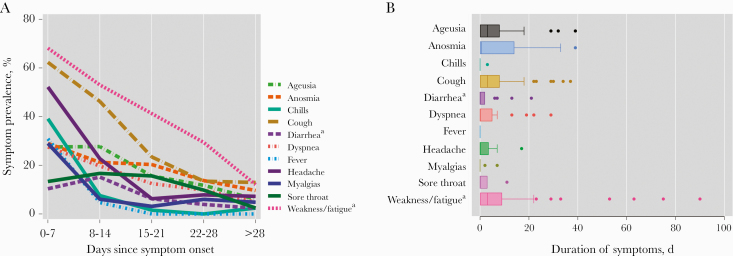
The most common symptoms during the first week since onset included weakness/fatigue (65.7%), cough (58.8%), headache (45.6%), chills (38.2%), and anosmia (27.9%) (Figure 2A). Repeated unexpected symptoms included a skin burning sensation (n = 3) and a smell of burning wood (n = 2). During the first month of illness, the prevalence of symptoms decreased but a substantial proportion of individuals still reported symptoms including weakness (13.6%) or a dry cough (13.6%) at least 28 days after symptom onset (Figure 2A and B).
Figure 2. .
A, Symptom prevalence by week of illness per a FLU-PRO questionnaire and additional COVID-19-specific questions and (B) box plots of duration of individual symptoms. aNot present in interim April 2020 CDC COVID-19 case definition. Abbreviations: CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019.
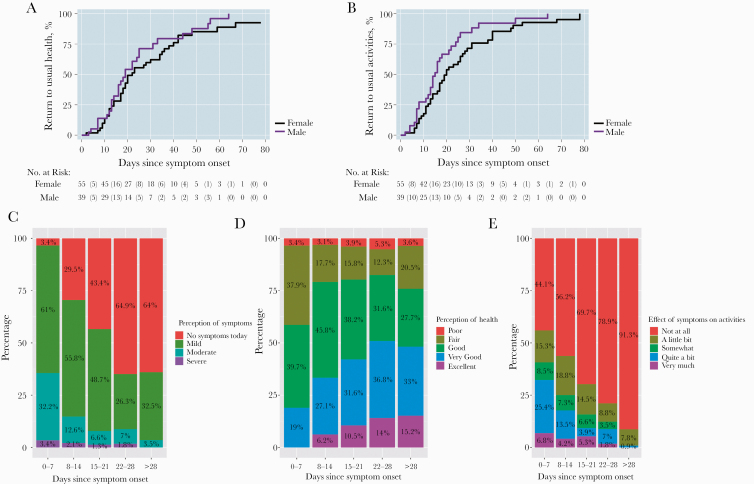
The effect of symptoms on activities of daily living and illness severity varied greatly at any given point and over time (Figure 3). Peak mean FLU-PRO scores occurred at a median (IQR) 10 (7–21) days after symptom onset. After peak illness, participants with symptoms returned to their usual health a median (IQR) of 20 (13–38) days from the onset of symptoms, and the median time to returning to usual activities (IQR) was 17 (11–28) days from symptom onset. While baseline factors of age or comorbid conditions were not associated with a delay in return to usual activities, female sex was associated with a delay in return to usual activities in an unadjusted Cox proportional hazards regression (hazard ratio, 1.6; 95% CI, 1.01–2.55; P = .046) (Figure 3A, B). These baseline factors, including biologic sex, were not associated with a return to a sense of usual health. Notably, while the majority (63.7%) of participants had no symptoms or only had mild symptoms during the first week of illness, a substantial proportion continued to have mild or moderate symptoms for over 1 month (Figure 3C–E). During the third and fourth week since symptom onset, only 53.2% and 66.0% of respondents had returned to their usual health, respectively. Among participants 28–99 days after symptom onset, 83.3% had returned to their usual health.
Figure 3. .
Kaplan-Meier curve of time to participants returning to usual activities (A) and to usual health (B) by biologic sex. Severity of disease during the first month of illness among those with symptomatic outpatient COVID-19 including (C) perception of disease, (D) perception of health, and (E) effect on usual activities. Abbreviation: COVID-19, coronavirus disease 2019.
Oxygen Saturation, Heart Rate, Temperature, and Subsequent Hospitalization
The initial median oxygen saturation values (SaO2) at rest (IQR) were 98.0% (96.0%–98.5%), and median SaO2 values during ambulation (IQR) were 97.0% (95.0%–98.0%), collected from 96 participants (Table 1). Pulse oximeters were received at a median of 8 days from symptom onset. Adherence to use decreased over time. There were 80 participants who reported results for up to 8 study days, and 54 participants reported results up to 15 study days from pulse oximeter receipt. The Pearson’s correlation coefficient between walking and ambulatory SaO2 was 0.61, with a larger difference between values noted at lower oxygen saturations (Supplementary Figure 2A). Resting SaO2 at ≤92% occurred among 9.7% (n = 9) of participants at a median (IQR) of 11.5 (10–14) days from symptom onset but only accounted for 3.4% of all SaO2 levels. Of the 9 hypoxic participants, 5 had FLU-PRO symptom results. Two out of 5 individuals had mild or no respiratory symptoms (Supplementary Figure 2B, C). Additionally, there were 8 participants with at least 1 low (≤92%) ambulatory SaO2 and 4 participants with at least 1 low resting home SaO2 who did not seek medical attention, despite prior guidance. One participant initially did not believe a resting SaO2 of 85%, initially unreported to the study team, and was subsequently hospitalized a few days later for respiratory failure.
During the study period, 13 (11.0%) participants, including 1 individual with 2 visits, presented to the emergency department (ED), and 9 (7.6%) required hospitalization (Supplementary Table 1). Low oxygen saturation (≤92%) was the leading factor for 5 participants being sent to the ED, followed by dyspnea (n = 4), diarrhea (n = 2), fever (n = 1), chest pain (n = 1), and elevated blood pressure (n = 1). The median time from symptom onset to hospitalization (IQR) was 11 (9–12) days.
Baseline demographics and initial study vital signs were evaluated for associations with subsequent hospitalization. Each year of age was associated with 9% increased odds of subsequent hospitalization (odds ratio [OR], 1.1; 95% CI, 1.00–1.19; P = .04; incidence rate ratio [IRR], 1.1; 95% CI, 1.00–1.17; P = .049). Other baseline demographics or comorbidities were not associated with an increased risk of subsequent hospitalization (Supplementary Figure 3).
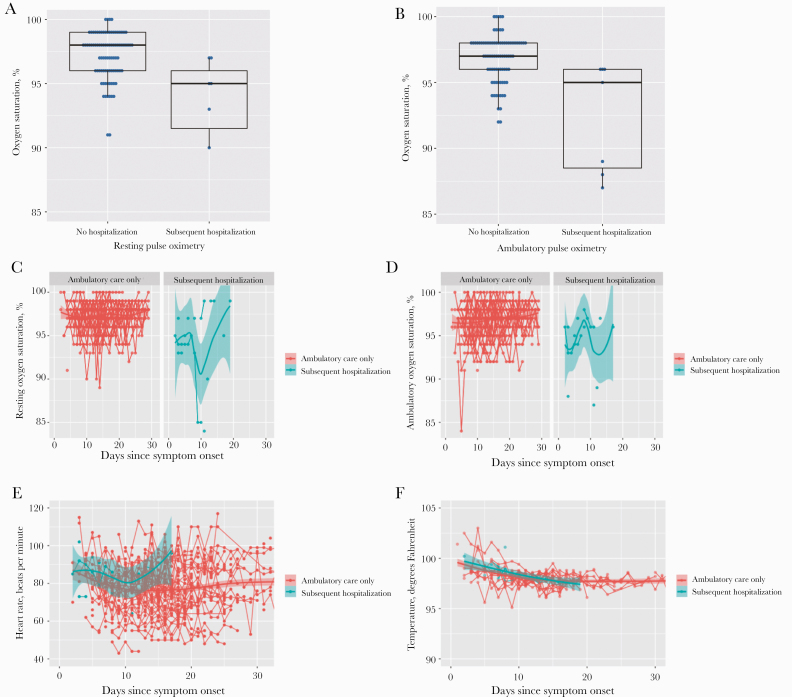
Among those who were subsequently hospitalized, the initial (study day 0) median SaO2 at rest (IQR) was 95.0% (90.0%–97.0%), and during ambulation it was 95.0% (88.0%–96.0%) (Table 1, Figure 4A, B). Temperature, heart rate, and SaO2 were plotted over time and stratified by the need for subsequent hospitalization (Figure 4C–F). However, after excluding 5 individuals who dropped out or were previously hospitalized, each percentage decrease in the initial resting SaO2 (n = 91) was associated with a 30.0% increased risk of a subsequent hospitalization (IRR, 1.3; 95% CI, 1.41–1.49; P = .004; OR, 1.7; 95% CI, 1.18–2.37; P = .004). While the initial ambulatory SaO2 was similarly associated with subsequent hospitalization (OR, 1.76; 95% CI, 1.24–2.48; P = .001), temperature (OR, 1.5 per degree Fahrenheit; 95% CI, 0.81–2.69; P = .20) and heart rate (OR, 1.0 per 10 beats per minute; 95% CI, 0.58–1.88; P = .90) were not. The AUROC for the initial resting SaO2 for predicting subsequent hospitalization was 0.86 (95% CI, 0.73–0.99). A negative likelihood ratio of 0 for subsequent hospitalization was observed among those with an initial resting oxygen saturation that went no lower than 97% (Supplementary Table 2).
Figure 4. .
Box plots of resting (A) and ambulatory (B) oxygen saturation (%) at enrollment among those who subsequently were hospitalized and those who were not during the study period. Outpatient vital signs over time stratified by subsequent hospitalization requirement including (C) resting oxygen saturation, (D) ambulatory oxygen saturation, (E) heart rate (beats per minute), and (F) temperature (degrees Fahrenheit).
DISCUSSION
This may be the first study to prospectively characterize the incidence, intensity, and duration of COVID-19 in an outpatient setting. Presenting symptoms were diverse, persistent, and uncommonly required hospitalization, especially when the initial home SaO2 levels were high (ie, ≥97%).
These findings support early observations from hospitalized patients that symptoms may persist after acute COVID-19 illness [6, 7, 21, 22]. Women had an increased risk of a protracted return to usual activities compared with men, consistent with a report about chronic COVID-19 symptoms [23]. Despite outpatient COVID-19 being considered generally mild, we also found that respiratory and systemic symptoms persisted for weeks, notably longer than with common respiratory viruses [17, 18]. Additionally, symptom scores did not peak until a median of 10 days after symptom onset. Our findings were consistent with a cross-sectional survey that found that 35% of outpatient COVID-19-positive respondents had not returned to their usual state of health between 2 and 3 weeks from diagnosis [6]. Additionally, cough, fatigue, and shortness of breath were present among 43%, 35%, and 29%, respectively, of those who initially reported symptoms [6]. In comparison, in our cohort cough was reported among 23.4%, weakness or fatigue among 41.3%, and shortness of breath among 12.5% during the third week of illness regardless of initial symptoms. Weakness or fatigue was the most pervasive symptom, and almost one-third of participants reported fatigue after 22–28 days of illness. Between 10% and 15% of participants continued to have some degree of weakness, cough, headache, or anosmia as long as a month or more after the onset of symptoms. The prolonged duration of loss of taste and smell has been previously noted in the study using telephone surveys and is consistent with our prospective findings [6]. Our results provide supportive evidence that COVID-19 frequently leads to prolonged symptoms.
While hospitalization was uncommon in our outpatient cohort, the initial resting oxygen saturation was predictive of subsequent hospitalization, suggesting a role for home pulse oximetry in outpatient management of COVID-19. High SaO2 values (>97%) had a high negative predictive value, and low values (<92%) were specific but not sensitive for subsequent hospitalization. We found no major difference in the diagnostic yield between resting and ambulatory SaO2 in this setting. The predictive value of outpatient oxygen saturation values has been previously described [5, 24]. One study provided pulse oximeters to participants with COVID-19 who presented to an ED or outpatient testing center; 29% required subsequent hospitalization. Low oxygen saturation detected on pulse oximetry was associated with hospitalization and more severe outcomes [24]. In light of the receipt of pulse oximetry devices a median of 8 days after symptom onset, the initial value, but perhaps not the longitudinal monitoring component, appeared to be useful for risk stratification. Longitudinal trends among subsequently hospitalized individuals were not observed due to the delay in receipt of the pulse oximeter. The device was received at a median of 8 days after symptoms, compared with the median peak symptoms at 10 days. After the first 2 weeks of illness, there was no obvious benefit to continued oxygen monitoring. Future research should evaluate the benefit of longitudinal oxygen monitoring starting earlier in illness (eg, <1 week).
Although in the present study pulse oximetry was predictive of hospitalization, this measure alone often was not sufficient. Some persons needed hospitalization for nonrespiratory symptoms (eg, diarrhea) that logically were not detected by lower oxygen saturation. In addition, no single oxygen saturation reading alone predicted outcome, as there was overlap in both the resting and ambulatory oxygen saturations of those who remained at home and those who were hospitalized. Therefore, pulse oximetry may be most useful as an adjunct to clinical monitoring of populations over 40 years of age or at-risk populations [5, 25].
While this is one of the largest prospective outpatient cohorts to characterize the clinical course of COVID-19, this study has several limitations. First, this study predominantly included older individuals to increase the statistical power for severe outcomes given the known association between age and hospitalization [12]. Symptoms including severity and duration of illness may differ considerably in younger individuals, and our results are more generalizable for persons of similar ages [26]. Outpatients have been previously found to be younger and have fewer comorbidities compared with hospitalized patients [27]. Second, the recruitment strategy, which used convenience sampling, may have skewed the study population. When comparing this outpatient cohort with a recent description of consecutively hospitalized patients in the same health network, race and ethnicity were similar, as 40% were Black (compared with 39%) and 16% were Latinx (compared with 15%) [28]. Unsurprisingly, this outpatient cohort was younger, more frequently female, and less frequently had diabetes and hypertension than the hospitalized population. The level of symptom severity or employment history (eg, health care workers) could have affected likelihood to participate in the study processes. A quarter of participants had symptom onset 10 days before enrollment, and therefore a proportion of participants may have been selected who had successfully passed a time window of disease severity. Individuals without active mobile phone access and individuals in living facilities were not enrolled due to the remote study procedures. Additionally, due to operational requirements, Spanish speakers were initially not enrolled proportionate to cases early after study initiation. Individuals without active mobile phone access and individuals in living facilities were not enrolled due to the contactless study procedures. Third, missing data from loss to follow-up or withdrawals during the study period could have skewed the longitudinal severity of results. For example, participants with milder illness could have been more likely to withdrawal during the course of the study, or people may not have been able to make a follow-up visit without compensation. While our results help elucidate the progression of outpatient COVID-19, recruitment and operational resources should prioritize inclusion of vulnerable populations in future outpatient COVID-19 cohort research.
The prospective cohort provides additional insight into the clinical progression of outpatient COVID-19 patients, who comprise the majority of patients with SARS-CoV-2 infection. Presenting symptoms were generally diverse and often persisted longer than expected for a respiratory virus. Hospitalization occurred among 7.6% and was associated with low home SaO2 values, supporting the utility of pulse oximetry as a supplemental tool for remote clinical decision-making. Given the diversity of manifestations of COVID-19, immunologic studies and longer-term follow-up of these patients are warranted to determine the extent of symptoms among those with persistent symptoms. There remains great uncertainty about the long-term effects of SARS-CoV-2 infection regardless of symptom severity.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Dr. John Powers, Leidos Biomedical, the National Institute for Allergy and Infectious Diseases (NIAID), and the National Institutes of Health for supplying the FLU-PRO Questionnaire.
Ambulatory COVID Study Team. Andrea L. Cox, MD, PhD, Chris D. Heaney, PhD, Sabra L. Klein, PhD, Shruti H. Mehta, PhD, Heba Mostafa, MBBCh, PhD, Andy S. Pekosz, PhD, Nora Pisanic, PhD, L. Leigh Smith, MD, Derek T. Armstrong, MHS, Razvan Azamfirei, MS, Brittany Barnaba, MS, Curtisha Charles, BSc, Taylor Church, BS, Weiwei Dai, PhD, Joelle Fuchs, BA, Abhinaya Ganesan, ScM, Justin Hardick, BS, Jeffrey Holden, MA, Jaylynn R. Johnstone, MPH, Kate Kruczynski, BS, Oyinkansola Kusemiju, MPH, Anastasia Lambrou, MSc, Lucy Li, MD, Kirsten Littlefield, MS, Manuela Plazas Montana, BS, Han-Sol Park, PhD, Christine B. Payton, BS, Caroline Popper, MD, Michelle Prizzi, BA, Carolyn J. Reuland, BS, Thelio Sewell, MS, Amanda Tuchler, BA, Rebecca L. Ursin, MS, Samantha N. Walch.
Financial support. This work was supported by the Sherrilyn and Ken Fisher Center for Environmental Infectious Diseases Discovery Program and the Johns Hopkins University School of Medicine COVID-19 Research Fund. Y.C.M. received salary support from the National Institutes of Health (grant numbers U54EB007958-12, U5411090366, U54HL143541-02S2, UM1AI068613).
Potential conflicts of interest. H.M. receives research funding from DiaSorin Molecular and Bio-Rad Laboratories. Y.C.M. receives research funding from Becton Dickinson, Quanterix, and Hologic and receives funding support to Johns Hopkins University from miDiagnostics. C.P. is the co-founder and president of Popper and Company. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This protocol and verbal consent were approved by the Johns Hopkins University School of Medicine Institutional Review Board. Due to the contagious nature of COVID-19 being studied under this protocol, obtaining signed informed consent forms for subjects enrolled in this study was not feasible or safe for study staff [13]. The study staff obtained verbal consent using a consent waiver with an alteration of the informed consent. All participants provided verbal consent according to a consent script that was provided in either English or Spanish, and a copy of the informed consent was sent to the participants. All procedures were in accordance with the ethical standards of the Helsinki Declaration of the World Medical Association.
Contributor Information
Ambulatory COVID Study Team:
Andrea L Cox, Chris D Heaney, Sabra L Klein, Shruti H Mehta, Heba Mostafa, Andy S Pekosz, Nora Pisanic, L Leigh Smith, Derek T Armstrong, Razvan Azamfirei, Brittany Barnaba, Curtisha Charles, Taylor Church, Weiwei Dai, Joelle Fuchs, Abhinaya Ganesan, Justin Hardick, Jeffrey Holden, Jaylynn R Johnstone, Kate Kruczynski, Oyinkansola Kusemiju, Anastasia Lambrou, Lucy Li, Kirsten Littlefield, Manuela Plazas Montana, Han-Sol Park, Christine B Payton, Caroline Popper, Michelle Prizzi, Carolyn J Reuland, Thelio Sewell, Amanda Tuchler, Rebecca L Ursin, and Samantha N Walch
References
- 1. Johns Hopkins Coronavirus Resource Center. Available at: https://coronavirus.jhu.edu/data/new-cases. Accessed 7 June 2020.
- 2. Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol 2020; 92:1902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun H, Jain A, Leone MJ, et al. CoVA: An acuity score for outpatient screening that predicts coronavirus disease 2019 prognosis. J Infect Dis 2021; 223:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tenforde MW, Kim SS, Lindsell CJ, et al. ; IVY Network Investigators; CDC COVID-19 Response Team; IVY Network Investigators Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March-June 2020. MMWR Morb Mortal Wkly Rep 2020; 69:993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alwan NA A negative COVID-19 test does not mean recovery. Nature 2020; 584:170. [DOI] [PubMed] [Google Scholar]
- 8. Jouffroy R, Jost D, Prunet B. Prehospital pulse oximetry: a red flag for early detection of silent hypoxemia in COVID-19 patients. Crit Care 2020; 24:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med 2020; 202:356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med 2020; 38:2243.e5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med July 21, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manabe YC, Reuland C, Yu T, et al. Self-collected oral fluid saliva is insensitive compared to nasal-oropharyngeal swabs in the detection of SARS-CoV-2 in outpatients. Open Forum Infect Dis 2020; XXX:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vyse AJ, Cohen BJ, Ramsay ME. A comparison of oral fluid collection devices for use in the surveillance of virus diseases in children. Public Health 2001; 115:201–7. [DOI] [PubMed] [Google Scholar]
- 15. Powers JH, Guerrero ML, Leidy NK, et al. Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza. BMC Infect Dis 2016; 16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu J, Powers JH 3rd, Vallo D, Falloon J. Evaluation of efficacy endpoints for a phase IIb study of a respiratory syncytial virus vaccine in older adults using patient-reported outcomes with laboratory confirmation. Value Health 2020; 23:227–35. [DOI] [PubMed] [Google Scholar]
- 17. Powers JH 3rd, Bacci ED, Guerrero ML, et al. Reliability, validity, and responsiveness of InFLUenza patient-reported outcome (FLU-PRO©) scores in influenza-positive patients. Value Health 2018; 21:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han A, Poon JL, Powers JH 3rd, et al. Using the influenza patient-reported outcome (FLU-PRO) diary to evaluate symptoms of influenza viral infection in a healthy human challenge model. BMC Infect Dis 2018; 18:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19) 2020 interim case definition, approved April 5, 2020. Available at: https://wwwn.cdc.gov/nndss/conditions/coronavirus-disease-2019-covid-19/case-definition/2020/. Accessed 29 July 2020.
- 21. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rimmer A Covid-19: impact of long term symptoms will be profound, warns BMA. BMJ 2020; 370:m3218. [DOI] [PubMed] [Google Scholar]
- 23. Davido B, Seang S, Tubiana R, de Truchis P. Post-COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect 2020; 26:1448–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah S, Majmudar K, Stein A, et al. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med 2020; 27:681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamer M, Gale CR, Kivimäki M, Batty GD. Overweight, obesity, and risk of hospitalization for COVID-19: a community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci U S A 2020; 117:21011–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scheuller HS, Park J, Lott L, et al. Comparison of clinical features in a population of basic military trainees versus the general Department of Defense beneficiary population presenting with influenza. Mil Med 2017; 182:e1917–21. [DOI] [PubMed] [Google Scholar]
- 27. Tenforde MW, Billig Rose E, Lindsell CJ, et al. ; CDC COVID-19 Response Team Characteristics of adult outpatients and inpatients with COVID-19—11 academic medical centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep 2020; 69:841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med 2021; 174:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.