Abstract
In this study, we report the history of a 40-year-old man with a primary cerebral abscess caused by Nocardia abscessus that led to the discovery of autoimmune pulmonary alveolar lipoproteinosis (anti-granulocyte-macrophage colony-stimulating factor [GM-CSF] autoantibodies). Anti-GM-CSF autoantibodies promote immunodeficiency and should be monitored to prevent opportunistic and disseminated infections and to diagnose asymptomatic pulmonary alveolar lipoproteinosis.
Keywords: Nocardia, Pulmonary alveolar proteinosis’ Anti-GM-CSF antibody
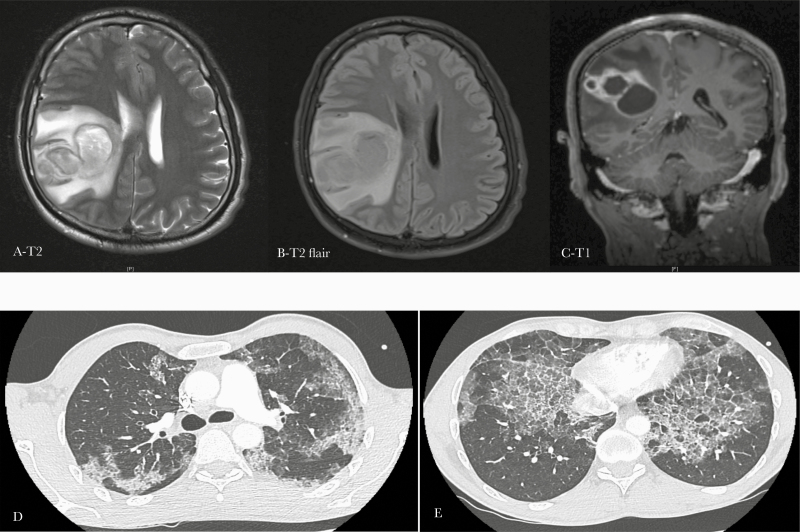
In April 2018, a 40-year-old man, who was an active smoker (10 pack-years), consulted the hospital for subacute left brachiofacial deficit and headaches. He had no medical history. He previously worked as an order picker and reported a former professional exposure to dust. On admission, he presented with moderate left facial paralysis and left brachial deficit (4/5). Pulmonary auscultation was normal. A voluminous right parietal lesion compatible with a cerebral abscess was identified on cerebral imaging and quickly drained by neurosurgeons (Figure 1). The patient underwent a full body computed tomodensitometry (CT) scan, which did not show any secondary infectious focus but did identify an unexpected diffuse interstitial lung disease with “crazy paving” aspect (Figure 1). Further pulmonary examinations showed a restrictive ventilatory disorder with a decrease in vital capacity and 60% decrease in total pulmonary capacity, associated with a severe alteration of alveolocapillary diffusion (DLCO at 31%).
Figure 1.
Cerebral and thoracic imaging of a 40-year-old man with cerebral nocardiosis and pulmonary alveolar proteinosis. (A–C). Magnetic resonance imaging showing a voluminous cerebral parietal abscess. (D–E) Thoracic computed tomodensitometry scan showing diffuse and bilateral interstitial syndrome with thickening of the interlobular septa and a “crazy paving” aspect, which is typically found in pulmonary alveolar lipoproteinosis.
Per-operative samples of surgical drainage showed partially necrotic polynuclear neutrophils in histopathology, with negative direct examination. Cultures returned positive after 72 hours for Nocardia spp. The matrix-assisted laser desorption ionization time-of-flight technique was used to identify N abscessus. Molecular biology performed on abscess samples to eliminate other pathogens such as aspergillus, mycobacterias, candidas, cryptococcus, histoplasma, and cysticercus was negative. Bronchoalveolar lavage (BAL) fluid was opalescent, microbiological culture and molecular biology searching for pneumocystis, aspergillus, mycobacteria, Streptococcus pneumoniae, mycoplasma, Bordetella pertussis, as well as for cytomegalovirus, herpes simplex virus, enterovirus, rhinovirus, respiratory syncytial virus, metapneumovirus, and influenzavirus were negative as well. Histopathologic BAL analysis revealed extracellular periodic acid-Schiff staining-positive material evocative of pulmonary alveolar lipoproteinosis. Less than 1% of lymphocytes were detected in the BAL fluid, with mainly T cells and an inverse CD4/CD8 ratio. Phenotypic and functional analyses of circulating lymphocytes did not reveal any obvious immunodeficiency: CD4+ T, CD8+ T, B, and natural killer cell counts were normal, as were mitogen-induced T-cell proliferation and Th1/Th2/Th17 cytokine production. Similarly, B cell function indicated by immunoglobulin production evaluation was normal. Anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibodies, evaluated by the functional method of TF1 cell line proliferation inhibition, were highly positive in the serum (titer of 155), confirming the neutralizing power of antibodies and therefore the autoimmune origin of pulmonary alveolar proteinosis (PAP) [1, 2].
The patient was treated with a combination of meropenem administered intravenously for 6 weeks and a high dose of trimethoprim-sulfamethoxazole relayed per os (800/160 mg 3 times a day) from the 7th day; treatment was continued for 1 year and then replaced with a secondary prophylactic regimen with sulfamethoxazole-trimethoprim at 800/160 mg once a day (ongoing treatment). The patient clinically improved, with total neurological recuperation and total regression of the cerebral abscess on cerebral CT scan control imaging performed in October 2019. Primary PAP was initially treated with dose escalation of recombinant GM-CSF (sargramostim [LEUKINE]) subcutaneous injection, at 500 µg per day. Despite excellent hematopoietic tolerance, recombinant GM-CSF was not effective enough, because the patient presented 3 respiratory distress syndromes during the year, requiring hospitalization in intensive care units and whole lung lavages. Inefficient LEUKINE treatment was interrupted and second-line rituximab was initiated with good tolerance and clinical stabilization. (The patient’s written consent was obtained. The design of the work has been approved by local ethical committees under the number of registration: 2016-024.)
In this study, we report the first case of N abscessus cerebral infection with anti-GM-CSF autoantibodies and documented PAP (Tables 1 and 2). In the literature, 3 cases of nocardial infection with anti-GM-CSF antibodies and documented PAP have been reported: 36 cases of PAP associated with nocardial infection without specifying the presence of anti-GM-CSF autoantibodies and 4 cases of nocardial infection with anti-GM-CSF autoantibodies without PAP were found (Tables 1 and 2). These observations highlight the promotive role of anti-GM-CSF autoantibodies in the occurrence of these 2 diseases, nocardial infection, and PAP.
Table 1.
Nocardiosis (Cerebral and/or Disseminated) Associated With Pulmonar Alveolar Proteinosis With or Whithout Anti-GM-CSF Autoantibodies
| Number of Patients/ Date/Country | Age/Sex | Infectious Focus | Species | Anti-GM-CSF Antibodies | Treatment | Evolution | Ref |
|---|---|---|---|---|---|---|---|
| 32 patients | 65% male | 75% pulmonar (n = 24) | Nocardia asteroides 19 (59%) | Not performed | Unspecified antibiotherapy (n = 20) Surgery (n = 6) |
41% died | 5 |
| 1950–2010 | 35% female | 19% (n = 6) cerebral | Nocardia brasiliensis 1 (3%) | ||||
| Worldwide | Mean age 35 | 6% other (n = 2) | Nocardia farcinica 1 (3%) | ||||
| Nocardia spp 11 (34%) | |||||||
| 1 patient | 37/male | Pulmonar | NA | Presence | NA | NA | 6 |
| 2010 | |||||||
| Japan | |||||||
| 2 patients | NA | NA | NA | NA | NA | NA | 7 |
| 1990–2010 China |
|||||||
| 1 patient | 50/male | Pulmonar | N farcinica | Not performed | Amikacin 6 weeks and TMP-SMX 6 months | Full recovery | 8 |
| 2014 | |||||||
| Spain | |||||||
| 1 patient | 42/male | Cerebral abscesses | N asteroids | Not performed | TMP-SMX, meropenem and amikacin 2 months, relayed TMP-SMX | No improvement | 9 |
| 2015 | |||||||
| Iran | |||||||
| 1 patient | 49/male | Cerebral abscess | N farcinica | Not performed | 12 months of AMC and minocycline | Full recovery | 10 |
| 2017 | |||||||
| Spain | |||||||
| 1 patient | NA | NA | NA | Not performed | Adapted antibiotherapy (not specified) | Full recovery | 11 |
| 2002–2016 | |||||||
| Brazil | |||||||
| 1 patient | 62/male | Pulmonar | N brasiliensis | Presence | Amikacin 6 weeks and TMP-SMX 6 months | Full recovery | 12 |
| 2020 | |||||||
| United States | |||||||
| 1 patient | 40/male | Cerebral abscess | Nocardia abscessus | Presence | Meropenem 6 weeks and TMP-SMX 12 months | Full recovery | Our case |
| 2018 | |||||||
| France |
Abbreviations: AMC, amoxicillin/clavulanate; GM-CSF, granulocyte macrophage colony-stimulating factor; NA, not available; Ref, reference; TMP-SMX, trimethoprim-sulfamethoxazole.
Table 2.
Reported Cases of Opportunistic Infections Associated With Anti-GM-CSF Autoantibodies Without Pulmonar Alveolar Proteinosis
| Infectious Agent | Number of cases Age/Sex | Infection Focus | Species | Anti-GM-CSF Antibodies | Presence of PAP | Treatment | Outcome | Ref | |
|---|---|---|---|---|---|---|---|---|---|
| Nocardiosis | 1 | 44/male | Cerebral | Nocardia paucivorans | Presence | Scanographic infiltrates but normal respiratory function tests, PAP diagnosis not retained | Amikacin and TMP-SMX 8 weeks, TMP-SMX and linezolide 8 weeks, then TMP-SMX alone | Full recovery | 13 |
| 2 | 73/male | -cutaneous, pulmonar, and subsequent cerebral nocadiosis-pulmonar aspergillosis | Nocardia spp Aspergillus fumigates | Presence | No evidence of PAP | Imipenem amikacin voriconazole, then TMP-SMX, AMC, voriconazole per os + subcutaneous GM-CSF | Neurologic relapse | ||
| 3 | 61/male | cerebral nocardiosis | Nocardia farcinica | Presence | No evidence of PAP | Imipenem amikacin IV 8 weeks and TMP-SMX and moxifloxacin | Neurologic relapse | ||
| 4 | 50/male | cerebral nocardiosis | N paucivorans | Presence | No evidence of PAP | 12 months of TMP-SMX, imipenem, and moxifloxacin | Full recovery | ||
| 5 | 52/female | cerebral and pulmonary nocardiosis and disseminated cryptococcosis | Nocardia asteroids | Presence | No evidence of PAP | NA | NA | ||
| Cryptoccocosis | 1 | 49/female | Meningitidis | Cryptococcus gattii | Presence | NA | NA | NA | 14 |
| 2 | NA/female | Meningitidis | C gattii | Presence | NA | NA | NA | ||
| 3 | NA/female | Meningitidis | C gattii | Presence | NA | NA | NA | ||
| 4 | NA/male | Meningitidis | C gattii | Presence | NA | NA | NA | ||
| 5 | NA/female | Meningitidis | C gattii | Presence | NA | NA | NA | ||
| 6 | NA/female | Meningitidis | C gattii | Presence | NA | NA | NA | ||
| 7 | NA/male | Meningitidis | C gattii | Presence | NA | NA | NA | ||
| 8 | 20/female | Meningitidis | Cryptococcus neoformans | Presence | Develop PAP 1 year later | AmphoB + 5-FC, relayed by FLC | Full recovery | 15 | |
| 9 | 31/female | Meningitides | C gattii | Presence | NA | AmphoB + 5-FC, relayed by FLC + 5-FC | Full recovery | ||
| 10 | 48/male | Cryptococcal meningitidis Pulmonar tuberculosis | C neoformans | Presence | NA | AmphoB, relayed by FLC | Full recovery | ||
| Antituberculous therapy | |||||||||
| 11 | 47/male | Meningitidis | C neoformans | Presence | Develop asymptomatic PAP 4 years later | AmphoB + FLC, relayed by FLC | Full recovery | ||
| 12 | 26/male | Meningitides | C gattii | Presence | NA | AmphoB + 5-FC | Full recovery | ||
| 13 | 34/male | Meningitides | C gattii | Presence | NA | AmphoB + 5-FC + therapeutic LP | Sequelae | ||
| 14 | 32/male | Meningitides | C gattii | Presence | NA | AmphoB + 5-FC + therapeutic LP | Sequelae | ||
| 15 | 48/male | Pulmonary cryptococcoma, and subsequent cerebral cryptococcosis | C gattii | Presence | Scanographic infiltrates but normal respiratory function tests, PAP diagnosis not retained | AmphoB + 5-FC, relayed by FLC | Full recovery | 16 | |
| 16 | 43/male | Solitary cerebral abscess | C gattii | Presence | NA | Surgically treated, AmphoB + 5-FC, relayed by several triazoles | Full recovery | ||
| 17 | 37/male | Disseminated | NA | Presence | No evidence of PAP | AmphoB + 5-FC + therapeutic LP | Death | 17 | |
| 18 | 40/male | Disseminated | NA | Presence | No evidence of PAP | AmphoB + 5-FC, relayed by FLC | Severe sequelae | ||
| 19 | 59/female | Ocular | NA | Presence | No evidence of PAP | Intraoculaire AmphoB relayed by voriconazole | Full recovery | ||
| 20 | 37/male | Meningitides | NA | Presence | No evidence of PAP | AmphoB + 5-FC 2 weeks, relayed by FLC | Full recovery |
Abbreviations: AMC, amoxicillin/clavulanate; AmphoB, amphotericin B; CNS, central nervous system; FLC, fluconazole; GM-CSF, granulocyte macrophage colony-stimulating factor; IgG, immunoglobulin G; IV, intravenous; LP, lumbar puncture; MXF, moxifloxacin; NA, not available; PAP, pulmonary alveolar proteinosis; Ref, reference; TMP-SMX, trimethoprim-sulfamethoxazole, 5-FC, 5-flucytosine.
Pulmonary alveolar proteinosis is mostly autoimmune (90% of cases), and in such cases, it is characterized by a high level of anti-GM-CSF autoantibodies, whereas hereditary PAP results in mutations in genes encoding the GM-CSF receptor [3].
Secondary infection is the most common and threatening complication of PAP, occurring in 5%–13% of cases and accounting for 10%–20% of deaths [3]. Patients with PAP are known to be more susceptible to bacterial, mycobacterial, and fungal infections such as nocardiosis, mycobacteriosis, aspergillosis, and cryptococcosis [3]. The association between PAP and opportunistic infection has been reported since the first description of the disease in 1958 by Rosen et al [4], with 2 cases of cryptococcosis and 2 cases of nocardiosis among the 27 patients described. More recently, a review of opportunistic infections occurring in 75 patients with PAP found 43% positivity for Nocardia spp infection, followed by mycobacterial and fungal infections representing 37% and 20% of the patients, respectively (Table 1) [5-12]. Disseminated or meningeal cryptococcal infections have led investigations to identify the presence of anti-GM-CSF autoantibodies in patients without a history of PAP (Table 2) [13-17]. Similarly, by screening the serum of 7 patients presenting with central nervous system or disseminated nocardiosis, Rosen et al [13] detected anti-GM-CSF autoantibodies in 5 of the 7 samples. None of the patients had PAP initially, and 2 developed PAP during follow-up replace by ref 13-17 (Table 2). Other cases of anti MG GM-CSF-CSF auto antibody without PAP have been described.
We decided to treat the patient with prolonged trimethoprim-sulfamethoxazole as a secondary prophylaxis because we considered the patient immunocompromised. In addition, whereas whole-lung lavage is still the gold standard for autoimmune PAP, subcutaneous and inhaled GM-CSF supplementations were reported to be beneficial [3]. In prospective studies, daily injection of GM-CSF was effective in 43% to 75% of patients at 1 year and 12 weeks, respectively. In addition, inhaled GM-CSF presents several advantages: reduced cost, reduced side effects, and 66% efficiency at 3 years. Two clinical trials evaluating the effect of inhaled GM-CSF on PAP patients are ongoing: IMPALA and PAGE [3]. More recently, rituximab has been proposed as a therapeutic option for the treatment of autoimmune PAP with controversial results. Some series of patients treated with rituximab showed PaO2, pulmonary function test, and chest CT scan lesion amelioration, whereas retrospective reports on 13 PAP patients did not support rituximab as a second-line therapy [3, 18]. Plasmapheresis has not shown promising results, and few cases of lung transplantation to treat severe PAP have been reported [3].
Granulocyte-macrophage colony-stimulating factor, a cytokine produced by T cells, B cells, macrophages, endothelial cells, and fibroblasts, is involved in proinflammatory functions such as the differentiation, adhesion, chemotaxis, and activation of inflammatory and immune cells such as monocytes, macrophages, neutrophils, microglia, and dendritic cells [19]. Granulocyte-macrophage colony-stimulating factor is also a hematopoietic growth factor that activates the proliferation of myeloid cells from bone marrow progenitors [19, 20]. Although overproduction of GM-CSF is associated with rheumatoid arthritis, multiple sclerosis, juvenile myelomonocytic leukemia, and chronic myelomonocytic leukemia, GM-CSF deficiency induces a lack of maturation of alveolar macrophages and accumulation of surfactant in the alveolar space, leading to PAP [3]. Anti-GM-CSF antibodies neutralize and clear GM-CSF in cases of PAP, which could induce an immune deficiency favoring opportunistic diseases [21]. Indeed, GM-CSF deficiency causes impaired antigen presentation, and reductions in dendritic cell numbers in nonlymphoid tissues, as well as in phagocytosis and bactericidal activities of neutrophils, promoting immunodeficiency [21]. Thus, the role of GM-CSF is not limited to the lungs and seems to be decisive in the host’s defense against pathogens, especially against opportunistic infections such as nocardia. On the basis of our experience and the research developed here, we therefore propose to test all patients with cerebral or disseminated nocardiosis for immunodeficiency with at least serum protein electrophoresis, immunophenotyping of circulating lymphocytes, presence and neutralizing activity of anti GM-CSF antibodies, and chest CT scan to diagnose asymptomatic pulmonary alveolar lipoproteinosis.
In conclusion, the presence of anti-GM-CSF autoantibodies should be considered an underdiagnosed immunodeficiency. Systematic screening of these autoantibodies in patients with nocardial, fungal, or mycobacterial infection will allow us to characterize this immunodeficiency and prevent the outbreak of disseminated infectious and pulmonary diseases.
Acknowledgments
Financial support. This work was funded by URMITE, IHU Méditerranée Infection. This work was also supported by the French Government under the “Investissements d’avenir” (Investments for the Future) programme managed by the Agence Nationale de la Recherche (reference: Méditerranée Infection 10-IAHU-03). Finally, this work was supported by Région Provence-Alpes-Côte d’Azur and European funding Fonds Européen de Développement Régional-Plateformes de Recherche et d’Innovation Mutualisées Méditerranée Infection.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Uchida K, Nakata K, Trapnell BC, et al. High-affinity autontibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 2004; 103:1089–98. [DOI] [PubMed] [Google Scholar]
- 2. Nakata K, Sugi T, Kuroda K, et al. Validation of a new serum granulocyte-macrophage colony-stimulating factor autoantibody testing kit. ERJ Open Res 2020; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jouneau S, Ménard C, Lederlin M. Pulmonary alveolar proteinosis. Respirology 2020; resp.13831. [DOI] [PubMed] [Google Scholar]
- 4. Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med 1958; 258:1123–42. [DOI] [PubMed] [Google Scholar]
- 5. Punatar AD, Kusne S, Blair JE, et al. Opportunistic infections in patients with pulmonary alveolar proteinosis. J Infect 2012; 65:173–9. [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi S, Takayanagi N, Tokunaga D, et al. A case of pulmonary alveolar proteinosis which initially deteriorated rapidly with exacerbation of pulmonary nocardiosis, responded promptly to treatment of the pulmonary nocardiosis. Nihon Kokyuki Gakkai Zasshi 2010; 48:580–3. [PubMed] [Google Scholar]
- 7. Huang H, Lu ZW, Xu ZJ. A clinical analysis of 9 cases of pulmonary alveolar proteinosis with secondary infection. Zhonghua Nei Ke Za Zhi 2011; 50:216–20. [PubMed] [Google Scholar]
- 8. Garmilla Ezquerra P, Gómez Roman J, Garcia de la Fuente C, Nan Nan D. Alveolar proteinosis in an immunocompetent patient with previous Legionella and Nocardia infections. Rev Clin Esp (Barc) 2014; 214:e1–3. [DOI] [PubMed] [Google Scholar]
- 9. Shirani K, Poulsen AN, Hakamifard A. Nocardial brain abscess in a patient with pulmonary alveolar proteinosis. Adv Biomed Res 2015; 4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carrasco García de León S, González AH, Rivas NV, Gómez JJB. Brain abscess due to Nocardia infection in an immunocompetent patient with asymptomatic pulmonary alveolar proteinosis. Acta Neurol Belg 2019; 119:281–3. [DOI] [PubMed] [Google Scholar]
- 11. Athayde RAB de, Arimura FE, Kairalla RA, et al. Characterization and outcomes of pulmonary alveolar proteinosis in Brazil: a case series. J Bras Pneumol 2018; 44: 231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ekici S, Malur A, Thomassen MJ, et al. Utilization of LC-MS to determine monoclonal gammopathy-associated granulocyte macrophage colony stimulating factor antibody and novel treatment of pulmonary alveolar proteinosis. J Appl Lab Med 2020; 5:394–400. [DOI] [PubMed] [Google Scholar]
- 13. Rosen LB, Rocha Pereira N, Figueiredo C, et al. Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin Infect Dis 2015; 60:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saijo T, Chen J, Chen SC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio 2014; 5:e00912–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosen LB, Freeman AF, Yang LM, et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol 2013; 190:3959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stevenson B, Bundell C, Mulrennan S, et al. The significance of anti-granulocyte-macrophage colony-stimulating factor antibodies in cryptococcal infection: case series and review of antibody testing. Intern Med J 2019; 49:1446–50. [DOI] [PubMed] [Google Scholar]
- 17. Kuo CY, Wang SY, Shih HP, et al. Disseminated cryptococcosis due to anti-granulocyte-macrophage colony-stimulating factor autoantibodies in the absence of pulmonary alveolar proteinosis. J Clin Immunol 2017; 37:143–52. [DOI] [PubMed] [Google Scholar]
- 18. Soyez B, Borie R, Menard C, et al. Rituximab for auto-immune alveolar proteinosis, a real life cohort study. Respir Res 2018; 19:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Becher B, Tugues S, Greter M. GM-CSF: from growth factor to central mediator of tissue inflammation. Immunity 2016; 45:963–73. [DOI] [PubMed] [Google Scholar]
- 20. Uchida K, Beck DC, Yamamoto T, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med 2007; 356:567–79. [DOI] [PubMed] [Google Scholar]
- 21. Piccoli L, Campo I, Fregni CS, et al. Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis. Nat Commun 2015; 6:7375. [DOI] [PMC free article] [PubMed] [Google Scholar]