Abstract
Background
Currently, there is still a lack of effective biomarkers for the recurrence monitoring and survival prognosis assessment of hepatocellular carcinoma (HCC) patients with alpha-fetoprotein (AFP)-negative (≤20 ng/mL) after radical resection.
Methods
The clinicopathological data of 606 patients (303 in the AFP-negative group and 303 in the AFP-positive group) who underwent radical resection of HCC were analyzed retrospectively.
Results
The gamma-glutamyl transpeptidase to lymphocyte count ratio (GLR) of patients in the AFP-negative group was lower than that in the AFP-positive group (p <0.001). The GLR level of the early-recurrence group was higher than that of the non-early-recurrence group (p =0.003). GLR had fair accuracy in predicting the early-recurrence of HCC patients [c-index=0.654 (95% CI=0.606–0.702); AUC=0.681 (95% CI=0.625–0.733)]. Univariate analysis showed that patients with tumor size <5 cm, no microvascular invasion, single tumor, no metastasis, BCLC stage 0–A, no recurrence, and GLR ≤45.0 had longer disease-free survival (DFS) and overall survival (OS) among AFP-negative HCC patients. In addition, multivariate Cox proportional hazards regression analysis showed that tumor size <5 cm (p =0.003), no recurrence (p <0.001), and GLR <45.0 (p <0.001) were independent predictors of longer OS.
Conclusion
GLR may be a potential indicator for early recurrence monitoring and prognosis evaluation in HCC patients with AFP-negative after radical resection.
Keywords: hepatocellular carcinoma, AFP-negative, GLR, prognosis, early-recurrence
Introduction
Liver cancer is the fourth most common cause of death related to cancer globally, and hepatocellular carcinoma (HCC) accounts for the majority of primary liver cancer.1,2 Despite the rapid development of diagnosis and treatment of HCC and postoperative monitoring, the overall prognosis is still not optimistic due to the high recurrence rate and metastasis rate.3
Alpha fetoprotein (AFP) is the most commonly used detection biomarker in clinical diagnosis and postoperative monitoring of HCC.4 However, about 30–40% of patients pathologically diagnosed with HCC have a negative level of serum AFP, which limits the role of AFP in the diagnosis and prognosis monitoring of HCC patients.5–7 In addition, studies have shown that the postoperative recurrence rate and survival rate of AFP-negative HCC patients were better than that of AFP-positive patients,8,9 so the clinical studies on AFP-negative HCC patients have great clinical significance. Although, for AFP-negative HCC patients, previous studies indicated that C-reactive protein, D-dimer, pre-albumin, and some models constructed by biomarkers may be used as auxiliary indicators for HCC diagnosis,10–12 none of them have been used in clinical practice, so it is urgent to find available indicators to monitor and evaluate the prognosis of AFP-negative HCC patients.
The occurrence and development of HCC is a complex and multi-step process, involving continuous inflammatory damage, which includes hepatocyte necrosis and regeneration related to fibrosis deposition.13,14 Lymphocyte plays a key role in the anti-tumor activity of the human immune system, and lymphocyte infiltration usually indicates a favorable prognosis.15 Gamma-glutamyl transferase (GGT) is a cell surface enzyme, which can be used as a marker of many diseases including cancer (renal cell carcinoma, breast cancer, gastric cancer, lung cancer, etc.).16–18 GGT to lymphocyte ratio (GLR) has been proved to be a prognostic indicator after radical resection of nonfunctional pancreatic neuroendocrine tumors.19 In addition, in our previous study, we found that GLR has a better prediction ability in HCC patients with single tumor size (TS) <5 cm.20 However, whether there is a similar result in AFP-negative HCC patients, and whether there is potential significance in the diagnosis and postoperative monitoring of HCC, there are no relevant researches reported yet. This study intended to focus on the application value of GLR in AFP-negative HCC, so as to provide possible assistance for the clinical diagnosis and treatment of AFP-negative HCC.
Method
Research Objects and Data Collection
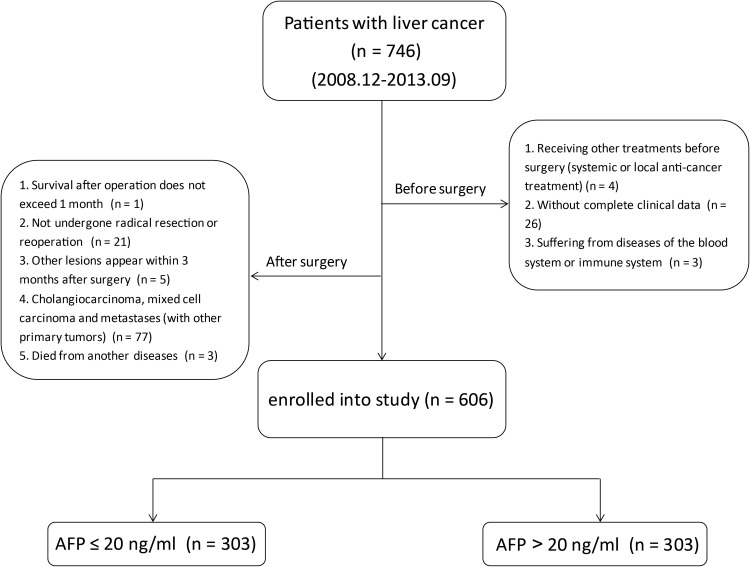
From December 2008 to September 2013, a total of 746 HCC patients were studied, and according to the exclusion criteria, 606 patients who received radical hepatectomy in the Affiliated Hospital of Guilin Medical University (Guilin, China) were enrolled in this study finally, among which half of them were AFP-negative and half were AFP-positive (Figure 1). All patients were diagnosed with HCC by pathological examination. The specific diagnosis criteria, surgical indications, and radical hepatectomy criteria of the patients were described in detail in our previous study.20 Radical resection was defined as all patients who underwent radical resection of HCC. The specific objectives were as follows: complete removal of all tumor nodules, and no residual tumor was found on the cutting edge according to histological examinations. Routine application of ultrasonography during operation and postoperative enhanced CT examination were conducted to ensure complete resection of HCC.21 Clinical data collection includes: personal history (age, gender, drinking history, smoking history, etc.), family history, hematological examinations (hepatitis B surface antigen (HBsAg), AFP, liver function, blood routine, blood biochemistry, etc.), imaging examinations (finishing at least one of them: ultrasonography (US), computerized tomography (CT), magnetic resonance imaging (MRI)), pathological examination results, and regular follow-up data, etc. In this study, all blood samples were collected before operation, and all hematologic examinations were completed in the laboratory of Affiliated Hospital of Guilin Medical University.
Figure 1.
Flow chart showing the inclusion process of eligible patients in the study.
The study was in accordance with the Declaration of Helsinki and has been approved by the research ethics committee of Affiliated Hospital of Guilin Medical University. Informed consent in written form was obtained from all patients.
Follow-Up
The specific principle of follow-up adopted in this study was the same as our previous study.20 The general methods were as follows: within the first 2 years after the operation, the blood routine, liver and kidney function, and abdominal ultrasonography were examined every 2 months, and chest X-ray film was taken every 6 months; after 2 years, the time interval of chest X-ray film remained unchanged, whereas the time interval of the other routine examinations was 3–6 months. If the reexamination results were abnormal, CT contrast-enhanced scanning or MRI examination should be performed to confirm tumor progression. The post-operative patients who were not reexaminated in our hospital on time were followed up by telephone. The last follow-up time was January 2020. Disease-free survival (DFS) refers to the time from radical surgery to tumor recurrence, death, or the last follow-up, while overall survival (OS) refers to the time from surgery to death or the last follow-up.
Receiver Operating Characteristic (ROC) Curve and Cut-Off Value
In this study, ROC curve was drawn according to whether the patients recurred within 1 year after operation. The ROC curve is a series of curves drawn with a positive rate (sensitivity) as ordinate axis and false positive rate (1-specificity) as abscissa axis according to different dichotomy. The area under curve (AUC) is between 0.5 and 1. The loser to 1.0 the AUC is, the more accurate the model is. In addition, The C-index of the GLR model is determined via rms package in R version 3.5.1 (https://www.r-project.org/). By analyzing the sensitivity and specificity of each point on the curve, we determined an optimal cut-off value, at which GLR had the best predictive ability on the recurrence of HCC patients with AFP-negative within 1 year after surgery.
Data Analysis
Continuous variables conforming to normal distribution were expressed as mean±standard deviation (SD) and evaluated by independent sample t-test, while classification data were compared by Pearson chi-square test or Fisher exact test. Kaplan-Meier method and log rank test were used to analyze and compare the difference of survival rate and recurrence rate among different risk groups. Univariate analysis and multivariate Cox proportional hazards regression analysis were used to determine the prognostic factors related to DFS and OS. All data were analyzed by SPSS 24.0 software, and P<0.05 was statistically significant.
Results
Analyses of Clinical Features of Patients
By comparing the clinical data of AFP-negative patients (n=303) and AFP-positive patients (n=303), we found that there was a significant difference in GLR between AFP-negative patients (58.36±51.18) and AFP-positive patients (95.07±87.11) (p <0.001). At the same time, we found statistically significant differences in age, hepatitis B surface antigen (HBsAg), microvascular invasion (MVI), lymphocyte count (LYMPH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), GGT, tumor size (TS), tumor stage, BCLC stage, and recurrence between AFP-negative and AFP-positive patients (Table 1).
Table 1.
Comparison of Clinicopathological Characteristics of AFP-Negative/Positive Patients
| Parameters | AFP Negative | AFP Positive | p-value |
|---|---|---|---|
| (n=303) | (n=303) | ||
| Gender: female/male (n) | 31/272 | 38/265 | 0.371 |
| Age (years) | 52.22±11.06 | 48.11±11.48 | <0.001* |
| HBsAg: negative/positive (n) | 63/240 | 36/267 | 0.003* |
| Family history: absent/present (n) | 264/39 | 269/34 | 0.533 |
| Drinking: absent/present (n) | 157/146 | 164/139 | 0.569 |
| Smoking: absent/present (n) | 162/141 | 174/127 | 0.206 |
| Cirrhosis: absent/present (n) | 28/275 | 20/283 | 0.229 |
| MVI: absent/present (n) | 242/61 | 190/113 | <0.001* |
| WBC (×109/L) | 6.61±2.43 | 6.35±2.32 | 0.172 |
| NEUT (×109/L) | 4.06±2.07 | 4.07±2.02 | 0.901 |
| LYMPH (×109/L) | 1.74±0.61 | 1.52±0.54 | <0.001* |
| Platelets (×109/L) | 183.28±83.24 | 184.18±84.15 | 0.895 |
| Albumin (g/L) | 39.42±4.91 | 39.03±4.79 | 0.314 |
| Globulin (g/L) | 30.74±5.64 | 30.67±6.05 | 0.887 |
| TBIL (μmol/L) | 14.92±10.51 | 16.03±12.01 | 0.622 |
| DBIL (μmol/L) | 5.41±3.20 | 6.30±4.74 | 0.783 |
| ALT (U/L) | 43.06±39.66 | 49.88±47.06 | 0.037* |
| AST (U/L) | 47.16±52.81 | 58.17±55.14 | 0.005* |
| GGT (U/L) | 89.11±70.65 | 125.18±95.93 | <0.001* |
| Tumor size (cm) | 7.57±6.35 | 9.13±5.10 | <0.001* |
| Tumor number: single/multiple (n) | 225/78 | 212/91 | 0.239 |
| Tumor differentiation: I/II/III (n) | 49/113/141 | 22/90/191 | <0.001* |
| BCLC stage: 0/A/B (n) | 20/148/135 | 8/117/178 | 0.001* |
| Early-recurrence: absent/present (n) | 250/53 | 226/77 | 0.0176* |
| GLR | 58.36±51.18 | 95.07±87.11 | <0.001* |
Note: *P-valueindicates statistically significant.
Abbreviations: n, number of patients; HBsAg, hepatitis B surface antigen; MVI, microvascular invasion; WBC, white blood cell; NEUT, neutrophil count, LYMPH, lymphocyte count; TBIL, total bilirubin; DBIL, direct bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase; BCLC, barcelona-clinic liver cancer; GLR, GGT-to-lymphocyte ratio.
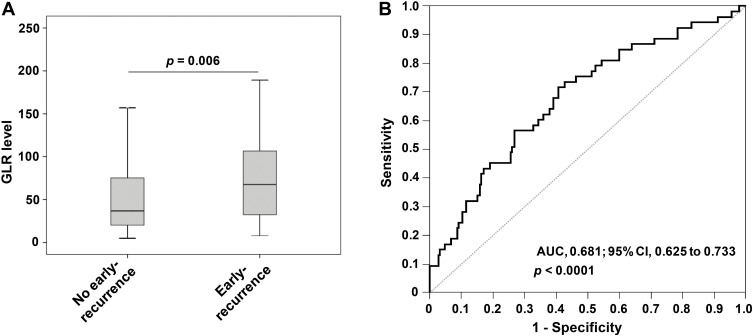
Three hundred and three HCC patients with AFP-negative were divided into the early-recurrence group (n=53) and the non-early-recurrence group (n=250) according to whether they had recurrence within 1 year after surgery. By analyzing and comparing the clinical characteristics of patients of the two groups, it was found that GLR (p =0.006) and GGT (p =0.030) had a significant difference between the early-recurrence group and the non-early-recurrence group (Table 2, Figure 2A).
Table 2.
Comparison of Clinicopathological Characteristics of No Early-Recurrence and Early-Recurrence Patients
| Parameter | No Early-Recurrence | Early-Recurrence | p-value |
|---|---|---|---|
| (n=250) | (n=53) | ||
| Gender: female/male (n) | 25/225 | 6/47 | 0.773 |
| Age (years) | 52.55±10.95 | 50.69±11.52 | 0.259 |
| HBsAg: negative/positive (n) | 52/198 | 11/42 | 0.994 |
| Family history: absent/present (n) | 217/33 | 47/6 | 0.711 |
| Drinking: absent/present (n) | 126/124 | 31/22 | 0.284 |
| Smoking: absent/present (n) | 133/117 | 29/24 | 0.841 |
| Cirrhosis: absent/present (n) | 20/230 | 8/45 | 0.105 |
| MVI: absent/present (n) | 197/53 | 45/8 | 0.314 |
| WBC (×109/L) | 6.66±2.44 | 6.36±2.37 | 0.418 |
| NEUT (×109/L) | 4.10±2.14 | 3.88±1.72 | 0.485 |
| LYMPH (×109/L) | 1.77±0.63 | 1.59±0.51 | 0.052 |
| Platelets (×109/L) | 180.65±78.37 | 191.91±88.76 | 0.357 |
| Albumin (g/L) | 39.11±5.23 | 40.49±4.42 | 0.075 |
| Globulin (g/L) | 30.67±5.76 | 31.06±5.06 | 0.650 |
| TBIL (μmol/L) | 15.19±13.72 | 13.78±6.23 | 0.507 |
| DBIL (μmol/L) | 5.62±4.42 | 5.05±2.81 | 0.355 |
| ALT (U/L) | 41.28±37.34 | 51.06±49.11 | 0.096 |
| AST (U/L) | 46.54±53.35 | 48.30±44.57 | 0.832 |
| GGT (U/L) | 84.50±66.65 | 107.03±76.50 | 0.030* |
| Tumor size (cm) | 7.38±6.64 | 8.50±4.73 | 0.241 |
| Tumor number: single/multiple (n) | 190/60 | 35/18 | 0.132 |
| Tumor differentiation: I/II/III (n) | 44/94/112 | 5/19/29 | 0.251 |
| BCLC stage: 0/A/B (n) | 18/124/108 | 2/24/27 | 0.443 |
| GLR | 54.66±48.84 | 75.83±58.43 | 0.006* |
Note: *P-value indicates statistically significant.
Abbreviations: n, number of patients; HBsAg, hepatitis B surface antigen; MVI, microvascular invasion; WBC, white blood cell; NEUT, neutrophil count, LYMPH, lymphocyte count; TBIL, total bilirubin; DBIL, direct bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, Gamma-glutamyl transpeptidase; BCLC, barcelona-clinic liver cancer; GLR, GGT to lymphocyte ratio.
Figure 2.
The role of GLR in the prediction and evaluation of early recurrence in different groups of HCC. (A) The scatter plot showed the difference in GLR level between the early-recurrence group and the non-early-recurrence group. (B) The ROC curve was performed to assess the role of GLR in predicting early recurrence of HCC.
The above results suggested that GLR level was related to recurrence in AFP-negative patients. Therefore, we further explored whether GLR level could be an indicator for monitoring of recurrence.
The Optimal Cut-off Value of GLR
By ROC curve analysis, AUC of GLR to predict the prognosis of AFP-negative HCC patients was 0.681 [95% confidence interval (CI)=0.625–0.733]. The optimal cut-off value for GLR was 45.0, with a sensitivity of 67.9% (95% CI=0.537–0.801) and a specificity of 60.8% (95% CI=0.544–0.669) (Figure 2B). The C-index of GLR calculated by R was 0.654 (95% CI=0.606–0.702). It suggested that GLR was significant in monitoring the early recurrence of AFP-negative HCC patients and had a certain predictive value. In addition, based on this cut-off value, patients were divided into GLR >45.0 group (n=141) and GLR ≤45.0 group (n=162).
Relationship Between GLR and Recurrence and Survival
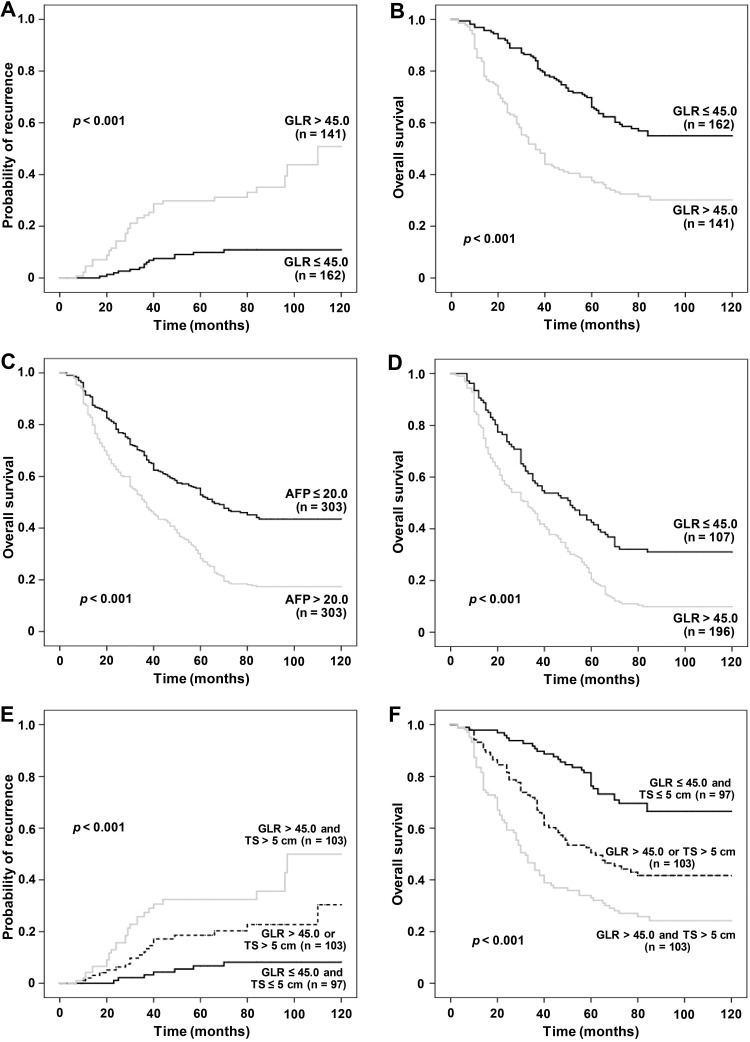
The GLR of the early-recurrence group was higher than that of the non-early-recurrence group (p =0.006, Figure 2A). Kaplan-Meier method was used to analyze the survival of patients, and it was found that the level of GLR was correlated with the prognosis of patients with AFP-negative HCC significantly. Postoperative recurrence rate of patients in the GLR ≤45.0 group was lower than that in the GLR >45.0 group (p <0.001, Figure 3A). In addition, postoperative OS in the GLR ≤45.0 group was longer than that in the GLR >45.0 group (p <0.001, Figure 3B). The mean postoperative OS (months) in the GLR ≤45.0 group (90.02, 95% CI=82.65–97.39) was higher than that in the GLR >45.0 group (62.68, 95% CI=56.32–69.05) (p <0.001, Table 3).
Figure 3.
The relationship of GLR level with recurrence rate and OS in AFP-negative HCC patients. Kaplan–Meier analysis revealed a significantly higher recurrence rate (A) and shorter OS (B) in AFP-negative HCC patients with GLR ≤45.0 than in those with GLR >45.0. The OS of AFP-negative patients was longer than that of AFP-positive patients (C). The OS of patients with GLR ≤45.0 was longer than that of patients with GLR >45.0 (D). The postoperative recurrence rate of AFP-negative patients was highest in the GLR >45.0 and TS >5 cm group, and lowest in the GLR ≤45.0 and TS ≤5 cm group (E), while the OS of patients was lowest in the GLR >45.0 and TS >5 cm group, and highest in the GLR ≤45.0 and TS ≤5 cm group (F).
Table 3.
Univariate Analysis of the Prognosis of Survival and Clinicopathologic Characteristics
| Clinical Characteristics | Category | No. of Patients | Disease-Free Survival (Months) | Overall Survival (Months) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | 95% CI | p-value | Mean | 95% CI | p-value | |||
| Gender | Female | 31 | 56.92 | 38.41–75.44 | 0.612 | 68.63 | 52.31–84.96 | 0.639 |
| Male | 272 | 61.99 | 55.81–68.17 | 72.77 | 67.41–78.11 | |||
| Age (years) | <60 | 228 | 59.03 | 52.34–65.72 | 0.182 | 70.06 | 64.21–75.91 | 0.147 |
| ≥60 | 75 | 68.97 | 57.05–80.90 | 79.49 | 69.37–89.62 | |||
| Family history | Absent | 264 | 60.44 | 54.13–66.72 | 0.243 | 70.84 | 65.33–76.35 | 0.194 |
| Present | 39 | 65.07 | 49.37–80.76 | 82.82 | 70.08–95.55 | |||
| HBsAg | Negative | 63 | 60.09 | 46.69–73.50 | 0.667 | 72.17 | 60.75–83.58 | 0.997 |
| Positive | 240 | 61.95 | 55.45–68.47 | 72.39 | 66.71–78.07 | |||
| Drinking | Absent | 157 | 63.58 | 55.31–71.85 | 0.613 | 74.39 | 67.33–81.46 | 0.468 |
| Present | 146 | 59.06 | 50.78–67.33 | 70.35 | 63.03–77.64 | |||
| Tumor size (cm) | <5 | 135 | 79.62 | 70.94–87.31 | <0.001* | 89.65 | 82.79–96.52 | < 0.001 |
| ≥5 | 168 | 47.55 | 40.36–54.73 | 58.59 | 51.97–65.21 | |||
| Cirrhosis | Absent | 28 | 56.15 | 35.77–76.52 | 0.325 | 67.68 | 50.13–85.23 | 0.610 |
| Present | 275 | 61.89 | 55.77–68.01 | 72.80 | 67.49–78.11 | |||
| Microvascular invasion | Absent | 242 | 65.13 | 58.53–71.73 | 0.015* | 76.83 | 71.31–82.34 | 0.001 |
| Present | 61 | 47.98 | 35.93–60.03 | 54.92 | 43.27–66.58 | |||
| Tumor number | Single | 225 | 65.53 | 58.71–72.73 | 0.017* | 76.35 | 70.53–82.16 | 0.009 |
| Multiple | 78 | 49.55 | 38.59–60.51 | 60.98 | 50.95–71.01 | |||
| Tumor differentiation | I–II | 162 | 74.01 | 65.83–80.35 | <0.001* | 85.81 | 79.80–91.06 | < 0.001 |
| III | 141 | 46.88 | 38.62–55.07 | 56.65 | 49.02–64.27 | |||
| BCLC stage | 0-A | 168 | 73.27 | 63.66–79.83 | <0.001* | 83.62 | 77.23–89.64 | < 0.001 |
| B | 135 | 47.92 | 39.95–55.16 | 57.12 | 49.71–64.58 | |||
| Early- recurrence |
Absent | 250 | 79.26 | 74.31–85.19 | < 0.001 | |||
| Present | 53 | 35.37 | 29.61–41.35 | |||||
| GLR | ≤ 45.0 | 162 | 79.75 | 70.26–89.23 | <0.001* | 90.02 | 82.65–97.39 | < 0.001 |
| > 45.0 | 141 | 50.89 | 43.86–57.91 | 62.68 | 56.32–69.05 | |||
Note: *P-value indicates statistically significant.
Abbreviations: HR, hazard ratio; CI, confidence interval; HBsAg, hepatitis B surface antigen; BCLC, barcelona-clinic liver cancer; GLR, gamma-glutamyl transpeptidase to lymphocyte count ratio.
Furthermore, we analyzed the postoperative survival of the 606 AFP-negative and AFP-positive patients, and found that the OS of AFP-negative patients was longer than that of AFP-positive patients (p <0.001, Figure 3C). After the analysis of the prognosis and survival of all patients (n=606), it was found that the OS of patients with GLR ≤45.0 group was longer than that of patients with GLR >45.0 group (p <0.001, Figure 3D).
Univariate and Multivariate Cox Regression Analyses Assessed the Risk of Postoperative Survival
Univariate analysis was conducted on the postoperative survival of 303 patients with AFP-negative HCC, and the results showed that TS (≥5 cm), microvascular invasion, multiple tumor, higher Barcelona Clinic Liver Cancer (BCLC) stage, and GLR >45.0 were important factors affecting OS and DFS. In addition, recurrence was an important factor affecting patients’ OS (Table 3). After adjusting for other risk predictors, multivariate Cox regression analysis of the above factors showed that TS ≥5 cm (HR=1.67; 95% CI=1.15–2.45; p =0.007) and GLR >45.0 (HR=1.73; 95% CI=1.19–2.58; p =0.001) could be used as independent predictors of DFS in AFP-negative HCC. In addition, TS ≥5 cm (HR=1.74; 95% CI=1.20–2.53; p =0.003), recurrence (HR=2.32; 95% CI=1.50–3.62; p <0.001), and GLR >45.0 (HR=1.91; 95% CI=1.35–2.83; p <0.001) were independent predictors of OS in AFP-negative HCC (Table 4).
Table 4.
Multivariate Cox Regression Analyses of the Prognosis of Survival and Clinicopathologic Characteristics
| Variables | Hazard Ratio (95% CI) | p-value |
|---|---|---|
| Disease-free survival | ||
| Tumor size, cm (≥5 vs <5) | 1.67 (1.15–2.45) | 0.007* |
| MVI (present vs absent) | 1.23 (0.78–1.96) | 0.374 |
| Tumor number (multiple vs single) | 1.31 (0.82–2.05) | 0.262 |
| Tumor differentiation (III vs I–II) | 1.27 (0.83–1.95) | 0.264 |
| BCLC stage (B vs 0-A) | 1.66 (0.96–2.81) | 0.072 |
| GLR (≥45.0 vs <45.0) | 1.73 (1.19–2.58) | 0.001* |
| Overall survival | ||
| Tumor size, cm (≥5 vs <5) | 1.74 (1.20–2.53) | 0.003* |
| MVI (present vs absent) | 1.19 (0.74–1.92) | 0.474 |
| Tumor number (multiple vs single) | 1.16 (0.73–1.86) | 0.531 |
| Tumor differentiation (III vs I–II) | 1.45 (0.96–2.17) | 0.080 |
| BCLC stage (B vs 0-A) | 1.28 (0.75–2.21) | 0.384 |
| Early-recurrence (present vs absent) | 2.32 (1.50–3.62) | <0.001* |
| GLR (≥ 45.0 vs < 45.0) | 1.91 (1.35–2.83) | <0.001* |
Note: *P-value indicates statistically significant.
Abbreviations: CI, confidence interval; MVI, microvascular invasion; BCLC, Barcelona-clinic liver cancer; GLR, gamma-glutamyl transpeptidase-to-lymphocyte count ratio.
The Prognostic Effect of GLR Combined with TS on the Prognosis of AFP-Negative HCC
The multivariate Cox proportional hazards regression analysis showed that GLR and TS were independent predictors of the prognosis of AFP-negative HCC. Therefore, we speculated that the combination of GLR and TS could better predict the prognosis of AFP-negative HCC. The AFP-negative patients were further divided into GLR >45.0 and TS >5 cm (group 1, n=103), GLR >45.0 or TS >5 cm (group 2, n=103), and GLR ≤45.0 and TS ≤5 cm (group 3, n=97). The postoperative prognosis of each group was compared, and it was found that the recurrence rate of patients was highest in the GLR >45.0 and TS >5 cm group, and lowest in the GLR ≤45.0 and TS ≤5 cm group (p <0.001, Figure 3E); while the postoperative OS of patients was shortest in the GLR >45.0 and TS >5 cm group, and longest in the GLR ≤45.0 and TS ≤5 cm groups (p <0.001, Figure 3F).
Discussion
It is well known that high recurrence rate is a major factor for poor postoperative prognosis of HCC patients, and early recurrence within 1 or 2 years after surgery is clinically common.22–25 In addition, studies have shown that the prognosis of early recurrence of HCC within 1 year after surgery was worse than that of patients with late recurrence or no recurrence significantly.25,26 Therefore, it is of obvious clinical significance to define the time node of postoperative recurrence as 1 year.
Serum AFP is the primary biomarker for the clinical screening, diagnosis, and postoperative recurrence monitoring of HCC, but there is a considerable proportion of HCC patients with AFP ≤20 ng/mL.4,5,27 In fact, detection of AFP level in such patients in follow-up increases the cost of detection and has limited clinical value, so AFP-negative HCC patients should be treated differently. We also found that the prognosis of AFP-negative HCC patients was better than that of AFP-positive HCC patients, which was consistent with the results of Zhang et al,9 further demonstrating the important clinical significance of prognosis prediction of AFP-negative HCC patients.
Currently, the diagnosis and postoperative monitoring of AFP-negative HCC mainly rely on ultrasonography, CT examination, and other imaging methods. However, due to the high cost and radiation of CT scanning, it is not recommended to repeat it in a short period of time, and ultrasonography is insufficient in the small lesions, so many researchers suggest using other biomarkers, such as hematological indicators as the ideal choice for postoperative recurrence monitoring.27–29 In addition, the TNM stage developed by the American joint committee on Cancer (AJCC) and BCLC stage are used for surgical evaluation and prognostic prediction in AFP-negative HCC patients widely. However, there are many controversies due to the dominant role of tumor load in TNM staging system,30 as the most common and comprehensive evaluation system for therapeutic methods selection and prognosis stratification in HCC patients, the BCLC staging system has been reported to have certain potential in predicting the prognosis of AFP-negative HCC patients.9 However, studies have shown that BCLC stage only had such good predictive value on patients with advanced HCC.31 It has not been reported to have surveillance potential for postoperative recurrence in AFP-negative patients either. Then, in this study, we found that the level of GLR was significantly higher in AFP-positive HCC patients than in AFP-negative patients. Based on the monitoring of early recurrence of AFP-negative HCC after operation, it was found that GLR had a high sensitivity and specificity in predicting the recurrence of HCC within 1 year after operation. Besides, the GLR was able to distinguish the early recurrence of patients to a certain extent via C-index. Meanwhile, we found that GLR can still be used as an independent predictor of DFS and OS in AFP-negative HCC patients, which was consistent with our previous study that GLR could be used as a prognostic predictor for postoperative HCC patients with TS ≤5 cm.20 Therefore, we speculated that GLR was necessarily related to the existence of AFP-negative liver cancer, which may be one of its potential markers.
Tumorigenesis is the result of the interaction between host characteristics and environmental factors, and lifestyle (drinking, smoking, etc.) has a profound impact on the development of cancer, including HCC.32 Globally, about 78% of HCC are caused by HBV or HCV infection.33 Among the cases involving in this study, HBV-related HCC was 79.2% (240/303), which further indicated that HBV infection was an important etiological factor for the occurrence of HCC in this region. Studies on antiviral therapy have shown that nucleoside or nucleotide analogs were related to disease progression and could reduce the incidence of HCC.34,35 For patients with increased HBV DNA level before hepatectomy, antiviral treatment after hepatectomy is significantly associated with the decrease of HCC recurrence rate.36 Some researchers have found that controlling the DNA level and shortening the duration of HBV were significantly associated with less recurrence and prolonged OS for HCC.37 The mechanism of benefits from antiviral therapy in HCC patients may be as follows: reducing the risk of HBV reactivation and further liver function damage, reducing mortality associated with liver failure, recovering from HBV-induced hepatitis and promoting liver tissue recovery, which provide patients with more opportunities to receive regional and systemic treatments, and prolong the OS.38,39
Meanwhile for HCC patients with AFP-negative who have undergone surgical resection, the trend of GLR level can be observed in regular follow-ups, therefore precautions and necessary interventions can be taken in advance to reduce recurrence.
However, there are some limitations in this study. As a retrospective analysis, the research object was from one single hospital, rather than a large-scale population. There is an impact of sample bias and a small number of patients can hardly reflect on the prognosis of all AFP-negative HCC. Although the number of samples is limited, what we focus on is clinical practice, so this study is still of great significance.
Conclusion
In conclusion, as a novel and readily available biomarker, GLR is an independent predictor of OS and DFS in AFP-negative HCC patients and is associated with recurrence significantly. Therefore, it suggests that clinicians should formulate treatment plans by considering the factors affecting the prognosis of HCC recurrence comprehensively, and develop appropriate personalized treatment plans for patients.
Acknowledgments
We are grateful to the patients who participated in this study and all the staff in the hepatobiliary surgery department of the Affiliated Hospital of Guilin Medical University for their support and cooperation in making this study completed successfully.
Funding Statement
This work was supported in part by the National Key Sci-Tech Special Project of China (No. 2018ZX10302207), the National Natural Science Foundation of China (No. 81772923) and the Science and Technology Planning Project of Guilin (No. 20190218-1).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Villanueva A, Longo DL. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;109(9):djx030. doi: 10.1093/jnci/djx030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S, Liu L, Yang S, et al. Preoperative serum alpha-fetoprotein and prognosis after hepatectomy for hepatocellular carcinoma. Br J Surg. 2016;103(6):716–724. doi: 10.1002/bjs.10093 [DOI] [PubMed] [Google Scholar]
- 5.Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101(3):524–532. doi: 10.1111/j.1572-0241.2006.00443.x [DOI] [PubMed] [Google Scholar]
- 6.Giannini EG, Marenco S, Borgonovo G, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology (Baltimore, Md). 2012;56(4):1371–1379. doi: 10.1002/hep.25814 [DOI] [PubMed] [Google Scholar]
- 7.Agopian VG, Harlander-Locke MP, Markovic D, Zarrinpar A, Kaldas FM, Cheng EY. Evaluation of patients with hepatocellular carcinomas that do not produce α-fetoprotein. JAMA Surg. 2017;152(1):55–64. doi: 10.1001/jamasurg.2016.3310 [DOI] [PubMed] [Google Scholar]
- 8.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi: 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 9.Zhang XF, Qi X, Meng B, Liu C, Yu L, Wang B. Prognosis evaluation in alpha-fetoprotein negative hepatocellular carcinoma after hepatectomy: comparison of five staging systems. Eur J Surg Oncol. 2010;36(8):718–724. doi: 10.1016/j.ejso.2010.05.022 [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Mao M, He Z, Zhang L, Li H, Lin J. Development and validation of a prognostic nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci. 2019;15(1):221–228. doi: 10.7150/ijbs.28720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.She S, Xiang Y, Yang M, et al. C-reactive protein is a biomarker of AFP-negative HBV-related hepatocellular carcinoma. Int J Oncol. 2015;47(2):543–554. doi: 10.3892/ijo.2015.3042 [DOI] [PubMed] [Google Scholar]
- 12.Jing W, Peng R, Zhu M, Lv S, Jiang S, Ma J. Differential expression and diagnostic significance of pre-albumin, fibrinogen combined with D-dimer in AFP-negative hepatocellular carcinoma. Pathol Oncol Res. 2020;26:1669–1676. doi: 10.1007/s12253-019-00752-8 [DOI] [PubMed] [Google Scholar]
- 13.Critelli R, Milosa F, Faillaci F, et al. Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study. Cell Death Dis. 2017;8(8):e3017. doi: 10.1038/cddis.2017.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramakrishna G, Rastogi A, Trehanpati N, Sen B, Khosla R, Sarin SK. From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer. 2013;2:367–383. doi: 10.1159/000343852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padera TP, Meijer EFJ, Munn LL. The Lymphatic system in disease processes and cancer progression. Annu Rev Biomed Eng. 2016;18(1):125–158. doi: 10.1146/annurev-bioeng-112315-031200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndrepepa G, Colleran R, Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin Chim Acta. 2018;476:130–138. doi: 10.1016/j.cca.2017.11.026 [DOI] [PubMed] [Google Scholar]
- 17.Xu T, Wang W, Zhai L, et al. Serum gamma-glutamyl transferase levels predict functional outcomes after aneurysmal subarachnoid hemorrhage. Biomed Environ Sci. 2017;30(3):170–176. doi: 10.3967/bes2017.024 [DOI] [PubMed] [Google Scholar]
- 18.Priolo C, Khabibullin D, Reznik E, Filippakis H, Ogórek B, Kavanagh TR. Impairment of gamma-glutamyl transferase 1 activity in the metabolic pathogenesis of chromophobe renal cell carcinoma. Proc Natl Acad Sci U S A. 2018;115(27):E6274–E6282. doi: 10.1073/pnas.1710849115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B, Zhan C, Wu J, Liu J, Zhou J, Zheng S. Prognostic significance of preoperative gamma-glutamyltransferase to lymphocyte ratio index in nonfunctional pancreatic neuroendocrine tumors after curative resection. Sci Rep-UK. 2017;7(1):13372. doi: 10.1038/s41598-017-13847-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao M, Qin W, Liao Y, Yao R, Yu J, Liao W. Prognostic value of gamma-glutamyl transpeptidase to lymphocyte count ratio in patients with single tumor size ≤ 5 cm hepatocellular carcinoma after radical resection. Front Oncol. 2019;9:347. doi: 10.3389/fonc.2019.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Ding T, He Q, et al. An in situ molecular signature to predict early recurrence in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57(2):313–321. doi: 10.1016/j.jhep.2012.03.027 [DOI] [PubMed] [Google Scholar]
- 22.Li T, Fan J, Qin LX, Zhou J, Sun HC, Qiu SJ. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011;18(7):1955–1963. doi: 10.1245/s10434-010-1540-z [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Huang Y, Chau G, Su C, Lai C, Lee P. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51(5):890–897. doi: 10.1016/j.jhep.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 24.Sohn W, Paik YH, Kim JM, Kwon CH, Joh JW, Cho JY. HBV DNA and HBsAg levels as risk predictors of early and late recurrence after curative resection of HBV-related hepatocellular carcinoma. Ann Surg Oncol. 2014;21(7):2429–2435. doi: 10.1245/s10434-014-3621-x [DOI] [PubMed] [Google Scholar]
- 25.Xing H, Sun L, Yan W, et al. Repeat hepatectomy for patients with early and late recurrence of hepatocellular carcinoma: a multicenter propensity score matching analysis. Surgery. 2019;11:005. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Li Q, Sun Y, Zheng H, Cui Y. Clinicopathologic features between multicentric occurence and intrahepatic metastasis of multiple hepatocellular carcinomas related to HBV. Surg Oncol. 2009;18(1):25–30. doi: 10.1016/j.suronc.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 27.Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A, Kanke F. Clinical utility of highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in hepatocellular carcinoma patients with alpha-fetoprotein < 20 ng/mL. Cancer Sci. 2011;102(5):1025–1031. doi: 10.1111/j.1349-7006.2011.01875.x [DOI] [PubMed] [Google Scholar]
- 28.Liu P, Lu D, Al-Ameri A, Wei X, Ling S, Li J. Glutamine synthetase promotes tumor invasion in hepatocellular carcinoma through mediating epithelial-mesenchymal transition. Hepatol Res. 2020;50(2):246–257. doi: 10.1111/hepr.13433 [DOI] [PubMed] [Google Scholar]
- 29.Bialecki ES, Ezenekwe AM, Brunt EM, Collins BT, Ponder TB, Bieneman BK. Comparison of liver biopsy and noninvasive methods for diagnosis of hepatocellular carcinoma. Clin Gastroenterol H. 2006;4(3):361–368. doi: 10.1016/S1542-3565(05)00977-8 [DOI] [PubMed] [Google Scholar]
- 30.Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909–922. doi: 10.1097/01.sla.0000254368.65878.da [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, González-Frutos C, Ciampi-Dopazo JJ, de-la-Cruz-Pérez G. The value of the barcelona clinic liver cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig. 2012;104(6):298–304. doi: 10.4321/S1130-01082012000600003 [DOI] [PubMed] [Google Scholar]
- 32.Saran U, Humar B, Kolly P, Dufour J-F. Hepatocellular carcinoma and lifestyles. J Hepatol. 2016;64(1):203–214. doi: 10.1016/j.jhep.2015.08.028 [DOI] [PubMed] [Google Scholar]
- 33.Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529–538. doi: 10.1016/j.jhep.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 34.Huang G, Li PP, Lau WY, et al. Antiviral therapy reduces hepatocellular carcinoma recurrence in patients with low HBV-DNA levels: a randomized controlled trial. Ann Surg. 2018;268(6):943–954. doi: 10.1097/SLA.0000000000002727 [DOI] [PubMed] [Google Scholar]
- 35.Kranidioti H, Manolakopoulos S, Khakoo SI. Outcome after discontinuation of nucleot(s)ide analogues in chronic hepatitis B: relapse rate and associated factors. Ann Gastroenterol. 2015;28(2):173–181. [PMC free article] [PubMed] [Google Scholar]
- 36.Fung J, Lai CL, Seto WK. Nucleoside/nucleotide analogues in the treatment of chronic hepatitis B. J Antimicrob Chemother. 2011;66(12):2715–2725. doi: 10.1093/jac/dkr388 [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Tian SL, Wang H, et al. Association of hepatitis B virus DNA level and follow-up interval with hepatocellular carcinoma recurrence. JAMA Netw Open. 2020;3(4):e203707. doi: 10.1001/jamanetworkopen.2020.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JL, Qian XJ, Ming Z, et al. Association of HBV DNA replication with antiviral treatment outcomes in the patients with early-stage HBV-related hepatocellular carcinoma undergoing curative resection. Chin J Cancer. 2016;35:28. doi: 10.1186/s40880-016-0089-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei Q, Tian H, Luo HX, et al. Better prognosis of hepatic resection combined with antiviral therapy for HBV-related hepatocellular carcinoma with BCLC stage B/C. Asian J Surg. 2017;40(6):453–462. doi: 10.1016/j.asjsur.2016.03.001 [DOI] [PubMed] [Google Scholar]