Abstract
Purpose
The purpose of this proposed research was to investigate a nano-formulation developed using self-nanoemulsifying drug delivery system (SNEDDS) to improve the pharmacodynamic potential of rosuvastatin by assisting its transportation through lymphatic circulation.
Methods
The utilized lipids, surfactants, and co-surfactants for SNEDDS were selected on the basis of solubility studies. The SNEDDS formulation was optimized by implementing a D-optimal mixture design, wherein the effect of concentration of Capmul MCM EP (X1), Tween 20 (X2) and Transcutol P (X3) as independent variables was studied on droplet size (Y1), % cumulative drug release (Y2) and self-emulsification time (Y3) as dependent variables. The optimized formulation was evaluated via in vitro parameters and in vivo pharmacodynamic potential in Wistar rats.
Results
The D-optimal mixture design and subsequent ANOVA application resulted in the assortment of the optimized SNEDDS formulation that exhibited a droplet size of nano range (14.91nm), in vitro drug release of >90% within 30 minutes, and self-emulsification time of 16 seconds. The in vivo pharmacodynamic study carried out using Wistar rats confirmed the better antihyperlipidemic potential of developed formulation in normalizing the lipidic level of serum in contrast to pure drug and marketed tablets.
Conclusion
This research reports the application of D-optimal mixture design for successful and systematic development of rosuvastatin-loaded SNEDDS with distinctly enhanced in vitro and in vivo performance in comparison to marketed formulation. Eventually, improved anti-hyperlipidemic efficacy was envisaged which might be attributed to increased drug solubility and absorption. Overall, this study shows the utility of SNEDDS for improving the dissolution rate and bioavailability of poor aqueous-soluble drugs. The present SNEDDS formulation could be a promising approach and alternative to conventional dosage form.
Keywords: SNEDDS, rosuvastatin, in vitro lipolysis, nanotechnology, D-optimal mixture design, pharmacodynamic study
Introduction
Hyperlipidemia is described as an increase in the concentration of lipids in the bloodstream, that is why it is also known as hypercholesterolemia or hypertriglyceridemia. Atherosclerosis and cardiovascular disease are its prime risks, which resulted in approximately 800,000 deaths in 2005 as the primary reason for death in the United States with yet increasing number of incidents. Rosuvastatin calcium (BCS II class drug) is a first-line therapy drug to treat hyperlipidemia, with better results than other statins.1 It is also known as a “super-statin” due to its most significant potential for antilipidemic conditions. Its low aqueous solubility is responsible for its slow dissolution rate and low bioavailability (20%). Thus, improvement in its dissolution can lead to its improved bioavailability.2
Nanotechnology has presented a novel perception to human knowledge and has led to innovation in the field of biological sciences including, diagnostics, food, drug delivery, biomaterials for implants or prosthesis, and the production of biomimetics appropriate for applications in the field of medicine.3 Nanotechnology is gaining significance because it concerns small size and targeted properties. Nanoscale formulations have a size range of 100–10000 times smaller than that of human cells. As a consequence of their small size and bigger surface area, they can easily interact with enzymes and receptors on the surface of the cell and inside the cell. After being administered to various regions of the body, they have the capability to “spot” disease at extremely low level and deliver medicament.4 Nanotechnology is the most promising technology over the last two decades and various efforts are ongoing to extend its applications in various streams of pharmaceutical sciences. Many nanoscale carriers have been recently explored for improving the therapeutic performance of drugs.5
Nanomedicine is the main and exhaustive field of research in nanotechnology and applicable widely for the diagnosis, prevention, and treatment of diseases.6 Nano drug designing has been investigated at broad level and these are the nano-formulations that are considered under novel advanced technology in Pharmaceutical Science. It has the potential to alter drug release profile, solubility, and bioavailability. Their main advantages are ability to target a specific site, reduction of toxicity, improvement of bioavailability, improving stability and reduction of dosing frequency by development of controlled release formulation. Therefore, it can be summed up that nanotechnology plays a vital role in the improvement of safety, patient compliance, efficacy and drug shelf life.7
Despite various novel innovations for the delivery of dynamic pharmacotherapeutic mixes, drug formulation through the oral route is viewed as the most common for patients of all ages.8 The high-dose requirements of some drugs have intensified the need to design nano-enabled drug delivery systems (DDSs), which have many benefits over conventional DDSs.9 Recently, attention has been focused toward the nanoscale manipulation of DDSs offering inimitable features for navigation of drug-vehicle and controlled drug release, which is often not achievable in conventional- (eg, free-form) and microscale-drug carriers.10 The current strategy of developing bio-polymeric SNEDDS has pronounced conviction of incorporating inherent features of no toxicity, biodegradability, biocompatibility, and high sensitivity. Recently, SNEDDS have been labeled as personalized health-care due to advancements in DDS for improved efficacy and least side-effects.
Self-nanoemulsifying drug delivery systems (SNEDDS) is an important approach that merges the advantages of lipid-based drug delivery system and nanotechnology. SNEDDS is a novel methodology in the delivery of drugs and tackles inadequacy associated with the delivery of BCS II class drugs.11,12 SNEDDS are depicted as transparent formulations comprised of oil, surfactant, and cosurfactant that creates droplets of ultrafine oil in water (o/w) nano-emulsion with a mean droplet range of < 100nm.13 They facilitate in having of greater solubilization limit, prompting the expansion of drug in oily phase.14,15 The ingredients present in these will encourage bioavailability (BA) of the drug not only by enhancing their permeability and solubility, but also by dodging their metabolism via liver and repressing P-glycoprotein efflux, alongside the capacity to encourage lymphatic assimilation of the drug. A few reports based on these formulations have proven possible enhancement of BA of different drugs.16–20
Nano-emulsions are emerging as very useful to improve performance of various biomedical systems based on drug delivery, food, cosmetics and pharmaceuticals because of their small size and useful properties such as high surface area per unit volume, robust dilution stability, optical transparency and tunable rheology.21 Due to small droplet size, nano-emulsions possess stability against sedimentation or creaming with Ostwald ripening that is the main mechanism of nano-emulsion breakdown. This is the main reason for their thermo-kinetic stability.22,23
The present study was planned to investigate the in vitro and in vivo evaluation of rosuvastatin-loaded SNEDDS formulation. In this study, SNEDDS were developed with Capmul MCM EP (oil), Tween 20 (surfactant), Transcutol (co-surfactant) and the formulation was optimized by implementation of D-optimal mixture design. The optimized SNEDDS formulation was characterized for globule size, zeta potential, DSC, FT-IR, and stability studies. Further, food-effect, in vitro and in vivo investigations were carried out. Transmission electron microscopy (TEM) and laser particle size analyzer were utilized to estimate shape and size of the resultant nano-emulsion. Because of these contemplations, the principle point of the current research was the utilization of optimization strategy for methodical product development that aids in accomplishing predictable quality and vigorous execution. Optimization strategy gives product and process understanding for consistent development. Experimental design was considered a key tool that maximizes data utilizing the least experimental work.24
Materials and Methodology
Materials
Pure rosuvastatin was a liberal sample from Sun Pharmaceutical Laboratories, Gurugram. Capmul MCM EP was sympathetically provided by IMCD, Delhi. Tween 20 was purchased from Croda, Transcutol P from Gattefosse, and Pancreatin from Loba Chemie. Rosufine 20 mg tablets of batch no. C-90061 were obtained from a drug store. Other ingredients and reagents utilized in the current research were of analytical grades.
Methodology
Investigation of Rosuvastatin’s Solubility in Various Ingredients
Considering the therapeutic dose strength of rosuvastatin, a higher medicament loading capacity of SNEDDS is required. When deciding on and plotting achievable emulsification milieu, it was important to determine solubility of rosuvastatin in various oils or surfactants.25
The super saturation solubility of rosuvastatin was examined in different ingredients for the selection of right ingredient which revealed high solubilizing potential and maximum drug loading for rosuvastatin. An excess amount of rosuvastatin was blended with every excipient (2 g) in a screw-topped glass vial. Vortex blender (Genius, India) was utilized for the best possible mixing of rosuvastatin with ingredients.26
For the blending of mixture, thermostatic control shaker (Calton, Germany) was utilized for 72 h at 100 rpm. After the collection of samples, centrifugation (Remi, Mumbai) was carried out for 30 minutes at 5000 rpm. The supernatant was gathered and 0.45μm membrane filters were utilized for filtering of solution. The amount of rosuvastatin was investigated utilizing UV-spectrophotometer (UV 1700, Shimadzu, Japan) at 242 nm. The examination was performed in triplicate.27 The self-emulsification limit of oil and surfactant was explored for picking their best combination. Each oil was titrated with 10 mL of every surfactant (10% w/w aqueous solution). We noted the volume of oil when it changed over emulsion clearness into cloudy transform and selected the blend that offered the most elevated amount of oil emulsified.28,29
Smix of 1:1 was prepared with every co-surfactant in order to choose the best combination and different preparations were formulated with selected oil and Smix. 520 mg of every preparation was mixed with purified water (500 mL) and we recorded the clarity of the resultant nano-emulsion.30
Plotting of Ternary Phase Diagram
This was constructed with each selected ingredient and all of them address a side of this diagram. Ternary mixes with the three different excipients were prepared, resulting in a total entirety of 1 g. Smix was mixed in four ratios 1:1, 2:1, 1:2 and 3:1 in order to choose the ratio with maximal region for nano-emulsion formation. Oil and explicit Smix ratios were mixed totally in 9 differing weight extents from 1:9 to 9:1 in different glass vials.31,32 The objective was that most outrageous extents were planned for the assessment to plot phase exactness restrictions made in this diagram.25 Its blueprints were made using “water dilution” technique. The development of nano-emulsion was observed as a clear/transparent solution that confirms the production of SNEDDS with low consistency of o/w nano-emulsion. The proportion of each ingredient (oil and Smix) was documented and presented in it. It was designed with Chemix software (Chemix Version 4.5).33,34
Optimization of SNEDDS
In recent times, various statistical designs have been used to even more expertly enhance strategy using less experimentation and estimate the significance of independent variables. There are various tools for statistical optimization. Among them, D-optimal mixture design is one of the most standard surface methodologies for their optimization since it confines the distinction identified with assessment of coefficient in model and conveys the perfect subset by considering measures for enhancing statistics grid determinant. In this design, complete arrangement of formulation is considered as 100%, while other designs do not consider it.35,36
The concentrations of Capmul MCM EP (X1), Tween 20 (X2) and Transcutol P (X3) were considered as independent variables for the formulation of SNEDDS with the least droplet size (Y1), maximum %CDR (Y2) and self-emulsification time (Y3) as dependent variables. The design of experiment (DoE) which aids in analyzing and recording the dependent variables (Y) as outcomes (Design Expert ® Software 10) was employed for harmonization of regression equation for calculation of the dependent variables.37 Three equations of regression and desirability index helped in the selection of the optimized SNEDDS formulation.
Evaluation of Globule Size
For this analysis, optimized SNEDDS formulation was diluted (1:100 w/v) with purified water and mixed for 1 minute prior to investigating this parameter. Zetasizer ZS of nano series; Malvern Instruments, Malvern, UK was utilized for the measurement of these parameters that is based on photon correlation spectroscopy.38
Dissolution Study
In this, 900 mL of pH 6.6±0.05 sodium citrate buffer media of 0.05M at 37°C±0.5°C was used in paddle type dissolution apparatus (Distek, USA) at 50 rpm. The capsules filled with SNEDDS (equivalent to 20 mg of rosuvastatin in capsule) were added into the cradle media ensuing to starting the revolution of paddle. Aliquots (5 mL) were collected after 30 minutes and examined via UV-spectrophotometer (UV 1700, Shimadzu, Japan) at λmax 242 nm. This analysis was performed in triplicate.39
Self-Emulsification Time
Based on the reported method, this study was done on every developed formulation. In brief, 1 g formulation of SNEDDS was incorporated in 500 mL of purified water and a magnetic stirrer (Remi, Mumbai) was used for agitation of 100 rpm. Time taken for development of nano-emulsion and dispersibility was recorded.40
Characterization
In view of enhancement results, optimized SNEDDS was picked to complete characterization and more evaluation parameters.
Differential Scanning Calorimetry
The thermochemical features of pure rosuvastatin, physical mixture (rosuvastatin + Capmul MCM EP + Tween 20 + Transcutol P) and rosuvastatin-loaded SNEDDS were characterized by differential scanning calorimetry (DSC) using DSC Q10 (TA Instruments, USA) at the heating rate of 10°C/min. For sample preparation, 5 mg of sample was kept in an aluminum pan and enclosed with an aluminum lid. The results of the analysis were evaluated in the range of 10-300°C. The peak melting temperature for each endothermic curve in the overlay thermogram was assessed automatically using TA Universal Analysis software.41
Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR investigations were carried out to assess whether any possible interaction was occurring among drug, oil, surfactant and co-surfactant. The samples were analyzed by Fourier Transform Infrared spectroscopy (Bruker Alpha, USA). A suitable quantity of pure rosuvastatin, physical mixture (rosuvastatin + Capmul MCM EP + Tween 20 + Transcutol P) and rosuvastatin-loaded SNEDDS was compressed for 5 min at 5 bars on a KBr press and the samples were scanned on the wavenumber range of 400–4400 cm−1.42
Test for % Transmittance
It was estimated for the detection of in vivo effect on the solubilization of the capacity of drug subsequent to dilution in the lumen of the gut. During their development for oral route, there are possibilities for a decline in solubilization capacity that will result in precipitation of the drug. 1 g of formulation was diluted with purified water (100 mL) and estimated at 242 nm utilizing UV spectrophotometer and performed in triplicate utilizing purified water as a blank.43
Robustness Dilution Test
In this, the formulation was explored to the different media such as pH 1.2 of 0.1 N HCl, pH 4.5 of Acetate buffer and pH 6.8 of phosphate buffer after 100 times dilution. After putting these formulations for 24 hours, they were evaluated for phase separation/drug precipitation by visual observation.44
Viscosity Estimation
This was measured by using Hyrdromotion viscometer (Brookfield Engineering, USA). This test was conducted to confirm the type of developed nano-emulsion, whether it was o/w type or w/o type nano-emulsion. The low viscosity of developed nano-emulsion confirms its o/w type of nano-emulsion and vice-versa.45
Estimation of Cloud Point
It was measured after 100-times dilution of SNEDDS (1 g) with purified water and set aside for a regular rise in water bath (Julabo, Germany) temperature at 10°C/minute increment.46
Analysis of Drug Content
The optimized formulation was diluted with methanol (10-times) and centrifugation was conducted for 30 minutes at 10,000 rpm. At that point, supernatant was diluted with methanol (2.5 times) and examined for drug content via UV spectrophotometer at 242 nm, performed in triplicate.47–49
Investigation of Droplet Size, Polydispersity Index (PDI) and Zeta Potential
Small size globule results in better emulsifying ability of the formulation. For this analysis, optimized SNEDDS formulation was diluted (1:100 w/v) with water and mixed for 1 minute prior to investigating these parameters. Zetasizer ZS nano series was utilized for the measurement of these parameters that is based on photon correlation spectroscopy. It was performed in triplicate and shown as mean.50,51
Drug Entrapment Efficiency
Entrapment efficiency (EE) of rosuvastatin SNEDDS was examined as per the procedure established by Ke et al. In brief, the content of free rosuvastatin was separated from SNEDDS by the ultrafiltration method (3500 Da) with centrifugation at 3000 g for 5 minutes followed by qualification using UV spectrophotometer at 242 nm. The EE was calculated as follows:
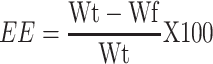 |
where Wt is the total amount of rosuvastatin in nano-emulsion and Wf is the amount of free rosuvastatin.52
Surface Morphology Determined via Transmission Electron Microscopy (TEM)
The morphology of the formed nano-emulsion (1:100 dilutions) was determined using TEM (JEM2100+ microscope JEOL, Japan). The nano-emulsion was negatively stained with 2% phosphotungstic acid, kept for 3 minutes at room temperature and allowed to dry before observation under the electron microscope.49
Dissolution (Multi-Media) Testing
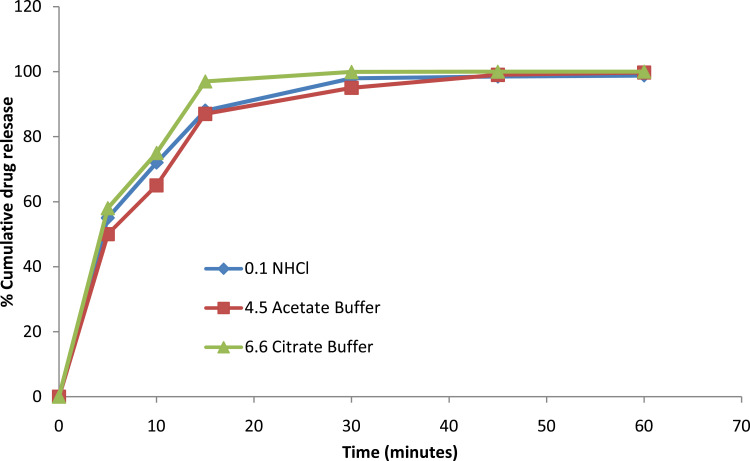
SNEDDS (filled in size “00” capsule) were placed in dissolution media of pH 1.2, 4.5 acetate and 6.6 citrate buffers at 37°C±0.5°C in paddle-type dissolution apparatus (Distek, USA). Samples (5 mL) were collected at a fixed interval of time; the equivalent amount of new fresh media was added after completion of every sampling. The samples were filtered with 0.45μm nylon Millipore membrane filters. The drug discharge rate was estimated utilizing UV spectrophotometer at 242 nm after proper dilution and performed in triplicate.53–55
Comparative Investigation of SNEDDS with Marketed Tablets and Pure Drug in Terms of Dissolution Profile
This investigation was carried out with pure rosuvastatin, SNEDDS and marketed formulation (Rosufine 20 with batch no. C-90061) in “USP Type-II dissolution assembly (Distek, USA) with 900 mL of 0.05M sodium citrate dissolution media of pH 6.6±0.05 at 50 rpm as specified by USFDA dissolution database“. A cellulose membrane dialysis sac (MWCO 12,000 g/mole; Sigma-Aldrich) was filled with equivalent volume of rosuvastatin-loaded optimized SNEDDS, marketed tablets and pure drug (rosuvastatin equivalent to 20 mg). These formulations were placed at the bottom of the vessels using a suitable sinker. On the basis of already established time intervals, 5 mL aliquots were collected and examined for drug release utilizing UV spectrophotometer.39
Dynamic in vitro Lipolysis Study for Investigation of Food Impact
The literature database confirmed that SNEDDS avoids food impact on the formulation by using drug release. For demonstrating this hypothesis, the dissolution of optimized SNEDDS was carried out in modified Fasted/Fed state simulated intestinal fluid V-2 (Fa/FeSSIF V-2) media to simulate an in vivo environment.
It is fundamental for superlative experimental relevance to perform in vitro examination of the drug to mirror in vivo condition as eagerly as could rationally be predicted. This examination is essential for two explicit justifications. Initially, measurement of level and rate of lipolysis that helps to investigate solubility and dispersion features of formulation. The second one is the analysis of post lipolysis to predict solubilized or precipitated form of the drug after completing the reaction. This model can reliably predict the limit of these preparations to improve oral retention of drugs with low solubility in the aqueous phase.
In this study, dynamic in vitro lipolysis examination was an interpretation of methodology as of late described by Mohsin.56 Each 550 mg of SNEDDS was supplemented with both media (36 mL), the formula of which is shown in Table 1.57,58 Compositions of Ca2+, bile salt (BS), phospholipids (PLs), and sodium chloride (NaCl) were linked to mimic representative concentration existing in Fa/FeSSIF V-2. Amid the early period of dispersion, pH 6.5/5.8 ± 0.05 was kept constant with NaOH or HCl.
Table 1.
Composition of Biorelevant Media Used During in vitro Lipolysis
| Chemicals | FaSSIF V-2 | FeSSIF V-2 |
|---|---|---|
| Sodium taurocholate (mM) | 3 | 10 |
| Glyceryl monooleate (mM) | - | 2 |
| Sodium oleate (mM) | - | 5 |
| Lecithin (mM) | 0.2 | 0.8 |
| Maleic acid (mM) | 19.12 | 55.02 |
| NaCl (mM) | 68.62 | 125.5 |
| NaOH (mM) | 34.8 | 81.65 |
| pH | 6.5 | 5.8 |
Abbreviations: FaSSIF V-2, fasted state simulated intestinal fluid V-2; FeSSIF V-2, fed state simulated intestinal fluid V-2.
The blending process was done by mixing on the magnetic stirrer with hot plate at 37°C to emulsify SNEDDS before adding the pancreatic extract solution having pancreatic lipase enzyme. Addition of 4 mL pancreatic concentrate [prepared by suspending 1 g of pancreatin powder in 5 mL of digestion buffer and vortex mixing for 15 minutes.59 For the collection of the supernatant of pH 6.5/5.8 having pancreatic lipase activity of 800 TBU/mL, ultracentrifugation (Remi, Mumbai) was conducted to digestion buffer that starts lipolysis, which was continued for the next 30 minutes. During lipolysis, the creation of unsaturated fats (FAs) brings about a rise in pH of media, NaOH solution of 0.2M was used for achieving constant pH of 6.8/5.8. The progress of drug discharge was observed by UV examination at 242 nm.60,61 The current technique was viewed as robust and assessed results were found to be reproducible by following this procedure.
Stability Study
50 capsules filled with SNEDDS (equivalent to 20.0 mg of rosuvastatin in each capsule) were kept in 60cc HDPE bottle. These bottles were put in an accelerated condition (40±2°C/75±5% RH) in the stability chamber (Thermolab, India) for six months subsequent to the sealing of these bottles. At periodic times, test samples were expelled and assessed for physical appearance, % drug discharge, and time of disintegration.62,63
Pharmacodynamic Investigation
It has been reported that rosuvastatin has dose-dependent pharmacodynamic impact and that is the reason for the execution of this in vivo investigation with a marketed tablet.
The ethical approval for this investigation of rosuvastatin SNEDDS was granted by Institutional Animal Ethical Committee (IAEC), Maharshi Dayanand University, India (Reg. no. 1767/RE/S/14/CPCSEA, vide reference no. 153-165 dated 14/12/2018). Male Wistar rats having weight 150–200 g were purchased from Lala Lajpat Rai University of Veterinary and Animal Sciences, Hisar. Animals were maintained as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPSEA), India.
All rats were set aside in plastic cage; six rats were accommodated in a single cage.
All rats were divided into 5 groups (total 30 rats; n=6 rat for each group), ie, control treatment group (CTG), placebo treatment group (PTG), reference treatment group (RTG), test (TTG) and marketed treatment group (MTG).
The impact of rosuvastatin SNEDDS formulation (TTG) on the lipidic profile was investigated by contrasting with rosuvastatin pure drug (RTG) and SNEDDS lacking rosuvastatin (PTG). Marketed, test, reference and placebo formulations were diluted with acacia solution (2.0%). Every group got a high-fat diet (mixture of dalda and coconut oil [3:2]) at a dose of 10 mL per kg per day body weight daily for a period of 4 weeks. TTG, RTG, MTG, and PTG were administered for 4 weeks with test, reference, marketed and placebo formulation, respectively. Test and reference formulations were administered through oral route at dose of 2 mg per kg per day of rosuvastatin. The blood samples were collected by a retro-orbital puncture method at a predetermined time interval, namely, before treatment and after 28 days in polo plain clot activator glass tubes.64 Ultracentrifugation was carried out for 10 min at 3000 rpm (Remi) for separation of serum and samples were analyzed for biochemical test such as CH, HDL and TGs level with in vitro diagnostic kits (Erba® Mannheim) and analyzer (Erba® with software Chem-5 Plus V2).65
For the determination of total cholesterol (CH), modified Roeschlau’s method was used. The method of Wako and the modification by McGowan and Fossati were used for the determination of triglycerides (TG). The method of Burstein was used for the determination of high-density lipoprotein (HDL).64 Total CH, TG VLDL and HDL (0 days and after 28 days) for each treatment group, ie, CTGs, PTGs, TTGs and RTGs were obtained.
The differences in the mean of various groups were analyzed by the implementation of one-way ANOVA with the Dunnet’s test in Graph Pad version 5.0 software. A p-value less than 0.05 is acceptable for statistical significance.
Result and Discussion
Investigation of Drug Solubility
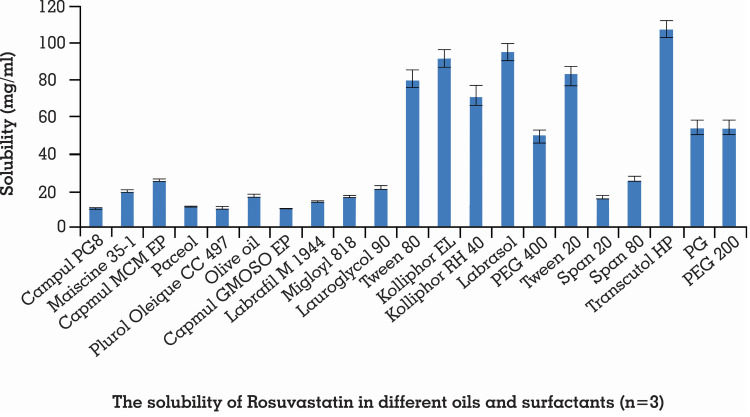
For assurance of stability of SNEDDS, solubility of the drug in excipients performs an important role since numerous formulations experience precipitation prior to encountering in situ solubilization. High drug solubilization is extremely important for expanding drug loading.66 Moreover, for development of rosuvastatin SNEDDS, its recommended sum ought to be soluble in its chosen ingredients with the least quantity of blend.67 The solubility of rosuvastatin in different excipients is demonstrated in Figure 1.
Figure 1.
Solubility of rosuvastatin in different oils and surfactants. Each value shows the mean ± SD (n=3).
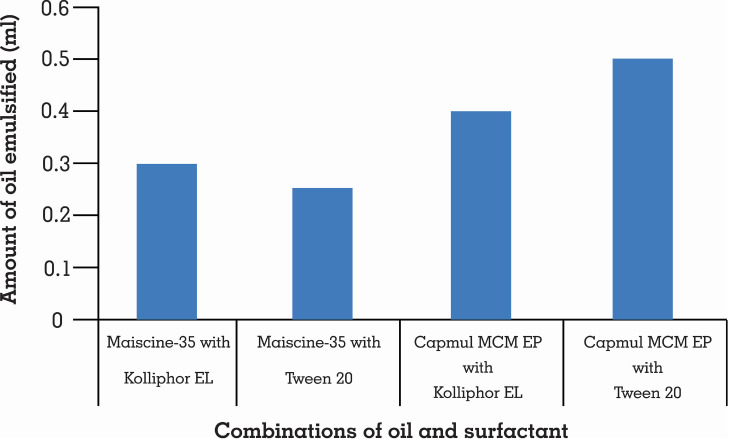
Self-emulsification relies upon the choice of an appropriate blend of excipients. This investigation demonstrated that Tween 20 with the highest amount of Capmul MCM EP had been emulsified, as demonstrated in Figure 2. That is the reason why Tween 20 and Capmul MCM EP combination was selected.
Figure 2.
Self-emulsification of different oils with selected surfactants (n=3).
Transcutol P indicated larger nano-emulsion area in contrast with Labraso,l as shown in Table 2. That is why it was chosen as a co-surfactant.
Table 2.
Nanoemulsion Region with Different Co-Surfactants
| %Oil | %Smix | Nanoemulsion Region | |
|---|---|---|---|
| Smix Tween 20: Transcutol P (1:1) | Smix Tween 20: Labrasol (1:1) | ||
| 10 | 90 | Yes | Yes |
| 20 | 80 | Yes | Yes |
| 30 | 70 | Yes | Yes |
| 40 | 60 | Yes | Yes |
| 50 | 50 | Yes | No |
| 60 | 40 | Yes | No |
| 70 | 30 | No | No |
| 80 | 20 | No | No |
| 90 | 10 | No | No |
The surfactant makes a coat around oil globules and decreases surface tension among the oily and aqueous phase. Further, their optimum quantity results in the improvement of the instant self-emulsification time. The rise in co-surfactant amount lessens the zone for development of emulsion, however, it has a negligible effect on decline in interfacial tension.68 A higher HLB value is important for making o/w type emulsion. These are utilized in rosuvastatin SNEDDS mostly to limit the surfactant quantity in the preparation.69 Transcutol P was mixed in preparation to expand the solubilization capacity of hydrophobic drugs.
Ternary Phase Diagram
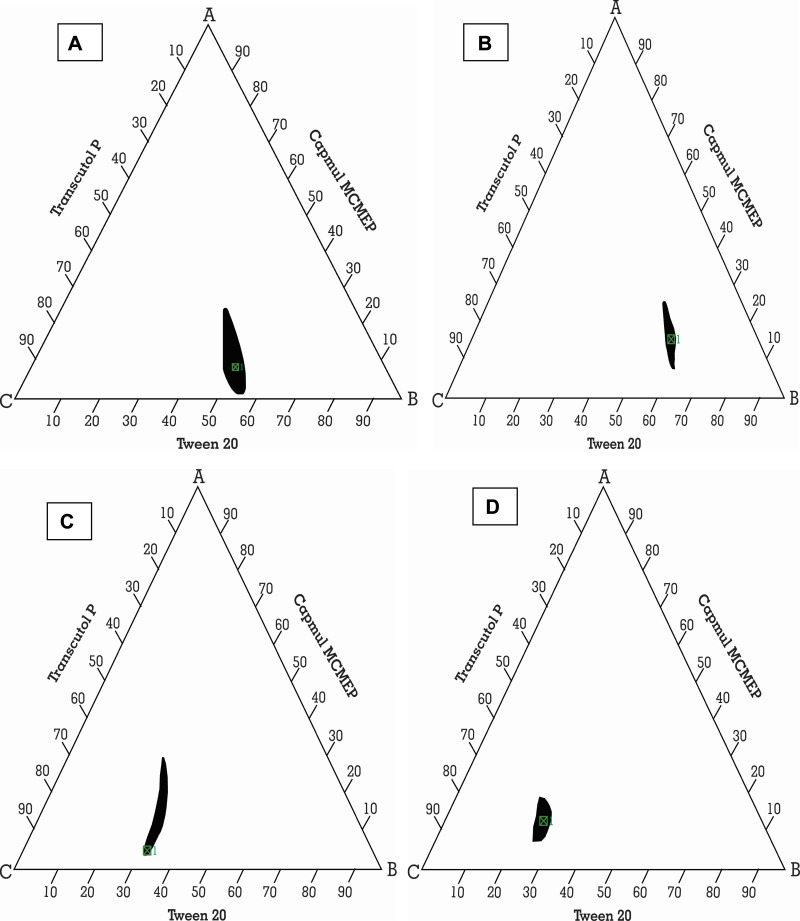
It was plotted to label self-nanoemulsifying area and select suitable concentrations of excipients for these formulations. This diagram plays a critical role in studying the phase behavior of developed nano-emulsions.70,71 It contains oil and Smix, where every vertex corresponds to 100% of those particular excipients. In Figure 3, the concealed zone introduced a transparent and lower viscosity nano-emulsion region in it.72
Figure 3.
Ternary phase diagram of Capmul MCMEP, tween 20 and transcutol P at Smix ratios (A) 1:1, (B) 2:1, (C) 1:2, and (D) 1:3. The colored region (black) represents nano-emulsion formation region.
It showed that a clear and transparent system is created when oil content is less than 20% (Figure S1). While biphasic systems are formed at higher concentration of oil and surfactants for development of clear nano-emulsion. However, rise in Tween 20 ratio in Smix (2:1) resulted in increase in nano-emulsion region. But, rise in concentration of Transcutol P (1:2 and 1:3) resulted in decline in nano-emulsion region. This may be due to creation of a layer around the oil globules by the Tween 20 that reduces the interfacial tension up to mark. But, Transcutol P had little impact on the interfacial tension. So, rise in Transcutol P concentration will not significantly reduce the interfacial tension and maintain the thermodynamic stability of the developed systems after the dilution.
D-Optimal Mixture Design for Optimization
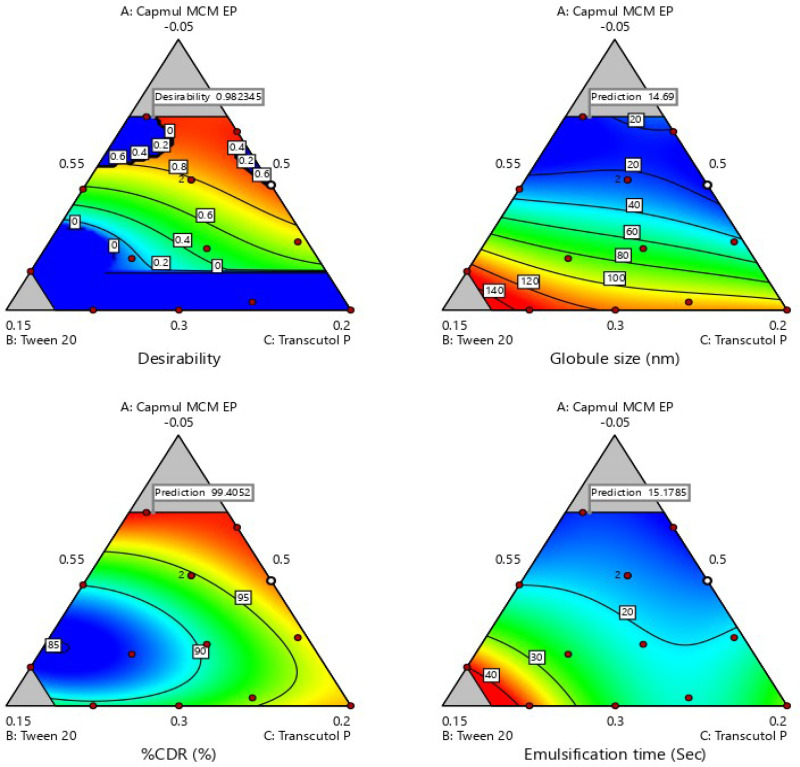
The effect of rosuvastatin SNEDDS factors (X1–X3) on globule size, %CDR and self-emulsification time was investigated utilizing the experimental design software. An optimized formula with the minimum globule size, maximum %CDR and minimum self-emulsification time was utilized. A mixture design was utilized for optimization of rosuvastatin SNEDDS with Design Expert® software. As exhibited in Table 3, 14 trial experiments were suggested with 2 center points. Y1 varied from 14.69–132.2 nm, Y2 from 86–99.5% and Y3 from 15–40 seconds. Equation 1, 2 and 3 explain the impact of various proportions of components on response Y1, Y2 and Y3.
Table 3.
Composition of Various SNEDDS Formulations Suggested by Design Expert® and Their Responses
| Formulation Code | Excipients Ratio | Y1 (nm) | Y2 (%) | Y3 (sec) | ||
|---|---|---|---|---|---|---|
| X1 (%) | X2 (%) | X3 (%) | ||||
| F1 | 0.05 | 0.4175 | 0.5325 | 17.89 | 98 | 16 |
| F2 | 0.14 | 0.5 | 0.36 | 14.69 | 99.5 | 15 |
| F3 | 0.3 | 0.24 | 0.46 | 130 | 91 | 38 |
| F4 | 0.28 | 0.40 | 0.32 | 104.7 | 94.5 | 22 |
| F5 | 0.3 | 0.5 | 0.2 | 114 | 97 | 30 |
| F6 | 0.13 | 0.42 | 0.45 | 23.56 | 94.5 | 18 |
| F7 | 0.21 | 0.49 | 0.30 | 43.41 | 96 | 21 |
| F8 | 0.23 | 0.31 | 0.46 | 87.2 | 87 | 26 |
| F9 | 0.13 | 0.42 | 0.45 | 24.24 | 95 | 18 |
| F10 | 0.14 | 0.31 | 0.55 | 28.07 | 90 | 20 |
| F11 | 0.3 | 0.33 | 0.37 | 125.2 | 92 | 25 |
| F12 | 0.22 | 0.39 | 0.39 | 63.94 | 90 | 20 |
| F13 | 0.06 | 0.5 | 0.44 | 15.89 | 99 | 14 |
| F14 | 0.25 | 0.2 | 0.55 | 132 | 86 | 40 |
Notes: Independent variable: X1 as oil percentage (Capmul MCM EP), X2 as surfactant percentage (Tween 20), and X3 as a co-surfactant percentage (Transcutol P). Dependent variables: globule size (Y1), %CDR (Y2) and self-emulsification time (Y3).
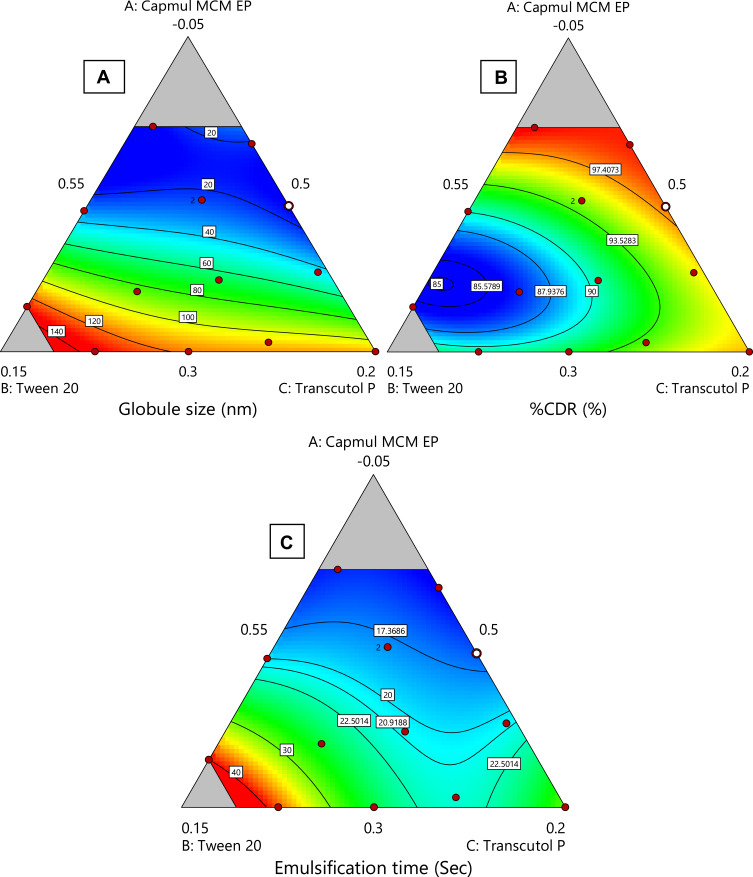
Contour plots (2-Dimensional) are shown in Figure 4 which elucidate effect of X1, X2 and X3 variables on Y1, Y2 and Y3 response. Response plots (3-Dimensional) which elucidate effect of X1, X2 and X3 variables on Y1, Y2 and Y3 response are shown in Figure S2.
Figure 4.
Various 2-D counter plots for response: (A) globule size, (B) % CDR, and (C) self-emulsification time.
It was found that globule size (Y1) increases with an increase in oil concentration and decreases the release rate of drug (Y2) and the time of self-emulsification (Y3) also rises. But, the rise in surfactant’s concentration resulted in a reduction of globule size and elevated release rate of drug and lessened time for self-emulsification. The raised concentration of Transcutol P resulted in an increase of the globule size because of inflow of a large number of Transcutol P molecules into the oily phase resulting in expansion of interfacial film and particle size.61 From 2-D and 3-D contours, it was summarized that a higher proportion of oily phase declined globule size significantly, while other excipients raised it up to a certain value in the formulation then it starts to increase. The same results were obtained for response Y2 and Y3. The result of ANOVA is given in Table 4.
Table 4.
Results of ANOVA
| Results of ANOVA | ||||||
|---|---|---|---|---|---|---|
| Response | Sum of Squares | df | Mean Square | F-value | p-value | Model |
| Y1 | 29,020.80 | 9 | 3224.53 | 89.87 | 0.0003 | Significant |
| Y2 | 239.80 | 9 | 26.64 | 114.41 | 0.0002 | Significant |
| Y3 | 841.79 | 9 | 93.53 | 328.64 | < 0.0001 | Significant |
The desirability index is employed for optimization of factors in a multi-response system that is based on transformation of all responses from various scales into a scale free value. Its values lie in the range of 0 and 1. Its value for SNEDDS was found to be 0.9832, as shown in Figure 5.
Figure 5.
Desirability index for optimized rosuvastatin-loaded SNEDDS.
This indicates that the model is significant. Three equations of regression (Equation S1, S2 and S3) and desirability index helped in the selection of the optimized formulation. From these two parameters, Formulation 2 (F-2) was found to be the optimized batch which contained rosuvastatin (20 mg), Capmul MCM EP (14%), Tween 20 (50%) and Transcutol P (36%) having a globule size of 14.69 nm, 99.5% dissolution within 15 minutes, and self-emulsification time of 15 seconds.
Characterization
DSC
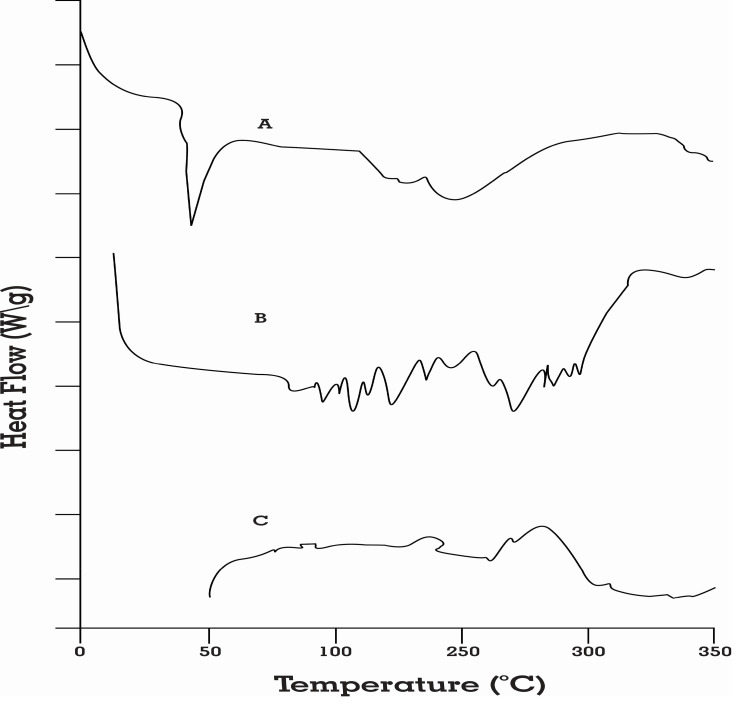
Thermodynamic methods were implemented for evaluating the thermal stress of pure drug and the excipients as well as their interactions during the development of formulation. DSC analysis confirmed the thermal changes of the formulations. Rosuvastatin had two endothermic peaks at 79.2°C and 131.6, as demonstrated in Figure 6A. Thermogram of physical mixture (rosuvastatin + Capmul MCM EP + Tween 20 + Transcutol P) showed a sharp endothermic peak at 131°C which confirms the transition of crystalline to liquid crystal form (Figure 6B). This might be the melting of rosuvastatin crystalline form change.31 The peaks of rosuvastatin and excipients disappeared in the DSC curve of optimized SNEDDS formulation due to the amorphous state (Figure 6C). These results indicated that rosuvastatin was completely dissolved in the blend of excipients and the interactions occurred among drug and excipients. The interactions between rosuvastatin and excipients contributed to entrapment of the rosuvastatin into the drug-loading system and development of rosuvastatin-loaded SNEDDS formulation.43
Figure 6.
DSC spectra of (A) pure rosuvastatin, (B) physical mixture of drug and excipients, and (C) optimized SNEDDS formulation.
FT-IR
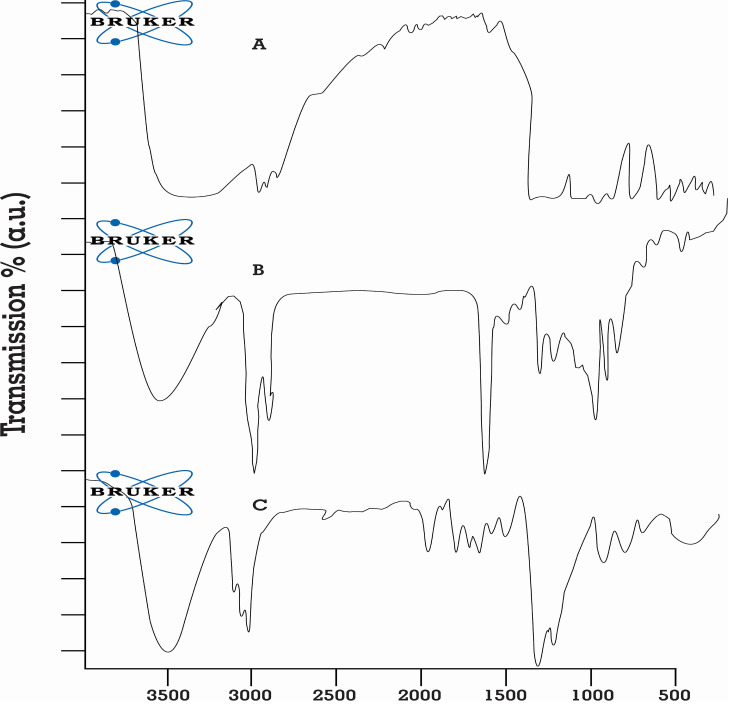
In FT-IR spectrum of the pure rosuvastatin, 3364.57 cm−1 was stretching vibration peak of aromatic N-H, 1174.19 cm−1 vibration peak of S=O, 965.20 cm−1 peak of aromatic C-H bending, 3392.14cm−1 was stretching vibration peak of carboxylic O-H, and 1547.59 cm−1 was stretching vibration peak of C=C, as shown in Figure 7A.
Figure 7.
FT-IR spectrum of (A) pure rosuvastatin, (B) physical mixture of drug and excipients, and (C) optimized SNEDDS formulation.
FT-IR spectrum of the physical mixture of rosuvastatin and excipients was in accordance with SNEDDS (Figures 7B and C), which was almost the superposition of FT-IR spectra of rosuvastatin and ingredients.
The main absorption peaks of rosuvastatin were observed in physical mixture and optimized SNEDDS formulation.40 In contrast, if specific interactions took place between drug and excipients, new peaks or a shift of existing peaks would appear. The overall FT-IR results proved no chemical interaction between drug and its excipients.52
% transmittance
It was estimated to assess the stability of SNEDDS formulation. It also offers some prediction for other formulation parameters such as uniformity and size of the globules. It was found to be 99.9±0.4% at 242 nm, which confirmed its transparency, reduced chances of drug precipitation, and improved solubilization limit subsequent to dispersion into buffering media.53
Test for Robustness Dilution
The creation of a homogeneous nano-emulsion from optimized SNEDDS is extremely important in different media since drug assimilation may be affected by in vivo precipitation. For this test, SNEDDS were dispersed in various media for simulating the in vivo milieu after 100 times dilution of this formulation. This formulation did not indicate drug precipitation, cloudiness or partition of phase even after 24 hours. This confirms that SNEDDS passed robustness dilution analysis. These results guaranteed the possibility of a consistent profile of drug release amid in vivo state.
Measurement of Viscosity
This parameter was evaluated with Brookfield hydromotion viscometer that resulted in 120±5 cps for optimized SNEDDS. This test was performed in triplicate. These results assure that this SNEDDS can be easily transferred or filled into capsule shells for their stockpiling.
Estimation of Cloud Point
It aids in looking at the effect of temperature on phase behavior of SNEDDS, which is one of the major problems identified with nano-emulsion, especially while utilizing non-ionic surfactants.
It is defined as the temperature above which SNEDDS clarity transforms into cloudiness. A perfect formulation ought to stay as a single phase with a clear solution at its stockpiling temperature and the temperature of its proposed use. It was a lot higher (65°C), demonstrating the stability of formulation at physiological temperature. At higher temperature, phase detachment can emerge on account of decreased solubility of surfactant in a fluid. It can decrease solubilization of drug and SNEDDS’ dependability because the cloud point ought to be more than 37°C.73
Evaluation of Drug Content
It was found to be 99.59±0.8% with UV spectrophotometer in optimized SNEDDS formulation that confirmed the dose accuracy of SNEDDS formulation.
Droplet Size, PDI, and Zeta Potential
Droplet size is one of the parameters that help in the evaluation of stability characteristics of nano-emulsion of SNEDDS. That results in enhanced retention of the drug and its better oral bioavailability. Its nano range size confirms larger interfacial surface zone for the absorption of the drug and upgraded bioavailability. Henceforth, its nano range may enable the efficient release of the drug. The droplet size of SNEDDS indicates that globules of the emulsion have nanometric range (14.69 nm) with PDI under 0.5, which demonstrates consistency in distribution of droplet size (Figure S3).74 The value of zeta potential was found to be - 4.09 mV. The obtained size of the formulations seems to be too small that kidney is capable of rapidly removing molecules from the vascular compartment mostly as the injected forms and therefore, renal excretion represents a desirable pathway for nano-emulsion removal with minimal catabolism or breakdown from the human body, to avoid the possible side effects.75
The stability of colloidal dispersion relies upon zeta potential value. For smaller droplets, its high value will confirm the stability of the formulation electrically since an increase in surface charge restricts the accumulation of droplets. At the point when potential is high, repulsion is more than attraction. This will result in a dispersed and deflocculated system which means the system will not break. In the current investigation, its value was reported to be negative because of the non-ionic surfactant that was present in the preparation. They make a negatively charged interface at neutral pH.76,77
Drug Entrapment Efficiency
EE is a vital indicator for examining the drug loading capacity of nano-pharmaceutics; the larger the value the better the drug loading capacity. The EE of rosuvastatin in optimized SNEDDS formulation was found to be 99.52%±0.86%.
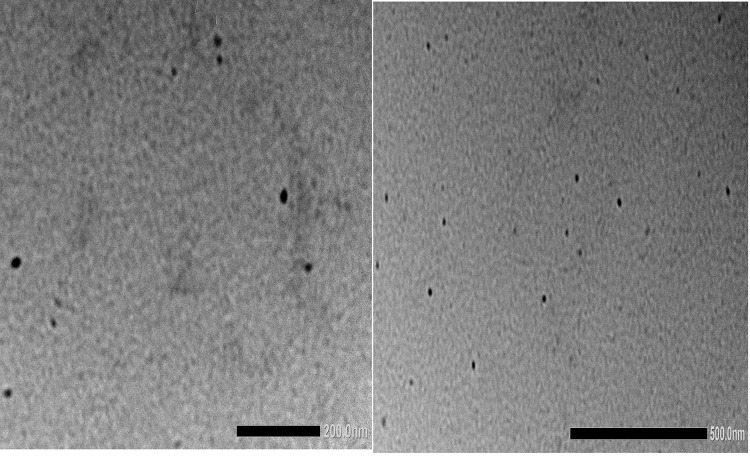
Surface Morphology Examined via Transmission Electron Microscopy
The microscopy of optimized SNEDDS formulation was examined using TEM micrographs. Transmission electron microscopy (TEM) revealed that optimized SNEDDS formulation exhibited a spherical shape structure with varying sizes of globules, as demonstrated in Figure 8. These results agreed with dynamic light scattering results, authenticating the achievability of SNEDDS formulation. The nanodroplets appeared dark, and the surroundings were found to be bright. Such findings prove the spontaneous formation of nanometer-range nanoemulsifying systems.78
Figure 8.
Transmission electron microscopy (TEM) of optimized SNEDDS formulation.
Dissolution (Multi-Media) Testing
The results of solubility study demonstrated good solubility of rosuvastatin in selected media (Table S1). Three dissolution media were used for the dissolution profile of optimized SNEDDS whose outcomes are depicted in Figure 9. From the results, it was inferred that drug release was > 80% in a short time in all media. However, a gentle decline or change in drug discharge was reported at pH 1.2 and 4.5. Overall SNEDDS formulation brought about amazingly enhanced drug discharge in multi-media dissolution testing.79 Drug release from SNEDDS was based on a simple diffusion process from a lipophilic liquid phase to an aqueous liquid phase.80
Figure 9.
Dissolution (multi-media) testing of rosuvastatin SNEDDS. Each value represents the mean ± SD (n=3).
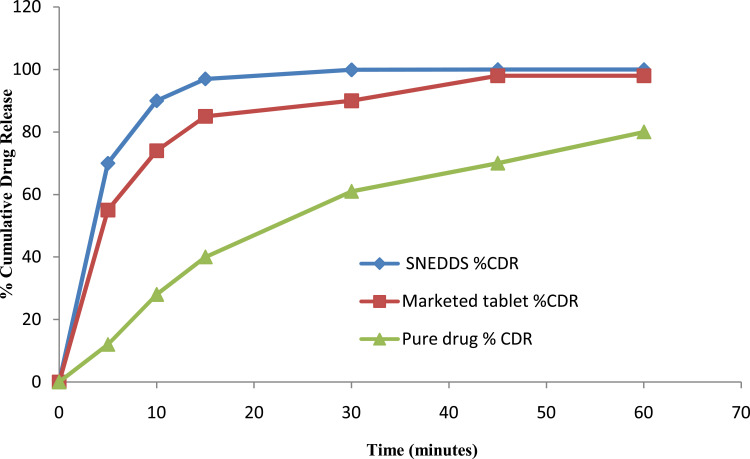
Comparative Investigation of SNEDDS with Pure Drug and Marketed Tablets in Terms of Dissolution Profile
This investigation was carried out with SNEDDS, marketed tablets, and pure drug. The reported results are summed up in Figure 10.
Figure 10.
Comparative dissolution study (pH 6.6 citrate buffer) of rosuvastatin-loaded SNEDDS, pure drug and marketed tablet. Each value represents the mean ± SD (n=3).
This investigation demonstrated that SNEDDS improved the dissolution rate of rosuvastatin up to 1.11 and 1.64 times compared to marketed tablets and pure drug respectively. These outcomes are identical or similar to results obtained by a number of other analysts/researchers. Alteration of the crystalline nature of the drug into an amorphous form is responsible for improved dissolution because this form is thermodynamically stable and SNEDDS offers conversion of solid-to-liquid phase easily. It is well recognized that the transformation of structure improves its disintegration pace to improve bioavailability.81 Another explanation is the presence of drug as a solubilized form inside nano-emulsion droplets and the suspending of drug in nanosized range in SNEDDS. All the results demonstrated quicker drug discharge than pure rosuvastatin in dissolution media.
Dynamic in vitro Lipolysis Study for Investigation of Food Impact
One of the most marvelous and insufficiently recognized capabilities of SNEDDS is that they “collaborate” with the content of GI that directly affects their potential. In the small intestine (SI), digestion of dietary TG is commonly very rapid and a variety of non-ionic esters behave as a substrate of pancreatic lipase or other esterase. This procedure helps in scattering of the drug in the presence of BS/PLs from formulation and improves their assimilation. Thus, assessment of the digestion of lipids can be crucial since they speculate the possibility of formulation for precipitation of drug in SI.
Changing the limit of solubilization that emerged all through this procedure was of incredible importance to assess food impact by methods of in vitro lipolysis.62 During this study, it was very important to establish whether there was some possibility of precipitation of drug within 30 minutes. The fasted condition outcomes authenticated that rosuvastatin existed in the solubilized form in SNEDDS that resulted in about 98.32±0.05% drug discharge. Similar results were reported under fed condition (98.52±0.05%) which indicates that SNEDDS formulation was capable of maintaining a solubilized form of rosuvastatin which is important for retention of the drug.
In this way, SNEDDS maintained a strategic distance from food impact in terms of drug release, which has been stated in various works of literature, and was found to be a correct hypothesis for SNEDDS. This proposes that SNEDDS conquered the impact of food on the release of drug. Along these lines, SNEDDS can improve patient compliance, particularly in patients who are not capable of consuming their drug with meals.
Stability Study
It was found that SNEDDS formulation was physically and chemically stable during six months of accelerated stability study at 40°C/75%RH. Results of the stability study for rosuvastatin SNEDDS are summarized in Table 5.
Table 5.
Stability Data of Optimized Rosuvastatin SNEDDS Formulation (n=6)
| Test Parameters | Initial | 40°C/75% RH/1M | 40°C/75% RH/3M | 40°C/75% RH/6M |
|---|---|---|---|---|
| Description | Whitish colored capsules containing clear liquid | Whitish colored capsules containing clear liquid | Whitish colored capsules containing clear liquid | Whitish colored capsules containing clear liquid |
| % CDR | 98.9±1.2 | 95.7±2.1 | 96.9±0.7 | 99.8±1.0 |
| Disintegration time | 5±0.5 min | 5±0.3 min | 6±1 min | 6±0.2 min |
Notes: %CDR-Percentage of cumulative drug release. Data are presented as the mean ± SD.
From these data, it was concluded that there was no considerable difference in rosuvastatin SNEDDS after six months of the accelerated stability study. Hence, it was inferred that rosuvastatin SNEDDS passed stability study.
Pharmacodynamic Studies
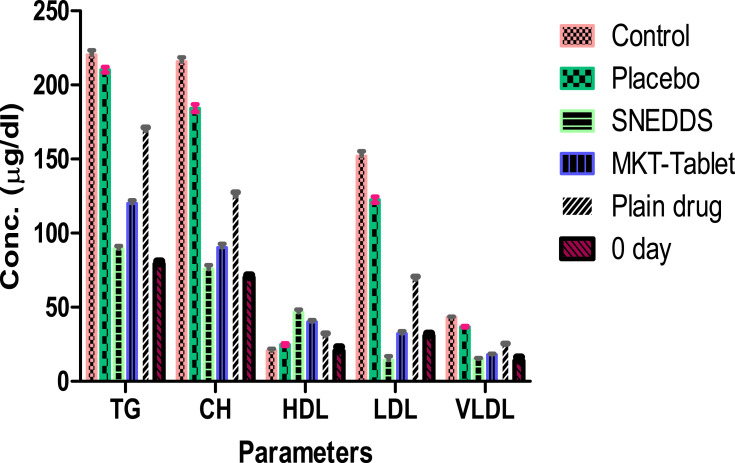
This investigation was conducted with rosuvastatin SNEDDS (F-2), marketed tablets and pure drug. The total CH, TG, and HDL results for each group are summarized in Table 6 and Figure 11. This study demonstrated a considerable variation in the percentage of parameters of CTG, PTG (no drug), MTG (rosuvastatin tablet 20 mg), RTG (pure drug) and TT (SNEDDS) group (p < 0.001).
Table 6.
Lipid Profile in Experimental Animals with Mean± Std. Deviation (n=6)
| Parameters | 0 Day | Control | Placebo | SNEDDS | Marketed Tablet | Pure drug |
|---|---|---|---|---|---|---|
| CH (µg/dl) | 70±1.8 | 215.7±2.88 | 205.3±2.58 | 75.83±2.64***$^^^ | 90.33±2.58**^^ | 125.2±2.48* |
| HDL (µg/dl) | 18.8±2.7 | 20.67±1.37 | 24.83±0.98 | 46.50±1.87***$^^^ | 40.00±1.41**^^ | 31.17±1.17* |
| LDL (µg/dl) | 30.7±2.7 | 151.9±3.4 | 122.6±2.12 | 14.17±2.85***$^^^ | 32.27±1.66***^^ | 68.97±1.86* |
| TG (µg/dl) | 79.3±1.9 | 220.3±3.14 | 210.3±1.86 | 89.50±1.87***$^^^ | 120.0±2.10**^^ | 169.5±1.87* |
| VLDL(µg/dl) | 13.9±2.5 | 43.13±0.58 | 36.87±0.52 | 15.17±0.53***$^^^ | 18.07±0.52***^^ | 25.03±0.50* |
Notes: Data are presented as the mean ± SD. ***p<0.001 (highly significant); **p<0.01 (significant); *p<0.05 (less significant) (*as compared to control). $p<0.01; ($ as compared to marketed tablet). ^^^p<0.001; ^^p<0.01; (^ as compared to pure drug). p<0.001 (highly significant); p<0.01 (significant); p<0.05 (less significant).
Abbreviations: CH, cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; VLDL, very low density lipoprotein.
Figure 11.
Results of comparative lipid profile testing. Each value represents the mean ± SD (n=6).
After the administration of a diet with a high quantity of fat for 28 days, all the groups showed a significant elevation of lipid content in the serum which indicates a hyperlipidemic condition in rats. After treatment of TTG, this group’s results showed remarkable variation in lipidic contents of the serum including CH, TG, HDL, LDL and VLDL as compared to the PTG, MTG and RTG as delineated in Figure 11. All the groups showed initiation of their pharmacodynamic impacts in fluctuating lipid content level in the serum after 28 days of the treatment (p<0.001).
It was summed up that TTG decreased the CH content level in serum more significantly in contrast to PTG, MTG and RTG as compared to CTG. A similar pattern was obtained for TG, LDL and VLDL contents. The percent expansion in HDL content level in serum of TTG was significantly more than PTG (p<0.001), MTG (p<0.01) and RTG (p<0.05) as compared to CTG.
This study established that the drug was highly effective when it was developed as a formulation of SNEDDS. This confirms that they were able to improve the therapeutic efficiency of rosuvastatin in contrast to the suspension of marketed tablets and pure drug. SNEDDS had a considerable impact on the lipid profile of rats after the experiment. Therefore, all these results confirmed that the tested formulation had superior in vivo potential compared to pure drug suspension in terms of pharmacodynamic response.
Conclusion
In this research, we developed SNEDDS with thermal stability in tropical conditions, capable of improving %CDR and in vivo pharmacodynamic potential of rosuvastatin as compared to pure drug and marketed tablets.82 DoE methodology was employed for the development of SNEDDS by the selection of an optimum concentration of excipients. It was illustrated that this type of formulation had a rapid rate of dissolution in contrast to marketed tablets and pure drug because of nano-range of droplet size and negative charge of zeta potential for this formulation. This will result in an expansion of surface area for greater drug release. After in vitro lipolysis study, it was concluded that there was no significant variation in the concentration of solubilized rosuvastatin under fasted and fed state. All the physicochemical evaluations were conducted in biorelevant media to better predict in vivo behavior. From this, it can be concluded that SNEDDS are able to avoid food’s effect in terms of drug release. SNEDDS also improved pharmacodynamics in the male rat compared to the suspension form of marketed tablets and pure drug. That is why SNEDDS was established: for the considerable improvement of bioavailability. This investigation involved the potential of SNEDDS for enhanced drug release profile, less food effect and better pharmacodynamics of the drug. All these outcomes suggest that SNEDSDS is an alternative approach for the delivery of rosuvastatin with superior bioavailability, patient compliance, and the least side effects.
Acknowledgment
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2020/96), King Saud University, Riyadh, Saudi Arabia.
Abbreviations
BCS, biopharmaceutical classification system; CH, cholesterol; CTG, control treatment group; DoE, design of experiment; Fa/FeSSIF V-2, fasted/fed state simulated intestinal fluid V-2; HDL, high-density lipoprotein; MTG, marketed treatment group; NaCl, sodium chloride; o/w, oil in water; PDI, polydispersity index; PLs, phospholipids; PTG, placebo treatment group; RTG, reference treatment group; SI, small intestine; SNEDDS, self-nanoemulsifying drug delivery system; TG, triglyceride; TTG, test treatment group.
Ethical Approval and Consent to Participate
This investigation was approved by IAEC under registration no. 1767/RE/S/14/CPCSEA; vide reference no. 153-165 dated 14/12/2018.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest.
References
- 1.Hwang Y-C. Response: comparison of the Efficacy of Rosuvastatin Monotherapy 20 mg with Rosuvastatin 5 mg and Ezetimibe 10 mg Combination Therapy on Lipid Parameters in Patients with Type 2 Diabetes Mellitus (Diabetes Metab J 2019;43:582–9). Diabetes Metab J. 2019;43(6):915–916. doi: 10.4093/dmj.2019.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alshora DH, Ibrahim MA, Elzayat E, Almeanazel OT, Alanazi F, Mukherjee A. Rosuvastatin calcium nanoparticles: improving bioavailability by formulation and stabilization codesign. PLoS One. 2018;13(7):e0200218. doi: 10.1371/journal.pone.0200218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YJ, Cha SH, Kim H, Choi SE, Cho S, Park Y. Diallyl disulphide-loaded spherical gold nanoparticles and acorn-like silver nanoparticles synthesised using onion extract: catalytic activity and cytotoxicity. Artif Cells Nanomed Biotechnol. 2020;48(1):948–960. doi: 10.1080/21691401.2020.1773485 [DOI] [PubMed] [Google Scholar]
- 4.Vashist A, Atluri V, Raymond A, et al. Development of multifunctional biopolymeric auto-fluorescent micro- and nanogels as a platform for biomedical applications. Front Bioeng Biotechnol. 2020;8:315. doi: 10.3389/fbioe.2020.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair M, Jayant RD, Kaushik A, Sagar V. Getting into the brain: potential of nanotechnology in the management of NeuroAIDS. Adv Drug Deliv Rev. 2016;103:202–217. doi: 10.1016/j.addr.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaushik A, Jayant RD, Bhardwaj V, Nair M. Personalized nanomedicine for CNS diseases. Drug Discov Today. 2018;23(5):1007–1015. doi: 10.1016/j.drudis.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colone M, Calcabrini A, Stringaro A. Drug delivery systems of natural products in oncology. Molecules. 2020;25(19):E4560. doi: 10.3390/molecules25194560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pal S, Nagy S, Bozo T, Kocsis B, Devay A. Technological and biopharmaceutical optimization of nystatin release from a multiparticulate based bioadhesive drug delivery system. Eur J Pharm Sci. 2013;49:258–264. [DOI] [PubMed] [Google Scholar]
- 9.Vashist A, Kaushik A, Vashist A, et al. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov Today. 2018;23(7):1436–1443. doi: 10.1016/j.drudis.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaushik A, Jayant RD, Sagar V, Nair M. The potential of magneto-electric nanocarriers for drug delivery. Expert Opin Drug Deliv. 2014;11(10):1635–1646. doi: 10.1517/17425247.2014.933803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas N, Holm R, Garmer M, Karlsson JJ, Mullertz A, Rades T. Supersaturated self-nanoemulsifying drug delivery systems (Super-SNEDDS) enhance the bioavailability of the poorly water-soluble drug simvastatin in dogs. AAPS J. 2013;15:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu CS, Chen L, Hu YN, et al. Self-microemulsifying drug delivery system for improved oral delivery and hypnotic efficacy of ferulic acid. Int J Nanomedicine. 2020;15:2059–2070. doi: 10.2147/IJN.S240449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gahlawat N, Verma R, Kaushik D. Recent developments in self-microemulsifying drug delivery system: an overview. Asian J Pharm. 2019;13(2):59–72. [Google Scholar]
- 14.Liu H, Mei J, Xu Y, et al. Improving The oral absorption of nintedanib by a self-microemulsion drug delivery system: preparation and in vitro/in vivo evaluation. Int J Nanomedicine. 2019;14:8739–8751. doi: 10.2147/IJN.S224044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suhair S, Al-Nimry A, Khouloud A, Bashar M. Solid self-nanoemulsifying drug delivery system filled in enteric coated hard gelatin capsules for enhancing solubility and stability of omeprazole hydrochloride. Pharm Dev Technol. 2020;25(5):588–600. doi: 10.1080/10837450.2020.1721536 [DOI] [PubMed] [Google Scholar]
- 16.Zeng L, Zhang YL. Development, optimization and in vitro evaluation of norcantharidin loaded self-nanoemulsifying drug delivery systems (NCTD-SNEDDS). Dev Technol. 2016;22(3):1–35. doi: 10.1080/10837450.2016.1219915 [DOI] [PubMed] [Google Scholar]
- 17.Kalantari A, Kósa D, Nemes D, Ujhelyi Z, Fehér F, Vecsernyés M. Self-nanoemulsifying drug delivery systems containing Plantago lanceolata-An assessment of their antioxidant and anti-inflammatory effects. Molecules. 2017;22(10):1773. doi: 10.3390/molecules22101773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alhasani KF, Kazi M, Ibrahim MA, Shahba AA, Alanazi FK. Self-nanoemulsifying ramipril tablets: A novel delivery system for the enhancement of drug dissolution and stability. Int J Nanomedicine. 2019;14:5435–5448. doi: 10.2147/IJNS203311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patki M, Giusto K, Gorasiya S, Reznik SE, Patel K. 17-α hydroxyprogesterone nanoemulsifying preconcentrate-loaded vaginal tablet: A novel non-invasive approach for the prevention of preterm birth. Pharmaceutics. 2019;11(7):335. doi: 10.3390/pharmaceutics11070335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alskär LC, Keemink J, Johannesson J. Impact of drug physicochemical properties on lipolysis-triggered drug supersaturation and precipitation from lipid-based formulations. Mol Pharm. 2018;15(10):4733–4744. doi: 10.1021/acs.molpharmaceut.8b00699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–2841. doi: 10.1039/c5sm02958a [DOI] [PubMed] [Google Scholar]
- 22.Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opinion in Colloid Interface Sci. 2005;10(3–4):102–110. doi: 10.1016/j.cocis.2005.06.004 [DOI] [Google Scholar]
- 23.Bazylińska U, Saczko J. Nanoemulsion-templated polylelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surf B Biointerfaces. 2016;137:191–202. doi: 10.1016/j.colsurfb.2015.07.056 [DOI] [PubMed] [Google Scholar]
- 24.Heshmati N, Cheng X, Eisenbrand G, Fricker G. Enhancement of oral bioavailability of E804 by self-nanoemulsifying drug delivery system (SNEDDS) in rats. J Pharm Sci. 2013;102:3792–3799. [DOI] [PubMed] [Google Scholar]
- 25.Gué E, Since M, Ropars S, Herbinet R, Pluart L, Malzert-Fréon A. Evaluation of the versatile character of a nanoemulsion formulation. Int J Pharm. 2016;498(1–2):49–65. [DOI] [PubMed] [Google Scholar]
- 26.Gamal W, Fahmy RH, Mohamed MI. Development of novel amisulpride-loaded liquid self-nanoemulsifying drug delivery systems via dual tackling of its solubility and intestinal permeability. Drug Dev Ind Pharm. 2017;43(9):1530–1548. [DOI] [PubMed] [Google Scholar]
- 27.ElShagea HN, ElKasabgy NA, Fahmy RH, Basalious EB. Freeze-dried self nanoemulsifying self-nanosuspension (SNESNS): A new approach for the preparation of a highly drug-loaded dosage form. AAPS PharmSciTech. 2019;20:258. doi: 10.1208/s12249-019-1472-2 [DOI] [PubMed] [Google Scholar]
- 28.Bharti D, Pandey P, Verma R, Kaushik D. Development and characterization of rosuvastatin loaded self-emulsifying drug delivery system. Appl Clinical Res Clinical Trials Reg Affairs. 2018;5:1–8. [Google Scholar]
- 29.Verma R, Mittal V, Kaushik D. Quality based design approach for improving oral bioavailability of valsartan loaded SMEDDS and study of impact of lipolysis on the drug diffusion. Drug Deliv Letters. 2018;8(2):130–139. [Google Scholar]
- 30.Lee JH, Kim HY, Cho YH, Koo TS, Lee GW. Development and evaluation of raloxifene-hydrochloride-loaded supersaturatable SMEDDS containing an acidifier. Pharmaceutics. 2018;10(78):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panigrahi KC, Patra N, Rao MEB. Quality by design enabled development of oral self-nanoemulsifying drug delivery system of a novel calcimimetic cinacalcet HCl using a porous carrier: in vitro and in vivo characterization. AAPS PharmSciTech. 2019;20:216. doi: 10.1208/s12249-019-1411-2 [DOI] [PubMed] [Google Scholar]
- 32.Johnson B, Nornoo AO, Zheng H, Lopes LB, Johnson-Restrepo B, Kannan K. Oral microemulsions of paclitaxel: in situ and pharmacokinetic studies. Eur J Pharm Biopharm. 2008;71(2):310–317. [DOI] [PubMed] [Google Scholar]
- 33.Patel MH, Mundada VP, Sawant KK. Novel drug delivery approach via self-microemulsifying drug delivery system for enhancing oral bioavailability of asenapine maleate: optimization, characterization, cell uptake, and in vivo pharmacokinetic studies. AAPS PharmSciTech. 2019;20(44):1–8. doi: 10.1208/s12249-018-1212-z [DOI] [PubMed] [Google Scholar]
- 34.Kim RM, Jang DJ, Kim YC, Yoon JH, Min KA. Flurbiprofen-loaded solid SNEDDS preconcentrate for the enhanced solubility, in vitro dissolution and bioavailability in rats. Pharmaceutics. 2018;10(4):247. doi: 10.3390/pharmaceutics10040247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Son HY, Chae BR, Choi JY, Shin DJ, Goo YT, Lee ES. Optimization of self-microemulsifying drug delivery system for phospholipid complex of telmisartan using D-optimal mixture design. PLoS One. 2018;13(12):1–17. doi: 10.1371/journal.pone.0208339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mura P, Furlanetto S, Cirri M, Maestrelli F, Marras AM, Pinzauti S. Optimization of glibenclamide tablet composition through the combined use of differential scanning calorimetry and D-optimal mixture experimental design. J Pharm Biomed Anal. 2005;37(1):65–71. doi: 10.1016/j.jpba.2004.09.047 [DOI] [PubMed] [Google Scholar]
- 37.Hosny KM, Aldawsari HM, Bahmdan RH, et al. Preparation, optimization, and evaluation of hyaluronic acid-based hydrogel loaded with miconazole self-nanoemulsion for the treatment of oral thrush. AAPS PharmSciTech. 2019;20(297):1–12. doi: 10.1208/s12249-019-1496-7 [DOI] [PubMed] [Google Scholar]
- 38.Eleftheriadis GK, Mantelou P, Karavasili C, Chatzopoulou P, Katsantonis D, Irakli M. Development and characterization of a self-nanoemulsifying drug delivery system comprised of rice bran oil for poorly soluble drugs. AAPS PharmSciTech. 2019;20:78. doi: 10.1208/s12249-018-1274-y [DOI] [PubMed] [Google Scholar]
- 39.Usfda. Available from: https://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_Search%20Results.cfm. Accessed January08, 2021.
- 40.Rangaraj N, Shah S, Maruthi AJ, et al. Quality by design approach for the development of self-emulsifying systems for oral delivery of febuxostat: pharmacokinetic and pharmacodynamic evaluation. AAPS PharmSciTech. 2019;20:267. doi: 10.1208/s12249-019-1476-y [DOI] [PubMed] [Google Scholar]
- 41.Rani R, Dahiya S, Dhingra D, et al. Antidiabetic activity enhancement in streptozotocin + nicotinamide-induced diabetic rats through combinational polymeric nanoformulation. Int J Nanomedicine. 2019;14:4383–4395. doi: 10.2147/IJN.S205319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alshehri S, Imam SS, Hussain A, et al. Flufenamic acid-loaded self-nanoemulsifying drug delivery system for oral delivery: from formulation statistical optimization to preclinical anti-inflammatory assessment. J Oleo Sci. 2020;69(10):1257–1271. doi: 10.5650/jos.ess20070 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Wen X, Dai Y, Xia Y. Mechanistic studies on the absorption enhancement of a self-nanoemulsifying drug delivery system loaded with norisoboldine-phospholipid complex. Int J Nanomedicine. 2019;14:7095–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahsan MN, Verma PR. Solidified self nano-emulsifying drug delivery system of rosuvastatin calcium to treat diet-induced hyperlipidemia in rat: in vitro and in vivo evaluations. Ther Deliv. 2017;8(3):125–136. doi: 10.4155/tde-2016-0071 [DOI] [PubMed] [Google Scholar]
- 45.Liao H, Gao Y, Lian C, et al. Oral absorption and lymphatic transport of baicalein following drug-phospholipid complex incorporation in self-microemulsifying drug delivery systems. Int J Nanomedicine. 2019;14:7291–7306. doi: 10.2147/IJN.S214883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu L, Qiao Y, Wang L, Guo J, Wang G, He W. A self-microemulsifying drug delivery system (SMEDDS) for a novel medicative compound against depression: a preparation and bioavailability study in rats. AAPS PharmSciTech. 2015;16(5):1051–1058. doi: 10.1208/s12249-014-0280-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoshtari S, Wen J, Alany RG. Formulation and physicochemical characterization of imwitor 308 based self microemulsifying drug delivery systems. Chem Pharm Bull. 2010;58:1332–1338. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal AG, Kumar A, Gide PS. Formulation of solid self-nanoemulsifying drug delivery systems using N-methyl pyrrolidone as cosolvent. Drug Dev Ind Pharm. 2015;41:594–604. [DOI] [PubMed] [Google Scholar]
- 49.Baloch J, Sohail MF, Sarwar HS, Kiani MH, Khan GM, Jahan S. Self-nanoemulsifying drug delivery system (SNEDDS) for improved oral bioavailability of chlorpromazine: in vitro and in vivo evaluation. Medicina. 2019;55(5):210. doi: 10.3390/medicina55050210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bang SP, Yeon CY, Adhikari N, Neupane S, Kim H, Lee DC. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS One. 2019;14(11):e0224805. doi: 10.1371/journal.pone.0224805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandey P, Ghimire G, Garcia J, et al. Single-entity approach to investigate surface charge enhancement in magnetoelectric nanoparticles induced by AC magnetic field stimulation. ACS Sens. 2020. doi: 10.1021/acssensors.0c00664 [DOI] [PubMed] [Google Scholar]
- 52.Ke Z, Hou X, Jia XB. Design and optimization of self-nanoemulsifying drug delivery systems for improved bioavailability of cyclovirobuxine D. Drug Des Devel Ther. 2016;10:2049–2060. doi: 10.2147/DDDT.S106356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alwadei M, Kazi M, Alanazi FK. Novel oral dosage regimen based on self-nanoemulsifying drug delivery systems for codelivery of phytochemicals – curcumin and thymoquinone. Saudi Pharm J. 2019;27:866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Enin HA. Self-nanoemulsifying drug-delivery system for improved oral bioavailability of rosuvastatin using natural oil antihyperlipdemic. Drug Dev Ind Pharm. 2015;41(7):1047–1056. doi: 10.3109/03639045.2014.983113 [DOI] [PubMed] [Google Scholar]
- 55.Jakab G, Fülöp V, Bozó T, Balogh E, Kellermayer N, Antal I. Optimization of quality attributes and atomic force microscopy imaging of reconstituted nanodroplets in baicalin loaded self-nanoemulsifying formulations. Pharmaceutics. 2018;10(4):275. doi: 10.3390/pharmaceutics [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohsin K. Design of lipid-based formulations for oral administration of poorly water-soluble drug fenofibrate: effects of digestion. AAPS PharmSciTech. 2012;13:637–646. doi: 10.1208/s12249-012-9787-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verma R, Kaushik D. Design and optimization of candesartan loaded self-nanoemulsifying drug delivery system for improving its dissolution rate and pharmacodynamic potential. Drug Deliv. 2020;1:756–771. doi: 10.1080/10717544.2020.1760961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosgaard MD, Sassene P, Rades T, Müllertz A. Development of a high-throughput in vitro lipolysis model for rapid screening lipid-based drug delivery systems. Eur J Pharm Biopharm. 2015;94:493–500. [DOI] [PubMed] [Google Scholar]
- 59.Xiao L, Yi T, Liu Y, Zhou Z. The in vitro lipolysis of lipid-based drug delivery systems: A newly identified relationship between drug release and liquid crystalline phase. Bio Med Res Int. 2016;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sassene P, Karen K, Williams HD, Mullertz A. Toward establishment of standardized in vitro tests for LbDDSs, Part 6: effect of varying pancreatin and calcium levels. Am Assoc Pharma Soci J. 2014;16(6):1344–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams HD, Anby MU, Sassene P, Kleberg K, Mullertz A, Porter JH. Toward the establishment of standardized in vitro tests for lipid-based formulations, Part 2. The effect of bile salt concentration and drug loading on the performance of type I, II, IIIA, IIIB, and IV formulations during in vitro digestion. Mol Pharm. 2012;101(9):3286–3300. [DOI] [PubMed] [Google Scholar]
- 62.Alshamsan A, Kazi M, Badran MM, Alanazi FK. Role of alternative lipid excipients in the design of self-nanoemulsifying formulations for fenofibrate: characterization, in vitro dispersion, digestion and ex vivo gut permeation studies. Front Pharmacol. 2018;9:1219. doi: 10.3389/fphar.2018.01219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Izham MHM, Hussin Y, Aziz MNM, Yeap SK, Rahman HS, Masarudin MJ. Preparation and characterization of self nano-emulsifying drug delivery system loaded with citral and its antiproliferative effect on colorectal cells in vitro. Nanomaterials. 2019;9(1028):1–18. doi: 10.3390/nano9071028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar A, Nanda A. Design and optimization of simvastatin self-microemulsifying drug delivery system for enhanced therapeutic potential. Asian J Pharm. 2018;12(1):S159–S165. [Google Scholar]
- 65.Borhade V, Nair H, Hegde D. Design and evaluation of self-microemulsifying drug delivery system (SMEDDS) of tacrolimus. AAPS Pharm Sci Tech. 2008;9:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parmar N, Singla N, Amin S, Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self-nanoemulsifying drug delivery system. Colloids Surf B: Biointerfaces. 2011;86:327–338.21550214 [Google Scholar]
- 67.Qi X, Wang L, Zhu J, Hu Z, Zhang J. Self-double-emulsifying drug delivery system (SDEDDS): A new way for oral delivery of drugs with high solubility and low permeability. Int J Pharm. 2011;409:245–251. [DOI] [PubMed] [Google Scholar]
- 68.Nepal PR, Han HK, Choi HK. Preparation and in vitro–in vivo evaluation of Witepsol H35 based self-nanoemulsifying drug delivery systems (SNEDDS) of coenzyme Q (10). Eur J Pharm Sci. 2010;39:224–232. [DOI] [PubMed] [Google Scholar]
- 69.Zhao Y, Wang C, Chow AH, Ren K, Gong T, Zhang Z. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of zedoary essential oil: formulation and bioavailability studies. Int J Pharm. 2010;383:170–177. [DOI] [PubMed] [Google Scholar]
- 70.Balakumar K, Raghavan CV, Selvan NT, Prasad RH, Abdu S. Self-nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B: Biointerfaces. 2013;112:337–343. [DOI] [PubMed] [Google Scholar]
- 71.Eltobshi AA, Mohamed EA, Abdelghani GM, Nouh AT. Self-nanoemulsifying drug-delivery systems for potentiated anti-inflammatory activity of diacerein. Int J Nanomedicine. 2018;13:6585–6602. doi: 10.2147/IJN.S178819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaudhri N. Formulation of amisulpride loaded nanoemulsion drug delivery system for the treatment of Schizophrenia. J Biomed Pharm Res. 2015;4(6):1–10. [Google Scholar]
- 73.Choi KO, Aditya NP, Ko S. Effect of aqueous pH and electrolyte concentration on structure, stability and flow behavior of non-ionic surfactant based solid lipid nanoparticles. Food Chem. 2014;147:239–244. [DOI] [PubMed] [Google Scholar]
- 74.Shakeel F, Haq N, Elbadry M, Alanazi FK, Alsarra IA. Ultra fine super self-nanoemulsifying drug delivery system (SNEDDS) enhanced solubility and dissolution of indomethacin. J Mol Liq. 2013;180:89–94. [Google Scholar]
- 75.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine. 2008;3(5):703–717. doi: 10.2217/17435889.3.5.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Sun C, Hao Y, Jiang T, Zheng L, Wang S. Mechanism of dissolution enhancement and bioavailability of poorly water soluble celecoxib by preparing stable amorphous nanoparticles. J Pharm Pharm Sci. 2010;13(4):589–606. doi: 10.18433/J3530J [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Zhi Z, Jiang T, Zhang J, Wang Z, Wang S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J Contr Release. 2010;145(3):257–263. [DOI] [PubMed] [Google Scholar]
- 78.Bello V, Mattei G, Mazzoldi P, et al. Transmission electron microscopy of lipid vesicles for drug delivery: comparison between positive and negative staining. Microsc Microanal. 2010;16(4):456–461. doi: 10.1017/S1431927610093645 [DOI] [PubMed] [Google Scholar]
- 79.Verma R, Kaushik D. Development, optimization, characterization and impact of in vitro lipolysis on drug release of telmisartan loaded SMEDDS. Drug Deliv Letters. 2019;9(9):330–340. doi: 10.2174/2210303109666190614120556 [DOI] [Google Scholar]
- 80.Bernkop-Schnürch A, Jalil A. Do drug release studies from SEDDS make any sense? J Control Release. 2018;271:55–59. doi: 10.1016/j.jconrel.2017.12.027 [DOI] [PubMed] [Google Scholar]
- 81.Kassem AA, Mohsen AM, Ahmed RS, Essam TM. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: design, optimization, in vitro and in vivo evaluation. J Mol Liquids. 2016;218(2):219–232. [Google Scholar]
- 82.Lindsay S, Dolores S, Marion M, Francisco BF, Auxiliadora D, Aikaterini L. Orally bioavailable and effective buparvaquone lipid based nanomedicines for visceral leishmaniasis. Mol Pharmaceutics. 2018;15:2570–2583. doi: 10.1021/acs.molpharmaceut.8b00097 [DOI] [PubMed] [Google Scholar]