Abstract
Background & objectives:
Medicinal plants like Swertia chirata are rich sources of different xanthones. This study was aimed to assess the cytotoxic potential of four most abundant xanthones present in S. chirata both in vivo and in vitro in Ehrlich ascites carcinoma (EAC), a mouse transplantable breast carcinoma cell line and two human breast carcinoma cell lines (MCF-7 and MDA-MB-231).
Methods:
Four xanthones derived from S. chirata namely 1-hydroxy-3,7,8-trimethoxyxanthone (XA), 1,8-dihydroxy-3,5-dimethoxyxanthone (XB), 1-hydroxy-3,5,8-trimethoxyxanthone (XC) and 1,5,8-trihydroxy-3-methoxyxanthone (XD) were used for determination of sub-lethal dose on the cell lines EAC, MCF-7, MDA-MB-231 and verified toxicity of sub-lethal dose on normal murine fibroblast cells. Cytotoxicity was measured in vivo and survivability of mice was plotted accordingly. Therapeutic efficacy of XD was evaluated both in vivo and in vitro by determination of lipid peroxidation (LPO), reactive oxygen species (ROS) generation and by quantitating the enzyme status (GSH, catalase, superoxide dismutase) in treated and untreated samples. DNA damage was evaluated using comet and DNA fragmentation assays. Furthermore, apoptotic effect was analyzed by flow cytometry and validated by TUNEL assay and Western blotting.
Results:
Among all the xanthones tested XD showed IC50 at the lowest dose, and normal cells were unaffected at this dose. Survivability of mice increased significantly when treated with XD compared to other xanthones and cisplatin. Significantly increased ROS and LPO were found in cancer cells as a result of XD treatment which was unaltered in normal cell line. XD induced DNA damage and apoptosis in cancer cell lines.
Interpretation & conclusions:
Our experimental data indicate that XD may potentially act as a chemotherapeutic agent by enhancing ROS in breast cancer cells thereby leading to apoptosis.
Keywords: Apoptosis, breast cancer, cell death, chirata, cytotoxicity, xanthones
Phytochemicals are believed to have the natural antioxidant potential to be considered for evaluation as preventive and therapeutic agents for several diseases, including cancer. Swertia chirata Buch. Ham (Gentianaceae), one of the oldest known medicinal herbs used as a source of Ayurvedic drug Chirata, has been in use for the treatment of liver disorders and is shown to have hepatoprotective effects1. Chemical analysis of chirata extracts have been undertaken by many to assess the compound(s) responsible for medicinal activity2. Important chemical compounds isolated and characterized from S. chirata include xanthones, dimeric xanthones, monoterpene glycosides and alkaloids3. Earlier we reported anti-carcinogenic and anti-tumour efficacy of the crude extract of S. chirata which was the bitter component amarogentin4. The xanthones derived from the aerial part of the plant are 1-hydroxy-3, 7. 8-trimethoxyxanthone (XA), 1,8-dihydroxy-3,5-dimethoxyxanthone (XB), 1-hydroxy-3, 5, 8-trimethoxyxanthone (XC) and 1, 5, 8-trihydroxy-3-methoxyxanthone (XD)5,6. This study was focused to evaluate their anti-tumour property on two different carcinoma models, Ehrlich ascites carcinoma (EAC), a mouse transplantable breast carcinoma cell line and two human breast carcinoma cell lines [Luminal A subtype MCF-7 (ER+ PR+ HER2+) and Luminal B subtype triple-negative MDA-MB-231 (ER− PR− HER2−)]7,8.
Material & Methods
This study was conducted in the department of Cancer Chemoprevention and Oncogene Regulation, Chittaranjan National Cancer Institute (CNCI), Kolkata, India. The study protocol was approved by the Institutional Animal Ethics Committee, CNCI, Kolkata.
Test compound: The coarsely powdered aerial parts of S. chirata Buch. Ham (4 kg) were extracted with normal hexane in soxhlet apparatus for 72 h. The extract was concentrated to afford a yellowish thick liquid (250 ml). This liquid was kept in refrigerator for 24 h. A pale yellow amorphous solid (2.4 g) was separated out. The solid was filtered and purified by repeated crystallization from ethanol. Finally, yellow shining needle-shaped crystals (XD) were obtained (yield 0.52%) having a melting point (MP) of 270-271°C. After separation of the compound XD, the filtrate was concentrated and chromatographed over silica gel (60-100 mesh) column with the solvents of increasing polarity. N-hexanane-ethylacetate (9:1) eluents afforded the compound XA, MP 148°C (yield 0.053%). The eluents hexane-ethylacetate (4:1) yielded mixture of XB and XC. Rechromatography over silica gel column of the mixture separated two compounds. After crystallization from n-hexane-acetone mixture XB showed MP of 185°C (yield 0.021%) and XC exhibited 203-204°C MP (yield 0.065%). All these compounds were identified by detailed spectral analyses (ultraviolet, IR, PMR, mass spectrometry)9.
Experimental animals: Adult (7-8 wk old) Swiss albino female mice (25±2 g body wt) were bred in animal colony of CNCI were used for this study. They were maintained at control temperature (23±2°C) and humidity (55±10%) under alternating light and dark conditions (12:12 h). Mice were fed with standard food pellet diet (EPIC rat and mice pellet from Kalyani Feed Milling Plant, Kalyani, West Bengal), and drinking water was provided regularly ad libitum.
Tumour cells
In vivo: EAC cells were maintained in Swiss albino mice by weekly intraperitoneal transplantation of 1×106 viable tumour cells suspended in isotonic phosphate-buffered solution (PBS). The EAC cells were isolated from the peritoneal cavity of tumour-bearing mice the ascetic fluid was collected in sterile petri dishes and incubated at 37°C for two hours. The cells of macrophage lineage adhered to the bottom of the petri dishes. The non-adherent population was aspirated out gently and repeatedly washed with PBS. These cells were characterized as EAC by Wright staining owing to their morphology and viability was assessed to be 95 per cent by trypan blue dye exclusion10. The viable EAC cells were used for further experiments11.
In vitro: The cell lines used were MDA-MB-231, MCF-7 and mouse fibroblast (obtained from the institutional facility). The cells were cultured in Dulbecco's Modified Eagle Medium (pH 7.4) containing 10 per cent FBS (Gibco, USA) supplemented with HEPES [4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid] (Sigma-Aldrich, USA) and penicillin-streptomycin at 37°C in an air jacketed CO2 (5%) incubator and cells were allowed to get attached for 24 h before treatment12.
Experimental groups for the determination of most efficacious xanthones
In vivo: The Swiss albino female mice were divided into 37 groups of six mice (n=6) each. Six doses of each xanthone (5, 10, 20, 30, 40 and 50 μM) were given to mice in experimental groups (6 groups/xanthone) and a group which received intraperitoneal injection of phosphate-buffered saline (PBS) served as control. Cisplatin (Sigma-Aldrich, USA) was taken as control as an established chemotherapeutic drug and mangosteen (Sigma-Aldrich, USA) was considered as an established xanthone with known anticancer property. Mice in each group were injected with tumour cells (1×106 cells/mouse) intraperitoneally. The day of tumour cell inoculation was counted as day 0 and no treatment was given on that day. Mice were distributed in the following groups: (i) EAC control group (EAC): Mice were treated with PBS by intraperitoneal injections from day 1 to 10; (ii) Only XA-treated groups: Mice were treated only with compound XA (5-50 μM) 24 h after tumour inoculation from day 1 to 10; (iii) Only XB-treated groups: Mice were treated with compound XB (5-50 μM) 24 h after tumour inoculation from day 1 to 10; (iv) Only XC-treated groups: Mice were treated with compound XC (5-50 μM) 24 h after tumour inoculation from day 1 to 10; (v) Only XD-treated groups: Mice were treated with compound XD (5-50 μM) 24 h after tumour inoculation from day 1 to 10; (vi) Only mangosteen-treated groups: Mice were treated with compound mangosteen (5-50 μM) 24 h after tumour inoculation from day 1 to 10; and (vii) Only cisplatin-treated groups: Mice were treated with compound cisplatin (5-50 μM) 24 h after tumour inoculation from day 1 to 10.
In vitro: MCF-7 and MDA-MB-231 cell lines were treated with xanthone (XA to XD), mangosteen and cisplatin at doses of 1 to 50 μM. Normal mouse fibroblast was taken as normal cell line which also received treatment as described above.
Determination of most safe and efficacious compound
In vivo determination of half maximal inhibitory concentration (IC50) dose: The effects of xanthones were compared against a control group where mice were treated with PBS. After 10 days of treatment, mice were sacrificed to collect total cells from the peritoneum and cells viability was checked by trypan blue exclusion method10. IC50 values of each xanthones were determined in respect to EAC control group. In EAC control group, the viability was considered as 100 per cent.
In vitro determination of IC50 dose: The effect of xanthones on cellular cytotoxicity was measured in both normal and cancer cell lines by MTT assay using EZcountTM MTT Cell Assay Kit from HiMedia, Mumbai, using manufacturer's protocol.
Sample collection: Before euthanasia, all mice were fasted for 4 h and the blood samples were collected by cardiac puncture in two sets of microcentrifuge tubes. One set was left to clot and the serum was separated by centrifugation at 2000×g for 15 min and stored at −20°C until analysis. The other set was heparinized and used for studies of haematological parameters.
Haematological parameters: Blood haemoglobin level was determined according to the Sahli's method13. Haematological parameters, i.e., red blood cell (RBC), white blood cell (WBC) and platelet were measured by automated haematology analyzer (KX-21, Sysmex, Japan). Liver toxicity markers such as serum alkaline phosphatase (ALP), aspartate transaminase (AST), alanine aminotransferase (ALT) level and kidney toxicity markers such as urea and creatinine were analyzed by automated clinical chemistry analyzer (AU400, Olympus, Japan) according to the manufacturer's protocol.
Survivability study: Survivability assay was performed to study the effect of xanthones, cisplatin and mangosteen on lifespan of EAC-bearing mice at respective IC50 dosages (n=10). The survivability was determined as follows: ILS%=T−C/C×100 (where ILS=increased life span, T=number of days the treated mice survived, C=number of days the untreated mice survived)12. Kaplan-Meir survival analysis was done using GraphPad Prism 5.05 (GraphPad software, San Diego, CA, USA).
Chronic toxicity study with therapeutic dosage of XD: Chronic cytotoxicity was performed with therapeutic dosage of XD, i.e., 8 μM (data not shown). The ALT, ALP, urea and creatinine levels in blood of normal mice were evaluated after 40 days of treatment. The drugs were given by oral administration of the non-toxic doses and 0.9 per cent normal saline was used to treat mice in the control group.
Determination of liver glutathione-S-transferase (GST) & glutathione peroxidas e (GPx) activities: The activity of GST was measured in the cytosol of liver cells after 15 days of treatment following the method described by Habig et al14. The enzyme activity was determined from the increase in absorbance at 340 nm with 1-chloro-2-4-dinitrobenzene (CDNB) as the substrate and specific activity of the enzyme expressed as formation of CDNB-GSH conjugate per minute per mg of protein. Glutathione peroxidase activity was also determined after 15 days of treatment in the post-mitochondrial fraction by the methods described by Bermingham et al15 and Pagliaet al16. The decrease in absorbance following addition of H2O2 was recorded at 340 nm. Enzyme activity was expressed as nanomoles of NADPH utilized per minute per mg protein using molar extinction co-efficient at 340 nm as 6200/m/cm. Activity of catalase (CAT) in liver was estimated by the method described by Johansson et al17. The enzyme activity was determined using spectrophotometer at 250 nm wavelength and expressed as unit/mg protein where the unit is the amount of enzyme that liberates half the peroxide oxygen from H2O2 in 100 sec at 25°C. Superoxide dismutase (SOD) activity was determined by quantification of pyrogallol autoxidation inhibition by the established protocol and expressed as unit/mg18,19.
Experimental groups for evaluation of therapeutic efficacy of most active xanthone compound
In vivo: The mice were divided into four groups containing six mice (n=6) in each group. Mice of each group were injected with tumour cells (1×106 cells/mouse) intraperitoneally. Mice were distributed in the following groups: EAC control group (Control): Mice were treated with water by intraperitoneal injections from day 1 to 10; and XD-treated: Mice were treated only with compound XD (8 μM) 24 h after tumour inoculation from day 1 to 10. EAC cells were collected from the peritoneal cavity of both XD-treated and XD-untreated mice after 10 days of treatment. The cells were collected, washed in PBS, counted in haemocytometer and then taken for further analysis.
In vitro: In vitro study was performed with the three cell lines among which MCF-7 and MDA-MB-231 were cancer and mouse fibroblast was normal cell line. Two groups were taken for each cell line: Control: did not receive any treatment; and XD-treated: treated at IC50 for respective cancer cell lines. For normal cell line treatment was done at 40 μM which was a higher dose showing toxicity to cancer cell lines (MCF-7 and MDA-MB-231). Mouse fibroblast cells were seeded in 90 mm petri dish and after 24 h of seeding time XD was given and after 48 h cells were taken for analysis.
Effect of XD on intracellular reactive oxygen species (ROS) generation of different cell lines: The ROS in EAC was measured by 2'-7'-dichlorofluorescin diacetate (DCFH-DA) method20. EAC cells 1×106 were incubated with DCFH-DA (10 μM) for 30 min at 37°C in dark. The fluorescence intensity of DCFH was measured using a spectrofluorimeter (Cary, Varian, Australia). The possible DNA damage induced by enhanced ROS as a result of XD treatment was detected using the alkaline single cell gel electrophoresis (comet assay) following a established protocol with slight modification21. The viability of cells was measured in each group and approximately 104 cells/slide was taken for the assay. A 10 μl aliquot of freshly prepared single cell suspension was mixed with one per cent low melting agarose and layered on the half frosted slides pre-coated with normal agarose. A third layer of 0.5 per cent low melting agarose was layered on the top of the second layer. The cells were lysed overnight at 4°C in lysis buffer containing 2.5 M NaCl, 100 mM ethylenediaminetetraacetic acid disodium salt (Na2-EDTA), 10 mM Tris buffer, one per cent Triton X-100 and 10 per cent dimethyl sulfoxide (pH 10.0). After lysis, the slides were subjected to electrophoresis in electrophoresis buffer (1 mM Na2-EDTA and 0.3 M NaOH, pH 13.1) for 30 min at 300 mA and 20 V. After electrophoresis, the slides were neutralized with neutralizing buffer (0.4 M Tris buffer, pH 7.5). The microscopic slides were dried at room temperature and stained with ethidium bromide in water (30 μg/ml; 50 μl/slide)21. Examination was done under a fluorescence microscope Leica DM 4000B (Leica Microsystems, Germany) with imaging system. Komet 5.5 software22 was used to take the photomicrograph of cells and to determine the length of the comet tail. A total of 150-200 cells selected randomly (5-7 zones/slide) in each slide were counted (4 slides/mice in each group) to determine the number of damaged cells, and the per cent of damaged cells was calculated using the following formula:
Damaged cell (%) = Number of damaged cells/total number of cells counted × 100
The results were expressed as the per cent of cells with tail (tailed cells) and average tail length due to DNA migration in each group.
DNA was collected from MCF-7, MDA-MB-231 and EAC cells according to the protocol described by Yoshida et al23. The fragmentation assay was performed using previously described protocol24, and the gel was stained with ethidium bromide and observed in chemidoc XRS+ (Bio-Rad Laboratories, USA) and photographed with image Lab 3 software (Bio-Rad Laboratories, USA).
Effect of XD on cell cycle of cancer cells: Deregulated cell cycle and suppressed apoptosis is a characteristic of cancer cells. Pattern of cell cycle distribution was analyzed in FACS calibur (B.D., USA) as per the previously described protocol25. Data were analyzed using Cell Quest Pro Software (Beckton and Dickinson, USA).
Confirmation of apoptosis by TUNEL assay: EAC cells were collected from mouse peritoneum washed in PBS and smear was drawn on slide and fixed in four per cent paraformaldehyde. MCF-7 and MDA-MB-231 cells were cultured on coverslip and fixed in paraformaldehyde. TUNEL assay was performed using a kit from Roche (In Situ Cell Death Detection Kit, Fluorescein, Roche Life Sciences, USA) according to manufacturer's protocol and observed under fluorescent microscope Leica DM4000 B (Leica Microsystems, Germany) and visualized with software Leica FW 4000 (Leica Microsystems, Germany).
Validation of apoptosis by Western blotting analysis & in silico bioavailability: Western blotting analysis was done by making whole cell extract of all the cell lines and calculating the Bax:Bcl-2 ratio according to previously described protocol26. In silico bioavailability analysis was done using SwissADME tool using chemical structure of XD27.
Statistical analysis: All data were represented as mean±standard deviation. All the experiments were repeated at least three times. Student's t test was performed for comparison between two groups.
Results
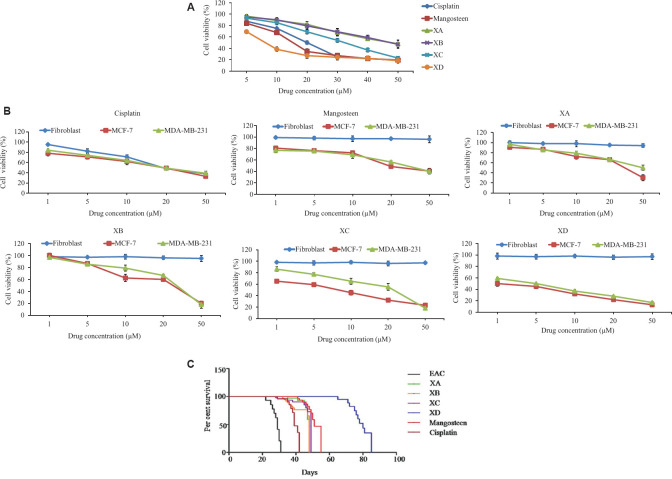
Selection of IC50 of different xanthones in vivo: Among the different compounds, XD was found to be most active with 50 per cent lethality of EAC cells at a very low concentration 8.1 μM followed by XC (32.35 μM), and XB and XA (48.53 μM). Mangosteen showed IC50 of 15 μM and cisplatin showed IC50 of 20 μM (Fig. 1A). Thus, all the xanthones were cytotoxic to EAC cells with varied efficacy but XD was found to be most active.
Fig. 1.
(A) IC50 determination in vivo in EAC cells by trypan blue dye exclusion assay against different xanthones and chemotherapeutic drug cisplatin. (B) MTT assay for IC50 determination of xanthones against MCF-7 and MDA-MB-231 cells. Data represented as mean±SD with significance P<0.001. (C) Kaplan-Meier survival analysis compared per cent survival of ascites bearing mice between EAC control group (black line) and different xanthones and cisplatin-treated groups at their respective IC50 dose. XD-treated group (blue line) showed the most increased survivability followed by mangosteen- (red line), XC- (violet line), XA- (green line), XB- (golden brown line) and cisplatin- (reddish black line) treated groups. Total number of mice in each group (n=10).
Dose determination in vitro: Similar to in vivo result, in vitro dose determination was done by MTT and XD was found to be the most effective for both the carcinoma cell lines with lowest IC501 μM for MCF-7 and 5 μM for MDA-MB-231 followed by XC (8 μM for MCF-7,25 μM for MDA-MB-231), XB (25 μM for MCF-7, 30 μM for MDA-MB-231) XA (30 μM for MCF-7, 50 μM for MDA-MB-231) and for mangosteen (18 μM for MCF-7 and 30 μM for MDA-MB-231). Normal murine fibroblast was found to be viable even at the highest concentration of all the xanthones. Cisplatin IC50 was found to be 20 μM but it was found to be toxic to normal fibroblast at this concentration (Fig. 1B).
Effect of xanthones on mouse: WBC and platelet counts were increased significantly in mice after development of ascitic carcinoma as compared to the normal mice (Table I). After treatment with xanthones at their respective IC50 dosage, only XD treatment could lower WBC and platelet counts back to normal level in ascitic carcinoma-bearing mice. There was significant change in total number of RBC count as well as haemoglobin level after development of ascitic carcinoma as compared to the normal mice. Cisplatin treatment caused lowering of RBC, Hb and platelets.
Table I.
Haematological parameters of EAC-bearing mice after treatment with different xanthones, mangosteen and cisplatin at IC50 doses
| Groups (Dose in µM) | WBC (103/µl) | RBC (106/µl) | Hb (g/dl) | Platelets (103/µl) |
|---|---|---|---|---|
| Normal | 14.8±0.7 | 4.88±0.8 | 9.3±0.3 | 309±9 |
| EAC | 92.8±2.6 | 2.88±0.7 | 2.3±0.8 | 856±8 |
| Cisplatin (20) | 35.8±0.8 | 2.7±0.6 | 2.1±0.4 | 200±2 |
| Mangosteen (15) | 20.3±0.3 | 3.15±0.6 | 8.5±0.7 | 326±1 |
| XA-treated (48) | 30.8±0.6 | 4.15±0.4 | 7.5±0.8 | 456±3 |
| XB-treated (48) | 24.6±0.6 | 4.75±0.3 | 7.4±0.5 | 596±4 |
| XC-treated (32.35) | 27.2±0.8 | 5.75±0.2 | 7.9±0.9 | 441±2 |
| XD-treated (8.1) | 17.2±0.2 | 5.05±0.5 | 10±2 | 341±8 |
Values represent mean±SD of six samples in each group. Comparative study of haematological (WBC, RBC, HGB, and PLT) of normal, EAC control, cisplatin-, mangosteen-, XA-, XB-, XC- and XD-treated groups of mice. Significant difference was observed in the haematological parameters of the XD-treated group compared with the EAC control group considering P<0.001. CBC, complete blood count; RBC, red blood cells; WBC, white blood cells; EAC, Ehrlich ascites carcinoma
AST, urea and creatinine levels were significantly (P<0.001) elevated in the mouse blood after development of ascitic carcinoma in comparison to the normal mice where ALT and AST level were significantly reduced. Liver and kidney toxicity parameters were almost back to normal after treatment with XD in tumour-bearing mice as compared to the normal mice whereas the other xanthones were not able to produce any significant changes. No significant difference in liver and kidney toxicity parameters was seen after 14 days of xanthone treatment at highest dosage, i.e., 50 μM. Mangosteen failed to alleviate the mice from EAC induced hepato and nephrotoxicity (Table II). Treatment with XD revealed maximum increase in life span of tumour-bearing mice (140%) followed by XC (81%), mangosteen (72%) XB (63%), XA (45%) and cisplatin (36.36%) (Fig. 1C).
Table II.
Modulation in hepatotoxicity and kidney toxicity parameters after treatment with different xanthones, mangosteen and cisplatin at IC50 dosage
| Groups (Dose in µM) | ALP (IU/l) | AST (IU/l) | ALT (IU/l) | Urea (mg/dl) | Creatinine (mg/dl) |
|---|---|---|---|---|---|
| Normal | 45.8±0.8 | 225±5 | 71±9 | 49±5 | 0.5±0.05 |
| EAC | 192.8±4.8 | 889±7 | 331±8 | 129±8 | 1.4±0.1 |
| Cisplatin (20) | 158±8 | 720±6 | 231±4 | 98±9 | 1.6±0.2 |
| Mangosteen (15) | 120±3 | 345±6 | 95±6 | 66±2 | 0.9±0.08 |
| XA-treated (48) | 103±3 | 350±1 | 91±3 | 71±8 | 1.7±0.09 |
| XB-treated (48) | 160±5 | 335±8 | 93±4 | 67±8 | 1.1±0.09 |
| XC-treated (32.35) | 106±2 | 355±9 | 85±5 | 79±8 | 1.2±0.09 |
| XD-treated (8.1) | 57.2±2 | 205±5 | 79±2 | 41±8 | 0.7±0.09 |
Values represent mean±SD of six samples in each group. Comparative study of biochemical parameters (ALP, AST, ALT, urea and creatinine) of normal, EAC control, cisplatin-, mangosteen-, XA-, XB-, XC- and XD-treated groups of mice. Significant difference was observed in the XD-treated group’s biochemical parameters compared with the EAC control group considering P<0.001. ALP, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine aminotransferase
Upon evaluating the chronic toxicity of XD it did not show any toxicity as all the parameters for liver and kidney toxicity lied within normal limit (data not shown). To evaluate the cytoprotective activity of XD, biochemical analyses for quantitation was performed of different phase II detoxifying enzymes and lipid peroxidation (LPO) in the liver cytosol of EAC ascites bearig mice. There was significant upregulation in expression of GSH, CAT and SOD after treatment with XD in comparison to the untreated group. A significant reduction of LPO was found after treatment with XD in comparison to control. Although all the xanthones modulated production of phase II enzymes (data not shown), the effect was most significant after treatment with XD at the sub lethal dosage (Table III).
Table III.
Modulation in antioxidative enzymes in liver cytosol of EAC-bearing mice after XD treatment
| Groups (Dose in µM) | GSH (nmol/mg) | GST (nmol/mg) | GPx (nmol/mg) | SOD (units/mg) | CAT (units/mg) | LPO (mol/mg) |
|---|---|---|---|---|---|---|
| Normal | 2.4±0.5 | 4.65±0.3 | 6.45±0.4 | 12±1.5 | 0.5±0.5 | 1±0.2 |
| Drug control | 2.6±0.3 | 8±0.6 | 8.28±0.6 | 17±6 | 0.4±0.06 | 1.6±0.09 |
| EAC | 0.95±0.08 | 1.75±0.5 | 2.5±0.08 | 5.29±0.8 | 1.4±0.1 | 15±1.2 |
| XD-treated (8.1) | 5.72±1 | 15±5 | 9.9±2 | 35±0.8 | 0.7±0.09 | 1.8±0.09 |
Values represent mean±SD of six samples in each group. Comparative study of modulation of detoxifying enzymes, endogenous LPO in liver cytosol of normal, EAC control and XD-treated groups of mice. Significant difference was observed in the antioxidative enzyme, and endogenous LPO levels of XD-treated group compared with the EAC control group considering P<0.001. GSH, glutathione; GST, glutathione-S-transferase; GPx, glutathione peroxidase; SOD, superoxide dismutase; LPO, lipid peroxidation; CAT, catalase
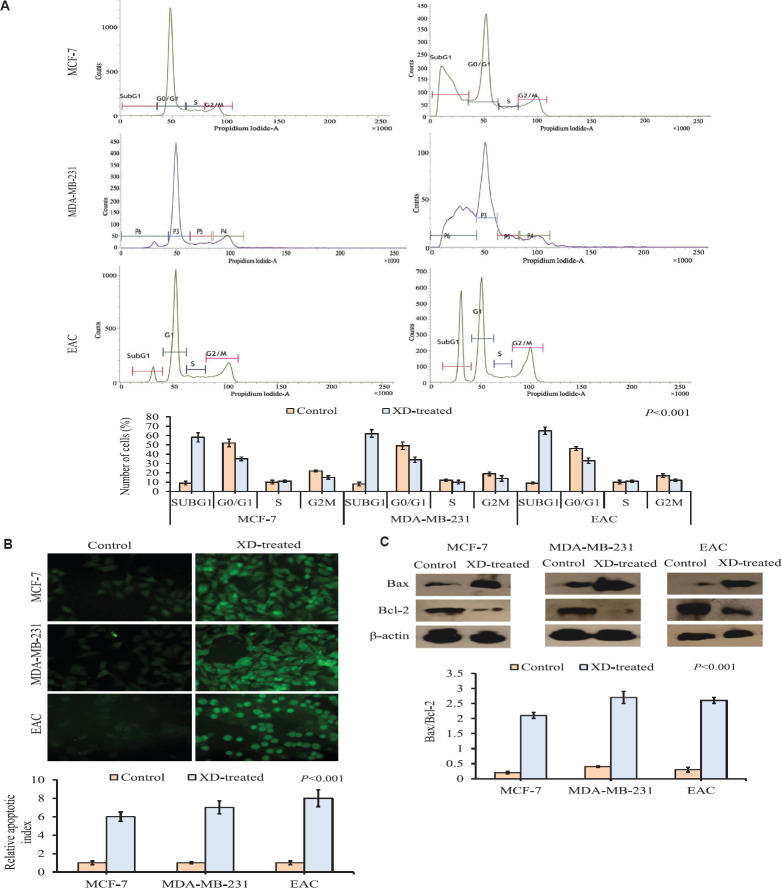
Therapeutic potential of XD by analysing oxidative stress both in vivo and in vitro: XD was found to be toxic against cancer (EAC) cells at a very low concentration (8.1 μM) and also on MCF-7 and MDA-MB-231 cell lines without affecting the normal cells (fibroblast) at the concentration of 1 and 5 μM, respectively. Therefore, to understand this phenomenon, the endogenous oxidative stress was measured by evaluating ROS generation after treatment with XD. ROS level was significantly (P<0.001) increased both in vivo and in vitro after treatment with XD in comparison to the untreated group which was taken as control. In case of normal fibroblast augmentation of ROS generation was not seen even after treatment with 50 μM of xanthone (Fig. 2A). The result of comet assay revealed the induction of DNA damage showing extended tail of damaged DNA in treated groups irrespective of cell lines both in vivo and in vitro keeping aside the normal cells. Cancer cells showed significant increase in per cent of damaged cells which were 54, 65 and 67 per cent, respectively (Fig. 2B). Validation of DNA damage was performed by DNA fragmentation assay. The result is also concordant with the previous data showing smearing pattern upon XD treatment (Fig. 2C).
Fig. 2.
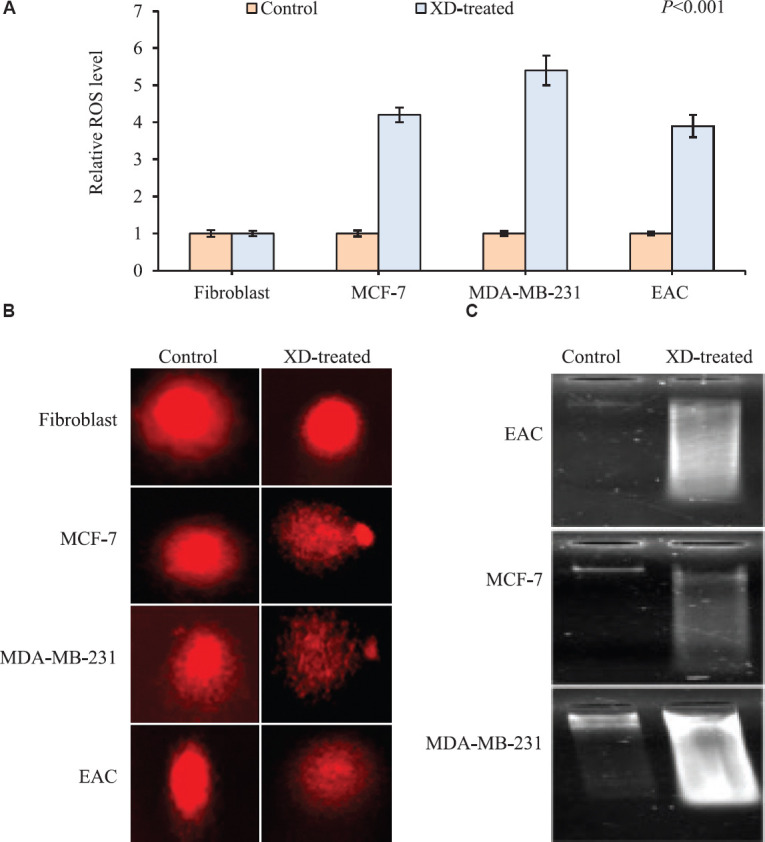
(A) Graphical representation of intracellular ROS generation upon XD treatment in cancer cells (EAC, MCF-7 and MDA-MB-231) at their respective IC50 dosage (8.1, 1 and 5 μM) and in normal cells (fibroblast) at highest dose of 40 μM by DCFH-DA assay. XD treatment caused a significant increase in intracellular ROS of cancer cells, whereas no induction in ROS was seen in normal cells. Data represented as mean±SD with significance P<0.001. (B) Representation of DNA damage upon XD treatment in cancer cells (EAC, MCF-7 and MDA-MB-231) at their respective IC50 dosage (8.1, 1 and 5 μM) and in normal cells (fibroblast) at the highest dose of 40 μM by comet assay. XD treatment caused significant DNA damage in cancer cells, as evidenced by growing tail length, whereas no DNA damage was seen in normal cells. (C) Representation of DNA damage upon XD treatment in cancer cells (EAC, MCF-7 and MDA-MB-231) at their respective IC50 dosage (8.1, 1 and 5 μM) and in normal cells (fibroblast) at the highest dose of 40 μM by agarose gel electrophoresis assay XD treatment caused significant DNA damage in cancer cells as evidenced by smearing pattern in all the three cancer cells.
Induction of apoptosis in vivo and in vitro after treatment: The indication of DNA damage mediated apoptosis was validated by cell cycle analysis. The increased cell population in the SubG0 stage of the cell cycle in experimental groups, which received XD treatment, gave a preliminary idea of apoptosis, as proved by TUNEL assay. The results showed that XD treatment could significantly (P<0.001) increase the SubG0 population in experimental groups in comparison to control after 10 days of treatment in vivo and 48 h in vitro (Fig. 3A). TUNEL assay exhibited significant apoptosis in experimental groups that received XD treatment compared to the control group in both in vivo and in vitro models (Fig. 3B).
Fig. 3.
(A) Cell cycle phase distribution analysis in experimental groups received XD treatment compared to control in EAC, MCF-7 and MDA-MB-231 cells. Graphical representation of cell cycle phase distribution showing the per cent of cells in each phase of the cell cycle. A significant (P<0.001) increase in SubG0 population in XD-treated group was observed compared to control group in all the three cancer cells. (B) Representation of in situ TUNEL assay (apoptotic population) in EAC, MCF-7 and MDA-MB-231 cells samples of the experimental groups, control and XD-treated. A significant upregulation in number of apoptotic cells of XD-treated group compared to the control group was observed in all the three cells. Representative microscopic photographs of TUNEL positive cells were brightly fluorescent. Photographs were taken under ×20 magnification of the fluorescent microscope. Scale bar 50 μM. (C) Expression of Bax and Bcl-2 by Western blot of control and XD-treated experimental groups of EAC, MCF-7 and MDA-MB-231 cells. Representative blots showed reduced Bcl-2 expression and increased Bax expression in the treated group compared with control in all the three cells. Densitometric analysis through graphical representation showed the relative expression of individual markers. The loading control β-actin normalized peak density. Data represented as mean±SD with significance P<0.001.
Further validation of apoptosis was found in Western blot analysis. The significant (P<0.001) augmentation of intracellular Bax:Bcl-2 ratio showed the stringent sensitivity of cancer cells toward apoptosis signal when treated with XD (Fig. 3C).
In silico bioavailability: This analysis indicated that XD was soluble in water.
Discussion
Traditional cancer chemotherapy which is offered globally is mostly toxic, causes cellular damage, and promotes serious side effects which are very often life-threatening. The present in vitro study with xanthone on two aggressive human breast cancer cell lines MCF-7 and MDA-MB-231 showed notable findings. All the xanthones exhibited potent inhibitory activity against cancer cell growth but XD came out to be most potent one with IC50 value at a lowest concentration both in vivo and in vitro without affecting the normal cells. This data strengthen the fact that XD is almost non-toxic to normal cells in contrast to its effect on cancer cells. Amarogentin, isolated from bitter part of S. chirata, was found to have anti-carcinogenic role on skin and liver carcinogenesis which was reported from our laboratory previously2,7. Other studies have revealed qualitative and quantitative assessment of several xanthone derivatives of S. chirata5. In this study, XD was found to be one of the most bioactive constituent among all other xanthones tested. In the toxicity assessment study on mice, XD showed significant cytoprotection in carcinoma-bearing mice as well as against conventional chemotherapeutic drug cisplatin induced toxicity (data not shown). XD showed activity better than mangosteen which is an established anticancer xanthone28. Carotenoids act as antioxidant in normal cells and pro-oxidant in cancer cells18,19. In concordance with this finding, amelioration of cisplatin induced intracellular level of ROS in normal mouse was found (data not shown) in contrast to significant induction of ROS generation in carcinoma-bearing mice on XD treatment. Our study exploited the dual characteristic of phytochemical like xanthone XD as both anti- and pro-oxidant. The resultant intracellular status of ROS was also reflected on programmed cell death. Indication of pro-apoptotic role of XD was found by assessing DNA damage through comet assay which was again validated by TUNEL assay and Western blotting. Extensive experimental studies revealed that Bax protein is an apoptosis promoting factor, whereas Bcl-2 is characterized as suppressor of apoptosis29. Therefore, the stringent regulatory function of these two proteins forms cellular 'Rheostat' of programmed cell death with enhanced intracellular ratio of Bax:Bcl-2 influencing cells' sensitivity toward apoptotic signal30. Our data showed a significant increased Bax:Bcl-2 ratio both in vivo and in vitro. On the basis of the background study and the study report we found, it can be stated that the effect of XD is so tuned that it possesses selective and preferential activity on cancer cell leaving normal cells unaltered. Our results corroborated that XD could ardently increase survivability of mice. Hence, the role of xanthone reinforces its potency that positively reflects on the enhanced period of patient's life span.
From the preclinical aspect, our investigation showed xanthone to be non-toxic to mice. More detailed toxicity studies are needed to be performed in other rodent and non-rodent species.
In conclusion, XD among the xanthones found in S. chirata exerted significant therapeutic potential by inducing ROS mediated apoptosis in breast cancer cells both in vivo and in vitro at a low dosage being non-toxic to normal cells. Therefore, XD may emerge as a key chemotherapeutic agent in the future, overcoming the limitations of conventional chemotherapy.
Acknowledgment
Authors acknowledge Dr Chandraditya Chakraborty of Dana Farber Cancer Institute, Boston, USA, for his valuable suggestion and Dr Sukta Das, Former Head, Department of Cancer Chemoprevention, Chittaranjan National Cancer Institute (CNCI), Kolkata, for encouragement.
Footnotes
Financial support & sponsorship: Authors acknowledge CNCI, Kolkata, for financial support.
Conflicts of Interest: None.
References
- 1.Aleem A, Kabir H. Review on Swertia chirata as traditional uses to its pyhtochemistry and phrmacological activity. J Drug Deliv Ther. 2018;8:73–8. [Google Scholar]
- 2.Saha P, Das S. Highlighting the anti-carcinogenic potential of an ayurvedic medicinal plant, Swertia Chirata. Asian Pac J Cancer Prev. 2010;11:1445–9. [PubMed] [Google Scholar]
- 3.Menković N, Šavikin-Fodulović K, Bulatović V, Aljančić I, Juranić N, Macura S, et al. Xanthones from Swertia punctata. Phytochemistry. 2002;61:415–20. doi: 10.1016/s0031-9422(02)00231-5. [DOI] [PubMed] [Google Scholar]
- 4.Kumar V, Van Staden J. A Review of Swertia chirayita (Gentianaceae) as a traditional medicinal plant. Front Pharmacol. 2015;6:308. doi: 10.3389/fphar.2015.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosal S, Sharma PV, Chaudhuri RK, Bhattacharya SK. Chemical constituents of the gentianaceae V: tetraoxygenated xanthones of Swertia chirata Buch.-Ham. J Pharm Sci. 1973;62:926–30. doi: 10.1002/jps.2600620614. [DOI] [PubMed] [Google Scholar]
- 6.Rawat B, Singh P, Negi JS. Chemical constituents and biological importance of Swertia: A review. Curr Res Chem. 2011;3:1–15. [Google Scholar]
- 7.Saha P, Mandal S, Das A, Das PC, Das S. Evaluation of the anticarcinogenic activity of Swertia chirata Buch.Ham, an Indian medicinal plant, on DMBA-induced mouse skin carcinogenesis model. Phytother Res. 2004;18:373–8. doi: 10.1002/ptr.1436. [DOI] [PubMed] [Google Scholar]
- 8.Westley B, Rochefort H. Estradiol induced proteins in the MCF7 human breast cancer cell line. Biochem Biophys Res Commun. 1979;90:410–6. doi: 10.1016/0006-291x(79)91250-6. [DOI] [PubMed] [Google Scholar]
- 9.Balasundari P, Singh SK, Kavimani S. Free radical scavenging of xanthones from Swertia chirata Buchham and tumor cell growth inhibition. Main Group Chem. 2005;4:177–85. [Google Scholar]
- 10.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol. 2015;111:A3B1–3. doi: 10.1002/0471142735.ima03bs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya A, Choudhuri T, Pal S, Chattopadhyay S, K Datta G, Sa G, et al. Apoptogenic effects of black tea on Ehrlich's ascites carcinoma cell. Carcinogenesis. 2003;24:75–80. doi: 10.1093/carcin/24.1.75. [DOI] [PubMed] [Google Scholar]
- 12.Segura JA, Barbero LG, Márquez J. Ehrlich ascites tumour unbalances splenic cell populations and reduces responsiveness of T cells to Staphylococcus aureus enterotoxin B stimulation. Immunol Lett. 2000;74:111–5. doi: 10.1016/s0165-2478(00)00208-x. [DOI] [PubMed] [Google Scholar]
- 13.Alstead S. Observations on Sahli's hæmoglobinometer. Postgrad Med J. 1940;16:278–86. doi: 10.1136/pgmj.16.178.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 15.Bermingham EN, Hesketh JE, Sinclair BR, Koolaard JP, Roy NC. Selenium-enriched foods are more effective at increasing glutathione peroxidase (GPx) activity compared with selenomethionine: A meta-analysis. Nutrients. 2014;6:4002–31. doi: 10.3390/nu6104002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 17.Johansson LH, Borg LA. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem. 1988;174:331–6. doi: 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- 18.Samudrala PK, Augustine BB, Kasala ER, Bodduluru LN, Barua C, Lahkar M. Evaluation of antitumor activity and antioxidant status of Alternanthera brasiliana against Ehrlich ascites carcinoma in Swiss albino mice. Pharmacognosy Res. 2015;7:66–73. doi: 10.4103/0974-8490.147211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheweita SA, Tilmisany AK. Cancer and phase II drug-metabolizing enzymes. Curr Drug Metab. 2003;4:45–58. doi: 10.2174/1389200033336919. [DOI] [PubMed] [Google Scholar]
- 20.Yotsu-Yamashita M, Kondo S, Segawa S, Lin Y-C, Toyohara H, Ito H, et al. Isolation and structural determination of two novel phlorotannins from the brown alga Ecklonia kurome Okamura, and their radical scavenging activities. Mar Drugs. 2013;11:165–83. doi: 10.3390/md11010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olive PL, Banáth JP. The comet assay: A method to measure DNA damage in individual cells. Nat Proto. 2006;1:23–9. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 22.Oxford Instruments - Andor. Komet Software. [accessed on August 4, 2019]. Available from: https://andoroxinstcom/products/komet-software/
- 23.Yoshida GJ. Emerging roles of Myc in stem cell biology and novel tumor therapies. J Exp Clin Cancer Res. 2018;37:173. doi: 10.1186/s13046-018-0835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahbar Saadat Y, Saeidi N, Zununi Vahed S, Barzegari A, Barar J. An update to DNA ladder assay for apoptosis detection. Bioimpacts. 2015;5:25–8. doi: 10.15171/bi.2015.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cossarizza A, Chang H-D, Radbruch A, Acs A, Adam D, Adam-Klages S, et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition) Eur J Immunol. 2019;49:1457–973. doi: 10.1002/eji.201970107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barua A, Choudhury P, Maity JK, Mandal SB, Mandal S, Saha P. Chemotherapeutic potential of novel non-toxic nucleoside analogues on EAC ascitic tumour cells. Free Radic Res. 2019;53:57–67. doi: 10.1080/10715762.2018.1551999. [DOI] [PubMed] [Google Scholar]
- 27.Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto K, Akao Y, Kobayashi E, Ohguchi K, Ito T, Tanaka T, et al. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J Nat Prod. 2003;66:1124–7. doi: 10.1021/np020546u. [DOI] [PubMed] [Google Scholar]
- 29.Inayat-Hussain SH, Chan KM, Rajab NF, Din LB, Chow SC, Kizilors A, et al. Goniothalamin-induced oxidative stress, DNA damage and apoptosis via caspase-2 independent and Bcl-2 independent pathways in Jurkat T-cells. Toxicol Lett. 2010;193:108–14. doi: 10.1016/j.toxlet.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frankfurt OS, Krishan A. Apoptosis-based drug screening and detection of selective toxicity to cancer cells. Anticancer Drugs. 2003;14:555–61. doi: 10.1097/00001813-200308000-00008. [DOI] [PubMed] [Google Scholar]