Abstract
Non-tuberculous mycobacteria (NTM) are ubiquitously present in the environment, but NTM diseases occur infrequently. NTM are generally considered to be less virulent than Mycobacterium tuberculosis, however, these organisms can cause diseases in both immunocompromised and immunocompetent hosts. As compared to tuberculosis, person-to-person transmission does not occur except with M. abscessus NTM species among cystic fibrosis patients. Lung is the most commonly involved organ, and the NTM-pulmonary disease (NTM-PD) occurs frequently in patients with pre-existing lung disease. NTM may also present as localized disease involving extrapulmonary sites such as lymph nodes, skin and soft tissues and rarely bones. Disseminated NTM disease is rare and occurs in individuals with congenital or acquired immune defects such as HIV/AIDS. Rapid molecular tests are now available for confirmation of NTM diagnosis at species and subspecies level. Drug susceptibility testing (DST) is not routinely done except in non-responsive disease due to slowly growing mycobacteria (M. avium complex, M. kansasii) or infection due to rapidly growing mycobacteria, especially M. abscessus. While the decision to treat the patients with NTM-PD is made carefully, the treatment is given for 12 months after sputum culture conversion. Additional measures include pulmonary rehabilitation and correction of malnutrition. Treatment response in NTM-PD is variable and depends on isolated NTM species and severity of the underlying PD. Surgery is reserved for patients with localized disease with good pulmonary functions. Future research should focus on the development and validation of non-culture-based rapid diagnostic tests for early diagnosis and discovery of newer drugs with greater efficacy and lesser toxicity than the available ones.
Keywords: Diagnosis, non-tuberculous mycobacteria pulmonary disease, NTM, NTM extrapulmonary disease, treatment
Introduction
Non-tuberculous mycobacteria (NTM) are known by several names including environmental mycobacteria, atypical mycobacteria or anonymous mycobacteria, mycobacteria other than Mycobacterium tuberculosis (Mtb) (MOTT) and its close relatives, M. africanum, M. bovis, M. canetti, M. caprae, M. pinnipedii and M. leprae1. These organisms are ubiquitous in the environment and have been isolated from air, soil, dust, plants, natural and drinking water sources including biofilms, wild animals, milk and food products2,3. NTM are characterized by a thin peptidoglycan layer surrounded by a thick outer lipid-rich coating that enables NTM attachment to rough surfaces and by offering resistance to antibiotics and disinfectants, helping NTM survival in low oxygen and carbon concentrations and in other adverse conditions4. Based on their growth characteristics from the subculture, NTM are divided into rapidly growing mycobacteria (RGM; <7 days) and slowly growing mycobacteria (SGM; ≥7 days)5 (Table I). At present, there is no evidence for the latency of NTM6. Taxonomy of the genus Mycobacterium includes about 200 species and 13 subspecies7,8,9.
Table I.
Common non-tuberculous mycobacteria (NTM) species causing human diseases
| Slowly growing NTM (showing growth in ≥7 days on subculture) |
| 1. Photochromogens (produce pigment on exposure to light) Mycobacterium kansasii |
| M. marinum |
| 2. Scotochromogens (produce pigment when grown in dark) |
| M. scrofulaceum |
| 3. Non-chromogens (growth not pigmented) |
| M. avium complex (MAC) |
| M. avium |
| M. intracellulare |
| M. chimaera |
| M. ulcerans |
| M. xenopi |
| M. simiae |
| M. malmoense |
| M. szulgai |
| M. haemophilum |
| Rapidly growing NTM (showing growth in <7 days on subculture) |
| M. abscessus |
| M. abscessus subspecies abscessus |
| M. abscessus subspecies bolletii |
| M. abscessus subspecies massiliense |
| M. fortuitum |
| M. chelonae |
Source: Ref. 5
In high tuberculosis (TB)-burden countries, diagnosis of NTM is rarely made because of lack of awareness among healthcare providers about the NTM diseases and poor access to adequate laboratory resources including mycobacterial culture and molecular methods for identification or speciation10. In these resource-limited settings, there is a heavy reliance on smear microscopy for the diagnosis of TB, and the diagnosis of NTM is frequently missed and these patients are empirically treated as drug-sensitive and -resistant TB11.
Epidemiology
NTM disease burden
Table II describes the distribution of various NTM species in the environment2,3. Table III details the major differences between NTM and Mtb1,2,3,4,5,6,9,12,13,14,15,16,17,18,19,20. Although recent reports regarding the transmission of M. abscessus and M. massiliense have not proven person-to-person transmission, but these are highly suggestive of indirect transmission among cystic fibrosis (CF) patients21. Systematic reporting of NTM diagnosis is not done because the disease is not notifiable to public health authorities in several countries10. NTM lung infection rates, defined as individuals with NTM-positive cultures and those with defined NTM pulmonary disease (NTM-PD), increase with age22 and differ considerably among various countries23,24,25. Many studies have suggested an increase in the prevalence rates of NTM over the last four decades22,25,26,27,28,29,30,31,32,33,34,35,36. The data from the USA suggest that the current prevalence of NTM-positive culture ranges between 1.4 and 6.6/100,000 individuals26, whereas UK data suggest that NTM-positive culture incidence has increased from 4/100,000 to 6.1/100,000 individuals between 2007 and 201235. A study from Canada has reported a significant increase in the prevalence of NTM-PD from 29.3 cases/100,000 in 1998-2002 to 41.3/100,000 individuals tested in 2006-201036. Several factors that have contributed to this increase in the incidence and prevalence are listed in Box I37,38. Published reports on rate of NTM isolation from several countries are summarized in Table IV22,29,30,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63.
Table II.
Environmental niches of non-tuberculous mycobacteria (NTM)
| Types of sources | Sources | Commonly isolated NTM |
|---|---|---|
| Natural water sources | Streams, rivers, lakes, ponds and seawater | MAC, Mycobacterium fortuitum, M. chelonae, M. kansasii, M. gordonae, M. xenopi, M. marinum |
| Man-made water sources | Drinking water supply pipelines | MAC, M. kansasii, M. gordonae, M. xenopi, M. abscessus, M. fortuitum, M. chelonae, M. scrofulaceum, M. szulgai |
| Cold and hot water tanks | ||
| Hot tubs, indoor and outdoor pools | ||
| Household plumbing, showerheads and faucets | ||
| Hospital plumbing and water supply | ||
| Ice machines and commercial ice | ||
| Bottled drinking water | ||
| Aerosols | Showers, hot-tubs, humidifiers, indoor swimming pools, heater-cooler units in hospitals | MAC, M. kansasii, M. gordonae, M. abscessus |
| Other sources* | Natural soil dust, potting soil, peat moss and domestic dust | MAC, M. fortuitum, M. chelonae, M. kansasii |
Table III.
Differences between non-tuberculous mycobacteria (NTM) and Mycobacterium tuberculosis (Mtb)
| Characteristics | NTM | Mtb |
|---|---|---|
| Nomenclature | NTM have several names: MOTT, atypical mycobacteria, anonymous mycobacteria and environmental mycobacteria. The preferred name is NTM. | Mtb is an important member of MTBC responsible for human TB. Other members include M. africanum, M. bovis, M. canettii, M. caprae and M. pinnipedii. |
| NTM species distribution | Nearly 200 species are described using DNA sequencing (a new species is defined as >1% difference in nucleotides); NTM species have regional variation due to climatic and geographical factors. | Mtb strains [Beijing (most pathogenic), Cameroon, CAS, EAI, Haarlem, LAM, Manu (Indian), and S] have geographical variation. |
| Biochemical tests | No single biochemical test is available for the diagnosis of NTM species. Some of the NTM species show positive results with niacin accumulation test (M. simiae, M. chelonae), nitrate reduction test (M. ulcerans, M. szulgai, M. fortuitum, M. smegmatis, M. kansasii), catalase test (M. fortuitum, M. chelonae, M. abscessus, M. ulcerans, M. szulgai, M. kansasii), citrate utilization test (M. chelonae, M. smegmatis), urea hydrolysis test (M. kansasii, M. marinum, M. simiae, M. szulgai, M. scrofulaceum), McConkey agar (without crystal violet) (M. fortuitum, M. abscessus) test and tellurite reduction (M. avium, M. intracellulare, M. simiae, M. fortuitum, M. abscessus). | Mtb is niacin positive, reduces nitrate and is negative for heat-stable catalase test. |
| Microscopic morphology | Absence of characteristics serpentine cords in acid-fast smears. | Characteristic serpentine cording seen as rope-like aggregates in which long axis of the bacilli is parallel to the long axis of the cord in acid-fast smears. |
| Growth characteristics in cultures | Rapidly growing (<7 days) and slowly growing (≥7 days) mycobacteria, growth rates are slower than other bacteria (Pseudomonas aeruginosa and Escherichia coli). | Mtb are slowly growing mycobacteria and take ~2 wk to grow. Ordinary bacteria may take ~20 min to 12-24 h in the laboratory. Mtb colonies are rough, cauliflower-like and light buff in colour. |
| Differential identification | Difficult to differentiate NTM from Mtb only on the basis of positive acid-fast smear. Culture is important in differentiating from P. aeruginosa, Staphylococcus aureus, Nocardia, Aspergillus and Sporothrix, etc. | Both smear and culture should be done. |
| Transmission | Person-to-person transmission does not occur except for M. abscessus among cystic fibrosis patients. | Mtb is highly transmissible through airborne route especially in PTB with cavitary disease and high bacillary loads. |
| Route of entry | Infection occurs mainly by inhalation, ingestion or direct inoculation. Airborne NTM are a major source of entry for NTM-PD. In advanced HIV/AIDS, gut colonization with subsequent haematogenous dissemination occurs. | Smaller cough droplet nuclei (<1-10 µM) carrying Mtb reach terminal bronchioles and alveoli and establish infection. |
| Pathogenicity potential | Opportunistic organisms | Highly pathogenic and obligate parasites |
| Virulence | Generally, NTM have low virulence. | Highly virulent |
| M. kansasii is more virulent among NTM. | ||
| Latent infection | No evidence of latent NTM infection | Systematic data are available regarding LTBI especially in low TB-burden countries. |
| Efforts should be made to differentiate between LTBI and active disease in high TB burden settings. | ||
| Case notification | It is not essential to notify laboratory confirmed, newly diagnosed NTM cases. NTM disease notification is practiced only in a few countries. | Systematic TB notification is encouraged and the global TB report is published annually on a regular basis by the World Health Organization. |
| Pulmonary: extrapulmonary disease proportions | Pulmonary: Extrapulmonary 80-90%: 10-20% in HIV-negative. Disseminated NTM disease occurs in severely immunocompromised individuals such as advanced HIV/AIDS. | Pulmonary 80-85%: extrapulmonary 15-20% in HIV-negative and pulmonary 40-50%: extrapulmonary 50-60% in HIV/AIDS. |
| Risk factors | NTM-PD usually occurs in individuals with pre-existing lung disease or in those with quantitatively impaired mucociliary function or in individuals who are heterozygous for CFTR mutations. | TB can involve both healthy and destroyed lungs. Risk factors include: malnutrition, tobacco smoking, chronic alcohol intake, diabetes mellitus, overcrowding, HIV/AIDS, head or neck cancer, leukaemia, or Hodgkin’s disease, drugs including corticosteroids, TNF-α inhibitors or receptor blocker. |
| Lady Windermere syndrome occurs in post-menopausal non-smoking females with nodular-bronchiectasis, several skeletal abnormalities, increased adiponectin and decreased leptin and oestrogen levels, abnormalities in fibrillin gene, high prevalence of gastroesophageal reflux disease and increased susceptibility to NTM infections. | ||
| NTM species predilection for various organs | Pulmonary: MAC, M. kansasii, M. xenopi, M. malmoense, M. abscessus, M. fortuitum M. simiae | No such predilection for body organs is known in TB. |
| Skin: M. ulcerans, M. marinum, M. abscessus, M. chelonae, M. fortuitum | ||
| Soft tissues: M. chelonae and M. fortuitum | ||
| Lymphadenitis: MAC but can occur with other NTM species also. | ||
| Disseminated NTM disease: Most commonly due to MAC but other species can also produce disseminated disease. | ||
| Radiographic patterns in MAC-pulmonary disease | Three types of radiographic patterns occur in MAC NTM-PD: | PTB Primary complex (usually in children) |
| Cavitary: In elderly smokers with COPD patients. | Progressive pulmonary disease | |
| NB: Predominantly in post-menopausal non-smoking females; bilateral bronchiectasis, multiple nodules and tree-in-bud appearance on HRCT, some may also have small cavitary lesions. | Post-primary PTB: Cavitary, atelectasis, consolidation Miliary PTB | |
| Hypersensitivity pneumonitis-like NTM pulmonary disease due to MAC and M. immunogenum. | Sequelae such as fibrotic and calcified lesions | |
| Clinical relevance of NTM isolates in respiratory specimens | Clinical relevance of isolated NTM species versus activity of the underlying pulmonary disease should be assessed. Colonization in the host and contamination in the laboratory must be ruled out. Causality association of the particular isolated NTM species with the pulmonary disease should be carefully established before starting the treatment. | Mtb produces both latent TB infection and active disease. Active TB disease must be ruled out appropriately before starting the treatment. |
| Drug susceptibility testing (DST) | DST for NTM is controversial because of poor correlation between in vitro DST pattern and in vivo treatment response and outcomes. According to CLSI (2018) guidelines16, initial and recurrent MAC and M. kansasii be tested for DST. | Universal DST should be performed and treatment should be carried out as per sensitivity profile of Mtb. DS-TB, H monoresistance, MDR-TB and XDR-TB should be treated with as per National Guidelines, and tolerance of drugs. |
| Both phenotypic and genotypic DST are performed. | ||
| For MAC, perform DST against macrolides (clarithromycin as a class agent) and amikacin; for M. kansasii, against rifampicin and clarithromycin. | ||
| RGM species (and subspecies) show different drug resistance patterns and DST should be selectively tested for various antibiotics (macrolides, amikacin, tobramycin, imipenem, trimethoprim-sulphamethoxazole, doxycycline, minocylcine, tigecyline, cefoxitin linezolid) DST, erm (41) gene status should be done in M. abscessus. | ||
| Information about erm (41) gene and phenotypic DST for clarithromycin should be done on days 3-5 and 14 in case of M. abscessus. | ||
| Treatment | ATS (2007)17 and BTS (2017)1 ATS/ERS/ESCMID/IDSA18 guidelines on NTM diseases should be followed. | National guidelines should be followed for treatment of drug sensitive and drug-resistant TB. |
| Treatment outcomes | Treatment outcomes differ among NTM species and subspecies. | Globally, treatment outcomes in case of drug-sensitive TB are good. Treatment of drug-resistant TB is still a challenge and global rate of successful treatment is 56% only. With newer drug regimen(s), treatment success rates are likely to improve in future. |
| Prevention | Exposure to NTM from the environmental sources especially household water systems, hospital settings and soil should be avoided. In HIV/AIDS patients (CD4 T-cells counts <50/μl), antimicrobial prophylaxis includes administration of azithromycin (1200 mg/weekly) or clarithromycin (500 mg twice daily) or rifabutin (300 mg/day) along with antiretroviral drugs till CD4 cell count is >100 cells/μl for three months. | Exposure to smear positive PTB should be avoided to halt TB transmission. Chemoprophylaxis for latent TB infection (active TB disease must be ruled out in high TB-burden countries), various treatment options include: isoniazid daily for 6 or 9 months, or combination of rifapentine and isoniazid once weekly for 12 wk or combination of rifampicin and isoniazid daily for 3-4 months or rifampicin alone daily for four months. |
| Vaccines | No vaccine is available at present | BCG vaccine is recommended in high TB burden countries to prevent severe form of TB (miliary and central nervous system TB); newer TB vaccines such as M72/AS01, M. vaccae, MVA85A etc., are in clinical trials. M72/AS01 was significantly protective against TB disease in a Phase IIb trial in Kenya20. |
Disseminated disease: Involvement of two or more non-contiguous body sites through haematogenous route. Note: Underlying oesophageal disease must be ruled out in NTM-PD due to RGM especially M. fortuitum. NTM-PD, NTM-pulmonary disease; MTBC, Mtb complex; CAS, Central Asian strain; EAI, East African Indian strain; LAM, Latin American-Mediterranean strain; COPD, chronic obstructive pulmonary disease; SGM, slowly growing mycobacteria; RGM, rapidly growing mycobacteria; CLSI, Clinical and Laboratory Standards Institute; ATS, American Thoracic Society; BTS, British Thoracic Society; ATS/ERS/ESCMID/IDSA, American Thoracic Sciety European Respiratory Society European Society of Clinical Microbiology and Infectious Diseases Infectious Diseases Society of America; erm, erythromycin ribosome methylation; MOTT, mycobacteria other than TB; LTBI, latent TB infection; CFTR, cystic fibrosis transmembrane conductance regulator; TNF, tumor necrosis factor; MAC, Mycobacterium avium complex; NB, nodular/bronchiectatic; HRCT, high-resolution computed tomography; MDR, multidrug resistant; XDR, extensively drug resistant; PTB, pulmonary TB; BCG, bacille Calmette-Guerin. Source: Refs 1,2,3,4,5,6,9,12,13,14,15,16,17,18,19,20
Box I.
Factors contributing to increased non-tuberculous mycobacteria burden
| 1. Genetic evolution in NTM due to mutations leading to increased virulence |
| 2. Environmental and climatic changes due to increased human-manufactured infrastructure |
| 3. Changes in host immunity due to increased life expectancy and immunocompromised population |
| 4. Increased incidence of chronic lung disease |
| 5. Decreasing herd immunity due to declining TB burden especially in high-income countries |
| 6. Widespread availability of CT scanning and laboratory infrastructure for NTM diagnosis |
| 7. Increasing awareness among medical personnel about NTM disease |
| 8. Sharp rise in NTM publications by laboratories and practicing physicians |
CT, computed tomography; NTM, non-tuberculous mycobacteria. Source: Ref. 38
Table IV.
Global prevalence of pulmonary non-tuberculous mycobacteria (NTM) isolation and NTM disease
| Zone | Countries | NTM isolation prevalence per 100,000 individuals | NTM disease prevalence per 100,000 individuals | Commonly isolated NTM species |
|---|---|---|---|---|
| North America | Canada36 | 22.2 | 9.08 | MAC, Mycobacterium xenopi, |
| USA | M. abscessus, M. fortuitum, | |||
| Oregon39 | 12.7 | 8.6 | M. chelonae, M. gordonae | |
| California39 | 191 | NR | MAC, M. kansasii, M. abscessus, | |
| Hawaii22 | 396 | NR | M. xenopi, M. fortuitum | |
| South America | Brazil40 | 1.31 | 0.25 | MAC, M. kansasii, M. abscessus, M. xenopi, M. fortuitum |
| Europe | Ireland41 | 1.9 | 0.2 | MAC, M. kansasii, M. xenopi, |
| Scotland42 | NR | 3.1 | M. malmoense, M. marinum, | |
| The United Kingdom43 | 2.9 | 1.7 | M. szulgai, M. gordonae, | |
| Denmark34 | 2.5 | 1.1 | M. abscessus, M. chelonae | |
| Netherlands44 | 6.3 | 1.4 | ||
| France45 | NR | 0.7 | ||
| Greece46 | 07 | 0.7 | ||
| Croatia47 | 5.3 | 0.75 | ||
| Oceania | Australia48 | 5.9 | 0.56 | MAC, M. kansasii, M. abscessus, |
| New Zealand49 | 3.7 | 0.56 | M. fortuitum, M. simiae | |
| Africa | Kenya50* | 1.7% | NR | MAC, M. abscessus, |
| Nigeria51* | 4.3% | NR | M. malmoense, M. marinum, | |
| Uganda52* | 4.3% | NR | M. xenopi, M. scrofulaceum, | |
| Burkina Faso53* | 20.6% | NR | M. simiae, M. gordonae | |
| Asia | Japan29 | 33-65 | NR | MAC, M. abscessus, M. fortuitum, |
| South Korea54 | 39.6 | NR | M. simiae, M. szulgai, M. chelonae, M. gordonae | |
| China55* | 6.3% | NR | ||
| Taiwan30 | 7.94 | NR | ||
| Singapore56* | 511 | NR | ||
| Iran57,58* | 0.7 to 8% | NR | ||
| India59,60,61,62,63* | 0.2 to 5.9% | 0.8% |
*Data presented in % is the isolation of NTM among TB suspected individuals in high TB burden countries Note: NTM isolation data for India provided from Refs 59-63 and disease prevalence from Ref. 61 NR, not reported; MAC, Mycobacterium avium complex
Details of 13 Indian studies published between 1985 and 2019 are summarized in Table V59,60,61,62,63,64,65,66,67,68,69,70,71. Most of these studies have reported NTM isolation rates from laboratories without describing clinical features and treatment details. Two studies were done exclusively on extrapulmonary specimens and 11 on both pulmonary and extrapulmonary specimens. NTM isolation prevalence varied between 0.38 and 23.7 per cent. Six of these 13 studies reported NTM prevalence ≤1 per cent among TB suspects. Almost all except one study have not provided treatment outcomes. Most of the studies (11/13) were hospital based and had selection bias. A large community-based study from south India conducted at four sites in the pre-HIV era has reported NTM isolation prevalence between 4.5 and 8.6 per cent in the sputum specimens. This variable NTM prevalence can be attributed to the following factors: (i) differences in study designs, (ii) standard American Thoracic Society (ATS) (2007)17 and British Thoracic Society (BTS) (2017)1 guidelines criteria were not followed in most of these studies, (iii) only laboratory-related NTM culture data have been reported, and (iv) most of the studies have not provided clinical details and treatment outcomes. Of the 13 studies, only two61,71 followed ATS guidelines (2007)17 and one of these reported treatment outcomes61. Future studies should report about extrapulmonary NTM diseases in addition to clinical details including treatment outcomes of various NTM diseases.
Table V.
Summary of Indian studies on non-tuberculous mycobacteria (NTM)
| Study details | Methods of NTM detection and identification and results | Identified NTM species | Limitations |
|---|---|---|---|
| North zone | |||
| Myneedu et al59, New Delhi Hospital-based prospective study (2009-2011) Total TB suspects=15,581 PTB=12,466 EPTB=3,115 HIV status: Not available |
ZN staining Liquid culture (MGIT 960) Biochemical tests Prevalence: 0.38% (60/15581) in TB suspects Other results: Pulmonary NTM: 45% (27/60) Extrapulmonary NTM: 55% (33/60) |
21 NTM species were identified, % (n) Mycobacterium simiae 11.3 (7) M.avium 9.7 (6) M. gordonae 8.1 (5) M. kansasii 8.1 (5) M. fortuitum 8.1 (5) Others: M. chelonae 8.1 (5), M. phlei 8.1 (5), M. terrae 6.4 (4), M. szulgai 3.2 (2), M. vaccae 3.2 (2), M. flavescens 3.2 (2), M. trivale 3.2 (2), M. malmoense, M. scrofulaceum, M. intracellulare, M. xenopi, M. ulcerans, M. tusciae, M. triplex, M. septicum, M. mucogenicum each 1.6 (1) |
Clinical relevance of isolated NTM is not established. HIV status of patients not provided. Molecular methods such as PCR and gene sequencing not performed for NTM species identification. Treatment details including outcomes not provided. |
| Jain et al60, New Delhi Hospital-based retrospective study (2011-2012) Total TB suspects=436 PTB=237 EPTB=199 HIV status: All negative |
ZN staining Liquid culture (MGIT 960) PNB-LJ culture ICA (SD MPT64TB Ag Kit) Multiplex-PCR Prevalence: 2.98% (13/436) Other results: Pulmonary NTM: 69.2% (9/13) Extrapulmonary NTM: 30.8% (4/13) |
M. kansasii 30 (4) M. chelonae 23.1 (3) M. xenopi 15.4 (2) M. scrofulaceum 7.7 (1) M. avium 7.7 (1) M. asiaticum 7.7 (1) M. fortuitum 7.7 (1) |
Retrospective study on culture isolates. Clinical relevance of isolated NTM not determined. Gene sequencing not used for speciation. Treatment details including outcomes not provided. |
| Maurya et al64, Lucknow, Uttar Pradesh Hospital-based prospective study (2015) EPTB suspects only=756 HIV status: Not available |
ZN staining Liquid culture (BacT/ALERT 3D) ICA (SD MPT64 TB Ag Kit) Biochemical tests LPA (CM/AS Kit) Prevalence: 8.2% (62/756) in EPTB suspects |
M. fortuitum 27.5 (17) M. intracellulare 20.9 (13) M. abscessus 14.6 (9) M. chelonae 12.9 (8) Others: MAC 8.1 (5), M. kansasii 4.8 (3), M. gordonae 3.2 (2), M. interjectum 3.2 (2) and other species 4.8 (3) |
Biased selection of population (EPTB suspects). Molecular techniques such as gene amplification and gene sequencing not used for NTM speciation. Treatment details including outcomes not provided. |
| Umrao et al65, Lucknow, Uttar Pradesh Hospital-based prospective study (2013-2015) TB suspects=4,620 HIV status: Available |
ZN staining Liquid culture (BacT/ALERT 3D) ICA (SD MPT64TB Ag Kit) Biochemical tests LPA (CM/AS Kit) Prevalence: 4.52% (263/4620) in TB suspects Pulmonary NTM: 79.1% (208/263) Extrapulmonary NTM: 20.9% (55/263) Other results: Three NTM patients were HIV positive |
M. abscessus 31.3 (82) M. fortuitum 22 (59) M. intracellulare 13.6 (36) M. chelonae 9.1 (24) M. avium 7.2 (19) M. interjectum 3.4 (9) M. simiae 3.4 (9) Others: M. gordonae 2.6 (7), M. scrofulaceum 1.9 (5), M. kansasii 1.9 (5), M. szulgai 1.7 (4), M. malmoense 0.7 (2), M. intermedium 0.7 (2) |
Gene sequencing not performed for NTM speciation. HIV status of the patients not provided. Clinical data of the patients not available. Treatment details including outcomes not provided. |
| Sairam et al66, New Delhi Hospital-based retrospective study (2015-2017) Total TB suspects=877 HIV status: Not available |
ZN staining GeneXpert MTB/RIF Culture Prevalence: 3.87% (34/877) in TB suspects Pulmonary NTM: 56% (19/877) Extrapulmonary NTM: 34% (15/877) |
M. intracellulare 23.5 (8) M. kansasii 20.5 (7) M. abscessus 14.7 (5) M. fortuitum 2.9 (1) M. chelonae 2.9 (1) M. interjectum 2.9 (1) Other include 11 isolates |
Details of methods of species identification not mentioned. HIV status of patients not provided. Data related to patients having actual disease not clear. Methods of NTM identification and speciation not clearly provided. Treatment details including outcomes not provided. |
| Sharma et al61, New Delhi Hospital-based prospective study (2014-2017) Total TB suspects=5,409 PTB=3,840 EPTB=1,569 HIV status: Available |
ZN and fluorochrome staining GeneXpert MTB/RIF Liquid culture (MGIT 960) ICA (SD MPT64TB Ag Kit) PNB-LJ culture LPA (CM/AS Kit) Mycolic acid analysis by HPLC 16S-23S rRNA ITS gene sequencing Prevalence: 0.7%(42/5409) Other results: Pulmonary NTM: 34 (81%) Extrapulmonary NTM: 8 (19%) M. simiae was repeatedly isolated in one patient with bronchial asthma, he was not treated because of absence of symptoms. One extrapulmonary NTM patient was HIV positive. |
Pulmonary NTM M. intracellulare 32.3 (11) M. abscessus 26.5 (9) M. simiae 14.7 (5) M. kansasii 11.8 (4) M. gordonae 8.8 (3) M. chimaera 2.9 (1) M. senegalense 2.9 (1) Extrapulmonary NTM M. abscessus 75 (6) M. intracellulare 12.5 (1) M. parascrofulaceum 12.5 (1) |
Multi-locus gene sequencing not performed to identify NTM to the subspecies level. |
| South zone | |||
| Paramasivan et al62, Thiruvallur, Tambaram, Madras city, Bangalore Community-based prospective study (1980-81) PTB suspects Thiruvallur: n=16,907 Tambaram: n=3,576 Madras city: n=24,121 Bangalore: n=12,909 HIV status: Pre-HIV era in India |
Solid culture (LJ medium) Biochemical tests Prevalence: 8.6% (1457/16,907): Thiruvallur 7.6% (270/3576): Tambaram 4.5% (1095/24,121): Madras city 4.5% (587/12,909): Bangalore |
Speciation for 1000 isolates from Thiruvallur was done M. avium/intracellulare 22.6 (226) M. terrae complex 12.5 (125) M. scrofulaceum 10.5 (105) M. fortuitum 7.6 (76) Others: M. flavescens 6.7 (67), M. gordonae 6.6 (66), M. chelonae 5.5 (55), M. vaccae 5.4 (54), M. phlei 3.4 (34), M. triviale 3.3 (33), M. smegmatis 1.9 (19), M. gastri 1.8 (18), M. asiaticum 1.5 (15), M. toakiense 1.1 (11), M. marinum 1 (10), M. malmoense 0.9 (9), M. kansasii 0.7 (7), M. szulgai 0.7 (7), M. haemophilum 0.6 (6), M. xenopi 0.5 (5), M. ulcerans 0.5 (5), M. aurum 0.5 (5), M. thermoresistable 0.2 (2), M. aichiense 0.2 (2), M. simiae 0.1 (1), M. thermophilum 0.1 (1), M. neoaurum 0.1 (1) |
Study done in pre-HIV era in India, therefore, it may not provide the true prevalence of NTM disease in the region. |
| Jesudason and Gladstone67, Vellore, Tamil Nadu Hospital-based prospective study (1999-2004) Total TB suspects=32,084 HIV status: Available |
ZN staining, Solid culture (LJ medium) Biochemical tests DST for rapidly growing NTM on Mueller-Hinton agar and for slow growing NTM on LJ medium was done Prevalence: 0.5% (173/32,084) among TB suspects Other results: Pulmonary NTM: 9.8% (17/173) Extrapulmonary NTM: 90.2% (156/173) 6 NTM patients were HIV positive |
Speciation was done only in 115 isolates M. chelonae 46 (53) M. fortuitum 41 (47) M. szulgai 2.6 (3) M. terrae 2.6 (3) Others: M. smegmatis 1.73 (2), M. scrofulaceum 0.9 (1), M. simiae 0.9 (1), M. flavescens 0.9 (1) and M. gordonae 0.9 (1) |
For NTM identification, newer molecular techniques such as gene probes, PCR and DNA sequencing not used. Clinical significance of isolated NTM not established. Data for pulmonary and extra-pulmonary NTM disease provided only for 115 patients. Treatment details including outcomes not provided. |
| Sivasankari et al68, Puducherry Hospital-based prospective study (2003-2004) Total TB suspects=635 PTB=337 EPTB=298 HIV status: Available |
ZN and fluorochrome staining Culture LJ medium Biochemical tests Prevalence: 0.8% (5/635) Other results: All patients had extrapulmonary NTM disease |
M. kansasii 60 (3) M. flavescens 20 (1) M. gordonae 20 (1) |
Molecular techniques such as HPLC, gene amplification and gene sequencing not used. Treatment details including outcomes not provided. |
| Radha Bai Prabhu et al69, Kancheepuram, Tamil Nadu Hospital-based prospective study (2008-2016) TB suspected tubal disease females=173 Only extrapulmonary specimens=urine, POD fluid and endometrial samples HIV status: Available |
ZN and fluorochrome staining Liquid culture (MGIT 960) Histopathological examination PCR Mycolic acid analysis by HPLC Prevalence: 23.7% (63/173) among tubal disease suspects Other results: 41 NTM isolates were associated with tubal disease |
M. chelonae 25.4 (16) M. fortuitum 6.3 (4) M. simiae 3.2 (2) M. kansasii 1.6 (1) M. intracellulare 1.6 (1) M. marinum 1.6 (1) |
Biased selection of population. Molecular methods for species identification not used Clinical relevance of isolated NTM species was not established. Treatment details including outcomes not provided. |
| West zone | |||
| Narang et al70, Wardha, Maharashtra Hospital-based prospective study (2001-2002) HIV-TB coinfection suspects=71 PTB=53 EPTB=14 (In 4 patients, information regarding pulmonary and extrapulmonary status was not available) HIV status: All positive |
Liquid culture (BACTEC 460TB) Biochemical tests Mycolic acid analysis by HPLC Prevalence: 8.4% (6/71) in HIV-TB suspected patients Other result Extrapulmonary NTM=6 |
MAC 50 (3) M. simiae 50 (3) |
Biased selection of the study population (HIV patients only). Molecular techniques not used for species identification. Clinical relevance of isolated NTM not discussed. Treatment details including outcomes not provided. |
| Shenai et al71, Mumbai, Maharashtra Hospital-based prospective study (2005-2008) Total TB suspects=14,627 HIV status: Available |
Liquid culture (MGIT 960) PNB-LJ culture NAP test (BACTEC 460 TB) RLBH assay of rpoB gene PCR-RE assay and gene sequencing Prevalence: 0.8% (127/14627) Other results: Pulmonary NTM: 81% (103/127) Extrapulmonary NTM: 19% (24/127) three NTM cases were HIV positive |
M. intracellulare 40 (32), M. simiae 35 (28), M. abscessus 59 (27), M. fortuitum 29 (19), M. kansasii 6 (5), M. gordonae 4 (3), M. szulgai 2 (2), M. avium 1 (1) Ten cases had mixed infection, 6 with Mtb and 4 had M. kansasii + M. fortuitum 1 (1), M. avium + M. kansasii 2 (2) and M. intracellulare + M. gordonae 1 (1) |
Sequencing of rpoB gene may lead to misidentification of NTM species. Multilocus gene sequencing would have given strength to the study. Treatment details including outcomes not provided. |
| Goswami et al63, Wardha, Maharashtra Community-based prospective survey (2007-2009) PTB suspects=6,445 HIV status: Not available |
Culture Biochemical tests DST by micro-broth dilution method Prevalence: 1% (65/6445) |
M. fortuitum 32.3 (21), M. gordonae 21.5 (14), M. avium 13.8 (9), M. flavescens 10.7 (7) Others: M. scrofulaceum 6.1 (4), M. chelonae 4.61 (3), M. abscessus 4.61 (3), M. kansasii 1.5 (1), M. simiae 1.5 (1), M. gastri 1.5 (1) and M. triviale 1.5 (1) |
Study was performed in PTB suspects only. How TB and NTM were distinguished not clear. HIV status of the patients not available. Gene sequencing for speciation not performed. Patients’ data not available. Treatment details including outcomes not provided. |
PTB, pulmonary TB; EPTB, extra PTB; LJ medium, Löwenstein-Jensen medium; ZN staining, Ziehl-Neelsen staining; DST, drug susceptibility testing; MGIT, mycobacteria growth indicator tube; PNB, p-nitrobenzoic acid; NAP, p-nitro-alpha-acetylamino-beta-hydroxypropiophenone; RLBH, reverse line blot hybridization; PCR-RE assay, polymerase chain reaction-restriction endonuclease assay; ICA, immunochromatographic assay; MPT64, mycobacterial protein 64 KD; LPA, line probe assay; 16S-23S rRNA ITS sequence, 16S-23S ribosomal RNA internal transcribed spacer sequence; MAC, Mycobacterium avium complex; POD, pouch of douglas; HPLC, high-performance liquid chromatography
Risk factors for NTM disease
Risk factors for NTM diseases vary according to the clinical type of NTM disease72,73,74. Various risk factors for NTM-PD are described in Box IIA72,73,74. Pre-existent lung disease is mostly present in these patients. In the absence of obvious structural lung disease, patients may have quantitatively impaired ciliary function or may be heterozygous for cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations75,76. Extrapulmonary NTM disease can occur due to breaches in skin or soft tissues or due to several nosocomial factors, which are detailed in (Box IIB)72,73,74. Disseminated NTM disease generally occurs in patients having primary or acquired immunodeficiency conditions. Certain environmental and organism-related factors such as water sources and reservoirs, and NTM growth characteristics in different climatic conditions, have also been reported as risk factors Box IIB72,73,74. In addition, habits, hobbies and profession of an individual may also increase the risk of having NTM disease74.
Box II.
(A and B): Risk factors for nontuberculous mycobacterial disease (A) Risk factors based on disease sites
| Pulmonary NTM disease | Extrapulmonary NTM disease (generally related to healthcare and commercial establishments) |
|---|---|
| Destroyed lungs due to TB or other diseases like pneumoconioses | Trauma (direct infection from environs) |
| Bronchiectasis (esp. middle lobe and lingula) due to any cause | Cosmetic surgeries |
| Chronic obstructive pulmonary disease | Prosthetic devices and implants |
| Cystic fibrosis-CFTR gene polymorphism* | Organ transplantation |
| Primary ciliary dyskinesia | Dental procedures and surgeries |
| Alpha 1 antitrypsin deficiency | Intramuscular or intradermal injection |
| Lung cancer | Joint injections |
| Thoracic skeletal abnormalities (kyphoscoliosis) | Invasive devices (e.g., pacemakers) |
| Lady Windermere syndrome† | Medical tourism (individuals infected with NTM visiting to some other country) |
| Gastroesophageal reflux disease‡ | |
| Pulmonary alveolar proteinosis | |
| Rheumatoid arthritis with lung involvement | |
| *NTM are isolated in sputum cultures of 3-19.5% of CF patients (majority are MAC). †High prevalence (26-44%) of NTM disease especially nodular-bronchiectatic type in nonsmoking postmenopausal white women who are taller and lean with scoliosis, pectus excavatum and mitral valve prolapse syndrome than their peers, ‡In gastroesophageal reflux disorders, RGM are commonly involved in the disease such as M. fortuitum. BMI: body mass index; CFTR: cystic fibrosis transmembrane receptor; MAC, Mycobacterium avium complex; CF, cystic fibrosis | |
| (B) Miscellaneous risk factors | |
| (i) Immunodeficiency states | |
| (a) Primary* | (b) Acquired |
| Anti-interferon γ-antibodies (blocking of interferon γ-interleukin-12 pathway) | HIV/AIDS status (CD4 counts <50 cells/µl) |
| Anti GM-CSF antibodies (impaired local immunity) | Use of biologics (anti-TNF agents and TNF receptor blockers) |
| NEMO mutations (impaired signal transduction from Toll-like receptors, interleukin-1, and TNFα) | Use of immunosuppressive agents and steroids |
| STAT1 deficiency (low systemic immunity) | |
| IL12 mutations (reduced T-cells and natural killer cells stimulation) | |
| CYBB mutations (decreased bactericidal activity) | |
| GATA2 gene mutations (impaired hematopoietic, lymphatic, and vascular development) | |
| (ii) Environmental factors | |
| (a) Household and lifestyle factors | (b) Climatic and bacterial population factors |
| Soil exposure | Larger water surface area |
| Showers and hot tubs | Higher mean daily potential evapotranspiration |
| Municipal water supply | Higher copper soil levels (helps mycobacteria to form biofilms) |
| Kitchen sink biofilms, ice machines, refrigerator taps | Higher sodium soil levels (more nutrition for mycobacteria) |
| Indoor swimming pool use in past 4 months | Lower manganese soil levels (manganese inhibits mycobacterial growth) |
| Outdoor swimming pool use for at least once a month | Lower top soil depth (high nutrition for mycobacteria due to low vegetation) |
| Infection from spa, Jacuzzi, whirlpool footbath, saunas, pedicure procedures | |
*These mutations are rare and associated with disseminated NTM disease. GM- CSF, granulocyte macrophage colony stimulating factor; NEMO, nuclear factor κB essential modulator; STAT1, Signal transducer and activator of transcription 1 (for disseminated infection); IL-12, interleukin-12; TNF, tumor necrosis factor; CYBB, cytochrome b-245 beta. Source: Refs 72,73,74
Immunopathogenesis of NTM disease
In addition to lung, the most common organ involved affected by NTM, localized and disseminated NTM infections can occur73. Patients with disseminated NTM infections (defined as involvement of two or more non-contiguous body organs) usually have underlying generalized immune defect such as HIV/AIDS, and 2-8 per cent of these patients may have concurrent pulmonary involvement77. Identification of the underlying immune defect is crucial for early diagnosis, treatment and prevention. Patients with NTM disease and underlying primary immunodeficiencies typically present in their childhood or adulthood, whereas those with acquired immunodeficiencies can present at any age (Table VI)73.
Table VI.
Primary and acquired immune deficiencies associated with disseminated non-tuberculous mycobacterial (NTM) infection
| Immunodeficiency | Inheritance | Disease onset | BCG infection | Systematic Salmonella infection | Other possible infection | Granuloma formation | Response to antimicrobial | Indication for immunotherapy | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| Early onset | |||||||||
| IFNGR1/R2 | |||||||||
| Complete | AR | Infancy/early childhood | Yes | Yes | Listeriosis, herpes virus, respiratory syncytial virus, parainfluenza virus infections, TB | No | Very poor | No | Poor |
| Partial | AR | Late childhood | Yes | Yes | TB | No report | Favourable | Variable | Good |
| Partial | AR | Late childhood/adolescence | Yes | Yes | Histoplasmosis, TB | Yes | Favourable | Yes | Good |
| IL12B | AR | Infancy/early childhood | Yes (97%) | Yes (25%) | CMC, disseminated TB, nocardia, Klebsiella spp. infection | Yes | Favourable | Yes | Fair |
| IL12RB1 | AR | Early childhood | Yes (76%) | Yes (43%) | TB, CMC (24%), Klebsiella spp. infection | Yes | Favourable | Yes | Fair |
| STAT1 LOF | |||||||||
| Complete | AR | Infancy (die early without HSCT) | Yes | No | TB, fulminant viral infection (mainly herpes) | Yes | Poor | No | Poor |
| Partial | AR | Infancy/early childhood/adolescence | Yes | Yes (50%) | Severe, curable viral infection (mainly herpes) | No report | Favourable | Yes | Fair |
| Partial | AD | Infancy/early/childhood/adolescence | Yes | No | TB | Yes | Favourable | Yes | Good |
| IRF8 | AR | Infancy | Yes | No | CMC | Poorly formed | Poor | No | Poor |
| IRF8 | AD | Late infancy | Yes | No | No report | Yes | Favourable | No | Good |
| ISG15 | AR | Infancy | Yes | Yes | No report | No report | Favourable | Yes | Good |
| NEMO | XR | Early to late childhood | Yes | No | Invasive Hib infection TB | Yes | Variable | Yes | Fair |
| CYBB | XR | Infancy/early childhood | Yes | No | TB | Yes | Fair | No | Fair |
| Late onset | |||||||||
| GATA2 | AD | Late childhood/adulthood | No | No | HPV, CMV, EBV, Clostridium difficile infections, histoplasmosis, aspergillosis | Yes | Poor | Yes | Poor |
| Anti-IFN-γ antibodies | Acquired | Young adult to elderly | No | Yes | Salmonella spp., Penicillium spp., Histoplasma spp., Cryptococcus spp., B. pseudomallei, VZV, CMV infections | Yes | Poor | No | Fair |
AR, autosomal recessive; AD, autosomal dominant; CMC, chronic mucocutaneous candidiasis; LOF, loss of function; HSCT, haemopoietic stem cell transplantation; Hib, Haemophilis influenzae type b, HPV, human papillomavirus; CMV, cytomegalovirus; EBV, Epstein-Barr virus; VZV, varicella zoster virus; BCG, bacille Calmette-Guerin; B. pseudomallei, Burkholderia pseudomallei; IFN-γ, interferon-gamma; XR, X-linked recessive; IFNGR, interferon-gamma recapter; IL, interleukin; STAT, signal transducer and activator of transcription; IRF, interferon regulatory factor; ISG, interferon-stimulated genes; NEMO, nuclear factor kappa-light-chain-enhancer of activated B cells essential modulator; GATA, transcription factor implicated in early hematopoietic, lymphatic and vascular development. Note: Investigations for GATA2 deficiency should be done in patients with myelodysplastic syndrome and mycobacterial disease. Source: Reproduced with permission from Ref. 73
Antimycobacterial cell-mediated immunity requires a close interaction between myeloid and lymphoid cells (Fig. 1)73. Mononuclear phagocytes after engulfing mycobacteria secrete interleukin-12 (IL-12) which, in turn, stimulates T cells and NK (natural killer) cells through the IL-12 receptor (heterodimer of IL12RB1 and IL12RB2). A complex cascade is triggered by IL-12 receptors via TYK2 (tyrosine kinase) and JAK2 (Janus kinase) signals, leading to STAT-4 (signal transducer and activator of transcription) phosphorylation, homodimerization and nuclear translocation to induce interferon-gamma (IFN-γ) secretion (Fig. 1). IFN-γ binds to its receptor IFNG receptor (IFNGR) (heterodimer of IFNGR1 and IFNGR2) and leads to phosphorylation of JAK2, JAK1 and STAT1 and phosphorylated STAT1 (pSTAT1) homodimerisation. The pSTAT1 homodimer [IFN-γ activators (GAF)] binds to IFN-γ activation sequence which upregulates IFN-γ responsive gene transcription. This cascade leads to activation and differentiation of macrophages. As a result, upregulation of IL-12 and tumour necrosis factor-α (TNF-α) secretions facilitates granuloma formation. After these events, macrophages can kill intracellular mycobacteria being assisted by maturation of mycobacterial phagosome, nutrition deprivation and induction of autophagy, exposure to antimicrobial peptides and reactive oxygen species. The nuclear factor (NF) κB essential modulator-mediated pathway and oxidative burst from macrophages are also important to fight against NTM infection73. Genetic defects in any of these immune factors may disturb the cascade of protection against mycobacterial infection and may lead to disseminated NTM disease73. These immune defects have been summarized in Table VI73.
Fig. 1.
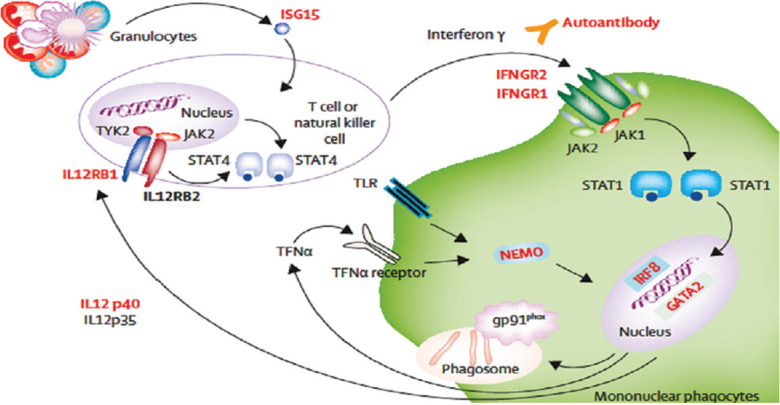
Host defence mechanisms against non-tuberculous mycobacteria (NTM). Defects leading to disseminated NTM infection are shown in red. ISG15, interferon-stimulated gene 15; IFNGR, interferon-gamma receptor; TYK, tyrosine kinase; JAK, Janus kinase; STAT, signal transducer and activator of transcription; IRF, interferon regulatory factor; GATA, transcription factor implicated in early haemopoietic, lymphatic, and vascular development; NEMO, nuclear factor kappa-light-chain-enhancer of activated B cells essential modulator; IL, interleukin; TNF, tumour necrosis factor; TLR, toll-like receptors. Source: Reproduced with permission from Ref. 73.
Clinical manifestations
The clinical manifestations of NTM disease are similar to those of TB and may pose a diagnostic challenge even to an experienced clinician. NTM disease is classified into four clinical types: (i) chronic PD, (ii) lymphadenopathy, (iii) skin and soft tissues, rarely, bones and joints, and (iv) disseminated disease73.
Chronic pulmonary disease (PD)
The ATS and Infectious Disease Society of America (IDSA), 200717, and BTS, 20171, ATS/ERS/ESCMID/IDSA18 have published guidelines to standardize the diagnosis and treatment of NTM diseases. While evaluating NTM suspects, the following criteria should be followed: (i) pulmonary symptoms, nodular or cavitary opacities on chest radiograph, or high-resolution computed tomography (CT) scan that shows multifocal bronchiectasis with multiple, small nodules; (ii) positive culture results from at least two separate expectorated sputum samples [if the results from the initial sputum samples are non-diagnostic, consider repeat sputum acid-fast bacilli (AFB) smear and culture]; single-positive NTM culture from CT-directed bronchoalveolar lavage or bronchial washing specimen from the affected lung segment of NTM suspect who cannot expectorate sputum or whose sputum is consistently culture-negative; and (iii) other disorders such as TB and fungal infections must be excluded1,17.
Patterns of NTM-PD: Chronic PD is the most common form of NTM disease. Three patterns of pulmonary involvement have been described17: (i) fibro-cavitary type, which usually occurs in the upper lobe with a history of smoking in an older male patient with pre-existent lung disease such as chronic obstructive pulmonary disease (COPD), bronchiectasis and CF (Fig. 2); (ii) nodular/bronchiectatic type of pattern occurring in post-menopausal, non-smoking females, predominantly having right middle lobe and left lingular bronchiectasis with a few lung nodules. This syndrome was described after the main character in Oscar Wilde's eponymous play as 'Lady Windermere syndrome'78, and was believed to occur from voluntary cough suppression79, however, subsequently, this hypothesis was discarded80. Other features include mitral valve prolapse, scoliosis and pectus excavatum; high prevalence of gastro-oesophageal reflux disease (GERD) (26-44%)81,82; increased adiponectin; decreased leptin and estrogen levels and abnormalities in fibrillin gene. Presence of all these features increases the susceptibility of these females to MAC infections83; and (iii) hypersensitivity pneumonitis-like NTM PD or 'hot tub lung' occurring due to exposure to aerosols from indoor hot tub. Various risk factors for NTM-PD are listed in Box IIA.
Fig. 2.
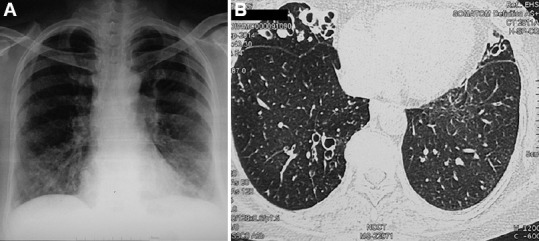
(A) Chest radiograph in a 62 yr old female with asthma, allergic bronchopulmonary aspergillosis and bronchiectasis. Mycobacterium simiae was isolated repeatedly from the sputum. (B) High-resolution computed tomography chest (axial section) showing bilateral bronchiectasis in the right middle lobe, lingula and lower lobes.
NTM species and NTM-PD: Because of variable virulence, it is important to identify NTM species and M. abscessus subspecies for the management of NTM-PD. It has been reported that only 25-60 per cent of patients with positive respiratory specimen fulfil clinical, radiographic and microbiological criteria of NTM-PD84. Patients in whom M. kansasii and M. malmoense are isolated from respiratory specimens frequently meet clinical disease criteria, as these NTM isolates are clinically highly relevant, whereas 40-60 per cent with MAC, M. abscessus and M. xenopi and <20 per cent of patients with M. chelonae and M. fortuitum meet clinical disease criteria44,85,86,87,88,89.
The potential to produce specific clinical type of lung disease also varies among NTM species. While M. kansasii, M. xenopi and M. malmoense commonly cause fibro-cavitary disease but rarely nodular-bronchiectatic disease17,44,90,91, MAC and M. abscessus cause both types of NTM-PDs and MAC and M. immunogenum cause hypersensitivity pneumonitis-like NTM-PD17.
MAC is the most common NTM isolated from respiratory secretions in patients with NTM-PD. While a single strain of MAC species is repeatedly isolated in the cavitary type, several strains of MAC species may occur simultaneously or the strain may change sequentially in nodular-bronchiectatic type92,93. Relapse versus new re-infection of MAC infection after treatment completion can be differentiated by MAC genotyping94.
According to one study from the USA, while tap water was the source of M. avium infection, soil was the source of M. intracellulare infection94. It has been suggested that patients with M. intracellulare lung disease present at a later stage with adverse prognosis than patients with M. avium lung disease, and M. chimaera is less virulent than M. avium and M. intracellulare95,96. Significant geographic variation exists in the distribution of NTM species in the USA; where M. avium complex was the most common species isolated in the South, M. abscessus/M. chelonae was proportionately higher in the West in one study97. MAC species also vary from region to region: while M. avium is dominantly found in South America and Europe, M. intracellulare is found in South Africa and Australia23. Recurrence rates in MAC-associated lung disease also differ among MAC species95.
The second common NTM species also has a geographical variation. While M. abscessus is the second most common cause of NTM-PD in the USA98, M. kansasii in some European countries including the UK, M. xenopi in some parts of Europe and Canada and M. malmoense in northern Europe are the second most common causes of NTM-PD99. M. kansasii, one of the slowly growing NTM, is most virulent98. About 80 per cent of NTM-PD due to RGM results from M. abscessus100. There are three subspecies of M. abscessus: (i) M. abscessus subsp. abscessus, which is the most common pathogen (45-65%), followed by (ii) M. abscessus subsp. massiliense (20-55%), and (iii) M. abscessus subsp. bolletii (1-18%)101. Patients with gastro-oesophageal disease may have NTM-PD due to RGM such as M. fortuitum17.
Clinical features: Respiratory symptoms and signs in NTM-PD vary depending on the clinical type. In the cavitary type, these may be severe due to the pre-existent underlying lung disease and include shortness of breath, cough with expectoration and haemoptysis, whereas patients with nodular-bronchiectasis have milder respiratory symptoms without pre-existing parenchymal lung disease and nagging cough may be prominent. Constitutional symptoms such as fever, anorexia, progressive fatigue, malaise and weight loss may be present especially in cavitary type of NTM-PD1,17. The clinical and radiographic presentation in M. kansasii PD is similar to Mtb and includes fever, cough with or without haemoptysis and chest pain, and chest X-ray often shows infiltrates and cavitary lesions17,102 (Fig. 3). Patients with hypersensitivity pneumonitis-like NTM-PD have subacute onset of respiratory symptoms involving young individuals without pre-existing lung disease and the prognosis is good17,103,104.
Fig. 3.
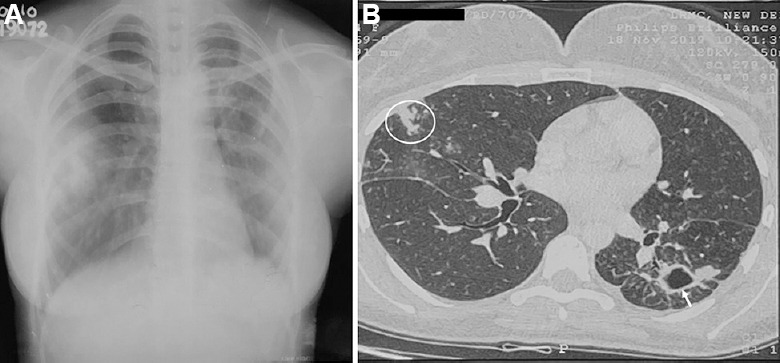
Chest radiograph in a 29 yr old female patient with Mycobacterium kansasii-pulmonary disease. (A) Chest X-ray reveals a cavitary lesion in the left lung. (B) Axial section in the high-resolution computed tomography scan demonstrates a cavity in the left lung (white arrow) and tree-in-bud appearance in the right lung (white circle).
Lymphadenitis
In low TB-burden countries, single-site lymphadenitis is the most common manifestation of NTM infection in younger children74,105. Solitary lymph node is usually localized to the submandibular or cervical region and rarely, can also involve other groups either singly or multiple such as axillary, inguinal region in the disseminated NTM disease in severely immunocompromised individuals106. The lymph node enlargement usually starts as a painless swelling and later in the advanced stage, the swelling becomes fluctuant with pus inside, which may later burst out with a sinus formation. Constitutional symptoms such as fever, weight loss and fatigue may be absent. Smear microscopy and culture may be negative because of paucibacillary nature of the disease17. Molecular tests may be used to establish the diagnosis. MAC is the most frequently isolated NTM species. There is an inverse relationship of TB incidence and NTM disease and in high TB-burden countries, Mtb is the most frequent cause of lymphadenitis in all ages106.
Skin, soft tissues and bone NTM infections
Three types of clinical presentations have been described: (i) Buruli ulcer (predominantly occurring in Uganda) or Bairnsdale ulcer disease (predominantly occurring in Australia), certain regional pockets in Latin America and China: it is a severe cutaneous disease due to M. ulcerans which progresses from nodular cutaneous lesions into large painless ulcers107. These organisms produce a toxin, mycolactone, which produces damage to the skin108. Early diagnosis and treatment is essential to minimize morbidity and costs and prevent long-term disability109; (ii) infection due to M. marinum is also known as fish-tank granuloma (previously known as swimming pool granuloma) and the infection can be acquired from swimming pools, cleaning of fish tanks or any other fish- or water-related activity110. Organisms usually gain access through skin cuts or abrasions111. It starts as a single papulonodular, verrucous or ulcerated granulomatous lesion over the hand and forearm that progresses to form multiple skin lesions in a sporotrichoid pattern - appearance which is similar to skin lesions due to Sporothrix schenckii and rarely, the underlying bone involvement occurs112; and (iii) localized skin and soft-tissue infections occurring due to RGM (M. abscessus, M. fortuitum and M. chelonae) at wound or injection sites113,114,115 (Figs. 4 and 5) and slowly growing mycobacteria in both immunocompromised and immunocompetent individuals115,116. These organisms gain access through skin breaks following trauma and surgical procedures, following the use of surgical instruments without autoclaving, during cosmetic surgery, pedicure and manicure procedures in beauty salons, surgical procedures involving placement of various implants, in mesh used for hernial site repair (Fig. 6), tattooing procedures following inoculation of contaminated ink containing M. haemophilum, intravenous punctures and lines, abscesses due to intramuscular injections through contaminated needles and use of tap water for skin cleaning112,113.
Fig. 4.
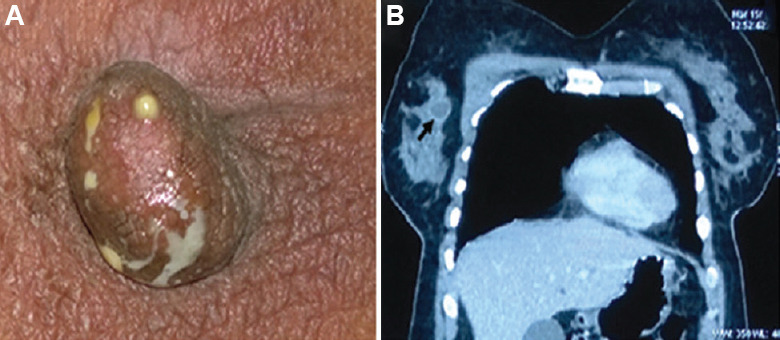
(A) A 35 yr old female presented with discharge from the right nipple, Mycobacterium abscessus was isolated from the pus on several occasions prior to treatment. (B) Computed tomography (CT)-chest showing enhancement of the margin of the abscess (black arrow) with intravenous contrast. Source: Reproduced with permission from Ref. 61.
Fig. 5.
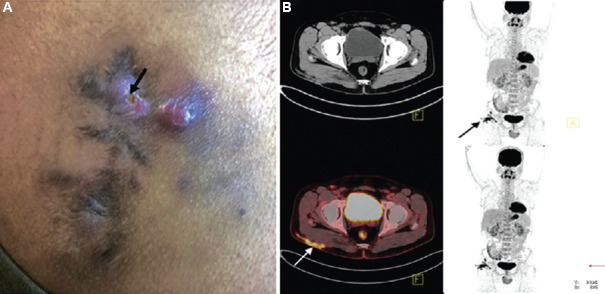
(A) Clinical photograph of a 30 yr old male, showing right-sided post-injection gluteal abscess (black arrow) in a patient with NTM infection. (B) Transaxial fused 18F-fluorodeoxyglucosepositron emission tomography-computed tomography (18F-FDG-PET-CT) image of the same patient, at the level of acetabulum showing FDG accumulation in the subcutaneous thickening and stranding (arrow) involving the underlying right gluteus muscle superficially in right gluteal region. Source: Reproduced with permission from Ref. 61.
Fig. 6.
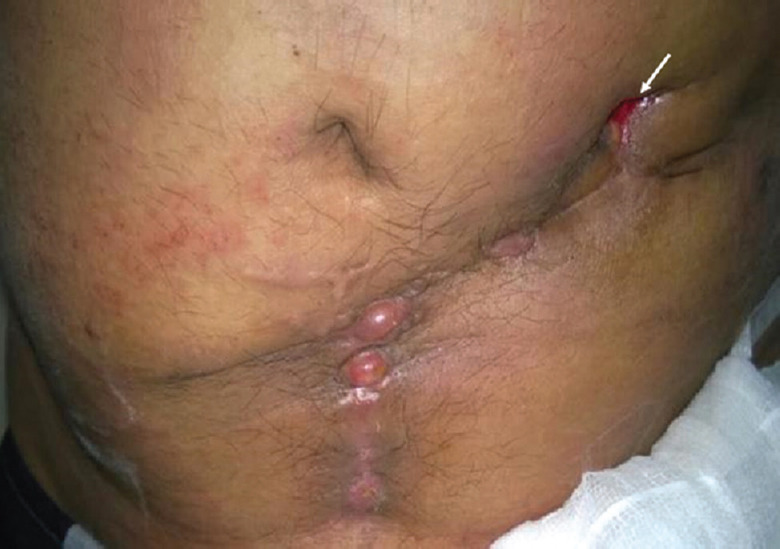
Clinical photograph of a 35 yr old male, showing discharging sinus (white arrow) in the abdominal wall in a patient infected with Mycobacterium abscessus following hernia repair with mesh. Source: Reproduced with permission from Ref. 61.
Disseminated NTM disease
Disseminated NTM disease due to MAC is frequent in HIV/AIDS especially in patients with CD4+ lymphocyte count <50 cells/μl. Isolated pulmonary involvement is rare in HIV/AIDS117. Pulmonary involvement occurs in 2.5-8 per cent of patients with disseminated MAC77. The portal of entry in these patients is believed to be through bowel118,119,120 and occasionally through lungs with subsequent haematogenous dissemination. MAC (predominantly M. avium) is the most common NTM species isolated in these patients17. These patients typically present with insidious onset of constitutional symptoms comprising fever with night sweats, weight loss, abdominal pain, diarrhoea and malaise17. They may have anaemia, hepatosplenomegaly and lymphadenopathy17. Somehow, disseminated NTM infections due to rapidly growing NTM (M. abscessus and M. fortuitum) are rare in HIV/AIDS patients121. Besides M. avium, less common NTM species such as M. genavense and M. simiae can also cause disseminated NTM disease in HIV/AIDS patients17.
M. kansasii can cause pulmonary involvement in HIV/AIDS patients at higher CD4+ counts, and its isolation should always be considered a potential pathogen17,122. Pulmonary involvement can also occur in other immunocompromised populations such as organ transplantation (6.5%)123, bone marrow (2.9%)124 and rarely liver and kidney transplantation. CF patients undergoing lung transplantation may develop life-threatening infection with M. abscessus124. Disseminated NTM infections can also occur in a few other rare settings (Fig. 7A-G) which will require appropriate investigations. These have been listed in (Box IIB)73. NTM, especially M. abscessus (Fig. 7) and M. fortuitum, may infect deep indwelling lines17,122. Anti-tumour necrosis factor-α agents (infliximab, etanercept and adalimumab) used to treat several diseases such as rheumatoid arthritis, psoriatic arthritis and inflammatory bowel disease can predispose to both TB and NTM diseases125. A good response to rituximab in disseminated MAC patients with interferon-gamma autoantibodies has also been reported126,127.
Fig. 7.
The patient, a 14 yr old male, had disseminated Mycobacterium intracellulare infection; no immune defect could be detected. He was successfully treated. (A) The magnetic resonance imaging scan shows osteomyelitis of foot bone (black arrow). (B) Black arrow shows healing of cutaneous lesion by keloid formation. (C) Upper part of thigh shows another healed skin lesion (black arrow). (D and E) Hypodense lesions in the spleen (white open circles) and peri-splenic abscess (white arrows). (F) Bilateral conglomerate necrotic axillary (extreme-left and -right arrows) and right paratracheal lymph nodes (long and short arrows in the centre of CT image), calcification is also noted in the lymph nodes. (G) Iliopsoas abscess on the right side (white asterisk). Source: Reproduced with permission from Ref. 61.
Diagnosis
Criteria for the diagnosis of NTM disease
Healthcare providers should carefully assess causality association of the isolated NTM species with patient's symptoms and signs. Approximately, one-third of NTM species are potentially pathogenic for humans128. Some of the common pathogenic NTM species are listed in Table VII2,3,10,129. It is possible that an individual with a particular NTM isolate may not have an active disease or the isolate may not be clinically relevant. While evaluating NTM suspects, the following criteria should be followed: (i) pulmonary symptoms, nodular or cavitary opacities on chest radiograph or high-resolution CT scan that shows multifocal bronchiectasis with multiple small nodules; (ii) positive culture results from at least two separate expectorated sputum samples (if the results from the initial sputum samples are non-diagnostic, consider repeat sputum AFB smear and culture; single-positive NTM culture from CT-directed bronchoalveolar lavage or bronchial washing specimen from the affected lung segment of NTM suspect who cannot expectorate sputum or whose sputum is consistently culture negative); and (iii) other disorders such as TB and fungal infections must be excluded1,17.
Table VII.
Clinically relevant non-tuberculous mycobacteria species
| Types of disease | Names of species |
|---|---|
| Pulmonary disease | MAC, M. kansasii, M. abscessus, M. xenopi, M. simiae, M. malmoense |
| Cervico-facial lymphadenitis | M. scrofulaceum, M. avium, M. malmoense, M. lentiflavum, M. bohemicum |
| Skin and soft tissue | M. ulcerans, M. marinum, M. abscessus, M. fortuitum, M. haemophilum, M. chelonae |
| Bone and joints | MAC, M. kansasii, M. abscessus, M. xenopi, M. goodii, M. terrae |
| Disseminated disease | M. avium, M. intracellulare, M. haemophilum, M. genavense |
Differential diagnosis
Because of similar clinical features and radiographic appearances, diseases such as TB, recurrent pulmonary aspirations, pneumonitis, bronchiectasis, histoplasmosis, aspergillosis and lung cancer should be considered in the differential diagnosis and should be appropriately ruled out. In the laboratory, the presence of Pseudomonas aeruginosa, Staphylococcus aureus, Nocardia and Aspergillus in the specimens must be carefully tested17. It is important to consider the differential diagnosis of Sporothrix schenckii infection in patients suspected to have skin and soft-tissue NTM disease due to M. marinum113.
Specimen collection, transportation and processing
A proper sample collection is crucial to establish a correct laboratory diagnosis of NTM disease. In case of NTM-PD patients, during collection of sputum, environmental and personal contamination should be avoided. To differentiate NTM-PD from occasional presence of NTM in tracheobronchial tract, at least 3 sputum specimens should be tested on separate occasions18. Sampling from extrapulmonary specimens should be obtained directly from the lesion or organ concerned130. Further, instruments used for sampling should be devoid of any contamination, especially in hospital settings. Storage and transportation of specimens should be done carefully130. Once the specimen reaches the laboratory, the process of decontamination should be done in fully sterilized set-up. As NTM are resistant to most of the common disinfectants, careful selection of disinfectants is necessary130. Various precautions for sample collection, transportation and laboratory processing are listed in Box III130.
Box III.
Essentials for Identification of non-tuberculous mycobacteria (NTM)
| Sample collection and transportation to the laboratory |
| For respiratory specimens, individuals should not rinse their mouths with tap water or other fluids before submitting the specimen. |
| Use a sterile, leak proof, disposable plastic container. Avoid waxed containers. Swabs are not recommended for the isolation of mycobacteria. |
| Collect specimens aseptically, reducing contamination with indigenous microbiota. |
| Collect initial specimens before antimicrobial therapy is started. |
| Three early morning specimens collected on three consecutive days are ideal. |
| For induced sputum, sterile hypertonic saline (3-5%) should be used. Avoid contamination with nebulizer reservoir water. |
| In case of BAL or bronchial wash, bronchoscope should be sterile, cleaned with suitable disinfectant not with tap water and saline used should be devoid of any micro-organism growth. (Lidocaine used during BAL procedure may inhibit growth of NTM). |
| While collection of extrapulmonary specimens, surgical instruments should be cleaned cautiously avoiding tap water or stored water. Formalin should not be used as transfer medium. |
| Once samples stored in container, it should not be opened until it reaches to the laboratory. |
| Store at 2-8°C (do not freeze) if transport is delayed more than one hour; should not be kept more than one week |
| Precautions in the laboratory |
| Effect of disinfectant depends on concentration of the disinfectant, duration of disinfection and mycobacterial load in solution or on surface. |
| Avoid use of chlorine, benzalkonium chloride, cetylpyridinium chloride, quaternary ammonium compounds, and phenolic- or glutaraldehyde-based disinfectants as NTM are resistant to these chemicals. |
| Use of tap water or stored distilled water should be avoided. |
| Use of 70% alcohol and 5% phenol as disinfectant is recommended for bench surface cleaning and biosafety filters. |
| Autoclaving (at 131°C under 15 psi pressure) of plasticware and glassware used in laboratory is strongly recommended. |
| Laboratory workers should look for contamination by other micro-organism such as Pseudomonas aeruginosa, Staphylococcus aureus, Nocardia, Aspergillus, etc. |
| Incubation temperature for every species may vary between 27-45°C and requires constant monitoring. |
| Selective drug susceptibility testing should be done. |
| Laboratory workers should be aware about the patient’s disease status and must co-ordinate the treating physician while reporting NTM species and subspecies. |
BAL, bronchoalveolar lavage. Source: Ref. 130
Laboratory diagnosis of NTM disease
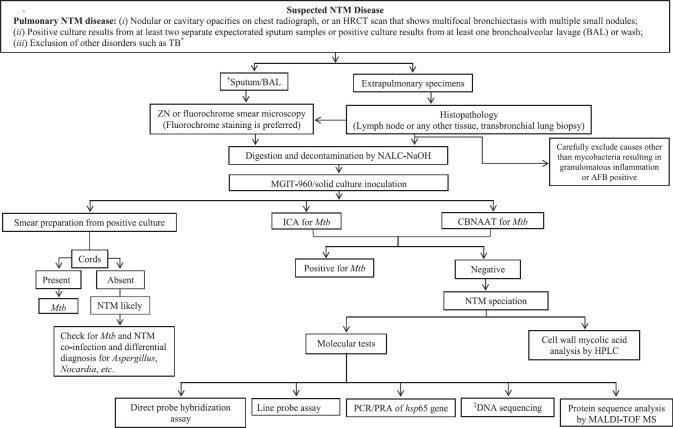
Figure 8 illustrates various steps for NTM isolation and identification in the laboratory. Initially, the specimens are simultaneously subjected to AFB (Ziehl-Neelsen or fluorochrome) staining and GeneXpert for Mtb detection. Samples that are positive on AFB staining and negative on GeneXpert are considered NTM suspects, and the culture for such specimens should be done. Most of the NTM are cultivable in Lowenstein-Jensen, Middle-brook and Dubos Broth and Agar. A novel agar-based medium, RGM medium, has been specifically developed for the isolation of rapidly growing NTM. It provides an alternative method for the recovery of NTM from respiratory specimens, particularly from CF patients, by offering a simple and rapid method for specimen processing131. For some NTM species, additional supplements (haemin for M. haemophilum and mycobactin J for M. paratuberculosis and M. genavense)130 are added in the culture medium for optimal growth. Incubation temperatures of 36±2°C for SGM and 28±2°C for RGM have been recommended18. Appropriate adjustments in the incubation temperature (M. xenopi: 42-45°C, M. ulcerans and M. marinum: 30°C) may be done for a few NTM species18,130. Some NTM species such as M. tilburgii which are not cultivable need to be tested directly from the specimen using molecular methods132. In patients with a high suspicion of NTM-PD but negative cultures, reassessment of decontamination procedures, use of supplemented media and molecular methods may be helpful18. Culture isolates of NTM-suspected specimens should be tested with Mtb-specific tests such as MPT64 antigen immunochromatographic test or GeneXpert, and if found negative, then it is likely to be NTM and thereafter its species identification should be done.
Fig. 8.
Diagnostic algorithm for detection of NTM disease. *According to Ref. 16, consecutive three sputum samples are obtained, positive results from at least two separate expectorated sputum samples confirms the diagnosis. †While sputum collection, the patient should not rinse mouth with municipal or untreated water. Spontaneous sputum should be collected or sputum should be induced if no sputum is produced by patient. ‡Whole genome sequencing (NGS) and multi-locus targeted gene sequencing of gene such as 16S rRNA, hsp65, rpoB, 16S-23S rRNA internal transcribed region (ITS), gyrB, danA, recA and secA. HRCT, high-resolution computed tomography; CSF, cerebrospinal fluid; ICA, immunochromatographic assay; CBNAAT, cartridge based nucleic acid amplification test; L-J, Lowenstein-Jensen media, HPLC: high-performance liquid chromatography, SGM, slowly growing mycobacteria; RGM, rapidly growing mycobacteria; DST, drug susceptibility testing; LPA, line probe assay; PNB: para-nitro benzoic acid; PCR/PRA, polymerase chain reaction/restriction endonuclease assay; MAC, Mycobacterium avium complex; MALDI-TOF MS, matrix-assisted laser desorption ionization-time of flight mass spectrometry. Source: Refs 1,17,130.
Earlier, several biochemical tests were done for NTM identification130 (Table VIII). These tests were cumbersome and time consuming and are obsolete now. High-performance liquid chromatography (HPLC)-based analysis of mycolic acid was used for NTM identification in the past. This method identifies slowly growing NTM species such as MAC and M. kansasii, but it is less specific in identifying RGM accurately130,133. It also has low discriminatory power to identify closely related SGM and RGM species130,133. These tests have now been replaced by molecular tests for NTM species and subspecies identification. These tests include polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis, gene probes and line probe assays (LPA)130 (Table VIII). These molecular tests though identify a limited number of NTM species, but fail to differentiate genetically closely related NTM species133.
Table VIII.
Laboratory methods for non-tuberculous mycobacteria (NTM) identification
| Method | Principle | Advantage(s) | Limitation(s) |
|---|---|---|---|
| Biochemical tests | Based on reaction products after niacin test, nitrate reduction, catalase activity, urease test, pyrazinamidase test, growth in the presence of p-nitrobenzoic acid, and hydrazide of thiophene 2-carboxylic acid | Low-cost tests and expert manpower not required | Time consuming and cumbersome tests; not useful for definitive species identification |
| HPLC | HPLC analysis of number of carbon atoms in mycolic acid found in the cell walls of NTM species | Cost of individual sample testing relatively inexpensive | Problematic for identification of rapidly-growing mycobacteria; limited ability to resolve some NTM groups/complexes |
| PCR-RFLP | Analysis of the band patterns of restricted hsp65 gene fragments which are specific for different NTM species | Specialized equipment not required | Time-consuming; analysis restricted to a small fraction of the genome; requires trained staff; different sequences may share identical RFLP patterns thus it is not useful for definitive species identification especially with newer species/subspecies |
| Nucleic acid probes | Binding of ester-labelled gene DNA probes complementary to 16S rRNA gene | Provide quick results, as analysis may be performed directly on clinical samples | Identifies M. avium, M. intracellulare, M. gordonae, M. kansasii only; shows a cross-reactivity between MAC species and other NTM species |
| LPA | Reverse hybridization of genetic probes | Nucleic acid amplification increases sensitivity; low implementation costs | Useful for species identification but there can be cross reactivity with similar species |
| Gene sequencing | TGS Sequencing of single conserved gene MSLT: multiple conserved gene sequencing and consensus analysis for NTM species identification WGS | Useful for definitive species identification for most clinically relevant species; detects previously unknown mutations. Provides more accurate results than single TGS. | Specificity depends upon selection of gene target; closely related NTM species may not be identified; requires costly specialized equipment. Requires skilled manpower; sequence analysis dependent upon updated and accurate database. |
| Sequencing of entire genome allows detection of different genetic variants within the same population; helpful in understanding geographical and environmental distribution of NTM; useful in studying disease outbreaks and transmission of NTM; also provides information about other features such as virulence and resistance to various antimicrobial agents. | Expensive; data analysis is cumbersome and difficult; drug-resistant variants may be undetected if the drug susceptible variants are in majority; currently available sequencing platforms have problems with analysis of microsatellites. | ||
| MALDI-TOF MS | Analysis of conserved protein sequences | Identifies almost 160 NTM species; most rapid NTM identification test; may identify other organisms such as Nocardia, fungi, thus useful for differential diagnosis | High initial cost; cannot differentiate between subspecies of M. abscessus and species within the MAC, M. fortuitum and M. mucogenicum groups; limited database at present |
HPLC, high-performance liquid chromatography; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism analysis; LPA, line probe assay; MALDI-TOF MS, matrix-assisted laser desorption time-of-flight mass spectrometry; rRNA, ribosomal RNA; TGS, targeted gene sequencing; MSLT, multi-locus sequence typing; WGS, whole genome sequencing; ITS, internal transcribed spacer; MAC, Mycobacterium avium complex. Source: Ref. 130
At present, DNA sequencing is the most accepted method for the identification and characterization of NTM species and subspecies134,135. These techniques include targeted gene sequencing and multi-locus sequence typing (MLST) that involve analysis of conserved genes such as rpoB, hsp65, 16S rRNA and 16S-23S rRNAinternal transcribed spacer (ITS) region134. Targeted sequencing of single gene may identify a reasonable number of NTM species but sometimes may not distinguish species having close genetic association. MLST is preferred as multiple conserved genes are sequenced with this technique and on the basis of consensus analysis of different gene sequences, NTM species are identified more accurately134.
Whole genome sequencing (WGS) is considered the gold standard for NTM species identification and is helpful in understanding the geographical and environmental distribution of NTM species. It is also useful to study healthcare-associated disease outbreaks and transmission134. WGS of NTM species can provide information on other characteristics such as virulence and resistance to various antimicrobial agents135,136. However, DNA sequencing is an expensive method and requires expertise130. This technique is not available in the routine laboratory set-up for NTM diagnosis in resource-limited countries130.
Matrix-assisted laser desorption/ionization-time of flight-mass spectrometry (MALDI-TOF-MS)-based analysis of conserved proteins is another technique available for NTM species identification137. MALDI-TOF-MS is considered the most rapid technique137 which identifies around 160 NTM species138. However, like other techniques, MALDI-TOF-MS also fails to identify closely linked NTM species130. Details of various NTM identifications methods130 are summarized in Table VIII.
Drug susceptibility testing (DST)
DST for NTM is controversial because of discrepancy between in vitro susceptibility and the treatment response101. DST should follow the Clinical and Laboratory Standards Institute (CLSI) guidelines16. CLSI recommends that phenotypic DST should be performed using broth microdilution method18. Both phenotypic and genotypic DST for MAC and M. kansasii are performed for initial and recurrent isolates. Acquired resistance for macrolide in MAC occurs due to point mutations in the 23S rRNA (rrl) gene and for amikacin due to mutations in 16S rRNA (rrs) gene (amikacin resistance is observed in MAC isolates cultured from sputum specimens of patients who were extensively exposed to the drug or related aminoglycosides)18. For MAC, DST against macrolides [clarithromycin is used as a class agent; minimum inhibitory concentration (MIC) cut-off: >32 μg/ml] and amikacin (MIC cut-off: >64 μg/ml for parenteral and >128 μg/ml for liposomal amikacin) and, for M. kansasii, DST against rifampicin (MIC >2 μg/ml) and clarithromycin are used (MIC ≥32 μg/ml)128. When M. kansasii is resistant against rifampicin, DST for amikacin, ciprofloxacin, doxycycline, linezolid, minocycline, moxifloxacin, rifabutin, and trimethoprim-sulfamethoxazole is recommended18. RGM species (and subspecies) show different drug resistance patterns1, and DST should be selectively done for the following antibiotics: macrolides, amikacin, tobramycin, imipenem, trimethoprim-sulphamethoxazole, doxycycline, minocycline, tigecycline, cefoxitin and linezolid1,65. Information on an active erm (41) gene is important in RGM (esp. in M. abscessus subspecies) as it can lead to inducible resistance to macrolides1,17. In M. abscessus subsp massiliense, the erm (41) gene is nonfunctional owing to a large deletion, thus rendering the strains macrolide susceptible. The erm (41) gene is non-functional in some M.abscessus subsp. abscessus due to presence of C instead of T at the nucleotide 28 (arginine 10 instead of tryptophan 10)18. Constitutive resistance to macrolides can occur due to mutation in 23S rRNA gene1. Table IX describes various conditions of macrolide resistance among M. abscessus subspecies. M. chelonae is resistant to cefoxitin and sensitive to tobramycin1.
Table IX.
Interpretation of extended clarithromycin susceptibility results for Mycobacterium abscessus
| Clarithromycin susceptibility (days 3-5) | Clarithromycin susceptibility (day 14) | Genetic implication | M. abscessus subspecies | Macrolide susceptibility phenotype |
|---|---|---|---|---|
| Susceptible | Susceptible | Dysfunctional erm (41) gene | M. abscessus. massiliense | Macrolide susceptible |
| Susceptible | Resistant | Functional erm (41) gene | M. abscessus. abscessus M. abscessus. bolletii | Inducible macrolide resistance |
| Resistant | Resistant | 23S ribosomal RNA point mutation | Any | High-level constitutive macrolide resistance |
Source: Reproduced with permission from Ref. 1
Treatment of NTM disease
Principles of treatment
Several guidelines have been published for the management of NTM diseases1,17,18,19,139. While ATS/ IDSA deals with both pulmonary and extrapulmonary NTM diseases, the US Cystic Fibrosis Foundation and European Cystic Fibrosis Society (ECFS) guidelines, 2016139, include consensus recommendations for the screening, investigation, diagnosis and management of NTM-PD in individuals with CF, and the BTS guidelines (2017)1 and ATS/ERS/ESCMID/IDSA guideline (2020)18 deal with NTM-PDs. The treating physician should be well versed with the prevalence of various NTM species in the geographical area of his/her practice1,17. Despite repeated isolation of NTM, laboratory contamination and colonization in the host must be ruled out. As MAC is the most common cause of NTM-PD worldwide, causality association of repeated NTM isolation in the respiratory specimens should be carefully established after reviewing clinical and radiographic features1,17. Subsequently, the underlying predisposing structural lung disease should be identified and its severity should be evaluated. NTM-PD should be stratified into mild to moderate (non-severe) and severe NTM-PD (Box IV) on the basis of patient's systemic signs and symptoms, chest radiographic appearances and microbiologic features (acid-fast smear status, bacillary load, mycobacterial culture, NTM species and subspecies characterization)1. The conventional microbiological outcomes are smear status, culture conversion and relapse1,140,141(Box V).
Box IV.
Definitions of mild-moderate and severe non-tuberculous mycobacteria (NTM) disease
| Mild-moderate (non-severe disease) |
| Mild-moderate symptoms |
| No signs of systemic illness |
| Absence of lung cavitation and extensive lung disease |
| AFB smear-negative in the pulmonary specimens |
| Severe disease |
| Presence of severe symptoms and signs of systemic illness |
| Presence of lung cavitation and extensive lung involvement |
| Pulmonary specimens positive for AFB smear |
AFB, acid-fast bacilli; NTM-PD, non-tuberculous mycobacterial pulmonary disease. Source: Ref. 1
Box V.
Definitions for microbiological outcomes in non-tuberculous mycobacterial (NTM) disease
| Culture conversion: Three consecutive negative mycobacterial sputum cultures collected over a minimum of three months, with the time of conversion being the date of the first of the three negative mycobacterial cultures. In patients unable to expectorate sputum, a single negative mycobacterial culture of a CT-directed bronchial wash is indicative of culture conversion |
| Recurrence: Two positive mycobacterial cultures following culture conversion. If available, genotyping may help distinguish relapse from reinfection |
| *Refractory disease: failure to culture-convert after six months of NTM treatment |
*Jhun et al140 defined refractory NTM-PD as persistent positive sputum cultures after at least 6 months of multidrug treatment instead of 12 month GBT. In addition, administration of ARIKAYCE plus GBT in patients with MAC pulmonary disease resulted sputum culture conversion by month 6 in 29% cases in comparison to 9% who were on GBT alone. GBT, guideline based treatment. Source: Ref. 1
The decision to start treatment should be made carefully as patients due to MAC remain stable without antibiotic treatment1,17. Early identification of certain clinical, radiographic and microbiological features that are associated with NTM-related progressive PD, is required. These include presence of severe symptoms, low body mass index (BMI) and poor nutritional status (esp. low albumin), lung cavitation, extensive disease, presence of comorbidity, elevated inflammatory markers, and positive AFB smears and isolation of more virulent NTM species18,94,142,143. Recent ATS/ERS/ESCMID/IDSA guideline (2020)18 suggests initiation of treatment rather than watchful waiting, especially in the context of positive AFB sputum smears and/or cavitary lung disease. Whereas, a watchful waiting is preferred in patients with mild signs and symptoms of disease, higher chances of drug intolerance and adverse drug reactions and NTM species less responsive to treatment (e.g.,M. abscessus). In such cases, treatment should be initiated after counselling the patient about potential adverse effects of antimicrobial therapy, the uncertainties surrounding the benefits of antimicrobial therapy, and the possibility for recurrence including reinfection (specifically in nodular-bronchiectatic disease setting). It is also recommended that treatment regimens should be designed by experts in the management of complicated NTM infections18.
NTM-PD is generally treated with a drug regimen, consisting of 3-4 antibiotics, administered either daily or thrice weekly depending on the severity of disease, patient's tolerance of drugs and occurrence of side effects, and the therapy is continued for at least 12 months following sputum conversion17,18. Table X summarizes the treatment durations of pulmonary and extrapulmonary NTM diseases144 due to different species.
Table X.
Durations of treatment for different non-tuberculous mycobacteria (NTM) diseases
| Site of NTM infection | Treatment duration/adjunct therapies | |
|---|---|---|
| Pulmonary | Twelve months after sputum culture becomes negative. | |
| Disseminated disease* | Twelve months after blood culture becomes negative. Secondary prophylaxis is required after this till CD4 count is >100 cells/μl for three months. |
|
| Lymphadenitis† | Surgery alone may be curative in children with NTM cervical lymphadenitis (i.e., MAC). Combination drug therapy is recommended when surgical debridement is not complete or in the setting of disseminated disease in an immunocompromised host. Duration of treatment is variable. In patients with single peripheral lymph node, surgical excision is the treatment of choice. In patients with disseminated disease, treatment duration is longer. | |
| Skin and soft tissue | Four to six months of combination therapy and adjunctive surgery may be done. | |
| Vertebral disease | Twelve months of drug treatment preferred and adjunctive surgery may be done. | |
| Other bone disease | Six to nine months of drug therapy and adjunctive surgery may be done. | |
| Catheter-associated bloodstream infection | Remove iv catheter, if possible. Treatment should be given 1-3 months depending on the immune status of the individual and NTM species. |
*Disseminated disease: Involvement of two or more organs through hematogenous spread. Lung involvement may or may not be present and pulmonary involvement occurs in 2.5-8% of patients with disseminated MAC disease in advanced HIV/AIDS. †In high TB burden countries, Mtb is the commonest cause of lymphadenitis. iv, intravenous. Source: Reproduced with permission from Ref. 144
A significant proportion of patients with NTM-PD discontinues the prescribed treatment because of lengthy duration and occurrence of side effects145. The treatment regimens vary depending on the isolation of NTM species, clinical phenotypes and drug susceptibility profiles, leading to varying therapeutic responses. The variable treatment responses are related to several factors such as NTM species (M. avium vs. M. abscessus) and subspecies (M. abscessus subsp. massiliense vs. M. abscessus subsp. abscessus), disease phenotype [fibrocavitary vs. nodular bronchiectatic (NB)] and the treatment regimen (drug treatment regimen with macrolide vs. without macrolide)146,147,148.
NTM-PD due to MAC is treated with a drug regimen comprising rifampicin (or rifabutin in HIV-positive individuals to avoid drug-drug interactions19), ethambutol and macrolide (azithromycin or clarithromycin; some patients tolerate azithromycin better)1,17. There is an in vitro synergy of antimycobacterial action between rifampicin and ethambutol as the latter destabilizes mycobacterial cell wall and facilitates rifampicin entry into the Mycobacteria to its target site, the RNA polymerase149,150. These two drugs also prevent development of macrolide resistance151. Neither isoniazid nor moxifloxacin is much active against MAC; clofazimine and amikacin are good alternatives. The BTS guidelines (2017)1 and ATS/ERS/ESCMID/IDSA guideline (2020)18 recommend intermittent three times-weekly treatment for non-cavitary (non-severe) MAC-PD due to potential benefits, better treatment adherence and comparable efficacy1,152. As per guidelines, intravenous or nebulized amikacin can be added as the fourth drug for the initial three months in patients with severe or macrolide-resistant MAC-PD1 (Table XI). The pooled treatment success rates in MAC-PD in the five systematic reviews ranged from 32 to 65 per cent, and 12 to 16 per cent of the enrolled patients had not completed treatment153,154,155,156,157.
Table XI.
Suggested antibiotic regimens for adults with Mycobacterium avium complex (MAC)-pulmonary disease
| MAC-pulmonary disease | Antibiotic regimen |
|---|---|
| Non-severe MAC-pulmonary disease (i.e., AFB smear-negative respiratory tract samples, no radiological evidence of lung cavitation or severe infection, mild-moderate symptoms, no signs of systemic illness) | Rifampicin 600 mg 3× per week and ethambutol 25 mg/kg 3× per week and Azithromycin 500 mg 3× per week or clarithromycin 1 g in two divided doses 3× per week antibiotic treatment should continue for a minimum of 12 months after culture conversion. |
| Severe MAC-pulmonary disease (i.e., AFB smear-positive respiratory tract samples, radiological evidence of lung cavitation/severe infection, or severe symptoms/signs of systemic illness) | Rifampicin 600 mg daily and ethambutol 15 mg/kg daily and azithromycin 250 mg daily or clarithromycin 500 mg twice daily and consider intravenous amikacin for up to three months or nebulized amikacin antibiotic treatment should continue for a minimum of 12 months after culture conversion. |
| Clarithromycin-resistant MAC-pulmonary disease | Rifampicin 600 mg daily and ethambutol 15 mg/kg daily and isoniazid 300 mg (+pyridoxine 10 mg) daily or moxifloxacin 400 mg daily and consider intravenous amikacin for up to three months or nebulized amikacin antibiotic treatment should continue for a minimum of 12 months after culture conversion. |
AFB, acid-fast bacilli. Source: Reproduced with permission from Ref. 1
Miwa et al158 in a preliminary open-label study compared three-drug regimen (clarithromycin, ethambutol and rifampicin) with two-drug regimen (clarithromycin and ethambutol) and demonstrated the rate of sputum culture conversion at 40.6 per cent with three-drug regimen versus 55 per cent with two-drug regimen, suggesting that two-drug regimen was not inferior to three-drug regimen. Further, the incidence of adverse events leading to treatment discontinuation was higher with the three-drug regimen (37.2 vs. 26.6%)158.
Koh et al159 evaluated 481 treatment-naïve patients with MAC lung disease who underwent antibiotic treatment for ≥12 months between January 2002 and December 2013. Nearly 58 per cent had non-cavitary NB disease, 17 per cent had cavitary NB disease and 25 per cent had fibrocavitary disease. The treatment outcomes and redevelopment of NTM lung disease after treatment completion differed by the clinical phenotype of MAC lung disease. Cavitary disease was independently associated with unfavourable outcomes. The NB form was an independent risk factor for the redevelopment of NTM lung disease. Of the 29 per cent of favourable outcomes, redevelopment of NTM lung disease occurred with the same MAC species in 55 per cent patients. In patients with recurrent MAC lung disease due to the same species, genotyping revealed that 74 per cent of cases were attributable to reinfection and 26 per cent to relapse159.
Addition of once-daily administration of amikacin liposome inhalation suspension (ALIS) (supplied in single-use vials delivering 590 mg amikacin to the nebulizer), also known as 'Arikayce' to standard guideline-based therapy (GBT) in adults with refractory MAC lung disease (with amikacin-susceptible MAC lung disease and MAC-positive sputum cultures despite at least six months of stable therapy considered to be macrolide-based multidrug treatment), has been reported141. Addition of ALIS to GBT for the treatment of refractory MAC lung disease achieved significantly greater culture conversion by six month than GBT alone. Respiratory adverse events (primarily dysphonia, cough and dyspnoea) were reported more (87.4%) in patients receiving ALIS+GBT than those receiving GBT alone (50%)141. Patients with limited and refractory MAC-PD should be considered for lung resection134.
Patients with clarithromycin-resistant MAC-PD should be treated with rifampicin, ethambutol and isoniazid or a quinolone and intravenous amikacin or nebulized amikacin (if intravenous amikacin is not tolerated or impractical to administer or is contraindicated) for initial three months1 (Table XI). The treatment of macrolide-resistant (MR) MAC-PD is challenging because of poor sputum culture conversion rates (15-36%) and high mortality rates at two year (9-15%) and five-year (47%)160,161. A recent systematic review and meta-analysis of nine studies reported poor treatment outcomes in MR-MAC-PD with overall 21 per cent sputum culture conversion rate and 10 per cent one-year all-cause mortality with no difference between NB and FC types of MR-MAC-PD162. Despite the combination of multiple antibiotics including ALIS and surgical resection, the treatment outcomes of MR-MAC-PD remained poor.
Patients with NTM-PD due to rifampicin-sensitive M. kansasii are treated with a treatment regimen similar to pulmonary TB1,17 comprising rifampicin, ethambutol and isoniazid along with pyridoxine for a fixed duration of 12 months instead of 12 months beyond culture conversion18. Even one-time isolation of M. kansasii from patient's sputum sample is considered pathogenic and should be treated immediately18 (Table XII). Because MICs (minimum inhibitory concentrations) of isoniazid are higher as compared to Mtb, therefore, macrolide (clarithromycin or azithromycin) is preferred over isoniazid for the treatment of M. kansasii163. Pyrazinamide is not recommended for M. kansasii pulmonary disease as the organismisnaturally resistant to pyrazinamide (a prodrug) due to reduced pyrazinamidase activity preventing conversion of the drug into pyrazinoic acid which is an active bactericidal compound164. Cure rates for rifampicin-sensitive M. kansasii have been >98 per cent17. Table XII describes treatment regimens for rifampicin-sensitive and rifampicin-resistant M. kansasii1.
Table XII.
Suggested antibiotic regimen for adults with Mycobacterium kansasii-pulmonary disease
| M. kansasii-pulmonary disease | Antibiotic regimen |
|---|---|
| Rifampicin-sensitive M. kansasii-pulmonary disease* | Rifampicin 600 mg daily and ethambutol 15 mg/kg daily and isoniazid 300 mg (with pyridoxine 10 mg) daily; or azithromycin 250 mg daily or clarithromycin 500 mg twice daily. Antibiotic treatment should continue for a minimum of 12 months after culture conversion. |
| Rifampicin-resistant M. kansasii-pulmonary disease* | Azithromycin 250 mg once daily or clarithromycin 500 mg twice daily and ethambutol 15 mg/kg daily and moxifloxacin 400 mg once daily; or isoniazid 300 mg once daily (with pyridoxine 10 mg) and ethambutol 15 mg/kg daily and moxifloxacin 400 mg once daily. |
*DST guided three-drug regimen from above mentioned antibiotic agents. Pyrazinamide is not recommended for M. kansasii pulmonary disease as the organism is naturally resistant to pyrazinamide (a prodrug) due to reduced pyrazinamidase activity preventing conversion of the drug into pyrazinoic acid which is an active bactericidal compound. Source: Adapted with permission from Refs 1, 18
Table XIII details the treatment of PD due to M. xenopi. While four-drug regimen (rifampicin, ethambutol, macrolide and moxifloxacin) is used to treat non-severe disease, intravenous amikacin or nebulized amikacin is added to the regimen as a fifth drug for severe disease1. In a retrospective matched cohort study comparing M. xenopi PD to MAC-PD, 24-month mortality was higher in M. xenopi-PD with comorbidities, especially COPD. Rifampicin was less frequently used in M. xenopi165.
Table XIII.
Suggested antibiotic regimens for adults with Mycobacterium xenopi-pulmonary disease
| M. xenopi-pulmonary disease | Antibiotic regimen |
|---|---|
| Non-severe M. xenopi-pulmonary disease (i.e., AFB smear-negative respiratory tract samples, no radiological evidence of lung cavitation or severe infection, mild-moderate symptoms, no signs of systemic illness) | Rifampicin 600 mg daily and ethambutol 15 mg/kg daily and azithromycin 250 mg/daily or clarithromycin 500 mg twice daily and moxifloxacin 400 mg daily or isoniazid 300 mg (+pyridoxine 10 mg) daily. Antibiotic treatment should continue for a minimum of 12 months after culture conversion. |
| Severe M. xenopi-pulmonary disease (i.e., AFB smear-positive respiratory tract samples, radiological evidence or lung cavitation/severe infection, or severe symptoms/signs of systemic illness) | Rifampicin 600 mg daily and ethambutol 15 mg/kg daily and azithromycin 250 mg/daily or clarithromycin 500 mg twice daily. Moxifloxacin 400 mg daily or isoniazid 300 mg (+pyridoxine 10 mg) daily and consider intravenous amikacin for up to 3 months or nebulized amikacin. Antibiotic treatment should continue for a minimum of 12 months after culture conversion. |
Source: Reproduced with permission from Ref. 1
Treatment response of macrolide-containing regimen in patients with M. malmoense NTM-PD is better than that of MAC or M. xenopi90. Table XIV provides the details of drug regimen. Treatment for other slowly growing NTM can be extrapolated from common NTM species. Isolation of M. simiae is rarely associated with true infection. Limited success is seen in M. simiae infection with rifampicin- and ethambutol-based drug regimen166, and a combination of amikacin and clofazimine may be used to construct a drug regimen to treat the infection167.
Table XIV.
Suggested antibiotic-regimens for adults with Mycobacterium malmoense-pulmonary disease
| M. malmoense-pulmonary disease | Antibiotic regimens |
|---|---|
| Non-severe M. malmoense-pulmonary disease (i.e., AFB smear-negative respiratory tract samples, no radiological evidence of lung cavitation or severe infection, mild-moderate symptoms, no signs of systemic illness) | Rifampicin 600 mg daily and ethambutol 15 mg/kg daily and azithromycin 250 mg/daily or clarithromycin 500 mg twice daily. Antibiotic treatment should continue for a minimum of 12 months after culture conversion. |
| Severe M. malmoense-pulmonary disease (i.e., AFB smear-positive respiratory tract sample, radiological evidence of lung cavitation/severe infection or sever symptoms/signs of systemic illness) | Rifampicin 600 mg daily and ethambutol 15 mg/kg daily and azithromycin 250 mg/daily or clarithromycin 500 mg twice daily and consider intravenous amikacin for up to 3 months or nebulised amikacin. Antibiotic treatment should continue for a minimum of 12 months after culture conversion. |
Source: Reproduced with permission from Ref. 1
The treatment details of PD due to M. abscessus are provided in Table XV, and antibiotic combination is administered according to the DST profile. In patients with M. abscessus, pulmonary disease is caused by strains with inducible and mutational macrolide resistance, a macrolide-based regimen is recommended if the drug is used as an immunomodulator (macrolide is not considered as active drug in multidrug regimen)18. A precise identification of subspecies along with information on erm (41) gene is important in M. abscessus infection1 because of a variable treatment response. The treatment outcomes among the three subspecies of M. abscessus differ due to erm (41) gene and inducible and constitutive resistance to macrolides1 (Table XVI). About 15 per cent of M. abscessus strains have a T to C mutation at position 28 in erm (41) gene, making them macrolide susceptible168.
Table XV.
Suggested antibiotic regimens for adults with Mycobacterium abscessus-pulmonary disease
| M. abscessus | Antibiotic regimen |
|---|---|
| Clarithromycin sensitive isolates | Initial phase: ≥1 month† Intravenous amikacin 15 mg/kg daily or 3×per week‡ and intravenous tigecycline 50 mg twice daily and where tolerated intravenous imipenem 1 g twice daily and where tolerated oral clarithromycin 500 mg twice daily or oral azithromycin 250-500 mg daily. Continuation phase: Nebulized amikacin‡ and oral clarithromycin 500 mg twice daily or azithromycin 250-500 mg daily and 1-3 of the following antibiotics guided by drug susceptibility results and patient tolerance: Oral clofazimine 50-100 mg daily¦ Oral linezolid 600 mg daily or twice daily Oral moxifloxacin 400 mg daily |
| Inducible macrolide-resistant isolates or constitutive macrolide-resistant isolates | Initial phase: ≥1 month†
Intravenous amikacin 15 mg/kg daily or 3× per week‡ and intravenous tigecycline 50 mg twice daily and where tolerated intravenous imipenem 1 g twice daily. Continuation phase: Nebulized amikacin‡ and 2-3 of the following antibiotics guided by drug susceptibility results and patient’s tolerance: Oral clofazimine 50-100 mg daily¦ Oral linezolid 600 mg daily or twice daily Oral moxifloxacin 400 mg daily |
†Due to the poor response rates in patients with inducible or constitutive macrolide-resistant isolates and the greater efficacy of antibiotics administered through the intravenous route, extending the duration of intravenous antibiotic therapy to 3-6 months in those who can tolerate it may be the most appropriate treatment strategy in this subgroup of patients. ‡Substitute intravenous/nebulized amikacin with an alternative antibiotic if the M. abscessus is resistant to amikacin (i.e., MIC >64 mg/l or known to have a 16S rRNA gene mutation conferring constitutive amikacin resistance). §Start clofazimine during the initial phase of treatment if tolerated as steady-state serum concentrations may not be reached until ≥30 days of treatment. Lower dose of intravenous tigecycline (25-50 mg once daily) may be given if not tolerated. Source: Adapted with permission from Ref. 1
Table XVI.
Drugs used in non-tuberculous mycobacteria (NTM) disease, monitoring and adverse drug reactions
| Drug | Dosing | Monitoring | Serious adverse effects |
|---|---|---|---|
| Clarithromycin (oral) or iv infusion (500 mg twice daily through a large proximal vein if not tolerated orally) | 500 mg twice daily or 500 mg PO twice daily TIW | Monitor QTc prolongation if administered with drugs having potential to prolong QTc, audiograms at baseline, one month, and then every three months; inhibits hepatic metabolism of several agents including rifabutin and some protease inhibitors. Drug levels need not be monitored. Avoid concomitant use of ivabradine, ticagrelor, decrease dose of rifabutin if co-administered with clarithromycin. Increases plasma concentrations of antileptics, phenytoin, carbamazepine (monitor plasma levels), ciclosporin, linezolid (monitor drug level), sirolimus and tacrolimus; coumarins: warfarin; theophylline |
GI disturbances including taste perversion, headache, QTc prolongation especially when co-administered with drugs that have the potential to prolong the QT interval, ototoxicity, dermatological: (toxic epidermal necrolysis and Stevens-Johnson syndrome) hepatic dysfunction, Clostridium difficile-induced diarrhoea. |
| Azithromycin (oral) | 250-500 mg daily | Monitor QTc prolongation if administered with other drugs having potential to prolong QTc; audiogram at baseline, one month, and then every three months | GI disturbances, QTc prolongation when administered with drugs having potential to increase QTc, ototoxicity, hepatitis |
| Ethambutol (oral) | 15 mg/kg per day or 25- 30 mg/kg thrice weekly. Target level 2-6 mg/l; drug levels routinely not measured; only in special situations like renal impairment and poor treatment response. | Crcl ≥30 ml/min: no dose adjustment; Crcl <30 ml/min: 15-25 mg thrice weekly; baseline eye examination and monthly visual acuity tests/colour discrimination tests (Ishihara). Baseline and every three months. Funduscopic monitoring. |
Dose dependent optic (retrobulbar) neuropathy (>30 mg/kg/day or 15-25 mg/kg in CKD); generally, reverses on prompt discontinuation; red-green colour blindness; risk increases with concurrent use of isoniazid; hyperuricemia. |
| Rare: interstitial nephritis, cholestatic jaundice, neutropenia and thrombocytopenia, reversible cutaneous hypersensitivity disappearing on desensitisation | |||
| Rifampicin (oral) | <50 kg: 450 mg once daily or >50 kg: 600 mg once daily (should be taken 30-60 min before food or 2 h after food) | Monitor LFTs, including ALT, AST, alkaline phosphatase, and bilirubin levels | Red/orange discoloration of secretions, GI disturbances, hepatitis, hypersensitivity (fever, rash) |
| Rifabutin (oral) | Routinely 300 mg daily, rarely 450 mg; may administer thrice weekly | Monitor LFTs, including ALT, AST, alkaline phosphatase, and bilirubin levels | Red/orange discolouration of secretions; GI disturbances, loss of taste, hypersensitivity, polyarthralgia, polymyalgia, anterior uveitis and leukopenia (in combination with clarithromycin) |
| Isoniazid (oral) | 5 mg/kg per day (maximum of 300 mg) | Monitor LFTs including ALT and AST levels in patients at risk | Hypersensitivity reaction, hepatitis, peripheral neuropathy, haematological abnormalities (agranulocytosis, megaloblastic anaemia, thrombocytopenia), psychosis (rare) drug induced lupus (rare), arthralgia, rhabdomyolysis |
| Amikacin (intravenous) | 15 mg/kg once daily for 5 days (Monday-Friday) or 15-25 mg thrice weekly. Consider starting with 8-10 mg/kg per day for the elderly and patient with mild renal impairment and titrate upward to goal Cmax. |
Target Cmax 25-35 μg/ml for daily dose and >35-45 μg/ml with thrice weekly administration. Audiometry should be done at baseline and subsequently monthly. A final audiometry should be done 2 months after the final dose. Monitor renal functions weekly in first month, twice weekly in second month and fortnightly thereafter. Preferably avoid or dose adjustment required in CKD. |
Nephrotoxicity: Higher chances in old age and with prolonged use. Ototoxicity: auditory>vestibular; ototoxicity includes hearing loss, loss of balance and tinnitus. Hearing loss occurs first and is detected by audiometric testing. Ototoxicity in audiogram is defined as 20 dB loss from baseline at any one test frequency or a 10 dB loss at any two adjacent test frequencies. Hearing loss is usually permanent. Vertigo, loss of balance and tinnitus. |
| Amikacin (inhalation) Arikayce (liposome inhalation) | 250 mg/ml solution diluted with 3 ml of 0.9% sodium chloride daily, can be increased to 500 mg once daily depending on patient’s tolerance. In patient with reactive airways disease, inhaled bronchodilators can be administered prior to administration to reduce the risk of wheezing and coughing. Oral inhalation, used in a limited and specific population of patients. Use Arikayce vials only with Lamira Nebulizer system. The recommended dosage in adults is once daily oral inhalation of the contents of one 590 mg/8.4 ml of Arikayce vial. Pre-treatment with inhaled bronchodilator should be considered in patients with a history of hyperactive airway disease. |
Observe amikacin trough and creatinine levels after 1-2 wk of therapy, then repeat in one month; audiogram at baseline and then in one month; if all normal, then creatinine and amikacin trough levels, and audiograms every three months. Arikayce use should be reserved for those adults who have limited or no alternative treatment options, for the treatment of MAC lung disease as part of a combination antibacterial drug regimen. This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by month 6. Arikayce has only been studied in refractory MAC lung disease (patient who did not achieve negative sputum cultures after minimum of 6 consecutive mo of multidrug background regimen therapy) |
Dysphonia, respiratory concerns (bronchiectasis exacerbation, dyspnoea); watch for systemic adverse effects as well. |
| Arikayce related increased risk of respiratory adverse events include, common: dysphonia (50%) and coughing (30%), and uncommon: hypersensitivity pneumonitis, haemoptysis, bronchospasm, exacerbation of underlying pulmonary disease. Other adverse reactions include ototoxicity, nephrotoxicity, neuromuscular blockade and embryo-foetal toxicity when administered to a pregnant woman. | |||
| Linezolid (oral or intravenous) | 600 mg daily; may decrease dose to 300 mg after 3-6 months | Careful monitoring for haematological toxicity, lactic acidosis, peripheral and optic neuropathy (often reversible); pyridoxine 100 mg can be administered to prevent haematological toxicity; to prevent serotonin syndrome, avoid tyramine rich food items and medications known to raise serotoin production; monitor CBC count with differential count weekly for 2 wk, then twice weekly. | Haematological toxicity, lactic acidosis, myelosuppression, peripheral and optic neuropathy and serotonin syndrome. Haematological toxicity (early) and lactic acidosis may occur in a few weeks to months whereas neurological toxicity occurs after 3-4 months (late) |
| Levofloxacin (oral) | 500-1000 mg daily | Consider ECG monitoring if additional risk factors present; Dose adjustment required in CKD. Crcl ml/min=750-1000 mg daily, Crcl <30 ml/min=750-1000 mg thrice weekly |
GI upset, dizziness, hypersensitivity, photosensitivity, headache, insomnia, tendinitis, tendon rupture, peripheral neuropathy, CNS effects, headache, agitation, depression, paranoia, seizures, QTc prolongation on ECG |
| Moxifloxacin (oral) | 400 mg daily | Consider ECG monitoring if additional risk factors present; no dose adjustment is required in CKD; hepatobiliary excretion; avoid concomitant use of antacids with aluminium sucralfate, phosphate binders, calcium, iron, or aluminium containing medications to avoid malabsorption | Tendinitis, tendon rupture, peripheral neuropathy, CNS effects, QTc prolongation on ECG |
| Doxycycline (oral) | 100 mg twice daily | Monitor clinical symptoms of the patient | GI disturbances, photosensitivity |
| Minocycline (oral) | 100 mg twice daily | Monitor clinical symptoms of the patient | GI disturbances, photosensitivity, hyperpigmentation of the skin and CNS effects |
| Trimethoprim/sulphamethoxazole (oral) | One double-strength twice or thrice daily | Monitor potassium at baseline, 2 wk, 12 wk then monthly | GI disturbances, cytopenia, renal failure, hyperkalemia |
| Bedaquiline (oral) | 400 mg daily 2 wk and subsequently 200 mg thrice weekly for next 22 wk | Administration with food increases bioavailability; baseline ECG, then 2, 12, 24 wk after initiation to monitor QTc prolongation esp. in combination with clarithromycin, clofazimine and flouroquinolones; stop drug if QTc>500 ms; monitor serum calcium, magnesium and potassium | QTc prolongation, nausea, arthralgia, headache, subjective fever, anorexia |
| Clofazimine (oral) | 50-100 mg daily | ECG monitoring is required if used in combination with bedaquiline, flouroquinolones and macrolides (clarithromycin or azithromycin); monitor serum magnesium, potassium and calcium levels for QTc prolongation correct low levels before stopping the offending drugs ; not used in pregnancy and severe hepatic insufficiency; skin hyperpigmentation can prevented by applying sunscreen and lubricants | GI disturbances, dermatological discoloration: pink to brownish-black skin; discoloration appears within 4 wk and disappears after 6-10 months of the discontinuation, cornea, retina and urine; acne flare within 1-4 wk, ichthyosis and dry skin, QTc prolongation |
| Tobramycin (intravenous) | 5-7 mg/kg per 24 h daily | Obtain peak level 2 and 6 h post dose until therapeutic goal back-extrapolated Cmax 10 the tobramycin MIC, along with undetectable trough. Observe weekly CBC count, creatinine level, tobramycin troughs weekly (should remain <1.2 mg/ml); baseline and monthly audiograms and vestibular function tests | Nephrotoxicity, ototoxicity |
| Imipenem/cilastin (intravenous) | 1g every 12 h (preferred). May consider 500 mg every 12 h for small, frail, or elderly patients |
Monitor serum creatinine level, CBC count with differential count, ALT/AST levels weekly; dose adjustment required in CKD | GI disturbances, seizures, rash, cytopenia |
| Tigecycline (intravenous) | 100 mg loading dose, subsequently 25-50 mg once daily (consider lower dose of 25 mg in case of intolerance to higher dosing) | Obtain serum creatinine level, CBC count, ALT/AST levels weekly; monitor INR and reduce warfarin dose | GI disturbances, hepatitis, prolonged aPTT, prolonged PT |
| Tedizolid (intravenous) | 200 mg every 24 h | Monitor CBC count weekly 2 wk, then twice weekly | Myelosuppression, peripheral neuropathy, serotonin syndrome |
| Cefoxitin (intravenous) | Preferred: 1-2 g every 6-8 h Alternative: 3 g every 12 h | Weekly CBC count monitoring with differential count, creatinine level, and ALT level | Rash, neutropenia, thrombocytopenia |
ALT, alanine transaminase; AST, aspartate transaminase; CBC, complete blood cell; CKD, chronic kidney disease; CNS, central nervous system; Crcl dB, decibel on audiogram; ECG, electrocardiogram; GI, gastrointestinal; LFT, liver function test; MAC, Mycobacterium avium complex; MIC, minimum inhibitory concentration; mo, months; PO, oral; TWI, three times per week; aPTT, activated partial thromboplastin time, PT, prothrombin time; NTM-PD, non-tuberculous mycobacterial pulmonary disease. Source: Refs 1,17,144,190
A systematic review and meta-analysis of the studies on the effect of chemotherapy on pulmonary M. abscessus with macrolide-containing regimens reported adverse microbiological outcomes with frequent recurrences according to the subspecies169. A good outcome was defined as sustained sputum culture conversion (SSCC) without relapse. Macrolide-containing regimens achieved SSCC in only 34 per cent (77/233) patients with new M. abscessus subsp. abscessus vs. 54 per cent (117/141) in those with M. abscessus subsp. massiliense. In refractory disease, SSCC was achieved in 20 per cent of patients, which was not significantly different across subspecies. The proportion of patients with good outcomes (SSCC rate without relapse) was 23 per cent (52/223) with M. abscessus subsp. abscessus versus 84 per cent (118/141) with M. abscessus subsp. massiliense disease. The pooled sputum culture conversion rate was 20 per cent (95% confidence interval, 7-36%), which on follow up after stopping therapy for 12 months was not significantly different across the mycobacterial species. Overall, disease recurrence in M. abscessus subsp. abscessus-infected patients was 40 per cent versus seven per cent in M. abscessus subsp. massiliense-infected patients. The odds ratio of recurrence in M. abscessus subsp. abscessus-infected versus M. abscessus subsp. massiliense-infected patients was 6.2169.
In patients with lung infection due to M. fortuitum, the underlying GERD should be carefully evaluated and treated17,170. Surgical excision is the treatment of choice for younger children with cervicofacial lymphadenitis due to NTM105,171. Treatment of the skin disease due to M. marinum depends on the extent of lesions, hence drug regimen comprising rifampicin and ethambutol or ethambutol and clarithromycin is administered for a single small lesion, whereas triple-drug regimen of rifampicin, ethambutol and a macrolide is used for severe disease172,173,174. Adjunctive surgical debridement is recommended for the underlying bone and joint involvement. Eight-week drug regimens of rifampicin with either clarithromycin or quinolone are administered for the treatment of M. ulcerans skin disease175,176. Disseminated skin and subcutaneous abscesses caused by RGM can be treated with two-drug regimen based on DST results for four months17 in addition to surgical debridement177,178. For M. fortuitum infection, drug regimen may include a combination of co-trimoxazole, tobramycin, imipenem, doxycycline and fluoroquinolones17; M. chelonae infection is treated with two-drug combinations of tobramycin, linezolid, macrolides and imipenem17,177,178. M. abscessus infections may be treated with a combination of the following antibiotics: amikacin, linezolid, cefoxitin, macrolides and imipenem based on DST results17,18. The utility of macrolides depends on erm (41) gene functional status (Table IX).
Recent recommendations for treating disseminated MAC disease in HIV/AIDS patients are provided in Box VI19. Non-steroidal anti-inflammatory drugs (NSAIDS) may be used in HIV patients experiencing moderate-to-severe symptoms of immune reconstitution inflammatory syndrome (IRIS), and short-term course of corticosteroids for 4-8 wk can be used if symptoms persist.
Box VI.
Measures for preventing non-tuberculous mycobacteria (NTM)
| Measures to reduce health care-and hygiene-associated NTM disease |
| Avoid the following |
| Exposure of injection sites, intravenous catheters and surgical wounds to tap water and tap water-derived fluids |
| Cleaning of endoscopes with tap water |
| Contamination of clinical specimens with tap water and ice |
| Use of benzalkonium chloride as a skin disinfectant prior to local injections |
| Household and personal measures |
| Avoid using saunas, hot tubs or any water with an aerator. Hot water usage should be done in proper ventilation |
| Replacement of shower heads at regular intervals; temperature of water heater should be ≥54.4°C |
| Sterilized water should be used in humidifiers; avoid ultrasonic humidifiers |
| Take steps to reduce GERD; avoid foods that may trigger it and avoid vulnerable body positions that may cause aspiration |
| NTM-associated hypersensitivity lung disease |
| Ensure regular cleaning of indoor pools, hot tubs and hot water pipes |
GERD, gastroesophageal reflux disease. Source: Ref. 10
Inhaled antibiotics for NTM-PD
Similar to TB treatment, drug treatment regimens comprising 3-4 drugs are used for treating NTM-PD for longer periods with high discontinuation rates (9-39%) due to significant side effects145,154,179,180. Use of inhaled drugs has demonstrated successful treatment outcomes in bronchial asthma, COPD and Pseudomonas aeruginosa infections in CF patients while achieving higher drug concentrations at the disease site without developing significant systemic side effects at the same time155. Similar approach can be considered in NTM-PD to deliver higher drug concentrations to the infected lungs with minimal extrapulmonary exposure to avoid adverse events. Inhaled amikacin along with other oral drugs is already used in patients with severe NTM-PD1,180,181,182. Development of inhaled clofazimine suspension for administration via nebulizer device in NTM-PD treatment is in progress180. In addition, studies using inhaled recombinant granulocyte-macrophage colony-stimulating factor and exogenous nitric oxide gas are in progress to evaluate their antibacterial effect on M. abscessus183.
Non-pharmacologic treatment of pulmonary NTM disease
In addition to pharmacological therapy, other non-pharmacological measures can be tried for treating the underlying lung disease184 These include techniques for mucus clearance such as nebulization using hypertonic saline, aerobic exercises, chest physiotherapy, postural drainage, use of oscillating positive expiratory pressure devices and high-frequency chest wall oscillation. Intake of balanced diet containing adequate calories and proteins to maintain ideal body weight is essential in the management of NTM diseases185. Following recovery, patients should avoid exposure to minimize re-infection from environmental sources such as hot tubs, use of tap water in humidifiers and continuous positive airway pressure units, use of specialized filtration systems in household plumbing and exposure to soil and dust.
Surgical intervention
Surgery may be considered in carefully selected individuals with NTM-PD. These patients should have localized structural lung disease and good pulmonary functions without having impaired gas exchange1,17,162. The role of a pulmonary and/or infectious disease specialist, a respiratory therapist and a nutrition expert is crucial for a successful surgical outcome186. A review of retrospective anatomic lung resection for NTM-PD in 236 consecutive patients revealed minimal mortality and morbidity and reported that 80 per cent of patients had MAC-PD and had received DST-guided antibiotic treatment prior to surgery187. Data from the annual survey between 2008 and 2012 by the Japanese Association for Thoracic Surgery (JATS) have demonstrated a steady increase in the number of NTM surgeries188. In patients with extrapulmonary NTM disease, surgical intervention may be required through aggressive debridement or removal of implanted material189. Surgical excision is the treatment of choice in patients with solitary peripheral lymph node involvement due to NTM, especially in children105,106,189.
Monitoring of drug toxicities
Drugs used for the treatment of NTM diseases are associated with several adverse events especially in elderly individuals and HIV/AIDS patients with multisystem involvement. During follow up, patients should be carefully monitored for side effects1,144,190. Table XVI provides the details of adverse events and laboratory monitoring.
Prevention
Box VI provides details of various preventive measures to reduce NTM disease in different settings, especially those due to contamination of disinfectants, ice, wounds, injection sites, catheters, endoscopes, etc., can be prevented by proper sterilization2,3. Avoiding the use of tap water is considered a key step to prevent NTM infections in the hospital settings. Further, patients undergoing cardiac surgery and transplants should receive extra attention10. Besides different drug regimens, certain non-pharmacological options are available which can help in improving the quality of life in patients with NTM-PD. Chest physiotherapy can be helpful in improving lung functions and mucociliary clearance, especially in cavitary disease, CF and bronchiectasis. Breathing exercises including aerobic activity such as yoga are generally believed to be helpful in pulmonary rehabilitation160. Besides drug therapy, exposure to NTM, especially from household plumbing and water sources, should be avoided. NTM transmission can be prevented by increasing water temperature to ≥54°C (130°F) and changing shower heads regularly10. Patients with GERD should be advised to avoid foods that may trigger it and avoid vulnerable body positions that may cause repeated aspirations.
Patients should be advised to pay special attention to maintain adequate calorie intake and body mass index especially if surgical intervention is contemplated. Monitoring of pre-albumin level can serve as a useful marker of nutrition184. In some individuals along with antibiotic regimen, probiotic therapy can be helpful.
Box VII details recommendations for preventing disseminated MAC disease19 and includes indications for initiating, discontinuing and restarting primary prophylaxis. Disseminated MAC disease must be carefully ruled out before starting drugs for primary prophylaxis. While azithromycin (1200 mg PO once weekly) or clarithromycin (500 mg PO twice daily) are preferred drugs, rifabutin (300 mg PO daily) is an alternative drug for primary prophylaxis provided that the active TB has been ruled out.
Box VII.
Recommendations for treating and preventing disseminated Mycobacterium avium complex (MAC) disease
| Treating Disseminated MAC Disease |
| Preferred therapy |
| At least 2 drugs as initial therapy to prevent or delay emergence of resistance |
| Clarithromycin 500 mg PO twice daily (AI) plus ethambutol 15 mg/kg PO daily or |
| Azithromycin 500-600 mg (AII) plus ethambutol 15 mg/kg PO daily when drug interactions or intolerance precludes the use of clarithromycin |
| Note: Testing of susceptibility to clarithromycin or azithromycin is recommended. |
| Alternative therapy |
| Some experts would recommend addition of a third or a fourth drug for people with HIV with high mycobacterial loads (i.e., >2 log cfu/ml of blood), or in the absence of effective ART |
| The third or fourth drug options may include: |
| Rifabutin 300 mg PO daily (dose adjustment may be necessary based on drug-drug interactions) |
| or |
| A fluoroquinolone (e.g., levofloxacin 500 mg PO daily or moxifloxacin 400 mg PO daily), or |
| An injectable aminoglycoside (e.g., amikacin 10-15 mg/kg iv daily or streptomycin 1 gm iv or im daily) |
| Chronic maintenance therapy (secondary prophylaxis): Same as treatment regimens |
| Criteria for discontinuing chronic maintenance therapy |
| Completed at least 12 month therapy |
| No signs and symptoms of MAC disease |
| Have sustained (>6 months) CD4 count >100 cells/μl in response to ART |
| Indication for restarting secondary prophylaxis |
| CD4 <100 cells/µl |
| Other considerations |
| NSAIDs may be used for people with HIV who experience moderate to severe symptoms attributed to IRIS |
| If IRIS symptoms persist, a short-term course (four weeks-eight weeks) of systemic corticosteroid (equivalent to prednisone 20-40 mg) can be used |
| Preventing first episode of disseminated MAC disease (primary prophylaxis) |
| Primary prophylaxis is not recommended for adults and adolescents who immediately initiate ART. Indications for initiating primary prophylaxis |
| Not on fully suppressive ART, and |
| CD4 count |
| Preferred therapy |
| Azithromycin 1200 mg PO once weekly or Clarithromycin 500 mg PO BID or azithromycin 600 mg PO twice weekly |
| Alternative therapy |
| Rifabutin 300 mg PO daily (BI) (dose adjustment may be necessary based on drug-drug interactions) |
| Note: Active TB should be ruled out before starting rifabutin. Indication for discontinuing primary prophylaxis |
| Initiation of effective ART indication for restarting primary prophylaxis |
| CD4 count <50 cells/µl (only if not fully suppressive ART) ARTIII |
ART: antiretroviral therapy, ARV, antiretroviral; BID, twice daily; CD4:CD4 T lymphocyte; cfu, colony-forming units; im, intramuscular; IRIS, immune reconstitution inflammatory syndrome; iv, intravenous; NSAIDs, non-steroidal anti-inflammatory drugs; PO, orally. Source: Ref. 19
Future prospects
Future studies should be directed to understand the role of risk factors for developing NTM-PD so that the benefit of screening can be offered to high risk individuals for early diagnosis and treatment. As growing evidence has established human-to-human transmission of M. abscessus among CF patients, further research should be done to study mechanisms contributing to patient-to-patient transmission of other NTM species to prevent further spread. Newer non-culture-based methods should be developed for early identification and speciation of NTM from respiratory specimens as the present methods rely heavily on mycobacterial culture for identification and further characterization of NTM species, causing significant delay in treatment. Future research should focus on understanding the role of DST in predicting treatment outcomes in NTM as currently the role of DST is controversial and limited only to a few situations in the management of NTM. Studies should also focus on understanding the pathogenic potential of various NTM species and subspecies to facilitate decision-making in treatment as there are significant knowledge gaps at present. Efforts should be made to follow progression of inflammatory lung disease systematically and to study treatment outcomes after timely intervention to develop and validate newer drugs and besides conventional routes of drug administration, potential use of drugs through inhalation route should be explored. Less toxic and more effective drug treatment regimens administered for short periods should be developed for the treatment of NTM-PD especially due to M. abscessus as these NTM species respond poorly to treatment with frequent relapses occurring after stopping the treatment.
Footnotes
Financial support & sponsorship: The first author (SKS) is sponsored by JC Bose Fellowship of the Science & Engineering Research Board (SERB No. SB/S2/ JCB-04/2013) of the Ministry of Science & Technology, Government of India. The second author (VU) is a Junior Research Fellow in the Department of Molecular Medicine at Jamia Hamdard University, Delhi, supported by Dr SK Sharma through SERB JC Bose Fellowship.
Conflicts of Interest: None.
References
- 1.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society Guideline for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD) BMJ Open Respir Res. 2017;4:e000242. doi: 10.1136/bmjresp-2017-000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falkinham JO., 3rd Environmental sources of nontuberculous mycobacteria. Clin Chest Med. 2015;36:35–41. doi: 10.1016/j.ccm.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Honda JR, Virdi R, Chan ED. Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front Microbiol. 2018;9:2029. doi: 10.3389/fmicb.2018.02029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkinham JO., 3rd Challenges of NTM drug development. Front Microbiol. 2018;9:1613. doi: 10.3389/fmicb.2018.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Runyon EH. Anonymous mycobacteria in pulmonary disease. Med Clin North Am. 1959;43:273–90. doi: 10.1016/s0025-7125(16)34193-1. [DOI] [PubMed] [Google Scholar]
- 6.Cha SB, Jeon BY, Kim WS, Kim JS, Kim HM, Kwon KW, et al. Experimental reactivation of pulmonary Mycobacterium avium complex infection in a modified Cornell-like murine model. PLoS One. 2015;10:e0139251. doi: 10.1371/journal.pone.0139251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tortoli E, Fedrizzi T, Meehan CJ, Trovato A, Grottola A, Giacobazzi E, et al. The new phylogeny of the genus Mycobacterium: The old and the news. Infect Genet Evol. 2017;56:19–25. doi: 10.1016/j.meegid.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Fedrizzi T, Meehan CJ, Grottola A, Giacobazzi E, Fregni Serpini G, Tagliazucchi S, et al. Genomic characterization of nontuberculous mycobacteria. Sci Rep. 2017;7:45258. doi: 10.1038/srep45258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parte AC. LPSN - List of Prokaryotic names with standing in Nomenclature (bacterio.net), 20 years on. Int J Syst Evol Microbiol. 2018;68:1825–9. doi: 10.1099/ijsem.0.002786. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin SL, Larsen SE, Ordway D, Cassell G, Coler RN. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases. PLoS Negl Trop Dis. 2019;13:e0007083. doi: 10.1371/journal.pntd.0007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarro YD, Kone B, Diarra B, Kumar A, Kodio O, Fofana DB, et al. Simultaneous diagnosis of tuberculous and non-tuberculous mycobacterial diseases: Time for a better patient management. Clin Microbiol Infect Dis. 2018;3 doi: 10.15761/CMID.1000144. doi: 1015761/CMID1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filliol I, Driscoll JR, Van Soolingen D, Kreiswirth BN, Kremer K, Valétudie G, et al. Global distribution of Mycobacterium tuberculosis spoligotypes. Emerg Infect Dis. 2002;8:1347–9. doi: 10.3201/eid0811.020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhalla GS, Sarao MS, Kalra D, Bandyopadhyay K, John AR. Methods of phenotypic identification of non-tuberculous mycobacteria. Pract Lab Med. 2018;12:e00107. doi: 10.1016/j.plabm.2018.e00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteiro PHT, Martins MC, Ueki SYM, Giampaglia CMS, Telles MADS. Cord formation and colony morphology for the presumptive identification of Mycobacterium tuberculosis complex. Braz J Microbiol. 2003;34:171–4. [Google Scholar]
- 15.Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67:813–6. doi: 10.1093/cid/ciy584. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. Susceptibility testing of mycobacteria, Nocardiae, and other aerobic actinomycetes. 3rd ed. Wayne, PA: CLSI; 2018. [PubMed] [Google Scholar]
- 17.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 18.Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71:905–13. doi: 10.1093/cid/ciaa1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. [accessed on May 18, 2020]. Available from: http://aidsinfonihgov/contentfiles/lvguidelines/adult_oipdf .
- 20.World Health Organization. WHO preferred product characteristics for new tuberculosis vaccines. Geneva: WHO; 2018. [Google Scholar]
- 21.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science. 2016;354:751–7. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in US Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185:881–6. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42:1604–13. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 24.Shao Y, Chen C, Song H, Li G, Liu Q, Li Y, et al. The epidemiology and geographic distribution of nontuberculous mycobacteria clinical isolates from sputum samples in the eastern region of China. PLoS Negl Trop Dis. 2015;9:e0003623. doi: 10.1371/journal.pntd.0003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson RM NTM working group at Queensland TB Control Centre and Queensland Mycobacterial Reference Laboratory. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16:1576–83. doi: 10.3201/eid1610.091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prevots DR, Shaw PA, Strickland D, Jackson LA, Raebel MA, Blosky MA, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–6. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin Chest Med. 2015;36:13–34. doi: 10.1016/j.ccm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simons S, van Ingen J, Hsueh PR, Van Hung N, Dekhuijzen PN, Boeree MJ, et al. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis. 2011;17:343–9. doi: 10.3201/eid1703100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morimoto K, Iwai K, Uchimura K, Okumura M, Yoshiyama T, Yoshimori K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc. 2014;11:1–8. doi: 10.1513/AnnalsATS.201303-067OC. [DOI] [PubMed] [Google Scholar]
- 30.Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000-2008. Emerg Infect Dis. 2010;16:294–6. doi: 10.3201/eid1602.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jing H, Wang H, Wang Y, Deng Y, Li X, Liu Z, et al. Prevalence of nontuberculous mycobacteria infection, China, 2004-2009. Emerg Infect Dis. 2012;18:527–8. doi: 10.3201/eid1803.110175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou MP, Clements AC, Thomson RM. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect Dis. 2014;14:279. doi: 10.1186/1471-2334-14-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary Referral Hospital in South Korea. Tuberc Respir Dis(Seoul) 2013;75:199–204. doi: 10.4046/trd.2013.75.5.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andréjak C, Thomsen VØ, Johansen IS, Riis A, Benfield TL, Duhaut P, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: Incidence and prognostic factors. Am J Respir Crit Care Med. 2010;181:514–21. doi: 10.1164/rccm.200905-0778OC. [DOI] [PubMed] [Google Scholar]
- 35.Shah NM, Davidson JA, Anderson LF, Lalor MK, Kim J, Thomas HL, et al. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007-2012. BMC Infect Dis. 2016;16:195. doi: 10.1186/s12879-016-1521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998-2010. Emerg Infect Dis. 2013;19:1889–91. doi: 10.3201/eid1911.130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adelman MH, Addrizzo-Harris DJ. Management of nontuberculous mycobacterial pulmonary disease. Curr Opin Pulm Med. 2018;24:212–9. doi: 10.1097/MCP.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 38.Rivero-Lezcano OM, González-Cortés C, Mirsaeidi M. The unexplained increase of nontuberculous mycobacteriosis. Int J Mycobacteriol. 2019;8:1–6. doi: 10.4103/ijmy.ijmy_18_19. [DOI] [PubMed] [Google Scholar]
- 39.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacteria disease prevalence and risk factors: A changing epidemiology. Clin Infect Dis. 2009;49:e124–9. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 40.Zamarioli LA, Coelho AG, Pereira CM, Nascimento AC, Ueki SY, Chimara E. Descriptive study of the frequency of nontuberculous mycobacteria in the Baixada Santista region of the state of São Paulo, Brazil. J Bras Pneumol. 2008;34:590–4. doi: 10.1590/s1806-37132008000800008. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy MP, O'Connor TM, Ryan C, Sheehan S, Cryan B, Bredin C. Nontuberculous mycobacteria: Incidence in Southwest Ireland from 1987 to 2000. Respir Med. 2003;97:257–63. doi: 10.1053/rmed.2003.1431. [DOI] [PubMed] [Google Scholar]
- 42.McCallum AD, Watkin SW, Faccenda JF. Non-tuberculous mycobacterial infections in the Scottish Borders: Identification, management and treatment outcomes - a retrospective review. J R Coll Physicians Edin. 2011;41:294–303. doi: 10.4997/JRCPE.2011.403. [DOI] [PubMed] [Google Scholar]
- 43.Moore JE, Kruijshaar ME, Ormerod LP. Increasing reports of nontuberculous mycobacteria in England, Wales and Northern Ireland, 1995-2006. BMC Public Health. 2010;10:612. doi: 10.1186/1471-2458-10-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Ingen J, Bendien SA, de Lange WC, Hoefsloot W, Dekhuijzen PN, Boeree MJ, et al. Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region, The Netherlands. Thorax. 2009;64:502–6. doi: 10.1136/thx.2008.110957. [DOI] [PubMed] [Google Scholar]
- 45.Dailloux M, Abalain ML, Laurain C, Lebrun L, Loos-Ayav C, Lozniewski A, et al. Respiratory infections associated with nontuberculous mycobacteria in non-HIV patients. Eur Respir J. 2006;28:1211–5. doi: 10.1183/09031936.00063806. [DOI] [PubMed] [Google Scholar]
- 46.Gerogianni I, Papala M, Kostikas K, Petinaki E, Gourgoulianis KI. Epidemiology and clinical significance of mycobacterial respiratory infections in Central Greece. Int J Tuberc Lung Dis. 2008;12:807–12. [PubMed] [Google Scholar]
- 47.Jankovic M, Samarzija M, Sabol I, Jakopovic M, Katalinic Jankovic V, Zmak L, et al. Geographical distribution and clinical relevance of non-tuberculous mycobacteria in Croatia. Int J Tuberc Lung Dis. 2013;17:836–41. doi: 10.5588/ijtld.12.0843. [DOI] [PubMed] [Google Scholar]
- 48.Haverkort F. National atypical mycobacteria survey, 2000. Commun Dis Intell Q Rep. 2003;27:180–9. [PubMed] [Google Scholar]
- 49.Freeman J, Morris A, Blackmore T, Hammer D, Munroe S, McKnight L. Incidence of nontuberculous mycobacterial disease in New Zealand, 2004. N Z Med J. 2007;120:U2580. [PubMed] [Google Scholar]
- 50.Nyamogoba HD, Mbuthia G, Mining S, Kikuvi G, Biegon R, Mpoke S, et al. HIV co-infection with tuberculous and non-tuberculous mycobacteria in western Kenya: Challenges in the diagnosis and management. Afr Health Sci. 2012;12:305–11. doi: 10.4314/ahs.v12i3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aliyu G, El-Kamary SS, Abimiku A, Brown C, Tracy K, Hungerford L, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013;8:e63170. doi: 10.1371/journal.pone.0063170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asiimwe BB, Bagyenzi GB, Ssengooba W, Mumbowa F, Mboowa G, Wajja A, et al. Species and genotypic diversity of non-tuberculous mycobacteria isolated from children investigated for pulmonary tuberculosis in rural Uganda. BMC Infect Dis. 2013;13:88. doi: 10.1186/1471-2334-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Badoum G, Saleri N, Dembélé MS, Ouedraogo M, Pinsi G, Boncoungou K, et al. Failing a re-treatment regimen does not predict MDR/XDR tuberculosis: is “blind” treatment dangerous? Eur Respir J. 2011;37:1283–5. doi: 10.1183/09031936.00144710. [DOI] [PubMed] [Google Scholar]
- 54.Lee H, Myung W, Koh WJ, Moon SM, Jhun BW. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007-2016. Emerg Infect Dis. 2019;25:569–72. doi: 10.3201/eid2503.181597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu X, Liu P, Liu G, Zhao L, Hu Y, Wei G, et al. The prevalence of non-tuberculous mycobacterial infections in mainland China: Systematic review and meta-analysis. J Infect. 2016;73:558–67. doi: 10.1016/j.jinf.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 56.Lim AYH, Chotirmall SH, Fok ETK, Verma A, De PP, Goh SK, et al. Profiling non-tuberculous mycobacteria in an Asian setting: characteristics and clinical outcomes of hospitalized patients in Singapore. BMC Pulm Med. 2018;18:85. doi: 10.1186/s12890-018-0637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nasiri MJ, Dabiri H, Fooladi AAI, Amini S, Hamzehloo G, Feizabadi MM. High rates of nontuberculous mycobacteria isolation from patients with presumptive tuberculosis in Iran. New Microbes New Infect. 2017;21:12–7. doi: 10.1016/j.nmni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davari M, Irandoost M, Sakhaee F, Vaziri F, Sepahi AA, Rahimi Jamnani F, et al. Genetic diversity and prevalence of nontuberculous mycobacteria isolated from clinical samples in Tehran, Iran. Microb Drug Resist. 2019;25:264–70. doi: 10.1089/mdr.2018.0150. [DOI] [PubMed] [Google Scholar]
- 59.Myneedu VP, Verma AK, Bhalla M, Arora J, Reza S, Sah GC, et al. Occurrence of non-tuberculous Mycobacterium in clinical samples - a potential pathogen. Indian J Tuberc. 2013;60:71–6. [Google Scholar]
- 60.Jain S, Sankar MM, Sharma N, Singh S, Chugh TD. High prevalence of non-tuberculous mycobacterial disease among non-HIV infected individuals in a TB endemic country - experience from a tertiary center in Delhi, India. Pathog Glob Health. 2014;108:118–22. doi: 10.1179/2047773214Y.0000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma SK, Sharma R, Singh BK, Upadhyay V, Mani I, Tripathi M, et al. A prospective study of non-tuberculous mycobacterial disease among tuberculosis suspects at a tertiary care centre in north India. Indian J Med Res. 2019;150:458–67. doi: 10.4103/ijmr.IJMR_194_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paramasivan CN, Govindan D, Prabhakar R, Somasundaram PR, Subbammal S, Tripathy SP. Species level identification of non-tuberculous mycobacteria from South Indian BCG trial area during 1981. Tubercle. 1985;66:9–15. doi: 10.1016/0041-3879(85)90048-0. [DOI] [PubMed] [Google Scholar]
- 63.Goswami B, Narang P, Mishra PS, Narang R, Narang U, Mendiratta DK. Drug susceptibility of rapid and slow growing non-tuberculous mycobacteria isolated from symptomatics for pulmonary tuberculosis, Central India. Indian J Med Microbiol. 2016;34:442–7. doi: 10.4103/0255-0857.195375. [DOI] [PubMed] [Google Scholar]
- 64.Maurya AK, Nag VL, Kant S, Kushwaha RA, Kumar M, Singh AK, et al. Prevalence of nontuberculous mycobacteria among extrapulmonary tuberculosis cases in tertiary care centers in Northern India. Biomed Res Int. 2015;2015:465403. doi: 10.1155/2015/465403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Umrao J, Singh D, Zia A, Saxena S, Sarsaiya S, Singh S, et al. Prevalence and species spectrum of both pulmonary and extrapulmonary nontuberculous mycobacteria isolates at a tertiary care center. Int J Mycobacteriol. 2016;5:288–93. doi: 10.1016/j.ijmyco.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Sairam B, Gogia A, Kakar A, Byotra SP. 781.Non-tuberculous mycobacterium: often a missed entity. Open Forum Infect Dis. 2018;5(Suppl 1):S280. [Google Scholar]
- 67.Jesudason MV, Gladstone P. Non tuberculous mycobacteria isolated from clinical specimens at a tertiary care hospital in South India. Indian J Med Microbiol. 2005;23:172–5. doi: 10.4103/0255-0857.16589. [DOI] [PubMed] [Google Scholar]
- 68.Sivasankari P, Khyriem AB, Venkatesh K, Parija SC. Atypical mycobacterial infection among HIV seronegative patients in Pondicherry. Indian J Chest Dis Allied Sci. 2006;48:107–9. [PubMed] [Google Scholar]
- 69.Radha Bai Prabhu T, Pandiyan N, Sujatha N, Jawahar MS. Significance of isolating non-tuberculous mycobacterial organisms in infertile women with tubal disease: An observational study. BJOG. 2019;126(Suppl 4):66–71. doi: 10.1111/1471-0528.15814. [DOI] [PubMed] [Google Scholar]
- 70.Narang P, Narang R, Mendiratta DK, Roy D, Deotale V, Yakrus MA, et al. Isolation of Mycobacterium avium complex and M. simiae from blood of AIDS patients from Sevagram, Maharashtra. Indian J Tuberc. 2005;52:21–6. [Google Scholar]
- 71.Shenai S, Rodrigues C, Mehta A. Time to identify and define non-tuberculous mycobacteria in a tuberculosis-endemic region. Int J Tuberc Lung Dis. 2010;14:1001–8. [PubMed] [Google Scholar]
- 72.Adjemian J, Olivier KN, Seitz AE, Falkinham JO, 3rd, Holland SM, Prevots DR. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. 2012;186:553–8. doi: 10.1164/rccm.201205-0913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15:968–80. doi: 10.1016/S1473-3099(15)00089-4. [DOI] [PubMed] [Google Scholar]
- 74.Jeon D. Infection source and epidemiology of nontuberculous mycobacterial lung disease. Tuberc Respir Dis (Seoul) 2019;82:94–101. doi: 10.4046/trd.2018.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fowler CJ, Olivier KN, Leung JM, Smith CC, Huth AG, Root H, et al. Abnormal nasal nitric oxide production, ciliary beat frequency, and Toll-like receptor response in pulmonary nontuberculous mycobacterial disease epithelium. Am J Respir Crit Care Med. 2013;187:1374–81. doi: 10.1164/rccm.201212-2197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jang MA, Kim SY, Jeong BH, Park HY, Jeon K, Kim JW, et al. Association of CFTR gene variants with nontuberculous mycobacterial lung disease in a Korean population with a low prevalence of cystic fibrosis. J Hum Genet. 2013;58:298–303. doi: 10.1038/jhg.2013.19. [DOI] [PubMed] [Google Scholar]
- 77.Kalayjian RC, Toossi Z, Tomashefski JF, Jr, Carey JT, Ross JA, Tomford JW, et al. Pulmonary disease due to infection by Mycobacterium avium complex in patients with AIDS. Clin Infect Dis. 1995;20:1186–94. doi: 10.1093/clinids/20.5.1186. [DOI] [PubMed] [Google Scholar]
- 78.Reich JM. In Defense of Lady Windermere Syndrome. Lung. 2018;196:377–9. doi: 10.1007/s00408-018-0122-x. [DOI] [PubMed] [Google Scholar]
- 79.Kumfer AM, Edriss H. Lady Windermere Syndrome. The Chronicles. 2017;5:22–32. [Google Scholar]
- 80.Reich JM. Cough suppression disorders spectrum. Respir Med. 2014;108:413–5. doi: 10.1016/j.rmed.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 81.Koh WJ, Lee JH, Kwon YS, Lee KS, Suh GY, et al. Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest. 2007;131:1825–30. doi: 10.1378/chest.06-2280. [DOI] [PubMed] [Google Scholar]
- 82.Thomson RM, Armstrong JG, Looke DF. Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest. 2007;13:1166–72. doi: 10.1378/chest.06-1906. [DOI] [PubMed] [Google Scholar]
- 83.Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend Med. 2010;7:5–18. doi: 10.1016/j.genm.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 84.Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis. 2016;45:123–34. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 85.Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997-2003. Thorax. 2007;62:661–6. doi: 10.1136/thx.2006.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O'Brien RJ, Geiter LJ, Snider DE., Jr The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987;135:1007–14. doi: 10.1164/arrd.1987.135.5.1007. [DOI] [PubMed] [Google Scholar]
- 87.van Ingen J, Boeree MJ, de Lange WC, Hoefsloot W, Bendien SA, Magis-Escurra C, et al. Mycobacterium xenopi clinical relevance and determinants, the Netherlands. Emerg Infect Dis. 2008;14:385–9. doi: 10.3201/eid1403.061393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Ingen J, de Zwaan R, Dekhuijzen RP, Boeree MJ, van Soolingen D. Clinical relevance of Mycobacterium chelonae-abscessus group isolation in 95 patients. J Infect. 2009;59:324–31. doi: 10.1016/j.jinf.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 89.van Ingen J, Hoefsloot W, Dekhuijzen PN, Boeree MJ, van Soolingen D. The changing pattern of clinical Mycobacterium avium isolation in the Netherlands. Int J Tuberc Lung Dis. 2010;14:1176–80. [PubMed] [Google Scholar]
- 90.Hoefsloot W, Boeree MJ, van Ingen J, Bendien S, Magis C, de Lange W, et al. The rising incidence and clinical relevance of Mycobacterium malmoense: A review of the literature. Int J Tuberc Lung Dis. 2008;12:987–93. [PubMed] [Google Scholar]
- 91.Wallace RJ, Jr, Zhang Y, Brown BA, Dawson D, Murphy DT, Wilson R, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998;158:1235–44. doi: 10.1164/ajrccm.158.4.9712098. [DOI] [PubMed] [Google Scholar]
- 92.Lim H-J, Park CM, Park YS, Lee J, Lee S-M, Yang S-C, et al. Isolation of multiple nontuberculous mycobacteria species in the same patients. Int J Infect Dis. 2011;15:e795–8. doi: 10.1016/j.ijid.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Wallace RJ, Jr, Zhang Y, Brown-Elliott BA, Yakrus MA, Wilson RW, Mann L, et al. Repeat positive cultures in Mycobacterium intracellulare lung disease after macrolide therapy represent new infections in patients with nodular bronchiectasis. J Infect Dis. 2002;186:266–73. doi: 10.1086/341207. [DOI] [PubMed] [Google Scholar]
- 94.Nishiuchi Y, Iwamoto T, Maruyama F. Infection Sources of a common non-tuberculous mycobacterial pathogen, Mycobacterium avium Complex. Front Med (Lausanne) 2017;4:27. doi: 10.3389/fmed.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boyle DP, Zembower TR, Reddy S, Qi C. Comparison of clinical features, virulence, and relapse among Mycobacterium avium complex species. Am J Respir Crit Care Med. 2015;191:1310–7. doi: 10.1164/rccm.201501-0067OC. [DOI] [PubMed] [Google Scholar]
- 96.Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017;49:1600537. doi: 10.1183/13993003.00537-2016. [DOI] [PubMed] [Google Scholar]
- 97.Horne D, Skerrett S. Recent advances in nontuberculous mycobacterial lung infections. F1000Res. 2019;8:F1000 Faculty Rev–1710. doi: 10.12688/f1000research.20096.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wassilew N, Hoffmann H, Andrejak C, Lange C. Pulmonary disease caused by non-tuberculous mycobacteria. Respiration. 2016;91:386–402. doi: 10.1159/000445906. [DOI] [PubMed] [Google Scholar]
- 99.Griffith DE, Girard WM, Wallace RJ., Jr Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271–8. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 100.Koh WJ, Stout JE, Yew WW. Advances in the management of pulmonary disease due to Mycobacterium abscessus complex. Int J Tuberc Lung Dis. 2014;18:1141–8. doi: 10.5588/ijtld.14.0134. [DOI] [PubMed] [Google Scholar]
- 101.Ryu YJ, Koh WJ, Daley CL. Diagnosis and treatment of nontuberculous mycobacterial lung disease: Clinicians' perspectives. Tuberc Respir Dis (Seoul) 2016;79:74–84. doi: 10.4046/trd.2016.79.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fjällbrant H, Akerstrom M, Svensson E, Andersson E. Hot tub lung: an occupational hazard. Eur Respir Rev. 2013;22:88–90. doi: 10.1183/09059180.00002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Larsson LO, Polverino E, Hoefsloot W, Codecasa LR, Diel R, Jenkins SG, et al. Pulmonary disease by non-tuberculous mycobacteria - clinical management, unmet needs and future perspectives. Expert Rev Respir Med. 2017;11:977–89. doi: 10.1080/17476348.2017.1386563. [DOI] [PubMed] [Google Scholar]
- 104.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210–20. doi: 10.3978/j.issn.2072-1439.2013.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhattacharya J, Mohandas S, Goldman DL. Nontuberculous mycobacterial infections in children. Pediatr Rev. 2019;40:179–90. doi: 10.1542/pir.2018-0131. [DOI] [PubMed] [Google Scholar]
- 106.Deveci HS, Kule M, Kule ZA, Habesoglu TE. Diagnostic challenges in cervical tuberculous lymphadenitis: A review. North Clin Istanb. 2016;3:150–5. doi: 10.14744/nci.2016.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Portaels F, Silva MT, Meyers WM. Buruli Ulcer. Clin Dermatol. 2009;27:291–305. doi: 10.1016/j.clindermatol.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 108.Deshayes C, Angala SK, Marion E, Brandli I, Babonneau J, Preisser L, et al. Regulation of mycolactone, the Mycobacterium ulcerans toxin, depends on nutrient source. PLoS Negl Trop Dis. 2013;7:e2502. doi: 10.1371/journal.pntd.0002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sakyi SA, Kennedy MP, Aboagye SY, Darko Otchere I, Yeboah-Manu D. Clinical and laboratory diagnosis of Buruli ulcer disease: A systematic review. Can J Infect Dis Med Microbiol. 2016;2016:5310718. doi: 10.1155/2016/5310718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petrini B. Mycobacterium marinum: Ubiquitous agent of waterborne granulomatous skin infections. Eur J Clin Microbiol Infect Dis. 2006;25:609–13. doi: 10.1007/s10096-006-0201-4. [DOI] [PubMed] [Google Scholar]
- 111.Hashish E, Merwad A, Elgaml S, Amer A, Kamal H, Elsadek A, et al. Mycobacterium marinum infection in fish and man: Epidemiology, pathophysiology and management; a review. Vet Q. 2018;38:35–46. doi: 10.1080/01652176.2018.1447171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tirado-Sánchez A, Bonifaz A. Nodular lymphangitis (sporotrichoid lymphocutaneous infections). Clues to differential diagnosis. J Fungi(Basel) 2018;4:56. doi: 10.3390/jof4020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and soft tissue infections due to rapidly growing mycobacteria: Comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287–92. doi: 10.1001/archderm.142.10.1287. [DOI] [PubMed] [Google Scholar]
- 114.Misch EA, Saddler C, Davis JM. Skin and soft tissue infections due to nontuberculous mycobacteria. Curr Infect Dis Rep. 2018;20:6. doi: 10.1007/s11908-018-0611-3. [DOI] [PubMed] [Google Scholar]
- 115.Xu X, Lao X, Zhang C, Cao C, Ding H, Pang Y, et al. Mycobacterium avium skin and soft tissue infection complicated with scalp osteomyelitis possibly secondary to anti-interferon-γ autoantibody formation. BMC Infect Dis. 2019;19:203. doi: 10.1186/s12879-019-3771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Piersimoni C, Scarparo C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15:1351–8. doi: 10.3201/eid1509.081259. quiz 1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bauer J, Andersen AB, Askgaard D, Giese SB, Larsen B. Typing of clinical Mycobacterium avium complex strains cultured during a 2-year period in Denmark by using IS1245. J Clin Microbiol. 1999;37:600–5. doi: 10.1128/jcm.37.3.600-605.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MacDonell KB, Glassroth J. Mycobacterium avium complex and other nontuberculous mycobacteria in patients with HIV infection. Semin Respir Infect. 1989;4:123–32. [PubMed] [Google Scholar]
- 119.Damsker B, Bottone EJ. Mycobacterium avium-Mycobacterium intracellulare from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J Infect Dis. 1985;151:179–81. doi: 10.1093/infdis/151.1.179. [DOI] [PubMed] [Google Scholar]
- 120.Hill AR. Mycobacterial infections in AIDS. Can J Infect Dis. 1991;2:19–29. doi: 10.1155/1991/476503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gupta-Wright A, Kerkhoff AD, Meintjes G, Corbett EL. Urinary lipoarabinomannan detection and disseminated nontuberculous mycobacterial disease. Clin Infect Dis. 2018;66:158. doi: 10.1093/cid/cix735. [DOI] [PubMed] [Google Scholar]
- 122.Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36:91–9. doi: 10.1016/j.ccm.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chalermskulrat W, Sood N, Neuringer IP, Hecker TM, Chang L, Rivera MP, et al. Non-tuberculous mycobacteria in end stage cystic fibrosis: implications for lung transplantation. Thorax. 2006;61:507–13. doi: 10.1136/thx.2005.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blanc P, DSutronc H, Peuchant O, Dauchy FA, Cazanave C, Neau D, et al. Nontuberculous mycobacterial infections in a French Hospital: A 12-year retrospective study. PLoS One. 2016;11:e0168290. doi: 10.1371/journal.pone.0168290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Winthrop KL, Baxter R, Liu L, Varley CD, Curtis JR, Baddley JW, et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis. 2013;72:37–42. doi: 10.1136/annrheumdis-2011-200690. [DOI] [PubMed] [Google Scholar]
- 126.Browne SK, Zaman R, Sampaio EP, Jutivorakool K, Rosen LB, Ding L, et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood. 2012;119:3933–9. doi: 10.1182/blood-2011-12-395707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yeh YK, Ding JY, Ku CL, Chen WC. Disseminated Mycobacterium avium complex infection mimicking malignancy in a patient with anti-IFN-γ autoantibodies: A case report. BMC Infect Dis. 2019;19:909. doi: 10.1186/s12879-019-4564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Orduña P, Castillo-Rodal AI, Mercado ME, Ponce de León S, López-Vidal Y. Specific proteins in nontuberculous mycobacteria: New potential tools. Biomed Res Int. 2015;2015:964178. doi: 10.1155/2015/964178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18:392–407. doi: 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- 130.Forbes BA, Hall GS, Miller MB, Novak SM, Rowlinson MC, Salfinger M, et al. Practice guidelines for clinical microbiology laboratories: Mycobacteria. Clin Microbiol Rev. 2018;31:e00038–17. doi: 10.1128/CMR.00038-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stephenson D, Perry A, Appleby MR, Lee D, Davison J, Johnston A, et al. An evaluation of methods for the isolation of nontuberculous mycobacteria from patients with cystic fibrosis, bronchiectasis and patients assessed for lung transplantation. BMC Pulm Med. 2019;19:19. doi: 10.1186/s12890-019-0781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Palmore TN, Shea YR, Conville PS, Witebsky FG, Anderson VL, Rupp Hodge IP, et al. “Mycobacterium tilburgii,” a newly described, uncultivated opportunistic pathogen. J Clin Microbiol. 2009;47:1585–7. doi: 10.1128/JCM.02385-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev. 2014;27:727–52. doi: 10.1128/CMR.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kwon YS, Daley CL, Koh WJ. Managing antibiotic resistance in nontuberculous mycobacterial pulmonary disease: Challenges and new approaches. Expert Rev Respir Med. 2019;13:851–61. doi: 10.1080/17476348.2019.1638765. [DOI] [PubMed] [Google Scholar]
- 135.Matsumoto Y, Kinjo T, Motooka D, Nabeya D, Jung N, Uechi K, et al. Comprehensive subspecies identification of 175 nontuberculous mycobacteria species based on 7547 genomic profiles. Emerg Microbes Infect. 2019;8:1043–53. doi: 10.1080/22221751.2019.1637702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Quan TP, Bawa Z, Foster D, Walker T, Del Ojo Elias C Rathod P, et al. Evaluation of whole-genome sequencing for mycobacterial species identification and drug susceptibility testing in a clinical setting: a large-scale prospective assessment of performance against line probe assays and phenotyping. J Clin Microbiol. 2018;56:e01480–17. doi: 10.1128/JCM.01480-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mediavilla-Gradolph MC, De Toro-Peinado I, Bermúdez-Ruiz MP, García-MartínezMde L, Ortega-Torres M, Quezel-Guerraz NM, et al. Use of MALDI-TOF MS for identification of nontuberculous mycobacterium species isolated from clinical specimens. Biomed Res Int. 2015;2015:854078. doi: 10.1155/2015/854078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kehrmann J, Schoerding AK, Murali R, Wessel S, Koehling HL, Mosel F, et al. Performance of Vitek MS in identifying nontuberculous mycobacteria from MGIT liquid medium and Lowenstein-Jensen solid medium. Diagn Microbiol Infect Dis. 2016;84:43–7. doi: 10.1016/j.diagmicrobio.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 139.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann JL, Nick JA, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71(Suppl 1):i1–22. doi: 10.1136/thoraxjnl-2015-207360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jhun BW, Yang B, Moon SM, Lee H, Park HY, Jeon K, et al. Amikacin inhalation as salvage therapy for refractory nontuberculous mycobacterial lung disease. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00011-18. pii: e00011-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198:1559–69. doi: 10.1164/rccm.201807-1318OC. [DOI] [PubMed] [Google Scholar]
- 142.Pan SW, Shu CC, Feng JY, Wang JY, Chan YJ, Yu CJ, et al. Microbiological persistence in patients with Mycobacterium avium complex lung disease: The predictors and the impact on radiographic progression. Clin Infect Dis. 2017;65:927–34. doi: 10.1093/cid/cix479. [DOI] [PubMed] [Google Scholar]
- 143.Kim SJ, Park J, Lee H, Lee YJ, Park JS, Cho YJ, et al. Risk factors for deterioration of nodular bronchiectatic Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis. 2014;18:730–6. doi: 10.5588/ijtld.13.0792. [DOI] [PubMed] [Google Scholar]
- 144.Shulha JA, Escalante P, Wilson JW. Pharmacotherapy Approaches in Nontuberculous Mycobacteria Infections. Mayo Clin Proc. 2019;94:1567–81. doi: 10.1016/j.mayocp.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 145.Balavoine C, Blanc FX, Lanotte P, Meurice JC, Andrejak C, Marchand-Adam S. Adverse events during treatment of nontuberculous mycobacterial lung disease: Do they really matter? Eur Respir J. 2018;52:PA2664. [Google Scholar]
- 146.Griffith D. Treatment of Mycobacterium avium complex (MAC) Semin Respir Crit Care Med. 2018;39:351–61. doi: 10.1055/s-0038-1660472. [DOI] [PubMed] [Google Scholar]
- 147.Strnad L, Winthrop KL. Treatment of Mycobacterium abscessus complex. Semin Respir Crit Care Med. 2018;39:362–76. doi: 10.1055/s-0038-1651494. [DOI] [PubMed] [Google Scholar]
- 148.Basille D, Jounieaux V. Treatment of other nontuberculous mycobacteria. Semin Respir Crit Care Med. 2018;39:377–82. doi: 10.1055/s-0038-1660473. [DOI] [PubMed] [Google Scholar]
- 149.van Ingen J, Boeree M, van Soolingen D, and Mouton J. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat. 2012;15:149–61. doi: 10.1016/j.drup.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 150.Kim HJ, Lee JS, Kwak N, Cho J, Lee CH, Han SK, et al. Role of ethambutol and rifampicin in the treatment of Mycobacterium avium complex pulmonary disease. BMC Pulm Med. 2019;19:212. doi: 10.1186/s12890-019-0982-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Griffith DE, Brown-Elliott BA, Langsjoen B, Zhang Y, Pan X, Girard W, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928–34. doi: 10.1164/rccm.200603-450OC. [DOI] [PubMed] [Google Scholar]
- 152.Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015;191:96–103. doi: 10.1164/rccm.201408-1545OC. [DOI] [PubMed] [Google Scholar]
- 153.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004;126:566–81. doi: 10.1378/chest.126.2.566. [DOI] [PubMed] [Google Scholar]
- 154.Xu HB, Jiang RH, Li L. Treatment outcomes for Mycobacterium avium complex: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2014;33:347–58. doi: 10.1007/s10096-013-1962-1. [DOI] [PubMed] [Google Scholar]
- 155.Kwak N, Park J, Kim E, Lee CH, Han SK, Yim JJ. Treatment outcomes of Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1077–84. doi: 10.1093/cid/cix517. [DOI] [PubMed] [Google Scholar]
- 156.Pasipanodya JG, Ogbonna D, Deshpande D, Srivastava S, Gumbo T. Meta-analyses and the evidence base for microbial outcomes in the treatment of pulmonary Mycobacterium avium-intracellulare complex disease. J Antimicrob Chemother. 2017;72(Supp 2):i3–19. doi: 10.1093/jac/dkx311. [DOI] [PubMed] [Google Scholar]
- 157.Diel R, Nienhaus A, Ringshausen FC, Richter E, Welte T, Rabe KF, et al. Microbiologic outcome of interventions against Mycobacterium avium complex pulmonary disease: A systematic review. Chest. 2018;153:888–921. doi: 10.1016/j.chest.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 158.Miwa S, Shirai M, Toyoshima M, Shirai T, Yasuda K, Yokomura K, et al. Efficacy of clarithromycin and ethambutol for Mycobacterium avium complex pulmonary disease. A preliminary study. Ann Am Thorac Soc. 2014;11:23–9. doi: 10.1513/AnnalsATS.201308-266OC. [DOI] [PubMed] [Google Scholar]
- 159.Koh WJ, Moon SM, Kim SY, Woo MA, Kim S, Jhun BW, et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J. 2017;50 doi: 10.1183/13993003.02503-2016. pii: 1602503. [DOI] [PubMed] [Google Scholar]
- 160.Moon SM, Park HY, Kim SY, Jhun BW, Lee H, Jeon K, et al. Clinical characteristics, treatment outcomes, and resistance mutations associated with macrolide-resistant Mycobacterium avium complex lung disease. Antimicrob Agents Chemother. 2016;60:6758–65. doi: 10.1128/AAC.01240-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Morimoto K, Namkoong H, Hasegawa N, Nakagawa T, Morino E, Shiraishi Y, et al. Macrolide-resistant Mycobacterium avium complex lung disease: Analysis of 102 consecutive cases. Ann Am Thorac Soc. 2016;13:1904–11. doi: 10.1513/AnnalsATS.201604-246OC. [DOI] [PubMed] [Google Scholar]
- 162.Park Y, Lee EH, Jung I, Park G, Kang YA. Clinical characteristics and treatment outcomes of patients with macrolide-resistant Mycobacterium avium complex pulmonary disease: A systematic review and meta-analysis. Respir Res. 2019;20:286. doi: 10.1186/s12931-019-1258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.DeStefano MS, Shoen CM, Cynamon MH. Therapy for Mycobacterium kansasii infection: Beyond 2018. Front Microbiol. 2018;9:2271. doi: 10.3389/fmicb.2018.02271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sun Z, Zhang Y. Reduced pyrazinamidase activity and the natural resistance of Mycobacterium kansasii to the antituberculosis drug pyrazinamide. Antimicrob Agents Chemother. 1999;43:537–42. doi: 10.1128/aac.43.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zaheen A, Hirama T, Mehrabi M, Brode SK, Marras TK. Clinical outcomes in Mycobacterium xenopi versus Mycobacterium avium complex pulmonary disease: A retrospective matched cohort study. Respir Med. 2020;167:105967. doi: 10.1016/j.rmed.2020.105967. [DOI] [PubMed] [Google Scholar]
- 166.van Ingen J, Totten SE, Heifets LB, Boeree MJ, Daley CL. Drug susceptibility testing and pharmacokinetics question current treatment regimens in Mycobacterium simiae complex disease. Int J Antimicrob Agents. 2012;39:173–6. doi: 10.1016/j.ijantimicag.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 167.van Ingen J, Totten SE, Helstrom NK, Heifets LB, Boeree MJ, Daley CL. In vitro synergy between clofazimine and amikacin in nontuberculous mycobacterial disease. Antimicrob Agents Chemother. 2012;56:6324–7. doi: 10.1128/AAC.01505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Koh WJ, Jeong BH, Kim SY, Jeon K, Park KU, Jhun BW, et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis. 2017;64:309–16. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 169.Pasipanodya JG, Ogbonna D, Ferro BE, Magombedze G, Srivastava S, Deshpande D, et al. Systematic review and meta-analyses of the effect of chemotherapy on pulmonary Mycobacterium abscessus outcomes and disease recurrence. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01206-17. pii: e01206-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Okamori S, Asakura T, Nishimura T, Tamizu E, Ishii M, Yoshida M, et al. Natural history of Mycobacterium fortuitum pulmonary infection presenting with migratory infiltrates: A case report with microbiological analysis. BMC Infect Dis. 2018;18:1. doi: 10.1186/s12879-017-2892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lindeboom JA, Kuijper EJ, Bruijnesteijn van Coppenraet ES, Lindeboom R, Prins JM. Surgical excision versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children: a multicenter, randomized, controlled trial. Clin Infect Dis. 2007;44:1057–64. doi: 10.1086/512675. [DOI] [PubMed] [Google Scholar]
- 172.Aubry A, Chosidow O, Caumes E, Robert J, Cambau E. Sixty-three cases of Mycobacterium marinum infection: Clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746–52. doi: 10.1001/archinte.162.15.1746. [DOI] [PubMed] [Google Scholar]
- 173.Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965–78. doi: 10.1517/14656566.8.17.2965. [DOI] [PubMed] [Google Scholar]
- 174.Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis. 2003;37:390–7. doi: 10.1086/376628. [DOI] [PubMed] [Google Scholar]
- 175.Chauty A, Ardant MF, Marsollier L, Pluschke G, Landier J, Adeye A, et al. Oral treatment for Mycobacterium ulcerans infection: Results from a pilot study in Benin. Clin Infect Dis. 2011;52:94–6. doi: 10.1093/cid/ciq072. [DOI] [PubMed] [Google Scholar]
- 176.O'Brien DP, McDonald A, Callan P, Robson M, Friedman ND, Hughes A, et al. Successful outcomes with oral fluoroquinolones combined with rifampicin in the treatment of Mycobacterium ulcerans: An observational cohort study. PLoS Negl Trop Dis. 2012;6:e1473. doi: 10.1371/journal.pntd.0001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wallace RJ, Jr, Tanner D, Brennan PJ, Brown BA. Clinical trial of clarithromycin for cutaneous (disseminated) infection due to Mycobacterium chelonae. Ann Intern Med. 1993;119:482–6. doi: 10.7326/0003-4819-119-6-199309150-00006. [DOI] [PubMed] [Google Scholar]
- 178.Wallace RJ, Swenson JM, Silcox VA, Bulen MG. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonae on the basis of in vitro susceptibilities. J Infect Dis. 1985;152:500–14. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- 179.Wallace RJ, Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, et al. Macrolide/Azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest. 2014;146:276–82. doi: 10.1378/chest.13-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Banaschewski B, Verma D, Pennings LJ, Zimmerman M, Ye Q, Gadawa J, et al. Clofazimine inhalation suspension for the aerosol treatment of pulmonary nontuberculous mycobacterial infections. J Cyst Fibros. 2019;18:714–20. doi: 10.1016/j.jcf.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 181.Yagi K, Ishii M, Namkoong H, Asami T, Iketani O, Asakura T, et al. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect Dis. 2017;17:558. doi: 10.1186/s12879-017-2665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Olivier KN, Shaw PA, Glaser TS, Bhattacharyya D, Fleshner M, Brewer CC, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc. 2014;11:30–5. doi: 10.1513/AnnalsATS.201307-231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yaacoby-Bianu K, Gur M, Toukan Y, Nir V, Hakim F, Geffen Y, et al. Compassionate nitric oxide adjuvant treatment of persistent Mycobacterium infection in cystic fibrosis patients. Pediatr Infect Dis J. 2018;37:336–8. doi: 10.1097/INF.0000000000001780. [DOI] [PubMed] [Google Scholar]
- 184.Basavaraj A, Segal L, Samuels J, Feintuch J, Feintuch J, Alter K, et al. Effects of chest physical therapy in patients with non-tuberculous mycobacteria. Int J Respir Pulm Med. 2017;4 doi: 10.23937/2378-3516/1410065. pii: 065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wakamatsu K, Nagata N, Maki S, Omori H, Kumazoe H, Ueno K, et al. Patients with MAC lung disease have a low visceral fat area and low nutrient intake. Pulm Med. 2015;2015:218253. doi: 10.1155/2015/218253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lu M, Fitzgerald D, Karpelowsky J, Selvadurai H, Pandit C, Robinson P, et al. Surgery in nontuberculous mycobacteria pulmonary disease. Breathe (Sheff) 2018;14:288–301. doi: 10.1183/20734735.027218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mitchell JD, Bishop A, Cafaro A, Weyant MJ, Marvin P. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg. 2008;85:1887–93. doi: 10.1016/j.athoracsur.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 188.Shiraishi Y. Current status of nontuberculous mycobacterial surgery in Japan: Analysis of data from the annual survey by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2016;64:14–7. doi: 10.1007/s11748-015-0594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wi YM. Treatment of extrapulmonary nontuberculous mycobacterial diseases. Infect Chemother. 2019;51:245–55. doi: 10.3947/ic.2019.51.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schlossberg D, editor. Tuberculosis and nontuberculous mycobacterial infections. 7th ed. Washington, DC: ASM Press; 2017. Nontuberculous mycobacteria -Overview; pp. 655–61. [Google Scholar]