Abstract
Background & objectives:
Being more efficient and widely used, limiting antigen (LAg)-avidity enzyme immunoassay (EIA) based on the recent infection testing algorithm (RITA) has been developed for differentiating recent and established HIV-1 infection. So far, LAg-avidity EIA has not been validated among the Indian population. Hence, the present study was planned to identify recent HIV infections in high risk patients in the North-West region of India using modified LAg-avidity RITA.
Methods:
Four hundred HIV-positive high risk patients registered on pre-antiretroviral therapy (ART) programme in the last one year, from five ART centres in North-Western States of India, were included for identifying the recent HIV infections. One hundred HIV-positive cases registered for pre-ART for greater than two years in ART centres were included for estimating false recent rate (FRR). Single-well LAg-avidity EIA-based modified RITA was used to identify recent HIV infection cases.
Results:
Of the 400 HIV-1-positive samples, 64 (16%) were found to have been infected within the past 130 days. The proportion of recent HIV infections was 16.8 per cent (18/107) among female sex workers, 10.7 per cent (9/84) among men who have sex with men and 17.7 per cent (37/209) among injecting drug users. The FRR was one per cent (1/100).
Interpretation & conclusions:
LAg-avidity EIA-based modified RITA provided good discrimination between recent and non-recent HIV infection, hence, it could be considered suitable for estimating HIV incidence in sentinel surveillance system in India.
Keywords: False recent rate, high risk groups, HIV, incidence, LAg-avidity, RITA
Human immunodeficiency virus (HIV) continues to be a major global public health issue. In 2019, with approximately 1.7 million new infections, an estimated 38 million people were living with HIV with a global HIV prevalence of 0.8 per cent among adults1. India has the third highest burden of HIV infection in the world2.
The determination of HIV incidence in a population is important to monitor the epidemic, to identify the target population for HIV prevention and care services and to evaluate the effectiveness of HIV prevention and treatment programmes3. Several methods for incidence measurement have been used in the past, including longitudinal studies, back calculation, p24 antigen enzyme immunoassay (EIA) and viral RNA testing. These methods are either difficult to perform or are costly or may not give accurate estimate4. Hence, avidity assays have been proposed to estimate HIV incidence. Limiting-antigen (LAg)-avidity EIA is commercially available single-well assay which is used to distinguish recent from long standing HIV infections for the purpose of estimating incidence in cross-sectional populations. Avidity measures the strength of the binding of immunoglobulin G antibodies with their corresponding antigen, a characteristic feature that enhances over a period of months in newly acquired infections5. Antibody avidity assays classically use urea or guanidine to extract low-avidity and low-affinity antibodies after antigen-antibody bonds are formed which can then be detected by a simple ELISA6. A new recombinant protein derived from immunodominant region of gp41 (rIDR-M) from all major subtypes and recombinants of HIV-1 group M has been developed7. This assay helps to identify sub-populations with high incidence for targeting resources and prevention efforts and to study avidity maturation of developing HIV antibodies in infected patients. There are some issues regarding identifying the recent HIV infection using LAg-avidity assay, as it cannot determine recent or long-term status of HIV-2-infected patients, because of lack of reactivity of rIDR-M with HIV-2 antibodies. In antiretroviral treatment, whether initiated early in infection or later is likely to interfere with normal maturation and/or persistence of HIV antibody, which may also affect its avidity7. Another alternative that has been proposed is based on the conclusion that in recent HIV infection there is an initial immunoglobulin isotype IgG3 response to p24. It takes about 120 days for isotype response to mature when isotype IgG3 is replaced by IgG1 isotype8. Although working efficiently with subtype B infections, the strength and efficacy of these assays are questionable with non-B subtypes such as the one predominant in India, sub-type C8,9,10. Hence, there is a need to develop algorithm for testing a combination of serological assays and clinical information for the detection of recent HIV infections for epidemiological investigations. Thus, the aim of this study was to use modified recent infection testing algorithm (RITA)11 based on LAg-avidity EIA for the identification of recently acquired HIV-1 infections among high risk individuals in North-West region of India and to calculate the false recent rate (FRR) of the algorithm.
Material & Methods
This study was conducted mainly in the department of Community Medicine, Postgraduate Institute of Medical Education & Research, (PGIMER), Chandigarh, India. The study protocol was approved by the Institutional Ethic Committee and written informed consent was obtained from all participants.
Female sex worker (FSW), men having sex with men (MSM) and injecting drug user (IDU) were enrolled in the study as per the inclusion and exclusion criteria, from five antiretroviral therapy (ART) centres of North-West India (Chandigarh, Amritsar, Jalandhar, Ludhiana and Rohtak). Only newly diagnosed HIV-positive patients who visited ART centres for the evaluation of their CD4 count were consecutively recruited from September 2013 to June 2016. The sample size of 400 HIV-positive cases registered for pre-ART in the past one year (2012) was considered to be sufficient for estimating recent HIV infection rate of 10 per cent with 95 per cent confidence and three per cent absolute precision. The sample size of 100 HIV-positive cases registered for more than two years was considered to be sufficient for estimating false recent HIV-positive rate (FRR) of 10 per cent with 30 per cent co-efficient of variance.
For identifying new HIV infections using RITA algorithm, HIV-positive patients registered for pre-ART in the past one year (2012) at the five ART centres of North-West India, who were ≥18 yr of age, with CD4+ count ≥200 cells/μl and who had not received any kind of ART prior to enrolment in the study were included for identifying new HIV infections using RITA. For the study of FRR, HIV-positive patients registered for pre-ART for more than two years in the ART centres, who have not received any kind of ART, who were ≥18 yr of age with CD4+ count was ≥200 cells/μl were included. Patients on directly observed treatment, short course (DOTS) for tuberculosis were excluded.
Prior to sample collection, permission was obtained from the National AIDS Control Organisation (NACO) and respective State AIDS Control Societies (SACS). Written informed consent was obtained from all the participants before enrolment. The counsellor was explained about inclusion and exclusion criteria of participants. The study participants were interviewed using semi-structured questionnaire. The information about socio-demographic characteristics, history of sexual and other behavioural risk factors and treatment history especially intake of antiretroviral drugs, CD4 cell counts was collected.
From each selected individual, 5 ml of venous blood was collected; and 2 ml blood was used for CD4 count estimation at respective ART centres. The remaining 3 ml blood was centrifuged to obtain plasma which was stored in separate vials at −80°C for determination of recent infection.
Estimation of recent HIV infection by RITA:In the avidity assay, anti-HIVgp41-specific antibodies interact with the recombinant immunodominant region of gp41 (rIDR-M) antigen. Modified RITA was used to identify recent HIV infections. The algorithm used in the study is presented in Figure 1.
Fig. 1.
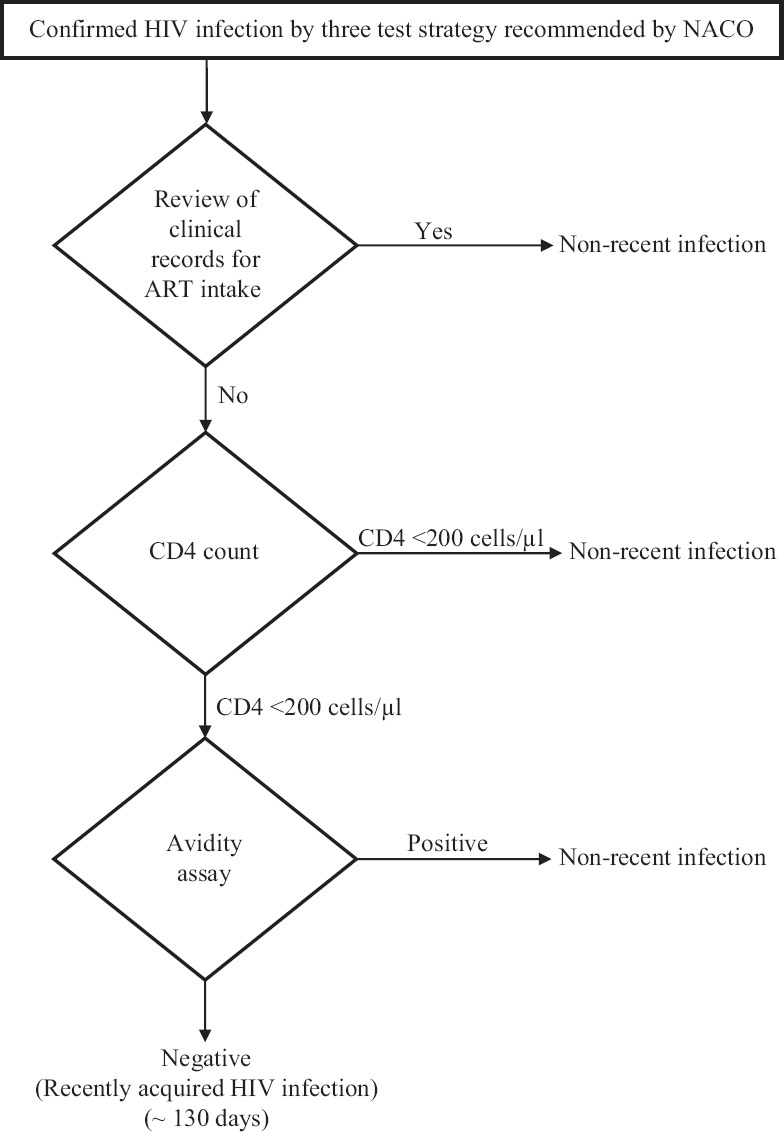
Modified recent infection testing algorithm (RITA). NACO, National AIDS Control Organisation; ART, antiretroviral therapy.
Limiting antigen (LAg)-avidity enzyme immunoassay: Recent HIV infection was detected using commercially available LAg-avidity assay kit (Maxim Biomedical Corporation, Inc., USA)7 as per the procedure described by manufacturer in the kit protocol. Assay controls [negative control (NC), calibrator (CAL), low-positive control (LPC) and high-positive control (HPC)] and HIV-positive specimens were diluted 1:101 (v/v) in specimen diluents. After dilution, 100 μl of the diluted specimens or controls were added to appropriate wells of antigen coated plates and incubated for 60 min at 37°C. NC was added in two wells, other controls (CAL, LPC and HPC) were added in three wells and specimens were added in singlet in the same plate. Plates were washed four times with 300 μl of phosphate-buffered saline Triton X-100 detergent (wash buffer) to remove unbound antibodies. A dissociation buffer (pH 3.0) was added to each well (100 μl/well) and incubated for 15 min at 37°C to dissociate low-avidity antibodies, if any. Following four washes, goat-anti-human IgG peroxidase (100 μl/well) was added to each well and incubated for 30 min at 37°C. Tetramethylbenzidine (TMB) substrate (100 μl/well) was added and incubated for 15 min at 25°C. Colour development was stopped by the addition of 1 N H2 SO4 (100 μl/well). The optical density (OD) was read at 450 nm with 650 nm as a reference. HIV-positive infections were classified as recent if OD was ≤1.5.
Statistical analysis:Socio-demographic characteristics according to high risk group (HRG) were presented using frequency and percentage. The association between HIV infection (long-term infection and recent infection) and demographic characteristics was tested using Chi-square test. Relation between the initial and the confirmatory run of the LAg-avidity EIA was tested using Pearson's correlation coefficient. Binomial regression was done to find association of the characteristics of respondents with recency of HIV infection. All statistical analysis were done using SPSS version 21.0 (IBM Corp., Armonk, NY, USA) at 0.05 level of significance.
Results
Socio-demographic characteristics: Of the, 400 HIV-1-positive HRG patients, most were IDUs (n=209, 52%) followed by FSWs (n=107, 27%) and MSMs (n=84, 21%). The socio-demographic characteristics of the study participants are shown in Table I. The mean age was 34.8 ±10 years. Three-fourth of them (n=293) were male and two-third were married. Majority (53%) of the respondents were either illiterate or studied up to primary class. Most of the MSMs (99%) had travelled outside their district as compared to IDUs (91%) and FSWs (80%). Average frequency of travel outside the district was three in the past two years, and it did not differ in various HRGs. The average stay during travel outside the district was maximum among FSWs (3.3 days) as compared to MSMs (2.9 days) and IDUs (2.8 days).
Table I.
Socio-demographic characteristics according to high-risk group (n=400)
| Variables | IDU, n (%) | FSW, n (%) | MSM, n (%) | Total, n (%) |
|---|---|---|---|---|
| Age (yr) | ||||
| 15-25 | 30 (15) | 29 (27) | 21 (25) | 80 (20) |
| 26-35 | 85 (40) | 35 (33) | 42 (50) | 162 (41) |
| 36-45 | 58 (28) | 26 (24) | 18 (21) | 102 (26) |
| 46-55 | 24 (11) | 10 (9) | 1 (1) | 35 (9) |
| >55 | 12 (5.7) | 7 (7) | 2 (2) | 17 (5) |
| Gender | ||||
| Male | 209 (100) | 0 | 84 (100) | 293 (73) |
| Female | 0 | 107 (100) | 0 | 107 (27) |
| Marital status | ||||
| Never married | 58 (28) | 26 (24) | 25 (30) | 109 (27) |
| Married | 130 (62) | 72 (68) | 54 (64) | 256 (64) |
| Divorced/separated/widowed | 21 (10) | 9 (8) | 5 (6.0) | 35 (9) |
| Literacy status | ||||
| Illiterate | 52 (25) | 42 (20) | 6 (7) | 100 (25) |
| Literate and 5th standard | 64 (31) | 24 (22) | 24 (29) | 112 (28) |
| 6th-10th standard | 45 (21) | 20 (19) | 21 (25) | 86 (22) |
| 11th to graduation | 41 (20) | 21 (20) | 28 (33) | 90 (23) |
| Post-graduation | 7 (3) | 0 | 5 (6) | 12 (3.0) |
| Travel history | ||||
| Travelled outside district in the past two years | 190 (91) | 86 (80) | 83 (99) | 359 (89) |
| CD4 count in recent infections (cells/µl) | ||||
| 200-500 | 30 (15.5) | 13 (14.I) | 8 (9.9) | 51 (13.9) |
| >500 | 7 (43.8) | 5 (33.3) | 1 (33.3) | 13 (38.2) |
FSW, female sex worker; MSM, men who have sex with men; IDU, injecting drug user
Standardization of limiting antigen (LAg)-avidity assay
Initial run: All 400 samples were initially run singularly on the LAg-avidity EIA. The CAL and controls (NC, LPC and HPC) were tested in triplicate on every plate and the median values were used to calculate the normalized OD (ODn). Samples with initial ODn >2.0 were considered long-term seroconversion but those with ODn value ≤2.0 were tested again in triplicate in the confirmatory run.
Confirmatory run: A total of 146 of the 400 samples with ODn ≤2.0 in the initial run were retested in triplicate by the LAg assay to confirm their status. The median values for the triplicate values were used to calculate ODn. All the samples with ODn of ≤1.5 were considered to be recently infected with HIV corresponding to mean seroconversion duration of 130 days. Of the 146 retested samples, 64 were found to be recent HIV infection. The correlation coefficient between the initial and the confirmatory runs was 0.97 (Fig. 2), demonstrating a high degree of reproducibility of the test procedure.
Fig. 2.
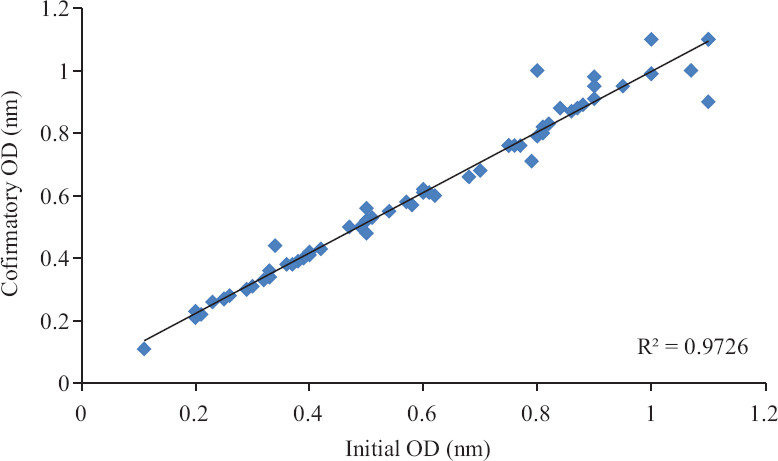
Concordance of the initial versus the confirmatory run of the limiting antigen (LAg)-avidity EIA. OD, optical density.
Recent HIV infection: Among the 400 respondents, 336 (84%) were identified as having long-term infection and the remaining 64 (16%) were identified as having recent HIV infections. The proportion of recent HIV infections was 16.8 (18/107) among FSWs, 10.7 (9/84) among MSM and 17.7 per cent (37/209) among IDU.
The characteristics of those having recent infection and long-term infection are presented in Table II. There was no significant difference in the socio-demographic characteristics between those having recent and long-term HIV infection. Among those who had recent HIV infection, the median CD4 count was 332.5 cells/μl (interquartile range (IQR): 257-491) compared to 287 cells/μl (IQR: 243-420) among those with long-term infection (Fig. 3). Binomial logistic regression was used, keeping recent infection (recent infection=1 and long-term infection=0) as dependent variable and risk groups (1=MSM, 2=FSW, 3=IDU) and CD4 counts (1=200-500, 2=>500) as independent variables. Compared to MSM group the odd ratio for FSW and IDU was 1.4 and 1.7, respectively, but the association was found to be non-significant. Odd ratio for CD4 count of >500 cells/μl was found to be 3.7 compared to 200-500 cells/μl and this association was significant (P<0.01).
Table II.
Characteristic of those having recent and long-term human immunodeficiency virus infection detected by LAg-avidity assay
| Characteristics | Long-term infection, n (%) | Recent infection, n (%) | P |
|---|---|---|---|
| Age (yr) | |||
| 15-25 | 68 (20.0) | 11 (17.0) | 0.2 |
| 26-35 | 136 (41.0) | 26 (41.0) | |
| 36-45 | 80 (23.0) | 22 (34.0) | |
| 46-55 | 30 (9.0) | 4 (6.0) | |
| >55 | 22 (7.0) | 1 (2.0) | |
| Gender | |||
| Male | 247 (74.0) | 45 (70.0) | 0.6 |
| Female | 89 (27) | 19 (29.7) | |
| Marital status | |||
| Never married | 65 (19.0) | 14 (21.9) | 0.1 |
| Married | 264 (79.0) | 46 (71.9) | |
| Divorced/separated/widowed | 7 (2.0) | 4 (6.3) | |
| Literacy status | |||
| Illiterate | 84 (25.0) | 15 (23.0) | 0.3 |
| Literate and 5th standard | 94 (28.0) | 19 (30.0) | |
| 6th-10th standard | 74 (22.0) | 16 (25) | |
| 11th to graduation | 82 (24.0) | 12 (19.0) | |
| Post-graduation | 2 (0.6) | 2 (3.0) | |
| Travel history | |||
| Travelled outside the district in the last two years | 299 (89) | 59 (92.0) | 0.4 |
Fig. 3.
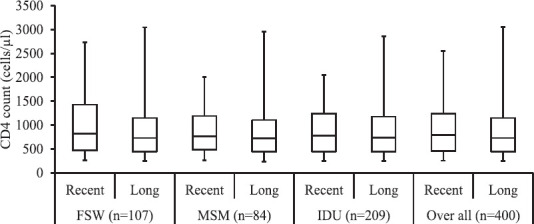
Boxplot of CD4 count among patients with recent and long-term HIV infection according to high risk groups (n=400). FSW, female sex worker; MSM, men who have sex with men; IDU, injecting drug user.
False recent rate (FRR): The median CD4 counts among these 100 samples were 374 cells/μl. One sample was found to be recent infection by LAg-avidity EIA, the proportion of samples misclassified as recent positive was found to be 0.01 (95% CI: 0.05-4.8).
Discussion
The HIV-1 LAg-avidity EIA used in this study was developed by the Centers for Disease Control and Prevention (CDC), to address some of the shortcomings that existed in previous incidence assays. Lag-avidity exploits a multi-subtype recombinant gp41 protein, which broadens its application for determining HIV incidence for various sub-types including A, B, C, D and E12.
In the present study, all the samples were found to be HIV-1, subtype C. This fact was corroborated by previous studies conducted in India13,14, which reported that the most common subtype of HIV-1 in Indian subcontinent was subtype C15. In the present study, of the 400 HIV positive, treatment naive HRGs, 16 per cent were found to be recently infected within the past four months. The higher proportions of recent infections were seen among IDUs, followed by FSWs showing that active transmission was going in these key population groups. The recent infection was least in MSMs. The reason for higher proportion of recently infected cases among IDUs could be the study samples which were collected mainly from North-Western States (Punjab, Haryana and Chandigarh) of India where an increasing HIV prevalence among IDUs was reported. Integrated Biological Behavioral Surveillance (IBBS) 2014-2015 reported the prevalence of 9.7 per cent (95% CI: 6.6-14.2) among IDUs in Punjab and Chandigarh and 7.3 per cent (95% CI: 5.4-9.7) in Haryana and Himachal Pradesh16.
The CD4 cell count may indicate the recency of HIV infection. In this study, the overall median CD4 count among recent HIV infection cases was 332.5 cells/μl. It is a common observation among HIV-infected people that CD4 counts halve within eight weeks of an initial count; about 25 per cent average variation is found from the mean over this period17. There is an ambiguity in the anticipated CD4 count within the first six months or year of infection, which may clarify why the CD4 count among likely recent HIV infection is not higher enough or similar to people who are HIV negative. It is established that CD4 counts may decrease during seroconversion18. According to the RITA used in this study, individuals who had CD4 count below 200 cells/μl were classified as having a long-standing infection and LAg-avidity assay was performed only on those having CD4 cell count more than or equal to 200 cells/μl. The purpose of including the additional information such as CD4 count in the RITA was to reduce the FRR by assisting in identifying cases with long-standing infection.
In the current study it was found that MSMs had travelled more often outside from their current district in the past two years as compared to IDUs and FSWs. Migration and mobility are more common among recent cases of HIV as compared to long-term infections. Mobility status, however, varies by districts, age at sexual debut with a male, sexual identities, marital status and main source of income19,20. In the study performed by Ramesh et al21 in southern India, it was observed that of the 1608 MSMs, about 26 per cent were mobile. Of these, three-fourths had travelled to different districts of a State (56%), and one-fifth (20%) among different States. Higher proportion of mobile MSMs as compared to non-mobile MSM reported involvement in unprotected sex with any male partner. The FRR obtained in the present study was similar to the studies conducted elsewhere with larger sample size. A study conducted by Moyo et al22 in Botswana utilizing the LAg-avidity assay with an assay cut-off of 1.5 ODn units, found FRR to be 0.97 per cent. A study by Shah et al23 in Vietnam using BED-CEIA and the LAg-avidity EIA, the LAg-avidity EIA proportion FRR was 1.2 per cent and the BED-CEIA FRR was 1.7 per cent. They concluded that the LAg-avidity EIA FRR was lower than the BED-CEIA FRR. The FRR can be influenced by various factors such as HIV-1 subtype, geographical area, presence of long-term non-progresses and extent of the use of ARV in the area. The FRR being a part of the HIV recency algorithm affects the estimated HIV incidence. Hence, lack of local FRR estimates can be held accountable for existing uncertainty in the estimation of the HIV incidence. FRR and mean duration of recent infection (MDRI) are supposed to be crucial parameters for the evaluation of incidence assays24. Key requirements of an HIV incidence assay are high reproducibility and a low FRR when applied across different populations and viral clades. The MDRI should ideally be between 6 and 12 months.
In conclusion, the LAg-avidity EIA may be a potential assay with consistent mean duration of recency in different populations and subtypes.
Acknowledgment
Authors thank Dr Bharat S. Parekh from Centers for Disease Control and Prevention, Atlanta, USA, for his valuable guidance for interpretation of results.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Joint United Nations Programme on HIV/AIDS. Global HIV & AIDS statistics - 2019 fact sheet. Geneva: UNAIDS; 2020. [accessed on July 2, 2020]. Available from: https://wwwunaidsorg/en/resources/fact-sheet . [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS. Ending AIDS: Progress towards the 90-90-90 targets. Geneva: UNAIDS; 2017. [accessed on July 17, 2019]. Available from: https://wwwunaidsorg/sites/default/files/media_asset/Global_AIDS_update_2017_enpdf . [Google Scholar]
- 3.Karita E, Price M, Hunter E, Chomba E, Allen S, Fei L, et al. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AID S. 2007;21:403–8. doi: 10.1097/QAD.0b013e32801481b7. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford GW, Schwarcz SK, McFarland W. Surveillance for incident HIV infection: New technology and new opportunities. J Acquir Immune Defic Syndr. 2000;25(Suppl 2):S115–9. doi: 10.1097/00042560-200012152-00005. [DOI] [PubMed] [Google Scholar]
- 5.Vauquelin G, Charlton SJ. Exploring avidity: Understanding the potential gains in functional affinity and target residence time of bivalent and heterobivalent ligands. Br J Pharmacol. 2013;168:1771–85. doi: 10.1111/bph.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla A, Murphy G, Donnelly C, Booth CL, Johnson M, Parry JV, et al. Human immunodeficiency virus (HIV) antibody avidity testing to identify recent infection in newly diagnosed HIV type 1 (HIV-1)-seropositive persons infected with diverse HIV-1 subtypes. J Clin Microbiol. 2007;45:415–20. doi: 10.1128/JCM.01879-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maxim HIV-1 LAg-Avidity EIAMaxim Biomedical, Inc. 2012. [accessed on July 17, 2019]. Available from: http://www.maximbio.com/img/insert/920013-product-insert.pdf .
- 8.Parekh BS, Hu DJ, Vanichseni S, Satten GA, Candal D, Young NL, et al. Evaluation of a sensitive/less-sensitive testing algorithm using the 3A11-LS assay for detecting recent HIV seroconversion among individuals with HIV-1 subtype B or E infection in Thailand. AIDS Res Hum Retroviruses. 2001;17:453–8. doi: 10.1089/088922201750102562. [DOI] [PubMed] [Google Scholar]
- 9.Kothe D, Byers RH, Caudill SP, Satten GA, Janssen RS, Hannon WH, et al. Performance characteristics of a new less-sensitive HIV-1 EIA for use in estimating HIV seroincidence. J AcquirImmune Defic Syndr. 2003;33:625–34. doi: 10.1097/00126334-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 10.Young CL, Hu DJ, Byers R, Vanichseni S, Young NL, Nelson R, et al. Evaluation of a sensitive/less-sensitive testing algorithm using the bioMerieux Vironostika-LS assay for detecting recent HIV-1 subtype B9 or E infection in Thailand. AIDS Res HumRetroviruses. 2003;19:481–6. doi: 10.1089/088922203766774522. [DOI] [PubMed] [Google Scholar]
- 11.Wilson KM, Johnson EI, Croom HA, Richards KM, Doughty L, Cunningham PH, et al. Incidence immunoassay for distinguishing recent from established HIV-1 infection in therapy-naive populations. AIDS. 2004;18:2253–9. doi: 10.1097/00002030-200411190-00005. [DOI] [PubMed] [Google Scholar]
- 12.Selleri M, Orchi N, Zaniratti MS, Bellagamba R, Corpolongo A, Angeletti C, et al. Effective highly active antiretroviral therapy in patients with primary HIV-1 infection prevents the evolution of the avidity of HIV-1-specific antibodies. J Acquir Immune Defic Syndr. 2007;46:145–50. doi: 10.1097/QAI.0b013e318120039b. [DOI] [PubMed] [Google Scholar]
- 13.Neogi U, Bontell I, Shet A, De Costa A, Gupta S, Diwan V, et al. Molecular epidemiology of HIV-1 subtypes in India: Origin and evolutionary history of the predominant subtype C. PLoS One. 2012;7:e39819. doi: 10.1371/journal.pone.0039819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddappa NB, Dash PK, Mahadevan A, Jayasuryan N, Hu F, Dice B, et al. Identification of subtype C human immunodeficiency virus type 1 by subtype-specific PCR and its use in the characterization of viruses circulating in the southern parts of India. J Clin Microbiol. 2004;42:2742–51. doi: 10.1128/JCM.42.6.2742-2751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seth P. Evolution of HIV-1 in India. Indian J Virol. 2010;21:3–7. doi: 10.1007/s13337-010-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National AIDS Control Organisation. Integrated biological and behavioural surveillance. New Delhi: Ministry of Health and Family Welfare, Government of India; 2015. [accessed on July 17, 2019]. Available from: http://nacogovin/sites/default/files/IBBS%20Report%202014-15pdf . [Google Scholar]
- 17.Lodi S, Phillips A, Touloumi G, Geskus R, Meyer L, Thiébaut R, et al. Time from human immunodeficiency virus seroconversion to reaching CD4+ cell count thresholds <200, <350, and <500 cells/mm[3]: Assessment of need following changes in treatment guidelines. Clin Infect Dis. 2011;53:817–25. doi: 10.1093/cid/cir494. [DOI] [PubMed] [Google Scholar]
- 18.Karon JM, Song R, Brookmeyer R, Kaplan EH, Hall HI. Estimating HIV incidence in the United States from HIV/AIDS surveillance data and biomarker HIV test results. Stat Med. 2008;27:4617–33. doi: 10.1002/sim.3144. [DOI] [PubMed] [Google Scholar]
- 19.Saggurti N, Jain AK, Sebastian MP, Singh R, Modugu HR, Halli SS, et al. Indicators of mobility, socio-economic vulnerabilities and HIV risk behaviours among mobile female sex workers in India. AIDS Behav. 2012;16:952–9. doi: 10.1007/s10461-011-9937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deane KD, Parkhurst JO, Johnston D. Linking migration, mobility and HIV. Trop Med Int Health. 2010;15:1458–63. doi: 10.1111/j.1365-3156.2010.02647.x. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh S, Mehrotra P, Mahapatra B, Ganju D, Nagarajan K, Saggurti N. The effect of mobility on sexual risk behaviour and HIV infection: A cross-sectional study of men who have sex with men in Southern India. Sex Transm Infect. 2014;90:491–7. doi: 10.1136/sextrans-2013-051350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyo S, Kotokwe KP, Mohammed T, Boleo C, Mupfumi L, Chishala S, et al. Short communication: low false recent rate of limiting antigen-avidity assay combined with HIV-1 RNA data in Botswana. AIDS Res Hum Retroviruses. 2017;33:17–8. doi: 10.1089/aid.2016.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah NS, Duong YT, Le LV, Tuan NA, Parekh BS, Ha HT, et al. Estimating false-recent classification for the limiting-antigen avidity EIA and BED-capture enzyme immunoassay in Vietnam: Implications for HIV-1 incidence estimates. AIDS Res Hum Retroviruses. 2017;33:546–54. doi: 10.1089/AID.2016.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim AA, Hallett T, Stover J, Gouws E, Musinguzi J, Mureithi PK, et al. Estimating HIV incidence among adults in Kenya and Uganda: A systematic comparison of multiple methods. PLoS One. 2011;6:e17535. doi: 10.1371/journal.pone.0017535. [DOI] [PMC free article] [PubMed] [Google Scholar]


