Abstract
CRLX101 is a nanoparticle-drug conjugate with a camptothecin payload. We assessed the toxicity and pathologic complete response (pCR) rate of CRLX101 with standard neoadjuvant chemoradiotherapy (CRT) in locally advanced rectal cancer. A single-arm study was conducted with a 3+3 dose escalation phase Ib followed by phase II at the maximum tolerated dose (MTD). Thirty-two patients were enrolled with 29 (91%) patients having T3/4 and 26 (81%) N1/2 disease. In phase Ib, no patient experienced a dose limiting toxicity (DLT) with every other week dosing, while 1/9 patients experienced a DLT with weekly dosing. The weekly MTD was identified as 15 mg/m2. The most common grade 3–4 toxicity was lymphopenia, with only 1 grade 4 event. pCR was achieved in 6/32 (19%) patients overall and 2/6 (33%) patients at the weekly MTD. CRLX101 at 15 mg/m2 weekly with neoadjuvant CRT is a feasible combination strategy with an excellent toxicity profile.
Keywords: nanoparticle, camptothecin, neoadjuvant, rectal cancer, maximum tolerated dose, pathologic complete response
Graphical Abstract
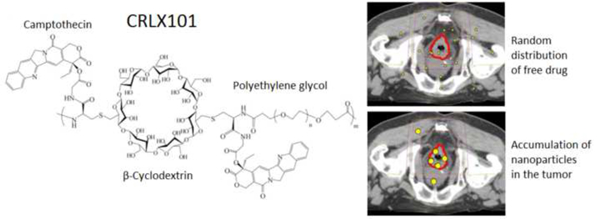
CRLX101 is a novel nanoparticle-drug conjugate of camptothecin. In a Phase Ib/II study of CRLX101 with standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer, the maximum tolerated dose of CRLX101 was found to be 15 mg/m2 weekly. CRLX101 was well-tolerated with 1 grade 4 toxicity (lymphopenia), and a pathologic complete response seen in 19% of patient in the overall cohort and 33% of patients at the maximum tolerated dose. Combination strategies with CRLX101 in rectal cancer is a proof-of-concept to enhance the therapeutic ratio.
Background
In the United States, approximately 40,000 patients are newly-diagnosed with rectal cancer and 22,000 patients die from the disease each year [1]. Neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision is the standard of care for stage II-III rectal cancer. With radiosensitizing fluoropyrimidine, pathologic complete response (pCR) rates range between 8 and 21% [2–7]. Achieving a pCR is associated with improved disease free and overall survival [8–10], and it can increase the chance of organ preservation including potentially forgoing extensive surgical resection [11–13]. Unfortunately, multiple recent efforts to improve on the efficacy of CRT with the addition of oxaliplatin [5,7,14,15], bevacizumab [16–18], and cetuximab [19–21] have failed to show any clear improvement in pCR rates or other clinically meaningful endpoints.
The overlapping toxicity of radiation and chemotherapy has been a major impediment to advances in radiosensitizing chemotherapy. Nanoparticles offer a potential for an enhanced therapeutic window to mitigate this concern while synergizing with concurrent daily radiation therapy (RT), due to their optimized drug delivery characteristics facilitating preferential accumulation in tumors, lower drug doses in and toxicities to normal tissues, and controlled payload delivery [22–25].
CRLX101 is an investigational nanoparticle-drug conjugate containing the payload camptothecin, which is a potent inhibitor of topoisomerase I and HIF-1α, as well as a radiosensitizer [26]. Camptothecin conjugated to the cyclodextrin-based polymer allows self-assembly into 30–40 nm diameter nanoparticles, which preferentially accumulates in the permeable tumor tissue with leaky vasculature [27]. Camptothecin is subsequently released gradually inside tumor cells with plasma half-life of approaching 24 hours vs. 2 hours for the naked drug [28], allowing for prolonged exposure and sustained inhibitory effects, while reducing toxicity to normal tissues. Camptothecin in its original formulations was not well tolerated, and its development was discontinued at phase II trials in the 1970s. Irinotecan, a derivative of camptothecin, has been shown to be effective against colorectal cancer, but has been associated with significant gastrointestinal toxicity when used concurrently with RT [29–31]. On the other hand, CRLX101 has been tested in multiple phase I and II studies, showing a favorable toxicity profile [32–34]. In colorectal cancer cell lines and murine xenograft models, CRLX101 and RT proved more effective than 5-fluorouracil (5-FU) and RT, with the greatest efficacy seen with the combination of CRLX101, 5FU, and RT, an effect mediated by inhibiting DNA repair and HIF-1α pathway activation [26].
In this context, the purpose of this phase Ib/II study was to assess the maximum tolerated dose and toxicity profile of CRLX101 in combination with standard neoadjuvant capecitabine and RT in locally advanced rectal cancer, and to evaluate the efficacy of the addition of CRLX101 as measured by the pCR rate in this cohort.
Methods
Patients
Adult patients with clinical T3–4N0 or clinical T1–4N+, pathologically-confirmed rectal cancer and ECOG performance status of ≤ 2 were eligible. During the phase Ib dose-finding portion, patients with metastatic rectal cancer were permitted if their primary site met eligibility requirements and CRT was recommended as initial therapy for palliation by the multidisciplinary tumor board. Exclusion criteria included patients with baseline ≥ grade 2 diarrhea, prior pelvic RT, or prior treatment with a topoisomerase I inhibitor. Patients whose laboratory values did not meet the following criteria were also excluded: Hemoglobin ≥ 10 g/dL (male); ≥9 g/dL (female); absolute neutrophil count ≥ 1500 /mm3; platelets ≥ 100,000/ mm3; alanine aminotransferase and aspartate aminotransferase < 2.5 upper limit of normal; total bilirubin < 1.5 upper limit of normal; creatinine clearance ≥ 50 mL/min; INR ≤2. All patients provided written informed consent before participating in any study related procedures. The study was approved by the institutional review board at each site of the 6 participating sites, and registered with clinicaltrials.gov (NCT02010567).
Study Design
This open label phase Ib/II study was initially designed to determine the MTD and recommended phase II dose (RP2D) of CRLX101 in combination with CRT given on weeks 1, 3, and 5. Following establishment of the RP2D, enrollment to the phase II portion proceeded. However, after 14 of a planned 31 patients in the initial phase of the Simon two-stage designed phase II portion were enrolled, data emerged about the safety of weekly dosing of CRLX101 as a single agent [35]. Given the minimal toxicity seen with CRLX101 and CRT to that point, the protocol was amended to include a second phase Ib dose escalation scheme of weekly dosing.
Both every other week and weekly CRLX101 dosing phase Ib portions utilized a standard 3 patient cohort dose escalation design. First, 3 patients began at dose level 1 (12 mg/m2). If no dose limiting toxicities (DLTs) were observed after completion of the DLT window (CRT + 2 weeks), 3 patients were enrolled at dose level 2 (15 mg/m2). However, if a single patient experienced a DLT, enrollment to that cohort was to be expanded to 6. If a total of 1/6 patients experienced DLT, enrollment would proceed to dose level 2. If ≥ 2/6 patients experienced DLT, the MTD was exceeded at that dose level, and enrollment to dose level −1 would proceed. If MTD was not exceeded in any cohort, dose level 2 was deemed the RP2D. DLT was defined as pre-specified grade 3–4 toxicities, toxicities requiring a dose reduction of CRLX101 or capecitabine, and toxicities requiring RT interruption ≥5 days.
Treatment
All patients were treated with standard CRT including RT to a total dose of 50.4 Gy in 1.8 Gy daily fractions and concurrent capecitabine 825 mg/m2 PO twice daily on Monday through Friday for the duration of radiotherapy. Capecitabine doses were rounded to the nearest dose using both 150 mg and 500 mg tablets.
CRLX101 was administered IV over 60 minutes on weeks 1, 3, 5 (initial phase Ib and phase II) or weekly (subsequent phase Ib). All patients received premedication with dexamethasone, and 5-HT3, H1, and H2 antagonists to minimize potential hypersensitivity reactions. One liter of normal saline IV was administered before and after CRLX101 to minimize risk of cystitis.
Following CRT, surgical resection occurred a minimum of 6 weeks following neoadjuvant CRT. Adjuvant chemotherapy was recommended, but not specified per protocol.
Endpoints
The primary endpoint of phase Ib every other week and weekly portions was the MTD of CRLX101 when added to standard CRT. The primary endpoint of the phase II every other week dosing portion was pCR rate. Secondary endpoints included the toxicity profile using the Common Terminology Criteria for Adverse Events version 4 (CTCAE v4), pathologic response rate, disease-free survival (DFS), and overall survival (OS). Pathologic complete response was defined as no gross or microscopic tumor at the primary site and lymph nodes. Moderate response and minimal response were defined as minimal residual disease (single cells or small groups of cancer cells) and residual cancer outgrown by fibrosis, respectively.
Statistical analysis
The phase II portion of the study was designed with 80% power and a one-sided α of 5% to detect a 15% improvement in pCR rate to 35% compared to the null hypothesis of 20% using a Simon two-stage mini-max design [36]. For the first stage, 31 patients were to be enrolled, and if ≤ 6 patients achieved a pCR, the study would have been stopped for futility. However, decision was made by Cerulean to withhold further funding of the study after completion of the weekly phase Ib. This decision was made independently of efficacy signal. Standard descriptive statistics of patient characteristics, acute toxicities, and pathologic response rates with their 95% confidence intervals (CIs) were reported. Due to insufficient number of events, DFS and OS were not analyzed for this report. Statistical analyses were performed using SAS statistical software, version 9.4, from the SAS Institute, Inc., in Cary, NC.
Results
Patient characteristics
A total of 32 patients were enrolled on the trial from 6 institutions from March, 2014 to July, 2016 (Figure 1). The median age was 59 (range 40–88), 69% were male, 81% were Caucasian, and nearly all had an ECOG performance status of 0–1 (Table 1). In regards to stage, 91% of patients had clinical T3 or T4 disease, 53% had clinical N1 disease, and 28% had clinical N2 disease.
Figure 1.

Consort Diagram
Table 1.
Patient characteristics
| Characteristic | N (%) |
|---|---|
| Median Age (range) | 59 (40–88) |
| Sex | |
| Male | 22 (69%) |
| Female | 10 (31%) |
| Race | |
| Caucasian | 26 (81%) |
| African American | 5 (16%) |
| Missing | 1 (3%) |
| Performance status | |
| 0 | 18 (56%) |
| 1 | 12 (27%) |
| 2 | 1 (3%) |
| Missing | 1 (3%) |
| Clinical stage | |
| T2N1 | 1 (3%) |
| T2N2 | 1 (3%) |
| T3N0 | 6 (19%) |
| T3N1 | 15 (47%) |
| T3N2 | 6 (19%) |
| T4N1 | 1 (3%) |
| T4N2 | 1 (3%) |
| TxN2 | 1 (3%) |
Toxicity
The dose escalation schema and the number of patients enrolled on the every other week and weekly CRLX101 phase Ib cohorts are shown in Table 2. For every other week dosing, 9 patients completed treatment on phase Ib without a DLT, and the MTD and RP2D was identified as 15 mg/m2. Subsequently, 14 additional patients were successfully treated on phase II of the study at the RP2D. One patient with high grade obstruction at diagnosis stopped therapy due to bowel obstruction requiring emergent diverting ostomy.
Table 2.
Phase I Dose Cohorts and Dose Limiting Toxicity
| CRLX101 Schedule | Dose | N | DLT |
|---|---|---|---|
| Week 1, 3, 5 | 12 mg/m2 | 3 | None |
| 15 mg/m2 | 6 | None | |
| Weekly | 12 mg/m2 | 3 | None |
| 15 mg/m2 | 6 | 1: Skin desquamation with 6 day treatment break | |
In the setting of emerging data presented in abstract form demonstrating the weekly (vs. biweekly) MTD of single agent CRLX101 to be 15 mg/m2 during our trial [35], the protocol was amended to assess weekly dosing of CRLX101. In the weekly dose escalation, no patient experienced a DLT at 12 mg/m2 weekly. One of 6 patients experienced a DLT at 15 mg/m2, consisting of moist skin desquamation requiring RT delay for 6 days. The MTD and RP2D of weekly CRLX101 was identified as 15 mg/m2.
Across all doses and schedules, no patient required a dose reduction of CRLX101 or capecitabine. The most common adverse events deemed to be at least possibly treatment related were fatigue (61%) and lymphopenia (45%) (Table 3). Thirteen of 32 patients (41%) experienced a ≥ grade 3 toxicity on the trial, 5 of which were non-hematologic including one case each of grade 3 colitis, diarrhea, hypophosphatemia, rectal obstruction, and radiation dermatitis. Severe lymphopenia was the only severe event occurring in more than one patient, including 8 (25%) with grade 3 and 1 (3%) with grade 4 events, though these did not result in any clinically significant events. No patient experienced severe cystitis. Although direct comparison across arms was not performed given the small sample size, there was no suggestion that weekly dosing was associated with significantly increased toxicity.
Table 3.
Treatment Related Adverse Events*
| All Grades | Grade 1^ | Grade 2^ | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Adverse event | N (%) | N (%) | N (%) | N (%) | N (%) |
| Fatigue | 19 (59%) | 13 (41%) | 6 (19%) | - | - |
| Lymphopenia | 14 (44%) | 1 (3%) | 4 (13%) | 8 (25%) | 1 (3%) |
| Anemia | 12 (38%) | 9 (28%) | 3 (9%) | - | - |
| Diarrhea | 12 (38%) | 8 (25%) | 3 (9%) | 1 (3%) | - |
| Leukopenia | 12 (38%) | 7 (22%) | 4 (13%) | 1 (3%) | - |
| Nausea | 11 (34%) | 11 (34%) | - | - | - |
| Urinary frequency | 8 (25%) | 8 (25%) | - | - | - |
| Cystitis | 7 (22%) | 6 (19%) | 1 (3%) | - | - |
| Hypophosphatemia | 7 (22%) | 2 (6%) | 4 (13%) | 1 (3%) | - |
| Neutropenia | 7 (22%) | 6 (19%) | 1 (3%) | - | - |
| Anorexia | 5 (16%) | 3 (9%) | 2 (6%) | - | - |
| Abdominal pain | 5 (16%) | 4 (13%) | 1 (3%) | - | - |
| Constipation | 5 (16%) | 3 (9%) | 2 (6%) | - | - |
| Thrombocytopenia | 5 (16%) | 5 (16%) | - | - | - |
| Urinary urgency | 5 (16%) | 4 (13%) | 1 (3%) | - | - |
| Hypocalcemia | 4 (13%) | 4 (13%) | - | - | - |
| Hyponatremia | 4 (13%) | 4 (13%) | - | - | - |
| Rectal pain | 4 (13%) | - | 4 (13%) | - | - |
| Urinary tract pain | 4 (13%) | 3 (9%) | 1 (3%) | - | - |
| Alanine aminotransferase increase | 3 (9%) | 2 (6%) | 1 (3%) | - | - |
| Aspartate aminotransferase increase | 3 (9%) | 2 (6%) | 1 (3%) | - | - |
| Hypertension | 3 (9%) | 2 (6%) | 1 (3%) | - | - |
| Hypoalbuminemia | 3 (9%) | 2 (6%) | 1 (3%) | - | - |
| Hypomagnesemia | 3 (9%) | 3 (9%) | - | - | - |
| Radiation dermatitis | 3 (9%) | 1 (3%) | 1 (3%) | 1 (3%) | - |
| Vomiting | 3 (9%) | 3 (9%) | - | - | - |
| Blood bilirubin increased | 2 (6%) | - | 2 (6%) | - | - |
| Dry skin | 2 (6%) | 2 (6%) | - | - | - |
| Hypermagnesemia | 2 (6%) | 2 (6%) | - | - | - |
| Hypokalemia | 2 (6%) | 1 (3%) | 1 (3%) | - | - |
| Palmar-plantar erythrodysesthesia | 2 (6%) | 2 (6%) | - | - | - |
| Rectal hemorrhage | 2 (6%) | 2 (6%) | - | - | - |
| Thrombotic thrombocytopenia purpura | 2 (6%) | 2 (6%) | - | - | - |
| Colitis | 1 (3%) | - | - | 1 (3%) | - |
| Rectal Obstruction | 1 (3%) | - | - | 1 (3%) | - |
Events deemed at least possibly related to treatment are reported by the worst grade per patient. There were no grade 5 events.
The following grade 1–2 adverse events occurred in a single patient (grade shown in parentheses): alkaline phosphatase increase (1), chills (1), dehydration (2), headache (1), hematuria (1), hemorrhoid (1), gastroesophageal reflux (1), gastrointestinal pain (1), infusion reaction (2), insomnia (1), non-cardiac chest pain (2), pain NOS (1), pain in skin (1), phlebitis (2), proteinuria (1), thromboembolic event (2), urinary disorders other (2), vaginal infection (2), vaginal pain (2), weight loss (1)
Pathologic response
Complete clinical and pathologic staging were available in 29 of the 32 patients. One patient had an unknown clinical T stage, one patient underwent local excision rather than resection and thus had unknown pathologic N stage, and one patient with metastatic disease did not undergo resection. Clinical to pathologic downstaging occurred in 15 of 30 (50%, 95% CI 31%, 69%) patients at the primary site, 17 of 25 (68%, 95% CI 46%, 85%) initially clinically node positive patients at the nodal site, and 20 of 29 (69%, 95% CI 49%, 85%) patients overall. Twelve of 25 (48%, 95% CI 28%, 69%) patients who were clinically node positive were found to be pathologically node negative at the time of surgical resection. Pathologic complete response was achieved in 6 of 31 (19%, 95% CI 7%, 37%) patients who had a surgical resection, with an additional 17 of 31 (55%, 95% CI 36%, 73%) experiencing a moderate response, defined as minimal residual disease on pathology (Table 4). At the CRLX101 weekly MTD of 15 mg/m2, 2 of 6 patients achieved a pCR.
Table 4.
Pathologic Response
| CRLX101 dosing schedule | Dose | N | pCR | Moderate response | Minimal response |
|---|---|---|---|---|---|
| Every other week | 12 mg/m2 | 3 | 1 (33%) | 2 (67%) | 0 |
| 15 mg/m2 | 19* | 3 (16%) | 11 (58%) | 5 (26%) | |
| Weekly | 12 mg/m2 | 3 | 0 | 3 (100%) | 0 |
| 15 mg/m2 | 6 | 2 (33%) | 2 (33%) | 2 (33%) | |
| Total | 30 | 6 (19%) | 17 (55%) | 7 (23%) | |
One patient did not undergo a surgical resection, one had a local excision with moderate response.
Discussion
In this phase Ib/II study of neoadjuvant CRT for locally advanced rectal cancer, we found that nano-camptothecin CRLX101 was well-tolerated with only a single dose limiting toxicity. Asymptomatic lymphopenia was the only grade 3 or 4 toxicity occurring in more than one patient, and the only grade 4 toxicity overall. Downstaging occurred in 69% of patients at the time of surgical resection, with almost half of the patients converting from clinical node-positive to pathologic node-negative disease. At the weekly MTD of CRLX101, pCR was achieved in 33% of patients.
Escalation of neoadjuvant CRT to improve both pathologic and clinical outcomes in locally advanced rectal cancer has been explored using various agents. Multiple, large randomized trials have assessed the role of adding oxaliplatin to standard fluoropyrimidine-based neoadjuvant CRT, especially in light of its role in adjuvant and metastatic settings. Although some trials have reported a modest improvement in pCR rate [37,38] and 3-year disease free survival [39] with the addition of oxaliplatin, the majority of randomized trials have shown no improvement in pathologic or clinical outcomes [5,7,14,15]. Furthermore, grade 3–4 toxicities have been consistently higher with regimens incorporating oxaliplatin; for example, on the STAR-01 trial, overall ≥ grade 3 toxicity for neoadjuvant treatment with and without oxaliplatin were 24% and 8%, respectively [5], similar to that observed on the ACCORD 12 trial of 25% and 11%, respectively [14]. Early phase single-arm trials incorporating irinotecan to neoadjuvant CRT saw ≥ grade 3 gastrointestinal toxicities in double digits [29–31] and no difference in pCR rates in a randomized phase II study [40]. As a result, addition of another chemotherapy agent beyond fluoropyrimidine has not become a standard in the neoadjuvant setting.
Alternative approaches of using targeted agents have yielded mixed results. Early phase studies of standard CRT plus cetuximab targeting the epidermal growth factor receptor have shown pCR rates of 8–11% [19,20,41] with increased rates of diarrhea and rash compared to standard CRT in a randomized phase II study [20]. Escalating neoadjuvant therapy with bevacizumab, a monoclonal antibody targeting the vascular endothelial growth factor, has also been investigated with promising pCR rates above 15% in single arm studies [17,18]. However, several published early phase trials have incorporated bevacizumab on a capecitabine/oxaliplatin backbone [18,42], which may limit their applicability given the unclear role of oxaliplatin in this setting.
There is a clear role for novel agents and combinations of neoadjuvant CRT for locally advanced rectal cancer. Escalation of radiosensitizing chemotherapy is most appropriate in patients with highest risk of locoregional recurrence such as T4 disease or tumor extending to or invading the mesorectal fascia. In addition, patients with a strong preference for a non-operative management may benefit from a more aggressive neoadjuvant chemoradiation approach to increase the chance of a pCR.
In the preclinical setting, CRLX101 was shown to be a potent radiosensitizer in colorectal cancer cell lines and xenograft models via inhibition of radiation-induced HIF1α activation [26], which provided a rationale for this current early phase clinical trial. Given the small numbers of patients in the biweekly and weekly dosing cohorts, we were not able to directly compare toxicity between cohorts or make any definite conclusions about the clinical efficacy of this combinatorial strategy. However, the excellent overall toxicity profile clearly demonstrates the potential of nanoparticle drug conjugates to improve the therapeutic window of otherwise intolerable regimens, such as the combination of camptothecin and radiation.
In conclusion, the addition of nanoparticle-drug conjugate CRLX101 to standard capecitabine-based CRT appears to be well tolerated in patients with locally advanced rectal cancer. The maximum tolerated dose and recommended phase 2 dose of weekly CRLX101 was determined to be 15 mg/m2.
Acknowledgments
This research was supported by Cerulean Pharma Inc.
Funding:
Cerulean Pharma Inc.
Footnotes
Conflict of Interest:
Sanoff: Research funding from Bayer, Merck
Moon: none
Moore: none
Boles: none
Bui: none
Blackstock: none
O’Neil: Research Funding from Bayer, Merck; Consulting from Bayer, Genentech, Merck
Subramaniam: Consulting from Bayer, Lexicon, Celgene
McRee: Research funding from Merck, InOvio, BVD; Consulting from Merck
Carlson: none
Lee: Consulting from Bayer
Tepper: none
Wang: Research funding from Cerulean
Clinicaltrials.gov registration NCT02010567
References
- 1.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA. Cancer J. Clin 62(4), 220–241 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med 351(17), 1731–1740 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Bosset J-F, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med 355(11), 1114–1123 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 27(31), 5124–5130 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 29(20), 2773–2780 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Park J, Yoon SM, Yu CS, Kim JH, Kim TW, Kim JC. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer 117(16), 3703–3712 (2011). [DOI] [PubMed] [Google Scholar]
- 7.O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 32(18), 1927–1934 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann. Surg. Oncol 18(6), 1590–1598 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 30(15), 1770–1776 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br. J. Surg 99(7), 918–928 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann. Surg 240(4), 711–717; discussion 717–718 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habr-Gama A, Perez RO, São Julião GP, Proscurshim I, Gama-Rodrigues J. Nonoperative approaches to rectal cancer: a critical evaluation. Semin. Radiat. Oncol 21(3), 234–239 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Maas M, Beets-Tan RGH, Lambregts DMJ, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 29(35), 4633–4640 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Gérard J-P, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 28(10), 1638–1644 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. J. Natl. Cancer Inst 107(11) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 27(18), 3020–3026 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys 76(3), 824–830 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Landry JC, Feng Y, Prabhu RS, et al. Phase II Trial of Preoperative Radiation With Concurrent Capecitabine, Oxaliplatin, and Bevacizumab Followed by Surgery and Postoperative 5-Fluorouracil, Leucovorin, Oxaliplatin (FOLFOX), and Bevacizumab in Patients With Locally Advanced Rectal Cancer: 5-Year Clinical Outcomes ECOG-ACRIN Cancer Research Group E3204. The Oncologist 20(6), 615–616 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horisberger K, Treschl A, Mai S, et al. Cetuximab in combination with capecitabine, irinotecan, and radiotherapy for patients with locally advanced rectal cancer: results of a Phase II MARGIT trial. Int. J. Radiat. Oncol. Biol. Phys 74(5), 1487–1493 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 30(14), 1620–1627 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Kim SY, Shim EK, Yeo HY, et al. KRAS mutation status and clinical outcome of preoperative chemoradiation with cetuximab in locally advanced rectal cancer: a pooled analysis of 2 phase II trials. Int. J. Radiat. Oncol. Biol. Phys 85(1), 201–207 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Wang AZ, Tepper JE. Nanotechnology in radiation oncology. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 32(26), 2879–2885 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi Y, Shao Z, Vang J, Kaidar-Person O, Wang AZ. Application of nanotechnology to cancerradiotherapy. Cancer Nanotechnol 7(1), 11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller SM, Wang AZ. Nanomedicine in chemoradiation. Ther. Deliv 4(2), 239–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werner ME, Foote MB, Wang AZ. Chemoradiotherapy of human tumors: novel approaches from nanomedicine. Curr. Pharm. Des 18(19), 2830–2837 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Tian X, Nguyen M, Foote HP, et al. CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1α. Cancer Res 77(1), 112–122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark AJ, Wiley DT, Zuckerman JE, et al. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proc. Natl. Acad. Sci. U. S. A 113(14), 3850–3854 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eliasof S, Lazarus D, Peters CG, et al. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc. Natl. Acad. Sci. U. S. A 110(37), 15127–15132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta VK, Cho C, Ford JM, et al. Phase II trial of preoperative 3D conformal radiotherapy, protracted venous infusion 5-fluorouracil, and weekly CPT-11, followed by surgery for ultrasound-staged T3 rectal cancer. Int. J. Radiat. Oncol. Biol. Phys 55(1), 132–137 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Klautke G, Feyerherd P, Ludwig K, Prall F, Foitzik T, Fietkau R. Intensified concurrent chemoradiotherapy with 5-fluorouracil and irinotecan as neoadjuvant treatment in patients with locally advanced rectal cancer. Br. J. Cancer 92(7), 1215–1220 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glynne-Jones R, Falk S, Maughan TS, Meadows HM, Sebag-Montefiore D. A phase I/II study of irinotecan when added to 5-fluorouracil and leucovorin and pelvic radiation in locally advanced rectal cancer: a Colorectal Clinical Oncology Group Study. Br. J. Cancer 96(4), 551–558 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss GJ, Chao J, Neidhart JD, et al. First-in-human phase 1/2a trial of CRLX101, a cyclodextrin-containing polymer-camptothecin nanopharmaceutical in patients with advanced solid tumor malignancies. Invest. New Drugs 31(4), 986–1000 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keefe SM, Hoffman-Censits J, Cohen RB, et al. Efficacy of the nanoparticle-drug conjugate CRLX101 in combination with bevacizumab in metastatic renal cell carcinoma: results of an investigator-initiated phase I-IIa clinical trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol 27(8), 1579–1585 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voss MH, Hussain A, Vogelzang N, et al. A randomized phase 2 trial of CRLX101 in combination with bevacizumab versus standard of care in patients with advanced renal cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol (2017). [DOI] [PubMed]
- 35.Lakhani N, Piha-Paul SA, Senderowicz A, et al. Evaluation of weekly dosing of CRLX101 alone and in combination with bevacizumab (bev) in patients (pts) with advanced solid tumors. Ann. Oncol 27(suppl_6), 393P (2016). [Google Scholar]
- 36.Simon R Optimal two-stage designs for phase II clinical trials. Control. Clin. Trials 10(1), 1–10 (1989). [DOI] [PubMed] [Google Scholar]
- 37.Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 13(7), 679–687 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 34(27), 3300–3307 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16(8), 979–989 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Mohiuddin M, Paulus R, Mitchell E, et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int. J. Radiat. Oncol. Biol. Phys 86(3), 523–528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velenik V, Ocvirk J, Oblak I, Anderluh F. A phase II study of cetuximab, capecitabine and radiotherapy in neoadjuvant treatment of patients with locally advanced resectable rectal cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol 36(3), 244–250 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Uehara K, Hiramatsu K, Maeda A, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 Phase II trial. Jpn. J. Clin. Oncol 43(10), 964–971 (2013). [DOI] [PubMed] [Google Scholar]


