Modern antiretroviral therapy (ART) has revolutionized the care and treatment of people living with HIV (PLWH), but the HIV/AIDS epidemic has also transformed our approach to chronic disease care in ways that extend beyond suppressing the virus with medications. In varied contexts throughout the world, clinicians, social workers, epidemiologists, activists, politicians and others have worked together to build systems for prevention of HIV transmission and ART treatment, eventually scientifically proving the power of treatment as prevention(1). Because having an undetectable HIV viral load in the blood makes transmission of the HIV virus virtually impossible (i.e. undetectable = untransmissable or “U=U”), HIV control programs now scrutinize each step of the HIV treatment cascade, from diagnosis to retention in care to provision of ART (Figure 1), with a goal of getting as many as possible to achieve viral suppression. Furthermore, with the advent of pre-exposure prophylaxis (PrEP)—the use of ART to prevent HIV infection—many are now focused on the upstream prevention cascade to get larger numbers of at-risk persons on PrEP. Despite continued room for improvement, the success of these efforts from a public health perspective are impressive.
Figure 1:
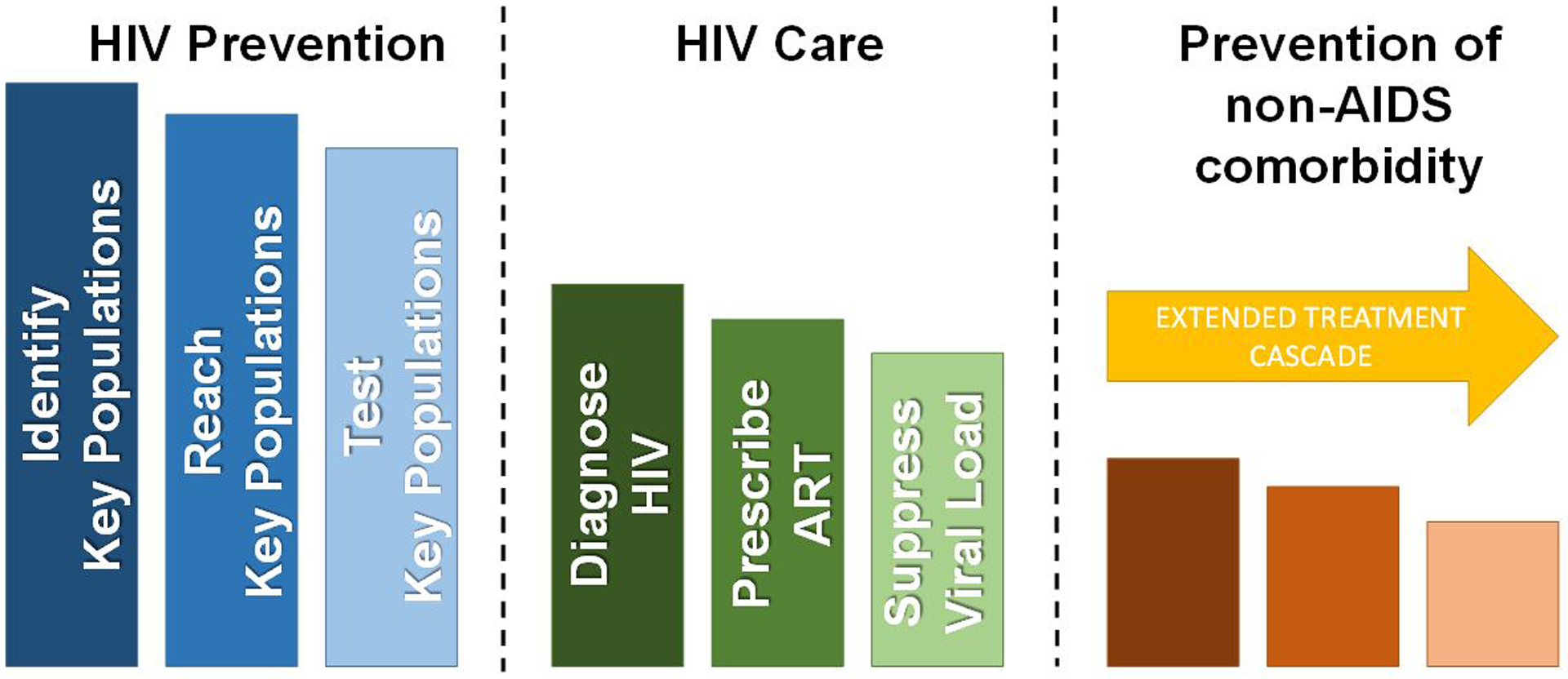
The extended treatment cascade for people living with HIV.
But what about ongoing care and treatment for PLWH who have achieved viral suppression on ART? What can be done to maintain the health gains achieved with ART, so that someone whose life has been prolonged by ART does not die of a heart attack? How can systems of care be re-engineered to focus on the prevention of non-AIDS comorbidity with the same enthusiasm as prevention of HIV transmission? Prevention of non-AIDS comorbidity should be a primary focus of treatment for those who achieve viral suppression on ART, who form a majority of PLWH active in care in the US(2). This requires an extension of the HIV treatment cascade as we have described (Figure 1)(3,4). Chief among the non-AIDS comorbidities is cardiovascular disease (CVD), along with cancer, liver disease, kidney disease, and neurocognitive disorders. PLWH may have up to twice the risk of atherosclerotic CVD events compared to HIV-negative persons even after adjustment for traditional risk factors that can be more common in PLWH (5), although some have argued that this relative hazard is probably decreasing over time with earlier access to ART and increased statin use(6). The most recent, large epidemiologic study of the modern treatment era (2011–2016), suggests that the risk does persist, though it is modestly lower than prior estimates [adj HR 1.29 (95% CI 1.18–1.40) for atherosclerotic CVD]. Traditional risk factors, chronic inflammation and immune activation, and individual ART drugs have all been associated with increased risk in numerous studies spanning over 2 decades(7). Although we now know a fair amount about the epidemiology and mechanisms of cardiovascular disease in PLWH(7), we know much less about how to implement changes in clinical care to promote cardiovascular health and prevent CVD in our patients with HIV. There is no shortage of effective interventions, ranging from diet and exercise to statins and PCSK9 inhibitors. Yet, implementing these interventions for PLWH may require special considerations.
The objective for this special issue of Progress in Cardiovascular Diseases is to highlight important work being done in this area. The first group of papers is from the National Institutes of Health (NIH)-funded consortium “ImPlementation REsearCh to DEvelop interventions for People Living with HIV (PRECLuDE)”. A second group of papers highlights special populations and considerations, including prevention of CVD for PLWH in low- and middle-income countries, prevention for under-represented minority populations in the US, prevention of stroke and heart failure, heart failure in PLWH with liver disease, and the importance of exercise.
The scope of papers collected here is unprecedented and illustrates the incredible opportunity we now have in this field to improve CVD outcomes for PLWH. This requires a bold vision of “prevention as treatment” for non-AIDS co-morbidity. This vision must be championed by clinicians, researchers, politicians, and peer advocates in the same way that they championed the quest to develop effective ART in the 1990’s and to disseminate it throughout the world in the 2000’s. An example of one such peer advocate is Jules Levin of the National AIDS Treatment Advocacy Project, who became one of the first patient advocates to serve on the writing group of an American Heart Association (AHA) Scientific Statement. In a supplement to the AHA Statement on HIV(7), Jules writes: “Our field remains consumed by HIV prevention and cure research, yet the possibility of a cure remains very difficult to achieve and even if possible, years away. Older aging PLWH suffering the devastating ravages of premature aging, senescence, and disability are suffering NOW and need help NOW. The comorbidities they are suffering from – including but not limited to cardiovascular diseases – need to be addressed NOW.”
Accelerating the translation of research on prevention as treatment for cardiovascular comorbidity to the HIV clinic context will require integration with cancer, liver, kidney, and neurocognitive disease care in a way that is efficient and scalable. The appropriate metrics must also be in place to measure exposures in the clinical setting—particularly for lifestyle factors like diet and exercise. It is not difficult, however, to envision how this might happen in HIV clinics, with supplemental funding from the Ryan White Care Act(8) and others. For example, the Center For AIDS Research Network of Integrated Clinical Systems (CNICS) integrates patient-reported outcomes across 8 large clinic systems in the United States(9). Additionally, the HIV community is already sensitized to the importance of treatment cascade metrics. Thus, it is not a stretch to think that extended treatment cascade metrics (e.g. rates of blood pressure control) might one day be a required part of maintaining federal funding for HIV care.
In conclusion, we are optimistic about the future state of CVD preventive care for PLWH. After all, PLWH who have achieved suppression of the HIV virus in the blood on ART are by necessity engaged with a clinic home where the opportunity to provide high-quality preventive care may exceed that of the general population. Indeed, the context of HIV care may catalyze advances in CVD prevention more readily than has been the case to date for primary care in the non-HIV population, because of the robust infrastructure that exists with HIV clinics. A 35 year old man with HIV may potentially be more likely to get smoking cessation services, treatment for high blood pressure, and counseling with a dietician through his HIV clinic than a demographically similar 35 year old man without HIV who does not see any doctors because he doesn’t have a chronic disease. One can envision the HIV medical home as a model for highly integrated primary prevention care. In memory of all those who have been taken too early by HIV/AIDS and for the empowerment of all those who are now successfully living with the virus, we need to make it happen.
References
- 1.Cohen MS, Chen YQ, McCauley M et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selected National HIV Prevention and Care Outcomes in the United States, 2019.
- 3.Okeke NL, Webel AR, Bosworth HB et al. Rationale and design of a nurse-led intervention to extend the HIV treatment cascade for cardiovascular disease prevention trial (EXTRA-CVD). Am Heart J 2019;216:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longenecker CT. Vascular disease and aging in HIV: Time to extend the treatment cascade. Vasc Med 2018;23:476–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah ASV, Stelzle D, Lee KK et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV. Circulation 2018;138:1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein DB, Leyden WA, Xu L et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis 2015;60:1278–80. [DOI] [PubMed] [Google Scholar]
- 7.Feinstein MJ, Hsue PY, Benjamin LA et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation 2019;140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley H, Viall AH, Wortley PM, Dempsey A, Hauck H, Skarbinski J. Ryan White HIV/AIDS Program Assistance and HIV Treatment Outcomes. Clin Infect Dis 2016;62:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredericksen RJ, Tufano J, Ralston J et al. Provider perceptions of the value of same-day, electronic patient-reported measures for use in clinical HIV care. AIDS Care 2016;28:1428–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


