Abstract
Previously, we reported that N-methyl-N-nitrosourea (MNU)-induced mammary tumors could be established in mutant spontaneous dwarf rats (SDRs), which lack endogenous growth hormone (GH) by supplementing with exogenous GH, and almost all such tumors regressed upon GH withdrawal. When the highly inbred SDR line was outcrossed to wild-type (WT) Sprague-Dawley rats, MNU-induced mammary tumors could still be established in resulting outbred SDRs by supplementing with exogenous GH. However, unlike tumors in inbred SDRs, 65% of mammary tumors established in outbred SDRs continued growth after GH withdrawal. We further tested whether these tumors were more sensitive to doxorubicin than their WT counterparts. To accomplish this, MNU-induced mammary tumors were established in WT rats and in SDRs supplemented with exogenous GH. Once mammary tumors reached 1 cm3 in size, exogenous GH was withdrawn from SDRs, and the subset that harbored tumors that continued or resumed growth in the absence of GH were selected for doxorubicin treatment. Doxorubicin was then administered in 6 injections over 2 weeks at 2.5 mg/kg or 1.25 mg/kg for both the WT and SDR groups. The SDR mammary tumors that had been growing in the absence of GH regressed at both doxorubicin doses while WT tumors continued to grow robustly. The regression of SDR mammary tumors treated with 1.25 mg/kg doxorubicin was accompanied by reduced proliferation and dramatically higher apoptosis relative to the WT mammary tumors treated with 1.25 mg/kg doxorubicin. These data suggest that downregulating GH signaling may decrease the doxorubicin dose necessary to effectively treat breast cancer.
Keywords: mammary tumor, breast cancer, growth hormone, IGF-1, doxorubicin, MNU
Breast cancer (BCa) is the second most common cancer worldwide, with roughly 1.7 million new cases a year (1). It continues to be of upmost importance to find safe treatments for BCa as its survival rates remain stagnant, likely due to a lack of breakthrough treatment options in recent years (2). Common chemotherapeutic agents used to treat a variety of cancers, including BCa, exert the majority of their effects by inducing apoptosis in cancer cells, via both extrinsic and intrinsic apoptotic pathways (3). Insulin-like growth factor 1 (IGF-1) is an anti-apoptotic factor and thus can lead to chemoresistance, making it a common target of cancer research. In BCa, research has shown that higher levels of IGF-1 are correlated with higher rates of BCa and a decrease in the time required for tumor generation (4,5). As a result, multiple clinical studies have aimed to generate and study IGF-1 antagonists in an attempt to increase the efficacy of chemotherapeutic treatments (6-8). However, the promising results seen in preliminary lab testing have not shown the same benefit in humans, as blocking IGF-1 signaling alongside other treatments has shown to have no effect on cancer progression in clinical trials (9). This may be due to the fact that the majority of IGF-1 secretion is controlled by feedback mechanisms involving growth hormone (GH) signaling, a relationship commonly referred to as the GH/IGF-1 axis. The feedback mechanisms within the GH/IGF-1 axis imply that IGF-1 antagonists would trigger increased IGF-1 activity by altering the feedback mechanisms that normally keep IGF-1 activity in a normal range. This would be expected to reduce the impact of such inhibitors and may explain the failure IGF-1 inhibitors in human trials.
Laron syndrome is caused by mutations in the GH receptor (GHR) gene that disrupt the GH/IGF-1 axis and appear to protect these patients from cancer (10). In a study of 169 patients with Laron syndrome, none developed cancer while many of their relatives with an intact GH/IGF-1 axis did (11). Since these patients appear to be protected from cancer, research has focused on how GH signaling contributes to cancer progression. Available evidence suggests that much of the impact of GH on BCa progression is dependent on its regulation of IGF-1 (12). However, some actions of GH may be via direct activation of GHR in BCa cells. MCF10A mammary epithelial cells overexpressing either GH or IGF-1 receptor are completely transformed in vitro and in orthotopic xenografts in immunocompromised mice (13,14). In contrast, GH and estradiol treatment of T47D BCa cells stimulated proliferation in vitro primarily through the GH-mediated JAK2/STAT5 pathway rather than through IGF-1 receptor activation (15). Thus, by targeting GH directly, techniques can be generated that block effects from IGF-1 and GH by controlling GH signaling. In general, it is seen that higher GH levels lead to increased rates of BCa while models with impaired or nonfunctioning GH pathways have no incidence of BCa (4). A mouse model of Laron syndrome has been combined with the C3(1)/TAg mouse to show that disrupting GH signaling can inhibit estrogen-independent mammary carcinogenesis (16). Rodent models, including experimental rat mammary carcinogenesis, are widely used models of human cancer (17).
Rodent model-based research has also identified that GH can induce chemoresistance by blocking the effects of many common therapeutic agents. Doxorubicin is a common chemotherapeutic agent used in BCa treatment and functions by inducing apoptosis in cancer cells. Studies have shown that normal to high levels of GH can reduce the apoptotic effect of doxorubicin (18,19). As a result, it has been hypothesized that decreasing GH levels would increase the efficacy of chemotherapeutic agents while also reducing the dose necessary to create the same effect. For this study, we investigated if GH-deficient mammary tumors in spontaneous dwarf rats (SDRs) were more sensitive to doxorubicin than their wild-type (WT) counterparts. We observed GH-deficient mammary tumors exhibited increased regression, lower proliferation, and higher apoptosis upon doxorubicin treatment compared to WT tumors suggesting that targeting GH signaling may decrease the chemotherapeutic dose necessary to treat BCa.
Materials and Methods
Animals
All experiments involving animals were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (20) and under a protocol approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago.
The SDR strain is not commercially available and was bred at the Biologic Resources Laboratory, University of Illinois at Chicago. The SDR mutation arose in 1977 and had been bred apart from its founder strain, the Sprague-Dawley (21,22). The phenotype is due to a nonsense point mutation designated dr that results in an unstable messenger RNA transcript roughly half the size of the normal Gh messenger RNA transcript (23). To control for factors resulting from genetic drift, SDRs (dr/dr) were back crossed with WT Sprague-Dawley rats (+/+) purchased from Harlan Sprague-Dawley. The progeny of these mating pairs were genotyped as described previously (24). Rats homozygous for the WT GH gene (Gh+/+), referred to in this article as WT, or homozygous for the nonsense mutation (Ghdr/dr), referred to as SDR, were used for these studies. All rats were fed Teklad 8640 diet (Harlan Teklad, Madison, WI, USA), provided water ad libitum, and housed in a temperature- and humidity-controlled environment with a regular light-dark illumination cycle (6:00 am on, 7:00 pm off).
Bovine GH was kindly provided by Dr. Gregg Bogosian (Monsanto Co., St. Louis, MO, USA). The material used for the current studies was the zinc salt of recombinant bovine GH and not Posilac®, which is a pegylated form of bovine GH specifically formulated for use in the dairy industry. The GH was dissolved in 10 mm NaHCO3, 20 mm NaOH, and 1 mm EDTA (pH 12). Rats were injected twice daily beginning at 3 weeks of age (approximately 9:00 am and 6:00 pm every day) with buffer or 150 μg GH.
At 21 days of age, SDRs began receiving supplemental GH via daily intraperitoneal (IP) injection. At 4 weeks of age, all animals received a single IP injection of N-methyl-N-nitrosourea (MNU; Ash-Stevens, Detroit, MI, USA; 50 mg/kg) dissolved in 0.85% saline acidified with acetic acid. Beginning 4 weeks after MNU injection, all animals were palpated weekly. Mammary tumor size and tumor location for each rat were recorded throughout the study. Tumor measurements were made with a digital caliper and tumor volume was calculated using the following formula: tumor volume = (π/6) × (larger diameter) × (smaller diameter)2. Tumors were named by their ventral location as left (L), right (R), or middle (M) and their mammary pad number from anterior (1) to posterior (6). Note that more than 1 tumor could erupt from the same mammary pad; these tumors were amended with “−1.” When an animal’s largest tumor reached a volume of 1 cm3, GH administration was halted in SDRs. Tumors were monitored to identify the subset of SDRs with tumors that exhibited growth during a 1- to 2-week GH washout period. After the GH washout, animals began treatment with 6 IP injections of 2.5 mg/kg or 1.25 mg/kg doxorubicin hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) over the course of 2 weeks. The same treatment protocol was used for WT rats when an animal’s largest tumor reached 1 cm3.
Blood was collected for analysis of serum IGF-I prior to GH withdrawal, 1 day prior to doxorubicin treatment, and at the time of tissue collection. Blood was collected from tail snip during the study and from trunk blood at the time of tissue collection. The blood samples were centrifuged at 1500 × g for 20 min, and serum was harvested and stored at −80°C until analysis by radioimmunoassay using a commercially available kit (Nichols Institute Diagnostics, San Clemente, CA, USA). All animals were sacrificed by CO2 asphyxiation in accordance with guidelines set forth by the American Medical Veterinary Association (25).
Histology and immunohistochemistry
Portions of each tumor were fixed in 10% neutral-buffered formalin, embedded in paraffin, and sectioned (5 µm). Sections were stained with hematoxylin and eosin (H&E) for histopathology assessment. The presence of hemosiderin staining was scored for 2 H&E sections per tumor by a blinded observer using a semiquantitative scale (0 = no hemosiderin stained cells observed; 1 = a few isolated hemosiderin stained cells observed; 2 = at least 1 cluster of multiple hemosiderin stained cells observed; 3 = multiple clusters of hemosiderin stained cells observed). Cell proliferation was measured via Ki67 immunohistochemistry staining. Apoptosis was assessed by the terminal deoxynucleotide-transferase-mediated deoxyuridine triphosphate-digoxigenin nick end-labeling (TUNEL) immunofluorescent assay using the Promega DeadEnd™ Colorimetric TUNEL System (G7131; Madison, WI, USA). Sections were dewaxed in Citrisolv and rehydrated through graded ethanol. For antigen retrieval, the sections were boiled in Vector Citrate-based Unmasking Solution (H-330; Burlingame, CA, USA) and cooled to room temperature. For Ki67 analysis, slides were incubated in 3% H2O2 to quench the reaction, followed by blocking, incubation with the Ki67 primary antibody (26), a series of phosphate-buffered saline plus polysorbate 20 washes, and incubation in biotinylated goat anti-rabbit immunoglobin G secondary antibody (27). Slides were stained with the Vector Vectastain ABC Elite kit (PK6100; Burlingame, CA, USA) and counterstained with hematoxylin. For TUNEL analysis, slides were refixed in 10% phosphate-buffered saline buffered formalin and incubated at 37ºC with biotinylated nucleotide mix. Slides were then incubated in Invitrogen eBioscienceTM Streptavidin-FITC (11-4317-87; Carlsbad, CA, USA) before mounting with Vectashield Antifade Mounting Medium with DAPI (H-1200; Burlingame, CA, USA). After Ki67 slides were stained, images were blinded and analyzed in ImageJ Java image processing program via the Cell Counter and counted manually. Positively stained cells were counted as well as a total count. For TUNEL analysis, images were blinded and imported into Adobe Photoshop. Threshold was adjusted to show all positively stained cells and all stained cells separately. These black-and-white threshold-adjusted images were imported into ImageJ and a pixel count was conducted to analyze apoptotic levels.
Statistical analysis
Prior to the statistical analysis, tumors from SDR animals were assessed for post-GH tumor shrinkage. All tumors that shrank in the period after GH supplementation concluded, but before the start of doxorubicin treatment, were omitted from the data used to generate the model-estimated tumor growth curves and subsequent analysis of doxorubicin-treated tumors. This was assessed by comparing the first post-GH volume measurement to the corresponding final predoxorubicin volume measurement. Five tumors, all in the 1.25 mg/kg dosage group, met this criterion and were not considered in the analysis.
Experimental groups consisted of 3 to 8 animals for histological analysis; figure images are representative of each treatment group. For all histological stains, a Welch’s t test was conducted to identify differences among means. Results are reported as mean ± standard error of the mean. A difference of P < 0.05 was considered significant. Statistical analyses of histological stains were performed using GraphPad Prism (8.4.3) statistical software (GraphPad Software, San Diego, CA, USA).
To estimate the tumor growth curves, a generalized linear mixed model (GLMM) using a Gaussian distribution and log link-function was fit to the data using the “lme4” package in R (V 4.0.2), where the initial timepoint (day 0) corresponded to when the animal began doxorubicin hydrochloride treatment (28). The fixed effects portion of the model was parameterized such that an intercept and slope were directly estimated for the four treatment groups (WT/SDR rats, 1.25/2.5 doxorubicin dosage). The random effects portion of the model was structured such that a random slope and intercept were estimated at the tumor level. A higher level random effect at the animal level was estimated to be zero; hence, it did not benefit model fit and was subsequently left out of the model. Because tumor volumes below a mechanically detectable threshold could not be measured accurately, they were omitted from the original data instead of being encoded as zero. This decision could possibly effect parameter estimation; however, because it is unknown which data values were below this threshold (and subsequently omitted). The impact of this decision could not be statistically assessed.
A graphical analysis of the residuals suggested that all GLMM assumptions were adequately met. The overall model error, within tumor error, and the estimated random effects were all found to be sufficiently homoscedastic and Gaussian in distribution. After model fitting, Wald-type 95% confidence intervals were estimated for all fixed-effect parameters.
Results
Most mammary tumors established in outbred SDR rats can grow in the absence of GH
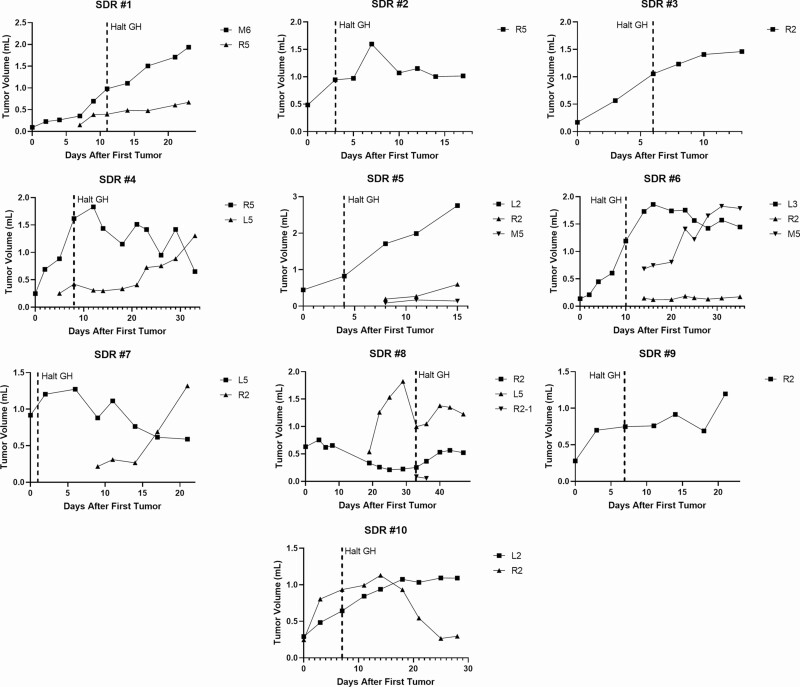
The SDR’s point mutation in Gh renders an inactive protein product (23). MNU-induced mammary tumor development has previously been established in SDRs exogenously treated with bovine GH (29). Furthermore, removing GH supplementation from MNU-treated SDRs resulted in regression of almost all tumors in the highly inbred rat strain on which the SDR mutation had been maintained for more than 30 years (29). To further test whether this GH dependence was also a feature of mammary tumors with a more varied genetic background, the highly inbred SDR line was outcrossed to WT Sprague-Dawley rats. Subsequent crosses established rats homozygous for the SDR mutation with the outbred genetics characteristic of Sprague-Dawley rats. In these rats, MNU-induced mammary tumors could still be established by supplementing with exogenous GH. However, unlike tumors in inbred SDR mutant rats, 24 out of 37 (65%) of mammary tumors established in outbred SDR rats continued or resumed growth after GH withdrawal. When GH was halted, some tumors continued to grow, new tumors emerged, and some tumors regressed (Fig. 1 and data not shown).
Figure 1.
Growth curves of MNU-induced mammary tumors in SDRs treated with supplemental GH from first appearance of tumor up to doxorubicin treatment start. When GH was halted, (indicated by the dashed line), some tumors continued to grow or resumed growth after an initial regression [SDRs #1, 2, 3, 4 (L5), 5 (L2), 9, 10, and 11 (L2)], new tumors emerged [SDRs #5 (R2, M5), 7 (R2, M5), and 8 (R2)], and some tumors regressed [SDRs #4 (R5), 6, 7(L3), 8 (L5), 9 (R2-1), and 11 (R2)].
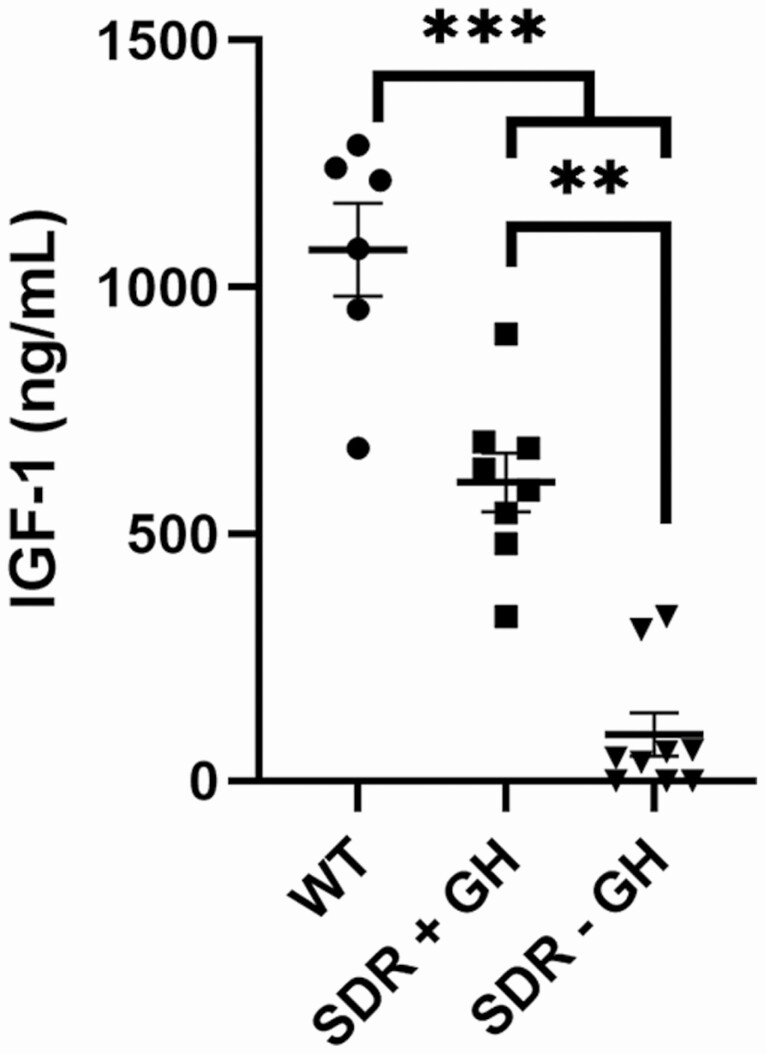
Mammary tumor growth occurred in SDRs with low serum IGF-1
Serum IGF-1 levels were measured for carcinogen exposed rats. The highest level of IGF-1 was present in the WT rats. An intermediate level was observed SDRs treated with bovine GH, and very low levels were observed in GH supplemented SDRs after the 2-week GH washout period (Fig. 2).
Figure 2.
Blood was collected for analysis of serum IGF-I from WT rats at time of sacrifice (WT), SDRs treated with bovine GH (SDR + GH), and SDRs treated with bovine GH after GH withdrawal but before doxorubicin treatment (SDR − GH). Asterisks indicate significant difference in mean (**P < 0.01, ***P < 0.001; analysis of variance).
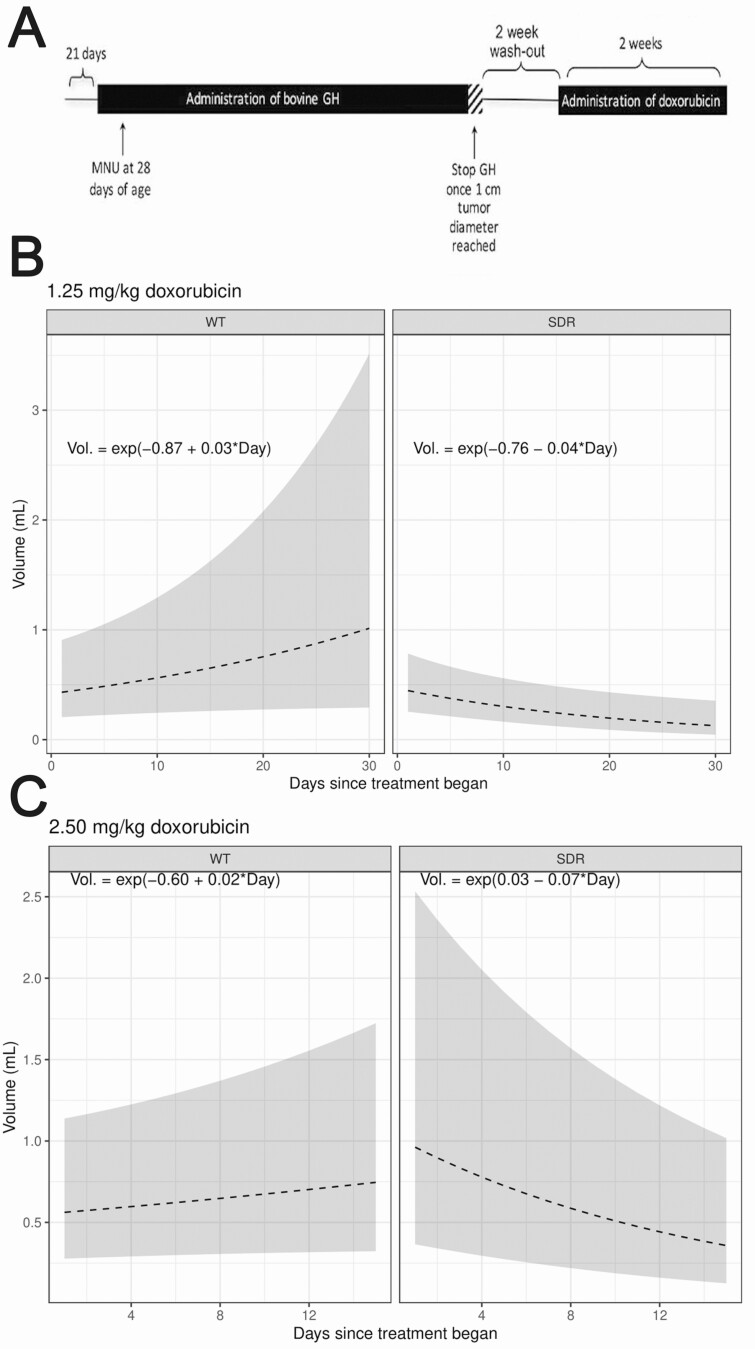
Antitumor efficacy of doxorubicin was enhanced in SDR tumors that grew or persisted after GH withdrawal
After MNU-induced mammary tumors grew to 1 cm3, doxorubicin was administered at 2.5 mg/kg or 1.25 mg/kg to treat WT rats and SDRs following a 2-week GH washout period (experimental design for SDRs shown in Fig. 3A). For SDR tumors, the effects of doxorubicin were assessed only for tumors where the first post-GH withdrawal volume measurement was less than or equal to the corresponding final pre-doxorubicin volume measurement. In model-estimated tumor growth curves for rats treated with 1.25 mg/kg doxorubicin, tumor volume regression only appeared in SDRs and not in WT rats over the course of doxorubicin treatment (Fig. 3B). At this dosage, WT rat tumors grew over the course of treatment at a mean rate of 2.99% [95% confidence interval (C)I = (−0.11%, 6.19%)] per day from their 0.42 cm3 initial volume while SDR tumors had an average −4.24% [95% CI = (−7.1%, −1.28%)] decrease in tumor size from an initial 0.47 cm3 initial volume, this difference in growth rates was found to be significant (P < 0.0001). The estimated GLMM equations can be seen overlaid on Fig. 3B. As an example interpretation, a rat in the WT 1.25 mg/kg doxorubicin group will have an expected day-20 tumor volume of e−0.87 + 0.03×20 = 0.76cm3. At a 2.5 mg/kg doxorubicin dose, WT rat tumors grew over the course of treatment at a mean rate of 2.05% [95% CI = (−0.76%, 4.93%)] per day from their 0.55 cm3 initial volume while SDR tumors had an average −6.82% [95% CI = (−9.65%, −3.91%)] decrease in tumor size from an initial 1.03 cm3 initial volume, this difference in growth rates was found to be significant (P < 0.0001) with estimated GLMM equations overlaid on Fig. 3C.
Figure 3.
Tumor volumes in experimental rats were modeled based on observable tumor volumes. (A) An outline of the experimental design used for this study. (B) Model-estimated tumor growth curves, along with approximate 95% CIs, are shown for both WT (n = 7 tumors) and SDR (n = 11 tumors) type rats treated with 1.25 mg/kg doxorubicin. (C) Model-estimated tumor growth curves, along with approximate 95% CIs, are shown for both WT (n = 6 tumors) and SDR (n = 4 tumors) type rats treated with 2.5 mg/kg doxorubicin. At both doses, SDR tumors had an overall decreasing tumor trajectory while the WT tumors grew over the observed time period, this difference in slopes was found to be statistically significant (P < 0.0001).
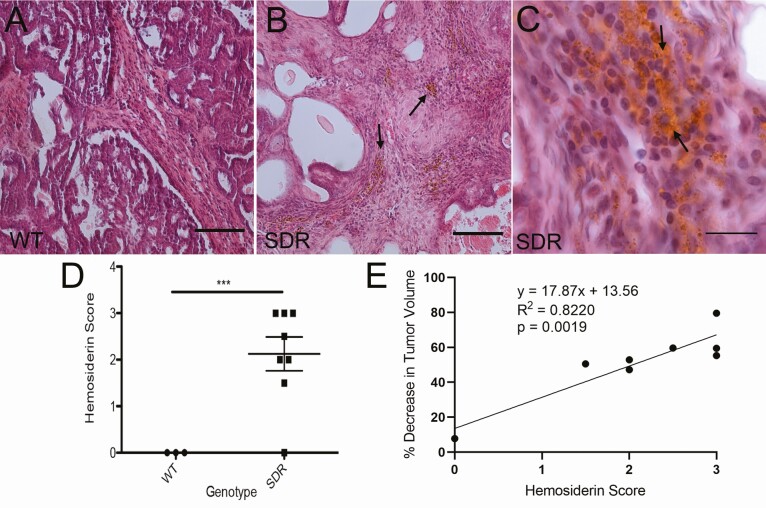
Tumor histology H&E staining revealed cancer lesions and hemosiderin deposits in SDRs
Histological examination of the mammary tumors following doxorubicin treatment revealed an assortment of mammary carcinomas. Most samples possessed papillary and cystic features and appeared solid in nature. This histologic variability is common in rat models and is probably not informative with respect to the biology of an individual tumor or its response to treatment. Some desmoplastic changes in the stroma were apparent in all groups, which may be indicative of local invasion. Signs of hemosiderin deposits were identified in SDRs treated with 1.25 mg/kg but not in WT rats (Fig. 4A-4C). Semiquantitative scoring of hemosiderin deposition shows an increase in SDRs compared to WT rats (Fig. 4D). There was a positive correlation between hemosiderin score and the extent of tumor regression in response to 1.25 mg/kg doxorubicin treatment in SDRs (Fig. 4E).
Figure 4.
H&E staining of the mammary tumors in WT (A) and SDRs (B and C) treated with 1.25 mg/kg doxorubicin showed evidence of hemosiderin deposits (golden-brown stain) in SDRs indicated by arrows in B and C. (D) Blinded semiquantitative hemosiderin stain scoring indicates a complete lack of hemosiderin in WT rats (n = 3) with extensive evidence in SDRs (n = 8; ***P < 0.001; Student’s t-test). (E) Simple linear regression analysis shows a positive correlation between hemosiderin score and tumor volume decrease over the course of treatment in SDRs treated with 1.25 mg/kg doxorubicin. Scale bars in A and B = 100 µm. Scale bar in C = 20 µm.
Doxorubicin caused decreased proliferation and increased apoptosis in SDR tumors
To determine the cause of the differential change in tumor volume between the 2 groups treated with 1.25 mg/kg doxorubicin, we assessed the extent of proliferation and apoptosis in the mammary tumors. Ki67 was used to identify proliferative cells via immunohistochemistry (Fig. 5A and 5B). SDRs had a lower percentage of cells expressing the proliferation marker Ki67 than WT rats (Fig. 5C). Similarly, TUNEL immunofluorescent staining was used to identify apoptotic cells in mammary tumors. Due to the fluorescent nature of this assay, a different counting method was employed (see Histology and Immunohistochemistry Methods). Using 4′,6-diamidino-2-phenylindole (DAPI) as a stain for general nuclei, the proportion of fluorescent green pixels to general pixels was used as a means of assessing positive staining (Fig. 6A-6D). Inversely to Ki67, the proportion of TUNEL-positive cells was higher in SDRs compared to WT rats treated with the same concentration of doxorubicin (Fig. 6E).
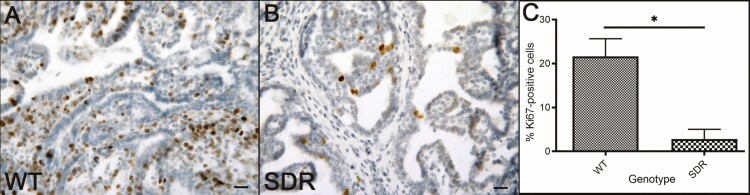
Figure 5.
Ki67 immunohistochemistry staining is a marker of proliferative cells. (A and B) Representative Ki67-stained images of mammary tumors treated with 1.25 mg/kg doxorubicin in WT (A) and SDR (B) are shown. Scale bar = 50µm. (C) Blinded cell counting shows a decrease in Ki67-positive cells in mammary tumors found in SDRs (n = 6) relative to WT (n = 3; *P < 0.05; Student’s t-test).
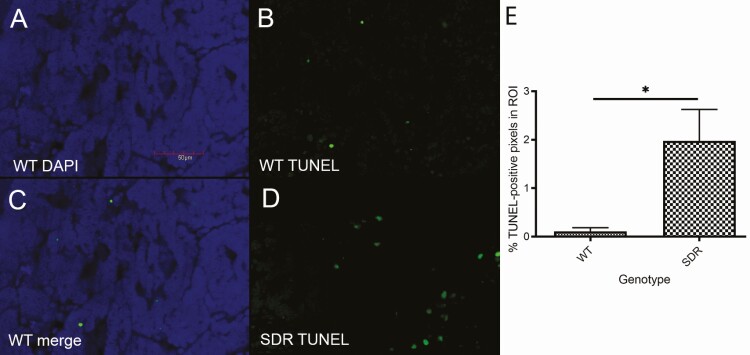
Figure 6.
Immunofluorescent TUNEL staining is a marker of apoptotic cells. DAPI stain (A), TUNEL stain (B), and merged image (C) are shown for mammary tumors from WT rats treated with 1.25 mg/kg doxorubicin. Representative TUNEL stain (D) is also shown for mammary tumors treated with 1.25 mg/kg doxorubicin in SDRs. Scale bar = 50µm. (E) Blinded cell stain analysis showed an increase in TUNEL-positive cells for mammary tumors from SDRs (n = 6) treated with 1.25 mg/kg doxorubicin compared to WT (n = 3) treated with 1.25 mg/kg doxorubicin (*P < 0.05; Student’s t-test).
Discussion
Previous studies have highlighted that strategies that target the GH/IGF-1 axis could be used to treat even advanced mammary cancers (16,24,29-33). SDRs, which are normally resistant to MNU-induced mammary carcinogenesis, will form MNU-induced mammary tumors when supplemented with exogenous GH. In these earlier studies, almost all of the tumors induced in this way regressed when GH supplementation was halted (29). Data in the current study suggest that the requirement for GH signaling for mammary tumor growth and survival was at least partially due to the unique genetic background of SDR rats. The SDR strain harbors a nonsense mutation in Gh at the Exon 4 splice site that alters amino acids 71-99 and introduces a premature stop codon at position 100, producing a truncated version of the GH protein unable to bind to GHRs. This mutation arose in 1977 in a Sprague-Dawley rat and had been subsequently inbred as a unique strain for almost 30 years prior to being used for the earlier mammary tumor experiments. For the current study, the highly inbred SDR line was outcrossed to WT Sprague-Dawley rats. Subsequent crosses established rats homozygous for the SDR mutation with the outbred genetics characteristic of Sprague-Dawley rats. These outbred rats were still susceptible to MNU-induced mammary tumorigenesis when supplementing with exogenous GH. However, unlike mammary tumors in inbred SDR rats, 65% of mammary tumors continued or resumed growth after GH withdrawal (Fig. 1 and data not shown). Furthermore, this was not due to continued IGF-1 production in the absence of exogenous GH as serum IGF-1 levels were very low after GH washout (Fig. 2) and similar to levels seen in previous studies using inbred SDR rats (29). Building on these findings, we investigated whether a lack of GH signaling rendered tumors that continued to grow in the absence of GH signaling more vulnerable to conventional chemotherapy.
Rats harboring tumors exhibiting GH-independent tumors were treated with doxorubicin, an effective chemotherapeutic agent used to treat human BCa (34,35). Model-estimated tumor growth curves show that tumors in WT rats continued to grow in spite of doxorubicin at either 2.5 mg/kg or 1.25 mg/kg (Fig. 3). However, tumors that had been growing after GH washout in SDR rats regressed upon doxorubicin treatment at either 2.5 mg/kg or 1.25 mg/kg (Fig. 3). Histologic and molecular analysis of tumors further clarified differences between WT and SDR tumors.
H&E staining displayed expected cancerous lesions in tumors from both genetic groups along with signs of hemosiderin deposition only in the SDR group (Fig. 4). Hemosiderin deposits are thought to reflect macrophage infiltration in response to chemotherapy treatment having previously been identified in BCa as a metabolic biomarker for tumor associated macrophage infiltration in response to immunotherapy—likely due to enhanced permeability and retention within vascularized mammary tumors with low macrophage specificity (36). Further experiments will need to assess if the hemosiderin deposits found in this study are a biomarker for macrophage infiltration in rat mammary tumors due to a response to chemotherapy, GH withdrawal, or a combination of both. A previous study that evaluated mammary tumors that regressed upon GH withdrawal did not note hemosiderin staining, but this was also not an explicit endpoint in the study (29). The current study did not evaluate tumor histology following GH withdrawal but before doxorubicin treatment, and archival tissues from the earlier study that focused solely on GH withdrawal were not available to determine if hemosiderin deposits might arise due to GH withdrawal alone. The positive correlation between hemosiderin score and tumor volume decrease noted in Fig. 4E supports the use of hemosiderin as a marker of tumor response to chemotherapy.
Doxorubicin treated SDR mammary tumors also had reduced proliferation as measured by Ki67 labeling index (Fig. 5) and had a greater extent of apoptosis as was determined by TUNEL assay (Fig. 6). As doxorubicin functions by increasing the apoptotic rate in mammary tumors, a greater apoptotic rate and reduced proliferation in SDRs supports decreasing GH signaling as a strategy to increase the efficacy of doxorubicin treatment and the lower dose necessary to treat mammary tumors.
The MNU-induced rat mammary cancer model is a well-characterized and highly reproducible model in terms of treatment timing, dosing, and methodology (37,38). The model’s resulting tumors reflect histology and hormone receptor expression that are found in many cases of human BCa (37,39,40). Some investigators have suggested that the MNU-induced model is not suitable for some studies due to variable tumor incidence (41), but other studies—including this study—show that mammary tumors are highly reproducible after a single MNU administration (42). Other preclinical models of mammary cancer include those that utilize carcinogens such as 7,12-dimethylbenzathracene (38), transgenic mouse mammary tumor virus and whey acidic protein that upregulate the expression of some oncogenes (43), and patient-derived xenografts in immunocompromised mice (44). All these models have advantages and limitations. The MNU model of mammary cancer was best suited for the current studies due to its high reproducibility, simple induction regimen, and its compatibility with the SDR model of GH disruption. However, human BCa is notable for having several molecular subtypes, for example, those defined based on differences in the expression of estrogen receptor, progesterone receptor, and/or human epidermal growth factor receptor 2 (45). One limitation of the current study is that the tumors induced by MNU do not fully model molecular differences that distinguish different human BCa subtypes (46). However, our results fit with previous studies using other models that also indicated targeting the GH/IGF-1 axis reduces tumor growth and further shows that disrupting the GH/IGF-1 axis increases sensitivity of GH-independent tumors to chemotherapeutic agents (47). Further analysis of tumors persisting past GH withdrawal can reveal the mechanism behind this independence. These and previous data indicate that GH could induce chemoresistance and thus decrease the efficacy of common chemotherapeutic agents (19). The US Food and Drug Administration–approved drug, pegvisomant, acts as a GHR antagonist. The drug was originally indicated for the use in acromegalic giants to maintain normal circulating IGF-1 levels through the disruption of the GH/IGF-1 axis. However, co-opting this drug for its use in treating diseases such as cancer has been repeatedly investigated. For instance, mice treated with pegvisomant showed a reduction in mammary tumor cell growth in vivo (30). Elsewhere, endometrial cancer cell xenograft growth is slowed when treated with pegvisomant in combination with radiation therapy (48). The data from the current study support the use of pegvisomant and other GHR antagonists in treating mammary tumors—specifically in conjunction with doxorubicin—to lower the treatment dose. This would be highly desirable as doxorubicin has proven to be cardiotoxic at typical dosing levels and therapy has sometimes led to lethal toxicity (49-51).
Acknowledgments
The authors thank James Shull for histopathology consultation.
Financial Support: Grant R21CA238105 from the National Institutes of Health/National Cancer Institute (NIH/NCI).
Author Contributions: DDL and SMS conceived the animal study design. DDL, CJU, PCM, and SMS conceived experiment design. DDL, CJU, ML, PDA, and PCM collected data and carried out experiments. CJU and CAL analyzed data. CJU, ML, and CAL organized and created figures. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Additional Information
Disclosure Summary: The authors have no conflicts of interest to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. [DOI] [PubMed] [Google Scholar]
- 3. Jan R, Chaudhry GE. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull. 2019;9(2):205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Subramani R, Nandy SB, Pedroza DA, Lakshmanaswamy R. Role of growth hormone in breast cancer. Endocrinology. 2017;158(6):1543-1555. [DOI] [PubMed] [Google Scholar]
- 5. ter Braak B, Siezen C, Speksnijder EN, et al. Mammary gland tumor promotion by chronic administration of IGF1 and the insulin analogue AspB10 in the p53R270H/ +WAPCre mouse model. Breast Cancer Res. 2015;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffmann-La Roche. . A study to evaluate the biological activity of R1507 in women with operable breast cancer. ClinicalTrials.gov Digital Repository. July 2009-December 2010. https://ClinicalTrials.gov/show/NCT00882674.
- 7. Hoffmann-La Roche. . A multiple ascending dose study of R1507 in children and adolescents with advanced solid tumors. ClinicalTrials.gov Digital Repository. December 2007-December 2011. https://ClinicalTrials.gov/show/NCT00560144.
- 8. Hoffmann-La Roche. . A study of R1507 in combination with multiple standard chemotherapy treatments in patients with advanced solid tumors. ClinicalTrials.gov Digital Repository. February 2009-December 2012. https://ClinicalTrials.gov/show/NCT00811993.
- 9. Hoffmann-La Roche. . A study of R1507 in combination with letrozole in postmenopausal women with advanced breast cancer. ClinicalTrials.gov Digital Repository. January 2009-March 2010. https://clinicaltrials.gov/ct2/show/NCT00796107.
- 10. Janecka A, Kołodziej-Rzepa M, Biesaga B. Clinical and molecular features of laron syndrome, a genetic disorder protecting from cancer. In Vivo. 2016;30(4):375-381. [PubMed] [Google Scholar]
- 11. Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164(4):485-489. [DOI] [PubMed] [Google Scholar]
- 12. Kleinberg DL, Wood TL, Furth PA, Lee AV. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev. 2009;30(1):51-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim HJ, Litzenburger BC, Cui X, et al. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol. 2007;27(8):3165-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu T, Starling-Emerald B, Zhang X, et al. Oncogenic transformation of human mammary epithelial cells by autocrine human growth hormone. Cancer Res. 2005;65(1):317-324. [PubMed] [Google Scholar]
- 15. Felice DL, El-Shennawy L, Zhao S, et al. Growth hormone potentiates 17β-estradiol-dependent breast cancer cell proliferation independently of IGF-I receptor signaling. Endocrinology. 2013;154(9):3219-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Mehta RG, Lantvit DD, et al. Inhibition of estrogen-independent mammary carcinogenesis by disruption of growth hormone signaling. Carcinogenesis. 2007;28(1): 143-150. [DOI] [PubMed] [Google Scholar]
- 17. Russo J. Significance of rat mammary tumors for human risk assessment. Toxicol Pathol. 2015;43(2):145-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minoia M, Gentilin E, Molè D, et al. Growth hormone receptor blockade inhibits growth hormone-induced chemoresistance by restoring cytotoxic-induced apoptosis in breast cancer cells independently of estrogen receptor expression. J Clin Endocrinol Metab. 2012;97(6):E907-E916. [DOI] [PubMed] [Google Scholar]
- 19. Zatelli MC, Minoia M, Molè D, et al. Growth hormone excess promotes breast cancer chemoresistance. J Clin Endocrinol Metab. 2009;94(10):3931-3938. [DOI] [PubMed] [Google Scholar]
- 20.Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. National Academies Press; 1996. [PubMed] [Google Scholar]
- 21. Okuma S. Study of growth hormone in spontaneous dwarf rat. Nippon Naibunpi Gakkai Zasshi. 1984;60:1005-1014. [DOI] [PubMed] [Google Scholar]
- 22. Okuma S. Spontaneous dwarf rat. Exp Anim. 1980;29:301-305. [Google Scholar]
- 23. Takeuchi T, Suzuki H, Sakurai S, Nogami H, Okuma S, Ishikawa H. Molecular mechanism of growth hormone (GH) deficiency in the spontaneous dwarf rat: detection of abnormal splicing of GH messenger ribonucleic acid by the polymerase chain reaction. Endocrinology. 1990;126(1):31-38. [DOI] [PubMed] [Google Scholar]
- 24. Swanson SM, Unterman TG. The growth hormone-deficient Spontaneous Dwarf rat is resistant to chemically induced mammary carcinogenesis. Carcinogenesis. 2002;23(6):977-982. [DOI] [PubMed] [Google Scholar]
- 25.American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. American Veterinary Medical Association; 2020. [Google Scholar]
- 26. RRID:AB_302459, https://scicrunch.org/resolver/AB_302459.
- 27. RRID:AB_2313606, https://scicrunch.org/resolver/AB_2313606.
- 28. Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. [Google Scholar]
- 29. Shen Q, Lantvit DD, Lin Q, et al. Advanced rat mammary cancers are growth hormone dependent. Endocrinology. 2007;148(10):4536-4544. [DOI] [PubMed] [Google Scholar]
- 30. Divisova J, Kuiatse I, Lazard Z, et al. The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF-7 breast cancer xenograft growth. Breast Cancer Res Treat. 2006;98(3):315-327. [DOI] [PubMed] [Google Scholar]
- 31. Litzenburger BC, Creighton CJ, Tsimelzon A, et al. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti–IGF-IR therapy. Clin Cancer Res. 2011;17(8):2314-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeng X, Sachdev D, Zhang H, Gaillard-Kelly M, Yee D. Sequencing of type I insulin-like growth factor receptor inhibition affects chemotherapy response in vitro and in vivo. Clin Cancer Res. 2009;15(8):2840-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Z, Levine KM, Bahreini A, et al. Upregulation of IRS1 enhances IGF1 response in Y537S and D538G ESR1 mutant breast cancer cells. Endocrinology. 2018;159(1):285-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330(18):1253-1259. [DOI] [PubMed] [Google Scholar]
- 35. Bonadonna G, Zambetti M, Valagussa P. Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes: ten-year results. JAMA. 1995;273(7):542-547. [PubMed] [Google Scholar]
- 36. Leftin A, Ben-Chetrit N, Joyce JA, Koutcher JA. Imaging endogenous macrophage iron deposits reveals a metabolic biomarker of polarized tumor macrophage infiltration and response to CSF1R breast cancer immunotherapy. Sci Rep. 2019;9(1):857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson HJ, McGinley JN, Rothhammer K, Singh M. Rapid induction of mammary intraductal proliferations, ductal carcinoma in situ and carcinomas by the injection of sexually immature female rats with 1-methyl-1-nitrosourea. Carcinogenesis. 1995;16(10):2407-2411. [DOI] [PubMed] [Google Scholar]
- 38. Thompson HJ. Methods for the induction of mammary carcinogenesis in the rat using either 7,12-Dimethylbenz[α]anthracene or 1-Methyl-1-Nitrosourea. In: Ip MM, Asch BB, eds. Methods in Mammary Gland Biology and Breast Cancer Research. Springer US; 2000:19-29. [Google Scholar]
- 39. Thompson HJ, Adlakha H, Singh M. Effect of carcinogen dose and age at administration on induction of mammary carcinogenesis by 1-methyl-1-nitrosourea. Carcinogenesis. 1992;13(9):1535-1539. [DOI] [PubMed] [Google Scholar]
- 40. Thompson HJ, McGinley JN, Wolfe P, Singh M, Steele VE, Kelloff GJ. Temporal sequence of mammary intraductal proliferations, ductal carcinomas in situ and adenocarcinomas induced by 1-methyl-1-nitrosourea in rats. Carcinogenesis. 1998;19(12):2181-2185. [DOI] [PubMed] [Google Scholar]
- 41. Perse M, Cerar A, Injac R, Strukelj B. N-methylnitrosourea induced breast cancer in rat, the histopathology of the resulting tumours and its drawbacks as a model. Pathol Oncol Res. 2009;15(1):115-121. [DOI] [PubMed] [Google Scholar]
- 42. McCormick DL, Adamowski CB, Fiks A, Moon RC. Lifetime dose-response relationships for mammary tumor induction by a single administration of N-methyl-N-nitrosourea. Cancer Res. 1981;41(5):1690-1694. [PubMed] [Google Scholar]
- 43. Hutchinson JN, Muller WJ. Transgenic mouse models of human breast cancer. Oncogene. 2000;19(53):6130-6137. [DOI] [PubMed] [Google Scholar]
- 44. Dobrolecki LE, Airhart SD, Alferez DG, et al. Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev. 2016;35(4):547-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alvarado A, Lopes AC, Faustino-Rocha AI, et al. Prognostic factors in MNU and DMBA-induced mammary tumors in female rats. Pathol Res Pract. 2017;213(5):441-446. [DOI] [PubMed] [Google Scholar]
- 47. Perez R, Schally AV, Popovics P, et al. Antagonistic analogs of growth hormone-releasing hormone increase the efficacy of treatment of triple negative breast cancer in nude mice with doxorubicin; A preclinical study. Oncoscience. 2014;1(10):665-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Evans A, Jamieson SM, Liu DX, Wilson WR, Perry JK. Growth hormone receptor antagonism suppresses tumour regrowth after radiotherapy in an endometrial cancer xenograft model. Cancer Lett. 2016;379(1):117-123. [DOI] [PubMed] [Google Scholar]
- 49. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879. [DOI] [PubMed] [Google Scholar]
- 50. Zhao L, Zhang B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci Rep. 2017;7:44735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710-717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.