Abstract
Magnetic Resonance Spectroscopy is a popular approach to probe brain chemistry in schizophrenia (SZ), but no consensus exists as to the extent of alterations. This may be attributable to differential effects of populations studied, brain regions examined, or antipsychotic medication effects. Here, we measured neurometabolites in the anterior cingulate cortex (ACC) and hippocampus, two structurally dissimilar brain regions implicated in the SZ pathophysiology. We enrolled 61 SZ with the goal to scan them before and after six weeks of treatment with risperidone. We also scanned 31 matched healthy controls twice, six weeks apart. Using mixed effect repeated measures linear models to examine the effect of group and time on metabolite levels in each voxel, we report an increase in hippocampal glutamate+glutamine (Glx) in SZ compared to controls (p= 0.043), but no effect of antipsychotic medication (p= 0.330). In the ACC, we did not find metabolite alterations or antipsychotic medication related changes after six weeks of treatment with risperidone. The coefficients for the discriminant function (differentiating SZ from HC) in the ACC were greatest for NAA (−0.83), and in the hippocampus for Glx (0.76), the same metabolites were associated with greater treatment response in patients at trend level. Taken together, our data extends the existing literature by demonstrating regionally distinct metabolite alterations in the same patient group and suggests that antipsychotic medications may have limited effects on metabolite levels in these regions.
Keywords: Magnetic Resonance Spectroscopy (MRS), glutamate, hippocampus, anterior cingulate cortex, treatment response, schizophrenia
1. INTRODUCTION
Magnetic Resonance Spectroscopy (MRS), an imaging technique that allows measurement of neurometabolites in vivo, has become an increasingly popular approach to probe brain chemistry in schizophrenia, but no consistent patterns have emerged as to the extent of neurometabolite alterations in the illness. Nonetheless, the literature suggests that a number of metabolites are abnormal (Iwata et al., 2018; Kraguljac et al., 2012a; Merritt et al., 2016; Poels et al., 2014), that aberrations may be variable across brain regions (Gallinat et al., 2016; Merritt et al., 2016), and that these are potentially affected by antipsychotic medications (Bustillo et al., 2002; Bustillo et al., 2008; de la Fuente-Sandoval et al., 2013; Egerton et al., 2017; Egerton et al., 2018; Kegeles et al., 2012; Kraguljac et al., 2012b; Kraguljac et al., 2013; Szulc et al., 2011) and illness progression (Keshavan et al., 2000; Marsman et al., 2013).
Here, we measured N-acetyl-aspartate (NAA), choline (Cho), and glutamate+glutamine (Glx) in the anterior cingulate cortex (ACC) and hippocampus, two regions of the brain that are dissimilar in the ratio of excitatory and inhibitory neurons (Heckers and Konradi, 2015), have been implicated in the pathophysiology of schizophrenia (Kraguljac et al., 2012b; Lahti et al., 1995a; Lahti et al., 2006), and are thought to be functionally modulated by antipsychotic medication (Kraguljac et al., 2016; Lahti et al., 2003). We scanned a large group of patients with schizophrenia and schizoaffective disorder (SZ) before and after six weeks of treatment with risperidone and in a group of matched healthy controls (HC) twice, approximately six weeks apart to investigate neurometabolite abnormalities and antipsychotic medication effects. We chose risperidone because it is commonly prescribed, now available as generic medication and thus one of the more affordable second generation antipsychotic medications in the US, and is considered one first line medications for treatment in schizophrenia (Moore et al., 2007), specifically in first episode patients (Robinson et al., 2015).
Based on the existing literature (Bustillo et al., 2008; Egerton et al., 2018; Grosic et al., 2014; Kraguljac et al., 2013; Szulc et al., 2011), we hypothesized that (1) hippocampal Glx would be elevated in unmedicated SZ compared to HC, but would normalize after six weeks of treatment, and (2) that ACC NAA would not be altered in unmedicated SZ, but decrease after antipsychotic treatment. We also explored the use of linear discriminant analyses to examine their utility in discriminating unmedicated SZ and HC, and their value in predicting treatment response in SZ.
2. EXPERIMENTAL MATERIAL AND METHODS
2.1. Study design
Unmedicated SZ were recruited from the emergency room, inpatient units, and outpatient clinics at the University of Alabama at Birmingham (UAB). HC matched 1:2 to SZ on age, sex, and parental occupation were recruited by advertisements. Approval was obtained from the UAB Institutional Review Board and written informed consent was obtained prior to enrolment and after subjects were deemed competent to provide consent (Carpenter et al.).
Diagnoses were established by review of medical records, the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994), and consensus of two board certified psychiatrists (ACL and NVK). The Brief Psychiatric Rating Scale (BPRS) was used to assess symptom severity (Woerner et al., 1988).
Subjects were excluded if they had major neurological or medical conditions, history of head trauma with loss of consciousness, substance use disorders (excluding nicotine) within six months of imaging, were pregnant or breastfeeding, or had MRI contraindications. Those with a positive drug screen were excluded. HC with a personal or family history in a first-degree relative of a psychiatric disorder were excluded.
Subjects who were medication naïve or had been off antipsychotic medications for at least two weeks (determined by self report) were enrolled in a six-week trial of risperidone using a flexible dosing regimen. Spectroscopy was done prior to treatment (off medication), and after six weeks of medication. Risperidone was prescribed by ACL and NVK; it was started at 1-3 milligrams and titrated in 1-2 milligram increments; dosing was based on therapeutic and side effects. Use of concomitant medications was permitted as clinically indicated. Compliance was monitored with pill counts at each visit. HC were scanned twice, with an average interscan interval of 57.5 days. Of the 92 subjects enrolled, one HC and 12 SZ dropped out prior to the second scan. One SZ was excluded from analyses due to gross anatomical abnormalities in the structural scan. Data included here partially overlaps with prior reports by our group (Hutcheson et al., 2015; Kraguljac et al., 2014; Kraguljac et al., 2013; Reid et al., 2010). Data from 35 unmedicated SZ measuring hippocampus metabolites were not included in prior reports. Additionally, no data from unmedicated SZ measuring ACC metabolites or longitudinal data were included in prior reports.
2.2. Image acquisition
Imaging was performed on a 3T head-only scanner (Magnetom Allegra, Siemens). A high-resolution structural scan was acquired (MPRAGE, TR/TE/TI=2300/3.93/1100msec, 1mm isotropic voxels).
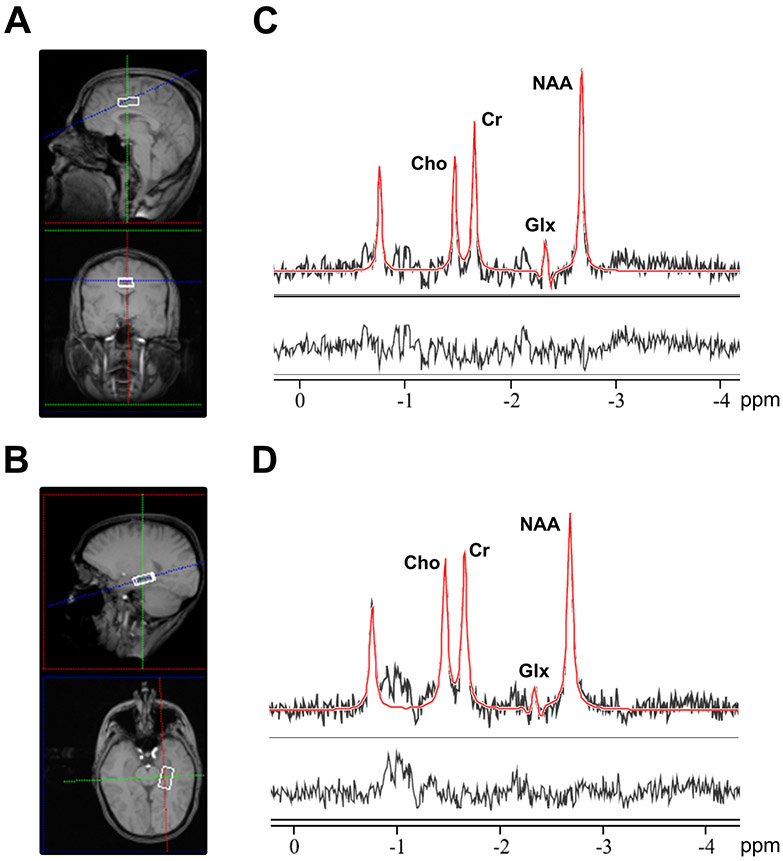
A series of T1-weighted anatomical scans (TR/TE= 250/3.48ms, 5mm slice thickness) were acquired to aid placement of the bilateral ACC (2.7x2x1cm3) and left hippocampus spectroscopy voxel (2.7x1.5x1cm3), as previously described (Hutcheson et al., 2012; Kraguljac et al., 2014; Kraguljac et al., 2013; Reid et al., 2010). For an illustration of voxel placement and example spectra, see Figure 1. Voxel placement for the second acquisition was guided by an image of the voxel placement during the first scan. After manual shimming, CHESS pulses were used to suppress the water signal. Spectra were acquired using a PRESS sequence (TR/TE= 2000/80ms to optimize the glutamate signal (Schubert et al., 2004) and minimize macromolecule contribution; 1200 Hz spectral bandwidth; 1024 points; 256 averages in the ACC and 640 averages in the hippocampus). To limit effects of nicotine intoxication or withdrawal, participants were allowed, but not encouraged, to smoke up to one hour before acquisition of images. Spectral acquisition failed for one SZ in the ACC at baseline, for one SZ in the ACC at endpoint, for one HC and three SZ in the hippocampus at baseline, and for four SZ in the hippocampus at week six.
Figure 1.
Illustration of voxel placement and example spectrum. A. Voxel placement in the anterior cingulate cortex (2.7x 2x 1cm3). B. Voxel placement in the left hippocampus (2.7x 1.5x 1cm3). C. Example spectra obtained from the anterior cingulate cortex. D. Example spectra obtained from the left hippocampus. For C and D, the black line represents averaged spectra, the red line is an overlay of the spectral fit, the bottom line is the residual. Cho, choline; Cr, creatine; Glx, glutamate + glutamine; NAA, N-acetylaspartate; ppm, parts per million.
2.3. MRS data processing
MRS data were quantified in the time domain with AMARES in jMRUI (version 5.2); prior knowledge derived from in vitro and in vivo metabolite spectra was included in the model, as reported elsewhere (Kraguljac et al., 2012b; Kraguljac et al., 2013). Exclusion criteria were (1) failure of fitting algorithm and (2) Cramer-Rao lower bounds (CRLB) >20%. Hippocampus Glx spectra of one SZ at baseline and two SZ at week six were excluded based on these criteria. Metabolites were quantified with respect to creatine; for the sake of brevity they will be referred to as Glx, NAA, and Cho hereafter. Structural scans were segmented into grey matter (GM), white matter (WM), and cerebrospinal fluid to calculate voxel tissue content, using in house Matlab codes.
2.4. Statistical analyses
Mixed effect repeated measures linear models were used to investigate the effects of group and time on neurometabolites in each voxel. Voxel GM fraction (GM%/ GM%+ WM% (Caprihan et al., 2015)) was also included in the model. Both a model assuming a common variance for HC and SZ as well as a model that allowed for separate variances for HC and SZ were considered. Akaike’s information criterion was used to select the final model.
For linear discriminant analyses, we included subjects who had a complete baseline dataset (all neurometabolites in the ACC and hippocampus; HC: n=30; SZ: n=55). Treatment response data (defined as [BPRS total baseline – BPRS total week 6]/ BPRS total at baseline *−100) were available for 45 SZ. Linear discriminant analysis was used to determine whether baseline metabolite values could discriminate between HC and SZ and predict change in symptoms after six weeks of treatment with risperidone.
3. RESULTS
3.1. Demographics, clinical characteristics
HC and SZ did not differ in gender, age, or parental socioeconomic status (Table 1). Forty SZ were medication naïve, and 20 had been off antipsychotic medications for an average of 22.02 months. BPRS scores significantly decreased after six weeks of treatment with risperidone; the average dose at that time was 3.83 +/− 1.66mg per day.
Table 1:
Demographics and Clinical Measuresa
| SZ (n= 61) | HC (n= 31) | t/X2 | p value | |
|---|---|---|---|---|
| Gender (% male) | 72.1 | 70.96 | 0.014 | .91 |
| Age | 27.84 (8.69) | 28.58 (9.81) | 0.372 | .71 |
| Parental Occupationb | 7.18 (5.67) | 5.84 (4.46) | 14.911 | .31 |
| Smoking status (% smokers) | 62.29 | 29.03 | 9.101 | < .01 |
| Diagnosis | ||||
| Schizophrenia | 50 | |||
| Schizoaffective Disorder | 8 | |||
| Schizophreniform Disorder | 1 | |||
| Psychosis not otherwise specified | 2 | |||
| Prior antipsychotic treatment | ||||
| Antipsychotic naive | 40 | |||
| Antipsychotic free interval (months)c | 22.02 (43.33) | |||
| BPRSd | ||||
| Total | ||||
| Baseline | 49.53 (9.95) | |||
| Week 6e | 31.67 (9.09)f | |||
| Positive | ||||
| Baseline | 14.60 (4.65) | |||
| Week 6g | 6.84 (2.93)h | |||
| Negative | ||||
| Baseline | 7.05 (3.13) | |||
| Week 6g | 5.76 (2.48)h | |||
| RBANSi | ||||
| Total index | 71.14 (14.37) | 90.29 (11.72) | 6.337 | < .01 |
| Immediate memory | 77.52 (16.59) | 95.65 (13.47) | 5.203 | < .01 |
| Visuospatial | 72.38 (15.78) | 82.97 (16.89) | 2.925 | < .01 |
| Language | 83.52 (13.29) | 97.06 (15.79) | 4.254 | < .01 |
| Attention span | 79.32 (19.47) | 96.10 (17.63) | 3.976 | < .01 |
| Delayed memory | 73.32 (19.29) | 92.71 (7.32) | 6.702 | < .01 |
SZ, schizophrenia; HC, healthy control
Mean (SD) unless indicated otherwise
Ranks determined from Diagnostic Interview for Genetic Studies (1 – 18 scale); higher rank (lower numerical value) corresponds to higher socioeconomic status
Antipsychotic free interval is denoted only for those with prior antipsychotic exposure (n= 20)
Brief Psychiatric Rating Scale (1 – 7 scale); positive (conceptual disorganization, hallucinatory behavior, and unusual thought content); negative (emotional withdrawal, motor retardation, and blunted affect)
n= 49
t= 13.352; p< .01
t= 11.030; p< .01
t= 3.515; p< .01
Repeatable Battery for the Assessment of Neuropsychological Status
3.2. MRS measurements
Mixed effects repeated measures analysis.
Of the models for mixed effects repeated measures considered, the model assuming a common variance for HC and SZ had the best fit. After controlling for time and GM fraction, SZ had significantly higher hippocampus Glx compared to HC (p= 0.043). None of the other metabolites, spectral quality indices or voxel GM fraction in either region differed between groups. When an interaction between group (HC and SZ) and time (baseline and week 6) was added to the model, this interaction was not statistically significant (p= 0.330, Figure 2A and 2B). None of the other metabolites, spectral quality indices or voxel GM fraction in either region changed across time, and no group by time interactions for neurometabolites were observed (Table 2).
Figure 2.
Neurometabolite measurements. A. Glutamate+glutamine (Glx) in the hippocampus in healthy controls (HC) and patients with schizophrenia or schizoaffective disorder (SZ). Hippocampus Glx was significantly higher in SZ compared to HC (p= 0.043). Dots represent individual measurements, line represents group mean. B. Hippocampus Glx at baseline and week six, top depicts measurements in HC, bottom depicts measurements in SZ, lines represent individual subjects. No effects of time were observed. C. Receiver operating characteristic curve with area under the curve of 0.821.
Table 2:
Neurometabolites, Spectral Quality, and Tissue Fraction
| Baseline | Week 6 | |||
|---|---|---|---|---|
| HC | SZ | HC | SZ | |
| Neurometabolites | ||||
| ACC | ||||
| NAA | 1.39 (0.11) | 1.36 (0.10) | 1.37 (0.11) | 1.34 (0.09) |
| Glx | 0.71 (0.08) | 0.70 (0.07) | 0.71 (0.07) | 0.70 (0.07) |
| Cho | 0.82 (0.07) | 0.82 (0.11) | 0.80 (0.66) | 0.81 (0.08) |
| Hippocampus | ||||
| NAA | 1.27 (0.10) | 1.29 (0.14) | 1.29 (0.16) | 1.30 (0.16) |
| Glx | 0.60 (0.09) | 0.65 (0.09) | 0.63 (0.12) | 0.65 (0.11) |
| Cho | 0.88 (0.11) | 0.91 (0.12) | 0.86 (0.13) | 0.90 (0.12) |
| Spectral quality indicesa | ||||
| ACC | ||||
| NAA CRLB | 3.50 (0.67) | 3.50 (0.82) | 3.35 (0.35) | 3.44 (0.59) |
| Glx CRLB | 6.62 (1.21) | 6.77 (0.17) | 6.51 (0.93) | 6.76 (0.13) |
| Cho CRLB | 2.59 (0.41) | 2.62 (0.55) | 2.49 (0.26) | 2.59 (0.50) |
| FWHM | 4.80 (0.63) | 4.82 (0.78) | 4.97 (0.75) | 5.01 (0.81) |
| SNR | 11.32 (1.33) | 10.97 (1.50) | 11.59 (1.41) | 11.14 (1.41) |
| Hippocampus | ||||
| NAA CRLB | 3.63 (0.78) | 3.88 (0.95) | 3.62 (0.74) | 4.00 (1.00) |
| Glx CRLB | 9.93 (2.82) | 10.80 (2.98) | 9.73 (2.26) | 10.81 (2.41) |
| Cho CRLB | 2.91 (0.52) | 3.17 (0.76) | 2.91 (0.56) | 3.20 (0.64) |
| FWHM | 8.19 (1.47) | 8.40 (1.76) | 7.98 (1.18) | 8.63 (1.57) |
| SNR | 12.46 (1.38) | 11.93 (1.64) | 12.42 (1.74) | 11.91 (1.51) |
| Voxel tissue fractionb | ||||
| ACC | ||||
| grey matter (%) | 73.99 (3.51) | 73.99 (4.71) | 74.07 (6.28) | 73.86 (5.34) |
| white matter (%) | 13.11 (4.48) | 14.47 (0.54) | 13.67 (6.46) | 14.50 (5.65) |
| Hippocampus | ||||
| grey matter (%) | 62.51 (4.39) | 63.08 (5.56) | 62.38 (5.75) | 61.04 (5.34) |
| white matter (%) | 34.16 (4.83) | 33.74 (5.97) | 34.37 (5.79) | 36.00 (5.04) |
None of the spectral indices differed between groups at either timepoint, or across timepoints in either group (all p> .90 after correction for multiple comparisons)
Voxel tissue fraction did not differ between groups at either timepoint, or across timepoints in either group (all p> .90 after correction for multiple comparisons)
Abbreviations: HC: Healthy control; SZ: Patient with schizophrenia, schizoaffective disorder, or schizophreniform disorder; ACC: Anterior cingulate cortex; NAA: N-acetyl-aspartate; Glx: Glutamate+glutamine; Cho: Choline; CRLB: Cramer rao lower bounds; FWHM: Full width half maximum; SNR: Signal to noise ratio
We found no correlations between the duration of the antipsychotic-free interval and neurometabolite levels in currently unmedicated patients.
We also compared metabolite measurements in never-treated and currently unmedicated patients, and found that NAA was higher in never-treated patients compared to controls in both the ACC and hippocampus at baseline, and that ACC Glx was higher in medication-naïve patients compared to those with prior exposure. Additionally, change in ACC NAA differed between medication-naïve patients and currently unmedicated patients, where NAA increased in the former group, but decreased in the latter. However, none of these results survived corrections for multiple comparisons (supplemental Table, supplemental Figure 1).
Discriminant analysis.
Following analyses were based on baseline metabolite values. The coefficients for the discriminant function in the ACC were greatest for NAA (−0.83; coefficient for Glx was 0.04 and for Cho was −0.27), and in the hippocampus for Glx (0.76; coefficient for NAA was 0.29 and for Cho was 0.33). The canonical discriminant function showed a trend towards statistical significance (F= 1.95; p= 0.08). The ROC curve for the discriminant function shown in Figure 2C had an area under the curve of 0.821. When leave-one-out cross-validation was applied, the area under the ROC curve decreased to 0.614 (supplemental Figure 2). When GM fraction was taken into account, the discriminant function was not statistically significant (F= 1.29; p= 0.27). Linear regression was used to determine whether a single neurometabolite at baseline could predict treatment response. None of the metabolites reached statistical significance, but lower NAA in the ACC and higher Glx in the hippocampus were associated with greater treatment response at trend level (Table 3).
Table 3.
Linear regression results by baseline metabolite. For all models the outcome is percent decrease in BPRS; BPRS and gray matter fraction at baseline are included as covariates in all models.
| Parameter Estimates | Model Fit Statistics | ||||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | p | R2 | F statistic |
F p- value |
||
| ACC | NAA | −43.28 | 23.30 | 0.07 | 0.14 | 2.27 | 0.095 |
| Glx | −42.76 | 33.53 | 0.21 | 0.11 | 1.61 | 0.201 | |
| Cho | −35.03 | 23.77 | 0.15 | 0.12 | 1.81 | 0.161 | |
| Hippocampus | NAA | −12.01 | 15.87 | 0.45 | 0.08 | 1.16 | 0.335 |
| Glx | 50.17 | 25.21 | 0.05 | 0.15 | 2.37 | 0.084 | |
| Cho | −20.06 | 17.27 | 0.25 | 0.10 | 1.44 | 0.245 | |
ACC: Anterior cingulate cortex; NAA: N-acetyl-aspartate; Glx: Glutamate+glutamine; Cho: Choline
4. DISCUSSION
To our knowledge, this is the largest longitudinal multi-voxel MRS study examining ACC and hippocampus neurometabolites in unmedicated SZ and effects of short-term antipsychotic treatment. We report increased hippocampal Glx, but no other metabolite abnormalities in SZ, and no changes in metabolites with antipsychotic treatment. Linear discriminant analyses showed ROC area under the curve of 0.82 indicates good discrimination ability for metabolites. Metabolites with the greatest power to discriminate between HC and SZ were ACC NAA and hippocampus Glx, the same metabolites that were predictive of treatment response at trend level.
We did not observe group differences in NAA or Cho levels in either brain region, but confirmed our previously reported increase in hippocampal Glx in unmedicated SZ in an extended sample size (Kraguljac et al., 2013). The elevation is similar in magnitude to that seen in healthy volunteers who receive sub-anaesthetic doses of ketamine (Kraguljac et al., 2017b), a non-competitive n-methyl-D-aspartate receptor (NMDAR) blocker commonly used as a model for schizophrenia (Kraguljac et al., 2017a; Lahti et al., 1995a; Lahti et al., 1995b; Lahti et al., 2001), suggesting that this abnormality may be related to NMDAR hypofunction (Javitt, 2012; Olney and Farber, 1995). Interestingly, we did not find Glx alterations in the ACC, which is in agreement with prior studies in medicated (Coughlin et al., 2015; Reid et al., 2010; Taylor et al., 2017) and unmedicated patients (Egerton et al., 2018). It also is consistent with the theory that the hippocampus may be more vulnerable to shifts in the excitation/ inhibition balance compared to the ACC because the vast majority of its neurons are glutamatergic pyramidal cells, which markedly differs from the cortex where the number of pyramidal cells and GABAergic interneurons is more similar (Heckers and Konradi, 2015).
Several lines of evidence describe complex interactions between the dopamine and glutamate systems, and identify glutamate receptor complexes as potentially important indirect targets for D2 receptor blockers (Del'guidice and Beaulieu, 2008; Hanaoka et al., 2003; Krzystanek et al., 2015). Additionally, single nucleotide polymorphisms in glutamate-related genes have been implicated in antipsychotic drug response (Bishop et al., 2015; Stevenson et al., 2016). Taken together, the literature suggests that antipsychotic medications may affect glutamate. However, we did not observe a significant decrease in Glx after six weeks of treatment with risperidone in either voxel, which stands in contrast with Szulc et al, who report a decrease in temporal lobe Glx after four weeks of treatment with a variety of antipsychotic medications (Szulc et al., 2011), de la Fuente-Sandoval who found a reduction in striatal glutamate after four weeks of risperidone treatment, and a recent report by Egerton et al who showed a reduction in glutamate in the ACC after four weeks of treatment with amisulpride (Egerton et al., 2018). It is possible that the discrepancy is due to differential effects of various antipsychotic medications on the glutamate system. Alternatively, risperidone may affect glutamate levels only in a subset of patients, and we were therefore unable to detect changes in this endophenotypically heterogeneous group of subjects. It is also possible that shifts in glutamatergic neurotransmission may be too subtle to be captured with proton MRS which measures tissue metabolites rather than compartment specific levels.
Here, we did not observe risperidone related changes in NAA. Several studies also report no change in NAA with short and longer term antipsychotic medications (Bustillo et al., 2008; Grosic et al., 2014; Szulc et al., 2011), while others find NAA increases in the dorsolateral prefrontal cortex but no other brain regions with short term treatment (Bertolino et al., 2001) and decreases in NAA within the first year of antipsychotic treatment (Bustillo et al., 2002). Taken together, evidence suggests that long term antipsychotic treatment (or illness chronicity) rather than short term treatment may affect neuronal viability to an extent that can be captured with MRS. Two studies examining drug effects on Cho found no changes in this metabolite (de la Fuente-Sandoval et al., 2013; Gan et al., 2014), which is also consistent with our findings.
Linear discriminant analysis, which is designed to separate classes, distinguished unmedicated SZ from HC at trend level. The area under the curve of 0.82 suggests this biomarker panel may have utility in separating patients from controls. ACC NAA and hippocampal Glx had the greatest discriminant power, and were associated with response to risperidone after six weeks of treatment. At first glance, the association between low cortical NAA and good response to treatment appears counter-intuitive and stands in contrast with a study reporting an association between lower prefrontal NAA in first episode patients and poorer functional outcomes 18 months later (Wood et al., 2006). It is striking however, that greater deviations in both ACC NAA and hippocampus glutamate in our sample appear to be linked to better treatment response. One could speculate that these features may point towards functional conditions, characterized by a disequilibrium in the excitation/ inhibition balance and a deprived neuronal state, which have the potential for restoration.
Our findings have to be interpreted in context of a number of strengths and limitations. We enrolled a large group of unmedicated SZ, many of them without prior antipsychotic exposure and a group of HC matched on key demographic characteristic in this prospective longitudinal trial. To minimize variance in the data, we used a single antipsychotic medication. We assessed adherence using pill counts and self-report, but future studies would benefit from monitoring risperidone blood levels to confirm compliance. We also used self-report to determine the off medication interval in those with prior antipsychotic exposure. Ideally, we would have had access to an additional source such as a family member or pharmacy record that would have corroborated this timeframe. We chose a six week interval for antipsychotic treatment as it is a commonly recommended timeframe to determine treatment response in clinical practice. However, we cannot rule out that longer antipsychotic exposure could have affected neurometabolite levels. Spectroscopy acquisition parameters were optimized for glutamate, and results were corrected for partial volume effects, but because we did not obtain unsuppressed water spectra, we used creatine as a reference (at the time the study was designed, referencing metabolites to creatine was the most common method of metabolite quantification). While meta-analytic evidence suggests a lack of alterations in creatine in schizophrenia (Kraguljac et al., 2012a), some do report abnormalities (Ongur et al., 2009). We thus cannot rule out that Glx alterations reported here may in part be driven by altered creatine. The Glx peak reported here is a combination of neuronal, glial, and synaptic glutamate and glutamine in the voxel, it is therefore not possible for us to definitively attribute alterations to aberrant glutamatergic neurotransmission. Because it is ethically not permissible to withhold known effective medications from patients, we did not include a placebo group. To account for possible effects of time on metabolites, we scanned healthy controls twice; however, due to the considerable side effect burden of antipsychotic medications, we did not include a group of healthy controls with prolonged exposure to risperidone.
In summary, we report evidence of hippocampus glutamate alterations in this profoundly disabling neuropsychiatric disorder, but did not detect effects of risperidone on any metabolites. Large, prospective studies mapping the neuro-metabolome and effects of dopamine D2 receptor blockers in this clinically and endophenotypically heterogeneous illness may be important in advancing rational drug development and identification of subtypes of patients who would most benefit from these drugs.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the UAB neuroimaging community for their support, especially their flexibility in making same day scanning for this protocol possible.
Footnotes
CONFLICT OF INTEREST
Dr. Lahti has received an investigator initiated grant from Janssen Pharmaceuticals. All other authors declare no conflicts of interest, including relevant financial interests, activities, relationships, and affiliations.
REFERENCES
- Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, Weinberger DR, 2001. The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biological psychiatry 49(1), 39–46. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Reilly JL, Harris MS, Patel SR, Kittles R, Badner JA, Prasad KM, Nimgaonkar VL, Keshavan MS, Sweeney JA, 2015. Pharmacogenetic associations of the type-3 metabotropic glutamate receptor (GRM3) gene with working memory and clinical symptom response to antipsychotics in first-episode schizophrenia. Psychopharmacology 232(1), 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo JR, Lauriello J, Rowland LM, Thomson LM, Petropoulos H, Hammond R, Hart B, Brooks WM, 2002. Longitudinal follow-up of neurochemical changes during the first year of antipsychotic treatment in schizophrenia patients with minimal previous medication exposure. Schizophrenia research 58(2-3), 313–321. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Jung R, Brooks WM, Qualls C, Hammond R, Hart B, Lauriello J, 2008. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 33(10), 2456–2466. [DOI] [PubMed] [Google Scholar]
- Caprihan A, Jones T, Chen H, Lemke N, Abbott C, Qualls C, Canive J, Gasparovic C, Bustillo JR, 2015. The Paradoxical Relationship between White Matter, Psychopathology and Cognition in Schizophrenia: A Diffusion Tensor and Proton Spectroscopic Imaging Study. Neuropsychopharmacology 40(9), 2248–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter WT Jr., Gold JM, Lahti AC, Queern CA, Conley RR, Bartko JJ, Kovnick J, Appelbaum PS, 2000. Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry 57(6), 533–538. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Tanaka T, Marsman A, Wang H, Bonekamp S, Kim PK, Higgs C, Varvaris M, Edden RA, Pomper M, Schretlen D, Barker PB, Sawa A, 2015. Decoupling of N-acetyl-aspartate and glutamate within the dorsolateral prefrontal cortex in schizophrenia. Current molecular medicine 15(2), 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Stephano S, Favila R, Diaz-Galvis L, Alvarado-Alanis P, Ramirez-Bermudez J, Graff-Guerrero A, 2013. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 70(10), 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del'guidice T, Beaulieu JM, 2008. Messing up with traffic: different effects of antipsychotic agents on glutamate receptor complexes in vivo. Molecular pharmacology 73(5), 1339–1342. [DOI] [PubMed] [Google Scholar]
- Egerton A, Bhachu A, Merritt K, McQueen G, Szulc A, McGuire P, 2017. Effects of Antipsychotic Administration on Brain Glutamate in Schizophrenia: A Systematic Review of Longitudinal 1H-MRS Studies. Front Psychiatry 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Broberg BV, Van Haren N, Merritt K, Barker GJ, Lythgoe DJ, Perez-Iglesias R, Baandrup L, During SW, Sendt KV, Stone JM, Rostrup E, Sommer IE, Glenthoj B, Kahn RS, Dazzan P, McGuire P, 2018. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre (1)H-MRS study (OPTiMiSE). Mol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Gallinat J, McMahon K, Kuhn S, Schubert F, Schaefer M, 2016. Cross-sectional Study of Glutamate in the Anterior Cingulate and Hippocampus in Schizophrenia. Schizophrenia bulletin 42(2), 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan JL, Cheng ZX, Duan HF, Yang JM, Zhu XQ, Gao CY, 2014. Atypical antipsychotic drug treatment for 6 months restores N-acetylaspartate in left prefrontal cortex and left thalamus of first-episode patients with early onset schizophrenia: A magnetic resonance spectroscopy study. Psychiatry research 223(1), 23–27. [DOI] [PubMed] [Google Scholar]
- Grosic V, Folnegovic Grosic P, Kalember P, Bajs Janovic M, Rados M, Mihanovic M, Henigsberg N, 2014. The effect of atypical antipsychotics on brain N-acetylaspartate levels in antipsychotic-naive first-episode patients with schizophrenia: a preliminary study. Neuropsychiatric disease and treatment 10, 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka T, Toyoda H, Mizuno T, Kikuyama H, Morimoto K, Takahata R, Matsumura H, Yoneda H, 2003. Alterations in NMDA receptor subunit levels in the brain regions of rats chronically administered typical or atypical antipsychotic drugs. Neurochemical research 28(6), 919–924. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C, 2015. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res 167(1-3), 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson NL, Reid MA, White DM, Kraguljac NV, Avsar KB, Bolding MS, Knowlton RC, den Hollander JA, Lahti AC, 2012. Multimodal analysis of the hippocampus in schizophrenia using proton magnetic resonance spectroscopy and functional magnetic resonance imaging. Schizophr Res 140(1-3), 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson NL, Sreenivasan KR, Deshpande G, Reid MA, Hadley J, White DM, Ver Hoef L, Lahti AC, 2015. Effective connectivity during episodic memory retrieval in schizophrenia participants before and after antipsychotic medication. Hum Brain Mapp 36(4), 1442–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata Y, Nakajima S, Plitman E, Mihashi Y, Caravaggio F, Chung JK, Kim J, Gerretsen P, Mimura M, Remington G, Graff-Guerrero A, 2018. Neurometabolite levels in antipsychotic-naive/free patients with schizophrenia: A systematic review and meta-analysis of (1)H-MRS studies. Prog Neuropsychopharmacol Biol Psychiatry 86, 340–352. [DOI] [PubMed] [Google Scholar]
- Javitt DC, 2012. Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophrenia bulletin 38(5), 911–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC, 2012. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69(5), 449–459. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Stanley JA, Pettegrew JW, 2000. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings--part II. Biol Psychiatry 48(5), 369–380. [DOI] [PubMed] [Google Scholar]
- Kraguljac NV, Carle M, Froelich MA, Tran S, Yassa MA, White DM, Reddy A, Lahti AC, 2017a. Mnemonic discrimination deficits in first-episode psychosis and a ketamine model suggests dentate gyrus pathology linked to N-methyl-D-aspartate receptor hypofunction Biol Psychiatry: CNNI in press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kraguljac NV, Frolich MA, Tran S, White DM, Nichols N, Barton-McArdle A, Reid MA, Bolding MS, Lahti AC, 2017b. Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatry 22(4), 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti AC, 2012a. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res 203(2-3), 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC, 2012b. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 37(12), 2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Hadley J, Reid MA, Lahti AC, 2014. Hippocampal-parietal dysconnectivity and glutamate abnormalities in unmedicated patients with schizophrenia. Hippocampus 24(12), 1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Hadley N, Hadley JA, Ver Hoef L, Davis E, Lahti AC, 2016. Aberrant Hippocampal Connectivity in Unmedicated Patients With Schizophrenia and Effects of Antipsychotic Medication: A Longitudinal Resting State Functional MRI Study. Schizophr Bull 42(4), 1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Reid MA, Lahti AC, 2013. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry 70(12), 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzystanek M, Bogus K, Palasz A, Krzystanek E, Worthington JJ, Wiaderkiewicz R, 2015. Effects of long-term treatment with the neuroleptics haloperidol, clozapine and olanzapine on immunoexpression of NMDA receptor subunits NR1, NR2A and NR2B in the rat hippocampus. Pharmacological reports : PR 67(5), 965–969. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Medoff DR, Tamminga CA, 1995a. Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport 6(6), 869–872. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA, 2003. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biological psychiatry 53(7), 601–608. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA, 1995b. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13(1), 9–19. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R, 2006. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology 31(1), 221–230. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA, 2001. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology 25(4), 455–467. [DOI] [PubMed] [Google Scholar]
- Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE, 2013. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Bull 39(1), 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK, 2016. Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA Psychiatry 73(7), 665–674. [DOI] [PubMed] [Google Scholar]
- Moore TA, Buchanan RW, Buckley PF, Chiles JA, Conley RR, Crismon ML, Essock SM, Finnerty M, Marder SR, Miller DD, McEvoy JP, Robinson DG, Schooler NR, Shon SP, Stroup TS, Miller AL, 2007. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry 68(11), 1751–1762. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI Jr., Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T, 1994. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of general psychiatry 51(11), 849–859; discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB, 1995. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52(12), 998–1007. [DOI] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF, 2009. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry research 172(1), 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels EM, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Dargham A, Girgis RR, 2014. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res 152(2-3), 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS, Knowlton RC, den Hollander JA, Lahti AC, 2010. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry 68(7), 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Schooler NR, John M, Correll CU, Marcy P, Addington J, Brunette MF, Estroff SE, Mueser KT, Penn D, Robinson J, Rosenheck RA, Severe J, Goldstein A, Azrin S, Heinssen R, Kane JM, 2015. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry 172(3), 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert F, Gallinat J, Seifert F, Rinneberg H, 2004. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage 21(4), 1762–1771. [DOI] [PubMed] [Google Scholar]
- Stevenson JM, Reilly JL, Harris MS, Patel SR, Weiden PJ, Prasad KM, Badner JA, Nimgaonkar VL, Keshavan MS, Sweeney JA, Bishop JR, 2016. Antipsychotic pharmacogenomics in first episode psychosis: a role for glutamate genes. Translational psychiatry 6, e739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulc A, Galinska B, Tarasow E, Waszkiewicz N, Konarzewska B, Poplawska R, Bibulowicz D, Simonienko K, Walecki J, 2011. Proton magnetic resonance spectroscopy study of brain metabolite changes after antipsychotic treatment. Pharmacopsychiatry 44(4), 148–157. [DOI] [PubMed] [Google Scholar]
- Taylor R, Osuch EA, Schaefer B, Rajakumar N, Neufeld RW, Theberge J, Williamson PC, 2017. Neurometabolic abnormalities in schizophrenia and depression observed with magnetic resonance spectroscopy at 7 T. BJPsych open 3(1), 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerner MG, Mannuzza S, Kane JM, 1988. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull 24(1), 112–117. [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Lambert M, Conus P, Velakoulis D, Stuart GW, Desmond P, McGorry PD, Pantelis C, 2006. Prediction of functional outcome 18 months after a first psychotic episode: a proton magnetic resonance spectroscopy study. Archives of general psychiatry 63(9), 969–976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.