Abstract
Cerebellopontine angle (CPA) tumours, are diverse pathologically with regard to the site of tumour origin and displacement of the neurovascular structures. In general CPA tumours are divided into acoustic and non-acoustic tumours. The aim of the present study was to see the spectrum of lesions at cerebellopontine angle and their historadiological correlation. A retrospective analysis of 122 cases of CPA tumors diagnosed in the Department of Pathology, IHBAS was done from January 2004 to August 2019. There were 56 males and 66 females in the age group of 8–68 years. Clinical history ranged from 10 days to 5 years. Clinical symptoms included hearing deficit, visual loss, facial nerve dysfunction, nausea, vomiting, headache, seizures, unsteadiness and disequilibrium. Historadiological correlation was found in 112 cases (91%).In our series of CPA tumors, nonacoustic tumors were more frequent than in previous studies. We found that presence of intrameatal fraction is not exclusive only for schwannomas. There can be a rare occurrence of medullobastoma/small round cell tumor in CP angle. The final pathological verification can at times give unexpected results. Immunohistochemistry did not have a significant role in diagnosis of cerebellopontine angle tumors.
Keywords: Histo-radiological, Correlation, Cerebellopontine, Angle, Tumors
Introduction
The cerebellopontine angle (CPA) is an anatomical space bound anterolaterally by the posterior aspect of the petrous temporal bone and posteromedially by the cerebellum and pons. It contains important vascular structures and cranial nerves bathed in cerebrospinal fluid (CSF). It is centred by the internal auditory canal (IAC) and is traversed by V cranial nerve to the IX–X–XI cranial nerve complex [1].Tumours of the CPA account for 10% of all intracranial tumours; can be of native origin or can be an extension of lesion from adjacent structures [2]. Tumours which are native to CPA can arise from various anatomical structures, includes primary origin from internal auditory meatus, pontocerebellar cistern and lateral recess of the 4th ventricle, temporal bone, brain stem, or cerebellar nervous tissue [3, 4]. Most (80%) of the cerebellopontine angle tumors are vestibular schwannomas. The rest of the tumors are meningiomas, epidermoids, arachnoid cysts; other rare tumors are trigeminal schwannomas, facial nerve schwannomas, exophytic brainstem gliomas, secondaries and choroid plexus papillomas [5]. Computerized tomography (CT) and magnetic resonance imaging (MRI) are the primary modalities for diagnosis of cerebellopontine lesions. MRI is considered superior in differentiating the different types of CPA masses. The high contrast resolution and multiplanar capabilities of MR helps to delineate shape and margins, extent, mass effect, intensity at MR imaging, enhancement and adjacent bone reaction. The main aim of radiological diagnosis is to assess the relation of the tumour to IAC, the brain stem and cerebellar hemispheres. The second line basic information is if the lesion is extra or intracerebral. [6].
The aim of the present study was to see the spectrum of lesions at cerebellopontine angle and their historadiological correlation.
Materials and Methods
A retrospective analysis of 122 cases of CPA tumors diagnosed in the department of pathology, Institute of Human Behaviour and Allied Sciences, IHBAS was done from January 2004 to August 2019. The patients were operated on in neurosurgery departments of IHBAS and Guru Teg Bahadur hospital. Radiological diagnosis was done at various diagnostic centres across the city and adjoining areas. MR imaging of the brain was performed in sagittal, axial and coronal planes. GRE (Gradient recalled echo), TSE (Turbo spin echo) and FLAIR (Fluid attenuated inversion recovery) sequences performed to obtain T1 and T2 weighted images were reviewed. Diffusion weighted images and post contrast images were also analysed. Histological reporting was done in the department of Pathology, IHBAS and diagnoses were reviewed by minimum of two pathologists. Haemotoxylin and Eosin stained sections were examined, immunohistochemistry and special stains were performed wherever required.
Results
There were 56 males and 66 females in the age group of 8–8 years (Tables 1, 2). Clinical history ranged from 10 days to 5 years. Clinical symptoms included hearing deficit, visual loss, facial nerve dysfunction, nausea, vomiting, headache, seizures, unsteadiness and dysequilbrium (Table 3). Diameter of the tumors ranged from 1.3 to 6.2 cm. On radiology, there were 73 cases of schwannoma, 15 cases of meningioma, 23 cases of epidermoid cyst, 2 cases of arachnoid cyst, 2 cases of tuberculoma, 1 case of cyst, 1 case of glioma, 1 case of infarct, and 5 cases were no definite radiological opinion was rendered. However, a detailed radiological investigation could be traced back in 57 cases. On histopathological examination, there were 73 cases of schwannoma, 14 cases of meningioma, 26 cases of epidermoid cyst, 1 case of arachnoid cyst, 2 cases of metastatic carcinoma, 1 case of neuroglial cyst, 1 case of medulloblastoma, 1 case of infarct, 1 case of choroid plexus papilloma, I case of high grade glioma and in one case a possible diagnosis of parasitic cyst was rendered. Historadiological correlation was found in 112 cases (91%).
Table 1.
Distribution of tumors according to age
| Tumor type | 0–10 | 10–20 | 20–30 | 30–40 | 40–50 | 50–60 | 60–70 | 70–80 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Schwannoma | 6 | 18 | 24 | 10 | 9 | 7 | 74 | ||
| Meningioma | 2 | 1 | 3 | 4 | 3 | 1 | 14 | ||
| Epidermoid cyst | 4 | 5 | 10 | 4 | 1 | 2 | 26 | ||
| Arachnoid cyst | 1 | 1 | |||||||
| Metastatic carcinoma | 2 | 2 | |||||||
| Neuroglial cyst | 1 | 1 | |||||||
| Medulloblastoma | 1 | ||||||||
| Infarct | 1 | 1 | |||||||
| Choroid plexus papilloma | 1 | 1 | |||||||
| High grade glioma | 1 | 1 | |||||||
| Parasitic cyst | 1 | 1 |
Table 2.
Distribution of Cases according to gender
| Schwannoma | Meningioma | Epidermoid cyst | Arachnoid cyst | Metastatic carcinoma | Neuroglial cyst | Medulloblastoma | Infarct | Choroid plexus pailooma | High grade glioma | Parsitic cyst | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 56) | 36 | 01 | 13 | 00 | 02 | 01 | 01 | 01 | 00 | 00 | 01 |
| Female (n = 66) | 37 | 13 | 13 | 01 | 00 | 00 | 00 | 00 | 01 | 01 | 00 |
Table 3.
Distribution of cases according to clinical presentation
| Schwannoma | Meningioma | Epidermoid cyst | Arachnoid cyst | Metastatic carcinoma | Neuroglial cyst | Medulloblastoma | Infarct | Choroid plexus papilloma | High grade glioma | Parasitic cyst | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual loss | 15 | 1 | 4 | 1 | |||||||
| Hearing loss | 45 | 7 | 6 | 1 | |||||||
| Headache | 9 | 11 | 2 | 1 | 1 | 1 | 1 | ||||
| Nausea/vomiting | 8 | 3 | 3 | 1 | 1 | 1 | |||||
| Weakness | 4 | 2 | |||||||||
| Facial hypoesthesia | 6 | 1 | |||||||||
| Tinnitus | 4 | 1 | |||||||||
| Vertigo | 2 | 5 | |||||||||
| Cerebellar signs | 2 | 1 | 1 | 1 | |||||||
| Seizures | 1 |
Out of the 73 cases in which radiological diagnosis of schwannoma was made, a detailed radiological investigation was available in 39 cases. There was homogeneous enhancement in 35 cases while 4 cases were isogeneously enhancing after contrast administration. Brainstem and cerebellar compression were seen in 37 cases. On T1 weighted images, 33 cases were hypointense and 6 were isointense. On T2 weighted images, 35 cases were hyperintense, 3 were isointense and 1 was hypointense. Intrameatal widening was seen in 38 cases and 28 cases were associated with hydrocephalus. Histologically, 61 cases showed the classical Antony A and Antony B areas and hyalinized vessels.
Out of the 23 cases in which radiological diagnosis of epidermoid cyst was made, a detailed radiological investigation was available in 11 cases. All the cases were homogeneously non enhancing after contrast administration. Seven cases were hypointense to isointense on T1 weighted images, 3 were isointense and 1 was hypointense. Eight cases were hyperintense on T2 weighted images, 2 were isointense and 1 was hypointense. Ten cases showed evidence of brainstem and 9 cases showed signs of cerebellar compression. One case was associated with hydrocephalus.
Out of the 14 cases in which radiological diagnosis of meningioma was made, a detailed radiological investigation was available in 6 cases. Homogeneous enhancement was seen in 2 cases, while 4 cases were isogeneously enhancing after contrast administration. On T1 weighted images, 4 cases were hypointense and 2 were isointense. On T2 weighted images, 3 cases were hyperintense 2 were isointense and 1 was hypointense. Brainstem and cerebellar compression was seen in 4 cases. One case was associated with hydrocephalus. Histologically the cases were of the following subtype: transitional (11), rhabdoid (1), atypical (1) and angiomatous (1) meningioma.
There were 6 cases of recurrent schwannoma, and 1 case each of recurrent meningioma and epidermoid cyst in our study. There were 2 cases of MISME lesion(multiple intracranial schwannoma meningioma ependymoma).
In one patient, lesions of both epidermoid cyst and CPA meningioma were present.
Discussion
Cerebellopontine angle tumours, are diverse pathologically with regard to the site of tumour origin and displacement of the neurovascular structures. In general CPA tumours are divided into acoustic and non-acoustic tumours [7].
Vestibular schwannomas (VS) account for 70–80% of all CPA lesions, meningiomas 5–12% and epidermoid cysts 2–6%, other lesions, which accounts each ≤ 1% [8].
In our series the CPA tumours were more frequent in women which was similar to the previous studies [4, 5].
The most common CPA tumour was typically vestibular schwannoma. It represented 60% of all tumours, so this frequency was lower than in the other described groups (72–80%) [6, 7].
On MRI, VS are typically T1 isointense and T2 hyper intense. VS demonstrate enhancement in post contrast images which can be either homogeneous or heterogeneous. Furthermore, 5–15% of VS may have a cystic appearance. Small VS are usually homogeneously enhancing and composed predominantly of highly cellular and organized Antoni type A regions histologically, whereas larger VS tend to be heterogeneously enhancing or and composed predominantly of hypocellular, loosely organized Antoni B regions or may demonstrate a mixed type A or type B pattern [9, 10].
In a study by Singh et al., all cases of Schwannoma were hypointense on T1 weighted image, while on T2 weighted image, 88% cases were hyperintense and 12% cases showed mixed signal intensity. Homogeneous contrast enhancement was seen in 53% cases whereas 47% cases showed inhomogeneous contrast enhancement [11].
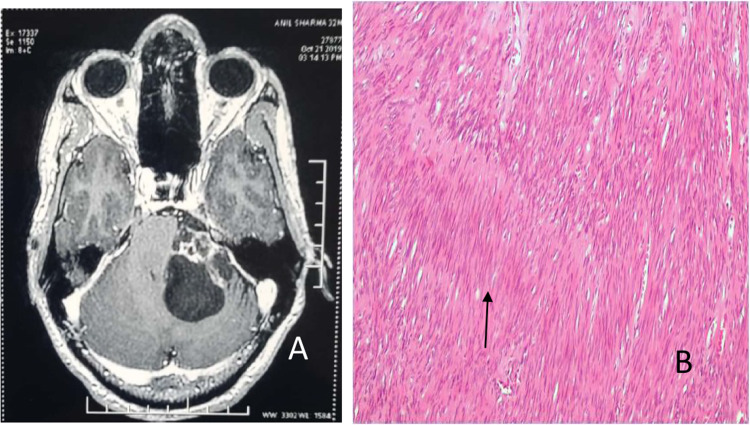
In our study, 80% cases of VS were hypointense on T1 and 20% cases were isointense on T1 T1 weighted image. On T2 weighted image, 89% cases were hyperintense, 8% cases were isointense and 3% cases were hypointense homogeneous contrast enhancement was seen in 85% cases while 7% cases showed isogeneous contrast enhancement (Fig. 1a, b).
Fig. 1.
a Axial post contrast image shows large extra axial left CPA mass showing heterogeneous post contrast enhancement in the solid component and thin peripheral enhancement with thin internal septations in the cystic component. b Spindle shaped cells arranged in palisades forming vercay bodies (↑)
The second most common tumor in our study was epidermoid cyst (21%).This was a deviation from the previous studies where meningioma is the second most common CPA tumor followed by epidermoid cyst [1]. On MRI, epidermoid cysts are T1 hypointense and T2 hyperintense, mimicking a fluid-filled cyst. Epidermoid cysts are mildly hyperintense on FLAIR and strikingly hyperintense on diffusion-weighted imaging (DWI). This helps in distinction from arachnoid cyst which has similar findings as epidermoid cyst on T1 and T2 images. Arachnoid cysts will be suppressed on FLAIR and will have very low signal intensity on DWI [12, 13].
73% cases of CPAepidermoid cysts were hypointense to isointense on T1 weighted image while 17% were isointense. On T2 weighted image, 50% cases were hyperintense, 34% were isointense and 16% were hypointense.
The third most common CPA tumor in our study were meningiomas (12%). Meningiomas are usually isointense with the cortex on all sequences, and strongly enhance after contrast injection, often homogeneously. Dural tail sign, which represents the intense enhancement of the non neoplastic thickened tumoral dura is a common finding in meningiomas [1, 14].
Three cases of meningioma were misdiagnosed as Schwannoma radiologically. All of them had an intrameatal extension with no clear distinction of vestibulocochlear nerve. Preoperative distinction of small intracanalicular meningiomas from schwannomas usually remains difficult. Clinical symptoms of both of them are similar with difficulty in hearing being the most common presenting symptom. The only subtle difference is that facial nerve symptoms are more likely to occur with meningiomas than with vestibular schwannomas when the size is small. Radiologically too, both the tumours have similar presentations.
Both lesions are isointense to hypointense on T1-weighted MR images and are of variable signal intensity on T2-weighted MR images. They also both brightly enhance after administration of contrast medium [15, 16]. There was one case of medulloblastoma which was misdiagnosed as meningioma radiologically (Fig. 2a–f) Medulloblastomas with dural base can mimick meningiomas radiologically and pose a diagnostic dilemma. There are no pathognomonic features on MRI to distinguish between the two lesions [17].
Fig. 2.
a Axial post contrast image shows heterogeneous moderately enhancing mass lesion in right cerebellar hemisphere and right CP cistern. b Diffusion weighted image shows restriction in the solid component of the lesion. c Round to oval pleomorphic cells with hyperchromatic nuclei arranged in nodules and nests separated by fibrous septae. d Ki 67 proliferation index is 80–85% in areas showing highest proliferation. e Cytoplasmic and membranous positivity for beta- catenin is seen
Another discordance in histordiological correlation was seen in the cases of metastatic carcinoma. Two cases of metastatic carcinoma were misdiagnosed as tuberculoma radiologically on MR images (Table 4). Both tuberculoma and metastasis can manifest as solitary or multiple ring-enhancing intra-axial lesions that are difficult to differentiate by conventional magnetic resonance imaging (MRI), Measuring regional cerebral blood volume (rCBV) obtained by T2*-weighted dynamic contrast-enhanced perfusion MRI can help in differentiating intracranial tubercular mass lesions and metastases. The mean rCBV ratio between the lesion periphery and normal white matter is inferior to one for tubercular lesions and greater than five for metastases [18].
Table 4.
Discordance in histo-radiological diagnosis
| S. no. | Histopathology no. | Radiological diagnosis | Histopathological diagnosis |
|---|---|---|---|
| 1. | R-69/04 (50/M) | Tuberculoma | Metastatic adenocarcinoma |
| 2. | R-181/04 (9/M) | Schwannoma | Meningioma |
| 3. | R-81/05 (40/M) | Meningioma | Medulloblastoma |
| 4. | R-79/06 (24/F) | Schwannoma | Meningioma |
| 5. | R-22/10 (50/M) | Tuberculoma | Metastatic carcinoma |
| 6. | H-32/10 (30/F) | Schwannoma | Meningioma |
| 7. | H-85/12 (42/M) | Meningioma/subarachnoid cyst | Neuroglial cyst |
| 8. | H-80/13 (24/M) | Schwannoma | ? parasitic cyst |
| 9. | R-43/17 (26/F) | Meningioma | Schwannoma |
| 10. | R-124/18 (24/F) | Meningioma | Schwannoma |
Conclusion
In our series of CPA tumors, nonacoustic tumors were more frequent than in previous studies. Presence of intrameatal fraction is not exclusive only for schwannomas, meningiomas with an intrameatal fraction is common. In our study, Medulloblastoma in the CPA also had an intrameatal component. There can be a rare occurrence of medullobastoma/small round cell tumor in CP angle. Immunohistochemistry did not have a significant role in diagnosis of cerebellopontine angle tumors. The final pathological verification can at times give unexpected results.
Authors Contribution
SC Concept, Guarantor; SL Design, Definition of intellectual content, Literature search, Data acquisition, Data analysis, Statistical analysis; IP Manuscript preparation, Manuscript editing, Manuscript review.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bonneville F, Savatovsky J, Chiras J. Imaging of cerebellopontine angle lesions:an update. Part 1: enhancing extra-axial lesions. EurRadiol. 2007;17:2472–2482. doi: 10.1007/s00330-007-0679-x. [DOI] [PubMed] [Google Scholar]
- 2.Bonneville F, Sarrazin JL, Marsot-Dupuch K, Iffenecker C, Cordoliani YS, Doyon D, et al. Unusual lesions of the cerebellopontine angle: a segmental approach. Radiographics. 2001;21:419–438. doi: 10.1148/radiographics.21.2.g01mr13419. [DOI] [PubMed] [Google Scholar]
- 3.Beaman FD, Kransdorf MJ, Menke D. Schwannoma:radiologic-pathologic correlation. Radiographics. 2004;24:1477–1481. doi: 10.1148/rg.245045001. [DOI] [PubMed] [Google Scholar]
- 4.Brackmann DE, Kwartler JA. A review of acoustic tumors. Am J Otol. 1990;11:216–232. [PubMed] [Google Scholar]
- 5.Smirniotopoulos JG, Yue NC, Rushing EJ. Cerebellopontine angle masses: radiologic-pathologic correlation. Radiographics. 1993;13:1131–1147. doi: 10.1148/radiographics.13.5.8210595. [DOI] [PubMed] [Google Scholar]
- 6.Zamani AA. Cerebellopontine angle tumors: role of magnetic resonance imaging. Top Magn Reson Imaging. 2000;11:98–107. doi: 10.1097/00002142-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Joarder MA, Karim AKMB, Sujon SI, Akhter N, Waheeduzzaman M, Joseph V, et al. Surgical outcomes of cerebellopontine angle tumors in 34 cases. Pulse. 2015;8:8–14. doi: 10.3329/pulse.v8i1.28095. [DOI] [Google Scholar]
- 8.Moffat DA, Ballagh RH. Rare tumours of the cerebellopontine angle. Clin Oncol. 1995;7:28–41. doi: 10.1016/S0936-6555(05)80632-6. [DOI] [PubMed] [Google Scholar]
- 9.Nikdokht F. Imaging of vestibular Schwannoma and other cerebellopontine angle tumors. Oper Tech Otolaryngol. 2014;25:87–95. doi: 10.1016/j.otot.2013.11.011. [DOI] [Google Scholar]
- 10.Gomez Brouchet A, Delisle MB, Cognard C, Bonak A, Charlet J, Deguire O, et al. Vestibular schwannomas: correlation between magnetic resonance imaging and histopathologic appearance. OtolNeurotol. 2001;22:79–86. doi: 10.1097/00129492-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Singh K, Singh MP, Thukral CL, Rao K, Singh K, Singh A. Role of magnetic resonance imaging in evaluation of cerebellopontine angle Schwannomas. Indian J Otolaryngol Head Neck Surg. 2015;67:21–27. doi: 10.1007/s12070-014-0736-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao PY, Osborn A, Smirniotopoulos J, Harris CP. Epidermoid tumor of the cerebellopontine angle. Am J Neuroradiol. 1992;13:863–872. [PMC free article] [PubMed] [Google Scholar]
- 13.Le Garlantezec C, Vidal VF, Guerin J, Bebear JP, Liguoro D, Darrouzet V. Management of cerebellopontine angle meningiomas and the posterior part of the temporal bone Report on 44 cases. Rev Laryngol Otol Rhinol. 2005;126:81–89. [PubMed] [Google Scholar]
- 14.Asaoka K, Barrs DM, Sampson JH, McElveen JT, Tucci DL, Fukushima T. Intracanalicular meningioma mimicking vestibular Schwannoma. Am J Neuroradiol. 2002;23:1493–1496. [PMC free article] [PubMed] [Google Scholar]
- 15.Caylan R, Falcioni M, De Donato G, Ferrara S, Russo A, Taibah A. Intracanalicular meningiomas. Otolaryngol Head Neck Surg. 2000;122:147–150. doi: 10.1016/S0194-5998(00)70166-5. [DOI] [PubMed] [Google Scholar]
- 16.Dinh DH, Clark SB, Whitehead M, Amedee R, Bhattacharjee MB. Intracanalicular meningioma. South Med J. 2000;93:618–621. doi: 10.1097/00007611-200093060-00018. [DOI] [PubMed] [Google Scholar]
- 17.Furtado SV, Venkatesh PK, Dadlani R, Reddy K, Hegde AS. Adult medulloblastoma and the “dural-tail” sign: rare mimic of a posterior petrous meningioma. Clin Neurol Neurosurg. 2009;111:540–543. doi: 10.1016/j.clineuro.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee S, Saini J, Kesavadas C, Arvinda HR, Jolapara M, Gupta AK. Differentiation of tubercular infection and metastasis presenting as ring enhancing lesion by diffusion and perfusion magnetic resonance. J Neuroradiol. 2009;37:167–171. doi: 10.1016/j.neurad.2009.08.005. [DOI] [PubMed] [Google Scholar]