Abstract
Purpose
The aim of this prospective study (ClinicalTrials.gov: NCT01880203) was to evaluate the diagnostic and prognostic value of a 7-panel mutation testing in the aspirates of thyroid nodules with indeterminate cytology (IC).
Methods
Eligible patients had a thyroid nodule ≥15 mm with IC (Bethesda III–V) for which surgery had been recommended. Detection of BRAF and RAS mutations was performed using pyrosequencing and RET/PTC and PAX8/PPARγ rearrangements using Real-Time quantitative reverse transcription‐polymerase chain reaction (RT-PCR).
Results
Among 131 nodules with IC, 21 (16%) were malignant including 20 differentiated cancers and one thyroid lymphoma. Molecular abnormalities were identified in 15 nodules with IC corresponding to 10 malignant and 5 benign tumours. BRAF mutation was detected in 4 nodules all corresponding to classic PTC, and PAX8/PPARγ rearrangement in 2 HCC. In contrast, RAS mutation was identified in eight nodules, of which four were malignant, and one RET/PTC3 rearrangement in a follicular adenoma. This data resulted in an accuracy of 88%, sensitivity of 48%, specificity of 95%, positive-predictive value of 67%, and negative-predictive value of 91%. After a 56 month’s follow-up, the proportion of excellent response was similar in patients with molecular alterations (67%) and those without (60%).
Conclusions
By increasing the overall risk of cancer from 16 to 67% in mutated nodules and by diminishing it to 9% in wild-type, this study confirms the relevance of the 7-panel mutation testing in the diagnostic of nodules with IC. Genetic testing, however, did not predict outcome in the cancer patient subgroup.
Keywords: Thyroid nodules, Diagnosis, Prognosis, Mutation, Thyroid cancer
Introduction
At least one woman out of two after 50 years old has a thyroid nodule. Owing to their frequency and the wide use of neck ultrasound (US), the clinical management of thyroid nodules has become a public health issue. Facing a thyroid nodule, the clinician has to recognize functional autonomy and to evaluate the risk of cancer. Although thyroid nodules are common, only a small fraction (~5%) corresponds to malignant tumours which are generally of good prognosis. Assessing the risk of cancer is mainly based on thyroid cytology after fine needle aspiration biopsy (FNAB) using the Bethesda classification [1]. This is a good, if not a perfect method for identifying patients with thyroid cancer. The main limitation is represented by indeterminate cytology (IC) which occurs in up to 25% of cases and does not allow differentiating between benign and malignant nodules. Indeterminate cytology comprises Bethesda class III (atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS)), class IV (follicular neoplasm/suspicious for follicular neoplasm (FN) or Hürthle cell neoplasm (FN/SFN)) and at a lesser degree, class V (suspicious for malignancy (SM)). Surgery is strongly recommended in Bethesda V associated with a high risk of cancer up to 75%. In clinical practice, surgery still remains a usual option for Bethesda III or IV nodules although the risk of cancer does not exceed 20–25%. As a result, ~80% of operations can be a posteriori considered as useless in those categories. Given the potential morbidity of thyroid surgery for parathyroid glands and recurrent nerve, and the necessity of hormonal treatment after total thyroidectomy, a more accurate preoperative assessment is needed to better select patients for surgery. This trend towards a risk-adapted approach, personalized therapy and treatment de-escalation was strengthened in the 2015 ATA guidelines [2]. Improving the characterization of nodules would allow surveillance in patients with likely benign nodules and enable a shift to the most appropriate surgical procedure in those with malignant nodules also taking into account the assumed prognosis of cancer. From this point of view, although the presence of somatic mutations, particularly BRAF mutation [3], has been shown to be associated with more aggressive disease, the prognostic impact of molecular testing in the aspirates of IC nodules on the long-term outcome of patients with thyroid cancer has not been evaluated so far.
In the last decade, tools of molecular analysis or imaging have been developed to improve the diagnosis of thyroid nodules. Investigating the presence of somatic mutations, genomic rearrangements or gene fusions in thyroid FNA specimens has been shown relevant in nodules with IC [4]. Testing for single mutations such as BRAF V600E has a high specificity for cancer but low sensitivity [5]. Testing for a limited panel of mutations (such as BRAF, RAS, RET/PTC, PAX8/PPARγ) enables to increase sensitivity while maintaining a good specificity [6]. More recently, ThyroSeq v2.1 next-generation sequencing (NGS) multi-gene panel of molecular markers has been shown to provide high sensitivity, 90.9 % and specificity, 92.1% in Bethesda III nodules [7]. Sophistication of ThyroSeq v3 improves sensitivity at 94.1% while moderately lowers specificity at 81.6% and enables to avoid 82% of unnecessary surgeries in patients with histologically proven benign nodules [8]. Also, recent studies in patients with IC nodules using genomic sequencing classifier have reported sensitivity ranging from 91 to 100% and specificity from 68 to 93% [9, 10].
Besides molecular testing, efforts have been made to look for imaging methods in capacity to refine diagnosis of nodules with IC. Recently, we reported the results of a prospective bicentric study designed to assess the relevance of US and shear wave elastography. Both methods failed to discriminate benign and malignant nodules [11]. The other objective of this study was to evaluate in the same patients the diagnostic and prognostic values of a 7-panel mutation testing on the indeterminate cytological specimens. We present here the results of that study.
Patients and methods
Patients
The study protocol was approved by the Local Ethics Committee (Ref. 2012–35, Comité de protection des personnes Nord-Ouest III) and the French Health Authorities (Ref 130213B-22). This trial is registered as ID-RCB 2012-A01313–40, ClinicalTrials.gov NCT01880203. It was conducted according to the provisions of the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference of Harmonization. Written informed consent was obtained from all patients.
As previously described [11], eligible patients had a thyroid nodule ≥15 mm with IC according to Bethesda classification in the six months before inclusion, for whom surgery had been recommended. Indeterminate cytology included class III, IV and V sub-categories and was confirmed by an experienced cytologist working in the other participating centre.
Fine needle aspiration (FNA)
The FNA procedures were conducted under US guidance into the nodule of interest. The study protocol provided for two dedicated passes of FNA washed in a tube containing nucleic acid preservative solution (RNA protect®, QIAGEN™) which was frozen at −20 °C until analysis.
Nucleic acids extraction
Total RNA and DNA were extracted from FNA samples using AllPrep DNA/RNA Micro Kit (QIAGEN™) according to the manufacturer’s protocol. The amount of total RNA and DNA was determined by spectrophotometry using NanoVue (GE Health Care Bio-Science, Piscataway, NJ, USA) and used as template for RT-PCR and PCR amplification.
Detection of point mutations
DNA was amplified by polymerase chain reaction (PCR) using the following primers (BRAF: forward: 5′-biotin- CTTCATAATGCTTGCTCTGATAGG-3′, reverse: 5′-GGCCAAAAATTTAATCAGTGGAA-3′; HRAS: forward: 5′-ATTGATGGGGAGACGTGCCTGTTG-3′, reverse: 5′-biotin- TACTGGTCCCGCATGGCGCTGT-3′; KRAS: forward: 5′- CATGTTCTAATATAGTCACATTTTCAT-3′, reverse: 5′- biotin- AGCTGTATCGTCAAGGCACTCTT-3′; NRAS: forward: 5′- GCAAATACACAGAGGAAGCCTTCG-3′, reverse: 5′-biotin-GGCCAAAAATTTAATCAGTGGAA-3′;) with a product size of 226 bp, 77 bp, 121 pb and 137 bp respectively. DNA was amplified using the Quantitec Multiplex PCR NoROX kit (QIAGEN™) according to the manufacturer’s protocol. The cycling conditions were: 1- for BRAF and KRAS: 95 °C for 15 min, 45 cycles of 95 °C for 20 s, 60 °C for 30 s and 72 °C for 30 s, final extension at 72 °C for 20 min; 2- for NRAS: 95 °C for 10 min, 30 cycles of 94 °C for 20 s, 70°C for 20 s with a decrease of 0.5 °C per cycle and 72 °C for 45 s, 19 cycles of 94 °C for 20 s, 50°C for 20 s and 72 °C for 45 s, final extension at 72 °C for 10 min; 3- for HRAS: 95 °C for 10 min, 40 cycles of 95 °C for 30 s, 69 °C for 45 s and 72 °C for 30 s, final extension at 72 °C for 10 min.
Mutation detection of BRAF codons 600 and 601 (sequencing primer: 5′-CCACTCCATCGAGATT-3′), HRAS codon 61 (sequencing primer: 5′-TCCTGGATACCGCCG-3′), KRAS codons 12 and 13 (sequencing primer: 5′-CTTGTGGTAGTTGGAGCT-3′), NRAS codon 61 (sequencing primer: 5′-GACATACTGGATACAGCT-3′) using the Pyrosequencing PyroMark™ Q24 system was done following the manufacturer’s instructions.
Detection of rearrangements
Real-time quantitative reverse transcription‐polymerase chain reaction (RT-PCR) mixture was prepared using 40 nM of each primer set and probes as previously described [12], 2X Quantitec Probe RT-PCR master Mix (QIAGEN™), Quantitec RT Mix (QIAGEN™) and 10 ng of RNA in a final reaction volume of 25 µL according to the manufacturer’s protocol. Reverse transcription two-step PCR thermal cycling for cDNA amplification and real-time data acquisition were performed with a 7500 FAST (Thermofisher™) Real-Time PCR System using the following cycle conditions: a reverse transcription step of 50 °C for 30 min, a cDNA denaturation step of 95 °C for 15 min followed by 50 cycles amplification of 94 °C for 15 s and 60 °C for 1 min. Negative control (no cDNA) and positive controls (RNA from tumours or cell lines known to carry a particular rearrangement was used as a positive control.) were cycled in parallel with each run. To decrease the likelihood of false negatives, GAPDH was amplified in parallel for each sample. Fluorescence data were analysed by the 7500 Fast Dx software and expressed as Ct, the number of cycles needed to generate a fluorescent signal above a predefined threshold. Baseline and threshold values were set by the 7500 Fast Dx software. Samples with a delta Ct inferior to 10 have been considered as positive.
Surgery and histological examination
Thyroid surgery was performed in each participating institution and consisted in either lobectomy or total thyroidectomy. The surgeon oriented the resected specimen and localized the nodule for pathological diagnosis with the support of the descriptive diagram. The 2004 World Health Organization criteria were used for diagnosis [12].
Initial treatment and follow-up for patients with differentiated thyroid cancer
The initial treatment for patients with differentiated thyroid cancer (DTC) was a combination of thyroid surgery, with or without neck dissection, and treatment with radioactive iodine (RAI). This treatment was discussed in a multidisciplinary team and was not affected by the presence or absence of molecular markers in FNAB samples.
After initial treatment, patients were assessed at 9–12 months and then annually. The response to treatment was evaluated according to the ATA 2015 guidelines [2]. Excellent response was defined by negative imaging and either suppressed thyroglobulin (Tg) <0.2 ng/mL or TSH-stimulated Tg <1 ng/mL, biochemical incomplete response by negative imaging and suppressed Tg ≥1 ng/mL or stimulated Tg ≥10 ng/mL or rising anti-Tg antibodies (TgAb) levels, structural incomplete response by structural or functional evidence of disease with any Tg level, with or without TgAb, and indeterminate response by nonspecific findings on imaging studies, with non-stimulated Tg between 0.2 and 1 ng/mL or stimulated Tg between 1 and 10 ng/mL, or TgAb stable or declining in the absence of structural or functional disease.
Statistical analysis
Patient characteristics and patient subgroups were compared using the Wilcoxon or Kruskal-Wallis test (continuous variables) and chi-square or Fisher’s exact test (nominal variables), as appropriate. All tests were two-sided, and a p value < 0.05 was considered statistically significant. Analyses were performed with R (version 3.4.0). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were computed to assess the diagnostic performances of the 7-panel testing. As an exploratory analysis, the link between diagnostic performances and cancer prevalence, PTC rate and BRAF mutated PTC rate in previous studies and ours was assessed through a linear model.
Results
Patient characteristics
As previously described [11], 140 patients were initially enroled in this study. Since nine patients were secondarily excluded (consent withdrawal, n = 4; spontaneous nodule shrinkage, n = 1, surgery cancelled for comorbidities, n = 1; surgery performed outside the participating centres, n = 3), 131 patients were assessable.
Cytological and pathological data
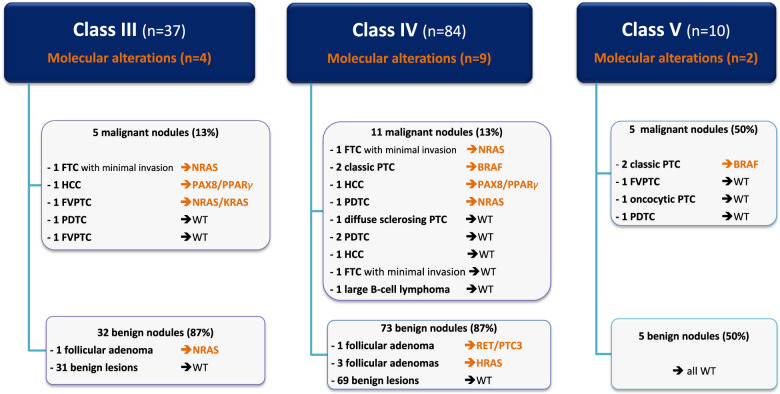
From a cytological point of view, 37 patients (28%) had a nodule scored class III, 84 (64%) class IV and 10 (8%) class V (Fig. 1). Central review confirmed the IC status in all 131 nodules.
Fig. 1.
Molecular alterations in patients with malignant or benign nodules for each cytological subgroup (Bethesda III, IV and V). WT wild-type, PTC papillary thyroid cancer, FVPTC papillary thyroid cancer with follicular variant, FTC follicular thyroid carcinoma, HCC Hürthle-cell carcinoma, PDTC poorly differentiated thyroid carcinoma
Among 131 nodules, 21 (16%) were pathologically confirmed as malignant and 110 were benign. Malignant nodules included 9 PTC (classic variant, n = 4; follicular variant [FVPTC], n = 3; oncocytic variant, n = 1; diffuse sclerosing variant, n = 1), 3 follicular thyroid carcinomas (FTC) with minimal invasion, 3 Hürthle-cell carcinoma (HCC), 5 poorly differentiated carcinomas (PDTC) and one large B-cell lymphoma. In the classes III, IV and V, the rates of cancer were 13%, 13% and 50%, respectively. Benign nodules included 61 follicular adenomas, 26 nodular hyperplasia, 15 oncocytic adenomas, 6 other pathological diagnoses (1 multinodular goiter, 1 Grave’s disease, 1 trabecular hyalinized adenoma, 3 lymphocytic thyroiditis) and 2 tumours of uncertain malignant potential. The median size of the 131 nodules was 30 mm (range, 15–71). According to EU-TIRADS classification [13], there were 52 (40%) nodules scored Tirads 3, 33 (25%) Tirads 4 and 46 (35%) Tirads 5 with no significant differences between malignant and benign nodules as previously reported [11].
Molecular analysis on cytological specimens
Figure 1 shows the molecular alterations in patients with malignant or benign nodules for each cytological subgroup. Molecular abnormalities were identified in 15/131 nodules including 4 BRAF mutations, 8 RAS mutations (4 NRAS, 3 HRAS, 1 with both KRAS and NRAS mutations), 1 RET/PTC3 rearrangement and 2 PAX8/PPARγ rearrangements. Ten (47.6%) of the 21 malignant nodules presented molecular abnormalities vs 5 (4.5%) of the 110 benign nodules (p < 0.001). BRAF mutation was identified in 4 nodules all corresponding to classic PTC. PAX8/PPARγ rearrangements were also associated with an HCC in the two patients where they were found. In contrast, RAS mutation was detected in 8 nodules, among which 4 were malignant (2 FTC, 1 FVPTC and 1 PDTC). Last, one RET/PTC3 rearrangement was detected in a follicular adenoma.
Overall, molecular analysis had an accuracy of 88%, a sensitivity of 48%, a specificity of 95%, a PPV of 67% and a NPV of 91% (Table 1). Therefore, while the probability of cancer before testing in nodules with IC was 16%, the probability after testing increased to 67% in mutated-nodules and decreased to 9% in wild-type ones. There was no significant difference between the Bethesda III, IV and V subgroups in terms of accuracy, sensitivity, specificity, PPV or NPV.
Table 1.
Test performances in the whole cohort of patients, and in the Besthesda III, IV and V groups
| All | Bethesda III | Bethesda IV | Bethesda V | |||||
|---|---|---|---|---|---|---|---|---|
| n = 131 | n = 37 | n = 84 | n = 10 | |||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| True prevalence | 16 | [10–23] | 14 | [5–29] | 13 | [7–22] | 50 | [19–81] |
| Sensitivity | 48 | [26–70] | 60 | [15–95] | 45 | [17–77] | 40 | [5–85] |
| Specificity | 95 | [90–99] | 97 | [84–100] | 95 | [87–98] | 100 | [48–100] |
| Positive predictive value (PPV) | 67 | [38–88] | 75 | [19–99] | 56 | [21–86] | 100 | [16–100] |
| Negative predictive value (NPV) | 91 | [84–95] | 94 | [80–99] | 92 | [83–97] | 62 | [24–91] |
| Accuracy | 88 | [81–93] | 92 | [78–98] | 88 | [79–94] | 70 | [35–93] |
Outcome of patients with thyroid cancer
The patient with thyroid lymphoma and a patient with an NRAS-mutated FTC who was long-lost after initial treatment were excluded from the prognostic analysis. Of the 19 remaining DTC patients, 9 had a mutated tumour and 10 a wild-type tumour (Table 2). At 9–12 months after initial therapy, a similar proportion of patients with mutated and wild-type cancers presented excellent response (55% (5/9) vs 40% (4/10); p = 0.66). Three of 19 DTC patients received additional treatments because of persistent or recurrent disease, two with mutated tumour and one with wild-type. At last visit, after a median follow-up of 56 months (16–81), the proportion of excellent response was similar in patients with molecular alterations and those without (67% (6/9) vs 60% (6/10); p = 1).
Table 2.
Outcome of patients with malignant nodules
| Age (Yr) | Sex (F/M) | Cytology Bethesda | Pathology | Mutation/ Rearrangement | Date of surgery | Initial treatment (surgery, RAI) | pTNM | ATA status at 9–12 months | Additional treatment (surgery, RAI, other) | Date of last visit | ATA status at last visit |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | F | III | HCC | PAX8/PPARγ | Jul-2015 | TT, RAI | pT2NxM0 | ER | None | Jan-2020 | ER |
| 32 | F | III | FVPTC | NRAS/KRAS | Feb-2015 | TT + CND, RAI | pT1bN1aM0 | IR | None | Jun-2017 | ER |
| 54 | F | IV | HCC | PAX8/PPARγ | Dec-2013 | TT (2), RAI | pT3NxM0 | ER | None | Feb-2020 | ER |
| 49 | F | IV | Classic PTC | BRAF | Jan-2014 | TT (2), RAI | pT2mNxM0 | ER | None | May-2019 | ER |
| 64 | F | IV | PDTC | NRAS | Jan-2015 | TT (2), RAI | pT3mNxM0 | IR | −1st reintervention for LN recurrence in Jan-2018–2nd reintervention for LN and subcutaneous recurrence in Jan-2020 | Feb-2020 | SIR |
| 42 | F | IV | Classic PTC | BRAF | May-2015 | TT + CND | pT1bN0M0 | ER | None | Dec-2019 | IR |
| 37 | F | IV | FTC minimal invasion | NRAS | Sep-2015 | TT (2), RAI | pT3NxM0 | ER | None | Oct-2018 | ER |
| 51 | M | V | Classic PTC | BRAF | Oct-2013 | TT + CND, RAI | pT4aN1aM0 | SIR | Neck and mediastinum external irradiation in Jun-2014 | Feb-2020 | ER |
| 49 | F | V | Classic PTC | BRAF | Feb-2014 | TT(2) + CND, RAI | pT2 N1a M0 | IR | None | Feb-2020 | IR |
| 47 | F | III | PDTC | WT | Jun-2013 | TT(2) + CND, RAI | pT3N0M0 | ER | None | Feb-2020 | ER |
| 63 | F | III | FVPTC | WT | Mar-2015 | TT(2), RAI | pT2NxM0 | ER | None | May-2019 | ER |
| 39 | F | IV | Diffuse sclerosing PTC | WT | Jun-2014 | TT + CND, RAI | pT3N1aM0 | IR | None | Mar-2019 | ER |
| 68 | M | IV | PDTC | WT | Mar-2014 | TT, RAI | pT3NxM0 | IR | 2nd RAI treatment for Tg increase in Dec-2019 | Dec-2019 | SIR |
| 83 | M | IV | PDTC | WT | Mar-2014 | TT (2), RAI | pT2NxM0 | ER | None | Feb-2020 | ER |
| 36 | F | IV | HCC | WT | Mar-2015 | TT(2) + CND | pT2N0M0 | ER | None | Mar-2019 | ER |
| 36 | F | IV | FTC minimal invasion | WT | Jun-2015 | TT(2), RAI | pT2NxM0 | IR | None | Mar-2020 | ER |
| 76 | F | V | FVPTC | WT | Jan-2014 | TT | pT2NxM0 | IR | None | Apr-2018 | IR |
| 59 | F | V | Oncocytic PTC | WT | Jul-2014 | TT | pT1aN0M0 | IR | None | Oct-2015 | IR |
| 80 | F | V | PDTC | WT | Oct-2014 | TT + CND, RAI | pT3N0M0 | BIR | None | Dec-2017 |
Deatha BIR |
WT wild-type, PTC papillary thyroid cancer, FVPTC PTC with follicular variant, FTC follicular thyroid carcinoma, HCC Hürthle-cell carcinoma, PDTC poorly differentiated thyroid carcinoma, TT total thyroidectomy, TT (2) total thyroidectomy in two times, CND central neck dissection, RAI radioiodine treatment, ER excellent response, IR indeterminate response, BIR biochemical incomplete response, SIR structural incomplete response
aThe patient died of metastatic colic carcinoma and presented with evidence of BIR just before
Comparison of present data with previous studies
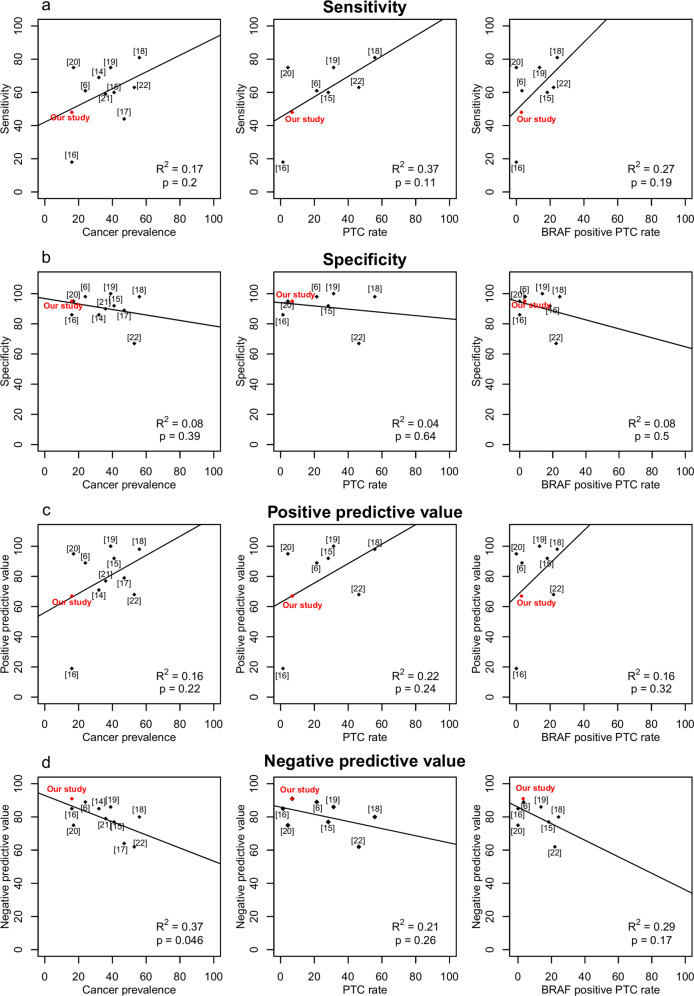
The analysis of the present data with previously reported studies [6, 14–22] is shown in Table 3 and in Fig. 2. In each study, we extracted the nodules with AUS/FLUS, FN/SFN or SM cytology, or Bethesda classes III, IV and V, or with “indeterminate” cytology. Only nodules operated on were taken into account for analysis. The number of cases in each study varied from 23 to 513, cancer prevalence from 16 to 56%, PTC rate from 1 to 56% and rate of BRAF positive PTC tumours from 0 to 24%. Sensitivity ranged from 18 to 81%, specificity from 86 to 100%, PPV from 19 to 100% and NPV from 64 to 91%. The sensitivity (48%) and PPV (67%) estimated in our series were generally lower than in other studies while NPV (91%) was slightly higher.
Table 3.
Analysis of the present data in comparison with that of the literature
| Reference 1st author, yr, [ref. number] | Cytology (n in each subcategory) | Operated nodules, n | Malignant nodules, n (%) | PTC, n (%) | BRAF positive PTC, n (%) | Test performance, % | |||
|---|---|---|---|---|---|---|---|---|---|
| Se. | Sp. | PPV | NPV | ||||||
| Nikiforov 2009 [19] | FLUS (21), FN (23), SM (7) | 51 | 20 (39%) | 16 (31%) | 7 (14%) | 75 | 100 | 100 | 86 |
| Cantara 2010 [18] | Indeterminate (41), SM (54) | 95 | 53 (56%) | 53 (56%) | 23 (24%) | 81 | 98 | 98 | 80 |
| Nikiforov 2011 [6] | AUS/FLUS (247), FN/SFN (214), SM (52) | 513 | 121 (24%) | 110 (21%) | 17 (3%) | 61 | 98 | 89 | 89 |
| Beaudenon-Huibregtse 2014 [17] | AUS/FLUS (22), FN/SFN (19), SM (12) | 53 | 25 (47%) | na | na | 44 | 89 | 79 | 64 |
| Eszlinger 2014 [16] | Indeterminate | 141 | 22 (16%) | 2 (1%) | 0 (0%) | 18 | 86 | 19 | 85 |
| Eszlinger 2015 [15] | Thy 3 (163), Thy 4 (39) | 202 | 83 (41%) | 57 (28%) | 37 (18%) | 60 | 92 | 92 | 77 |
| Labourier 2015 [14] | III (58) IV (51) | 109 | 35 (32%) | na | na | 69 | 86 | 71 | 85 |
| Bongiovanni 2015 [20] | FN/SFN (23) | 23 | 4 (17%) | 1 (4%) | 0 (0%) | 75 | 95 | 95 | 75 |
| Mancini 2012 [21] | Thy 3 (38), Thy 4 (9) | 47 | 17 (36%) | na | na | 59 | 90 | 77 | 79 |
| Bellevicine 2020 [22] | AUS/FLUS (86), FN/SFN (34), SM (57) | 177 | 93 (53%) | 82 (46%) | 39 (48%) | 63 | 67 | 68 | 62 |
| Present study | III (37), IV (84), V (10) | 131 | 21 (16%) | 9 (7%) | 4 (3%) | 48 | 95 | 67 | 91 |
III, IV, V: for Bethesda class III, IV or V
Thy 3, equivalent to FN/SFN; Thy 4, equivalent to SM
AUS/FLUS atypia of undetermined significance/follicular lesion of undetermined significance, FN/SFN follicular neoplasm/suspicious for follicular neoplasm (FN) or Hürthle cell neoplasm, SM suspicious for malignancy
Fig. 2.
Test performances (a, sensitivity; b, specificity; c, PPV; d, NPV) according to cancer prevalence, PTC rate and BRAF positive PTC rate in previous studies (6, 12, 14–20) and in the present study. The quality of the linear model adjustment is displayed on each graph (R2 and p value)
Although not significant, data shows a trend to a link between diagnostic performances of genetic testing and cancer prevalence, PTC rate and proportion of PTC harbouring BRAF mutations in each cohort, especially regarding sensitivity and PPV (Fig. 2).
Discussion
This prospective bicentric study confirms the clinical relevance of a 7-panel mutational testing in the characterization of cytologically indeterminate nodules. Molecular testing shows a good specifity (95%), a more limited sensitivity (48%) and an acceptable accuracy (88%). In this series of patients with a 16% pre-test probability of cancer, a 67% PPV and 91% NPV mean that the post-test probability increased to 67% in mutated-nodules and decreased to 9% in wild-type ones.
The impact of a 7-panel mutational testing on the diagnosis of IC nodules has been reported in previous studies [6, 14–22]. As shown in Fig. 2, the analysis of the present data and the literature shows that our sensitivity (48%) and PPV (67%) was generally lower than in previous studies while NPV (91%) was moderately higher. These studies were conducted between 2009 and 2018, and included nodules with variable proportions of AUS/FLUS, FN/SFN or SM cytology, using Bethesda classification or not, resulting in a wide range of cancer prevalence from 16 to 56%. Similarly, the PTC rates were highly variable ranging from 1 to 56% as well as the proportions of BRAF mutated PTC tumours from 0 to 24%. Given its frequency, the presence of BRAF mutations significantly affects the results of the 7-panel testing in IC nodules. BRAF mutation is characteristic of PTC but only a part of them (45 to 80%) are BRAF mutated. This variable proportion is linked to pathological variants [23], classic PTC tumours being more often BRAF positive than other PTC variants, and to the geographical origin of patients with Koreans showing very high proportions of BRAF mutated PTC tumours [24]. The quite low sensitivity observed in the present study (48%) can be explained by a 16% cancer prevalence and a 7% PTC rate at the low end of the expected range. Nevertheless, the histological distribution of the study group is consistent with what we could expect from IC, namely a combination of classic PTC (19%), FVPTC (14%), FTC (14%), and HCC (14%) and PDTC (24%). As cancer prevalence also impacts NPV and PPV [25], this could have resulted in “underestimating” a 67% PPV and “overestimating” a 91% NPV.
The prevalence of PAX8/PPARγ rearrangements is generally limited in IC nodules with no cases reported in some studies [15, 18] and only one in others [17, 19]. Our data confirms that PAX8/PPARγ rearrangements are cancer specific. Nevertheless, whereas PAX8/PPARγ rearrangements were identified in three FVPTC and one FTC in Nikiforov’s study [6], in our series there were associated with two patients with HCC. The findings in the three HCC of our cohort (i.e., two cases with PAX8/PPARγ rearrangements and one wild-type tumour) were quite unexpected. Indeed, the prevalence of PAX8/PPARγ rearrangements is generally low in HCC, estimated at 5% in the review by Maximo et al [26] although rates up to 27% have already been reported [27]. The association between RET/PTC rearrangements and HCC is more prevalent and has been estimated at 35% [26]. Comprehensive analysis of the molecular landscape of HCC has very recently been achieved showing that these tumours exhibit a wide range of recurrent mutations, notably of the mitochondrial genome and high DNA copy-number alterations [28, 29].
In contrast, and as expected, RET/PTC rearrangements and RAS mutations presented more limited diagnostic values. No RET/PTC rearrangements were found in malignant tumours and one RET/PTC3 was detected in a benign nodule. The presence of RET/PTC rearrangements is possible in benign nodules and a recent systematic review in 2239 benign lesions from 38 studies showed a prevalence of RET/PTC rearrangements ranging from 0% to 68% [30]. A study performed in PTC tumours also suggests that the variability in the rate of RET/PTC rearrangement could also be related to the use of different detection methods and tumour genetic heterogeneity [31]. With respect to RAS mutations, they were detected in eight nodules, half of them corresponding to malignant lesions (2 FTC, 1 FVPTC, 1 PDTC) leading to a 50% PPV. Although comprehensive pathological data was available in a few previous studies [6, 15, 16], true-positive RAS mutations were observed mainly in FVPTC or FTC, and sometimes in classic PTC, HCC or PDTC. False positives were found in all previous studies, and the estimated PPV for RAS testing ranged from 13 to 92% [6, 15, 16]. As in previous studies, mutations were present in follicular adenomas. No non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) was found in our series. Recently, it has been reported that a substantial proportion (47%) of NIFTP could harbour NRAS mutations [32].
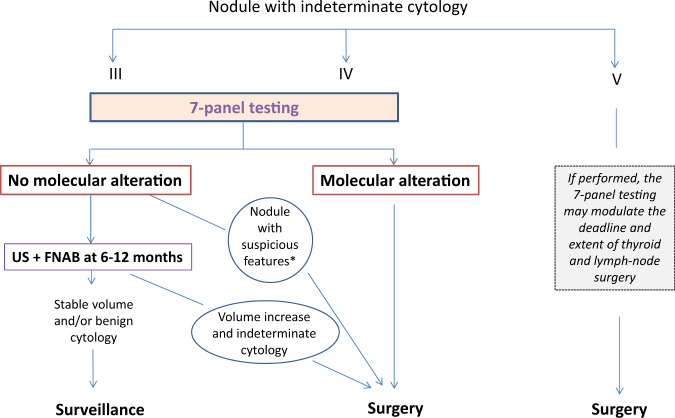
Based on our data and that of literature, a flow chart for the use of 7-panel testing to guide clinical decision in patients with IC nodules is proposed in Fig. 3. Given the generally high risk of cancer in Bethesda V nodules, and limited sensitivity of the 7-panel testing, surgery is recommended in all Bethesda V patients. If performed, genetic testing may modulate the extent of thyroid and lymph-node surgery. The similar performances of the 7-panel testing in Bethesda III and IV nodules suggest using it in both categories of patients. Surgery appears to be recommended when a molecular alteration is detected, particularly BRAF or PAX8/PPARγ which are highly specific for cancer. In the absence of molecular alteration, a US and FNAB control at 6–12 months could be performed, except for nodules suspicious of cancer for other reasons, e.g., history of radiation, serum calcitonin increase or EU-Tirads 5, which should be operated on. The absence of volume progression at 1 year, all the more if associated with a benign cytology, would allow continuing spaced monitoring.
Fig. 3.
Proposal for the use of 7-panel testing to guide clinical decision in patients with cytologically indeterminate nodules
An issue that has not been resolved to date is whether 7-panel molecular testing of cytology specimens could help predict long-term outcomes in the cancer patient subgroup. Our data show that the outcome of patients with or without molecular alterations was not different, suggesting that mutational testing in FNAB with 7-gene panel may play a minimal role in identifying high-risk cancers. Again, since the BRAF mutation is the most common alteration associated with aggressive behaviour in PTC [3], the fairly low rates of PTC and BRAF-mutated PTC in our series may have contributed to such negative results. Above all, there is evidence pointing out that the presence of multiple molecular alterations or mutations such as those of TERT [33], TP53 or PIK3CA [34, 35], that are not included in the 7-gene panel, have a higher prognostic value. In any event, this prognostic analysis has been performed in a limited number of patients and must be confirmed in larger series.
The strengths of the present study are its prospective design, the confirmation of IC by an independent review, the histological gold standard and the ability to assess the prognostic value of the mutational testing with a significant follow-up. The study also presents some limitations. One is that certain mutations of the RAS genes, notably HRAS codon 13 mutations previously reported in anaplastic thyroid cancer, and KRAS codon 61 in both PTC and PDTC, were not analyzed. This may have underestimated the number of positive cases although these mutations are uncommon [35]. Above all, a limitation was not having access to NGS multi-gene panel such as ThyroSeq v3 [36] or other recently reported technologies [37–39]. The use of multi-gene NGS panels makes it possible to analyse more than one hundred genes and to detect different classes of genetic alterations, including mutations, insertions and deletions, gene fusions, gene expression alterations and copy number variations to improve diagnostic accuracy and potentially prognostic value of genetic testing on cytologically indeterminate nodules. When the study was launched, the NGS multi-gene panel was not available. The cost of NGS panels remains a critical problem, raised in the same way by the genomic tests currently available. In countries or institutions subject to financial constraints, and before the widespread use of NGS technics, this would support a relevant role in clinical practice for a small panel of genes.
In conclusion, this prospective study confirms that the 7-panel mutation testing is a simple and low-cost tool to help the clinician manage patients with cytologically indeterminate nodules. In the subgroup of cancer patients, however, mutational testing has not been shown to have a significant prognostic value.
Acknowledgements
We are indebted to all clinical research associates working in each centre, especially Chantal Rieux, Theary Cheav and Paul Ihout. We are also grateful to Helen Lapasset for her assistance in reviewing the manuscript.
Funding
The study was supported by grants from the “Fondation de l’Avenir” and the French Society of Endocrinology.
Compliance with ethical standards
Ethical approval
This study was approved by the Local Ethics Committee and the French Health Authorities, and was conducted according to the provisions of the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference of Harmonization. Written informed consent was obtained from all patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baloch ZW, Livolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, Vielh P, DeMay RM, Sidawy MK, Frable WJ. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn. Cytopathol. 2008;36:425–437. doi: 10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 2.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O’Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paschke R, Cantara S, Crescenzi A, Jarzab B, Musholt TJ, Sobrinho SM. European thyroid association guidelines regarding thyroid nodule molecular fine-needle aspiration cytology diagnostics. Eur. Thyroid J. 2017;6:115–129. doi: 10.1159/000468519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SK, Hwang TS, Yoo YB, Han HS, Kim DL, Song KH, Lim SD, Kim WS, Paik NS. Surgical results of thyroid nodules according to a management guideline based on the BRAFV600E mutation status. J. Clin. Endocrinol. Metab. 2011;96:658–664. doi: 10.1210/jc.2010-1082. [DOI] [PubMed] [Google Scholar]
- 6.Nikiforov YE, Ohori NP, Hodak SP, Carty SE, Lebeau SO, Ferris RL, Yip L, Seethala RR, Tublin ME, Stang MT, Coyne C, Johnson JT, Stewart AF, Nikiforova MN. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metab. 2011;96:3390–3397. doi: 10.1210/jc.2011-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, Gooding WE, Lebeau SO, Ohori NP, Seethala RR, Tublin ME, Yip L, Nikiforova MN. Impact of the multi-gene thyroseq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid. 2015;25:1217–1223. doi: 10.1089/thy.2015.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steward DL, Carty SE, Sippel RS, Yang SP, Sosa JA, Sipos JA, Figge JJ, Mandel S, Haugen BR, Burman KD, Baloch ZW, Lloyd RV, Seethala RR, Gooding WE, Chiosea SI, Gomes-Lima C, Ferris RL, Folek JM, Khawaja RA, Kundra P, Loh KS, Marshall CB, Mayson S, McCoy KL, Nga ME, Ngiam KY, Nikiforova MN, Poehls JL, Ringel MD, Yang H, Yip L, Nikiforov YE. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol. 2019;5:204–212. doi: 10.1001/jamaoncol.2018.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel KN, Angell TE, Babiarz J, Barth NM, Blevins T, Duh QY, Ghossein RA, Harrell RM, Huang J, Kennedy GC, Kim SY, Kloos RT, Livolsi VA, Randolph GW, Sadow PM, Shanik MH, Sosa JA, Traweek ST, Walsh PS, Whitney D, Yeh MW, Ladenson PW. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg. 2018;153:817–824. doi: 10.1001/jamasurg.2018.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endo M, Nabhan F, Porter K, Roll K, Shirley LA, Azaryan I, Tonkovich D, Perlick J, Ryan LE, Khawaja R, Meng S, Phay JE, Ringel MD, Sipos JA. Afirma gene sequencing classifier compared with gene expression classifier in indeterminate thyroid nodules. Thyroid. 2019;29:1115–1124. doi: 10.1089/thy.2018.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardet S, Ciappuccini R, Pellot-Barakat C, Monpeyssen H, Michels JJ, Tissier F, Blanchard D, Menegaux F, de RD, Lefort M, Reznik Y, Rouxel A, Heutte N, Brenac F, Leconte A, Buffet C, Clarisse B, Leenhardt L. Shear wave elastography in thyroid nodules with indeterminate cytology: results of a prospective bicentric study. Thyroid. 2017;27:1441–1449. doi: 10.1089/thy.2017.0293. [DOI] [PubMed] [Google Scholar]
- 12.Pathology and genetics of tumours of endocrin organs. Word Health Organization Classification of Tumours. Lyon 2004: IARC Press (2011)
- 13.Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur. Thyroid J. 2017;6:225–237. doi: 10.1159/000478927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labourier E, Shifrin A, Busseniers AE, Lupo MA, Manganelli ML, Andruss B, Wylie D, Beaudenon-Huibregtse S. Molecular testing for miRNA, mRNA, and DNA on fine-needle aspiration improves the preoperative diagnosis of thyroid nodules with indeterminate cytology. J. Clin. Endocrinol. Metab. 2015;100:2743–2750. doi: 10.1210/jc.2015-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eszlinger M, Piana S, Moll A, Bosenberg E, Bisagni A, Ciarrocchi A, Ragazzi M, Paschke R. Molecular testing of thyroid fine-needle aspirations improves presurgical diagnosis and supports the histologic identification of minimally invasive follicular thyroid carcinomas. Thyroid. 2015;25:401–409. doi: 10.1089/thy.2014.0362. [DOI] [PubMed] [Google Scholar]
- 16.Eszlinger M, Krogdahl A, Munz S, Rehfeld C, Precht Jensen EM, Ferraz C, Bosenberg E, Drieschner N, Scholz M, Hegedus L, Paschke R. Impact of molecular screening for point mutations and rearrangements in routine air-dried fine-needle aspiration samples of thyroid nodules. Thyroid. 2014;24:305–313. doi: 10.1089/thy.2013.0278. [DOI] [PubMed] [Google Scholar]
- 17.Beaudenon-Huibregtse S, Alexander EK, Guttler RB, Hershman JM, Babu V, Blevins TC, Moore P, Andruss B, Labourier E. Centralized molecular testing for oncogenic gene mutations complements the local cytopathologic diagnosis of thyroid nodules. Thyroid. 2014;24:1479–1487. doi: 10.1089/thy.2013.0640. [DOI] [PubMed] [Google Scholar]
- 18.Cantara S, Capezzone M, Marchisotta S, Capuano S, Busonero G, Toti P, Di Santo A, Caruso G, Carli AF, Brilli L, Montanaro A, Pacini F. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J. Clin. Endocrinol. Metab. 2010;95:1365–1369. doi: 10.1210/jc.2009-2103. [DOI] [PubMed] [Google Scholar]
- 19.Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, Fagin JA, Falciglia M, Weber K, Nikiforova MN. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J. Clin. Endocrinol. Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 20.Bongiovanni M, Molinari F, Eszlinger M, Paschke R, Barizzi J, Merlo E, Giovanella L, Fasolini F, Cattaneo F, Ramelli F, Mazzucchelli L, Frattini M. Laser capture microdissection is a valuable tool in the preoperative molecular screening of follicular lesions of the thyroid: an institutional experience. Cytopathology. 2015;26:288–296. doi: 10.1111/cyt.12226. [DOI] [PubMed] [Google Scholar]
- 21.Mancini I, Pinzani P, Pupilli C, Petrone L, De Feo ML, Bencini L, Pazzagli M, Forti G, Orlando C. A high-resolution melting protocol for rapid and accurate differential diagnosis of thyroid nodules. J. Mol. Diagn. 2012;14:501–509. doi: 10.1016/j.jmoldx.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Bellevicine C, Sgariglia R, Migliatico I, Vigliar E, D’Anna M, Nacchio MA, Serra N, Malapelle U, Bongiovanni M, Troncone G. Different qualifiers of AUS/FLUS thyroid FNA have distinct BRAF, RAS, RET/PTC, and PAX8/PPARg alterations. Cancer Cytopathol. 2018;126:317–325. doi: 10.1002/cncy.21984. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J. Clin. Endocrinol. Metab. 2012;97:4559–4570. doi: 10.1210/jc.2012-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong AR, Lim JA, Kim TH, Choi HS, Yoo WS, Min HS, Won JK, Lee KE, Jung KC, Park DJ, Park YJ. The frequency and clinical implications of the BRAF(V600E) mutation in papillary thyroid cancer patients in korea over the past two decades. Endocrinol. Metab. 2014;29:505–513. doi: 10.3803/EnM.2014.29.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vargas-Salas S, Martinez JR, Urra S, Dominguez JM, Mena N, Uslar T, Lagos M, Henriquez M, Gonzalez HE. Genetic testing for indeterminate thyroid cytology: review and meta-analysis. Endocr. Relat. Cancer. 2018;25:R163–R177. doi: 10.1530/ERC-17-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maximo V, Lima J, Prazeres H, Soares P, Sobrinho-Simoes M. The biology and the genetics of Hurthle cell tumors of the thyroid. Endocr. Relat. Cancer. 2012;19:R131–R147. doi: 10.1530/ERC-11-0354. [DOI] [PubMed] [Google Scholar]
- 27.de Vries MM, Celestino R, Castro P, Eloy C, Maximo V, van der Wal JE, Plukker JT, Links TP, Hofstra RM, Sobrinho-Simoes M, Soares P. RET/PTC rearrangement is prevalent in follicular Hurthle cell carcinomas. Histopathology. 2012;61:833–843. doi: 10.1111/j.1365-2559.2012.04276.x. [DOI] [PubMed] [Google Scholar]
- 28.Gopal RK, Kubler K, Calvo SE, Polak P, Livitz D, Rosebrock D, Sadow PM, Campbell B, Donovan SE, Amin S, Gigliotti BJ, Grabarek Z, Hess JM, Stewart C, Braunstein LZ, Arndt PF, Mordecai S, Shih AR, Chaves F, Zhan T, Lubitz CC, Kim J, Iafrate AJ, Wirth L, Parangi S, Leshchiner I, Daniels GH, Mootha VK, Dias-Santagata D, Getz G, McFadden DG. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in hurthle cell carcinoma. Cancer Cell. 2018;34:242–255. doi: 10.1016/j.ccell.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganly I, Makarov V, Deraje S, Dong Y, Reznik E, Seshan V, Nanjangud G, Eng S, Bose P, Kuo F, Morris LGT, Landa I, Carrillo Albornoz PB, Riaz N, Nikiforov YE, Patel K, Umbricht C, Zeiger M, Kebebew E, Sherman E, Ghossein R, Fagin JA, Chan TA. Integrated genomic analysis of hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell. 2018;34:256–270. doi: 10.1016/j.ccell.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Najafian A, Noureldine S, Azar F, Atallah C, Trinh G, Schneider EB, Tufano RP, Zeiger MA. RAS mutations, and RET/PTC and PAX8/PPAR-gamma chromosomal rearrangements are also prevalent in benign thyroid lesions: implications thereof and a systematic review. Thyroid. 2017;27:39–48. doi: 10.1089/thy.2016.0348. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J. Clin. Endocrinol. Metab. 2006;91:3603–3610. doi: 10.1210/jc.2006-1006. [DOI] [PubMed] [Google Scholar]
- 32.Kim M, Jeon MJ, Oh HS, Park S, Kim TY, Shong YK, Kim WB, Kim K, Kim WG, Song DE. BRAF and RAS mutational status in noninvasive follicular thyroid neoplasm with papillary-like nuclear features and invasive subtype of encapsulated follicular variant of papillary thyroid carcinoma in Korea. Thyroid. 2018;28:504–510. doi: 10.1089/thy.2017.0382. [DOI] [PubMed] [Google Scholar]
- 33.Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, Castro P, Prazeres H, Lima J, Amaro T, Lobo C, Martins MJ, Moura M, Cavaco B, Leite V, Cameselle-Teijeiro JM, Carrilho F, Carvalheiro M, Maximo V, Sobrinho-Simoes M, Soares P. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014;99:E754–E765. doi: 10.1210/jc.2013-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haroon Al Rasheed MR, Xu B. Molecular alterations in thyroid carcinoma. Surg. Pathol. Clin. 2019;12:921–930. doi: 10.1016/j.path.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, Schweppe RE, Fishbein L, Ross JS, Haugen BR, Bowles DW. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin. Cancer Res. 2018;24:3059–3068. doi: 10.1158/1078-0432.CCR-18-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikiforova MN, Mercurio S, Wald AI, Barbi de MM, Callenberg K, Santana-Santos L, Gooding WE, Yip L, Ferris RL, Nikiforov YE. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018;124:1682–1690. doi: 10.1002/cncr.31245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sponziello M, Brunelli C, Verrienti A, Grani G, Pecce V, Abballe L, Ramundo V, Damante G, Russo D, Lombardi CP, Durante C, Rossi ED, Straccia P, Fadda G, Filetti S. Performance of a dual-component molecular assay in cytologically indeterminate thyroid nodules. Endocrine. 2020;68:458–465. doi: 10.1007/s12020-020-02271-y. [DOI] [PubMed] [Google Scholar]
- 38.Ablordeppey KK, Timmaraju VA, Song-Yang JW, Yaqoob S, Narick C, Mireskandari A, Finkelstein SD, Kumar G. Development and analytical validation of an expanded mutation detection panel for next-generation sequencing of thyroid nodule aspirates. J. Mol. Diagn. 2020;22:355–367. doi: 10.1016/j.jmoldx.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 39.De Biase D, Acquaviva G, Visani M, Sanza V, Argento CM, De LA, Maloberti T, Pession A, Tallini G. Molecular diagnostic of solid tumor using a next generation sequencing custom-designed multi-gene panel. Diagnostics. 2020;10:250. doi: 10.3390/diagnostics10040250. [DOI] [PMC free article] [PubMed] [Google Scholar]