Abstract
It is well known that the heart and kidney and their synergy is essential for hemodynamic homeostasis. Since the early XIX century it has been recognized that cardiovascular and renal diseases frequently coexist. In the nephrological field, while it is well accepted that renal diseases favor the occurrence of cardiovascular diseases, it is not always realized that cardiovascular diseases induce or aggravate renal dysfunctions, in this way further deteriorating cardiac function and creating a vicious circle. In the same clinical field, the role of venous congestion in the pathogenesis of renal dysfunction is at times overlooked. This review carefully quantifies the prevalence of chronic and acute kidney abnormalities in cardiovascular diseases, mainly heart failure, regardless of ejection fraction, and the consequences of renal abnormalities on both organs, making cardiovascular diseases a major risk factor for kidney diseases. In addition, with regard to pathophysiological aspects, we attempt to substantiate the major role of fluid overload and venous congestion, including renal venous hypertension, in the pathogenesis of acute and chronic renal dysfunction occurring in heart failure. Furthermore, we describe therapeutic principles to counteract the major pathophysiological abnormalities in heart failure complicated by renal dysfunction. Finally, we underline that the mild transient worsening of renal function after decongestive therapy is not usually associated with adverse prognosis. Accordingly, the coexistence of cardiovascular and renal diseases inevitably means mediating between preserving renal function and improving cardiac activity to reach a better outcome.
Keywords: Cardiovascular disease, Heart failure, Venous congestion, Worsening renal function, Acute kidney injury, Chronic kidney disease
Introduction
The heart and kidney are essential for cardiovascular (CV) homeostasis. Cardiac activity provides blood and oxygen to all the organs of the body, whereas the kidney plays a key role in the maintenance of fluid, electrolyte and acid–base equilibrium, in hemoglobin synthesis as well as in the clearance of metabolic waste products. Maintenance of hemodynamic homeostasis depends on many complex and delicate interactions between the heart and kidney [1]. This interaction is fine-tuned by neurohormonal activity, including renin–angiotensin–aldosterone system (RAAS), sympathetic nervous system (SNS) and atrial natriuretic peptides (ANP).
In the early 1800s, Richard Bright described for the first time the association between cardiac and kidney diseases [2], confirmed one century later [3]. Today, there is more awareness of the renal consequences of cardiovascular disorders (CVD) and vice versa, as well as of the accelerated progression of both organ failures influenced by the bidirectional heart-kidney interactions. The frequent coexistence of CV and kidney diseases has led to a proposal of cardiorenal syndromes (CRS) defined as “a complex pathophysiological disorder of the heart and the kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ” [4]. This classification provides a clinically oriented descriptive definition, however not yet tested in clinical practice or in clinical trials [5–7].
Even though renal dysfunctions in CVD are mainly the result of hemodynamic changes and neurohormonal activation, in clinical practice cardiorenal interactions are more complex for many reasons. In fact, among them the coexistence of CV and renal dysfunction often may be the result of shared traditional CV risk factors, such as hypertension, diabetes mellitus, obesity, lipid disorders and smoking, or of non-traditional CV risk factors such as inflammation, bone and mineral disorders, anemia and malnutrition [7–9].
In the nephrological field, while it is well known that when kidney diseases are the primary event, they favor the occurrence of CVD, it is less appreciated that when CVD are the initiating event they induce or aggravate renal dysfunctions that in turn are associated with further CV deterioration. In addition, in the clinical nephrology community the pathophysiology of renal dysfunctions in CVD is traditionally associated with reduced arterial renal perfusion and the role of renal congestion is at times overlooked.
In this review, renal dysfunctions in CVD, their renal and cardiac consequences and their related mechanisms will be discussed in the following dedicated sections.
CVD and renal consequences
CV abnormalities, even subclinical ones, are frequently associated with a preexistent or de novo chronic kidney disease (CKD): i.e., estimated glomerular filtration rate (eGFR) < 60 ml/min and/or albuminuria or proteinuria, which can progress to end stage renal disease (ESRD) and favor CV morbidity and mortality (so-called chronic CRS). However, in acute CVD it is often difficult to discriminate the preexisting chronic renal abnormalities from the acute renal dysfunctions [10]. In fact, acute CVD are frequently associated also with acute worsening of renal function (WRF) or acute kidney injury (AKI) (so called acute CRS), even if their incidence is not rare in chronic CVD.
Association of CVD with baseline CKD
CKD is found in 6–12% of the general population [11] but is up to at least five times more frequent in patients with CVD. The prevalence of CKD is a little lower in clinical trials that usually exclude more severe CKD (serum creatinine ≥ 2–3 mg/dl), so population-based studies provide more reliable data. In analyzed papers, glomerular filtration rate (GFR) is estimated by Cockroft and Gault, MDRD or CKD-EPI formulae [12–14].
The prevalence of baseline GFR < 60 ml/min has been reported in around 40–60% of patients with chronic heart failure (CHF) with both preserved or reduced ejection fraction [15–23], and in about 30–40% of patients with stable coronary artery disease (CAD), cerebrovascular or peripheral artery disease (PAD) [24, 25]. In these patients CKD usually precedes or coincides with the “onset” of heart failure (HF). Results of individual studies on the so-called chronic CRS are either meta-analyzed or reviewed [26–28].
The prevalence of GFR < 60 is similar or even higher in acute CVD, particularly in decompensated acute heart failure (AHF, 50–70%) [29–34], in acute coronary syndromes (ACS, 25–50%) [35–38], or in strokes (25–30%) [39, 40]. Again, the results of many studies are either meta-analyzed or reviewed [27, 28].
The prevalence of abnormal albuminuria or proteinuria is high in CHF (25–50%) [20, 41–44], and in ACS (15–20%) [45] relative to the general population (7%) [46]. Interestingly, even if in most patients albuminuria/proteinuria were associated with GFR < 60, many albuminuric or proteinuric patients had GFR ≥ 60, thus increasing the dimension of CKD [20, 42, 44, 47].
Progression of preexistent CKD or de novo CKD in CVD
In patients with baseline CKD, the presence of stable CVD or subclinical CV abnormalities [left ventricular hypertrophy (LVH), augmented intima-media thickness or aortic calcifications] was associated with a more rapid progression of CKD, leading even to ESRD [48–53].
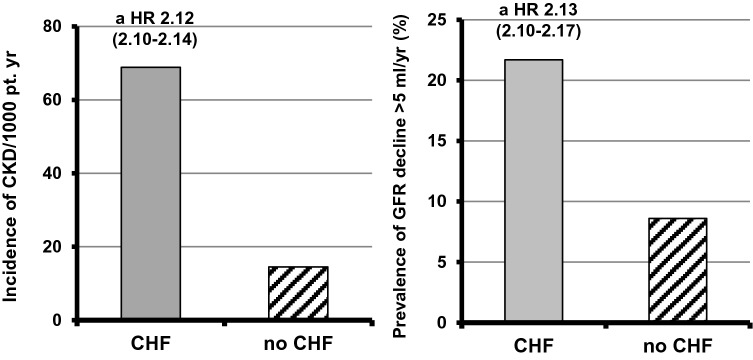
Elsayed and colleagues [48] firstly demonstrated that stable CVD are also independently associated with increased development of new CKD, an observation that was recently confirmed in CHF in a very large cohort of patients with normal basal GFR [54] (Fig. 1). Also subclinical abnormalities of the heart (i.e., LVH) or PAD were significantly associated with a faster decline in eGFR with the occurrence of de novo CKD [50, 54–57].
Fig. 1.
Incidence of CKD or GFR decline > 5 ml/min/year in patients with chronic heart failure (CHF) (156,743) or without CHF (3,414,122) (f up 3.6 year)
(Drawn from data by George LK et al. Circ Heart Fail 2017 [54])
Increased CV morbidity and mortality by CKD in CVD
GFR < 60 and its decline are independently associated with new CV events, rehospitalization and short- and long-term mortality in CHF [15–23, 26, 28, 58] and in chronic CAD, cerebrovascular disease or PAD [24, 25]. Also in AHF, ACS or acute stroke, GFR < 60 is significantly and independently associated with rehospitalization and short- and long-term mortality [29–33, 35–37, 39, 40]. The same was true for albuminuria or proteinuria in CHF [20, 41–44, 47] or ACS [45] even after adjusting for GFR. In summary, in CVD, CKD is frequently the most powerful predictor of morbidity and mortality, particularly when also the ratio of blood urea nitrogen to creatinine is higher than the normal range [59, 60].
Incidence of WRF or AKI in CVD and their consequences
In CVD, in addition to baseline CKD, WRF or AKI are frequently observed mainly in hospitalized patients. WRF is arbitrarily defined as acute serum creatinine increased by ≥ 0.5 mg/dl or alternatively by ≥ 0.3 mg/dl sometimes associated with a creatinine increase by ≥ 25% [6, 61–66]. AKI is defined according to RIFLE, AKIN or KDIGO criteria [67–69]. In AHF, the use of different criteria seems to provide similar results in identifying acute renal dysfunction, its severity and also its capacity in predicting mortality [70, 71].
These acute complications are reported in about 15% of hospitalized patients with CHF [18, 28, 72] and more frequently in AHF (10–50%) [28, 34, 71, 73–79]. WRF/AKI are also reported in ACS (10–20%) [35, 37, 38, 63, 70, 80]. About 1/3–2/3 of acute renal dysfunctions are transient [10, 34, 76, 77, 80–83].
In patients with CVD, not only baseline CKD, but also acute renal events are independently associated with rehospitalization and short- and long-term mortality in the above reported studies. Nevertheless, not all increases in serum creatinine have the same meaning and prognosis. In fact, in persistent WRF/AKI, the greater the severity of renal dysfunction, the greater the increase in mortality [10, 34, 35, 78, 81–83], even though in recent studies persistent WRF was significantly associated with mortality only in patients with residual congestion [73, 76, 78] (Fig. 2). Transient WRF was also associated with mortality, though this outcome occurred less often than in patients with persistent WRF [34, 35, 77, 81, 82]. In other studies, transient WRF was not significantly associated with increased mortality [10, 76, 83] particularly when mild [35].
Fig. 2.
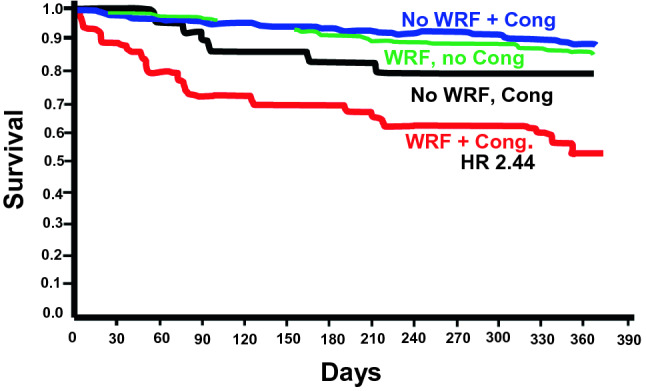
One-year death or urgent heart transplantation in acute heart failure (AHF) (594 patients) on the basis of worsening renal function (WRF) and signs of congestion at discharge
(Adapted from Metra M et al. Circ Heart Fail 2012 [73])
Interestingly, a transient, mild WRF/AKI after patient decongestion may reflect adequate treatment and not necessarily a worsening of prognosis [73–76, 78, 84, 85] as frequently happens for WRF early after the initiation of RAAS inhibitors [86, 87]. Notably, one study found that even mild WRF/AKI were associated with long-term ESRD [63].
Pathophysiology of renal dysfunctions in CVD and their consequences
CKD in subclinical CVD or coronary, cerebrovascular and peripheral artery disease without heart failure (HF)
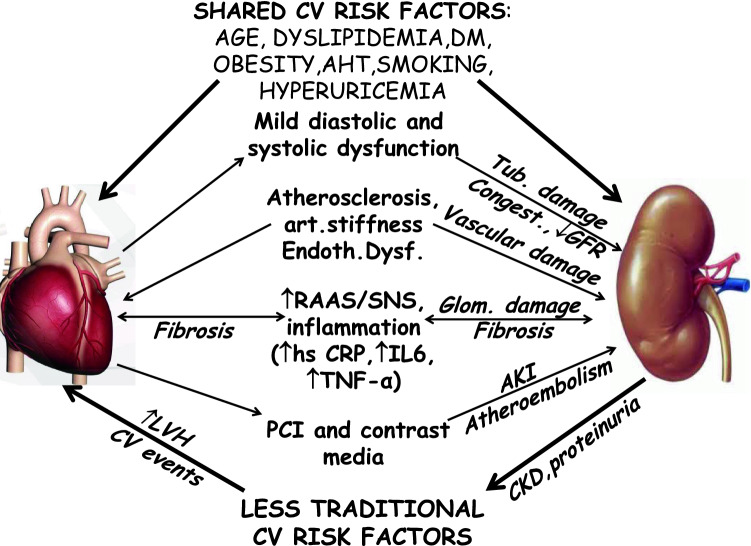
Mechanisms of de novo CKD in these patients are poorly understood (Fig. 3). In long-term LVH (particularly if concentric) or stable CVD, shared traditional CV risk factors are frequently present [8, 48].
Fig. 3.
Pathophysiology of cardiorenal syndrome in subclinical cardiovascular disorders (CVD) or coronary, cerebrovascular and peripheral artery diseases without heart failure (HF)
In addition, endothelial dysfunction, arterial stiffness and arteriosclerosis may affect renal vasculature [48, 49, 55, 88, 89]. In these patients, hemodynamic mechanisms, even an initial mild systolic and diastolic dysfunction, could have a consistent role [48, 50, 90, 91]. They can determine mild renal hypoperfusion and congestion with consequent subclinical inflammation and neurohormonal activation with tubular damage, glomerular (and also cardiac) fibrosis and proteinuria [50, 55]. An additional contribution could derive from conventional drug toxicity, and/or from occasional percutaneous interventions and contrast media [35, 36, 38, 48, 81]. Once CKD has been established, less traditional (CKD dependent) CV risk factors (Table 1) and the underuse of cardioprotective drugs and procedures can have an important role in worsening heart function [7, 9, 36, 38].
Table 1.
Less traditional cardiovascular (CV) risk factors more frequent in chronic kidney disease (CKD)
| Hyperactivation of RAAS |
| Sympathetic over-reactivity |
| Insufficient pressure-natriuresis (with consequent volume overload, arterial hypertension, venous congestion and heart failure) |
| CKD-related mineral and bone disorders( CKD-MBD): ↑ P, ↑ FGF23,↓ Kloto, ↑ PTH, ↑ propensity for vascular calcifications, Vitamin D deficiency |
| Endothelial dysfunction and nitric oxide inhibition |
| Atherosclerosis, intima media thickness, arterial stiffness |
| Inflammation (↑ CRP,↑ TNF-α, ↑ fibrinogen, ↑ Cytokines) and malnutrition |
| Accumulation of uremic toxins (ADMA, p-cresyl sulfate, indoxyl-sulfate, indole-3 acetic acid, trimethylamine N-oxide, etc.) |
| Hyperhomocysteinemia |
| Anemia (↓ EPO, iron depletion) |
| ↑ Uric acid levels |
| Low or extremely high bicarbonate levels |
| Uremic dyslipidemia |
ADMA asymetric dimethylarginine, CRP C-reactive protein, FGF23 fibroblast growth factor 23, EPO erythropoietin, PTH parathyroid hormone, RAAS renin–angiotensin–aldosterone system, TNF-α tumor necrosis factor-α
Renal dysfunctions in HF
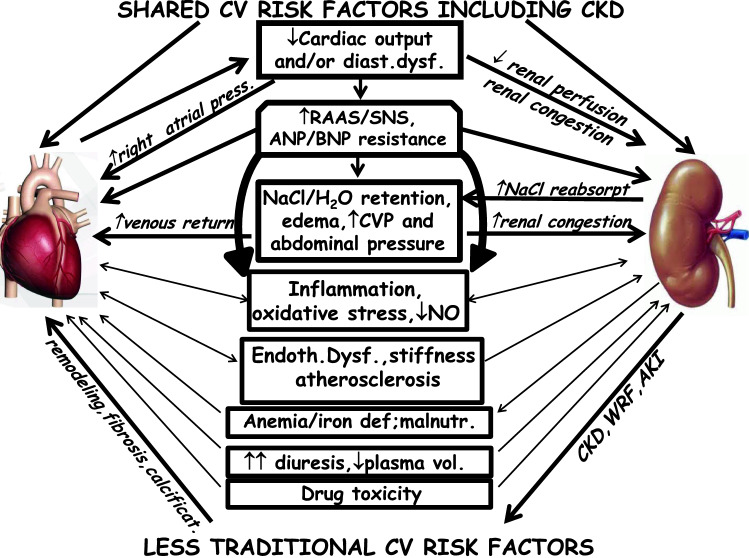
In HF the pathophysiology of renal dysfunction is complicated and multifactorial [6, 9, 66, 92–102] (Fig. 4).
Fig. 4.
Pathophysiology of cardiorenal syndrome in heart failure (HF)
Six categories of factors mainly contribute to renal and also cardiac outcomes in HF:
shared traditional CV and renal risk factors;
hemodynamic abnormalities due to systolic and/or diastolic dysfunction and congestion;
impaired atrial contribution to diastolic ventricular filling in the case of atrial fibrillation
SNS activation and the triggering of the RAAS and vasopressin;
other factors such as inflammation, atherosclerosis, arterial stiffness and endothelial dysfunction, anemia ± iron deficiency, malnutrition, drug and procedure toxicity, in particular diuretic excess, and underuse of cardioprotective drugs;
less traditional CV risk factors associated with CKD, including low GFR, (Table 1) and with vascular and valvular calcifications further worsening the heart condition.
GFR is determined by the pressure gradient between glomerular capillaries and the Bowman space according to the formula: GFR = Kf[Pgc − Pbc] − [πgc − πbc] where Kf = filtration constant, Pgc = capillary hydrostatic pressure, Pbc = Bowman hydrostatic pressure, πgc = capillary oncotic pressure and πbc = Bowman oncotic pressure. According to this relationship, GFR is commonly reduced when Pgc is reduced (hypotension, low renal perfusion) and/or Pbc is increased (ureteral obstruction, renal congestion) [103, 104].
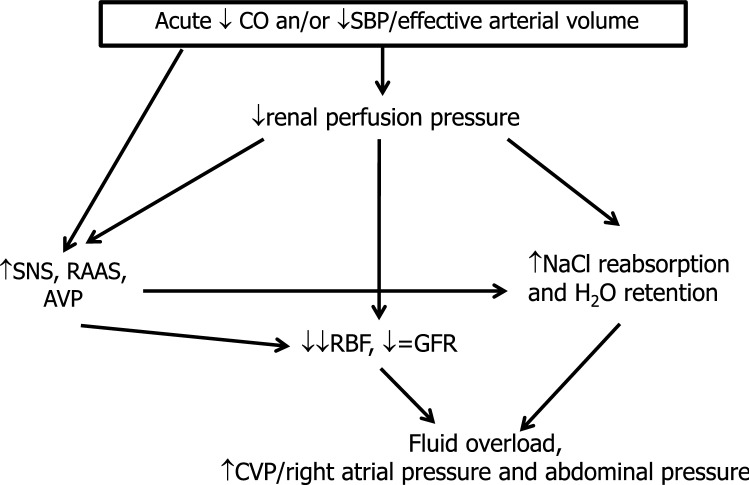
According to the “low flow” or “forward failure” theory, in patients with HF with severe reduction of cardiac output, particularly when systolic blood pressure (SBP)/ effective arterial volume are reduced, renal perfusion pressure and renal blood flow (RBF) are reduced as well as GFR. SNS, RAAS, non-osmotic vasopressin and NO depletion are the most important mediators of intrarenal mechanisms of adaptation (Fig. 5) [6, 9, 92, 94–96, 105–109].
Fig. 5.
Impact of acute reduction in cardiac output (CO) and/or in systolic blood pressure (SBP)/effective arterial volume on renal function in heart failure (HF) (forward mechanism). AVP arginine vasopressin, CVP central venous pressure, GFR glomerular filtration rate, RAAS renin–angiotensin–aldosterone system, RBF renal blood flow, SNS sympathetic nervous system
Interestingly, in mild reduction of cardiac output, GFR is maintained at an almost constant rate by an increased filtration fraction through intrinsic renal autoregulatory mechanisms such as afferent vasodilatation and predominant vasoconstriction of the efferent arteriolae with a secondary increase in postglomerular resistance. Both afferent vasodilatation and efferent vasoconstriction increase capillary hydrostatic pressure thereby counteracting the reduced renal perfusion. However, in severe reduction of cardiac output, vasoconstriction of also the afferent arteriolae ensues with an increase in preglomerular resistance, and the renal autoregulatory capacity is exhausted with a marked decrease in glomerular perfusion pressure and GFR. In this setting, non-hemodynamic factors such as inflammatory cytokine release, oxidative stress and endothelial dysfunction worsen the hemodynamic disorders and cooperate in further alterations of GFR.
The above-reported activation of neurohormonal axis directly and indirectly enhances also tubular reabsorption of NaCl and water, thus worsening fluid overload and congestion even in the presence of only mild reduction in cardiac output [108, 110–112]. Eventually, acute renal dysfunctions or even acute tubular necrosis could occur; tubulo-interstitial fibrosis and glomerulosclerosis resulting in worsening of renal function in CKD patients, leading to ESRD could be long-term consequences [94, 106, 113]. Thus, while the kidneys help to maintain homeostasis in healthy subjects, in HF they contribute to worsening CRS. Interestingly, similar responses are seen in HF with normal or increased cardiac output where neurohormonal adaptation, salt reabsorption and consequent blood volume expansion initially preserve renal perfusion [114].
Recent clinical data have shown that in persistent mild CHF or even in severe or acute cases, low cardiac output (“forward failure”) is not the major determinant of renal abnormalities but a great role is played by “backward failure”; this is particularly evident in right ventricular failure and/or in tricuspid regurgitation [115–118]. In fact, in HF, no correlation has been found between cardiac index and the reduction in GFR which is more closely associated with elevated central venous pressure or right atrial pressure even if their relationships are complex particularly in AHF [6, 96, 99, 100, 104, 119–128].
Interestingly, renal congestion detected by intraparenchymal Doppler venous pattern shows an independent and incremental role in predicting a worse outcome in CHF outpatients [129, 130] and perhaps WRF/AKI [131–133]. Moreover, it has been shown that backward failure impairs GFR preferentially in the presence of forward failure including low SBP [118, 119, 134–137].
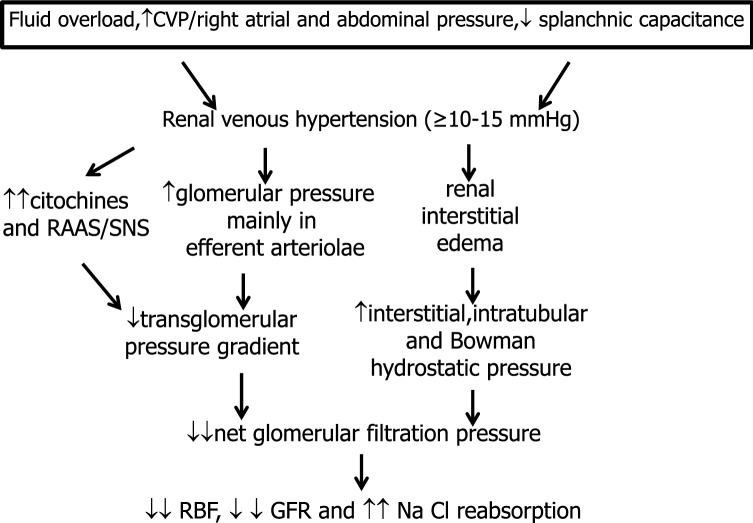
Venous congestion in HF depends on fluid overload and/or cardiac dysfunction sometimes with the contribution of a transient decreased splanchnic capacity independent of fluid overload [109, 128, 138–140]. From half to two thirds of patients with AHF experienced clinical signs of congestion and/or no significant loss of weight during hospitalization and both were associated with significant adjusted increase in mortality [121, 123, 141–147] and in WRF [119, 121–123, 148].
Venous congestion affects renal veins where an increase in pressure ≥ 10–15 mmHg further alters glomerular hemodynamics, renal resistances, NaCl reabsorption and renin and inflammatory cytokine release [149–154]. Furthermore, in severe HF, the increased intra-abdominal pressure secondary to visceral edema and ascites further increases renal venous pressure as well as neurohormonal activation with consequent additional deterioration of GFR and sodium and water excretion [6, 94, 125, 128, 150, 155–159].
In fact, it was already demonstrated that humans with CHF have renal venous hypertension [151]: renal vein pressure was about 25 cm H2O (15–33) versus control values of 15 cm H2O (0.8–18). In those patients afferent, efferent and total renal resistance were markedly increased according to constriction of glomerular arteriolae, and both RBF and GFR were substantially decreased [151]. Interestingly, Bradley et al. in those years showed that in healthy subjects the experimental increase of renal vein pressure from 3–8 mmHg to 14–22 mmHg decreases urine flow and both RBF and GFR [150].
In the same period, several studies in experimental animals, in which renal venous pressure was increased to values observed in Maxwell’s patients, confirmed the reduction in urine flow, NaCl excretion, RBF and also GFR after a substantial increase in renal venous pressure [149, 160, 161]. These data were subsequently confirmed in non oliguric animals [162–164]. Notably, results in acute experiments are confirmed in dogs with renal vein hypertension of 3–4 weeks of duration [163]. In other experimental studies conducted in the same period it was shown that the increase in renal vein pressure similar to values observed in CHF, linearly increased interstitial, intratubular and Bowman hydrostatic pressure [153, 164–168], as well as renal vascular resistance in non denervated kidneys [169–171]. Similar experimental increases in renal venous pressure increased renin and aldosterone release [152, 156, 172, 173] as well as proteinuria [149, 173].
Recently, a reduction in RBF and GFR and an increase in interstitial hydrostatic pressure were observed in the congested kidney with a novel rat model of renal congestion [174]. Three days of renal congestion induced glomerular and tubular interstitial injury triggered by pericyte loss [174].
In summary, in HF, forward and backward mechanisms frequently coexist and are strictly interconnected. The importance of congestion for explaining renal dysfunction is in part reported in many reviews [6, 93–96, 102, 104, 125, 127, 128, 139, 158, 159, 175] even though the mechanisms of the adverse effects of congestion on renal function are not fully elucidated. Clinical and physiological data reported in humans and also in experimental animals allow us to substantiate the concept that renal venous hypertension, together with SNS/RAAS activation, increases glomerular pressure in the efferent pole of glomerular capillaries (thus decreasing the A-V pressure gradient), and favors interstitial edema of the encapsulated kidney with an increase in interstitial, intratubular and Bowman hydrostatic pressure. As a consequence, the net filtration pressure is further reduced and consequently so is GFR; moreover, NaCl reabsorption is further increased and a vicious cycle is generated thereby worsening both cardiac and renal function (Figs. 5, 6). The renal effects of congestion are particularly evident in the presence of reduced cardiac output and/or SBP.
Fig. 6.
Impact of congestion on kidney function in heart failure (HF) (backward mechanism). CVP central venous pressure, GFR glomerular filtration rate, RAAS renin–angiotensin–aldosterone system, RBF renal blood flow, SNS sympathetic nervous system
In these patients, even a mild reduction in cardiac output also increases the pressure in the right atrium, the ratio between right atrial pressure to pulmonary capillary wedge pressure and also increases the venous return owing to the fluid overload. Accordingly, the right cardiac filling pressure is further increased and the left ventricle is relatively underfilled (in diastole) with consequent further impairment of forward output [98, 118, 137, 176, 177].
As reported above (Table 1), also CV risk factors due to CKD or vascular or valvular calcification contribute to cardiac and renal damage and mortality [7–9].
Acute renal dysfunctions (WRF/AKI) in CVD
In CVD, WRF/AKI, particularly if persistent, are markers of a severe setting in which both severe HF and CKD frequently coexist, which makes patients particularly vulnerable to acute renal dysfunctions [18, 35, 73, 81, 178–181]. So, all the mechanisms reported above to explain renal dysfunction are involved, in particular decreased renal perfusion, venous congestion with increased right atrial pressure and renal venous pressure, neurohormonal activation and inflammation. Other predisposing factors frequently detected by multivariable analysis are baseline CKD, diabetes, hypertension, vascular disease, old age and anemia [18, 35, 77, 79–81, 148, 178–180, 182, 183].
Among precipitating factors, the worsening of congestion, too little fluid loss or vice versa diuretic excess, a substantial decrease in SBP, nephrotoxic agents or percutaneous interventions with contrast media have to be considered [10, 18, 73–77, 79, 84, 85, 179, 180, 183–186].
How can AKI/WRF generate long-term mortality? First, they may be markers of CVD severity [18, 73, 181]. Second, when persistent, they may worsen CVD through fluid overload, anemia, neurohormonal activation and inflammation [6, 60, 70, 154]. Third, they can favor long-term ESRD further worsening CVD [63, 187, 188].
Therapeutic approaches for treatment of HF with renal dysfunctions
The goal of treatment is to counteract the major modifiable pathophysiological abnormalities. So, the main target is to fight against hemodynamic abnormalities and to preserve euvolemia, pressure homeostasis and renal function. Another important goal is to avoid the underuse of CV drugs and interventions in patients with moderate to severe CKD [189–191]. In addition, it is necessary to differentiate WRF/AKI due to aggressive decongestion that is frequently transitory and with benign prognosis, from persistent dysfunctions. Finally, these patients should be treated early by a team involving cardiologists and nephrologists [191].
Treatment of fluid overload and congestion
Ideally, congestion is prevented by initial salt (and water in hyponatremia) restriction [191, 192]. Diuretics are commonly used to treat fluid overload and renal congestion. Their dose must be tailored to not consistently exceed the interstitial mobilization of fluids to the vascular space [the so-called “plasma refill rate” (PRR)] which is continuously changing and in a relatively steady state condition is about 2.5–7 ml/min in hemodialyzed patients, varying with body size, capillary permeability, lymphatic flow, regional blood flow, serum protein levels and duration of decongestion [193–196]. A clinical surrogate of changes in PRR could be hemoconcentration regarding compounds (i.e., hemoglobin) or cells (i.e., red blood cells) confined in the intravascular compartment [194]: at a given moment, an increase in hematocrit or hemoglobin indicates that the removal of intravascular fluids exceeds PRR. In clinical practice, despite the evidence that hemoconcentration is associated with better outcomes, the removal of intravascular fluids consistently greater than PRR is unwise. In fact, the diuretic doses must be adequately tailored to avoid severe hypovolemia, hypotension, a further increase in RAAS activation, a further reduction of renal perfusion and GFR, and electrolyte disorders. Decongestion is associated with reduced mortality [74, 75, 84, 85, 197–199], and transient WRF after decongestion frequently has no negative impact on the prognosis [73–76, 78, 84, 85, 200]; indeed, late decongestion can offset the negative effects of WRF [75] (Fig. 7). Also the reduction in abdominal pressure was associated with a significant reduction in serum creatinine [157, 201].
Fig. 7.
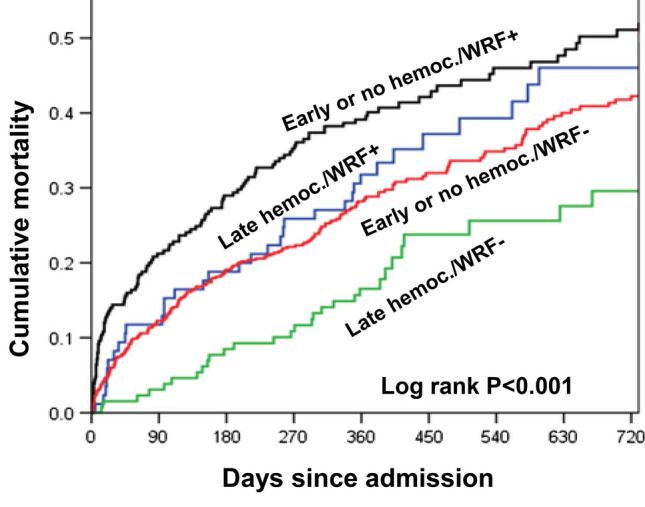
Mortality according to hemoconcentration and worsening renal function (WRF) in 1019 patients with acute heart failure (AHF)
(Adapted from Breidthardt T et al. Eur J Heart Fail 2017 [75])
Loop diuretics (furosemide or torasemide in furosemide-resistant patients) are the cornerstone of treatment. In severe CHF, the association of thiazide-like diuretics, such as metolazone and/or potassium-sparing diuretics (“sequential nephron blockade”) are often used to overcome the increased distal sodium reabsorption due to the chronic use of loop diuretics. In diuretic-resistant patients with metabolic alkalosis the association of acetazolamide to loop diuretics is particularly effective [190, 202].
Theoretically, congestion can also be reduced by increasing splanchnic vascular capacitance by ACE inhibitors (ACE-I) and/or β-blockers [140].
SGTL-2 inhibitors have recently shown important results in preventing hospitalization in diabetic patients with HF together with a reduction in the progression of renal disease. They have a diuretic effect (through a reduction of proximal sodium reabsorption and osmotic diuresis) and paradoxically a reduction of RAAS hyperactivity; in addition they inhibit cardiomyocyte Na/H exchanger and increase myocardial energetics [203–205]. Many trials were designed to evaluate the SGTL-2 effect in HF patients also without diabetes [203] and, interestingly, in November 2019 the DAPA-HF first demonstrated a reduction in CV mortality and HF hospitalization by SGTL-2 inhibitors in non-diabetic patients [203, 206].
In patients resistant to combined diuretic therapy, sodium and fluid retention were reduced by extracorporeal ultrafiltration with optimal results in most studies [207–209] also owing to the predictability of the amount of fluid removal and to the removal of cytokines and of isotonic fluids instead of hypotonic fluids which occurs with diuretics [97, 207, 208]. However, other trials obtained contrasting results [210, 211], also due to limitations in their design and conduction [212].
In the presence of severe congestion and stage IIIb, IV or V CKD, peritoneal dialysis could be a good therapeutic option to control both volume overload and uremic toxins and to enhance quality of life [213, 214].
Neurohormonal blockers
International guidelines strongly recommend ACE-I or angiotensin receptor blockers (ARB) or mineralocorticoid-receptor antagonists (MRA) and β-blockers to improve survival and prevent hospitalization; recently, also angiotensin receptor-neprilysn inhibitors (ARNI) such as sacubitril-valsartan have been recommended [190–192]. In an updated network meta-analysis [215] in CHF better results on survival have been obtained by ACE-I plus MRA plus β-blockers or by ARNI plus MRA plus β-blockers. ACE inhibitors, ARB, MRA and ARNI improve renal perfusion and sodium and water retention counteracting the hemodynamic and neurohormonal imbalance and long-term cardiac and renal fibrosis that further deteriorate both cardiac and renal function [216]. These drugs initially worsen GFR (increase serum creatinine ~ 30%) particularly when SBP decreases to less than 80–90 mmHg; however, later changes in GFR over time could be lower than in controls as well as the risk of mortality [86, 87, 106]. These associations can be used also in the presence of CKD with special attention firstly to hyperkalemia in patients with less than 30 ml/min of GFR and secondly to maintain SBP not lower than 80–90 mm/Hg [190, 217]. They can also be used in patients with WRF/AKI without hemodynamic instability or hypotension, reducing the dose until renal function improves. Theoretically, neurohormonal blockers can have an additional favorable effect in congested patients augmenting splanchnic capacitance [109, 128, 138–140].
Inotropic and vasopressor drugs
In refractory AHF patients with reduced ejection fraction and systolic blood pressure ≥ 90 mm/Hg, low dose dopamine, a renal vasodilator [218], could be useful to improve RBF and GFR even in the presence of WRF/AKI. In fact, dopamine infused at 1–5 μg/Kg/min with low/medium doses of diuretics maintains stable or increases GFR and reduces the incidence of WRF relative to high doses of diuretics ± dopamine [219–223]. Unfortunately, these data were overlooked in recent guidelines [190–192].
In refractory AHF patients with severe reduction of ejection fraction and with systolic blood pressure ≥ 85 mm/Hg, the infusion of levosimendan, an inotropic drug with arterial and venous dilatation properties, was associated with an improvement of GFR [224, 225], reduced mortality [226], and an increased risk of CV adverse events [192, 226]. Other inotropes seem associated with increased mortality [191, 192].
Drugs counteracting nontraditional CV risk factors
Among them, in HF, the correction of anemia through intravenous iron improves NYAA class and symptoms, and reduces hospitalization [227]; a recent meta-analysis showed a significant reduction also in mortality [228]. Anemic patients with GFR less than 30–45 ml must be treated with erythropoietin, avoiding overtreatment at all times.
Promising results are also reported in chronic CRS with Cinacalcet that reduces FGS-23 levels [229, 230].
Limitations
The present review has however some limitations. First, in CRS it is sometimes difficult: to understand the temporal causality of renal dysfunction; to highlight the role of traditional CV risk factors in simultaneously determining both cardiac and renal disorders; to distinguish their role from the direct contribution of CVD; and to discriminate the preexisting CKD from the acute renal dysfunction. In addition, studies are heterogeneous for many aspects such as selection bias, different inclusion criteria, formulae to estimate GFR and definition of acute renal dysfunction. Second, the effectiveness of diagnostic procedures to better predict risk is not completely understood. Third, mechanisms of adverse renal effects of congestion and the role of other factors such as inflammation, endothelial dysfunction and neurohormonal activation are not fully clarified. Moreover, renal congestion cannot be directly measured. Fourth, the ratio between long-term risks and benefits of therapeutic interventions, particularly on renal congestion, is not fully understood. Furthermore, data on PRR in CRS are lacking. Fifth, there is no strong clinical evidence of appropriate treatment. The few clinical trials that do exist are frequently retrospective, come from a single center and/or exclude severe renal dysfunction. Accordingly, large prospective trials are needed to better understand pathophysiological mechanisms and clinical results in CRS.
Conclusions
In conclusion (Table 2), baseline CKD and AKI/WRF are frequently observed in patients with chronic and acute HF; both chronic and acute renal dysfunction are usually associated with a poor clinical outcome. CVD represent one of the most important causes of renal dysfunction. Renal congestion is a major contributing factor to renal dysfunction in HF. Finally, therapeutic principles for the treatment of CRS are described.
Table 2.
Conclusions
| 1. In CVD: |
| Baseline CKD is up to 5 times more frequent than in the general population; |
| When CKD is present at baseline, the GFR decline is faster than in the general population, leading even to ESRD; |
| de novo CKD is frequently found; |
| CKD (GFR < 60 and/or albuminuria/proteinuria) is one of the most important factors independently predicting new CV events, rehospitalization, and mortality; |
| WRF/AKI occur in 50% of patients with AHF, slightly less frequently in CHF and ACS, and have a poor prognosis only if persistent and/or connected with residual congestion; transient WRF after decongestion may not result in a worse prognosis; |
| 2. CVD may be considered one of the most important contributory causes of renal disease owing to the enormous diffusion of clinical and subclinical CVD and the high prevalence and incidence of renal abnormalities |
| 3. Among the mechanisms of renal dysfunction in CVD, venous congestion is the most important; arterial renal hypoperfusion plays an important role particularly in acute severe reduction of cardiac output and/or of SBP |
| 4. Therapeutic approaches for the treatment of HF with renal dysfunctions: |
| Treat fluid overload and congestion, and pay little attention to mild transient increases in serum creatinine after hemoconcentration; |
| Counteract hemodynamic and neurohormonal imbalance by RAAS inhibitors and/or β-blockers; |
| Infuse low dose dopamine in selected refractory AHF patients; |
| Counteract less traditional CV risk factors; |
| Avoid the underuse of CV drugs and interventions in CKD; |
| Early detect CRS for its effective management by a team involving cardiologists and nephrologists |
CVD cardiovascular disease, CKD chronic kidney disease, GFR glomerular filtration rate, ESRD end stage renal disease, WRF worsening renal function, AKI acute kidney injury, AHF acute heart failure, CHF chronic heart failure, ACS acute coronary syndrome, SBP systolic blood pressure, HF heart failure, RAAS renin angiotensin aldosterone system, CV cardiovascular, CRS cardiorenal syndrome
Acknowledgements
We thank Mrs. Alessandra Bozomo, Personal Assistant, Genoa, Italy, for technical assistance and Mr. Justin Rainey, English lecturer, University of Genoa, Italy, for English revision.
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not applicable because this study is a review.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boudoulas KD, Boudoulas H. Cardiorenal interrelationship. Cardiology. 2011;120:135–138. doi: 10.1159/000334407. [DOI] [PubMed] [Google Scholar]
- 2.Bright R. Cases and observations illustrative of renal disease accompanied with the secretion of albuminous urine. Guy’s Hosp Rep. 1836;II:1–43. [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis T. A clinical lecture on paroxysmal dyspnea in cardiorenal patients: with special references to “cardiac” and “uraemic” asthma: delivered at University College Hospital, London, November 12th, 1913. Br Med J. 1913;29:1417–1420. doi: 10.1136/bmj.2.2761.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. JACC. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Braam B, Joles JA, Danishwar AH, Gaillard CA. Cardiorenal syndrome-current understanding and future perspectives. Nat Rev Nephrol. 2014;10:48–55. doi: 10.1038/nrneph.2013.250. [DOI] [PubMed] [Google Scholar]
- 6.Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;12:610–623. doi: 10.1038/nrneph.2016.113. [DOI] [PubMed] [Google Scholar]
- 7.Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, et al. The systemic nature of CKD. Nat Rev Nephrol. 2017;13:344–358. doi: 10.1038/nrneph.2017.52. [DOI] [PubMed] [Google Scholar]
- 8.Zoccali C. Cardiorenal risk as a new frontier of nephrology: research needs and areas for intervention. Nephrol Dial Transplant. 2002;17(S11):50–54. doi: 10.1093/ndt/17.suppl_11.50. [DOI] [PubMed] [Google Scholar]
- 9.Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, et al. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol. 2013;9:99–111. doi: 10.1038/nrneph.2012.279. [DOI] [PubMed] [Google Scholar]
- 10.Aronson D, Burger AJ. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J Card Fail. 2010;16:541–547. doi: 10.1016/j.cardfail.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 11.De Nicola L, Zoccali C. Chronic kidney disease prevalence in the general population: heterogeneity and concerns. Nephrol Dial Transplant. 2016;31:331–335. doi: 10.1093/ndt/gfv427. [DOI] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1986;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- 16.Ezekowitz J, McAlister FA, Humphries KH, Norris CM, Tonelli M, et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6427 patients with heart failure and coronary artery disease. JACC. 2004;44:1587–1592. doi: 10.1016/j.jacc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 17.McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol. 2004;15:1912–1919. doi: 10.1097/01.asn.0000129982.10611.4c. [DOI] [PubMed] [Google Scholar]
- 18.de Silva R, Nikitin NP, Witte KK, Rigby AS, Goode K, et al. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: contributing factors and relationship to prognosis. Eur Heart J. 2006;27:569–581. doi: 10.1093/eurheartj/ehi696. [DOI] [PubMed] [Google Scholar]
- 19.Waldum B, Westheim AS, Sandvik L, Flønaes B, Grundtvig M, et al. Renal function in outpatients with chronic heart failure. J Card Fail. 2010;16:374–380. doi: 10.1016/j.cardfail.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Smith DH, Thorp ML, Gurwitz JH, McManus DD, Goldberg RJ. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013;6:333–342. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosselmann H, Gislason G, Gustafsson F, Hildebrandt PR, Videbaek L, et al. Incidence and predictors of end-stage renal disease in outpatients with systolic heart failure. Circ Heart Fail. 2013;6:1124–1131. doi: 10.1161/CIRCHEARTFAILURE.113.000553. [DOI] [PubMed] [Google Scholar]
- 22.Van Deursen VM, Urso R, Laroche C, Damman K, Dahlström U, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16:103–111. doi: 10.1002/ejhf.30. [DOI] [PubMed] [Google Scholar]
- 23.Löfman I, Szummer K, Hagerman I, Dahlström U, Lund LH, et al. Prevalence and prognostic impact of kidney disease on heart failure patients. Open Heart. 2016;3:e000324. doi: 10.1136/openhrt-2015-000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernaudo D, Coll R, Sánchez Muñoz-Torrero JF, Pascual MT, García-Díaz AM, et al. Renal function and short-term outcome in stable outpatients with coronary, cerebrovascular or peripheral artery disease. Atherosclerosis. 2013;229:258–262. doi: 10.1016/j.atherosclerosis.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Lacroix P, Aboyans V, Desormais I, Kowalsky T, Cambou JP, et al. Chronic kidney disease and the short-term risk of mortality and amputation in patients hospitalized for peripheral artery disease. J Vasc Surg. 2013;58:966–971. doi: 10.1016/j.jvs.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. JACC. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 27.Cleland JG, Carubelli V, Castiello T, Yassin A, Pellicori P, et al. Renal dysfunction in acute and chronic heart failure: prevalence, incidence and prognosis. Heart Fail Rev. 2012;17:133–149. doi: 10.1007/s10741-012-9306-2. [DOI] [PubMed] [Google Scholar]
- 28.Damman K, Valente MA, Voors AA, O’Connor CM, van Veldhuisen DJ, et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 29.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Ismailov RM, Goldberg RJ, Lessard D, Spencer FA. Decompensated heart failure in the setting of kidney dysfunction: a community-wide perspective. Nephron Clin Pract. 2007;107:c147–c155. doi: 10.1159/000110035. [DOI] [PubMed] [Google Scholar]
- 31.Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Ide T, et al. Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD) Circ J. 2009;73:1442–1447. doi: 10.1253/circj.cj-09-0062. [DOI] [PubMed] [Google Scholar]
- 32.Blair JE, Pang PS, Schrier RW, Metra M, Traver B, et al. Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur Heart J. 2011;32:2563–2572. doi: 10.1093/eurheartj/ehr238. [DOI] [PubMed] [Google Scholar]
- 33.Kajimoto K, Sato N, Keida T, Sakata Y, Takano T. Associations of anemia and renal dysfunction with outcomes among patients hospitalized for acute decompensated heart failure with preserved or reduced ejection fraction. Clin J Am Soc Nephrol. 2014;9:1912–1921. doi: 10.2215/CJN.04400514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnamoorthy A, Greiner MA, Sharma PP, DeVore AD, Johnson KW, et al. Transient and persistent worsening renal function during hospitalization for acute heart failure. Am Heart J. 2014;168:891–900. doi: 10.1016/j.ahj.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg A, Hammerman H, Petcherski S, Zdorovyak A, Yalonetsky S, et al. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J. 2005;150:330–337. doi: 10.1016/j.ahj.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 36.Nagashima M, Hagiwara N, Koyanagi R, Yamaguchi J, Takagi A, et al. Chronic kidney disease and long-term outcomes of myocardial infarction. Int J Cardiol. 2013;167:2490–2495. doi: 10.1016/j.ijcard.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 37.Choi JS, Kim MJ, Kang YU, Kim CS, Bae EH, et al. Association of age and CKD with prognosis of myocardial infarction. Clin J Am Soc Nephrol. 2013;8:939–944. doi: 10.2215/CJN.06930712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mody P, Wang T, McNamara R, Das S, Li S, Chiswell K, et al. Association of acute kidney injury and chronic kidney disease with processes of care and long-term outcomes in patients with acute myocardial infarction. Eur Heart J Qual Care Clin Outcomes. 2018;4:43–50. doi: 10.1093/ehjqcco/qcx020. [DOI] [PubMed] [Google Scholar]
- 39.Tsagalis G, Akrivos T, Alevizaki M, Manios E, Stamatellopoulos K, et al. Renal dysfunction in acute stroke: an independent predictor of long-term all combined vascular events and overall mortality. Nephrol Dial Transplant. 2009;24:194–200. doi: 10.1093/ndt/gfn471. [DOI] [PubMed] [Google Scholar]
- 40.El Husseini N, Fonarow GC, Smith EE, Ju C, Schwamm LH, et al. Renal dysfunction is associated with poststroke discharge disposition and in-hospital mortality: findings from get with the guidelines-stroke. Stroke. 2017;48:327–334. doi: 10.1161/STROKEAHA.116.014601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374:543–550. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]
- 42.Masson S, Latini R, Milani V, Moretti L, Rossi MG, et al. Prevalence and prognostic value of elevated urinary albumin excretion in patients with chronic heart failure: data from the GISSI-Heart Failure trial. Circ Heart Fail. 2010;3:65–72. doi: 10.1161/CIRCHEARTFAILURE.109.881805. [DOI] [PubMed] [Google Scholar]
- 43.Niizeki T, Takeishi Y, Sasaki T, Kaneko K, Sugawara S, et al. Usefulness of albuminuria as a prognostic indicator in patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2013;111:1180–1186. doi: 10.1016/j.amjcard.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 44.Miura M, Sakata Y, Miyata S, Nochioka K, Takada T, et al. Prognostic impact of subclinical microalbuminuria in patients with chronic heart failure. Circ J. 2014;78:2890–2898. [PubMed] [Google Scholar]
- 45.Åkerblom A, Clare RM, Lokhnygina Y, Wallentin L, Held C, et al. Albuminuria and cardiovascular events in patients with acute coronary syndromes: results from the TRACER trial. Am Heart J. 2016;178:1–8. doi: 10.1016/j.ahj.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Inker LA, Grams ME, Levey AS, Coresh J, Cirillo M, et al. Relationship of estimated GFR and albuminuria to concurrent laboratory abnormalities: an individual participant data meta-analysis in a global consortium. Am J Kidney Dis. 2019;73:206–217. doi: 10.1053/j.ajkd.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anand IS, Bishu K, Rector TS, Ishani A, Kuskowski MA, et al. Proteinuria, chronic kidney disease, and the effect of an angiotensin receptor blocker in addition to an angiotensin-converting enzyme inhibitor in patients with moderate to severe heart failure. Circulation. 2009;120:1577–1584. doi: 10.1161/CIRCULATIONAHA.109.853648. [DOI] [PubMed] [Google Scholar]
- 48.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, et al. Cardiovascular disease and subsequent kidney disease. Arch Intern Med. 2007;167:1130–1136. doi: 10.1001/archinte.167.11.1130. [DOI] [PubMed] [Google Scholar]
- 49.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, et al. Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis. 2009;204:298–303. doi: 10.1016/j.atherosclerosis.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsioufis C, Kokkinos P, Macmanus C, Thomopoulos C, Faselis C, et al. Left ventricular hypertrophy as a determinant of renal outcome in patients with high cardiovascular risk. J Hypert. 2010;28:2299–2309. doi: 10.1097/HJH.0b013e32833d95fe. [DOI] [PubMed] [Google Scholar]
- 51.Ravera M, Noberasco G, Signori A, Re M, Cannavò R, et al. Left-ventricular hypertrophy and renal outcome in hypertensive patients in primary-care. Am J Hypert. 2013;26:700–707. doi: 10.1093/ajh/hps100. [DOI] [PubMed] [Google Scholar]
- 52.Li LC, Lee YT, Lee YW, Chou CA, Lee CT. Aortic arch calcification predicts the renal function progression in patients with stage 3 to 5 chronic kidney disease. Biomed Res Internat. 2015;2015:131263. doi: 10.1155/2015/131263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paoletti E, De Nicola L, Gabbai FB, Chiodini P, Ravera M, et al. Associations of left ventricular hypertrophy and geometry with adverse outcomes in patients with CKD and hypertension. Clin J Am Soc Nephrol. 2016;11:271–279. doi: 10.2215/CJN.06980615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.George LK, Koshy SKG, Molnar MZ, Thomas F, Lu JL, et al. Heart failure increases the risk of adverse renal outcomes in patients with normal kidney function. Circ Heart Fail. 2017;10:e003825. doi: 10.1161/CIRCHEARTFAILURE.116.003825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park M, Shlipak MG, Katz R, Agarwal S, Ix JH, et al. Subclinical cardiac abnormalities and kidney function decline: the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol. 2012;7:1137–1144. doi: 10.2215/CJN.01230212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zelnick LR, Katz R, Young BA, Correa A, Kestenbaum BR, et al. Echocardiographic measures and estimated GFR decline among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2017;70:199–206. doi: 10.1053/j.ajkd.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu S, Rifkin DE, Criqui MH, Suder NC, Garimella P, et al. Relationship of femoral artery ultrasound measures of atherosclerosis with chronic kidney disease. J Vasc Surg. 2018;67:1855–1863. doi: 10.1016/j.jvs.2017.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan NA, Ma I, Thompson CR, Humphries K, Salem DN, et al. Kidney function and mortality among patients with left ventricular systolic dysfunction. J Am Soc Nephrol. 2006;17:244–253. doi: 10.1681/ASN.2005030270. [DOI] [PubMed] [Google Scholar]
- 59.Aronson D, Mittleman MA, Burger AJ. Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am J Med. 2004;116:466–473. doi: 10.1016/j.amjmed.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Matsue Y, van der Meer P, Damman K, Metra M, O'Connor CM, et al. Blood urea nitrogen-to-creatinine ratio in the general population and in patients with acute heart failure. Heart. 2017;103:407–413. doi: 10.1136/heartjnl-2016-310112. [DOI] [PubMed] [Google Scholar]
- 61.Gotlieb SS, Abraham W, Butler J, Forman DE, Loh E, et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 62.Smith GL, Vaccarino V, Kosiborod M, Lichtman JH, Cheng S, et al. Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail. 2003;9:13–25. doi: 10.1054/jcaf.2003.3. [DOI] [PubMed] [Google Scholar]
- 63.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 64.Damman K, Tang WH, Testani JM, McMurray JJ. Terminology and definition of changes renal function in heart failure. Eur Heart J. 2014;35:3413–3416. doi: 10.1093/eurheartj/ehu320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheerin NJ, Newton PJ, Macdonald PS, Leung DY, Sibbritt D, et al. Worsening renal function in heart failure: the need for a consensus definition. Int J Cardiol. 2014;174:484–491. doi: 10.1016/j.ijcard.2014.04.162. [DOI] [PubMed] [Google Scholar]
- 66.Núñez J, Miñana G, Santas E, Bertomeu-González V. Cardiorenal syndrome in acute heart failure: revisiting paradigms. Rev Esp Cardiol. 2015;68:426–435. doi: 10.1016/j.rec.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, et al. Acute kidney injury network: acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure definition, outcome measures, animals model, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kellum JA. Section 2: AKI definition. Kidney Int Suppl. 2012;2:19–36. doi: 10.1038/kisup.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168:987–995. doi: 10.1001/archinte.168.9.987. [DOI] [PubMed] [Google Scholar]
- 71.Roy AK, Mc Gorrian C, Treacy C, Kavanaugh E, Brennan A, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med. 2013;3:26–37. doi: 10.1159/000347037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pimentel R, Couto M, Laszczyńska O, Friões F, Bettencourt P, et al. Prognostic value of worsening renal function in outpatients with chronic heart failure. Eur J Intern Med. 2014;25:662–668. doi: 10.1016/j.ejim.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Metra M, Davison B, Bettari L, Sun H, Edwards C. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- 74.Greene SJ, Gheorghiade M, Vaduganathan M, Ambrosy AP, Mentz RJ, et al. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Eur J Heart Fail. 2013;15:1401–1411. doi: 10.1093/eurjhf/hft110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breidthardt T, Weidmann ZM, Twerenbold R, Gantenbein C, Stallone F, et al. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur J Heart Fail. 2017;19:226–236. doi: 10.1002/ejhf.667. [DOI] [PubMed] [Google Scholar]
- 76.Fudim M, Loungani R, Doerfler SM, Coles A, Greene SJ, et al. Worsening renal function during decongestion among patients hospitalized for heart failure: findings from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial. Am Heart J. 2018;204:163–173. doi: 10.1016/j.ahj.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 77.Kang J, Park JJ, Cho YJ, Oh IY, Park HA, et al. Predictors and prognostic value of worsening renal function during admission in HFpEF versus HFrEF: data from the KorAHF (Korean Acute Heart Failure) registry. J Am Heart Assoc. 2018;7:e007910. doi: 10.1161/JAHA.117.007910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metra M, Cotter G, Senger S, Edwards C, Cleland JG, et al. Prognostic significance of creatinine increases during an acute heart failure admission in patients with and without residual congestion: a post hoc analysis of the PROTECT data. Circ Heart Fail. 2018;11:e004644. doi: 10.1161/CIRCHEARTFAILURE.117.004644. [DOI] [PubMed] [Google Scholar]
- 79.Sato Y, Yoshihisa A, Oikawa M, Nagai T, Yoshikawa T. Prognostic impact of worsening renal function in hospitalized heart failure patients with preserved ejection fraction: a report from the JASPER registry. J Card Fail. 2019;25:631–642. doi: 10.1016/j.cardfail.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Goldberg A, Kogan E, Hammerman H, Markiewicz W, Aronson D. The impact of transient and persistent acute kidney injury on long-term outcomes after acute myocardial infarction. Kidney Int. 2009;76:900–906. doi: 10.1038/ki.2009.295. [DOI] [PubMed] [Google Scholar]
- 81.Choi JS, Kim YA, Kim MJ, Kang YU, Kim CS, et al. Relation between transient or persistent acute kidney injury and long-term mortality in patients with myocardial infarction. Am J Cardiol. 2013;112:41–45. doi: 10.1016/j.amjcard.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 82.Logeart D, Tabet JY, Hittinger L, Thabut G, Jourdain P, et al. Transient worsening of renal function during hospitalization for acute heart failure alters outcome. Int J Cardiol. 2008;127:228–232. doi: 10.1016/j.ijcard.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Lanfear DE, Peterson EL, Campbell J, Phatak H, Wu D, et al. Relation of worsened renal function during hospitalization for heart failure to long-term outcomes and rehospitalization. Am J Cardiol. 2011;107:74–78. doi: 10.1016/j.amjcard.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darwash W, Chirmicci S, Solomonica A, Wattad M, Kaplan M, et al. Discordance between hemoconcentration and clinical assessment of decongestion in acute heart failure. J Card Fail. 2016;22:680–688. doi: 10.1016/j.cardfail.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 86.Damman K, Solomon SD, Pfeffer MA, Swedberg K, Yusuf S, et al. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM-study programme. Eur J Heart Fail. 2016;18:1508–1517. doi: 10.1002/ejhf.609. [DOI] [PubMed] [Google Scholar]
- 87.Beldhuis IE, Streng KW, Ter Maaten JM, Voors AA, van der Meer P, et al. Renin-angiotensin system inhibition, worsening renal function, and outcome in heart failure patients with reduced and preserved ejection fraction: a meta-analysis of published study data. Circ Heart Fail. 2017;10:e003588. doi: 10.1161/CIRCHEARTFAILURE.116.003588. [DOI] [PubMed] [Google Scholar]
- 88.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 89.Gutierrez E, Flammer AJ, Lerman LO, Elízaga J, Lerman A, et al. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J. 2013;34:3175–3181. doi: 10.1093/eurheartj/eht351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Simone G, Kitzman DW, Palmieri V, Liu JE, Oberman A, et al. Association of inappropriate left ventricular mass with systolic and diastolic dysfunction: the HyperGEN study. Am J Hypertens. 2004;17:828–833. doi: 10.1016/j.amjhyper.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 91.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. JACC. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 92.Bock J, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 93.Aronson D. Cardiorenal syndrome in acute decompensated heart failure. Expert Rev Cardiovasc Ther. 2012;10:177–189. doi: 10.1586/erc.11.193. [DOI] [PubMed] [Google Scholar]
- 94.Braam B, Cupples WA, Joles JA, Gaillard C. Systemic arterial and venous determinants of renal hemodynamics in congestive heart failure. Heart Fail Rev. 2012;17:161–175. doi: 10.1007/s10741-011-9246-2. [DOI] [PubMed] [Google Scholar]
- 95.Sinkeler SJ, Damman K, van Veldhuisen DJ, Hillege H, Navis G. A re-appraisal of volume status and renal function impairment in chronic heart failure: combined effects of pre-renal failure and venous congestion on renal function. Heart Fail Rev. 2012;17:263–270. doi: 10.1007/s10741-011-9233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Anand IS. Cardiorenal syndrome: a cardiologist's perspective of pathophysiology. Clin J Am Soc Nephrol. 2013;8:1800–1807. doi: 10.2215/CJN.04090413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Valika A, Costanzo MR. The acute cardiorenal syndrome type I: considerations on physiology, epidemiology, and therapy. Curr Heart Fail Rep. 2014;11:382–392. doi: 10.1007/s11897-014-0224-6. [DOI] [PubMed] [Google Scholar]
- 98.Sarnak MJ. A patient with heart failure and worsening kidney function. Clin J Am Soc Nephrol. 2014;9:1790–1798. doi: 10.2215/CJN.11601113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437–1444. doi: 10.1093/eurheartj/ehv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takahama H, Kitakaze M. Pathophysiology of cardiorenal syndrome in patients with heart failure: potential therapeutic targets. Am J Physiol Heart Circ Physiol. 2017;313:H715–H721. doi: 10.1152/ajpheart.00215.2017. [DOI] [PubMed] [Google Scholar]
- 101.Thind GS, Loehrke M, Wilt JL. Acute cardiorenal syndrome: mechanisms and clinical implications. Cleve Clin J Med. 2018;85:231–239. doi: 10.3949/ccjm.85a.17019. [DOI] [PubMed] [Google Scholar]
- 102.Di Nicolo’ P. The dark side of the kidney in cardio-renal syndrome: renal venous hypertension and congestive kidney failure. Heart Fail Rev. 2018;23:291–302. doi: 10.1007/s10741-018-9673-4. [DOI] [PubMed] [Google Scholar]
- 103.Tucker BJ, Blintz RC. An analysis of the determinants of nephron filtration rate. Am J Physiol. 1977;232:F477–F483. doi: 10.1152/ajprenal.1977.232.6.F477. [DOI] [PubMed] [Google Scholar]
- 104.Tsuruya K, Eriguchi M. Cardiorenal syndrome in chronic kidney disease. Curr Opin Nephrol Hyper. 2015;24:154–162. doi: 10.1097/MNH.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 105.Schrier RM. Pathogenesis of sodium and water retention in high-output and low-output cardiac failure, nephrotic syndrome, cirrhosis, and pregnancy (1) NEJM. 1988;319:1065. doi: 10.1056/NEJM198810203191606. [DOI] [PubMed] [Google Scholar]
- 106.Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs. 1990;39(S4):10–21. doi: 10.2165/00003495-199000394-00004. [DOI] [PubMed] [Google Scholar]
- 107.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, et al. Endothelial dysfunction, arterial stiffness, and heart failure. JACC. 2012;60:1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 108.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511. doi: 10.1152/physrev.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mentz RJ, O'Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol. 2016;13:28–35. doi: 10.1038/nrcardio.2015.134. [DOI] [PubMed] [Google Scholar]
- 110.Anand IS, Ferrari R, Kalra GS, Wahi PL, Poole-Wilson PA, et al. Edema of cardiac origin. Studies of body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive cardiac failure. Circulation. 1989;80:299–305. doi: 10.1161/01.cir.80.2.299. [DOI] [PubMed] [Google Scholar]
- 111.Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, et al. The kidney in congestive heart failure: ‘are natriuresis, sodium, and diuretics really the good, the bad and the ugly?’. Eur J Heart Fail. 2014;16:133–142. doi: 10.1002/ejhf.35. [DOI] [PubMed] [Google Scholar]
- 112.Mullens W, Verbrugge FH, Nijst P, Tang WHW, et al. Renal sodium avidity in heart failure: from pathophysiology to treatment strategies. Eur Heart J. 2017;38:1872–1882. doi: 10.1093/eurheartj/ehx035. [DOI] [PubMed] [Google Scholar]
- 113.Burke M, Pabbidi MR, Farley J, Roman RJ. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol. 2014;12:845–859. doi: 10.2174/15701611113116660149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anand IS, Florea VG. High output cardiac failure. Curr Treat Options Cardiovasc Med. 2001;3:151–159. doi: 10.1007/s11936-001-0070-1. [DOI] [PubMed] [Google Scholar]
- 115.Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393–398. doi: 10.1016/j.amjcard.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maeder MT, Holst DP, Kaye DM. Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J Card Fail. 2008;14:824–830. doi: 10.1016/j.cardfail.2008.07.236. [DOI] [PubMed] [Google Scholar]
- 117.Testani JM, Khera AV, St John Sutton MG, Keane MG, Wiegers SE, et al. Effect of right ventricular function and venous congestion on cardiorenal interactions during the treatment of decompensated heart failure. Am J Cardiol. 2010;105:511–516. doi: 10.1016/j.amjcard.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guazzi M, Gatto P, Giusti G, Pizzamiglio F, Previtali I, et al. Pathophysiology of cardiorenal syndrome in decompensated heart failure: role of lung-right heart-kidney interaction. Int J Cardiol. 2013;169:379–384. doi: 10.1016/j.ijcard.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 119.Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–878. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 120.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, et al. Cardiorenal interactions: insights from the ESCAPE trial. JACC. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 121.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. JACC. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 122.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. JACC. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Damman K, Voors AA, Hillege HL, Navis G, Lechat P, et al. Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur J Heart Fail. 2010;12:974–982. doi: 10.1093/eurjhf/hfq118. [DOI] [PubMed] [Google Scholar]
- 124.Testani JM, Damman K. Venous congestion and renal function in heart failure … it’s complicated. Eur J Heart Fail. 2013;15:599–601. doi: 10.1093/eurjhf/hft060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gnanaraj JF, von Haehling S, Anker SD, Raj DS, Radhakrishnan J. The relevance of congestion in the cardio-renal syndrome. Kidney Int. 2013;83:384–391. doi: 10.1038/ki.2012.406. [DOI] [PubMed] [Google Scholar]
- 126.Hanberg JS, Sury K, Wilson FP, Brisco MA, Ahmad T, et al. Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. JACC. 2016;67:2199–2208. doi: 10.1016/j.jacc.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Afsar B, Ortiz A, Covic A, Solak Y, et al. Focus on renal congestion in heart failure. CKJ. 2016;9:39–47. doi: 10.1093/ckj/sfv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen X, Wang X, Honore PM, Spapen HD, Liu D. Renal failure in critically ill patients, beware of applying (central venous) pressure on the kidney. Ann Intensive Care. 2018;8:91. doi: 10.1186/s13613-018-0439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Iida N, Seo Y, Sai S, Machino-Ohtsuka T, Yamamoto M, et al. Clinical implications of intrarenal hemodynamic evaluation by doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4:674–682. doi: 10.1016/j.jchf.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 130.Puzzovivo A, Monitillo F, Guida P, Leone M, Rizzo CJ, et al. Renal venous pattern: a new parameter for predicting prognosis in heart failure outpatients. Cardiovasc Dev Dis. 2018;5:52. doi: 10.3390/jcdd5040052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nijst P, Martens P, Dupont M, Tang WHW, Mullens W. Intrarenal flow alterations during transition from euvolemia to intravascular volume expansion in heart failure patients. JACC Heart Fail. 2017;5:672–681. doi: 10.1016/j.jchf.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 132.de la Espriella-Juan R, Núñez E, Miñana G, Sanchis J, Bayés-Genís A, et al. Intrarenal venous flow in cardiorenal syndrome: a shining light into the darkness. ESC Heart Fail. 2018;5:1173–1175. doi: 10.1002/ehf2.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Komuro K, Seo Y, Yamamoto M, Sai S, Ishizu T, et al. Assessment of renal perfusion impairment in a rat model of acute renal congestion using contrast-enhanced ultrasonography. Heart Vessels. 2018;33:434–440. doi: 10.1007/s00380-017-1063-7. [DOI] [PubMed] [Google Scholar]
- 134.Hinschaw LB, Brake CM, Iampietro PF, Emerson TE., Jr Effect of increased venous pressure on renal hemodynamics. Am J Physiol. 1963;204:119–123. doi: 10.1152/ajplegacy.1963.204.1.119. [DOI] [PubMed] [Google Scholar]
- 135.Uthoff H, Breidthardt T, Klima T, Aschwanden M, Arenja N, et al. Central venous pressure and impaired renal function in patients with acute heart failure. Eur J Heart Fail. 2011;13:432–439. doi: 10.1093/eurjhf/hfq195. [DOI] [PubMed] [Google Scholar]
- 136.Ambrosy AP, Vaduganathan M, Mentz RJ, Greene SJ, Subačius H, et al. Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: insights from the efficacy of vasopressin antagonism in heart failure: outcome study with Tolvaptan (EVEREST) trial. Am Heart J. 2013;165:216–225. doi: 10.1016/j.ahj.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 137.Grodin JL, Drazner MH, Dupont M, Mullens W, Taylor DO. A disproportionate elevation in right ventricular filling pressure, in relation to left ventricular filling pressure, is associated with renal impairment and increased mortality in advanced decompensated heart failure. Am Heart J. 2015;169:806–812. doi: 10.1016/j.ahj.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011;4:669–675. doi: 10.1161/CIRCHEARTFAILURE.111.961789. [DOI] [PubMed] [Google Scholar]
- 139.Ross EA. Congestive renal failure: the pathophysiology and treatment of renal venous hypertension. J Card Fail. 2012;18:930–938. doi: 10.1016/j.cardfail.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 140.Fudim M, Hernandez AF, Felker GM. Role of volume redistribution in the congestion of heart failure. J Am Heart Assoc. 2017;6:e006817. doi: 10.1161/JAHA.117.006817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. JACC. 2003;41:1797–1804. doi: 10.1016/s0735-1097(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 142.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 143.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 144.Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, et al. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND-HF trial. JACC Heart Fail. 2017;5:1–13. doi: 10.1016/j.jchf.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fudim M, Parikh KS, Dunning A, DeVore AD, Mentz RJ, et al. Relation of volume overload to clinical outcomes in acute heart failure (From ASCEND-HF) Am J Cardiol. 2018;122:1506–1512. doi: 10.1016/j.amjcard.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rubio-Gracia J, Demissei BG, Ter Maaten JM, Cleland JG, O'Connor CM, et al. Prevalence, predictors and clinical outcome of residual congestion in acute decompensated heart failure. Int J Cardiol. 2018;258:185–191. doi: 10.1016/j.ijcard.2018.01.067. [DOI] [PubMed] [Google Scholar]
- 147.Cooper LB, Lippmann SJ, DiBello JR, Gorsh B, Curtis LH, et al. The Burden of congestion in patients hospitalized with acute decompensated heart failure. Am J Cardiol. 2019;124:545–553. doi: 10.1016/j.amjcard.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 148.Krumholz HM, Chen YT, Vaccarino V, Wang Y, Radford MJ, et al. Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am J Cardiol. 2000;85:1110–1113. doi: 10.1016/s0002-9149(00)00705-0. [DOI] [PubMed] [Google Scholar]
- 149.Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931;72:49–61. doi: 10.1113/jphysiol.1931.sp002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bradley SE, Bradley GP. The effect of increased intra-abdominal pressure on renal function in man. J Clin Invest. 1947;26:1010–1022. doi: 10.1172/JCI101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maxwell MH, Breed ES, Schwartz IL. Renal venous pressure in chronic congestive heart failure. J Clin Invest. 1950;29:342–348. doi: 10.1172/JCI102263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kastner PR, Hall JE, Guyton AC. Renal hemodynamic responses to increased renal venous pressure: role of angiotensin II. Am J Physiol. 1982;243:F260–F264. doi: 10.1152/ajprenal.1982.243.3.F260. [DOI] [PubMed] [Google Scholar]
- 153.Dilley JR, Corradi A, Arendshorst WJ. Glomerular ultrafiltration dynamics during increased renal venous pressure. Am J Physiol. 1983;244:F650–F658. doi: 10.1152/ajprenal.1983.244.6.F650. [DOI] [PubMed] [Google Scholar]
- 154.Colombo PC, Rastogi S, Onat D, Zacà V, Gupta RC. Activation of endothelial cells in conduit veins of dogs with heart failure and veins of normal dogs after vascular stretch by acute volume loading. J Card Fail. 2009;15:457–463. doi: 10.1016/j.cardfail.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Harman PK, Kron IL, McLachlan HD, Freedlender AE, Nolan SP. Elevated intra-abdominal pressure and renal function. Ann Surg. 1982;196:594–597. doi: 10.1097/00000658-198211000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bloomfield GL, Blocher CR, Fakhry IF, Sica DA, Sugerman HJ. Elevated intra-abdominal pressure increases plasma renin activity and aldosterone levels. J Trauma. 1997;42:997–1004. doi: 10.1097/00005373-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 157.Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? JACC. 2008;51:300–306. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 158.Mohamed H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011;22:615–621. doi: 10.1681/ASN.2010121222. [DOI] [PubMed] [Google Scholar]
- 159.Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. JACC. 2013;62:485–495. doi: 10.1016/j.jacc.2013.04.070. [DOI] [PubMed] [Google Scholar]
- 160.Selkurt EE, Hall PW, Spencer MP. Response of renal blood flow and clearance to graded partial obstruction of the renal vein. Am J Physiol. 1949;157:40–46. doi: 10.1152/ajplegacy.1949.157.1.40. [DOI] [PubMed] [Google Scholar]
- 161.Hall PW, Selkurt EE. Effects of partial graded venous obstruction on electrolyte clearance by the dog's kidney. Am J Physiol. 1951;164:143–154. doi: 10.1152/ajplegacy.1950.164.1.143. [DOI] [PubMed] [Google Scholar]
- 162.Wathen RL, Selkurt EE. Intrarenal regulatory factors of salt excretion during renal venous pressure elevation. Am J Physiol. 1969;216:1517–1524. doi: 10.1152/ajplegacy.1969.216.6.1517. [DOI] [PubMed] [Google Scholar]
- 163.Mullane JF, Gliedman ML. Effect of chronic experimental unilateral renal vein hypertension on renal hemodynamics, concentrating ability, urine flow, and sodium excretion. Surgery. 1969;66:368–374. [PubMed] [Google Scholar]
- 164.Burnett JC, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238:F279–F282. doi: 10.1152/ajprenal.1980.238.4.F279. [DOI] [PubMed] [Google Scholar]
- 165.Gottschalk CW. A comparative study of renal interstitial pressure. Am J Physiol. 1952;169:180–187. doi: 10.1152/ajplegacy.1952.169.1.180. [DOI] [PubMed] [Google Scholar]
- 166.Gottschalk CW, Mylle M. Micropuncture study of pressures in proximal tubules and peritubular capillaries of the rat kidney and their relation to ureteral and renal venous pressures. Am J Physiol. 1956;185:430–439. doi: 10.1152/ajplegacy.1956.185.2.430. [DOI] [PubMed] [Google Scholar]
- 167.Källskog O, Wolgast M. Effect of elevated interstitial pressure on the renal cortical hemodynamics. Acta Physiol Scand. 1975;95:364–372. doi: 10.1111/j.1748-1716.1975.tb10063.x. [DOI] [PubMed] [Google Scholar]
- 168.Fiksen-Olsen MJ, Strick DM, Hawley H, Romero JC. Renal effects of angiotensin II inhibition during increases in renal venous pressure. Hypertension. 1992;19(2 Suppl):II137–141. doi: 10.1161/01.hyp.19.2_suppl.ii137. [DOI] [PubMed] [Google Scholar]
- 169.Haddy FJ. Effect of elevation of intraluminal pressure on renal vascular resistance. Circ Res. 1956;4:659–663. doi: 10.1161/01.res.4.6.659. [DOI] [PubMed] [Google Scholar]
- 170.Haddy FJ, Scott J, Fleishman M, Emanuel D. Effect of change in renal venous pressure upon renal vascular resistance, urine and lymph flow rates. Am J Physiol. 1958;195:97–110. doi: 10.1152/ajplegacy.1958.195.1.97. [DOI] [PubMed] [Google Scholar]
- 171.Waugh WH, Hamilton WF. Physical effects of increased venous and extrarenal pressure on renal vascular resistance. Circ Res. 1958;6:116–121. doi: 10.1161/01.res.6.1.116. [DOI] [PubMed] [Google Scholar]
- 172.Kishimoto T, Maekawa M, Abe Y, Yamamoto K. Intrarenal distribution of blood flow and renin release during renal venous pressure elevation. Kidney Internat. 1973;4:259–266. doi: 10.1038/ki.1973.112. [DOI] [PubMed] [Google Scholar]
- 173.Doty JM, Saggi BH, Sugerman HJ, Blocher CR, Pin R, et al. Effect of increased renal venous pressure on renal function. J Trauma. 1999;47:1000–1003. doi: 10.1097/00005373-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 174.Shimada S, Hirose T, Takahashi C, Sato E, Kinugasa S, et al. Pathophysiological and molecular mechanisms involved in renal congestion in a novel rat model. Sci Rep. 2018;8:16808. doi: 10.1038/s41598-018-35162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dupont M, Mullens W, Tang WH. Impact of systemic venous congestion in heart failure. Curr Heart fail Rep. 2011;8:233–241. doi: 10.1007/s11897-011-0071-7. [DOI] [PubMed] [Google Scholar]
- 176.Alpert JS. The effect of right ventricular dysfunction on left ventricular form and function. Chest. 2001;119:1632–1633. doi: 10.1378/chest.119.6.1632. [DOI] [PubMed] [Google Scholar]
- 177.Drazner MH, Velez-Martinez M, Ayers CR, Reimold SC, Thibodeau JT, et al. Relationship of right- to left-sided ventricular filling pressures in advanced heart failure: insights from the ESCAPE trial. Circ Heart Fail. 2013;6:264–270. doi: 10.1161/CIRCHEARTFAILURE.112.000204. [DOI] [PubMed] [Google Scholar]
- 178.Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. JACC. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 179.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, et al. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. doi: 10.1016/j.ejheart.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 180.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, et al. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) Eur J Heart Fail. 2009;11:847–854. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 181.Lawson CA, Testani JM, Mamas M, Damman K, Jones PW, et al. Chronic kidney disease, worsening renal function and outcomes in a heart failure community setting: a UK national study. Int J Cardiol. 2018;267:120–127. doi: 10.1016/j.ijcard.2018.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chittineni H, Miyawaki N, Gulipelli S, Fishbane S, et al. Risk for acute renal failure in patients hospitalized for decompensated congestive heart failure. Am J Nephrol. 2007;27:55–62. doi: 10.1159/000099012. [DOI] [PubMed] [Google Scholar]
- 183.Voors AA, Davison BA, Felker GM, Ponikowski P, Unemori E, et al. Early drop in systolic blood pressure and worsening renal function in acute heart failure: renal results of Pre-RELAX-AHF. Eur J Heart Fail. 2011;13:961–967. doi: 10.1093/eurjhf/hfr060. [DOI] [PubMed] [Google Scholar]
- 184.Kazory A, Elkayam U. Cardiorenal interactions in acute decompensated heart failure: contemporary concepts facing emerging controversies. J Card Fail. 2014;20:1004–1011. doi: 10.1016/j.cardfail.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 185.Dupont M, Mullens W, Finucan M, Taylor DO, Starling RC, et al. Determinants of dynamic changes in serum creatinine in acute decompensated heart failure: the importance of blood pressure reduction during treatment. Eur J Heart Fail. 2013;15:433–440. doi: 10.1093/eurjhf/hfs209. [DOI] [PubMed] [Google Scholar]
- 186.Nijst P, Mullens W. The acute cardiorenal syndrome: burden and mechanisms of disease. Curr Heart Fail Rep. 2014;11:453–462. doi: 10.1007/s11897-014-0218-4. [DOI] [PubMed] [Google Scholar]
- 187.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 188.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, et al. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9:448–456. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Patel UD, Hernandez AF, Liang L, Peterson ED, LaBresh KA, et al. Quality of care and outcomes among patients with heart failure and chronic kidney disease: a Get With the Guidelines—Heart Failure Program study. Am Heart J. 2008;156:674–681. doi: 10.1016/j.ahj.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Damman K, Tang WH, Felker GM, Lassus J, Zannad F, et al. Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. JACC. 2014;63:853–871. doi: 10.1016/j.jacc.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 191.Rossignol P, Hernandez AF, Solomon SD, Zannad F. Heart failure drug treatment. Lancet. 2019;393:1034–1044. doi: 10.1016/S0140-6736(18)31808-7. [DOI] [PubMed] [Google Scholar]
- 192.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 193.Marenzi G, Lauri G, Grazi M, Assanelli E, Campodonico J, et al. Circulatory response to fluid overload removal by extracorporeal ultrafiltration in refractory congestive heart failure. JACC. 2001;38:963–968. doi: 10.1016/s0735-1097(01)01479-6. [DOI] [PubMed] [Google Scholar]
- 194.Boyle A, Sobotka PA. Redefining the therapeutic objective in decompensated heart failure: hemoconcentration as a surrogate for plasma refill rate. J Card Fail. 2006;12:247–249. doi: 10.1016/j.cardfail.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 195.Agar JW. Personal viewpoint: limiting maximum ultrafiltration rate as a potential new measure of dialysis adequacy. Hemodial Int. 2016;20:15–21. doi: 10.1111/hdi.12288. [DOI] [PubMed] [Google Scholar]
- 196.Mitsides N, Pietribiasi M, Waniewski J, Brenchley P, Mitra S. Transcapillary refilling rate and its determinants during haemodialysis with standard and high ultrafiltration rates. Am J Nephrol. 2019;50:133–143. doi: 10.1159/000501407. [DOI] [PubMed] [Google Scholar]
- 197.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR, et al. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. JACC. 2013;62:516–524. doi: 10.1016/j.jacc.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Oh J, Kang SM, Hong N, Youn JC, Han S, et al. Hemoconcentration is a good prognostic predictor for clinical outcomes in acute heart failure: data from the Korean Heart Failure (KorHF) registry. Int J Cardiol. 2013;168:4739–4743. doi: 10.1016/j.ijcard.2013.07.241. [DOI] [PubMed] [Google Scholar]
- 199.van der Meer P, Postmus D, Ponikowski P, Cleland JG, O'Connor CM, et al. The predictive value of short-term changes in hemoglobin concentration in patients presenting with acute decompensated heart failure. JACC. 2013;61:1973–1981. doi: 10.1016/j.jacc.2012.12.050. [DOI] [PubMed] [Google Scholar]
- 200.Rao VS, Ahmad T, Brisco-Bacik MA, Bonventre JV, Wilson FP, et al. Renal effects of intensive volume removal in heart failure patients with preexisting worsening renal function. Circ Heart Fail. 2019;12:e005552. doi: 10.1161/CIRCHEARTFAILURE.118.005552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mullens W, Abrahams Z, Francis GS, Taylor DO, Starling RC, et al. Prompt reduction in intra-abdominal pressure following large-volume mechanical fluid removal improves renal insufficiency in refractory decompensated heart failure. J Card Fail. 2008;14:508–514. doi: 10.1016/j.cardfail.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 202.Verbrugge FH, Dupont M, Bertrand PB, Nijst P, Penders J, et al. Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol. 2015;70:265–273. doi: 10.1080/ac.70.3.3080630. [DOI] [PubMed] [Google Scholar]
- 203.Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. JACC. 2018;72:1845–1855. doi: 10.1016/j.jacc.2018.06.040. [DOI] [PubMed] [Google Scholar]
- 204.McHugh KR, DeVore AD, Mentz RJ, Edmonston D, Green JB, et al. The emerging role of novel antihyperglycemic agents in the treatment of heart failure and diabetes: a focus on cardiorenal outcomes. Clin Cardiol. 2018;41:1259–1267. doi: 10.1002/clc.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lam CSP, Chandramouli C, Ahooja V, Verma S. SGLT-2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc. 2019;8:e013389. doi: 10.1161/JAHA.119.013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. NEJM. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 207.Bart BA, Boyle A, Bank AJ, Anand I, Olivari MT, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the relief for acutely fluid-overloaded patients with decompensated congestive heart failure (RAPID-CHF) trial. JACC. 2005;46:2043–2046. doi: 10.1016/j.jacc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 208.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. JACC. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 209.Marenzi G, Muratori M, Cosentino ER, Rinaldi ER, Donghi V, et al. Continuous ultrafiltration for congestive heart failure: the CUORE trial. J Card Fail. 2014;20:9–17. doi: 10.1016/j.cardfail.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 210.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. NEJM. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Costanzo MR, Negoianu D, Jaski BE, Bart BA, Heywood JT, et al. Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. JACC Heart Fail. 2016;4:95–105. doi: 10.1016/j.jchf.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 212.Grodin JL, Carter S, Bart BA, Goldsmith SR, Drazner MH, et al. Direct comparison of ultrafiltration to pharmacological decongestion in heart failure: a per-protocol analysis of CARRESS-HF. Eur J Heart Fail. 2018;20:1148–1156. doi: 10.1002/ejhf.1158. [DOI] [PubMed] [Google Scholar]
- 213.Lu R, Muciño-Bermejo MJ, Ribeiro LC, Tonini E, Estremadoyro C, et al. Peritoneal dialysis in patients with refractory congestive heart failure: a systematic review. Cardiorenal med. 2015;5:145–156. doi: 10.1159/000380915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Grossekettler L, Schmack B, Meyer K, Brockmann C, Wanninger R. Peritoneal dialysis as therapeutic option in heart failure patients. ESC Heart Fail. 2019;6:271–279. doi: 10.1002/ehf2.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Burnett H, Earley A, Voors AA, Senni M, McMurray JJ, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta-analysis. Circ Heart Fail. 2017;10:e003529. doi: 10.1161/CIRCHEARTFAILURE.116.003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hundae A, McCullough PA. Cardiac and renal fibrosis in chronic cardiorenal syndromes. Nephron Clin Pract. 2014;127:106–112. doi: 10.1159/000363705. [DOI] [PubMed] [Google Scholar]
- 217.Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in mild patients hospitalization and survival study in heart failure (EMPHASIS-HF) Circ Heart Fail. 2014;7:51–58. doi: 10.1161/CIRCHEARTFAILURE.113.000792. [DOI] [PubMed] [Google Scholar]
- 218.Elkayam U, Ng TM, Hatamizadeh P, Janmohamed M, Mehra A. Renal vasodilatory action of dopamine in patients with heart failure: magnitude of effect and site of action. Circulation. 2008;117:200–205. doi: 10.1161/CIRCULATIONAHA.107.737106. [DOI] [PubMed] [Google Scholar]
- 219.Cotter G, Weissgarten J, Metzkor E, Moshkovitz Y, Litinski I, et al. Increased toxicity of high-dose furosemide versus low-dose dopamine in the treatment of refractory congestive heart failure. Clin Pharmacol Ther. 1997;62:187–193. doi: 10.1016/S0009-9236(97)90067-9. [DOI] [PubMed] [Google Scholar]
- 220.Varriale P, Mossavi A. The benefit of low-dose dopamine during vigorous diuresis for congestive heart failure associated with renal insufficiency: does it protect renal function? Clin Cardiol. 1997;20:627–630. doi: 10.1002/clc.4960200709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Giamouzis G, Butler J, Starling RC, Karayannis G, Nastas J, et al. Impact of dopamine infusion on renal function in hospitalized heart failure patients: results of the Dopamine in acute decompensated heart failure (DAD-HF) trial. J Card Fail. 2010;16:922–930. doi: 10.1016/j.cardfail.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 222.Aziz EF, Alviar CL, Herzog E, Cordova JP, Bastawrose JH, et al. Continuous infusion of furosemide combined with low-dose dopamine compared to intermittent boluses in acutely decompensated heart failure is less nephrotoxic and carries a lower readmission at thirty days. Hellenic J Cardiol. 2011;52:227–235. [PubMed] [Google Scholar]
- 223.Triposkiadis FK, Butler J, Karayannis G, Starling RC, Filippatos G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in acute decompensated heart failure II (DAD-HF II) trial. Internat J Cardiol. 2014;172:115. doi: 10.1016/j.ijcard.2013.12.276. [DOI] [PubMed] [Google Scholar]
- 224.Ylmaz MB, Yalta K, Yontar C, Karadas F, Erdem A, et al. Levosimendan improves renal function in patients with acute decompensated heart failure: comparison with dobutamine. Cardiovasc Drugs Ther. 2007;21:431–435. doi: 10.1007/s10557-007-6066-7. [DOI] [PubMed] [Google Scholar]
- 225.Fedele F, Bruno N, Brasolin B, Caira C, D'Ambrosi A, et al. Levosimendan improves renal function in acute decompensated heart failure: possible underlying mechanisms. Eur J Heart Fail. 2014;16:281–288. doi: 10.1002/ejhf.9. [DOI] [PubMed] [Google Scholar]
- 226.Gong B, Li Z, Yat Wong PC. Levosimendan treatment for heart failure: a systematic review and meta-analysis. J Cardiothor Vasc Anesth. 2015;29:1415–1425. doi: 10.1053/j.jvca.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 227.Ponikovski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36:657–668. doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Failure. 2018;20:125–133. doi: 10.1002/ejhf.823. [DOI] [PubMed] [Google Scholar]
- 229.Evans M, Methven S, Gasparini A, Barany P, Birnie K, et al. Cinacalcet use and the risk of cardiovascular events, fractures and mortality in chronic kidney disease patients with secondary hyperparathyroidism. Sci Rep. 2018;8:2103. doi: 10.1038/s41598-018-20552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bellasi A, Morrone L, Mereu MC, Massimetti C, Pelizzaro E, et al. CKD-MBD management: what is the role of parathyroidectomy? Results from a nationwide survey in Italy. J Nephrol. 2018;31:585–591. doi: 10.1007/s40620-018-0481-7. [DOI] [PubMed] [Google Scholar]