Abstract
Objective
Retrospective and prospective memory deficits are associated with lower quality of life (QoL); however, there are no validated measures that comprehensively and directly assess the impact of memory problems on QoL. The Survey of Memory-Related Quality of Life (SMRQoL) was developed as a 30-item questionnaire to measure memory-related QoL.
Method
Both HIV+ (n = 195) and HIV− (n = 146) participants completed the SMRQoL, a neurocognitive research battery, and validated self-report questionnaires of memory, QoL, and mood. Participants were recruited into younger (age ≤ 40 years) and older (age ≥ 50 years) groups per the parent study design.
Results
The SMRQoL had a unidimensional factor structure and demonstrated measurement invariance across the HIV+ and HIV− participants. Analyses of 111 clinically stable participants (e.g., persons with no incident or remitting central nervous system disorders) who returned for a 14-month follow-up visit indicated that the SMRQoL had adequate test–retest stability. There was a significant interaction of age and HIV status on the SMRQoL, such that older HIV+ participants reported the lowest memory-related QoL. SMRQoL scores were associated with validated measures of mental and physical QoL, self-reported memory and cognitive symptoms, and performance-based memory and executive functions.
Conclusions
The SMRQoL shows evidence of reliability and validity as a measure of memory-related QoL that can be used to assess the impact of memory problems on everyday life, but future work is needed to demonstrate the measure’s incremental value in the context of diagnosis and treatment.
Keywords: Retrospective memory, Prospective memory, Everyday functioning, Neuropsychological assessment, Aging
Quality of life (QoL) is an important outcome in both clinical and research settings. According to Wilson and Cleary’s (1995) conceptual model, health-related QoL is comprised of five domains: (1) biological and physical variables; (2) symptom status, which includes a person’s subjective experience of physical and psychological health; (3) functional status, which includes the ability to complete tasks related to physical, social, role, and psychological functioning; (4) general health perceptions, which may be influenced by emotional distress and somatization; and (5) overall QoL, which includes subjective well-being and life satisfaction. These domains of QoL are influenced by various environmental factors, such as psychological, social, and economic support, as well as the individual’s personal motivation and values (Wilson & Cleary, 1995). Thus, QoL is a broad, multifaceted construct that can be influenced by several different factors and may differ greatly from one individual to another. Valid and reliable assessments of QoL are important for evaluating the clinical status of a variety of patient populations and the impact of interventions.
Neurocognitive functioning is one factor that may have a significant influence on an individual’s QoL. For example, deficits in retrospective episodic memory (i.e., memory for information from the past) might interfere with an individual’s ability to recall important work tasks or participate meaningfully in social activities, which could lead to altered health perceptions and lower QoL. Indeed, retrospective memory performance is associated with QoL among several clinical populations, including individuals with HIV disease (Tozzi et al., 2003), epilepsy (Hrabok, Sherman, Bello-Espinosa, & Hader, 2013), and severe mental illness (Fujii, Wylie, & Nathan, 2004). Subjective complaints of retrospective memory problems are also associated with QoL among community-dwelling older adults (Maki et al., 2014). Similarly, deficits in prospective memory (i.e., memory for future intentions, or the ability to “remember to remember”) may influence QoL. For example, an individual with poor prospective memory may forget to attend medical appointments or complete errands, which may affect their functional independence, health perceptions, and life satisfaction. Prospective memory is separable from retrospective memory at the level of theory (e.g., Heathcote, Loft, & Remington, 2015; McDaniel & Einstein, 2000), measurement (e.g., Gupta et al., 2010), neurobiology (e.g., Cona & Rothen, 2019; Woods et al., 2006), and everyday functioning (e.g., Scott et al., 2016; Woods, Iudicello, et al., 2008). Prospective memory symptoms and ability are associated with QoL in several populations, including HIV disease (Doyle et al., 2012), Parkinson’s disease (Pirogovsky, Woods, Filoteo, & Gilbert, 2012), and community-dwelling older adults (Woods et al., 2015). Given the strong and reliable associations between retrospective and prospective memory problems and lower QoL, directly assessing the extent to which these problems affect QoL in various clinical populations could help shape memory interventions to improve QoL.
However, few measures are available to specifically and directly examine the effect that memory problems may have on QoL. For example, the 36-item Short Form Survey (SF-36; Ware & Sherbourne, 1992), which is commonly used in research settings to measure health-related QoL, does not include any questions about memory. Some QoL questionnaires include a few questions about memory-related complaints, but the questions tend to be broad, do not usually query the direct impact of those memory symptoms on QoL, and rarely distinguish between retrospective and prospective memory. For example, measures like the World Health Organization Quality of Life assessment (WHOQOL-100; WHOQOL Group, 1998) and Patient-Reported Outcomes Measurement Information System (PROMIS; Fries, Bruce, & Cella, 2005) include general questions about problems with memory (e.g., “How would you rate your memory?” or “My memory has been as good as usual”). QoL questionnaires tailored to clinical populations who often demonstrate memory deficits more commonly include questions about memory. The Stroke Impact Scale version 3.0 (SIS; Duncan, Bode, Lai, Perera, & GAIN Americas Investigators, 2003) includes seven questions about memory and thinking. These items ask about problems with retrospective (e.g., “How difficult was it for you to remember things that people just told you?”) and prospective (e.g., “How difficult was it for you to remember to do things?”) memory, as well as concentration, processing speed, and problem-solving. The DEMQOL assessment of health-related QoL for individuals with dementia includes a subscale of six cognitive items that ask specifically about the impact of retrospective memory lapses on QoL (e.g., “How worried have you been about forgetting things that happened recently?”) in addition to questions about concentration and decision-making (Smith et al., 2005). The Neuro-QOL version 2.0, which includes a subscale of items designed to assess QoL related to cognition in neurological disorders, includes five questions about the frequency and severity of everyday memory problems (Gershon et al., 2012). However, these questionnaires do not comprehensively assess the downstream impact of memory lapses on QoL (e.g., are the memory lapses sufficiently frequent and severe to disrupt daily functioning, independent living, and well-being?).
To address these gaps in the literature, we developed the Survey of Memory-Related Quality of Life (SMRQoL), a 30-item questionnaire designed to comprehensively assess the effects of retrospective and prospective memory problems on a range of QoL domains, according to Wilson and Cleary’s (1995) model. In order to investigate the psychometric properties of this new measure of memory-related QoL, the questionnaire was given to a sample of HIV+ and seronegative adults. Adults with HIV disease demonstrate worse retrospective (e.g., Doyle et al., 2019; Reger, Welsh, Razani, Martin, & Boone, 2002) and prospective (e.g., Avci et al., 2018; Carey et al., 2006) memory performance compared to seronegative individuals and report more retrospective and prospective memory difficulties in daily life (Sheppard, Woods, Massman, & Gilbert, 2019; Woods et al., 2007). These deficits may be particularly pronounced among older HIV+ individuals, who perform worse on retrospective (e.g., Sacktor et al., 2007) and prospective (e.g., Avci, Loft, Sheppard, Woods, & The HNRP Group, 2016; Woods, Dawson, Weber, Grant, & The HNRC Group, 2010) memory tasks compared to their younger HIV+ counterparts. Individuals with HIV disease also report lower QoL than seronegative individuals do (e.g., Hays et al., 2000; Moore et al., 2013). Furthermore, older age is associated with lower health-related QoL among HIV+ adults (Pereira & Canavarro, 2011). Specifically, HIV and aging have been shown to have additive effects on QoL related to physical functioning and general health perceptions, as well as interactive effects on QoL related to emotional functioning (Morgan et al., 2012). Age may also modulate the effects of memory problems on QoL in HIV disease. Doyle et al. (2012) found that self-reported prospective memory complaints were associated with lower health-related QoL in both younger (≤40 years) and older (age ≥ 50 years) HIV-infected adults; however, time-based prospective memory performance was only associated with lower QoL in the younger participants.
The primary aim of the current study was to establish the psychometric properties of the SMRQoL by analyzing its factor structure, measurement invariance, internal consistency, test–retest stability, and convergent validity. We hypothesized that the SMRQoL would be comprised of two distinct factors corresponding to retrospective and prospective memory and demonstrate good measurement invariance and reliability. A secondary aim of the study was to investigate the effects of HIV and aging on memory-related QoL, as measured by the SMRQoL. We hypothesized that there would be an interaction of HIV and aging, whereby older HIV+ adults would demonstrate the lowest memory-related QoL compared to the other three groups (younger HIV−, older HIV−, and younger HIV+ adults). Finally, we expected that the SMRQoL would demonstrate convergent validity with other, well-validated measures of memory and QoL.
Methods
Participants
Participants were recruited from HIV clinics and the local community in Southern California. The sample included both HIV+ and HIV− adults, who were classified into younger (age ≤ 40 years) and older (age ≥ 50 years) groups (see also Avci et al., 2016; Morgan et al., 2012). HIV status was confirmed using standard western blot or point-of-care tests (MedMira Inc., Nova Scotia, Canada). Participants were excluded if they had a severe psychiatric (e.g., schizophrenia, bipolar disorder) or neurological (e.g., seizure disorder, stroke) disorder or an estimated verbal IQ score less than 70 on the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001). Additionally, participants were excluded if they met criteria for a substance use disorder within the past month, or if they tested positive on a breathalyzer or urine toxicology screen for illicit substances (except marijuana) on the day of testing. The final sample (N = 341) included 69 younger HIV− individuals, 77 older HIV− individuals, 85 younger HIV+ individuals, and 110 older HIV+ individuals. Demographic and medical data for the sample are provided in Table 1. This study was approved by the institutional review board, and all participants provided written, informed consent.
Table 1.
General descriptive data for the study sample (N = 341)
| Variable | Younger HIV− (n = 69) | Older HIV− (n = 77) | Younger HIV+ (n = 85) | Older HIV+ (n = 110) | p |
|---|---|---|---|---|---|
| Demographic | |||||
| Age (years) | 28.2 (6.1) | 56.7 (5.9) | 31.6 (5.3) | 56.3 (5.6) | <.001 |
| Education (years) | 13.9 (2.3) | 14.0 (3.0) | 12.7 (2.0) | 14.2 (2.6) | <.001 |
| Sex (% men) | 68.1 | 71.4 | 85.9 | 84.5 | .008 |
| Race/ethnicity | <.001 | ||||
| Caucasian (%) | 42.0 | 67.5 | 37.6 | 70.0 | |
| Hispanic (%) | 33.3 | 15.6 | 31.8 | 9.1 | |
| African American (%) | 18.8 | 15.6 | 25.9 | 20.0 | |
| Asian (%) | 5.8 | 0.0 | 4.7 | 0.0 | |
| Native American (%) | 0.0 | 1.3 | 0.0 | 1.0 | |
| Neurocognitive | |||||
| WTAR Verbal IQ | 101.9 (10.8) | 103.3 (10.9) | 99.5 (10.9) | 102.3 (10.9) | .169 |
| Global cognitive impairment (%) | 20.3 | 22.1 | 31.8 | 38.2 | .027 |
| Psychiatric and medical | |||||
| Lifetime affective diagnosis (%) | 27.5 | 45.5 | 71.8 | 68.2 | <.001 |
| Lifetime SUD (%) | 52.2 | 64.9 | 68.2 | 78.2 | .004 |
| POMS Depression/Dejection scale | 5.9 (7.1) | 7.2 (8.3) | 11.6 (14.0) | 11.0 (11.8) | .007 |
| Hepatitis C virus (%) | 2.9 | 21.1 | 4.8 | 31.8 | <.001 |
| HIV disease | |||||
| HIV duration (years) | — | — | 6.8 (5.7) | 16.8 (7.8) | <.001 |
| AIDS (%) | — | — | 35.7 | 66.4 | <.001 |
| CD4 count (cells/μl) | — | — | 590.7 (260.6) | 586.0 (305.5) | .527 |
| Nadir CD4 count (cells/μl) | — | — | 284.7 (192.3) | 181.9 (168.4) | <.001 |
| cART status (%) | — | — | 83.5 | 90.9 | .121 |
| Plasma RNA detectable (%) | — | — | 27.8 | 16.4 | .058 |
| Among subjects on cART (%) | — | — | 14.9 | 13.0 | .724 |
Note. Data represent M (SD) or %. WTAR = Wechsler Test of Adult Reading; SUD = substance use disorder; POMS = Profile of Mood States; AIDS = acquired immunodeficiency syndrome; CD4 = cluster of differentiation 4; cART = combination antiretroviral therapy. Bolded numbers indicate ps < .05.
A subset of participants (n = 172) returned for a follow-up evaluation to complete the same procedures approximately 1 year after their initial visit. This test–retest interval was intended to reflect common clinical practice in neuropsychology. Sixty-one of these participants were excluded from test–retest analyses due to clinically significant changes at the follow-up evaluation that could affect self-reported QoL (i.e., incident or remitting global neurocognitive impairment, incident or remitting major depressive disorder, incident substance use disorder, or a positive urine toxicology screen for illicit substances at follow-up). The final test–retest analyses therefore included 111 participants (19 younger HIV−, 32 older HIV−, 22 younger HIV+, and 38 older HIV+ individuals) with a mean follow-up interval of 14 months (M = 14.1, SD = 2.9). Descriptive data for these participants are provided in Table 2.
Table 2.
General descriptive data at the initial visit for test-retest participants (n = 111)
| Variable | Younger HIV− (n = 19) | Older HIV− (n = 32) | Younger HIV+ (n = 22) | Older HIV+ (n = 38) | p | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age (years) | 30.2 (6.9) | 56.3 (4.8) | 31.7 (5.1) | 56.4 (5.9) | <.001 | |
| Education (years) | 13.8 (2.0) | 14.6 (2.4) | 12.4 (1.6) | 14.4 (2.6) | .003 | |
| Sex (% men) | 57.9 | 65.6 | 77.3 | 81.6 | .208 | |
| Race/ethnicity | .114 | |||||
| Caucasian (%) | 52.6 | 68.7 | 45.4 | 65.8 | ||
| Hispanic (%) | 26.3 | 18.8 | 9.1 | 10.5 | ||
| African American (%) | 15.8 | 9.4 | 36.4 | 23.7 | ||
| Asian (%) | 5.3 | 0.0 | 9.1 | 0.0 | ||
| Native American (%) | 0.0 | 3.1 | 0.0 | 0.0 | ||
| Neurocognitive | ||||||
| WTAR Verbal IQ | 104.3 (8.3) | 106.4 (9.1) | 96.8 (11.5) | 103.7 (10.1) | .018 | |
| Global cognitive impairment (%) | 0.0 | 6.3 | 22.7 | 23.7 | .010 | |
| Psychiatric and medical | ||||||
| Lifetime affective diagnosis (%) | 52.6 | 46.0 | 72.7 | 63.2 | .232 | |
| Lifetime SUD (%) | 68.4 | 71.9 | 59.1 | 78.9 | .435 | |
| POMS Depression/Dejection scale | 7.5 (7.9) | 5.6 (5.2) | 9.6 (13.9) | 9.6 (12.7) | .876 | |
| Hepatitis C virus (%) | 0.0 | 12.5 | 9.1 | 34.2 | .002 | |
| HIV disease | ||||||
| HIV duration (years) | — | — | 7.2 (6.5) | 15.7 (7.8) | .001 | |
| AIDS (%) | — | — | 31.8 | 55.3 | .077 | |
| CD4 count (cells/μl) | — | — | 563.4 (215.2) | 565.0 (282.2) | .982 | |
| Nadir CD4 count (cells/μl) | — | — | 283.1 (210.3) | 214.5 (169.5) | .163 | |
| cART status (%) | — | — | 81.8 | 86.8 | .603 | |
| Plasma RNA detectable (%) | — | — | 35.0 | 23.7 | .364 | |
| Among subjects on cART (%) | — | — | 18.8 | 18.2 | .094 | |
Note. Data represent M (SD) or %. WTAR = Wechsler Test of Adult Reading; SUD = substance use disorder; POMS = Profile of Mood States; AIDS = acquired immunodeficiency syndrome; CD4 = cluster of differentiation 4; cART = combination antiretroviral therapy. Bolded numbers indicate ps < .05.
Kruskal–Wallis and X2 tests were used to compare the Table 2 characteristics of participants who were retained in test–retest analyses with those who were retained but excluded and those who were lost to follow-up. Findings revealed omnibus baseline group differences in age, sex, neurocognitive impairment, mood, and QoL (p < .05). Specifically, participants retained and included for the test–retest analyses were older than persons who were lost to follow-up. As compared to the group that was retained but excluded, persons in the test–retest analyses had higher nadir CD4 counts (HIV+ only), higher SMRQoL scores, and lower frequencies of men, neurocognitive impairment, and affective disorders at baseline (p < .05). The groups did not differ on any other variables in Table 2 (p > .05).
QoL Measures
Survey of Memory-Related Quality of Life
The Survey of Memory-Related Quality of Life (SMRQoL; see Appendix) is a 30-item questionnaire that was developed to assess the impact of memory problems on QoL. As both retrospective and prospective memory problems are associated with lower QoL (e.g., Doyle et al. 2012; Tozzi et al., 2003), questions about both types of memory were included in the SMRQoL. The SMRQoL includes 15 distinct items, each with a Part A, which asks whether the participant experiences lower QoL due to retrospective memory problems (“Remembering information or events from the past”) and a Part B, which asks whether the participant experiences lower QoL due to prospective memory problems (“Remembering to do things in the future”). Questions about retrospective and prospective memory problems were grouped together to allow side-by-side comparison of these constructs vis-à-vis the QoL items of interest. This format is used in other common neuropsychological questionnaires; for example, the Frontal Systems Behavior Scale (FrSBe; Grace & Malloy, 2001) asks participants to rate symptoms of executive functions, apathy, and disinhibition pre- and post-injury in a side-by-side fashion. Additionally, this format was thought to be particularly important given the subtle conceptual differences between retrospective and prospective memory, which might be difficult for lay persons to distinguish if they were probed separately. Thus, we expected that this side-by-side approach would give participants the greatest likelihood of being able to carefully weigh possible differences between the impact of prospective and retrospective memory symptoms on different aspects of QoL.
The 15 distinct items of the SMRQoL address five domains of QoL: general, physical, mental, social, and instrumental activities of daily living (IADLs). These domains were selected based on well-established models of QoL (e.g., Wilson & Cleary, 1995) in order to provide comprehensive coverage of the construct. All items were generated by the senior author and were reviewed by two neuropsychologists with expertise in aging, HIV, and everyday functioning. Three items per domain were constructed to represent each of the five QoL domains. Specifically, general QoL is assessed with items 3 (overall health and well-being), 7 (QoL), and 13 (difficulty maintaining health and well-being). Physical QoL is assessed with items 4 (physical needs), 10 (physical health), and 15 (personal grooming), and mental QoL is assessed with items 6 (embarrassment), 9 (sadness or depression), and 14 (nervousness or anxiety). Social QoL is assessed with items 2 (other people’s opinions), 8 (relationships with family and friends), and 12 (social interactions). Finally, QoL related to IADLs is assessed with items 1 (relying on others for help in daily life), 5 (trouble managing personal affairs), and 11 (difficulty following a doctor’s advice). Each item is rated on a scale from 1 (“Strongly Agree”) to 5 (“Strongly Disagree”). The total score is the sum of responses across the 30 items (range = 30–150), whereby higher scores indicate better memory-related QoL. Retrospective and prospective memory items on the SMRQoL were highly correlated (ρ = .94, p < .001)
36-Item Short Form Survey
All participants completed the SF-36 (Ware & Sherbourne, 1992), which is a widely used and well-validated questionnaire that assesses health-related QoL (sample Cronbach’s α = .88). Analyses were performed using sample-based Z-scores of the Physical Health and Mental Health Summary Scores from the SF-36.
Memory Measures
Performance-based memory
Participants completed three performance-based memory tests that assessed both prospective and retrospective memory. The research version of the Memory for Intentions Test (MIsT; Raskin, Buckheit, & Sherrod, 2010) is a well-validated and reliable laboratory measure of prospective memory performance (Woods, Moran, et al., 2008). In this test, participants are asked to perform eight different intentions while completing an ongoing word search. Participants are instructed to complete four time-based (e.g., “In two minutes, ask me what time this session ends today”) and four event-based (e.g., “When I hand you a red pen, sign your name on your paper”) intentions throughout the test. Participants also completed the California Verbal Learning Test–Second Edition (CVLT-II; Delis, 2000) and the Logical Memory subtest of the Wechsler Memory Scale–Third Edition (WMS-III; Wechsler, 1997b). In the CVLT-II, the examiner reads a list of 16 words to the participant, who is asked to repeat them over five trials (Trial 1–5 score). After reading an interference list, the examiner asks the participant to repeat the original 16-word list (Short Delay Free Recall). After a 20-min delay, participants are again asked to name as many words as they can from the original list (Long Delay Free Recall score). During the Logical Memory subtest, the examiner reads two short stories to the participant. The participant is asked to repeat the short stories immediately after hearing them (Logical Memory I Total Unit Score) and after a 20–30-min delay (Logical Memory II Total Unit Score). A performance-based delayed memory Z-score was derived by averaging the sample-based Z-scores of the MIsT Summary Score, CVLT-II Long Delay Free Recall raw score, and Logical Memory II Total Unit Score.
Self-report memory
Participants completed the Prospective and Retrospective Memory Questionnaire (PRMQ; Smith, Della Sala, Logie, & Maylor, 2000), which assesses memory symptoms in daily life. The PRMQ consists of 16 questions related to prospective and retrospective memory problems, which are rated on a scale from 1 (“Never”) to 5 (“Very Often”). A sum of responses is calculated (range = 16–80), whereby higher PRMQ scores indicate more everyday memory complaints. Scores were converted to sample-based Z-scores. Additionally, general cognitive complaints were assessed with the ‘Confusion or Bewilderment’ scale of the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971). The POMS Confusion/Bewilderment scale includes seven items that ask about cognitive symptoms within the past week. Each item is rated on a 5-point scale from 0 (“Not at All”) to 4 (“Extremely”). The POMS Confusion/Bewilderment scale is calculated as the sum of responses for the seven items (range = 0–28), whereby higher scores correspond to more cognitive complaints. A sample-based Z-score was calculated for the POMS Confusion/Bewilderment scale.
Other Neurocognitive Measures
In order to determine the relationship of the SMRQoL to other neurocognitive domains, a neuropsychological research battery was administered. Participants completed the Digit Span subtest of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler, 1997a), the Trailmaking Test (TMT) parts A and B (Reitan & Wolfson, 1985), the Tower of London Test (Drexel Version; Culbertson & Zillmer, 2001), the Grooved Pegboard Test (Kløve, 1963), and action (verb) fluency (Woods et al., 2005). A sample-based Z-score for attention and working memory was calculated using the Digit Span total raw score and CVLT-II Trial 1 raw score, and a Z-score for executive functions was calculated using TMT B, action fluency, and the Tower of London total moves score. A processing speed Z-score was calculated with TMT A and Tower of London total time, and a motor functioning Z-score was calculated using the dominant and non-dominant hand trials of the grooved pegboard test.
Global Neurocognitive Impairment
Global neurocognitive impairment was determined using the reliable and well-validated clinical ratings procedure from Woods et al. (2004) based on the participant’s normative performance in the domains of attention/working memory, executive functions, learning, retrospective memory, processing speed, and motor skills. Participants were assigned a clinical rating for each domain, ranging from 1 (above average, corresponding to T-scores ≥ 55) to 9 (severe impairment, corresponding to T-scores ≤ 19). Participants with ratings of 5 (mild impairment, corresponding to T-scores ≤ 40) or greater in at least two domains were considered to have global neurocognitive impairment (Woods et al., 2004).
Psychiatric Measures
Current affective distress was measured using the ‘Depression or Dejection’ scale of the POMS. This scale consists of 15 items that are rated from 0 (“Not at All”) to 4 (“Extremely”). Higher scores indicate more depressive symptoms (range = 0–60). The Composite International Diagnostic Interview (version 2.128; World Health Organization, 1998) was also administered to determine current and lifetime diagnoses of depression, anxiety, and substance use disorder. Participants were classified with an affective diagnosis if they met criteria for a current or lifetime diagnosis of depression or anxiety.
Data Analysis
Preliminary analyses
Parallel analysis and confirmatory factor analysis (CFA) were conducted to examine the structure of the SMRQoL. The analyses were computed separately for the HIV+ and HIV− participants. The parallel analysis was performed to examine dimensionality of the SMRQoL items. This analysis involved computing eigenvalues from a tetrachoric correlation matrix of items and comparing these eigenvalues with those obtained from a simulated tetrachoric matrix that is known to be unidimensional. The comparison of the eigenvalues from the real and simulated data indicated the degree of unidimensionality in the items. The parallel analysis was estimated in SAS software, Version 9.4 for Windows (SAS Institute, Inc., 2015). The CFA model with correlated residuals for paired items (e.g., Items 1A and 1B) was used to examine the latent structure of the SMRQoL. A graded response model was used to account for the polytomous response format of the SMRQoL items. CFA analyses were estimated in Mplus software, Version 7.2 (Muthén & Muthén, 2012). The weighted least square mean and variance adjusted (WLSMV) with a probit link, and the THETA parameterization were used to estimate unknown parameters. Model fit was examined using the root mean square error of approximation (RMSEA), comparative fit index (CFI), and TLI. Given smaller sample size, an acceptable fit was assumed for the models with RMSEA ≤ .08, CFI ≥ .95, and TLI ≥ .95 (Chen, Curran, Bollen, Kirby, & Paxton, 2008).
Measurement invariance
Measurement invariance across the HIV+ and HIV− groups was examined by testing the invariance of measurement parameters including factor loadings, thresholds, and residual variances. Testing measurement invariance involved fitting a series of increasingly restricted models: configural invariance (Model 1: equal pattern of factor loadings), metric invariance (Model 2: equal factor loadings, i.e., 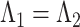 ), scalar invariance (Model 3: equal factor loadings and thresholds, i.e.,
), scalar invariance (Model 3: equal factor loadings and thresholds, i.e.,  and
and 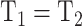 ), and residual variance invariance (Model 4: equal factor loadings, thresholds, and residual variances, i.e.,
), and residual variance invariance (Model 4: equal factor loadings, thresholds, and residual variances, i.e.,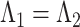 and
and 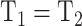 and
and 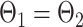 ; Millsap, 2011).
; Millsap, 2011).
Multiple Indicators and Multiple Causes model
A single-group Multiple Indicators and Multiple Causes (MIMIC) model with categorical factor indicators and a threshold structure using the THETA parameterization was estimated to examine the influence of HIV status and age group on the SMRQoL. Any demographic, neurocognitive, or psychiatric/medical variables from Table 1 that significantly differed across the four age and HIV groups and were significantly related to the SMRQoL factor scores were included as covariates in this model. The interaction of HIV status and age was included to determine whether the HIV effect is moderated by age.
Models were estimated in Mplus software, Version 7.2 (Muthén & Muthén, 2012). The WLSMV with a probit link and the THETA parameterization was used to estimate unknown parameters. Model fit indices, including RMSEA, CFI, and TLI, were used to examine the fit of any given model to the polychoric correlation matrix among the items for each group (Kenny, 2011). Difference in  was used to evaluate whether a particular model constraint was tenable. If the constraint was not tenable, modification indices were examined to determine a source of model misfit and to refit a model with relaxed constraints (Jöreskog & Sörbom, 2002).
was used to evaluate whether a particular model constraint was tenable. If the constraint was not tenable, modification indices were examined to determine a source of model misfit and to refit a model with relaxed constraints (Jöreskog & Sörbom, 2002).
Reliability
After an adjusted sum of SMRQoL responses was calculated based on the results of preliminary analyses, Cronbach’s alpha was used to determine the internal consistency of this adjusted sum at the initial visit. Test–retest analyses of the SMRQoL were calculated using the adjusted sum of SMRQoL responses for the 111 participants who remained clinically stable at the 14-month follow-up. First, test–retest stability was calculated using an intraclass correlation coefficient. A Wilcoxon signed-rank test was then conducted to determine whether there was a significant change in SMRQoL responses at follow-up. Next, a reliable change index (RCI; Jacobson & Truax, 1991) was calculated. A 90% confidence interval for significant change was generated using the RCI, adjusting for the mean 14-month change in SMRQoL responses. Reliability analyses were conducted using JMP Pro version 13.0.0 and IBM SPSS Statistics version 24.
Validity
Multiple regressions were run in JMP Pro version 13.0.0 to establish convergent validity of the SMRQoL with other measures of memory and QoL. Each neurocognitive and QoL measure was regressed on the SMRQoL factor score, controlling for age/HIV status group. Using a Bonferroni correction to correct for multiple comparisons, the critical alpha was set to .006.
Results
Preliminary Analyses
Endorsement rates
Frequency count for each item was examined before conducting the parallel analysis. In the HIV+ group, endorsement rates for “Strongly Agree” ranged from 1 to 11 participants, “Agree” ranged from 8 to 57 participants, “Neither Agree nor Disagree” ranged from 23 to 49 participants, “Disagree” ranged from 38 to 72 participants, and “Strongly Disagree” ranged from 54 to 101 participants. In the HIV− group, endorsement rates for “Strongly Agree” ranged from 0 to 3 participants, “Agree” ranged from 3 to 22 participants, “Neither Agree nor Disagree” ranged from 9 to 26 participants, “Disagree” ranged from 19 to 32 participants, and “Strongly Disagree” ranged from 78 to 108 participants. Because endorsement rates for “Strongly Agree” were very low (especially in the HIV− group), the “Strongly Agree” and “Agree” options were collapsed into one category for all analyses. Items were scored as 0 points if the participant endorsed “Agree” or “Strongly Agree,” 1 point if the participant responded “Neither Agree nor Disagree,” 2 points if the participant responded “Disagree,” and 3 points if the participant responded “Strongly Disagree.” Correlations for all 30 SMRQoL items are presented in Table 3.
Table 3.
Pearson correlation coefficients for the items of the Survey of Memory-Related Quality of Life (N = 341)
| 1A | 1B | 2A | 2B | 3A | 3B | 4A | 4B | 5A | 5B | 6A | 6B | 7A | 7B | 8A | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1.00 | ||||||||||||||
| 1B | .69 | 1.00 | |||||||||||||
| 2A | .52 | .53 | 1.00 | ||||||||||||
| 2B | .53 | .62 | .82 | 1.00 | |||||||||||
| 3A | .47 | .44 | .53 | .56 | 1.00 | ||||||||||
| 3B | .43 | .53 | .56 | .62 | .89 | 1.00 | |||||||||
| 4A | .47 | .40 | .52 | .55 | .61 | .59 | 1.00 | ||||||||
| 4B | .45 | .53 | .51 | .59 | .61 | .68 | .86 | 1.00 | |||||||
| 5A | .55 | .53 | .55 | .60 | .72 | .69 | .70 | .69 | 1.00 | ||||||
| 5B | .48 | .65 | .54 | .62 | .64 | .73 | .64 | .73 | .83 | 1.00 | |||||
| 6A | .47 | .46 | .54 | .56 | .63 | .61 | .50 | .50 | .68 | .59 | 1.00 | ||||
| 6B | .52 | .57 | .57 | .67 | .64 | .68 | .56 | .58 | .68 | .70 | .82 | 1.00 | |||
| 7A | .49 | .49 | .55 | .56 | .67 | .64 | .50 | .48 | .68 | .61 | .75 | .75 | 1.00 | ||
| 7B | .50 | .55 | .53 | .62 | .67 | .70 | .52 | .56 | .69 | .72 | .70 | .82 | .84 | 1.00 | |
| 8A | .47 | .49 | .58 | .56 | .61 | .55 | .51 | .50 | .68 | .58 | .67 | .60 | .76 | .71 | 1.00 |
| 8B | .46 | .52 | .55 | .65 | .59 | .59 | .49 | .53 | .61 | .65 | .60 | .69 | .69 | .82 | .83 |
| 9A | .40 | .43 | .49 | .49 | .60 | .57 | .39 | .39 | .61 | .54 | .62 | .59 | .69 | .66 | .64 |
| 9B | .44 | .49 | .57 | .63 | .59 | .61 | .40 | .46 | .60 | .62 | .60 | .67 | .67 | .76 | .61 |
| 10A | .49 | .46 | .52 | .53 | .69 | .64 | .51 | .53 | .68 | .59 | .66 | .61 | .74 | .71 | .75 |
| 10B | .46 | .53 | .54 | .62 | .68 | .70 | .54 | .58 | .66 | .70 | .61 | .68 | .71 | .78 | .67 |
| 11A | .51 | .49 | .52 | .53 | .60 | .59 | .61 | .59 | .71 | .63 | .61 | .62 | .67 | .66 | .66 |
| 11B | .52 | .57 | .51 | .61 | .58 | .62 | .58 | .61 | .68 | .71 | .56 | .63 | .62 | .68 | .61 |
| 12A | .53 | .49 | .56 | .56 | .61 | .60 | .48 | .48 | .70 | .60 | .71 | .63 | .76 | .68 | .70 |
| 12B | .53 | .59 | .58 | .63 | .60 | .63 | .54 | .56 | .67 | .72 | .64 | .71 | .69 | .76 | .67 |
| 13A | .43 | .41 | .45 | .49 | .63 | .60 | .56 | .57 | .67 | .60 | .60 | .58 | .71 | .66 | .65 |
| 13B | .45 | .50 | .46 | .56 | .57 | .60 | .56 | .60 | .61 | .67 | .52 | .59 | .61 | .70 | .58 |
| 14A | .51 | .45 | .48 | .51 | .59 | .55 | .42 | .41 | .62 | .54 | .69 | .65 | .71 | .63 | .61 |
| 14B | .52 | .52 | .56 | .60 | .61 | .62 | .45 | .48 | .60 | .63 | .62 | .73 | .69 | .75 | .59 |
| 15A | .40 | .41 | .45 | .48 | .53 | .51 | .51 | .53 | .61 | .55 | .51 | .49 | .55 | .53 | .61 |
| 15B | .37 | .45 | .48 | .49 | .45 | .51 | .48 | .53 | .55 | .60 | .43 | .48 | .48 | .55 | .54 |
| 8B | 9A | 9B | 10A | 10B | 11A | 11B | 12A | 12B | 13A | 13B | 14A | 14B | 15A | 15B | |
| 8B | 1.00 | ||||||||||||||
| 9A | .59 | 1.00 | |||||||||||||
| 9B | .68 | .85 | 1.00 | ||||||||||||
| 10A | .67 | .68 | .66 | 1.00 | |||||||||||
| 10B | .75 | .65 | .71 | .89 | 1.00 | ||||||||||
| 11A | .60 | .59 | .58 | .69 | .68 | 1.00 | |||||||||
| 11B | .65 | .56 | .64 | .64 | .73 | .87 | 1.00 | ||||||||
| 12A | .60 | .70 | .63 | .71 | .68 | .70 | .66 | 1.00 | |||||||
| 12B | .72 | .68 | .71 | .70 | .78 | .66 | .73 | .82 | 1.00 | ||||||
| 13A | .62 | .62 | .61 | .77 | .73 | .78 | .70 | .70 | .62 | 1.00 | |||||
| 13B | .67 | .57 | .65 | .70 | .79 | .73 | .78 | .60 | .73 | .84 | 1.00 | ||||
| 14A | .54 | .74 | .67 | .66 | .61 | .59 | .57 | .76 | .69 | .58 | .51 | 1.00 | |||
| 14B | .66 | .74 | .78 | .66 | .71 | .62 | .64 | .69 | .79 | .58 | .63 | .84 | 1.00 | ||
| 15A | .54 | .51 | .53 | .62 | .57 | .59 | .54 | .57 | .61 | .60 | .58 | .57 | .56 | 1.00 | |
| 15B | .56 | .47 | .55 | .52 | .59 | .55 | .60 | .53 | .64 | .54 | .63 | .48 | .58 | .90 | 1.00 |
Parallel analysis
As depicted in Fig. 1, the parallel analysis suggested that SMRQoL items were unidimensional and captured one underlying factor in the HIV+ and HIV− groups. In both groups, only one factor had an observed eigenvalue that was larger than the 95th percentile of the distribution of the expected eigenvalue obtained from the simulated data.
Confirmatory factor analysis
The one-factor CFA model provided an acceptable fit to the data for the HIV− group, RMSEA = .08, CFI = 0.99, TLI = 0.99, but not for the HIV+ group, RMSEA = .14, CFI = 0.96, TLI = 0.96. To improve model fit, Items 11A (“I have difficulty following my doctor’s advice because of my problems remembering information or events from the past”) and 11B (“I have difficulty following my doctor’s advice because of my problems remembering to do things in the future”) were removed from the model based on the modification indices from the Mplus software. This pair of items was highly correlated in the HIV+ group (r = .87), which suggests that the two items may be measuring the same construct. The models with Items 11A and 11B removed provided sufficient fit to the data in both the HIV+ group, RMSEA = .08, CFI = 0.98, TLI = 0.98, and the HIV− group, RMSEA = .08, CFI = 0.99, TLI = 0.98. Subsequent analyses, including measurement invariance, the MIMIC model, and reliability, and validity were computed using the remaining 28 SMRQoL items.
Measurement Invariance
The configural invariance model had an acceptable fit, RMSEA = .09, CFI = 0.98, TLI = 0.98, suggesting equivalent patterns of factor loadings across groups. The metric invariance model had a good fit, RMSEA = .07, CFI = 0.99, TLI = 0.99. The difference in 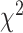 between the configural and metric models,
between the configural and metric models, 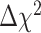 (27) = 36.81, p = .10, suggested that an equality constraint imposed on factor loadings was tenable. The scalar invariance was also tenable. The fit indices, RMSEA = .07, CFI = 0.99, TLI = 0.99, and
(27) = 36.81, p = .10, suggested that an equality constraint imposed on factor loadings was tenable. The scalar invariance was also tenable. The fit indices, RMSEA = .07, CFI = 0.99, TLI = 0.99, and 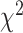 difference test between metric and scalar models,
difference test between metric and scalar models, 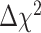 (83) = 104.36, p = .06, suggested that the threshold equality constraint did not significantly worsen the model fit. The model with non-equivalent residual variances (RMSEA = .08, CFI = 0.98, TLI = 0.98) provided significantly better fit to the data,
(83) = 104.36, p = .06, suggested that the threshold equality constraint did not significantly worsen the model fit. The model with non-equivalent residual variances (RMSEA = .08, CFI = 0.98, TLI = 0.98) provided significantly better fit to the data,  (28) = 41.56, p = .05, relative to the model with equivalent residual variances (RMSEA = .07, CFI = 0.99, TLI = 0.99), suggesting that the equality constraint that was imposed on the residual variances was not tenable. After relaxing residual variances for Item 6B (“I am embarrassed by my problems remembering to do things in the future”), the fit indices, RMSEA = .07, CFI = 0.99, TLI = 0.99, and
(28) = 41.56, p = .05, relative to the model with equivalent residual variances (RMSEA = .07, CFI = 0.99, TLI = 0.99), suggesting that the equality constraint that was imposed on the residual variances was not tenable. After relaxing residual variances for Item 6B (“I am embarrassed by my problems remembering to do things in the future”), the fit indices, RMSEA = .07, CFI = 0.99, TLI = 0.99, and 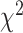 difference test,
difference test, 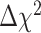 (27) = 37.35, p = .09, suggested lack of deterioration in model fit. Because the measurement model was invariant, it was permissible to estimate the MIMIC model in a subsequent step.
(27) = 37.35, p = .09, suggested lack of deterioration in model fit. Because the measurement model was invariant, it was permissible to estimate the MIMIC model in a subsequent step.
MIMIC Model
Of the potential demographic, neurocognitive, and psychiatric/medical covariates listed in Table 1, education, global neurocognitive impairment, affective diagnosis, lifetime substance use disorder, and hepatitis C virus infection were associated with the four age/HIV groups and SMRQoL factor scores and therefore met criteria for inclusion as covariates. Specifically, lower education, presence of an affective diagnosis, lifetime substance use disorder, global neurocognitive impairment, and hepatitis C virus infection were associated with lower SMRQoL factor scores (p < .01). Whereas more reported symptoms on the POMS Depression/Dejection scale were also associated with lower SMRQoL factor scores (p < .001), this variable was not included in the model because affective diagnosis was already present and those two variables were highly related to one another. The MIMIC model provided a good fit to the data, RMSEA = .07, CFI = 0.98, TLI = 0.97.
As expected, the HIV status × age interaction was statistically significant, b = −0.48, p = .03, over and above relevant covariates. As shown in Fig. 2, SMRQoL factor scores were lower for the HIV+ group relative to the HIV− group, though the magnitude of the difference between the two groups was greater for older compared to younger participants. Education, b = 0.05, p = .031, affective diagnosis, b = −0.70, p < .001, global neurocognitive impairment, b = −0.36, p = .002, and lifetime substance use disorder, b = −0.39, p = .001, were also significant predictors of SMRQoL factor scores, whereby individuals with fewer years of education, affective diagnoses, global neurocognitive impairment, and lifetime substance use disorders reported lower memory-related QoL. Post hoc Steel–Dwass tests to probe the interaction revealed that the older HIV+ group had significantly lower SMRQoL factor scores than the younger HIV+ group (d = .45, p = .011), the older HIV− group (d = 1.00, p < .001), and the younger HIV− group (d = 1.20, p < .001).
Reliability
Reliability analyses were conducted using the adjusted sum of SMRQoL responses across the 28 SMRQoL items (collapsing the “Strongly Agree” and “Agree” categories) for a possible adjusted SMRQoL score ranging from 0 (lowest memory-related QoL) to 84 (highest memory-related QoL). The adjusted SMRQoL sum demonstrated strong internal consistency at the initial visit (α = .98). The intraclass correlation coefficient for test–retest stability among the clinically stable participants was acceptable (see Table 4). A Wilcoxon signed-rank test among these participants indicated that there was no significant change in adjusted SMRQoL responses at the 14-month follow-up visit, S = 330.50, d = 0.10, p = .330. The adjusted sum of SMRQoL scores at baseline and follow-up, as well as the RCI 90% confidence intervals generated at an individual level in the test–retest sample, are presented in Table 4.
Table 4.
Fourteenth-month stability of the Survey of Memory-Related Quality of Life (SMRQoL) among clinically stable participants (n = 111)
| Initial visit | 14-month visit | 14-month change | |
|---|---|---|---|
| SMRQoL-adjusted sum M (SD) | 60.0 (23.6) | 62.4 (23.9) | 2.3 (17.7) |
| RCI 90% confidence interval | — | — | [−26, 31] |
| Intraclass correlation | — | — | .72 |
Note. RCI = reliable change index.
Validity
In separate regression analyses controlling for age/HIV status group, higher SMRQoL factor scores were significantly associated with higher physical QoL (b = 0.56, SE = 0.05, p < .001) and mental QoL (b = 0.72, SE = 0.05, p < .001) on the SF-36, fewer memory complaints on the PRMQ (b = −0.67, SE = 0.06, p < .001), and fewer reported symptoms on the POMS Confusion/Bewilderment scale (b = −0.64, SE = 0.06, p < .001). SMRQoL factor scores were also associated with performance-based delayed memory (b = 0.22, SE = 0.05, p < .001) and executive functions (b = 0.20, SE = 0.05, p < .001); however, they were not associated with measures of attention/working memory (b = 0.07, SE = 0.05, p = .209), processing speed (b = 0.10, SE = 0.05, p = .063), or motor functioning (b = 0.09, SE = 0.06, p = .137).
Discussion
Problems with retrospective and prospective memory are associated with lower QoL among a variety of clinical populations; however, no measures are currently available to directly and comprehensively assess memory-related QoL. The SMRQoL was developed as a 30-item self-report questionnaire to assess five domains of QoL related to problems with retrospective and prospective memory. Preliminary analyses indicated that some adjustments to the calculation of the SMRQoL score were necessary to achieve adequate model fit. Few participants endorsed the “Strongly Agree” option for questions about retrospective and prospective memory problems; therefore, the initial five Likert response options were scored on a four-point scale. Additionally, Items 11A and 11B, which ask about problems following a doctor’s advice due to memory problems, were removed from the CFA model in order to achieve adequate fit.
After making these adjustments, the one-factor CFA model provided a good fit to the data for the HIV+ and HIV− groups. Therefore, the hypothesis that the SMRQoL contained two factors, pertaining to retrospective memory and prospective memory, was not supported. The correlation between retrospective and prospective memory items on the SMRQoL was quite large (ρ = .94, p < .001), which may help to explain the one-factor structure of the questionnaire. Although prospective memory performance is separable from retrospective memory (e.g., Gupta et al., 2010), these two types of episodic memory are correlated among older adults (e.g., Kamat et al., 2014), and conceptual models agree that successful prospective memory depends on retrospective recall of the intention (McDaniel & Einstein, 2000). A prior factor analysis of the PRMQ also found that retrospective and prospective memory complaints represented two distinct but correlated constructs (Crawford, Smith, Maylor, Della Sala, & Logie, 2003). Therefore, it is likely that individuals who experience QoL problems related to retrospective memory failures would also have problems due to prospective memory. The item structure and prompts (i.e., side-by-side ratings of prospective and retrospective memory) may have also played a role in the SMRQoL’s unidimensional structure. It may be difficult for lay persons to appreciate the subtle differences in how retrospective and prospective memory affect their daily lives. Indeed, prospective memory is only singly dissociable from retrospective memory (i.e., the latter is necessary, but not sufficient for the former). Although we attempted to prevent this issue by providing examples of each type of memory in the SMRQoL instructions (see Appendix), it is possible that participants may require a more in-depth explanation and more salient examples of these subtle differences. The one-factor structure of the SMRQoL also did not support the existence of empirically dissociable subscales corresponding to the different domains of QoL (i.e., general, physical, mental, social, and IADL).
Nonetheless, results of psychometric analyses suggest that the SMRQoL can be used as a measure of overall memory-related QoL. The SMRQoL demonstrated adequate measurement invariance across the HIV+ and HIV− groups, providing initial evidence for its use as an appropriate measure of memory-related QoL in these populations. Analyses also indicated that the SMRQoL’s internal consistency was very strong at the initial visit, and test–retest stability was acceptable among the clinically stable participants. There was no significant change in SMRQoL responses over the 14-month follow-up interval. These results suggest that the SMRQoL is a reliable and consistent measure among both HIV+ and HIV− adults. That said, the test–retest analyses were conducted among the subset of clinically stable participants who returned for a 14-month follow-up visit, which allowed us to focus on the psychometric stability of the SMRQoL, but may have simultaneously limited the generalizability of the results. Most notably, participants included in stability analyses were older than persons lost to follow-up; they also had higher baseline SMRQoL scores and lower rates of both neurocognitive impairment and affective disorders as compared to persons who were retained but excluded due to clinical instability. Therefore, it is possible that individuals with lower memory-related QoL, neurocognitive disorders, or subjective cognitive impairment at baseline may demonstrate different trajectories of SMRQoL scores over time. Consideration of these baseline factors and responsible use of the confidence intervals displayed in Table 4 may be important for studies seeking to determine the natural course, the impact of incident and remitting primary and comorbid conditions (e.g., HIV-associated dementia, apathy, cardiovascular disease), and the effects of interventions (e.g., cognitive rehabilitation, pharmacotherapy) on SMRQoL scores in the setting of HIV disease.
In terms of its validity, results of the MIMIC model supported the hypothesis of an interaction between HIV status and age group on SMRQoL scores, which was not confounded by demographic, neurocognitive, psychiatric, and medical variables. Follow-up analyses revealed that the older HIV+ group endorsed significantly more memory-related QoL problems than the other groups at the level of a large effect size (d = 0.84). This finding is consistent with prior research that found that HIV disease and aging increase the risk of memory problems (e.g., Carey et al., 2006; Reger et al., 2002, Sacktor et al., 2007; Woods et al., 2007, 2009) and are associated with lower QoL (e.g., Hays et al., 2000; Moore et al., 2013; Piette et al., 1995). A prior study (Morgan et al., 2012) reported additive and interactive effects of HIV and aging on various domains of QoL on the SF-36; however, their study did not assess for memory-related problems. Therefore, the current study extends our understanding of HIV and aging by demonstrating interactive effects specifically on memory-related QoL. Findings extend prior work using separate measures of memory and QoL (e.g., Woods et al., 2015) and provide more direct evidence that individual perceptions of memory failures in daily life have apparent downstream effects on diverse aspects of QoL (i.e., mental, physical, social, and IADL).
The MIMIC model also found independent effects of education, global neurocognitive impairment, affective diagnosis, lifetime substance use disorder, and hepatitis C virus infection on SMRQoL factor scores. Findings are consistent with prior studies showing that these factors are associated with poorer memory and QoL (e.g., Barreira, Marinho, Bicho, Fialho, & Ouakinin, 2019; González-Saiz, Rojas, & Castillo, 2009; Scott et al., 2018). Future studies focusing on the combined effects of these variables in the context of HIV will be useful in understanding whether they have independent, additive, or synergistic effects on memory-related QoL (e.g., Thaler, Sayegh, Kim, Castellon, & Hinkin, 2015). Unfortunately, this study was not properly designed to test for additive or synergistic effects of HIV disease, psychiatric diagnoses, substance use, and cognitive impairment on memory-related QoL, as such an undertaking requires larger sample sizes and consideration of very specific covariates for each variable under study, which in this case was focused exclusively on the combined effects of HIV and aging. However, this is an important area for future work, particularly given that, for example, HIV and substance use may have interactive effects on memory and daily functioning (Blackstone et al., 2013; Rippeth et al., 2004).
The convergent validity of the SMRQoL is supported by its associations with related measures. Specifically, the SMRQoL was associated with well-validated self-report scales measuring physical and mental health-related QoL, retrospective and prospective memory symptoms, and cognitive complaints. Thus, there is evidence that the SMRQoL relates to the two broad constructs on which it converges (i.e., memory and QoL). The SMRQoL was also associated with performance-based measures of memory and executive functions, with the latter being acknowledged as an important aspect of “working with memory” (Moscovitch, 1992). Therefore, lower levels of memory ability and higher levels of executive dysfunction (i.e., cognitive flexibility, generativity, and planning) appear to amplify the extent to which perceived memory failures affect QoL. A recent meta-analysis concluded that the association between subjective and objective memory is small and inconsistent (Crumley, Stetler, & Horhota, 2014), which is consistent with studies specifically in prospective memory (e.g., Uttl & Kibreab, 2011; Woods et al., 2007). Self-reported retrospective and prospective memory symptoms in HIV can be strongly influenced by psychological factors, including depression, fatigue, and personality (e.g., Millikin, Rourke, Halman, & Power, 2003; Rourke, Halman, & Bassel, 1999; Uttl & Kibreab, 2011; Woods et al., 2007). Indeed, our results showed that the SMRQoL was significantly associated with self-reported depressive symptoms and the presence of an affective diagnosis. Nonetheless, subjective memory problems are still independently associated with everyday functioning outcomes in HIV+ (e.g., Woods, Iudicello, et al., 2008) and aging (e.g., Woods et al., 2014) populations. Additionally, despite a small-to-moderate correlation between attention/working memory and SMRQoL factor scores in the overall sample (ρ = .22, p < .001), this effect was not significant after controlling for age/HIV group (b = 0.07, p = .209). The lack of an independent association between attention/working memory and memory-related QoL was somewhat surprising, as those with poor attention and working memory might be expected to have more downstream difficulty with learning and memory in their everyday lives. However, our battery was heavy on verbal auditory attention (i.e., WAIS-III Digit Span, CVLT-II Trial 1) and did not include visual or higher-load working memory tasks (e.g., Paced Auditory Serial Addition Task, Corsi block-tapping test), which may be of interest to future studies in this area. Although the SMRQoL suffers from the same limitations as other self-report measures, it demonstrates convergent validity with established self-report measures of memory and QoL and may be helpful in assessing everyday functioning.
Although results provide preliminary support for the use of the SMRQoL as a reliable and valid measure of memory-related QoL, in the following, we highlight six interpretive and practical limitations. First, responses on the SMRQoL were collapsed from a 5-point scale to a 4-point scale. Collapsing response categories is based on an assumption that Likert scale items are not interval data (Beamish, 2004). Data are considered to be measured on an ordinal scale if the criteria of distinctiveness and ordering in magnitude are met, whereas an interval scale additionally requires equal intervals (Allen & Yen, 2002). The feature of equal intervals is not applicable to the SMRQoL, as one cannot assume equivalent differences between two numbers (response categories) on a Likert scale. Consequently, our data meet the criteria of an ordinal scale, which in turn allows for collapsing response categories. A second notable limitation of this study is that self-reported memory measures rely on metacognition, or insight into one’s own cognitive abilities. Individuals with HIV-associated neurocognitive disorders (HAND) demonstrate deficits in metamemory (Casaletto, Doyle, Weber, Woods, & The HNRP Group, 2014), which is unsurprising given the involvement of fronto-striato-thalamo-cortical circuits in HAND and the importance of frontal executive processes for metamemory (Rourke, Halman, & Bassel, 1999). Older adults with HAND may demonstrate even less awareness of their memory functioning. Therefore, future studies may wish to investigate the utility of informant- or clinician-report versions of the SMRQoL, although these methods are more common for assessments of cognitive symptoms or activities of daily living, rather than QoL, which by definition is a highly subjective psychological construct. A third limitation of this study is the long test–retest interval (14 months). Although there was no significant change in SMRQoL scores between the initial visit and follow-up, it is important to note that there was a large amount of variability in adjusted SMRQoL scores among the sample. Indeed, the RCI 90% confidence interval indicated that a participant’s score must decline more than 26 points, or increase more than 31 points, in order to constitute a significant difference. Although test–retest analyses were only calculated among participants who remained clinically stable at the follow-up visit, the large amount of heterogeneity in memory-related QoL may have precluded the detection of clinically significant changes in memory-related QoL. Future studies with shorter test-retest intervals would be helpful in clarifying the stability of the SMRQoL.
A fourth limitation is the unidimensional structure of the questionnaire which suggests that the SMRQoL measures overall memory-related QoL. Thus, it is unclear whether the SMRQoL’s approach to parsing out the different effects of prospective and retrospective memory on QoL provides added value (i.e., expanded content coverage and reliability) or is perhaps unnecessary and redundant. Clinicians and researchers must careful weigh the potential benefits and burdens of adding a moderately long questionnaire to a battery for use in persons with memory disorders, who may feel overwhelmed or confused by the number of probes. Future studies in populations with stronger dissociations between prospective and retrospective memory (e.g., lesion samples) may provide further information about the usefulness of these dimensions of the SMRQoL. A fifth, related limitation is that this study did not demonstrate the incremental value of comprehensively assessing both prospective and retrospective memory symptoms on QoL in the context of either diagnosis or treatment. One might therefore argue that assessing memory symptoms and QoL separately with existing tools is sufficient and that the added, albeit minimal, time and effort expended on the SMRQoL may not therefore be justified.
Sample characteristics represent a sixth limitation of this study. We investigated the psychometric properties of the SMRQoL in the setting of HIV disease; however, these properties may not directly apply to other clinical populations. Therefore, whereas the SMRQoL may be clinically useful in populations such as traumatic brain injury (TBI), further examination of its psychometric properties will be necessary. Also, 85.1% of the HIV+ participants and 69.9% of the HIV− participants in this sample were men, which may limit the generalizability of its findings, as recent research (Maki et al., 2018) has found that women with HIV disease demonstrate more neurocognitive impairment than men. It is possible, for example, that women might demonstrate more memory-related QoL problems than men or that they might demonstrate more of a dissociation between QoL problems due to prospective versus retrospective memory lapses. Therefore, further research may aim to study memory-related QoL among a larger sample of HIV+ women. Nevertheless, the current sample is fairly representative of the HIV+ population in the USA; for example, the Centers for Disease Control and Prevention (CDC, 2019) estimates that 77.3% of persons living with HIV are men. Moreover, we had a slightly higher proportion of HIV+ women in the test–retest sample (20%).
Despite these limitations, the SMRQoL shows early evidence as a reliable and valid questionnaire that allows for assessment of the impact of memory symptoms on aspects of QoL, which can be useful in both clinical and research settings. In clinical practice, providers commonly draw inferences about the functional consequences of memory deficits to inform clinical diagnoses and recommendations. In general, such comments are based on research showing that clinical measures of memory correlate with (and/or are independently predictive of) separate measures of functional problems; however, this requires a leap of faith, as functional problems are highly multifactorial, particularly in the setting of chronic disease and sociocultural disadvantage. It also potentially ignores an important aspect of evidence-based neuropsychological practice, which is consideration of patient values at the level of the individual (Chelune, 2010). Tools such as the SMRQoL might help “cut out the middle man” by directly probing individuals about a variety of ways that memory lapses may affect their QoL. Because the various domains of memory-related QoL were found to be highly correlated and correspond to one underlying factor, the measure can be used to provide an overall index of the impact of the patients’ memory problems on their everyday lives. Whereas the SMRQoL focuses specifically on memory, similar measures focusing on other aspects of cognition, including attention/executive functions, processing speed, and motor skills, would also be useful in helping clinicians assess the functional impact of these deficits and the neurocognitive domains that may be most meaningful or valuable to our patients. Although this study focused on HIV, the SMRQoL and similar measures could be useful in a variety of populations with memory deficits that affect daily life, such as TBI, dementia, or epilepsy. For example, individuals with moderate to severe TBI often exhibit clinical memory deficits that adversely affect IADLs, treatment outcomes, community integration, and QoL. Providers might then administer the SMRQoL periodically to assess the extent to which any memory deficits are affecting QoL longitudinally to determine the deployment and effectiveness of intervention strategies that align with the perception and values of the patient and family, as clinically indicated. Future research will be necessary to investigate the association of SMRQoL responses with functional outcomes, including activities of daily living, employment, and medication adherence.
Supplementary Material
Acknowledgements
The authors have no financial conflicts of interest related to this work. This study was supported by National Institutes of Health (NIH) grants R01-MH073419 and P30-MH62512. The authors are grateful to the UC San Diego HIV Neurobehavioral Research Program (HNRP) Group (I. Grant, PI) for their infrastructure support of the parent R01. In particular, we thank Donald Franklin, Dr Erin Morgan, Clint Cushman, and Stephanie Corkran for their assistance with data processing; Marizela Verduzco for her assistance with study management; Drs Scott Letendre and Ronald J. Ellis for their assistance with the neuromedical aspects of the parent project; and Dr. J. Hampton Atkinson and Jennifer Marquie Beck and their assistance with participant recruitment and retention. We also thank Drs Erin Morgan, Erica Weber, and Michael Weinborn for their input on the design of this questionnaire. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. The authors thank the study volunteers for their participation. Aspects of these data were presented at the 118th Annual Convention of the American Psychological Association (Division 40) in San Diego, CA, and the 47th Annual Meeting of the International Neuropsychological Society in New York, NY.
A. Appendix
A.1. Survey of Memory-Related Quality of Life (SMRQoL)
We all make minor memory mistakes from time to time in our daily lives. One way to think about memory problems is to divide them into two kinds: (1) remembering information or events from the past, and (2) remembering to do things in the future. Both of these kinds of memory can play an important role in our day-to-day lives. For example, think about the different things you need to remember to take your medications. Remembering the names and dosages of your medications is an example of remembering information or events from the past. Remembering to take your medications during the day is an example of remembering to do things in the future.
Please read the statements below and rate how much you agree or disagree with them as they relate to memory problems you may currently have on a daily basis.
| Strongly Disagree | Disagree | Neither Agree nor Disagree | Agree | Strongly Agree | |
|---|---|---|---|---|---|
| 1. I rely more on others to help me out in my daily life (e.g., work, chores, child care) because of my problems: | |||||
| 1a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 1b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 2. I believe that people think less of me because of my problems: | |||||
| 2a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 2b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 3. My overall health and well-being are worse because of my problems: | |||||
| 3a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 3b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 4. I have trouble meeting my basic physical needs (e.g., eating, sleeping, toileting) because of my problems: | |||||
| 4a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 4b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 5. I have trouble managing my personal affairs because of my problems: | |||||
| 5a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 5b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 6. I am embarrassed by my problems: | |||||
| 6a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 6b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 7. My quality of life suffers because of my problems: | |||||
| 7a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 7b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 8. My relationships with family and friends suffer because of my problems: | |||||
| 8a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 8b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 9. I sometimes feel sad or depressed because of my problems: | |||||
| 9a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 9b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 10. My physical health is worse because of my problems: | |||||
| 10a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 10b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 11. I have difficulty following my doctor’s advice because of my problems: | |||||
| 11a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 11b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 12. I have trouble with social interactions because of my problems: | |||||
| 12a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 12b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 13. It is difficult to maintain my general health and well-being because of my problems: | |||||
| 13a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 13b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 14. I sometimes feel nervous or anxious because of my problems: | |||||
| 14a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 14b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
| 15. I sometimes neglect my personal grooming (e.g., bathing, dressing) because of my problems: | |||||
| 15a. Remembering information or events from the past | 5 | 4 | 3 | 2 | 1 |
| 15b. Remembering to do things in the future | 5 | 4 | 3 | 2 | 1 |
FIGURE A1.

Parallel analysis. As depicted in this figure, the parallel analysis suggested that SMRQoL items were unidimensional and captured one underlying factor in the HIV+ and HIV− groups. In both groups, only one factor had an observed eigenvalue that was larger than the 95th percentile of the distribution of the expected eigenvalue obtained from the simulated data.
FIGURE A2.
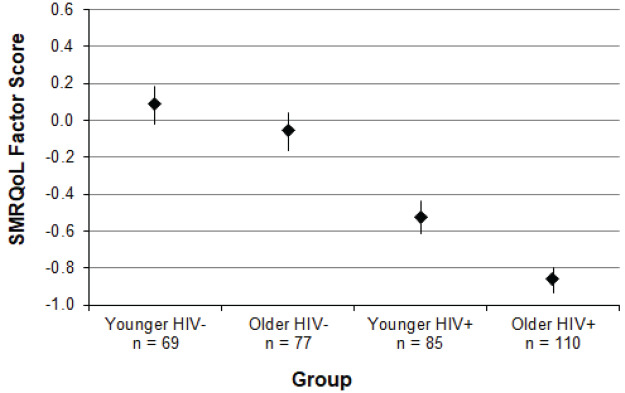
SMRQoL factor scores were lower for the HIV+ group relative to the HIV− group, though the magnitude of the difference between the two groups was greater for older compared to younger participants. Education, b = 0.05, p = .031, affective diagnosis, b = −0.70, p < .001, global neurocognitive impairment, b = −0.36, p = .002, and lifetime substance use disorder, b = −0.39, p = .001, were also significant predictors of SMRQoL factor scores, whereby individuals with fewer years of education, affective diagnoses, global neurocognitive impairment, and lifetime substance use disorders reported lower memory-related QoL. Post hoc Steel–Dwass tests to probe the interaction revealed that the older HIV+ group had significantly lower SMRQoL factor scores than the younger HIV+ group (d = .45, p = .011), the older HIV− group (d = 1.00, p < .001), and the younger HIV− group (d = 1.20, p < .001).
References
- Allen, M. J., & Yen, W. M. (2001). A review of basic statistical concepts In Introduction to measurement theory (1st ed., pp. 6–52). Waveland Press. [Google Scholar]
- Avci, G., Loft, S., Sheppard, D. P., Woods, S. P., & The HIV Neurobehavioral Research Program (HNRP) Group (2016). The effects of HIV disease and older age on laboratory-based, naturalistic, and self-perceived symptoms of prospective memory: Does retrieval cue type and delay interval matter? Aging, Neuropsychology, and Cognition, 23(6), 716–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci, G., Sheppard, D. P., Tierney, S. M., Kordovski, V. M., Sullivan, K. L., & Woods, S. P. (2018). A systematic review of prospective memory in HIV disease: From the laboratory to daily life. The Clinical Neuropsychologist, 32(5), 858–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreira, D. P., Marinho, R. T., Bicho, M., & Ouakinin, S. R. S. (2019). Psychosocial and neurocognitive factors associated with hepatitis C – Implications for future health and wellbeing. Frontiers in Psychology, 9, 2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamish, W. (2004). Consensus about program quality: An Australian study in early childhood special education. Griffith University, Queensland, Australia: Unpublished doctoral dissertation. [Google Scholar]
- Blackstone, K., Iudicello, J. E., Morgan, E. E., Moore, D. J., Franklin, D. R., Ellis, R. J. et al. (2013). HIV infection heightens concurrent risk of functional dependence in persons with chronic methamphetamine use. Journal of Addiction Medicine, 7(4), 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey, C. L., Woods, S. P., Rippeth, J. D., Heaton, R. K., Grant, I., & HIV Neurobehavioral Research Center (HNRC) Group (2006). Prospective memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology, 28(4), 536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto, K. B., Doyle, K. L., Weber, E., Woods, S. P., & The HIV Neurobehavioral Research Program (HNRP) Group (2014). Self-predictions of prospective memory in HIV-associated neurocognitive disorders: Evidence of a metamemory deficit. Archives of Clinical Neuropsychology, 29, 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2019). Estimated HIV incidence and prevalence in the United States, 2010-2016. HIV Surveillance Supplemental Report, 24(1), 1–89. [Google Scholar]
- Chelune, G. J. (2010). Evidence-based research and practice in clinical neuropsychology. The Clinical Neuropsychologist, 24(3), 454–467. [DOI] [PubMed] [Google Scholar]
- Chen, F., Curran, P. J., Bollen, K. A., Kirby, J., & Paxton, P. (2008). An empirical evaluation of the use of fixed cutoff points in RMSEA test statistic in structural equation models. Sociological Methods & Research, 36, 462–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona, G., & Rothen, N. (2019). Neuropsychological and physiological correlates of prospective memory In Rummel, J., & McDaniel, M. A. (Eds.), Prospective memory. New York: Routledge. [Google Scholar]
- Crawford, J. R., Smith, G., Maylor, E. A., Della Sala, S., & Logie, R. H. (2003). The Prospective and Retrospective Memory Questionnaire (PRMQ): Normative data and latent structure in a large non-clinical sample. Memory, 11(3), 261–275. [DOI] [PubMed] [Google Scholar]
- Crumley, J. J., Stetler, C. A., & Horhota, M. (2014). Examining the relationship between subjective and objective memory performance in older adults: A meta-analysis. Psychology and Aging, 29(2), 250–263. [DOI] [PubMed] [Google Scholar]
- Culbertson, W. C., & Zillmer, E. (2001). Tower of London-Drexel University (TOLDX). Multi-Health Systems.
- Delis, D. C. (2000). CVLT-II: California verbal learning test: adult version. Psychological Corporation. [Google Scholar]
- Doyle, K. L., Woods, S. P., McDonald, C. R., Leyden, K. M., Holden, H. M., Morgan, E. E. et al. (2019). Verbal episodic memory profiles in HIV-associated neurocognitive disorders (HAND): A comparison with Huntington’s disease and mesial temporal lobe epilepsy. Applied Neuropsychology: Adult, 26(1), 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, K., Weber, E., Atkinson, J. H., Grant, I., Woods, S. P., & HIV Neurobehavioral Research Program (HNRP) Group (2012). Aging, prospective memory, and health-related quality of life in HIV infection. AIDS and Behavior, 16(8), 2309–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, P. W., Bode, R. K., Lai, S. M., Perera, S., & Glycine Antagonist in Neuroprotection Americas Investigators (2003). Rasch analysis of a new stroke-specific outcome scale: The Stroke Impact Scale. Archives of Physical Medicine and Rehabilitation, 84(7), 950–963. [DOI] [PubMed] [Google Scholar]
- Fries, J. F., Bruce, B., & Cella, D. (2005). The promise of PROMIS: Using item response theory to improve assessment of patient-reported outcomes. Clinical and Experimental Rheumatology, 23, S53–S57. [PubMed] [Google Scholar]
- Fujii, D. E., Wylie, A. M., & Nathan, J. H. (2004). Neurocognition and long-term prediction of quality of life in outpatients with severe and persistent mental illness. Schizophrenia Research, 69, 67–73. [DOI] [PubMed] [Google Scholar]
- Gershon, R. C., Lai, J. S., Bode, R., Choi, S., Moy, C., Bleck, T. et al. (2012). Neuro-QOL: Quality of life item banks for adults with neurological disorders: Item development and calibrations based upon clinical and general population testing. Quality of Life Research, 21, 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Saiz, F., Rojas, O. L., & Castillo, I. I. (2009). Measuring the impact of psychoactive substance on health-realted quality of life: An update. Current Drug Abuse Reviews, 2, 5–10. [DOI] [PubMed] [Google Scholar]
- Grace, J., & Malloy, P. F. (2001). Frontal Systems Behavior Scale: FrSBe. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Gupta, S., Paul Woods, S., Weber, E., Dawson, M. S., Grant, I., & HIV Neurobehavioral Research Center (HNRC) Group (2010). Is prospective memory a dissociable cognitive function in HIV infection? Journal of Clinical and Experimental Neuropsychology, 32(8), 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays, R. D., Cunningham, W. E., Sherbourne, C. D., Wilson, I. B., Wu, A. W., Cleary, P. D. et al. (2000). Health-related quality of life in patients with human immunodeficiency virus infection in the United States: Results from the HIV Cost and Services Utilization Study. The American Journal of Medicine, 108(9), 714–722. [DOI] [PubMed] [Google Scholar]
- Heathcote, A., Loft, S., & Remington, R. W. (2015). Slow down and remember to remember! A delay theory of prospective memory costs. Psychological Review, 122(2), 376–410. [DOI] [PubMed] [Google Scholar]
- Hrabok, M., Sherman, E. M. S., Bello-Espinosa, L., & Hader, W. (2013). Memory and health-related quality of life in severe pediatric epilepsy. Pediatrics, 131, e525. [DOI] [PubMed] [Google Scholar]
- Jacobson, N. S., & Truax, P. (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59(1), 12–19. [DOI] [PubMed] [Google Scholar]
- Jöreskog, K. G., & Sörbom, D. (2002). LISREL 8: Structural equation modeling with the SIMPLIS command language. Chicago, IL: Scientific Software International. [Google Scholar]
- Kamat, R., Weinborn, M., Kellogg, E. J., Bucks, R. S., Velnoweth, A., & Woods, S. P. (2014). Construct validity of the Memory for Intentions Screening Test (MIST) in healthy older adults. Assessment, 21(6), 742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny, D. (2011, September 7). Multiple groups. Retrieved from http://davidakenny.net/cm/mgroups.htm.
- Kløve, H. (1963). Grooved pegboard. Lafayette, IN: Lafayette Instruments. [Google Scholar]
- Maki, P. M., Rubin, L. H., Springer, G., Seaberg, E. C., Sacktor, N., Miller, E. N. et al. (2018). Differences in cognitive function between women and men with HIV. Journal of Acquired Immune Deficiency Syndromes, 79(1), 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki, Y., Yamaguchi, T., Yamagami, T., Murai, T., Hachisuka, K., Miyamae, F. et al. (2014). The impact of subjective memory complaints on quality of life in community-dwelling older adults. Psychogeriatrics, 14(3), 175–181. [DOI] [PubMed] [Google Scholar]
- McDaniel, M. A., & Einstein, G. O. (2000). Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology, 14, S127–S144. [Google Scholar]
- McNair, D. M., Lorr, M., & Droppleman, L. F. (1971). Profile of mood states. San Diego, CA: Educational and Industrial Testing Service. [Google Scholar]
- Millikin, C. P., Rourke, S. B., Halman, M. H., & Power, C. (2003). Fatigue in HIV/AIDS is associated with depression and subjective neurocognitive complaints but not neuropsychological functioning. Journal of Clinical and Experimental Neuropsychology, 25(2), 201–215. [DOI] [PubMed] [Google Scholar]
- Millsap, R. (2011). Statistical approaches to measurement invariance. New York, NY: Routledge. [Google Scholar]
- Moore, R. C., Moore, D. J., Thompson, W., Vahia, I. V., Grant, I., & Jeste, D. V. (2013). A case-controlled study of successful aging in older adults with HIV. The Journal of Clinical Psychiatry, 74(5), e417–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, E. E., Iudicello, J. E., Weber, E., Duarte, N. A., Riggs, P. K., Delano-Wood, L. et al. (2012a). Synergistic effects of HIV infection and older age on daily functioning. Journal of Acquired Immune Deficiency Syndromes, 61(3), 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch, M. (1992). Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience, 4(3), 257–267. [DOI] [PubMed] [Google Scholar]
- Muthén, L. K., & Muthén, B. O. (2012). Mplus User’s Guide (7th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Pereira, M., & Canavarro, M. C. (2011). Gender and age differnces in quality of life and the impact of psychopathological symptoms among HIV-infected patients. AIDS and Behavior, 15(8), 1857–1869. [DOI] [PubMed] [Google Scholar]
- Piette, J., Wachtel, T. J., Mor, V., & Mayer, K. (1995). The impact of age on the quality of life in persons with HIV infection. Journal of Aging and Health, 7(2), 163–178. [DOI] [PubMed] [Google Scholar]
- Pirogovsky, E., Woods, S. P., Filoteo, J. V., & Gilbert, P. E. (2012). Prospective memory deficits are associated with poorer everyday functioning in Parkinson’s disease. Journal of the International Neuropsychological Society, 18(6), 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin, S., Buckheit, C., & Sherrod, C. (2010). Memory for Intentions Test (MIsT). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Reger, M., Welsh, R., Razani, J., Martin, D. J., & Boone, K. B. (2002). A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society, 8(3), 410–424. [DOI] [PubMed] [Google Scholar]
- Reitan, R. M., & Wolfson, D. (1985). The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. Reitan Neuropsychology, Vol. 4. [Google Scholar]
- Rippeth, J. D., Heaton, R. K., Carey, C. L., Marcotte, T. D., Moore, D. J., Gonzalez, R. et al. (2004). Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society, 10(1), 1–14. [DOI] [PubMed] [Google Scholar]
- Rourke, S. B., Halman, M. H., & Bassel, C. (1999). Neurocognitive complaints in HIV-infection and their relationship to depressive symptoms and neuropsychological functioning. Journal of Clinical and Experimental Neuropsychology, 21(6), 737–756. [DOI] [PubMed] [Google Scholar]
- Sacktor, N., Skolasky, R., Selnes, O. A., Watters, M., Poff, P., Shiramizu, B. et al. (2007). Neuropsychological test profile differences between young and old human immunodeficiency virus–positive individuals. Journal of Neurovirology, 13(3), 203–209. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Inc (2015). SAS® 9.4 In-Database Products: User’s Guide (Fifth ed.). Cary, NC: SAS Institute, Inc. [Google Scholar]
- Scott, J. C., Slomiak, S. T., Jones, J. D., Rosen, A. F. G., Moore, T. M., & Gur, R. C. (2018). Association of cannabis with cognitive functioning in adolescents and young adults. JAMA Psychiatry, 75(6), 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. C., Woods, S. P., Wrocklage, K. M., Schweinsburg, B. C., Southwick, S. M., & Krystal, J. H. (2016). Prospective memory in posttraumatic stress disorder. Journal of the International Neuropsychological Society, 22, 724–734. [DOI] [PubMed] [Google Scholar]
- Sheppard, D. P., Woods, S. P., Massman, P. J., & Gilbert, P. E. (2019). Frequency and correlates of subjective cognitive impairment in HIV disease. AIDS and Behavior, 23(3), 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, G., Della Sala, S., Logie, R. H., & Maylor, E. A. (2000). Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory, 8(5), 311–321. [DOI] [PubMed] [Google Scholar]
- Smith, S. C., Lamping, D. L., Banerjee, S., Harwood, R., Foley, B., Smith, P. et al. (2005). Measurement of health-related quality of life for people with dementia: Development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technology Assessment, 9(10), 1–93. [DOI] [PubMed] [Google Scholar]
- Thaler, N. S., Sayegh, P., Kim, M. S., Castellon, S. A., & Hinkin, C. H. (2015). Interactive effects of neurocognitive impairment and substance use on antiretroviral non-adherence in HIV disease. Archives of Clinical Neuropsychology, 30(2), 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi, V., Balestra, P., Galgani, S., Murri, R., Bellagamba, R., Narciso, P. et al. (2003). Neurocognitive performance and quality of life in patients with HIV infection. AIDS Research and Human Retroviruses, 19(8), 643–652. [DOI] [PubMed] [Google Scholar]
- Uttl, B., & Kibreab, M. (2011). Self-report measures of prospective memory are reliable but not valid. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Expérimentale, 65(1), 57–68. [DOI] [PubMed] [Google Scholar]
- Ware, J. E., Jr., & Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care, 473–483. [PubMed] [Google Scholar]
- Wechsler, D. (1997a). Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler, D. (1997b). WMS-III: Wechsler Memory Scale administration and scoring manual. Psychological Corporation. [Google Scholar]
- Wechsler, D. (2001). Wechsler Test of Adult Reading: WTAR. Psychological Corporation. [Google Scholar]
- Wilson, I. B., & Cleary, P. D. (1995). Linking clinical variables with health-related quality of life: A conceptual model of patient outcomes. JAMA, 273(1), 59–65. [PubMed] [Google Scholar]
- Woods, S. P., Carey, C. L., Moran, L. M., Dawson, M. S., Letendre, S. L., Grant, I. et al. (2007). Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Archives of Clinical Neuropsychology, 22(2), 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S. P., Dawson, M. S., Weber, E., Grant, I., & HIV Neurobehavioral Research Center (HNRC) Group (2010). The semantic relatedness of cue–intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology, 32(4), 398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S. P., Iudicello, J. E., Moran, L. M., Carey, C. L., Dawson, M. S., & Grant, I. (2008a). HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology, 22(1), 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S. P., Moran, L. M., Dawson, M. S., Carey, C. L., Grant, I., & HIV Neurobehavioral Research Center (HNRC) Group (2008b). Psychometric characteristics of the Memory for Intentions Screening Test. The Clinical Neuropsychologist, 22(5), 864–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S. P., Morgan, E. E., Marquie-Beck, J., Carey, C. L., Grant, I., Letendre, S. L. et al. (2006). Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cognitive and Behavioral Neurology, 19(4), 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S. P., Rippeth, J. D., Frol, A. B., Levy, J. K., Ryan, E., Soukup, V. M. et al. (2004). Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology, 26(6), 759–778. [DOI] [PubMed] [Google Scholar]
- Woods, S. P., Scott, J. C., Sires, D. A., Grant, I., Heaton, R. K., Tröster, A. I. et al. (2005). Action (verb) fluency: Test–retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society, 11(4), 408–415. [PubMed] [Google Scholar]
- Woods, S. P., Weinborn, M., Li, Y. R., Hodgson, E., Ng, A. R., & Bucks, R. S. (2015). Does prospective memory influence quality of life in community-dwelling older adults? Aging, Neuropsychology, and Cognition, 22(6), 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S. P., Weinborn, M., Maxwell, B. R., Gummery, A., Mo, K., Ng, A. R. J. et al. (2014). Event-based prospective memory is independently associated with self-report of medication management in older adults. Aging & Mental Health, 18(6), 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHOQOL Group (1998). The World Health Organization quality of life assessment (WHOQOL): Development and general psychometric properties. Social science & Medicine, 46(12), 1569–1585. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1998). Composite International Diagnostic Interview (CIDI, version 2.1). Geneva, Switzerland: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


