Abstract
Cysticercosis is a condition in which humans are infected by the larval form of Pork Tapeworm Taenia solium. Isolated lingual cysticercosis is a rare entity due to the high muscular activity and metabolic rate of tongue which prevents the development of larva. We present a series of three patients with isolated lingual cysticercosis. One patient was treated by medical management while the other two were treated with surgical excision followed by medical therapy. All patients are asymptomatic after repeated follow ups.
Keywords: Cysticercosis, Tongue, Oral cavity, Magnetic resonance imaging
Introduction
Cysticercosis is a condition in which humans are infected by the larval form of Pork Tapeworm Taenia solium. Humans act as both definitive and intermediate hosts of Taenia solium [1]. It can occur due to consumption of under cooked pork as well as through faeco-oral route [2]. Cysticercosis is the most common parasitic disease of the central nervous system [3]. However Extra neural cysticercosis, which is also very common, infects muscles, heart, lung, peritoneum, eye and subcutaneous tissue [4]. Isolated lingual cysticercosis is a rare entity due to the high muscular activity and metabolic rate of tongue which prevents the development of larva [5]. It can be a diagnostic dilemma due to resemblance with various other benign tumors.
We present a series of 3 patients with isolated lingual cysticercosis.
Case Series
Case 1
A 12 year old boy presented to the ENT outpatient department with a painless progressively increasing midline swelling in the anterior one-third of tongue since past 3 months (Fig. 1). The patient was asymptomatic. On intra oral examination the swelling was firm, non-tender, non-pulsatile with diffuse margins and normal overlying mucosa. There was no restriction in tongue mobility and no other similar swelling anywhere else in the body.
Fig. 1.
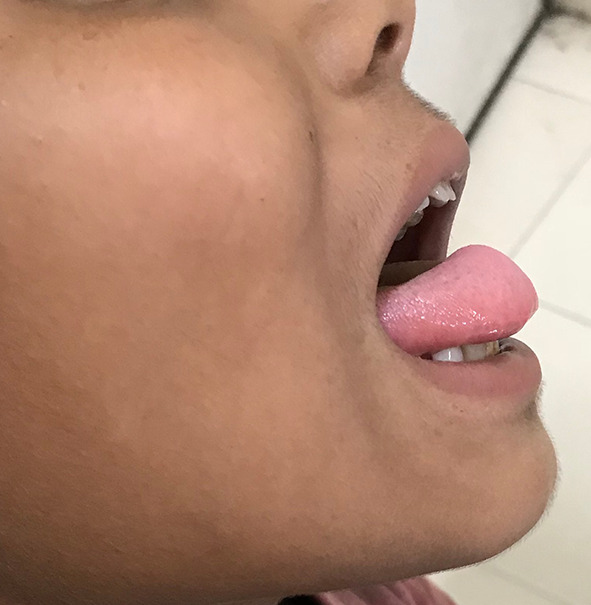
Clinical photograph showing swelling on the dorsal aspect of tongue
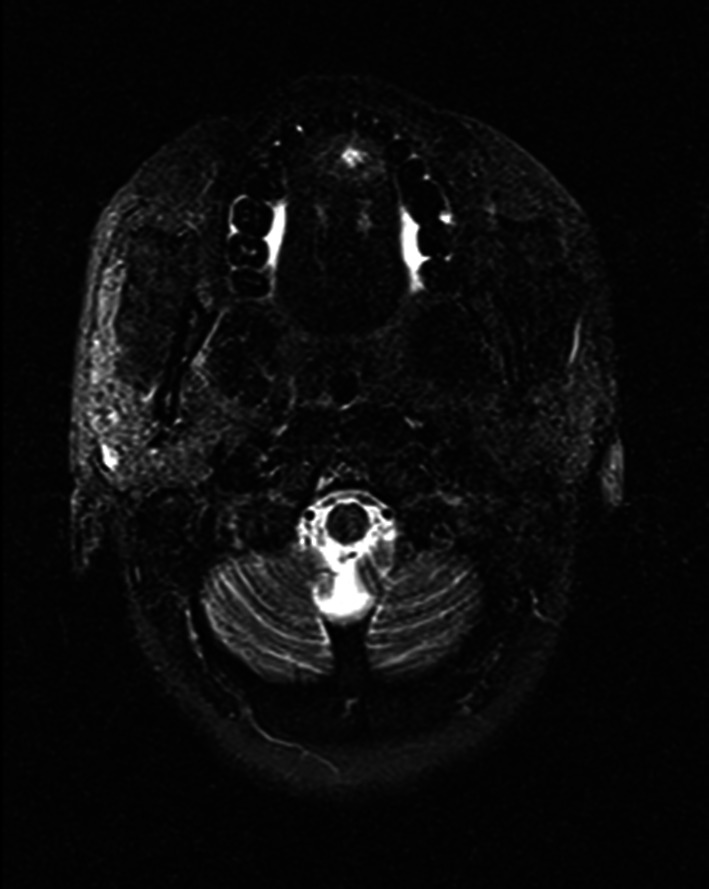
On fine needle aspiration cytology a provisional diagnosis of infected parasitic cyst was made. Magnetic resonance imaging (Fig. 2) showed subcentimetric T2 mildly hyperintense lesion in midline within the intrinsic muscles of tongue with small eccentric ill defined T2 hypointense nodule within the lesion suggestive of a scolex. It also helped exclude a similar lesion in brain and eyes. Patient was pretreated with Tab Prednisolone 30 mg OD for 5 days followed by Oral Albendazole 15 mg/kg which was given for 28 days.
Fig. 2.

T2 weighted MRI showing hupointense nodule suggestive of scolex
Case 2
A 25 year old female presented to ENT outpatient department with complaint of painless swelling on the undersurface of tongue (Fig. 3) since past 4 months with difficulty in eating. There was no history of fever or trauma. On examination the swelling measured 1 cm * 1 cm, was spherical, firm, non-tender with intact overlying mucosa. Tongue movements were not restricted and there was no palpable cervical lymphadenopathy. No abnormality was detected in rest of the Ear nose and Throat examination. On Fine needle aspiration Cytology showed inflammatory cells with significant eosinophils, occasional giant cells and a granuloma suggestive of infected parasitic cyst was made (Fig. 4). Magnetic resonance of imaging of tongue (Fig. 5) was done to confirm the diagnosis. MRI of head was done to exclude presence of cysticercosis in brain and eyes. A complete surgical excision was done under local anesthesia. Patient Received Oral Albendazole 15 mg/kg for 8 days postoperatively along with a short course of oral prednisolone. Regular review was done in the outpatient clinic to look for recurrence as well as neurological or ophthalmological signs.
Fig. 3.
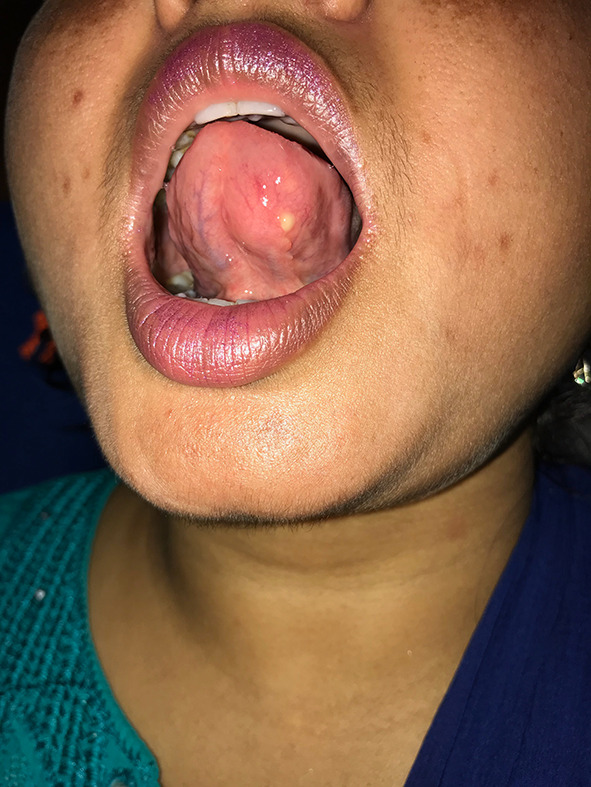
Clinical photograph showing lesion on the ventral aspect of tongue
Fig. 4.
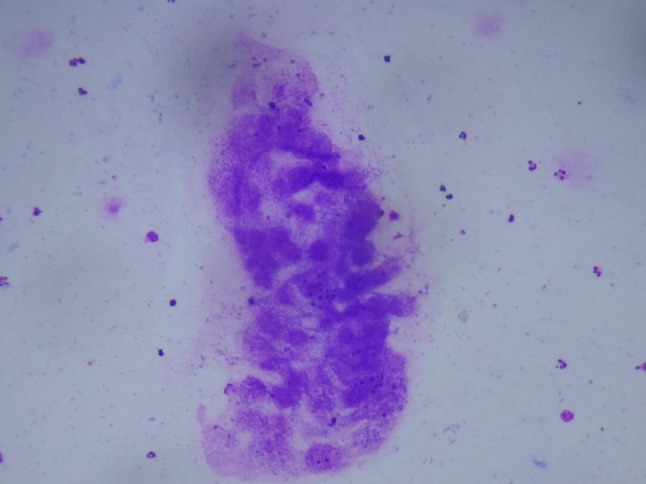
FNAC smear showing fragments of parasite (Geimsa stain) (10X)
Fig. 5.
T2 weighted MRI showing hyperintense lesion with perilesional edema in intrinsic muscles of tongue
Case 3
An 18 year old male came to the ENT OPD with a symptomless swelling on the dorsal aspect of anterior 1/3rd of tongue on the right side (Fig. 6). The swelling was present since past 9 months. It was a 1.5 cm × 1.5 cm firm, non tender, immobile swelling with diffuse margins and normal overlying mucosa. FNAC of the swelling was inconclusive. Patient underwent a complete surgical excision (Fig. 7) of the cyst and it was sent for histopathology. Histopathology report of the specimen was consistent with Cysticercus cellulosae. A non contrast CT scan of brain was done to rule out neurocysticercosis, which was normal. After a short course of low dose oral prednisolone patient received oral Albendazole 15 mg/kg and was symptom free after repeated follow ups.
Fig. 6.

Clinical photograph showing lesion on the Right dorsal aspect of tongue
Fig. 7.

Intraoperative photograph showing exposure of the well circumscribed lesion
Discussion
Cysticercosis refers to encystation of larva (Cysticercus cellulosae) of Taenia solium (pork tapeworm) in various tissues of the body [6]. Taenia solium is a hermaphrodite cestode for which human beings act as the only definitive hosts while both pigs and humans act as the intermediate hosts. Adult worm consists of the Scolex (head) and Proglottids (Caudal end).
Each proglottid contains 40,000–60,000 eggs which are released in faeces. Pigs become infected by ingesting eggs from ground contaminated by human faeces. The eggs, on being acted upon by gastric juices, release the embryo (oncosphere) which penetrate the intestinal wall and travels to various body tissues. These embryos then develop into larval form (cystercosis). Human beings acquire the larvae on ingesting uncooked pork, which ultimately develops into the adult worm in small intestine thereby completing the life cycle of Taenia solium [7].
Cysticercosis develops when humans, instead of pigs, become intermediate host by ingesting tapeworm eggs. This usually happens by consumption of faecally contaminated food and water or by faeco oral contamination as well as reflux of eggs into the stomach [8].
Clinical Spectrum of the disease depends on the site, stage of development and number of cysts. Neural cysticercosis has non specific signs and symptoms however acute symptomatic seizures is the most common presentation. Extra neural cysticercosis usually involves subcutaneous tissues and muscles presenting as clinical nodules. Intraorally they present as painless, subcutaneous nodules most commonly involving the tongue [9]. Differential diagnosis of lingual cysticercosis include mucocoele, reactive lesions like giant cell fibroma or focal fibrous hyperplasia, lipoma, granular cell myoblastoma, neurofibroma, neurilemmoma, vascular leiomyoma and benign salivary gland tumors [10].
Oral cavity and perioral involvement by Cysticercous larva is rare in humans. According to a series published by Singh et al. [6] in 2018 a total of 89 cases of oral cysticercosis (excluding masseter and parotid) had been reported in pubmed English literature, out of which 50.6% were in the tongue. Since then one more case has been reported, excluding the present series [11].
Definitive diagnosis can only be made by demonstration of Cysticercus cellulosae on histopathology. However preoperatively Fine needle aspiration cytology can make the diagnosis in 45–100% of the cases especially if specks of pearly white content, representing larval fragments in inflammatory background, are included in the aspirate [12]. Depending on the stage of presentation radiological investigations like ultrasonography may show the presence of larval forms inside cysts as an eccentric echogenic structure along with perilesional edema [13]. Computed tomography reveals cystic lesion with variable rim enhancement, perilesional edema and calcification. Although Magnetic resonance imaging may give a better visualization of active parasite, but it fails to detect calcification reliably [14]. CT and MRI are of great value in diagnosing neuro cysticercosis and it is important to emphasize that every case of oral cysticercosis should be thoroughly investigated to determine the involvement of multiple foci since there is a high incidence of such a feature [15]. Immunodetection of cysticercosis can be achieved in sera, cerebrospinal fluid and saliva using Enzyme Linked immunosorbent Assay (ELISA) or Enzyme Linked Immunoelectrotransfer Blot (ELIB) [16]. Taenia Solium cyst fluid antigen based lymphocyte transformation test (LTT), which is a more recent test, holds better diagnostic prospects than previously mentioned tests [14]. However it is important to remember that those living in endemic areas may have antibodies because of exposure rather than established infection [17].
Treatment of cysticercosis varies according to the symptoms as well as whether the lesion is amenable to excision. It is important to rule out involvement of vital organs like brain, optic nerve and heart as it may change the course of treatment as well as prognosis [6]. Treatment of oral cysticercosis involves surgical excision along with administration of antihelminthics like praziquental and albendazole [18]. Drugs should be used especially in symptomatic patients, disseminated cysticercosis and cases in which surgical treatment is risky or not possible, as in neurocysticercosis [19]. However cysticidal drugs may be associated with severe reactions such as local tissue swelling and generalized anaphylactic reaction due to massive antigen release. The use of Corticosteroids with or before their administration can help in decreasing the incidence of these complications [20].
Compliance with Ethical Standards
Conflict of interest
All the authors, Dr Gyanesh Sethi, Dr Daljeet Kaur, Dr Nikhil Arora and Dr Deepika Sethi declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Written informed consent was taken from the individual participants in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gyanesh Sethi, Email: gyan2s@gmail.com.
Daljeet Kaur, Email: drdaljeet@yahoo.com.
Nikhil Arora, Email: for_nikhilarora@yahoo.com.
Deepika Sethi, Email: dset17111978@gmail.com.
References
- 1.Garcia HH, Del Brutto OH. Taenia solium cysticercosis. Infect Dis Clin N Am. 2000;14(97–119):ix. doi: 10.1016/s0891-5520(05)70220-8. [DOI] [PubMed] [Google Scholar]
- 2.Schantz PM, Moore AC, Muñoz JL, et al. Neurocysticercosis in an orthodox Jewish community in New York City. N Engl J Med. 1992;327:692–695. doi: 10.1056/NEJM199209033271004. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt DK, Jordaan HF, Schneider JW, Cilliers J. Cerebral and subcutaneous cysticercosis treated with albendazole. Int J Dermatol. 1995;34:574–579. doi: 10.1111/j.1365-4362.1995.tb02959.x. [DOI] [PubMed] [Google Scholar]
- 4.Khare P, Chauhan N, Dogra R, et al. Isolated cysticercosis of tongue: a case report. Diagn Cytopathol. 2014;42:716–718. doi: 10.1002/dc.23109. [DOI] [PubMed] [Google Scholar]
- 5.Koteeswaran G, Mangala G, Kotasthane D, Tirou A. Cysticercosis of tongue: cytohistologic approach to diagnosis. J Oral Maxillofac Pathol. 2013;17:480. doi: 10.4103/0973-029X.125231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh A, Gautam P, Handa AC, Handa KK. Oral cysticercosis: a case series and review of literature. J Oral Maxillofac Surg. 2018;76:2572–2576. doi: 10.1016/j.joms.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Elias FM, Martins MT, Foronda R, et al. Oral cysticercosis: case report and review of the literature. Rev Inst Med Trop Sao Paulo. 2005;47:95–98. doi: 10.1590/s0036-46652005000200007. [DOI] [PubMed] [Google Scholar]
- 8.Saran RK, Rattan V, Rajwanshi A, et al. Cysticercosis of the oral cavity: report of five cases and a review of literature. Int J Paediatr Dent. 1998;8:273–278. doi: 10.1046/j.1365-263x.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- 9.Richa Ray J, Pattanayak S, Vibha An asymptomatic tongue nodule. Contemp Clin Dent. 2012;3:464. doi: 10.4103/0976-237X.107441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhal R, Bhagol A, Narwal A, et al. Asymptomatic swelling of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:159–162. doi: 10.1016/j.oooo.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Dube G, Choube A, Sachdeva N. Taenia solium: a rare expression in oral cavity. J Maxillofac Oral Surg. 2019;18:229–232. doi: 10.1007/s12663-018-1116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado-Azañero WA, Mosqueda-Taylor A, Carlos-Bregni R, et al. Oral cysticercosis: a collaborative study of 16 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2007;103:528–533. doi: 10.1016/j.tripleo.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Mittal A, Das D, Iyer N, et al. Masseter cysticercosis—a rare case diagnosed on ultrasound. Dentomaxillofac Radiol. 2008;37:113–116. doi: 10.1259/dmfr/31885135. [DOI] [PubMed] [Google Scholar]
- 14.Prasad KN, Prasad A, Verma A, Singh AK. Human cysticercosis and Indian scenario: a review. J Biosci. 2008;33:571–582. doi: 10.1007/s12038-008-0075-y. [DOI] [PubMed] [Google Scholar]
- 15.Lustmann J, Copelyn M. Oral cysticercosis. Review of the literature and report of 2 cases. Int J Oral Surg. 1981;10:371–375. doi: 10.1016/S0300-9785(81)80038-5. [DOI] [PubMed] [Google Scholar]
- 16.Flisser A, Plancarte A, Correa D, et al. New approaches in the diagnosis of Taenia solium cysticercosis and taeniasis. Ann Parasitol Hum Comp. 1990;65(Suppl 1):95–98. doi: 10.1051/parasite/1990651095. [DOI] [PubMed] [Google Scholar]
- 17.Garcia HH, Harrison LJ, Parkhouse RM, et al. A specific antigen-detection ELISA for the diagnosis of human neurocysticercosis. The cysticercosis working group in Peru. Trans R Soc Trop Med Hyg. 1998;92:411–414. doi: 10.1016/s0035-9203(98)91069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Souza PE, Barreto DC, Fonseca LM, et al. Cysticercosis of the oral cavity: report of seven cases. Oral Dis. 2000;6:253–255. doi: 10.1111/j.1601-0825.2000.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 19.Gupta DS, Goyal AK, Tandon PN, et al. Platyhelminthes in tongue—a rare case and review. J Oral Maxillofac Surg. 2012;70:2605–2609. doi: 10.1016/j.joms.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Park SY, Kong MH, Kim JH, Song KY. Disseminated cysticercosis. J Korean Neurosurg Soc. 2011;49:190–193. doi: 10.3340/jkns.2011.49.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]


