Abstract
OBJECTIVE:
Insomnia and depression have been inconsistently associated with inflammation. Age may be one important moderator of these associations. This study examined associations between insomnia and depression with inflammatory biomarkers in nurses and how these associations varied by age.
DESIGN:
Participants were 392 nurses ages 18-65 (Mage = 39.54 years ± 11.15, 92% female) recruited from two hospitals.
MAIN OUTCOME MEASURES:
Participants completed surveys to assess insomnia and depression symptoms. Serum samples were obtained and analyzed for inflammatory biomarkers interleukin-6 (IL-6), C-reactive protein (CRP), interleukin-1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α).
RESULTS:
Neither insomnia nor depression symptoms were associated with inflammatory biomarkers. Older age was associated with higher IL-1β, and age moderated the effects of depression symptoms on CRP and TNF-α: Greater depression symptoms were associated with higher CRP (b = .14, p = .017) and TNF-α (b = .008, p = .165) among older nurses only.
CONCLUSION:
Results suggest older nurses with higher depression symptoms may be at increased risk for elevated inflammation. Interventions should consider the role of age-related processes in modifying health and well-being. Given relatively low levels of depression in the current sample, future studies should replicate results in clinical and non-nurse samples.
Keywords: insomnia, depression, inflammation, C-reactive protein, age, nurses
Insomnia and depression are highly prevalent disorders with a large public health and economic impact (Kessler, 2012; Marcus, Yasamy, van Ommeren, Chisholm, & Saxena, 2012; Taylor, Lichstein, & Durrence, 2003; Wickwire, Shaya, & Scharf, 2016). They are also frequently comorbid and are considered strong risk factors for one another (Bei, Ong, Rajaratnam, & Manber, 2015; Harvey, 2011). Both insomnia and depression have been associated with increased risk for other chronic disease outcomes (Chapman, Perry, & Strine, 2005; Von Ruesten, Weikert, Fietze, & Boeing, 2012), including cardiovascular disease (Phillips & Mannino, 2007; Zhang, Chen, & Ma, 2018), cancer (Irwin, 2013; Nikendei et al., 2018; Samuelsson, Bovbjerg, Roecklein, & Hall, 2018), and metabolic dysregulation (Ayas et al., 2003). Further research is needed to understand the unique influences of insomnia and depression and their interactions with key demographic factors on disease risk.
There are multiple potential psychophysiological mechanisms linking insomnia and depression with more distal health outcomes. One potential mechanism may be elevated levels of systemic inflammation, which is prospectively implicated in many chronic diseases (Irwin, Chen et al., 2019; 2013), as well as in insomnia and depression. Systemic inflammation can be measured by assessing levels of inflammatory biomarkers (e.g., cytokines and acute phase proteins), which are small signaling molecules secreted from T- and B-lymphocytes and macrophages. Inflammatory biomarkers help the body regulate its response to infection, trauma, and injury to prevent further tissue damage (Smith, Das, Ray, & Banik, 2012). Although secretion of inflammatory markers is adaptive in the short term, responses can become dysregulated under certain conditions (e.g., chronic stress) leading to low-grade systemic inflammation that may increase disease risk (Cohen et al., 2012). Depression and insomnia patients often exhibit heightened sympathetic and β-adrenergic activation (Irwin et al., 1991; Vgontzas, Fernandez-Mendoza, Liao, & Bixler, 2013), which can increase production of pro-inflammatory cytokines (Goebel, Mills, Irwin, & Ziegler, 2000). Neurobiological pathways involved in depression and insomnia include pro-inflammatory cytokine signals that initiate inflammatory reactions in the brain and interfere with the activity of important behavior-modulating neurotransmitters, including norepinephrine, dopamine, and serotonin (Dantzer, O'Connor, Freund, Johnson, & Kelley, 2008; Irwin, 2001; Irwin, 2015).
Studies have shown that both insomnia and depression are associated with elevated plasma- and serum-based markers of inflammation, such as interluekin-6 (IL-6), C-reactive protein (CRP), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α; D’Antono & Bouchard, 2019; Eisenberger, Inagaki, Mashal, & Irwin, 2010; Howren, Lamkin, & Suls, 2009; Liukkonen et al., 2007; Mills et al., 2007; Von Känel et al., 2010; effect sizes: d = 0.15-0.25; r = 0.19). IL-6 has also been linked to increased sleep fragmentation (Hogan, Morrow, Smith, & Opp, 2003), shorter total sleep time (Meier-Ewert et al., 2004), and increased sleep onset latency (Irwin et al., 2004). A recent meta-analysis showed that self-reported insomnia symptoms were associated with higher levels of IL-6 (d = 0.10) and CRP (d = 0.10; Irwin, Olmstead, & Carroll, 2016). Similarly, major depressive disorder has been associated with increased nocturnal levels of IL-6 (r = 0.40; Lesperance, Frasure-Smith, Theroux, & Irwin, 2004; Motivala, Sarfatti, Olmos, & Irwin, 2005). A meta-analysis of 24 studies demonstrated that, compared to control participants, individuals with depression had higher levels of IL-6 (d = 1.78) and TNF-α (d = 3.97; Dowlati et al., 2010).
However, several other studies have shown null associations between insomnia and depression with pro-inflammatory biomarkers. For example, neither depression symptoms nor sleep disturbances (as measured by the Pittsburgh Sleep Quality Index) was associated with the inflammatory markers IL-1β, TNF-α, or CRP in a sample of middle-aged women (Bower et al., 2011). However, in this same sample, older age was a significant predictor of higher levels of soluble tumor necrosis factor-alpha receptor II (sTNF-RII; Bower et al., 2011). Further, one meta-analysis showed that depression was not associated with multiple inflammatory biomarkers, including IL-1β (Dowlati et al., 2010), and another meta-analysis showed that insomnia symptoms assessed via clinical interview were not associated with IL-6 (Irwin et al., 2016). In addition, when insomnia items have been partialed out of analyses, depression symptoms appear to be uncorrelated with inflammatory markers such as IL-6 (Motivala et al., 2005). Given that insomnia and depression are commonly comorbid, it is unclear if each independently predict inflammation, or if they represent a partially overlapping phenotype. Discrepant results highlight the need for clarification of the association between insomnia and depression with inflammatory markers, including the examination of potential moderators.
One likely moderator of associations among insomnia, depression, and inflammation is age. Previous studies suggest there are important and robust age differences in the prevalence and severity of insomnia and depression symptoms. Older adults are more likely to suffer from insomnia (Portela et al., 2015). The prevalence of insomnia increases 1.1-fold each decade of life (Morphy, Dunn, Lewis, Boardman, & Croft, 2007) and almost doubles for individuals 75 years and older (Morgan, 2003). Age is also correlated with the severity of insomnia, with older adults reporting worse insomnia symptoms (Lichstein, Durrence, Riedel, Taylor, & Bush, 2004; Ohayon, 2002; Sivertsen, Krokstad, Overland, & Mykletun, 2009). Furthermore, depression or the occurrence of depression symptomatology is a prominent condition among older people, with a significant impact on the well-being and quality of life (Alexopoulos, 2005; Mitchell & Harvey, 2014). Many studies have demonstrated that the prevalence of depression symptoms increases with age (Kennedy, 1996). Furthermore, increased age is also one of the strongest risk factors for elevated levels of systemic inflammation (Bektas, Schurman, Sen, & Ferrucci, 2018; Franceschi et al., 2000), with older adults showing higher levels of TNF-a, CRP, IL-1β and IL-6 (Franceschi & Campisi, 2014; Michaud et al., 2013). Therefore, it is plausible that the associations between insomnia and depression with inflammation may vary as a function of age, with older individuals exhibiting stronger associations between these variables. Importantly, no studies have tested simultaneous associations among these variables in samples at risk for high levels of depression and insomnia, such as nurses.
Nurses represent a particularly important population in which to examine the associations between insomnia, depression, and inflammation. National studies have shown that approximately 29.2% to 34.3% of nurses report chronic insomnia, a rate three to four times higher than that of the general population (Kageyama, Nishikido, Kobayashi, Oga, & Kawashima, 2001; Ohayon, 1997; Portela et al., 2015). An additional 18% to 32.4% of nurses report symptoms of depression (Letvak, Ruhm, & McCoy, 2012; Maharaj, Lees, & Lal, 2019), a rate approximately two times higher than the general population (Brody, Pratt, & Hughes, 2018). This high prevalence of insomnia and depression among nurses may be attributed to the demanding and stressful nature of their profession and highly variable work and sleep schedules (Kageyama et al., 2001; Øyane, Pallesen, Moen, Åkerstedt, & Bjorvatn, 2013). To inform prevention and intervention efforts, it is critical to understand how insomnia and depression are associated with biomarkers of health in nurses.
To address these gaps, this study examined both insomnia and depression as predictors of inflammatory biomarkers IL-6, CRP, TNF-α, and IL-1β in a large sample of nurses. We hypothesized that both insomnia and depression would be independently associated with elevated levels of these biomarkers. We also hypothesized that age would moderate these associations, such that older nurses would have stronger positive associations between insomnia and depression with inflammation.
Methods
Procedure
This study was part of a larger parent study investigating the effects of sleep patterns on antibody response to the influenza vaccine in nurses. All study procedures were approved by the University of North Texas and Medical City Plano Institutional Review Board. Participants were recruited from two regional hospitals through nursing staff presentations, notification through employee email systems, and flyers that directed them to an initial online consent form. Four-hundred and sixty-one nurses provided online consent and were asked to complete online Qualtrics surveys to collect demographic information and retrospective self-report estimates of recent health. Participants were then invited to enroll in the main portion of the study, which included an in-person informed consent approximately one month later. Seven days after providing informed consent, 393 participants reported to the hospital for a serum blood draw to assess inflammatory biomarkers. The average number of days between completion of the baseline surveys and the blood draw to assess inflammatory markers was 26.62 days (SD = 8.52, range = 7 to 50 days; median = 27 days).
Participants
Inclusion criteria were: 1) not yet vaccinated for the current season of influenza, 2) between the ages of 18 and 65, and 3) registered nurses actively working at least part-time at one of two regional hospitals. Exclusion criteria were: 1) currently pregnant or breastfeeding, or planning to become pregnant, or 2) having an egg allergy. Table 1 reports demographic characteristics for the entire sample and by age group. Generally, participants matched national demographics of nurses in the United States (Health Resources and Services Administration, 2013). The majority of participants were female (92%), white (78%), and non-Hispanic (89%). A substantial portion of the sample also self-reported as Asian (10%) or African American (7%). A majority (63%) of the sample was currently married and most had children (65%). A power analysis revealed that, for a multiple linear regression with nine variables in the model, a sample size of N = 392 (α = 0.05) yielded 89% power to detect a small to medium effect size of f2 = .05.
Table 1.
Participant Demographics for the Whole Sample and by Age Group
| Whole sample | Younger nurses (ages 18-35) |
Middle-aged nurses (ages 36-65) |
SMD | p-value | |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |||
| n | 392 | 165 | 224 | -- | -- |
| Age | 39.54 (11.15) | 29.20 (3.78) | 47.16 (8.26) | 2.796 | <0.001 |
| BMI | 27.28 (5.84) | 25.86 (5.27) | 28.33 (6.02) | 0.436 | <0.001 |
| PHQ-9 total | 3.63 (3.99) | 4.08 (4.24) | 3.28 (3.79) | 0.198 | 0.053 |
| SCI total | 21.66 (6.90) | 21.49 (6.68) | 21.82 (7.06) | 0.047 | 0.650 |
| IL-6 (pg/mL) | 2.19 (2.15) | 2.01 (2.19) | 2.29 (2.09) | 0.129 | 0.210 |
| CRP (ng/mL) | 30.57 (25.28) | 28.79 (27.48) | 31.81 (23.54) | 0.118 | 0.245 |
| IL-1β (pg/mL) | 0.08 (0.20) | 0.07 (0.21) | 0.09 (0.19) | 0.098 | 0.337 |
| TNF-α (pg/mL) | 1.33 (1.97) | 1.16 (0.57) | 1.46 (2.56) | 0.161 | 0.142 |
Note. SCI = Sleep Condition Indicator total score. PHQ-9 = Patient Health Questionnaire-9. IL-6 = interluekin-6. CRP = C-reactive protein. TNF-α = tumor-necrosis factor alpha. IL-1β = interleukin-1 beta. BMI = body mass index. SMD = standardized mean difference.
Measures
Depression (Patient Health Questionnaire-9; PHQ-9).
The PHQ-9 (Kroenke, Spitzer, & Williams, 2001) is a nine-item self-report measure to assess the severity of depression symptoms. It assesses both affective and somatic depression symptoms and corresponds to DSM diagnostic criteria for Major Depression Disorder (Kroenke et al., 2001). The PHQ-9 is summed to obtain a total score ranging from zero to 27, with greater scores indicating greater depression symptomatology. The PHQ-9 has been well-validated and demonstrated good sensitivity and specificity (88% for both) compared to a structured clinical interview (Kroenke et al., 2001). In the current study, the PHQ-9 demonstrated good internal consistency (α = .87). A PHQ-9 total score ≥ 10 is indicative of clinically significant depression (i.e., moderate to severe depressive symptoms; Kroenke et al., 2001).
Insomnia (Sleep Condition Indicator; SCI).
The SCI (Espie et al., 2014) is an eight-item self-report measure that evaluates insomnia symptoms according to DSM-5 diagnostic criteria. The SCI can be summed to obtain a total score ranging from zero to 32, with higher scores indicating better sleep. Nurses were classified as having probable insomnia disorder if they endorsed each of the primary DSM-5 criteria on the SCI (i.e., scores of ≤ 2 on each of the eight items; total scores of ≤ 16). In the original validation study, the SCI demonstrated good internal consistency (α = .86) and good concurrent validity with other measures of insomnia (i.e., PSQI and ISI; rs = .73 and .79, respectively; Espie et al., 2014). In the current study, the SCI demonstrated good internal consistency (α = .87).
Inflammation.
Serum blood draws were conducted by trained phlebotomists. All blood draws occurred between 7am and 12pm to control for circadian rhythmicity of inflammation. Samples remained stationary for 60 minutes to clot and were then centrifuged at 3000 rpms for 30 minutes and aliquoted into cryovials. Samples were temporarily frozen on dry ice and then frozen at −80°C until assayed. All inflammatory samples were assayed using high sensitivity enzyme-linked immunosorbent assays (ELISA) from R&D Systems, Inc. (Minneapolis, MN) within one year of collection. The lower limit of detection (LLD) for CRP was 0.010 ng/mL, 0.033 pg/mL for IL-1β, 0.022 pg/mL for TNF-α, and 0.039 pg/mL for IL-6. All IL-6 and TNF-α samples were within detectable limits. However, 123 (31%) were below the LLD for IL-1β and 1 (0.3%) was below the LLD for CRP. Examination of the standard curve revealed these were not missing data but were instead indicative of very low values. Therefore, in alignment with previous research (Newton et al., 2017; Szabo, Fernandez-Botran, & Newton, 2018), half the LLD was imputed (i.e., 0.0165 pg/mL for IL-1β and 0.005 ng/mL for CRP). Without half the LLD imputed, the intra-assay coefficients of variation (CVs) were 1.60% for CRP, 2.29% for IL-1β, 2.45% for TNF-α, and 3.26% for IL-6. Without half the LLD imputed, inter-assay CVs were 6.73% for CRP, 6.04% for IL-1β, 5.06% for TNF-α, and 7.21% for IL-6.
As expected, inflammatory biomarkers were not normally distributed (CRP skewness before half the LLD was imputed = 0.93, kurtosis = 3.22; IL-6 skewness = 3.47, kurtosis = 18.04; IL-1β skewness before half the LLD was imputed = 7.16, kurtosis = 63.43; TNF-α skewness = 12.76, kurtosis = 179.95). Therefore, IL-1β and CRP values were imputed for samples below the LLD, and then all biomarkers were either square root or log-10 transformed to resolve non-normality for each biomarker (square root CRP skewness after half the LLD was imputed = 0.21, kurtosis = 2.15; log-10 IL-6 skewness = 0.39, kurtosis = 3.32; log-10 IL-1β skewness after half the LLD was imputed = 0.35, kurtosis = 2.86; log-10 TNF-α skewness = 0.38, kurtosis = 13.68).
Baseline covariates.
At baseline, participants reported their age, gender, race, ethnicity, height, and weight. Body mass index (BMI) was calculated based on participants’ self-reported height and weight according to CDC guidelines (May, Freedman, Sherry, & Blanck, 2011).
Analysis Plan
Prior to analyses, all data were screened for missingness, outliers, and violation of assumptions (e.g., excessive skew). All analyses were run in R, an open source statistical program (R Core Team, 2013). Regression analyses were run using R base packages and the package sjPlot (Lüdecke, 2019), and all analyses covaried for age, gender, BMI, race, and ethnicity.
Results
Descriptive Results
Descriptives are provided in Table 1. Approximately 16.8% of the sample (n = 66) met cutoffs for insomnia based on the SCI, and 3.8% (n = 15) met cutoffs for depression based on the PHQ-9 (i.e., scores ≥ 10). Average IL-6 levels were 2.19 (SD = 2.15) pg/mL; CRP levels were 30.57 (SD = 25.28) ng/mL; IL-1β levels were 0.08 (SD = 0.20) pg/mL; and TNF-α levels were 1.33 (SD = 1.97) pg/mL, which are lower values than other similar, primarily female samples (Lakoski et al., 2006; Poole et al., 2013). When using a median split to compare younger nurses (ages 18-35) to middle-aged nurses (ages 36-65), mean inflammatory biomarker levels and insomnia and depression symptoms did not vary significantly by age (Table 1). Bivariate correlations among all study variables are shown in Table 2. Age was correlated with higher TNF-α, but was not correlated with depression, insomnia, or other inflammatory biomarkers. Depression symptoms were correlated with higher levels of insomnia symptoms (lower scores on the SCI indicates worse sleep), IL-6, and CRP. Insomnia symptoms were also correlated with higher IL-6 and CRP.
Table 2.
Bivariate Correlations
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Age | 1 | ||||||
| 2. BMI | .19** [.10, .29] |
||||||
| 3. PHQ9 Total | −.10 [−.19, .00] |
.20** [.10, .29] |
|||||
| 4. SCI Total | .01 [−.09, .11] |
−.21** [−.30, −.11] |
−.62** [−.68, −.55] |
||||
| 5. IL-6 (pg/mL) | .06 [−.04, .16] |
.35** [.26, .44] |
.10* [.01, .20] |
−.15** [−.25, −.05] |
|||
| 6. CRP (ng/mL) | .04 [−.06, .14] |
.48** [.40, .56] |
.13** [.04, .23] |
−.13* [−.22, −.03] |
.40** [.31, .48] |
||
| 7. IL-1β (pg/mL) | .07 [−.03, .17] |
.06 [−.04, .16] |
−.03 [−.12, .07] |
.01 [−.09, .11] |
.25** [.15, .34] |
.09 [−.01, .19] |
|
| 8. TNF-α (pg/mL) | .13* [.03, .23] |
.19** [.09, .28] |
.07 [−.03, .17] |
−.05 [−.15, .05] |
.19** [.10, .29] |
.11* [.01, .21] |
.01 [−.09, .11] |
Note. All biomarkers represent raw (non-transformed) values. M and SD are used to represent mean and standard deviation, respectively. Values in square brackets indicate the 95% confidence interval for each correlation. The confidence interval is a plausible range of population correlations that could have caused the sample correlation (Cumming, 2014).
indicates p < .05.
indicates p < .01. SCI = Sleep Condition Indicator (higher scores indicate better sleep). PHQ-9 = Patient Health Questionnaire-9. IL-6 = interluekin-6. CRP = C-reactive protein. TNF-α = tumor-necrosis factor alpha. IL-1β = interleukin-1 beta. BMI = body mass index.
Main Effects of Insomnia and Depression Symptoms on Inflammation
After controlling for all covariates, insomnia and depression symptoms were no longer significantly associated with any inflammatory biomarkers (Table 3). Results were similar when insomnia or depression symptoms were each examined separately with other covariates (see Supplementary Tables 1-4). Age continued to predict higher IL-1β (Table 3).
Table 3.
Main Effects of Insomnia and Depression Symptoms on Inflammatory Markers
| CRP (ng/mL) | IL-6 (pg/mL) | IL-1β (pg/mL) | TNF-α (pg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | p | Estimates | CI | p | Estimates | CI | p | Estimates | CI | p |
| (Intercept) | −0.47 | −2.07 – 1.13 | 0.562 | −0.35 | −0.57 – −0.13 | 0.002 | −2.07 | −2.50 – −1.64 | <0.001 | −0.28 | −0.45 – −0.11 | 0.002 |
| Insomnia | −0.00 | −0.04 – 0.04 | 0.941 | −0.01 | −0.01 – 0.00 | 0.056 | 0.00 | −0.01 – 0.01 | 0.517 | −0.00 | −0.00 – 0.00 | 0.772 |
| Depression | 0.02 | −0.04 – 0.09 | 0.461 | −0.00 | −0.01 – 0.00 | 0.326 | 0.00 | −0.02 – 0.02 | 0.847 | −0.00 | −0.01 – 0.01 | 0.647 |
| Age | −0.00 | −0.02 – 0.01 | 0.618 | 0.00 | −0.00 – 0.00 | 0.229 | 0.01 | 0.00 – 0.01 | 0.007 | 0.00 | −0.00 – 0.00 | 0.231 |
| Gender | −0.76 | −1.51 – −0.01 | 0.047 | −0.03 | −0.13 – 0.07 | 0.556 | 0.06 | −0.14 – 0.26 | 0.531 | 0.08 | 0.00 – 0.16 | 0.041 |
| Ethnicity | −0.40 | −1.07 – 0.27 | 0.241 | 0.04 | −0.06 – 0.13 | 0.450 | −0.04 | −0.22 – 0.14 | 0.682 | −0.07 | −0.14 – 0.00 | 0.056 |
| Race | 0.49 | −0.02 – 0.99 | 0.058 | 0.00 | −0.07 – 0.07 | 0.998 | 0.05 | −0.08 – 0.19 | 0.430 | 0.03 | −0.02 – 0.08 | 0.252 |
| BMI | 0.20 | 0.16 – 0.23 | <0.001 | 0.02 | 0.02 – 0.03 | <0.001 | 0.01 | −0.00 – 0.02 | 0.131 | 0.01 | 0.01 – 0.01 | <0.001 |
| Observations | 380 | 379 | 378 | 377 | ||||||||
| R2 / R2 adjusted | 0.267 / 0.253 | 0.213 / 0.198 | 0.037 / 0.018 | 0.097 / 0.079 | ||||||||
Note. IL-6 = interluekin-6. CRP = C-reactive protein. TNF-α = tumor-necrosis factor alpha. IL-1β = interleukin-1 beta. BMI = body mass index. Bold values indicate p < .05 associations. CRP was square-root transformed, and IL-6, IL-1β, and TNF-α were all log 10 transformed. Estimates represent unstandardized betas. CI = 95% confidence intervals. Race is coded as 0 = any other race, 1 = white. Ethnicity is coded as 1 = non-Hispanic/Latinx, 2 = Hispanic/Latinx.
Moderation by Age
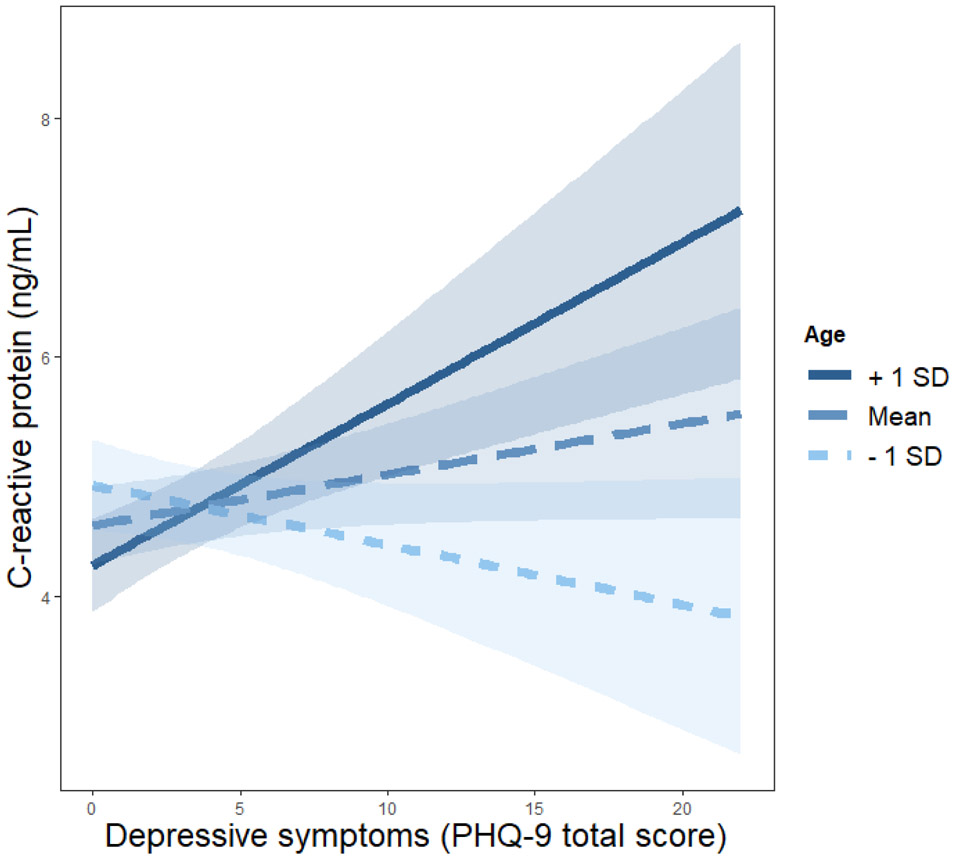
Age did not moderate the association between insomnia symptoms and any inflammatory markers, or associations between depression symptoms and IL-6 or IL-1β (Table 4). However, there was a significant interaction between age and depression symptoms with CRP levels (Table 4), such that only among older nurses (1 SD above the mean of age) were depression symptoms associated with higher CRP (b = .14, SE = .06, p = .017; Figure 1). For younger nurses, depression symptoms were not associated with CRP (mean age: b = 0.04, SE = 0.03, p = 0.215; 1 SD below the mean age: b = −0.05, SE = 0.05, p = 0.282; Figure 1). There also was a significant interaction between age and depression symptoms with TNF-α (Table 4), such that only among older nurses (1 SD above the mean of age) were depression symptoms associated with higher TNF-α (b = .008, SE = .006, p = .165; Figure 2). For younger nurses, depression symptoms were not associated with TNF-α (mean age: b = −0.0002, SE = 0.004, p = 0.953; 1 SD below the mean age: b = −0.009, SE = 0.005, p = 0.076; Figure 2).
Table 4.
Moderated Effect of Age on the Associations between Insomnia and Depression Symptoms and Inflammatory Markers
| CRP (ng/mL) | IL-6 (pg/mL) | IL-1β (pg/mL) | TNF-α (pg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | p | Estimates | CI | p | Estimates | CI | p | Estimates | CI | p |
| (Intercept) | 1.90 | −2.12 – 5.92 | 0.352 | 0.05 | −0.52 – 0.61 | 0.873 | −1.92 | −3.01 – −0.83 | 0.001 | 0.06 | −0.37 – 0.48 | 0.797 |
| Depression | −0.29 | −0.55 – −0.02 | 0.034 | −0.03 | −0.07 – 0.01 | 0.106 | −0.01 | −0.08 – 0.06 | 0.846 | −0.03 | −0.06 – −0.00 | 0.033 |
| Age | −0.07 | −0.16 – 0.03 | 0.186 | −0.01 | −0.02 – 0.01 | 0.221 | 0.00 | −0.02 – 0.03 | 0.802 | −0.01 | −0.02 – 0.00 | 0.171 |
| Insomnia | −0.06 | −0.21 – 0.09 | 0.423 | −0.02 | −0.04 – 0.00 | 0.068 | −0.00 | −0.04 – 0.04 | 0.915 | −0.01 | −0.03 – 0.00 | 0.163 |
| Gender | −0.73 | −1.47 – 0.02 | 0.056 | −0.03 | −0.13 – 0.08 | 0.595 | 0.06 | −0.14 – 0.27 | 0.525 | 0.09 | 0.01 – 0.16 | 0.033 |
| Ethnicity | −0.32 | −0.99 – 0.34 | 0.338 | 0.04 | −0.05 – 0.13 | 0.413 | −0.04 | −0.22 – 0.14 | 0.690 | −0.06 | −0.14 – 0.01 | 0.080 |
| Race | 0.49 | −0.01 – 0.99 | 0.056 | 0.00 | −0.07 – 0.07 | 0.947 | 0.06 | −0.08 – 0.19 | 0.424 | 0.03 | −0.02 – 0.09 | 0.236 |
| BMI | 0.19 | 0.15 – 0.23 | <0.001 | 0.02 | 0.02 – 0.03 | <0.001 | 0.01 | −0.00 – 0.02 | 0.136 | 0.01 | 0.01 – 0.01 | <0.001 |
| Depression x Age | 0.01 | 0.00 – 0.02 | 0.017 | 0.00 | −0.00 – 0.00 | 0.160 | 0.00 | −0.00 – 0.00 | 0.806 | 0.00 | 0.00 – 0.00 | 0.038 |
| Insomnia x Age | 0.00 | −0.00 – 0.01 | 0.386 | 0.00 | −0.00 – 0.00 | 0.162 | 0.00 | −0.00 – 0.00 | 0.775 | 0.00 | −0.00 – 0.00 | 0.160 |
| Observations | 380 | 379 | 378 | 377 | ||||||||
| R2 / R2 adjusted | 0.279 / 0.262 | 0.219 / 0.199 | 0.037 / 0.013 | 0.107 / 0.085 | ||||||||
Note. IL-6 = interluekin-6. CRP = C-reactive protein. TNF-α = tumor-necrosis factor alpha. IL-1β = interleukin-1 beta. BMI = body mass index. Bold values indicate p < .05 associations. CRP was square-root transformed, and IL-6, IL-1β, and TNF-α were all log 10 transformed. Estimates represent unstandardized betas. CI = 95% confidence intervals. Race is coded as 0 = any other race, 1 = white. Ethnicity is coded as 1 = non-Hispanic/Latinx, 2 = Hispanic/Latinx.
Figure 1. Moderated Effect of Age on the Association between Depression Symptoms and CRP.
Note. Simple slopes at 1 standard deviation (SD) below, at, and above the mean of age (M = 39.54, SD = 11.15). PHQ-9 = Patient Health Questionnaire-9.
Figure 2. Moderated Effect of Age on the Association between Depression Symptoms and TNF-α.
Note. Simple slopes at 1 standard deviation (SD) below, at, and above the mean of age (M = 39.54, SD = 11.15). PHQ-9 = Patient Health Questionnaire-9.
Discussion
Multiple studies have examined the associations among depression or insomnia with inflammation, but this is the first study to consider all three in the context of age in a sample of nurses. Although our fully-adjusted models did not indicate associations between insomnia or depression symptoms with inflammatory biomarkers, we found a significant association for older nurses, such that higher depression symptoms were associated with higher levels of pro-inflammatory biomarkers CRP and TNF-α. Our results indicate that older nurses may be at increased risk for elevated inflammation when their depression symptoms are high.
In contrast to existing literature (Eisenberger et al., 2010; Howren et al., 2009; Liukkonen et al., 2007; Mills et al., 2007; Von Känel et al., 2010), we did not find any main effects of insomnia or depression symptoms on inflammation in fully adjusted models. One possible explanation for this could be unique characteristics of our participants. Nurses may be particularly likely to engage in healthy lifestyle behaviors (e.g., engaging in regular exercise, abstaining from tobacco use) due to their health care knowledge (Fair, Gulanick, & Braun, 2009). These favorable lifestyle choices may decrease their systemic inflammation and act as a buffer against the inflammatory effects of depression or insomnia. Nurses also commonly work 12-hour shifts, with a majority of that time spent standing or involved in physically demanding tasks. The labor-intensive nature of their work could have a protective effect on their systemic inflammation and symptoms of depression and insomnia. Indeed, in our sample, insomnia and depression symptoms were quite low (16.8% had probable insomnia; 3.8% had probable depression, according to scale cutoffs) compared to other similar samples (Kageyama et al., 2001; Letvak et al., 2012; Maharaj et al., 2019; Portela et al., 2015). This likely led to a restriction of range in values and limited predictive power. Inflammatory biomarker levels also were considerably lower in our sample than other large, primarily female samples (Lakoski et al., 2006; Poole et al., 2013). These differences may be due to the relatively young age of our sample, and/or suggest that our sample may have been relatively healthier than average.
Furthermore, nurses have rotating work and sleep schedules, which means that, although all blood draws were performed during morning hours, all participants were not necessarily in the same phase of their sleep/wake cycle, which could impact levels of inflammatory biomarkers. For example, previous studies have shown that IL-6 and IL-1β have a circadian rhythm (Covelli et al., 1992; DeRijk et al., 1997; Vgontzas et al., 2005) and are sensitive to acute sleep loss (Chennaoui et al., 2011; Covelli et al., 1992; Vgontzas et al., 1999; Vgontzas et al., 2002). In contrast, CRP is relatively stable across time and does not exhibit a strong circadian rhythm (Meier-Ewert et al., 2001), which may be one explanation for why we observed significant effects for CRP but not IL-6 or IL-1β. In the current study, exploratory post hoc analyses revealed that a later wake time on the day of the blood draw was associated with higher IL-6 levels, but not IL-1β, TNF-α, or CRP levels. It is also important to note that there was an approximately one-month lag between the insomnia and depression assessment and the blood draw to assess inflammatory markers, which may explain the low correlations we observed between these measures. Future studies should examine insomnia and depression symptoms more proximal to inflammation assessments.
Another possible explanation for the differences between our findings and previous studies could be that other studies examined specific symptoms of insomnia (e.g., sleep fragmentation, total sleep time, sleep onset latency) or depression (e.g., mood, cognitive symptoms, neurovegetative symptoms), whereas the current study only focused on the total scores of these measures. Different dimensions of insomnia and depression could possibly be associated with specific inflammatory biomarkers, and our correlations may have been attenuated by using more holistic measures. For example, recent research has shown that only neurovegetative symptoms of depression (e.g., fatigue, appetite changes, weight fluctuations) may be associated with elevated inflammation (Majd, Saunders, & Engeland, 2019). Furthermore, there is evidence that specific sub-types of insomnia (e.g., insomnia with objective short sleep duration) pose greater risk for physiological dysregulation (including elevated inflammation; Fernandez-Mendoza et al., 2017; Fernandez-Mendoza et al., 2012). Future studies should continue to examine how specific symptoms and subtypes of insomnia and depression may be differentially related to inflammation.
Moderation by Age
Moderation analyses revealed that, for older nurses only, more severe depression symptoms were associated with higher levels of CRP and TNF-α. Previous studies have shown that age is one of the strongest risk factors for elevated inflammation (Franceschi & Campisi, 2014). In fact, chronic, low-grade inflammation (“inflammaging”) may be one of the defining characteristics of aging (Franceschi & Campisi, 2014). Age may also exacerbate symptoms of both insomnia (Lichstein et al., 2004; Sivertsen et al., 2009) and depression (Mirowsky & Ross, 1992), and older age may also be associated with longer duration of insomnia and depression symptoms. It is possible that younger adults with elevated depression may not have had symptoms for a sufficient length of time to elicit significant alterations in inflammation. Older nurses may also experience a greater level of work responsibility and household demands (e.g., caregiving) that increase chronic stress levels and render them particularly susceptible to elevated depression symptoms and inflammation. Therefore, it is possible that previous studies have failed to find significant associations among insomnia or depression and inflammation due to the exclusion of age as a moderating variable.
Potential Implications
The joint effect of depression and age on inflammation among nurses represents a particularly timely issue, as the nursing workforce in the United States is aging, and approximately one million nurses are currently over the age of 50 (Haddad, Annamaraju, & Toney-Butler, 2020). Depressive symptoms among nurses can influence nurses’ own health, their colleagues’ health, and the quality of care provided to their patients (Letvak et al., 2012). Screening nurses for depression and providing clinical treatment early on may be one way to proactively improve health before symptoms become exacerbated and possibly lead to further physiological dysregulation. Developing a better understanding of the unique psychological correlates of systemic inflammation among nurses may also inform more targeted and effective treatment and prevention efforts. For example, cognitive behavioral therapy for depression and other psychosocial interventions have been shown to lead to decreases in inflammation and improved immune function (Lopresti, 2017; Shields, Spahr, & Slavich, 2020). The results in the current study could also influence organizational policies to improve employee health and well-being. Employers could promote health among nurses by fostering a supportive environment in which their employees are encouraged to utilize their vacation time and sick days, provide psychoeducational training related to self-care, and other mental health resources such as free counseling services through their employers.
Limitations
Although this study has many unique strengths (e.g., large representative sample, validated measures of psychosocial health), there are some limitations warranting discussion. First, the current study only investigated some of the potential correlates of inflammation. There are likely other factors involved in the associations between insomnia, depression, age, and inflammation, such as medication use, underlying medical conditions, perceived stress, and/or work schedule. These factors warrant future investigation. Furthermore, given the cross-sectional, observational nature of this study, it is impossible to infer causality of the associations we observed. It is likely that elevated levels of inflammation may also cause depression symptoms. Indeed, studies have shown that exogenous administration of pro-inflammatory cytokines elicits depression-like symptoms (for a review, see Dantzer, 2001). Future longitudinal and experimental studies are needed to untangle the directionality of the effects observed. Finally, although our sample demographics generally matched national demographics of nurses in the U.S. (Health Resources and Services Administration, 2013), our sample was primarily female, limiting the ability to investigate potential sex and gender differences in these associations. Previous studies have demonstrated important gender differences in both sleep and inflammation, such that women may be more affected by the detrimental effects of sleep loss on inflammation (Prather, Epel, Cohen, Neylan, & Whooley, 2013; Suarez, 2008).
Conclusion
Nurses represent a critical, frontline part of the healthcare system and make up the largest segment of the healthcare profession (Haddad et al., 2020). The findings of the current study indicate older nurses with greater depression symptoms may be particularly at risk for elevated inflammation. As such, future studies examining the associations among insomnia, depression, and inflammation should consider the important role of age in modifying these associations. Future randomized clinical trials targeting insomnia and depression in nurses are necessary to more definitively determine if treating these disorders may improve inflammation in this population. Given the essential role that nurses play in maintaining patient health and safety, it is critical to better understand the unique correlates of their own health.
Supplementary Material
Acknowledgments
Conflicts of Interest and Source of Funding: No conflicts of interest. This research was supported by the National Institute of Allergy and Infectious Diseases under Grant 1R01AI128359-01 (PIs: Taylor & Kelly).
References
- Alexopoulos GS (2005). Depression in the elderly. The Lancet, 365(9475), 1961–1970. [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, … Hu FB (2003). A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes care, 26(2), 380–384. [DOI] [PubMed] [Google Scholar]
- Bei B, Ong JC, Rajaratnam SM, & Manber R (2015). Chronotype and improved sleep efficiency independently predict depressive symptom reduction after group cognitive behavioral therapy for insomnia. Journal of Clinical Sleep Medicine, 11(09), 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bektas A, Schurman SH, Sen R, & Ferrucci L (2018). Aging, inflammation and the environment. Experimental Gerontology, 105, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, & Cole SW (2011). Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology, 29(26), 3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DJ, Pratt LA, & Hughes JP (2018). Prevalence of Depression Among Adults Aged 20 and Over: United States, 2013-2016. NCHS data brief(303), 1–8. [PubMed] [Google Scholar]
- Chapman DP, Perry GS, & Strine TW (2005). The vital link between chronic disease and depressive disorders. Preventing chronic disease, 2(1), 480–486. [PMC free article] [PubMed] [Google Scholar]
- Chen P-Y, Qin L, Li G, Wang Z, Dahlman JE, Malagon-Lopez J, … Sun L (2019). Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis. Nature Metabolism, 1(9), 912–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, … Gomez-Merino D (2011). Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine, 56(2), 318–324. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, & Turner RB (2012). Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences, 109(16), 5995–5999. doi: 10.1073/pnas.1118355109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covelli V, Massari F, Fallacara C, Munno I, Jirillo E, Savastano S, … Lombardi G (1992). Interleukin-Ibeta and Beta-Endorphin Orcadian Rhythms are Inversely Related in Normal and Stress-Altered Sleep. International Journal of Neuroscience, 63(3-4), 299–305. [DOI] [PubMed] [Google Scholar]
- D’Antono B, & Bouchard V (2019). Impaired sleep quality is associated with concurrent elevations in inflammatory markers: Are post-menopausal women at greater risk? Biology of sex differences, 10(1), 34. doi: 10.1186/s13293-019-0250-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R (2001). Cytokine-induced sickness behavior: mechanisms and implications. Annals of the New York Academy of Sciences, 933(1), 222–234. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9(1), 46–56. doi: 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRijk R, Michelson D, Karp B, Petrides J, Galliven E, Deuster P, … Sternberg EM (1997). Exercise and circadian rhythm-induced variations in plasma cortisol differentially regulate interleukin-1b (IL-1b), IL-6, and tumor necrosis factor-a (TNF-a) production in humans: High sensitivity of TNF-a and resistance of IL-6. Journal of Clinical Endocrinology and Metabolism, 82, 2182–2192. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, & Lanctôt KL (2010). A Meta-Analysis of Cytokines in Major Depression. Biological Psychiatry, 67(5), 446–457. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, & Irwin MR (2010). Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity, 24(4), 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie CA, Kyle SD, Hames P, Gardani M, Fleming L, & Cape J (2014). The Sleep Condition Indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open, 4(3), e004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair JM, Gulanick M, & Braun LT (2009). Cardiovascular risk factors and lifestyle habits among preventive cardiovascular nurses. Journal of Cardiovascular Nursing, 24(4), 277–286. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Baker JH, Vgontzas AN, Gaines J, Liao D, & Bixler EO (2017). Insomnia symptoms with objective short sleep duration are associated with systemic inflammation in adolescents. Brain, Behavior, and Immunity, 61, 110–116. doi: 10.1016/j.bbi.2016.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, & Bixler EO (2012). Insomnia with objective short sleep duration and incident hypertension: The Penn State Cohort. Hypertension, 60(4), 929–935. doi: 10.1161/hypertensionaha.112.193268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, & De Benedictis G (2000). Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences, 908, 244–254. [DOI] [PubMed] [Google Scholar]
- Franceschi C, & Campisi J (2014). Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 69(Suppl_1), S4–S9. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Mills PJ, Irwin MR, & Ziegler MG (2000). Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: Differential effects and pathways. Psychosomatic Medicine, 62, 591–598. [DOI] [PubMed] [Google Scholar]
- Haddad LM, Annamaraju P, & Toney-Butler TJ (2020). Nursing shortage. In StatPearls [Internet]: StatPearls Publishing. [PubMed] [Google Scholar]
- Harvey AG (2011). Sleep and circadian functioning: critical mechanisms in the mood disorders? Annual Review of Clinical Psychology, 7, 297–319. [DOI] [PubMed] [Google Scholar]
- Health Resources and Services Administration. (2013). The U.S. nursing workforce: Trends in supply and education. Retrieved from https://bhw.hrsa.gov/sites/default/files/bhw/nchwa/projections/nursingworkforcetrendsoct2013.pdf
- Hogan D, Morrow JD, Smith EM, & Opp MR (2003). Interleukin-6 alters sleep of rats. Journal of Neuroimmunology, 137(1-2), 59–66. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, & Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine, 71(2), 171–186. [DOI] [PubMed] [Google Scholar]
- Irwin M (2001). Depression and immunity. In Ader R, Felten DL, & Cohen N (Eds.), Psychoneuroimmunology, Third Edition (Vol. 2, pp. 383–398). San Diego: Academic Press. [Google Scholar]
- Irwin M, Brown M, Patterson T, Hauger R, Mascovich A, & Grant I (1991). Neuropeptide Y and natural killer cell activity: Findings in depression and Alzheimer caregiver stress. FASEB Journal, 5, 3100–3107. [DOI] [PubMed] [Google Scholar]
- Irwin M, Rinetti G, Redwine L, Motivala S, Dang J, & Ehlers C (2004). Nocturnal proinflammatory cytokine-associated sleep disturbances in abstinent African American alcoholics. Brain, Behavior, and Immunity, 18(4), 349–360. [DOI] [PubMed] [Google Scholar]
- Irwin MR (2013). Depression and insomnia in cancer: prevalence, risk factors, and effects on cancer outcomes. Current Psychiatry Reports, 15(11), 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR (2015). Why sleep is important for health: A psychoneuroimmunology perspective. Annual Review of Psychology, 66, 143–172. doi: 10.1146/annurev-psych-010213-115205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, & Carroll JE (2016). Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biological Psychiatry, 80(1), 40–52. doi: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama T, Nishikido N, Kobayashi T, Oga J, & Kawashima M (2001). Cross-sectional survey on risk factors for insomnia in Japanese female hospital nurses working rapidly rotating shift systems. Journal Of Human Ergology, 30(1-2), 149–154. doi: 10.11183/jhe1972.30.149 [DOI] [PubMed] [Google Scholar]
- Kennedy GJ (1996). The epidemiology of late-life depression. In Kennedy GJ (Ed.), Suicide and depression in late life: Critical issues in treatment, research and public policy (pp. 23–37). New York: John Wiley and Sons. [Google Scholar]
- Kessler RC (2012). The costs of depression. Psychiatric Clinics, 35(1), 1–14. doi: 10.1016/j.psc.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D'Agostino RB Jr, & Herrington DM (2006). Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. American Heart Journal, 152(3), 593–598. [DOI] [PubMed] [Google Scholar]
- Lesperance F, Frasure-Smith N, Theroux P, & Irwin M (2004). The association between major depression and levels of soluble intercellular adhesion molecule 1, interleukin-6, and C-reactive protein in patients with recent acute coronary syndromes. The American Journal of Psychiatry, 161, 271–277. [DOI] [PubMed] [Google Scholar]
- Letvak S, Ruhm CJ, & McCoy T (2012). Depression in hospital-employed nurses. Clin Nurse Spec, 26(3), 177–182. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, & Bush AJ (2004). Epidemiology of sleep: Age, gender, and ethnicity: Psychology Press. [Google Scholar]
- Liukkonen T, Räsänen P, Ruokonen A, Laitinen J, Jokelainen J, Leinonen M, … Timonen M (2007). C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosomatic Medicine, 69(8), 756–761. [DOI] [PubMed] [Google Scholar]
- Lopresti AL (2017). Cognitive behaviour therapy and inflammation: A systematic review of its relationship and the potential implications for the treatment of depression. Aust N Z J Psychiatry, 51(6), 565–582. doi: 10.1177/0004867417701996 [DOI] [PubMed] [Google Scholar]
- Lüdecke D (2019). sjPlot: Data Visualization for Statistics in Social Science. R package version 2.7.2. doi: 10.5281/zenodo.1308157 [DOI] [Google Scholar]
- Maharaj S, Lees T, & Lal S (2019). Prevalence and risk factors of depression, anxiety, and stress in a cohort of Australian nurses. International journal of environmental research and public health, 16(1), 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majd M, Saunders EF, & Engeland CG (2019). Inflammation and the Dimensions of Depression: A Review. Frontiers in Neuroendocrinology, 100800. [DOI] [PubMed] [Google Scholar]
- Marcus M, Yasamy MT, van Ommeren M. v., Chisholm D, & Saxena S (2012). Depression: A global public health concern. [Google Scholar]
- May AL, Freedman D, Sherry B, & Blanck HM (2011). Obesity—United States, 1988–2008. MMWR, 60(01), 73–77. [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, & Mullington JM (2001). Absence of diurnal variation of C-reactive protein concentrations in healthy human subjects. Clinical Chemistry, 47(3), 426–430. [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, & Mullington JM (2004). Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology, 43(4), 678–683. doi: 10.1016/j.jacc.2003.07.050 [DOI] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, … Nourhashemi F (2013). Proinflammatory cytokines, aging, and age-related diseases. Journal of the American Medical Directors Association, 14(12), 877–882. [DOI] [PubMed] [Google Scholar]
- Mills PJ, von Känel R, Norman D, Natarajan L, Ziegler MG, & Dimsdale JE (2007). Inflammation and sleep in healthy individuals. Sleep, 30(6), 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky J, & Ross CE (1992). Age and depression. Journal of Health and Social Behavior, 187–205. [PubMed] [Google Scholar]
- Mitchell PB, & Harvey SB (2014). Depression and the older medical patient—When and how to intervene. Maturitas, 79(2), 153–159. [DOI] [PubMed] [Google Scholar]
- Morgan K (2003). Daytime activity and risk factors for late‐life insomnia. Journal of Sleep Research, 12(3), 231–238. [DOI] [PubMed] [Google Scholar]
- Morphy H, Dunn KM, Lewis M, Boardman HF, & Croft PR (2007). Epidemiology of insomnia: a longitudinal study in a UK population. Sleep, 30(3), 274–280. [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, & Irwin MR (2005). Inflammatory markers and sleep disturbance in major depression. Psychosomatic Medicine, 67(2), 187–194. [DOI] [PubMed] [Google Scholar]
- Newton TL, Fernandez-Botran R, Lyle KB, Szabo YZ, Miller JJ, & Warnecke AJ (2017). Salivary cytokine response in the aftermath of stress: An emotion regulation perspective. Emotion, 17(6), 1007–1020. doi: 10.1037/emo0000156 [DOI] [PubMed] [Google Scholar]
- Nikendei C, Terhoeven V, Ehrenthal JC, Maatouk I, Wild B, Herzog W, & Friederich HC (2018). Depression profile in cancer patients and patients without a chronic somatic disease. Psycho-oncology, 27(1), 83–90. [DOI] [PubMed] [Google Scholar]
- Ohayon M (2002). Epidemiology of insomnia: What we know and what we still need to learn. Sleep Medicine Reviews, 6, 97–111. doi: 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- Ohayon MM (1997). Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. Journal of Psychiatric Research, 31(3), 333–346. [DOI] [PubMed] [Google Scholar]
- Øyane NM, Pallesen S, Moen BE, Åkerstedt T, & Bjorvatn B (2013). Associations between night work and anxiety, depression, insomnia, sleepiness and fatigue in a sample of Norwegian nurses. PLoS ONE, 8(8), e70228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B, & Mannino DM (2007). Do insomnia complaints cause hypertension or cardiovascular disease? Journal of Clinical Sleep Medicine, 3(5), 489–494. [PMC free article] [PubMed] [Google Scholar]
- Poole EM, Lee I-M, Ridker PM, Buring JE, Hankinson SE, & Tworoger SS (2013). A prospective study of circulating C-reactive protein, interleukin-6, and tumor necrosis factor α receptor 2 levels and risk of ovarian cancer. American Journal of Epidemiology, 178(8), 1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela LF, Kröning Luna C, Rotenberg L, Silva-Costa A, Toivanen S, Araújo T, & Griep RH (2015). Job strain and self-reported insomnia symptoms among nurses: What about the influence of emotional demands and social support? BioMed research international, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Epel ES, Cohen BE, Neylan TC, & Whooley MA (2013). Gender differences in the prospective associations of self-reported sleep quality with biomarkers of systemic inflammation and coagulation: findings from the Heart and Soul Study. Journal of Psychiatric Research, 47(9), 1228–1235. doi: 10.1016/j.jpsychires.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/ [Google Scholar]
- Samuelsson LB, Bovbjerg DH, Roecklein KA, & Hall MH (2018). Sleep and circadian disruption and incident breast cancer risk: An evidence-based and theoretical review. Neuroscience & Biobehavioral Reviews, 84, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Spahr CM, & Slavich GM (2020). Psychosocial interventions and immune system function: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2020.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivertsen B, Krokstad S, Overland S, & Mykletun A (2009). The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J Psychosom Res, 67(2), 109–116. doi: 10.1016/j.jpsychores.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Smith JA, Das A, Ray SK, & Banik NL (2012). Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Research Bulletin, 87(1), 10–20. doi: 10.1016/j.brainresbull.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez EC (2008). Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain, Behavior, and Immunity, 22(6), 960–968. doi: 10.1016/j.bbi.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo YZ, Fernandez-Botran R, & Newton TL (2018). Cumulative trauma, emotion reactivity and salivary cytokine levels following acute stress in healthy women. Anxiety, Stress, and Coping, 20, 1–13. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Lichstein KL, & Durrence HH (2003). Insomnia as a health risk factor. Behavioral Sleep Medicine, 1(4), 227–247. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, & Chrousos GP (2005). IL-6 and its circadian secretion in humans. Neuroimmunomodulation, 12(3), 131–140. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Liao D, & Bixler EO (2013). Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Medicine Reviews, 17(4), 241–254. doi: 10.1016/j.smrv.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, … Chrousos GP (1999). Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab, 84, 2603–2607. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, … Chrousos GP (2002). Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism, 51(7), 887–892. doi: 10.1053/meta.2002.33357 [DOI] [PubMed] [Google Scholar]
- Von Känel R, Ancoli-Israel S, Dimsdale JE, Mills PJ, Mausbach BT, Ziegler MG, … Grant I (2010). Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology, 56(1), 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Ruesten A, Weikert C, Fietze I, & Boeing H (2012). Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS ONE, 7(1), e30972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickwire EM, Shaya FT, & Scharf SM (2016). Health economics of insomnia treatments: the return on investment for a good night's sleep. Sleep Medicine Reviews, 30, 72–82. doi: 10.1016/j.smrv.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, & Ma L (2018). Depression and cardiovascular disease in elderly: Current understanding. Journal of Clinical Neuroscience, 47, 1–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.