Abstract
Few classes of natural products have inspired as many chemists and biologists as have the iboga alkaloids. This family of monoterpenoid indole alkaloids includes the anti-addictive compound ibogaine as well as catharanthine, a precursor to the chemotherapeutic vinblastine. Despite being known for over 120 years, these small molecules continue to challenge our assumptions about biosynthetic pathways, catalyze our creativity for constructing complex architectures, and embolden new approaches for treating mental illness. This review will cover recent advances in both the biosynthesis and chemical synthesis of iboga alkaloids as well as their use as next-generation neurotherapeutics. Whenever appropriate, we provide historical context for the discoveries of the past decade and indicate areas that have yet to be resolved. While significant progress regarding their chemistry and pharmacology has been made since the 1960s, it is clear that the iboga alkaloids will continue to stoke scientific innovation for years to come.
Keywords: Iboga alkaloids, ibogaine, ibogamine, coronaridine, voacangine, catharanthine, total synthesis, biosynthesis, psychedelic, neural plasticity, addiction
toc
INTRODUCTION
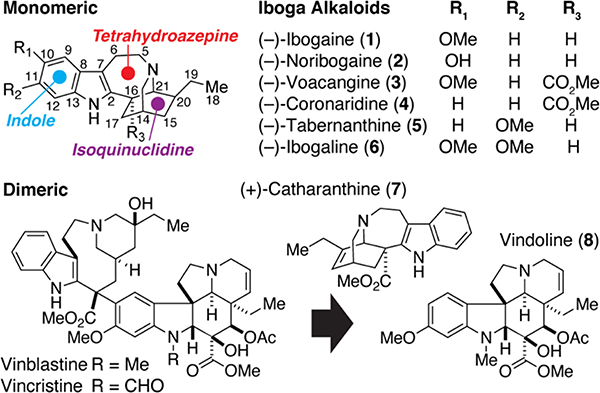
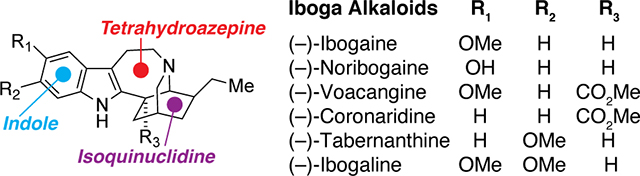
Ibogaine was first isolated in 1901,1 and its structure was deduced by Bartlett, Dickel, and Taylor in 1958.2 In 1960, its absolute stereochemistry was unambiguously assigned by X-ray crystallography.3 The defining features of the iboga architecture include an indole, a 7-membered tetrahydroazepine, and a bicyclic isoquinuclidine (Figure 1). Since ibogaine’s initial discovery, hundreds of alkaloids bearing structural and/or biosynthetic similarities to ibogaine have been identified. The chemical structures of some of the more common iboga alkaloids are highlighted in Figure 1 with the Le Men and Taylor numbering convention indicated.4 While this review will primarily focus on monomeric iboga alkaloids, dimeric structures containing at least one iboga component are also common and include the notable chemotherapeutics vincristine and vinblastine (Figure 1). For information on the chemistry and/or biology of this class of bisindole alkaloids, we point the reader to a recent review.5
Figure 1.
Structures of iboga alkaloids and related compounds
The unique structures of iboga alkaloids have captured the imagination of chemists for decades, while their unusual effects on the brain have challenged conventional ideas about treating substance use disorder. Though substantial progress has been made concerning the chemistry and neuropharmacology of these alkaloids, many unresolved problems and unanswered questions remain. Ibogaine—the prototypical iboga alkaloid with the most neurobiological data—still lacks a truly robust, scalable, enantioselective total synthesis. Moreover, its biological mechanism of action is completely opaque, pushing the limits of what traditional neuropharmacology is capable of explaining. Here, we summarize the history of iboga alkaloid isolation, biosynthesis, total synthesis, analog development, and neuropharmacology with particular emphasis placed on advances made in the past decade.
ISOLATION
Monoterpenoid indole alkaloids (MIA) of the iboga-type are found in a variety of plant species around the world, though historically, isolation of iboga alkaloids has generally been restricted to regions of West Africa.6 There are hundreds of iboga alkaloids, but the compounds shown in Figure 1 represent some of the most commonly reported in natural product isolation and total synthesis literature. Of these alkaloids, ibogaine has attracted significant attention due to its therapeutic potential (vide infra). However, the isolation of ibogaine from natural sources has been plagued by ethical and environmental challenges,7 as it only accounts for approximately 0.3% of the root bark weight in Tabernanthe iboga (Table 1). In contrast, significantly larger amounts of voacangine can be extracted from the root bark of Voacanga africana (~1.7% of the root bark). As a result, ibogaine is often produced via semi-synthesis starting from voacangine.10 Conversion of voacangine to ibogaine generally requires a two-step protocol involving saponification of the C16 methyl ester followed by acidification and heating to induce decarboxylation.7,8 In addition to ibogaine, Table 1 details several other common iboga alkaloids found in the Tabernanthe and Voacanga genera. Interestingly, catharanthine belongs to the opposite optical series compared to other reported iboga alkaloids and is exclusively found in the Catharanthus roseus plant species. The isolated compounds in Table 1 have sparked interest in more recent efforts to determine the alkaloid profiles of plants in the broader Apocynaceae family.
Table 1.
Approximate yields of iboga alkaloids isolated from the whole root bark of various sources. Percentages indicate the weight of the alkaloid free base relative to the weight of the plant source. TR = trace (< 0.01%). NR = Not Reported.
| Plant Species | Ibogaine | Ibogamine | Voacangine | Coronaridine | Catharanthine |
|---|---|---|---|---|---|
| T. iboga9,10,11,12 | 0.27–0.32 | 0.097–0.40 | 0.043–0.28 | NR | NR |
| V. africana13 | 0.25 | TR | 1.67 | TR | NR |
| T. arborea13,12 | 0.27 | 0.036 | 0.96 | 0.073 | NR |
| C. roseus14 | NR | NR | NR | NR | 0.003–0.099 |
| T. alba12 | 0.046–0.22 | 0.042–0.30 | 0.033–0.96 | 0.075–0.52 | NR |
| T. donnell-smithii12 | 0.069–0.74 | 0.028–0.032 | 0.21–0.44 | 0.046–0.23 | NR |
| T. amygdalifolia12 | 0.047 | 0.76–0.96 | 0.19–0.22 | 1.092–1.38 | NR |
NEWLY ISOLATED IBOGA ALKALOIDS (2010–2019)
Prior to 2010, iboga alkaloids were primarily isolated from plant species found in western parts of Africa.6 However, recent reports have noted the discovery of these alkaloids in plants from other regions of the world including Mexico, China, and Malaysia (Figure 2 and Table S1).15 Belonging to the Apocynaceae family, the Ervatamia genus (also called Tabernaemontana) has been found to produce a wide array of interesting iboga-type alkaloids with unique oxidation patterns and complex polycyclic frameworks. In 2014, Ye and co-workers isolated seven new iboga alkaloids from the Ervatamia officinalis plant species (9, 10, 11, 14, 18, 37, 49) along with ten previously known compounds including ibogaine, voacangine, and ibogaline.16 Primarily found in the Guangdong and Hainan Provinces of China, Ervatamia officinalis is rich in MIAs.17 Isolated ervaoffines A and B (9 and 10) were characterized using X-ray diffraction and electronic circular dichroism (ECD), and they represent the first iboga-type pseudoindoxyl alkaloids with a C2 spiro carbon configuration opposite that of known members of this class, such as iboluteine (177).
Figure 2.
Newly isolated iboga alkaloid during the period 2010–2020
In addition to ervaoffines A–D (9, 10, 11, 49), 14 and 37 were also isolated from Ervatamia officinalis (Figure 2).18 These newly isolated alkaloids are likely derived from ibogaine (1), which is a major constituent of Ervatamia plants. Indole oxidation would lead to intermediate 50, which could lose an equivalent of water through either Path A or Path B (Scheme 1). Subsequent pinacol rearrangements would ultimately lead to 9 and 11, respectively. In 2017, Ye and co-workers isolated several additional ervaoffine alklaoids (33, 34) from E. officinalis.19 The proposed biosynthesis of 33 and 34 involves the generation of a C5–C6 olefin in either ibogaine or coronaridine. Oxidative cleavage of the C5–C6 olefin to the corresponding dicarbonyl would produce an intermediate that could be transformed to 33 and 34 through oxidation followed by amidation or decarboxylation.
Scheme 1.
Proposed mechanism for the oxidative rearrangement of ibogaine to form ervaoffine A and C
The Ervatamia genus consists of approximately 120 species, which are primarily distributed in the subtropical and tropical regions of Australia and Asia. Phytochemical studies of this genus have led to the discovery of MIAs and bisindole alkaloids of various skeletal types.20,21 In 2015, Gao and co-workers isolated 12, 22, 23, and 43 from Ervatamia hainanensis (Figure 2).22 Absolute configurations were determined by various methods including X-ray diffraction and ECD spectroscopy. Interestingly, compounds 12, 22, and 23 contain an alcohol at C19, suggesting that 19-hydroxycoronaridine is likely a common precursor. Conodusine E (31) was also isolated and its biosynthesis likely involves oxidation of 19-hydroxycoronaridine. The newly isolated alkaloid 31 was shown to possess mild anti-inflammatory properties. In 2016, several additional alkaloids from E. hainanensis were isolated by Ye and co-workers (Figure 2).23 Similar to ervatamine G, 38, 39, 40, 47 are all substituted at the C3 position of the isoquinuclidine, with 39 representing the first example of a cyano-substituted oxindole alkaloid.
In 2016, Kam and co-workers mined the Malaysian plant Tabernaemontana corymbosa for iboga-type alkaloids.24 Using NMR, mass spectrometry, and X-ray diffraction analysis, they isolated and characterized the conodusine alkaloids (27, 29, 30, 31, 32) (Figure 2). Since then, other groups have reported the isolation of additional iboga alkaloids (21, 28, 25, 26, 10, 35) from T. corymbose.25,26,27 The biosynthetic origin of the unusual pyrrolidinone-containing compound 35 is still largely unknown.27 Alkaloids 25 and 26 possess unprecedented skeletons, possibly derived from a cleavamine precursor (Scheme 2).25 The proposed biosynthetic pathway to produce 25 and 26 likely starts from the oxidation of conodusine A (27) to 53. Next, fragmentation of the isoquinuclidine would produce 54. Hydrolysis of the iminium followed by enamine attack of the resulting aldehyde would produce 56. Nitrogen attack at the iminium and dehydration would yield 26 (Path A), while tautomerization followed by SN2′ reaction and dehydration (Path B) would yield 25. Though tabertinggine was only just recently discovered as an optically active natural product, the skeletal framework has been known since 1966 when Büchi inadvertently produced a related compound through an undesired rearrangement.28 Ultimately, he was able to use this intermediate to complete the first ever total synthesis of ibogaine (vide infra).
Scheme 2.
Proposed mechanism for the formation of tabertinggine and voatinggine
The plant species Tabernaemontana divaricata is yet another member of the Apocynaceae family that is rich in iboga alkaloids. Commonly found in the Yunnan and Guangxi Provinces of China, phytochemical investigations of T. divaricata have led to the isolation and characterization of 13, 36, 42, 44, 45, 46, and 48 (Figure 2 and Table S1).29,30,31 Most of these newly isolated alkaloids possess substitution at the C3 position of the isoquinuclidine. The biosynthesis of these compounds is likely to proceed through oxidation of simpler iboga alkaloids at C3 followed by C–C bond formation.
Initially believed to be found only in plants native to western Africa, iboga alkaloids are being discovered in a wide variety of Apocynaceae family members found around the world. These new sources may help to ensure that sufficient quantities of iboga alkaloids can be produced for future biological testing. In addition, the isolation of new iboga alkaloids provides important insight into novel biosynthetic pathways.
BIOSYNTHESIS OF IBOGA ALKALOIDS
Unlike in prokaryotes, the genes encoding metabolic pathways in plants are not typically clustered, making the full elucidation of alkaloid biosynthesis quite challenging. Typically, each individual plant-derived enzyme must be identified, cloned, and isolated to firmly establish a role in the synthesis of a particular alkaloid. Though several enzymes in the production of iboga alkaloids still remain elusive, our knowledge of iboga alkaloid biosynthesis has improved drastically over the past 15 years due in large part to the pioneering work of Sarah O’Connor, Vincenzo De Luca, and others.
Like all MIAs, the iboga alkaloids are derived from tryptamine (59), which is produced from the enzymatic decarboxylation of tryptophan (58) by tryptophan decarboxylase (TDC) (Scheme 3).32,33,34 The remainder of the iboga carbon skeleton can trace its origins to secologanin (62), an iridoid synthesized from isopentenyl pyrophosphate (60) and dimethylallyl pyrophosphate (61) via the non-mevalonate pathway (Scheme 3).35 Ten enzymes are required to produce 62,36,37,38,39,40,41,42 which subsequently reacts with 59 to form strictosidine (63). This critical Pictet-Spengler reaction is catalyzed by strictosidine synthase (STR),43,44 producing 63 as a single enantiomer (Scheme 3). Strictosidine (63) is a key intermediate en route to a number of indole alkaloids including those of the ajmalan, corynanthe, aspidosperma, quinoline, and iboga families.35
Scheme 3.
Biosynthesis of strictosidine
Many of the enzymes in T. iboga and C. roseus share a high degree of sequence homology, and thus, the biosynthetic pathways leading to ibogaine and catharanthine are quite similar until their late-stage divergence from dehydrosecodine (77). A number of the compounds en route to the iboga alkaloids are short-lived or produced in such small quantities that characterization via LC-MS has been challenging. As a result, many of the intermediates in the biosynthetic pathway to iboga alkaloids have been proposed based on the structures of their precursors and products. Whenever possible, we indicate which intermediates have actually been isolated.
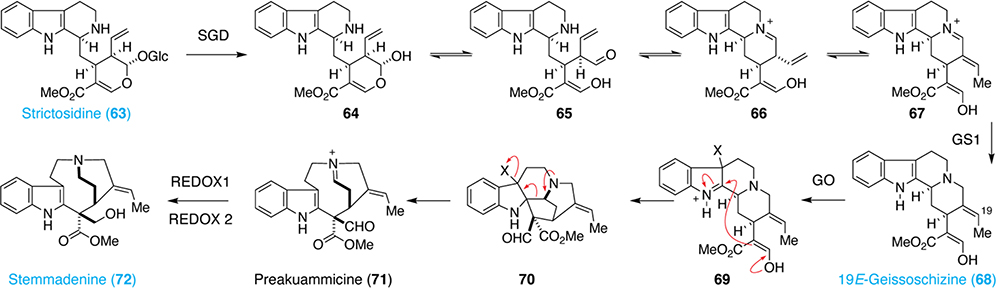
The first step in the biosynthesis of iboga alkaloids from strictosidine (63) involves glycosidic bond cleavage catalyzed by strictosidine β-deglucosidase (SGD) to produce the six-membered lactol 64 (Scheme 4).45,46 Opening of the lactol produces aldehyde 65, which rapidly condenses with the secondary amine to yield iminium 66. Isomerization to the more conjugated system produces 67, which is immediately reduced by geissoschizine synthase 1 (GS1) to produce 68.47 Oxidation of the indole by geissoschizine oxidase (GO) yields 69, which immediately undergoes intramolecular Mannich reaction followed by Grob fragmentation to yield preakuammicine (71).48 As 71 is unstable, the iminium is rapidly reduced by REDOX1. The identification of REDOX1 was made possible due to its sequence homology to GS1, another iminium reductase.48 Subsequent reduction of the aldehyde by REDOX2 produces the stable intermediate stemmadenine (72).48
Scheme 4.
Biosynthesis of stemmadenine. Compounds that have been isolated are highlighted in blue.
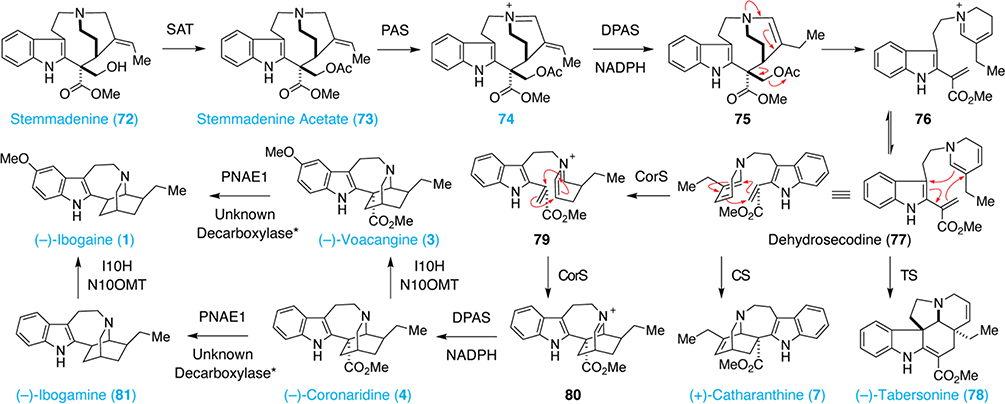
The conversion of stemmadenine (72) to dehydrosecodine (77) is a common pathway en route to both iboga and aspidosperma alkaloids (Scheme 5). First, stemmadenine (72) is acetylated by stemmadenine O-acetyltransferase (SAT) to afford stemmadenine acetate (73).48 Oxidation by precondylocarpine acetate synthase (PAS) produces iminium 74, which is reduced by dihydroprecondylocarpine acetate synthase (DPAS) in the presence of NADPH to yield the key enamine intermediate 75.49 Fragmentation produces iminium 76, which tautomerizes to the critical intermediate dehydrosecodine (77).49 Dehydrosecodine (77) represents the point of divergence for the aspidosperma and iboga biosynthetic pathways. However, it has never been isolated, presumably due to the unstable nature of the N-alkyl dihydropyridine. When O’Connor and co-workers subjected 73 to PAS and DPAS, they were able to isolate angryline (82) as a mixture of enantiomers (Figure 3).53 Angryline represents a stable precursor to dehydrosecodine (77) and is produced from an intramolecular Diels-Alder reaction between the iminium group of 77 and the vinylindole (Figure 3).
Scheme 5.
Biosynthesis of iboga and aspidosperma alkaloids. Compounds that have been isolated are highlighted in blue. *Compound 1 can be produced from 3 following slow, spontaneous decarboxylation. This reaction can be accelerated with heat. No spontaneous decarboxylation of 4 was observed even after heating.
Figure 3.
Racemic angryline gives rise to three chiral natural products through the intermediacy of achiral dehydrosecodine. Isolable compounds are highlighted in blue.
In the presence of tabersonine synthase (TS), dehydrosecodine (77) can undergo a Diels-Alder-like reaction to produce tabersonine (78).50 However, it is currently unclear if natural enzymes like TS are capable of catalyzing truly concerted Diels-Alder reactions.51 Reorientation of dehydrosecodine (77) reveals that it has the potential to undergo a second Diels-Alder-like reaction to form (+)-catharanthine (7) (Scheme 5). This reaction is likely to occur through a stepwise mechanism. When (+)-catharanthine (7) was incubated with catharanthine synthase (CS), the product of a retro Mannich reaction was observed, indicating that the forward reaction is likely to proceed through conjugate addition of the vinylogous enamine followed by Mannich reaction.52,53 Such stepwise formal Diels-Alder reactions are known to be favored with very electron-rich dienes, very electron-deficient dienophiles, or under conditions that stabilize the zwitterionic intermediate.54 In this case, the reaction must be templated by CS, as only one enantiomer is formed.49
One of the most intriguing aspects of iboga alkaloid biosynthesis is the fact that C. roseus and T. iboga produce natural products of the opposite enantiomeric series (e.g., (+)-catharanthine and (–)-coronaridine, respectively). One might assume that T. iboga generates (–)-catharanthine, which in principle could be reduced to yield (–)-coronaridine (7). However, (–)-catharanthine has never been isolated, suggesting that this pathway does not exist in nature. Instead, deuterium labeling studies have shown that coronaridine synthase (CorS) catalyzes the isomerization of 77 to 79 followed by a very unusual [4+2] cycloaddition to produce 80 (Scheme 5).50,53 While imino Diels-Alder reactions are well known,55the product 80 is formally an anti-Bredt olefin when drawn as the iminium resonance structure. This reactive species is immediately reduced by DPAS to afford (–)-coronaridine (4).50
When angryline (82) is exposed to TS, CS, or CorS/DPAS it produces tabersonine (78), (+)-catharanthine (7), and (–)-coronaridine (4), respectively.53 The formation of three distinct chiral natural products from a common achiral intermediate is a hallmark of iboga/aspidosperma biosynthesis (Figure 3)
Conversion of 4 to 10-hydroxycoronaridine is accomplished by the P450 enzyme ibogamine-10-hydroxylase (I10H) (Scheme 5).56 Methylation by noribogaine-10-O-methyltransferase (N10OMT) produces (–)-voacangine (3).56 Polyneudridine aldehyde esterase-like 1 (PNAE1) has been shown to convert both 3 and 4 to their corresponding carboxylic acids.50,57 However, the pathways converting these acids to ibogaine and ibogamine still have not been fully elucidated. The O’Connor group reported that the hydrolysis product of 3 does spontaneously decarboxylate to produce ibogaine.50 However, this reaction is slow, and heating is required to increase its rate. Furthermore, this transformation does not proceed when the hydrolysis product of 4 is heated. Therefore, it is likely that an unidentified decarboxylase(s) is responsible for the final step in the biosynthesis of ibogaine and ibogamine. Furthermore, ibogamine can be converted to ibogaine via I10H-mediated hydroxylation followed by N10OMT-catalyzed O-methylation.56 However, when noribogaine (2) is used as a substrate, N10OMT exhibits a low turnover rate suggesting that this transformation might actually be mediated by a currently unidentified O-methylase.
Tabersonine (78) is known to be hydroxylated at the C11 position by tabersonine 16-hydrolase (T16H); however, a similar hydroxylase(s) acting on the iboga scaffold to produce tabernanthine (5) or ibogaline (6) has not been identified. Through sequence homology, five additional P450s related to T16H and I10H were identified in T. iboga. These enzymes were cloned, but they did not catalyze the C11-hydroxylation of either coronaridine or ibogamine.56 Therefore, it is possible that tabernanthine (5) and ibogaline (6) are produced from an oxidase unrelated to the P450 family.
The enzymes that produce iboga and aspidosperma alkaloids have been shown to be somewhat promiscuous. An interesting study by the O’Connor group involved feeding substituted tryptamines to a C. roseus hairy root culture.58 Using LC-MS, they concluded that the unnatural tryptamine derivatives were incorporated into the scaffolds of the natural products. Feeding studies are only one approach for producing “unnatural” natural products. The O’Connor group has also expressed several prokaryotic halogenases or engineered halogenases, such as RebH, in C. roseus, leading to the production of chlorinated tabersonine analogs.59,60
HISTORICAL SYNTHESES OF IBOGA ALKALOIDS
Since Büchi’s pioneering synthesis of ibogaine in 1966,28 there have been numerous synthetic approaches to iboga alkaloids. For a comprehensive analysis of strategies prior to 2011, we refer the reader to an excellent review by Sinha and co-workers.61 Here, we focus on historical strategies for constructing the isoquinuclidine, tetrahydroazepine, and indole ring systems characteristic of this alkaloid family.
Construction of the Isoquinuclidine
The isoquinuclidine ring system represents a structural focal point for the iboga alkaloids.62 Methods to construct this [2.2.2] bicycle have centered around three fundamental strategies—cycloaddition, transannular cyclization, and radical rearrangement (Figures 4–6). The cycloaddition strategy was first successfully utilized by Büchi and co-workers (Figure 4).63,28 Their approach involved the reduction of nicotinamide pyridinium 83 with sodium borohydride to give a regioisomeric mixture of dienes 84 and 85. When this mixture was subjected to a Diels-Alder reaction with methyl vinyl ketone (MVK), only dihydropyridine 85 underwent cycloaddition, yielding isoquinuclidine 86 in 13% yield as a mixture of epimers over two steps. The authors postulated that the greater electron delocalization of 84 prevents cycloaddition with MVK.
Figure 4.
Cycloaddition approaches to the isoquinuclidine of iboga alkaloids
Figure 6.
Hodgson’s radical rearrangement approach to the isoquinuclidine cyclization approaches to the isoquinuclidine of iboga alkaloids
Most syntheses that have adopted this approach have opted to transform the substituent on the dienophile into the exo C20 ethyl group of the iboga alkaloids.28,64,65 This poses an obvious challenge given the inherent endo selectivity of most Diels-Alder reactions. To overcome this issue, Sames and co-workers took advantage of the acidic protons alpha to the carbonyl (Figure 4).65 By treating a 3:1 endo:exo mixture of the Diels-Alder adducts with base, they achieved epimerization to a thermodynamic ratio (2:3) of 88 and 89. After exo enrichment, conversion to the tosylhydrazones 90 and 91 allowed for separation via crystallization. Fukuyama and co-workers sought to avoid endo/exo diastereoselectivity issues altogether by performing the Diels-Alder reaction with a symmetrical dienophile (Figure 4).66 Treatment of trans-dibromide 92 with DABCO afforded the requisite dihydropyridine 93, which was then reacted with dimethyl methylenemalonate to give isoquinuclidine 94 in 94% yield over two steps.
In the approaches described above, the Diels-Alder reactions were uncatalyzed, leading to racemic mixtures of isoquinuclidines. Recently, Batey and co-workers reported an enantioselective Diels-Alder reaction of dihydropyridine 87 with acrolein in the presence of a valine-derived organocatalyst (Figure 4),67 leading to a formal synthesis of (+)-catharanthine. In 2006, Borschberg and co-workers reported another enantioselective synthesis of an iboga alkaloid, although their approach involved an intramolecular nitrone-olefin [3+2] cycloaddition (Figure 4).68,69 Key intermediate 96 was synthesized from L-glutamic acid and (2S)-but-3-en-2-ol. A crucial chirality transfer in the Ireland-Claisen rearrangement of a silyl ketene acetal afforded intermediate 96 in high diastereoselectivity. The subsequent 1,3-dipolar cycloaddition produced 97 in 67% yield.
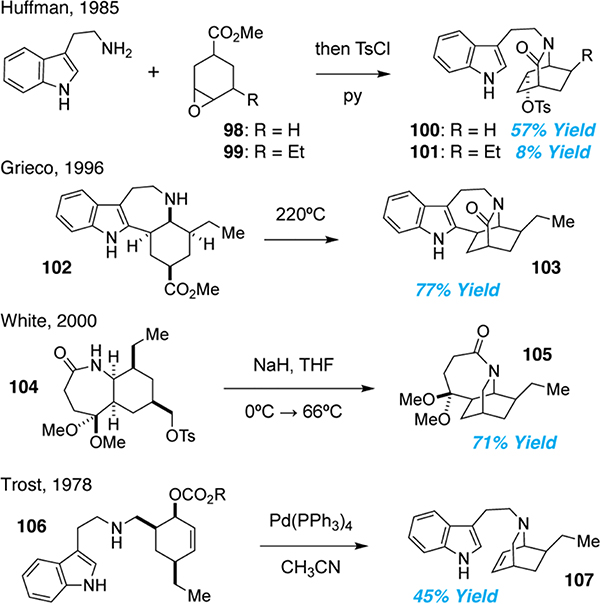
Another common approach to accessing the isoquinuclidine framework of iboga alkaloids involves the transannular cyclization of an amine derivative. Huffman and co-workers were the first to employ this strategy in 1965.70,71 Ring opening of cyclic epoxy ester 98 followed by transannular amidation produced the isoquinuclidine in a single step. Subsequent tosylation of the C16 alcohol afforded intermediate 100 in 57% yield over two steps (Figure 5). However, when this strategy was applied to the synthesis of ibogamine, the yields were greatly reduced owing to the complex mixture of diastereomeric products obtained during the synthesis of epoxide 99.72
Figure 5.
Transannular cyclization approaches to the isoquinuclidine of iboga alkaloids
Variants of the transannular cyclization strategy have involved the preassembly of the indole and/or tetrahydroazepine rings prior to formation of the isoquinuclidine (Figure 5). The approach taken by Grieco and co-workers mirrored that of Huffman and produced 103 in excellent yield.73 A hallmark of both of the Huffman and Grieco syntheses is the incorporation of an enolizable proton at what will become the bridgehead position, enabling both diastereomers to be converted to the desired isoquinuclidine.
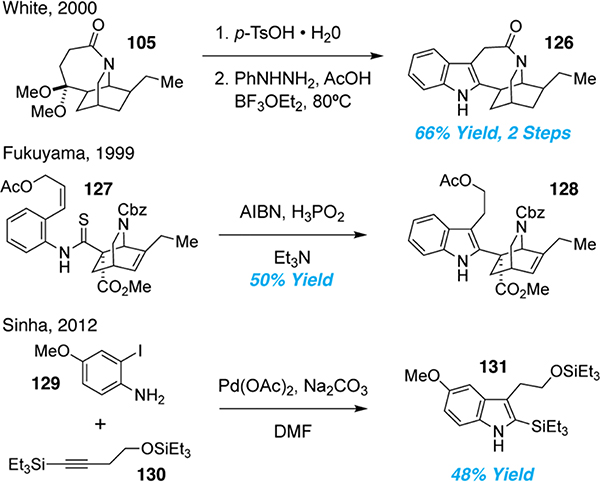
A related approach pioneered by Sallay established what would become the tetrahydroazepine of ibogamine prior to transannular alkylation forming 105.74 White and co-workers improved upon the racemic Sallay synthesis by constructing 104 using an asymmetric Diels-Alder reaction as the key step (Figure 5). Base-mediated cyclization afforded 105 in high yield, which was converted to (–)-ibogamine.75
In addition to classic acylation and alkylation reactions, several other transannular cyclizations have been utilized to access the isoquinuclidine core of iboga alkaloids. For example, Nagata and co-workers favored the use of a transannular aziridination reaction,76,77,78 while Trost and co-workers relied on Pd-catalyzed allylic alkylation.79,80 Trost’s 1978 synthesis of (+)-ibogamine was a landmark paper for the field (Figure 5). Use of a chiral auxiliary enabled an asymmetric Diels-Alder reaction (60% ee), and the product was subjected to reductive amination to produce 106. Treatment of 106 with Pd0 led to 107, which was converted to the natural product through a metal-mediated olefin arylation. Trost’s synthesis was the first to use a transition metal to access an iboga alkaloid, and it set precedent for many similar strategies to follow.
Generally, methods to form the isoquinuclidine of iboga alkaloids have started from either an acyclic, cyclic, or fused cyclic precursor. In 2005, Hodgson developed an alternative strategy starting from an existing bridged bicycle (Figure 6).81 An enantioselective desymmetrization of meso tropenone 108 gave radical precursor 109 in 46% yield over four steps. Reduction of the ketone with NaBH4 gave the corresponding bromohydrin, which was then transformed into 113 via homoallylic radical rearrangement. This one-pot procedure led to an efficient formal synthesis of (+)-ibogamine.
Construction of Tetrahydroazepine
The 7-membered tetrahydroazepine is a crucial structural element linking the indole and isoquinuclidine rings of the iboga alkaloids. Most syntheses have relied on one of three strategies for its construction—formation of the C2–C16 bond, C7–amine linkage, or ring-expansion. The C2–C16 bond disconnection was first explored by Nagata in 196876 and later exploited by Imanishi.82 The cyclization of 114 was achieved by refluxing in a stoichiometric amount of p-TsOH for a short duration. Treatment of the resulting tosylate with in situ generated AlH3 produced ibogamine (81) in reasonable yields (Figure 7). In 1985, Huffman employed an alternative strategy using tosylate 115. Lewis acid-mediated cyclization of 115 followed by reduction of the lactam gave 81 in 32% yield over two steps (Figure 7).
Figure 7.
Construction of the tetrahydroazepine through C2–C16 bond formation. Though Trost and co-workers did not report the number of equivalents of Pd that were used to effect cyclization, subsequent work by Sames and co-workers suggests that > 1 equivalent of Pd was likely necessary.
The C2–C16 bond disconnection was also key feature of Trost’s 1978 synthesis.79 Using a novel olefin arylation, Trost and co-workers were able to forge the C2–C16 bond from 107 using a mixture of palladium and silver (Figure 7). Reduction of the resulting organopalladium species afforded (+)-ibogamine in 43% yield. In 2015, Sames applied a slight modification of this C–H activation strategy by using a preformed palladium tetrafluoroborate catalyst instead of a combination of PdCl2(CH3CN)2 and AgBF4.65 This result suggests that silver is not directly involved in cyclization and serves merely to facilitate chloride exchange for a noncoordinating tetrafluoroborate, thus generating a more active catalyst. Though interesting, the methods developed by Trost and Sames suffered several drawbacks such as low yields and the need for stoichiometric or supra-stoichiometric amounts of palladium (1–2 eq). In 2012, Sinha employed a modified strategy using only catalytic amounts of palladium catalyst (Figure 7).64,83 Pre-functionalization of the indole C2 position with an iodide (116) enabled a reductive Heck reaction to be performed using only 10 mol% of Pd(OAc)2. This more economical approach towards the tetrahydroazepine ring system enabled the synthesis of ibogaine (1) in 66% yield.
Another popular strategy for constructing the tetrahydroazepine involves the introduction of a linker between the nitrogen of the isoquinuclidine and the C3 position of the indole (C7 based on the Le Men and Taylor numbering). Using a method developed by Kutney,84 Das and co-workers performed a debenzylation of a quaternary ammonium salt derived from 117 (Figure 8).85 Though they were able to isolate catharanthine, the route was low yielding as formation of the quaternary ammonium salt was challenging. Fukuyama and co-workers took a slightly different approach by deprotecting the isoquinuclidine prior to alkylation.66 They noticed that hydrogenolysis of 118 also resulted in the reduction of the endocyclic olefin. Instead, a mild and chemoselective deprotection using triethylsilane and palladium acetate afforded catharanthine in a single step. Interestingly, the desired intramolecular SN2 alkylation occurred readily following carbamate deprotection, presumably due to the highly rigidified nature of the intermediate amine.
Figure 8.
Construction of the tetrahydroazepine through C7–amine linkage
Sundberg and co-workers also chose to construct the tetrahydroazepine after the indole and isoquinuclidine rings had already been established (Figure 8).86 However, their approach involved the generation of a radical through irradiation of chloroacetamide 119. Radical cyclization afforded 120 in modest yield.
The final strategy used to construct the tetrahydroazepine involves ring expansion, and this was the method by which Büchi first synthesized ibogaine (Figure 9).28,63 Treatment of 121 with a mixture of Zn and AcOH resulted in reductive opening of the 6-membered ring. Following protonation at the γ-position, conjugate addition of the amine produced ibogaine in 57% yield. White and co-workers also synthesized the 7-membered ring through ring expansion. Beckman rearrangement of 124 yielded 125 in good yield.
Figure 9.
Construction of the 7-membered ring through ring expansion
Construction of the Indole
The indole ring system of the iboga alkaloids is biosynthetically derived from tryptophan, and most synthetic efforts toward the iboga alkaloids have started from tryptamine derivatives. For example, Büchi’s 1966 synthesis of ibogaine involved the coupling of an isoquinuclidine with indole acetic acid.28 More recently, alternative approaches to generating the indole have been explored (Figure 10). In 2000, White and co-workers installed the indole at a late-stage through a Fischer indole cyclization of 105 to afford 126 in 66% yield over two steps.75 Fukuyama and co-workers built the indole through radical cyclization of thioanilide 127.66 They noticed that standard tin hydride conditions failed to produce the desired product in acceptable yields. Instead, they found that a phosphorus-based hydrogen atom donor afforded the desired cyclization. Recently, Sinha and co-workers decided to synthesize the indole very early in their synthesis.64 Larock annulation of aniline 129 and alkyne 130 provided 131 in modest yield. Very few synthetic strategies towards iboga alkaloids have made the construction of the indole a focal point for the synthesis, perhaps because the 7-membered tetrahydroazepine and bicyclic isoquinuclidine ring systems are viewed as being more challenging to access.
Figure 10.
Construction of the indole
RECENT SYNTHETIC APPROACHES TO IBOGA ALKALOIDS (2010–2019)
In the past decade, several groups have focused on developing innovative methods to construct the isoquinuclidine ring system.87,88,89 Coldham and coworkers previously employed a tandem oxime alkylation/nitrone cycloaddition to access both the aspidosperma90,91 and daphniphyllum alkaloids.92 In 2019, they extended their methods for constructing bridge bicyclic [3.3.1] systems to the iboga alkaloids simply by adjusting the positions of the branch point (C5 vs C4) and the dipolarophile (internal vs terminal olefin) (Figure 11).87 Following hydrolysis of the dithiane in 137 and N–O bond reduction, Coldham and co-workers were able to intercept an intermediate previously accessed by Borschberg,69 completing a formal synthesis of 19-hydroxyibogamine.
Figure 11.
Coldham’s alkylation/cycloaddition strategy to iboga alkaloids
Asymmetric Syntheses of Iboga Alkaloids
Prior to 2010, most synthetic strategies to iboga alkaloids led to racemic mixtures, while the more modern approaches of the past decade have taken advantage of recent developments in asymmetric synthesis. Most asymmetric syntheses of iboga alkaloids focus on the enantioselective construction of the isoquinuclidine ring system. In 2019, Nemoto and coworkers showcased an elegant strategy focused on the desymmetrization of meso isoquinuclidine 143.88 To access the meso compound, they employed chemistry that they first reported in 201593 involving the formal insertion of a rhodium carbenoid into an amide C–N bond (Figure 12). Attack of the PMB-protected nitrogen in 138 on the rhodium carbenoid produced Rh-associated N-ylide 139. Subsequent Stevens rearrangement produced isoquinuclidine 141. Using computational chemistry, Nemoto and co-workers ruled out a step-wise mechanism for the Stevens rearrangement in favor of a concerted, asynchronous process.
Figure 12.
Nemoto’s asymmetric synthesis of 144. PG = protecting group.
Upon generation of diketone 141, an in situ reduction with DIBAL-H afforded 1,3-diol 142 with high diastereoselectivity. Desymmetrization of 142 with a chiral phosphoric acid catalyst proved unsuccessful due to a background reaction involving the basic nitrogen (Figure 12). While Boc protection of 143 showed slight improvement in enantioselectivity, protection with a strong electron withdrawing sulfonamide (i.e, Ns) afforded the desymmetrized product 144 in 40% yield and 78% ee. By optimizing the catalyst, solvent, and temperature, they were eventually able to obtain the nosyl-protected 144 in 74% yield and 94% ee, which they took on to complete a formal synthesis of (+)-catharanthine.
A similar enantioselective approach to the isoquinuclidine using an N-ylide intermediate was taken by Luo and coworkers in 2016 (Scheme 6).89 Their route started with 145, which was prepared from tryptamine in 3 steps (98% ee) via an asymmetric organocatalytic Pictet-Spengler reaction.94 Protection of the indole nitrogen, propargylation, and deprotection afforded diastereomers that were readily separated. Reduction of the desired diastereomer 146 produced 147, which was subjected to a one-pot procedure involving the gold-catalyzed conversion of the terminal alkyne to an α-chloromethyl ketone95 followed by intramolecular alkylation to yield 149 in 73% yield. The basicity of the tertiary amine poisoned the gold catalyst making acidification with TFA/MsOH necessary. Gram scale preparation of 149 was improved significantly by adding one equivalent of AgOTf, which promoted transannular alkylation of 148. The key Stevens rearrangement was accomplished through initial formation of enamine 150, furnishing the isoquinuclicine in modest yield. Interestingly, the use of N-methylmorpholine in place of piperidine failed to produce 153, suggesting that formation of the enamine was critical to the success of the Stevens rearrangement.
Scheme 6.
Luo’s 2016 asymmetric synthesis of (+)-ibogamine
Next, exocyclic olefin 154 was accessed through a Wittig reaction, and the (Z)-configuration was assigned by NOESY NMR. Hydrogenation of 154 with H2 and Pd/C gave (+)-epiibogamine (155) exclusively (97% yield). To solve this issue, Luo and co-workers employed hydrogen atom transfer (HAT) conditions originally developed by Baran and Shenvi,96,97,98 which produced a separable mixture of (+)-epiibogamine (155) and (+)-ibogamine (81) in 34% and 26% yield, respectively.
In 2012, Takayama and coworkers utilized an asymmetric Diels-Alder reaction to complete an enantioselective synthesis of voacangalactone (Scheme 7).99 Takayama’s approach incorporated elements from several previous strategies towards iboga alkaloids. First, 156 was synthesized through vinyl iodide coupling with a chiral oxazolidinone using Buchwald’s method.100 A highly diastereoselective Diels-Alder reaction with dimethyl methylenemalonate produced 157 in 97% yield. Removal of the oxazolidinone and TBS group followed by Cbz protection of the nitrogen yielded alcohol 158, which was mesylated and subjected to transannular alkylation. Using a strategy pioneered by Fukuyama and co-workers, 160 was converted to 161 through ester hydrolysis and subsequent iodolactonization.66 Reduction to the corresponding alcohol followed by radical-mediated deiodination and oxidation with DMP produced aldehyde 163, which was converted to the desired alkyne 164 through Seyferth-Gilbert homologation using the Ohira-Bestmann reagent. Sonagashira cross-coupling of 164 with 2-iodo-4-methoxyaniline produced 165 in 60% yield. Alkyne 165 was transformed into indole 166 using a Au-catalyzed cyclization first developed by Utimoto.101 The 7-membered ring was constructed through a series of steps that included indole acylation with oxalyl chloride, ester formation, Cbz-deprotection, and amide bond formation. Reduction of 168 with borane produced (–)-voacangalactone (169) in 56% yield. Takayama’s approach afforded optically pure voacangalactone in 3% overall yield and highlights the power of diastereoselective Diels-Alder reactions for the asymmetric construction of 6-membered rings.
Scheme 7.
Takayama’s 2012 asymmetric synthesis of (–)-voacangalactone
Synthesis of Post Iboga Alkaloids
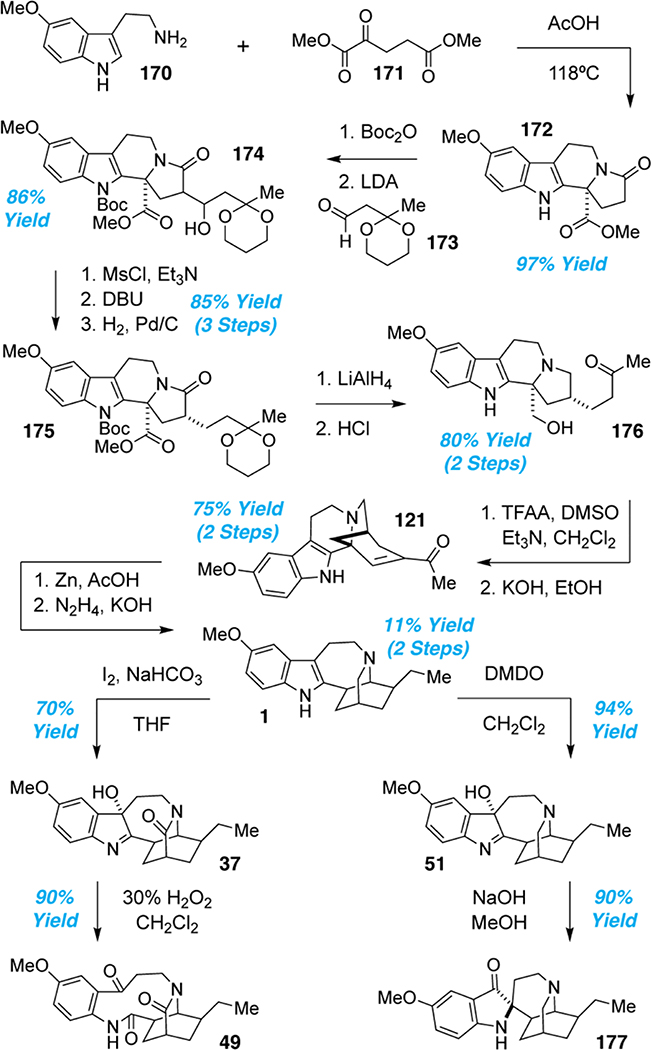
In 1966, Büchi and coworkers isolated an unusual rearrangement product (121) en route to ibogaine.28 It wasn’t until 2013 when it was realized that Büchi’s rearrangement product resembled the structure of the newly isolated natural product tabertinggine (25).25 Büchi’s pioneering efforts inspired a more recent strategy by She and co-workers enabling ibogamine to be accessed from tabertinggine (25).102 Moreover, they were able to access several additional iboga alkaloids from Büchi’s intermediate 121. Their synthesis commenced with the preparation of 172 via a one-pot sequence involving Pictet-Spengler reaction and intramolecular amidation of 5-MeO-tryptamine (170) (Scheme 8). After protection of the indole nitrogen with Boc2O, an aldol condensation with 173 produced 174 in excellent yield. The secondary alcohol was mesylated and eliminated to afford an α,β-unsaturated lactam that underwent diastereoselective hydrogenation. Treatment with LiAlH4 followed by HCl effectively reduced both the amide and ester while also removing the Boc group and hydrolyzing the ketal. Oxidation of primary alcohol 176 followed by intramolecular aldol condensation gave 121 in 75% yield over two steps. Reduction with zinc in acetic acid followed by Wolff-Kishner reduction gave (±)-ibogaine (1) in low yield (11%).
Scheme 8.
She’s 2016 racemic syntheses of (±)-1, (±)-37, (±)-49, (±)-51, and (±)-177
With the exception of the final step, this route to ibogaine was relatively high yielding, providing sufficient material to access several “post-iboga alkaloids”103 (e.g., 37, 49, 51, and 177) via oxidation of ibogaine (1) (Scheme 8). Ibogaine was successfully oxidized with DMDO from the less hindered face to produce 51. Base-mediated ring-contraction gave iboluteine (177) with the desired spiro configuration. An alternative oxidation of ibogaine with I2/NaHCO3 produced lactam 37. Further oxidation with H2O2 led to ring cleavage affording ervaoffine D (49) in excellent yield. She’s strategy not only enabled an efficient route to tabertinggine (25) and Büchi’s intermediate (121), it provided the first syntheses of compounds 37, 49, and 51.
In 2018, Han and coworkers reported a biosynthetically inspired conversion of (+)-catharanthine to various post-iboga alkaloids including (–)-voatinggine (26), (–)-tabertinggine (25), and (+)-dippinine B.104 A hallmark of Han’s strategy was the utilization of oxidative rearrangements to acquire structural diversification. Additionally, this pioneering work showcased a unique method for accessing the challenging pentacyclic core found in the chippiine and dippinine alkaloids.
Biosynthetic Approach to Iboga Alkaloids
A defining feature of iboga biosynthesis is the formation of multiple alkaloid families from a common dehydrosecodine (77) intermediate (Scheme 5). However, the dihydropyridine of dehydrosecodine is very unstable, making the implementation of a truly biomimetic synthesis quite challenging. In 2014, Oguri and co-workers found an elegant solution to this problem (Scheme 9).105 Their synthesis began with a Pictet-Spengler reaction between tryptamine and 178, followed by ring expansion106 to give 179 in 68% yield. Reduction of 179 with sodium cyanoborohydride and subsequent N-propargylation produced 180. Reaction of 180 with chiral oxazolidinone 181 gave a zwitterionic intermediate that underwent a regioselective Hoffmann elimination to afford dihydropyridine precursor 182. Upon treatment with a Cu(I) catalyst, a cascade transformation first produced dihydropyridine 183, which immediately underwent a diastereoselective Diels-Alder reaction to produce 184. An additional 4 steps were required to convert the C20 substituent into the ethyl group of (–)-catharanthine (7).
Scheme 9.
Oguri’s 2014 biomimetic synthesis of (–)-catharanthine
Of all the syntheses of iboga alkaloids (Table 2), the Oguri Group’s synthesis stands out because their biomimetic approach enabled them to access several alkaloid families from a common intermediate (Figure 13). Simply by changing R1, R2, and the reaction conditions, Oguri and co-workers could access either iboga-type (e.g., 186), ngouniensine-type (e.g., 187), aspidosperma-type (e.g., 188), or andranginine-type structures (e.g., 189) from 185. This landmark paper showcases how powerful biomimetic approaches can be for alkaloid total synthesis.
Table 2.
Total syntheses of iboga alkaloids. The order in which the indole (I), isoquinuclidine (Q), and tetrahydroazepine (A) rings were formed is indicated. A “ / ” indicates that two ring systems were formed in the same step. NR = not reported (i.e., not enough information was provided to calculate an overall yield or determine step count).
| Year | Group | Alkaloid(s) | Formation Sequence | Step Count | Overall Yield (%) |
|---|---|---|---|---|---|
| 1965 | Buchi | (±)-Ibogamine (81) | Q → I → A | 14 | 1.3 |
| 1966 | Buchi | (±)-Ibogaine (1) | Q → I → A | 15 | 0.2 |
| 1967 | Sallay | (±)-Ibogamine (81) | A → Q → I | 14 | NR |
| 1968 | Nagata | (±)-Ibogamine (81) | Q → I → A | 16 | 0.8 |
| 1978 | Trost | (+)-Ibogamine (81) | I → Q → A | NR* | NR* |
| 1981 | Hanaoka | (±)-Ibogamine (81) | Q → I → A | 17 | 3.9 |
| 1985 | Kuehne | (±)-Ibogamine (81) | I/Q → A | 10 | 2.6 |
| 1985 | Raucher | (±)-Catharanthine (7) | Q → I → A | 11 | 9.0 |
| 1991 | Herdeis | (±)-Ibogamine (81) | Q → I → A | 8 | 14 |
| 1996 | Grieco | (±)-Ibogamine (81) | I → Q → A | 9 | 7.0 |
| 1999 | Fukuyama | (±)-Catharanthine (7) | Q → I → A | 17 | 6.0 |
| 2000 | White | (−)-Ibogamine (81) | A → Q → I | 15 | 4.6 |
| 2001 | Kuehne | (−)-Coronaridine (4) | I → Q/A | 10 | NR |
| 2005 | Hodgson | (+)-Ibogamine (81) | Q → I → A | 11 | 2.0 |
| 2006 | Borschberg | (−)-19-hydroxyibogamine | Q → I → A | 20 | 1.9 |
| 2012 | Sinha | (±)-Ibogaine (1) (±)-Ibogamine (81) |
Q → I → A | 9 9 |
9.4 5.6 |
| 2012 | Takayama | (−)-Voacangalactone (169) | Q → I → A | 25 | 3.2 |
| 2014 | Oguri | (−)-Catharanthine (7) | I → Q/A | 10 | 2.8 |
| 2015 | Sames | (±)-Ibogamine (81) | Q → I → A | 9 | 7.3 |
| 2016 | Luo | (+)-Ibogamine (81) | I → Q/A | 12 | 4.2 |
| 2016 | She | (±)-Ibogaine (1) (±)-Ibogamine (81) (±)-Tabertinggine (25) (±)-37 (±)-51 (±)-Iboluteine (177) (±)-Ervaoffines D (49) |
I → Q/A | 12 12 10 13 13 14 14 |
4.6 6.0 41 3.2 4.3 3.9 2.9 |
Trost synthesized (+)-ibogamine from an intermediate in 4 steps (17% yield). However, the synthesis of this intermediate from simpler precursors was not detailed, and thus, we cannot provide an overall step count and yield for the Trost synthesis.
Figure 13.
Oguri’s intermediate 185 enables access to multiple families of alkaloids
BIOLOGICAL ACTIVITY OF IBOGAINE
The anti-addictive properties of ibogaine have been known since the 1960s, though this initial information was based entirely on anecdotal reports from heroin users. Since that time, several open-label and/or retrospective studies have suggested that ibogaine might be useful for treating substance use disorder (SUD) as it appears to reduce drug cravings, decrease symptoms of withdrawal, and prevent relapse.107,108,109,110,111 Moreover, rodent studies have confirmed the anti-addictive potential of ibogaine by demonstrating the natural product reduces drug self-administration, prevents drug-induced dopamine release in several brain regions, attenuates drug-induced conditioned place preference, and decreases signs of withdrawal.112,113,114 However, double-blind, placebo-controlled clinical trials firmly establishing the efficacy of ibogaine are still lacking. It is illegal to possess ibogaine in the United States, as it is classified as a schedule I drug. As a result, many people have sought treatment from informal clinics in countries where ibogaine is not regulated.115 For information on the history and pharmacology of ibogaine, we point the reader to several excellent reviews on these subjects.116,117,118,119
Despite ibogaine’s promising therapeutic efficacy, major safety concerns have tempered excitement for its clinical development. First and foremost, ibogaine is known to cause long-lasting hallucinations,118 and at very high doses it can lead to tremors and Purkinje cell death in rats.120 However, its cardiotoxicity has been the biggest concern. Ibogaine inhibits hERG potassium channels in the heart,121,122 with several deaths being linked to its adverse effects on heart function.123,124 Ibogaine is very nonpolar, as evidenced by the fact that it readily accumulates in adipose tissue,125 and it is well known that hERG inhibition is a major liability for many non-polar, basic amines.126 Previously, ibogaine was sold in France as a neurotherapeutic, however its adverse effects led to its removal from the market.116 Since that time, a major goal for the field has been to identify ibogaine congeners with similar therapeutic efficacies, but improved safety profiles.
SYNTHESIS OF IBOGALOGS
Ibogaine’s mechanism of action is poorly defined, which has severely inhibited drug discovery efforts using it as a lead structure. Ibogaine and its active metabolite noribogaine bind to a number of targets with only modest affinities including serotonin, opioid, acetylcholine, sigma, and NMDA receptors as well as serotonin, dopamine, and norepinephrine transporters (Table S2).116 Without an obvious target-based assay to drive structure-activity relationship (SAR) studies, medicinal chemistry efforts have focused on producing antiaddictive ibogaine analogs (i.e., ibogalogs) lacking major side effects. As in vivo addiction assays are costly and time consuming, very few ibogalogs have been tested in these models. However, a number of compounds with interesting structures and/or preliminary biological data have been reported (Figure 14 and Table S2).64,65,120,149,154,161,162,163,172,127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144. 145, 146, 147, 148
Figure 14.
Structures of several iboga analogs. Blue headings indicate analogs that have been tested in biological assays. Red headings indicate analogs that have not yet been tested in biological assays. Select IC50 values are indicated (μM). For complete biological testing details, see Table S2.
Early efforts focused on comparing the properties of ibogaine to those of other iboga alkaloids. With the exception of very bulky substituents (e.g., 192), indole substitution appears to have very little effect on NMDA receptor binding affinity (Table S2, 1–5).149 However, reducing the size of the C18 ethyl group decreases the NMDA receptor binding affinity substantially (Table S2, 190 and 191). While 192 is a weak NMDA receptor ligand, it displays comparable potency to ibogaine at the KOR. In contrast, the affinity of noribogaine for the KOR is nearly two orders of magnitude greater than that of ibogaine (Table S2),149 and it appears to be a G protein biased ligand.150 As noribogaine is a long-lived metabolite of ibogaine,151 and kappa ligands are known to have anti-addictive properties,152 noribogaine might play an important role in the anti-addictive properties of ibogaine.
The majority of iboga alkaloid SAR studies were made possible by the synthetic efforts of Martin Kuehne and co-workers. Perhaps the most exciting analog that they discovered was 18-methoxycoronaridine (18-MC, 193), which was synthesized in 13 steps.153 In collaboration with Stanley Glick’s pharmacology group, 18-MC was found to possess many of the same anti-addictive properties as ibogaine without causing tremors, cerebellar neurotoxicity, or inhibiting hERG channels154 (Table S2). By varying the ester or exchanging it for an amide, a number of 18-MC derivatives were synthesized.155 The binding affinities of these analogs for the α3β4-nicotinic receptor correlated to their effects on morphine self-administration, leading to the hypothesis that α3β4-nicotinic receptors are important for the anti-addictive properties of 18-MC.156 Few ibogalogs have demonstrated ibogaine-like therapeutic properties with minimal toxicity issues, making 18-MC quite unique.157,158 Currently, 18-MC is being tested in phase II clinical trials for treating opioid use disorder.
The majority of the analogs developed by Kuehne and co-workers contained all of the major structural features of iboga alkaloids—isoquinuclidine, tetrahydroazepine, and indole rings. As a result, they are quite difficult to synthesize. Thus far, relatively few function-oriented synthesis (FOS)159 efforts toward simplified iboga scaffolds have emerged. A few studies have disclosed biological data for ibogalogs lacking the tetrahydroazepine (Figure 14, Table S2, 195–197), while only a single report describes biological data for ibogalogs lacking the isoquinuclidine (Figure 14, Table S2, 198–202). To the best of our knowledge, none of these simplified ibogalogs have been tested using in vivo models of addiction.
In addition to 18-MC and the other iboga congeners described above, a number of very interesting analogs related to iboga alkaloids have been synthesized, but not tested for biological activity. These include 1) analogs developed by Sundberg where the tetrahydroazepine is contracted (203),160 expanded (204),161 or removed completely (205),160 2) ibogaine analogs developed by Sames and co-workers that lack the C2–C16 bond (206–207),65 and compounds developed by Sinha with C2 and C7 transposed (208–209).162,64
With the exception of one report in 1994,163 all of the SAR studies described above utilized the natural products or synthetic racemates. This single report claims to have tested the “R- and S-enantiomers” of ibogamine and coronaridine and found that these compounds produced similar effects on morphine self-administration. However, no asymmetric syntheses of (+)-coronaridine or (+)-ibogamine had been reported prior to this publication. In fact, the first asymmetric syntheses of (+)-coronaridine164 and (+)-ibogamine81 were reported by Kuehne and Hodgson in 2001 and 2005, respectively. Furthermore, ibogamine and coronaridine contain multiple stereogenic centers, making the designation of “R- and S-enantiomers” ambiguous (no description of their optical rotations was provided). We think that the “R- and S-enantiomers” described in this study are more likely the exo and endo epimers of the C20 position, both of which would be racemic compounds. If we are correct, then to the best of our knowledge, it is unknown if alkaloids from ibogaine’s antipodal series (e.g., catharanthine) have anti-addictive properties.
ADVANCES IN IBOGA NEUROBIOLOGY
In standard assays, ibogaine appears to have weak affinity for a large number of neuroreceptors, which has hindered efforts to define its mechanism of action.165,166 Moreover, its therapeutic effects are relatively long-lasting,167,168 which implicates neural plasticity mechanisms leading to the re-wiring of addiction-related neural circuitry. The first piece of evidence suggesting that ibogaine might promote neural plasticity was provided by Dorit Ron and co-workers. They demonstrated that ibogaine increases glial cell line-derived neurotrophic factor (GDNF) expression in the ventral tegmental area (VTA), and that infusion of ibogaine into the VTA of rodents decreases alcohol consumption.169 Recently, a study by Carrera and co-workers showed that ibogaine can impact brain-derived neurotrophic factor (BDNF) and GDNF signaling in several brain regions that play key roles in the pathophysiology of addiction.170 Neurotrophic factor signaling is well known to promote structural neural plasticity—the physical re-wiring of the brain.
Compounds capable of rapidly promoting structural neural plasticity are known as psychoplastogens.171 Recently, our group demonstrated that noribogaine, an active metabolite of ibogaine,172 is a potent psychoplastogen that increases cortical neuron dendritogenesis.173 Other psychoplastogens, including several psychedelic compounds, possess anti-addictive properties in the clinic that mirror the effects of ibogaine.174 By modifying addiction-related circuitry instead of simply blocking the targets of addictive substances, psychoplastogens like ibogaine have the potential to produce long-lasting anti-addictive effects across a wide range of substances (e.g., opioids, alcohol, stimulants, etc.) and represent a new approach to treating substance use disorders.
Currently, it is unknown if ibogalogs like 18-MC are psychoplastogenic. However, unlike ibogaine, 18-MC does not increase GDNF expression in SH-SY5Y cells or reduce alcohol self-administration following direct infusion into the VTA, suggesting that the mechanisms of the two compounds are likely different.175 Given the fact that a large number of novel ibogalogs have been developed (Table 4), these compounds should be re-evaluated using phenotypic neural plasticity assays173 in an effort to identify safer and more efficacious ibogaine analogs.
CONCLUSION
A number of advances have been made in the past 10 years regarding iboga chemistry and biology. We now know how the antipodal series of these alkaloids are generated from a common achiral precursor, and biomimetic approaches are enabling the rapid synthesis of several iboga family members. Furthermore, new clues have emerged regarding how iboga alkaloids might produce long-lasting neurotherapeutic effects. However, there are still a number of chemical and biological challenges that need to be addressed if we are to rationally engineer safe and effective medicines for treating neuropsychiatric disorders based on the iboga core structure.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funds from the National Institutes of Health (NIH) (R01GM128997 to DEO). We thank Daniel Nurco, James Fettinger, and members of the Olson Lab for helpful discussions.
BIOGRAPHIES

David E. Olson
David E. Olson studied chemistry and biology at Union College and received his Ph.D. in chemistry from Stanford University. After his graduate work, he completed postdoctoral training in neuroscience at the Broad Institute of MIT and Harvard. He is currently an assistant professor in the Department of Chemistry and the Department of Biochemistry & Molecular Medicine at the University of California, Davis as well as the president and chief scientific officer of Delix Therapeutics. His research interests broadly encompass the area of chemical neuroscience and include the synthesis of psychoactive natural products, the development of novel neurotherapeutics, and mechanistic neuropharmacology.

Rishab N. Iyer
Rishab N. Iyer received his B.S. in Chemistry from the University of Alabama in Tuscaloosa, AL, where he performed undergraduate research under Professor Anthony J. Arduengo III. He is currently a second-year graduate student in Professor David E. Olson’s laboratory at the University of California, Davis. His graduate studies are focused on the total synthesis of natural products from the iboga family.

David Favela
David Favela received his B.S. in Biochemistry from New Mexico State University. While there, he worked in Professor Michael Johnson’s lab studying the properties of metals encapsulated in reverse micelles. In 2014 he transitioned from inorganic chemistry to synthetic organic chemistry research working under Professor Amudhu Gopalan. He joined Professor David E. Olson’s research group at the University of California, Davis in 2018, and has been focusing on biomimetic strategies for the synthesis of monoterpene indole alkaloids.

Guoliang Zhang
Guoliang Zhang received his B.S. in chemistry from Jilin University in China before completing his M.S. degree in chemistry from Shanghai Institute of Organic Chemistry. In 2015, he joined the Olson Lab at the University of California, Davis to work on the total synthesis of iboga alkaloids and related analogs.
Footnotes
DECLARATION OF INTERESTS
DEO is the president and chief scientific officer of Delix Therapeutics, Inc.
REFERENCES
- 1.Dybowski J, Landrin E. Concerning Iboga, its excitement-producing properties, its composition, and the new alkaloid it contains, ibogaine. CR Acad. Sci 1901; 133:748. [Google Scholar]
- 2.Bartlett MF, Dickel DF, Taylor WI. The alkaloids of Tabernanthe iboga. Part IV. 1 The structures of ibogamine, ibogaine, tabernanthine and voacangine. J. Am. Chem. Soc, 1958, 80, 126–136. [Google Scholar]
- 3.Arai G, Coppola J, and Jeffrey G The Structure of Ibogaine. Acta Crystallogr., 1960, 13, 553–564. [Google Scholar]
- 4.Men JL; Taylor WI A uniform numbering system for indole Alkaloids. Experientia, 1965, 21, 508–510. [DOI] [PubMed] [Google Scholar]
- 5.Martino E; Casamassima G; Castiglione S; Cellupica E; Pantalone S; Papagni F; Rui M; Siciliano AM; Collina S Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorganic & Medicinal Chemistry Letters, 2018, 28, 2816–2826. [DOI] [PubMed] [Google Scholar]
- 6.Pope HG Tabernanthe Iboga: an African Narcotic Plant of Social Importance. Economic Botany, 1969, 23, 174–184. [Google Scholar]
- 7.Krengel F; Mijangos MV; Reyes‐Lezama M; Reyes‐Chilpa R Extraction and Conversion Studies of the Antiaddictive Alkaloids Coronaridine, Ibogamine, Voacangine, and Ibogaine from Two Mexican Tabernaemontana Species (Apocynaceae). Chemistry Biodivers, 2019, 16, e1900175. [DOI] [PubMed] [Google Scholar]
- 8.Janot M, Goutarel R, Derivatives of Ibogaine Alkaloids. US Pat. 2813873A, 1957.
- 9.Bouso JC; Fornís I; Vilamala MV; Loenen BD; Sainz-Cort A; Jiménez-Garrido DF; Santos RGD; Hallak JEC; Alcázar-Córcoles MÁ; Jenks CW An Analytical Study of Iboga Alkaloids Contained in Tabernanthe Iboga-Derived Products Offered by Ibogaine Treatment Providers. Arch. Clin. Psychiatry, 2020, 47, 51–54. [Google Scholar]
- 10.Jenks CW.; Extraction studies of Tabernanthe iboga and Voacanga africana. Nat Prod Lett, 2002, 16, 71–76. [DOI] [PubMed] [Google Scholar]
- 11.Dickel D; Holden C; Maxfield R; Paszek L; Taylor W The Alkaloids of Tabernanthe iboga. Part III.1 Isolation Studies. J. Am. Chem. Soc, 1958, 80, 123–125. [Google Scholar]
- 12.Krengel F; Chevalier Q; Dickinson J; Herrera-Santoyo J; Reyes-Chilpa R Metabolite Profiling of Anti-Addictive Alkaloids from Four Mexican Tabernaemontana Species and the Entheogenic African Shrub Tabernanthe iboga (Apocynaceae). Chem. Biodiversity, 2019, 16, e1800506. [DOI] [PubMed] [Google Scholar]
- 13.Krengel F; Dickinson J; Reyes-Chilpa R Quantitative Evaluation of a Mexican and a Ghanaian Tabernaemontana Species as Alternatives to Voacanga africana for the Production of Antiaddictive Ibogan Type Alkaloids. Chem. Biodiversity, 2020, 17, e2000002. [DOI] [PubMed] [Google Scholar]
- 14.Ferreres F; Pereira DM; Valentão P; Oliveira JM; Faria J; Gaspar L; Sottomayor M; Andrade PB Simple and Reproducible HPLC–DAD–ESI-MS/MS Analysis of Alkaloids in Catharanthus Roseus Roots. J. Pharm. Biomed. Anal, 2010, 51, 65–69. [DOI] [PubMed] [Google Scholar]
- 15.Lavaud C; Massiot G The Iboga Alkaloids. Prog. Chem. Org. Nat. Prod 2017, 105, 89–136. [DOI] [PubMed] [Google Scholar]
- 16.Tang BQ; Wang WJ; Huang XJ; Li GQ; Wang L; Jiang RW; Yang TT; Shi L; Zhang XQ; Ye WC Iboga-Type Alkaloids from Ervatamia officinalis. J. Nat. Prod, 2014, 77, 1839–1846. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier SW Alkaloids: Chemical and Biological Perspectives; John Wiley & Sons: New York, 1988; Vol. 6, Chapter 2, pp 7680 [Google Scholar]
- 18.Madinaveitia A; De La Fuente G; Gonzalez A The Absolute Configuration at C(7) of Voacangine Hydroxyindolenine. Helv. Chim. Acta, 1998, 81, 1645–1653. [Google Scholar]
- 19.Liu ZW; Tang BQ; Zhang QH; Wang WJ; Huang XJ; Zhang J; Shi L; Zhang XQ; Ye WC Ervaoffines E–G, three iboga-type alkaloids featuring ring C cleavage and rearrangement from Ervatamia officinalis. RSC Adv., 2017, 7, 21883–21889. [Google Scholar]
- 20.Kazumasa Z; Tomoko H; Takahiro H; Yusuke H; Koichiro K; Abdul R; Idha K; Noor CZ; Motoo S; Hiroshi M Biscarpamontamines A and B, an Aspidosperma−Iboga Bisindole Alkaloid and an Aspidosperma−Aspidosperma Bisindole Alkaloid, from Tabernaemontana sphaerocarpa. J. Nat. Prod, 2009, 72, 1686–1690. [DOI] [PubMed] [Google Scholar]
- 21.Bao MF; Yan JM; Cheng GG; Li XY; Liu YP; Li Y; Cai XH; Luo XD Cytotoxic Indole Alkaloids from Tabernaemontana divaricate. J. Nat. Prod, 2013, 76, 1406–1412. [DOI] [PubMed] [Google Scholar]
- 22.Zhang DB; Yu DG; Sun M; Zhu XX; Yao XJ; Zhou SY; Chen JJ; Gao K Ervatamines A-I, Anti-inflammatory Monoterpenoid Indole Alkaloids with Diverse Skeletons from Ervatamia hainanensis. J. Nat. Prod, 2015, 78, 1253–1261. [DOI] [PubMed] [Google Scholar]
- 23.Liu ZW; Huang XJ; Xiao HL; Liu G; Zhang J; Shi L; Jiang RW; Zhang XQ; Ye WC New iboga-type alkaloids from Ervatamia hainanensis. RSC Adv., 2016, 6, 30277–30284. [Google Scholar]
- 24.Nge CE; Chong K Nge CE; Chong KW; Thomas NF; Lim SH; Low YY; Kam TS Ibogan, Aspidosperman, Vincamine, and Bisindole Alkaloids from a Malayan Tabernaemontana corymbosa: Iboga Alkaloids with C-20α Substitution. J. Nat. Prod, 2016, 79, 1388–1399. [DOI] [PubMed] [Google Scholar]
- 25.Nge CE; Gan CY; Low YY; Thomas NF; Kam TS Voatinggine and Tabertinggine, Pentacyclic Indole Alkaloids Derived from an Iboga Precursor via a Common Cleavamine-Type Intermediate. Org. Lett, 2013, 15, 18, 4774–4777. [DOI] [PubMed] [Google Scholar]
- 26.Nge CE; Sim KS; Lim SH; Thomas NF; Low YY; Kam TS A Hexacyclic Iboga Derived Monoterpenoid Indole with a Contracted Tetrahydroazepine C-Ring and Incorporation of an Isoxazolidine Moiety, a Seco-Corynanthean, an Aspidosperma-Aspidosperma Bisindole with Anticancer Properties, and the Absolute Configuration of the Pyridopyrimidine Indole Alkaloid, Vernavosine. J. Nat. Prod, 2016, 79, 2709–2717. [DOI] [PubMed] [Google Scholar]
- 27.Lim KH; Raja VJ; Bradshaw TD; Lim SH; Low YY; Kam TS Ibogan, Tacaman and cytotoxic bisindole alkaloids from tabernaemontana. Cononusine, an iboga alkaloid with unusual incorporation of a pyrrolidone moiety. J. Nat. Prod, 2015, 78, 1129–1138. [DOI] [PubMed] [Google Scholar]
- 28.Büchi G; Coffen DL; Kocsis K; Sonnet PE; Ziegler FE The Total Synthesis of Iboga Alkaloids. J. Am. Chem. Soc, 1966, 88, 3099–3109. [Google Scholar]
- 29.Deng Y; Bao MF; Shi BB; Wu J; Cai XH Three New Indole Alkaloids from Tabernaemontana divaricata. Nat. Prod. Bioprospect, 2018, 8, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuwen H; Yuan Y; Hao X; He H; Zhang Y Two New Monoterpenoid Indole Alkaloids from Tabernaemontana divaricata. Nat. Prod. Res, 2019, 33, 2139–2144. [DOI] [PubMed] [Google Scholar]
- 31.Li XM; Jiang XJ; Wei GZ; Ren LH; Wang LX; Cheng XL; Wang F New Iboga-Type Indole Alkaloids from Tabernaemontana divaricata. Nat. Prod. Bioprospect, 2019, 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leete E Biogenesis of Rauwolfia alkaloids. II. Incorporation of tryptophan into serpentine and reserpine. Tetrahedron, 1961, 14, 35–41. [Google Scholar]
- 33.Battersby AR; Burnett AR; Parsons PG Partial synthesis and isolation of vincoside and isovincoside: Biosynthesis of the three major classes of indole alkaloids from the beta carboline system., Chem. Comm, 1968, 1282–1284. [Google Scholar]
- 34.De Luca V; Marineau C; Brisson N Molecular cloning and analysis of a cDNA encoding a plant tryptophan decarboxylase. Proc. Natl. Acad. Sci, 1989, 86, 2582–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor SE; Maresh JJ Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat. Prod. Rep 2006, 23, 532–472. [DOI] [PubMed] [Google Scholar]
- 36.Miettinen K; Dong L; Navrot N et al. The seco-iridoid pathway from Catharanthus roseus. Nat Comm, 2014, 5, 3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oudin A; Courtois M; Rideau M et al. The iridoid pathway in Catharanthus roseus alkaloid biosynthesis. Phytochem Rev., 2007, 6, 259–276. [Google Scholar]
- 38.Rai A; Smita S; Singh AK; Shanker K; Naegegowda DA Heteromeric and Homomeric Geranyl Diphosphate Synthases from Catharanthus roseus and Their Role in Monoterpene Indole Alkaloid Biosynthesis. Mol. Plant, 2013, 6, 1531–1549. [DOI] [PubMed] [Google Scholar]
- 39.Simkin AJ; Miettinen K; Claudel P; Burlat V; Guirimand G; Courdavault V; Papon N; Meyer S; Godet S; St-Pierre B; Giglioli-Guivarc’H N; Fischer MJ; Memelink J; Clastre M Characterization of the Plastidial Geraniol Synthase from Madagascar Periwinkle Which Initiates the Monoterpenoid Branch of the Alkaloid Pathway in Internal Phloem Associated Parenchyma. Phytochemistry, 2013, 85, 36–43. [DOI] [PubMed] [Google Scholar]
- 40.Geu-Flores F; Sherden NH; Courdavault V; Burlat V; Glenn WS; Wu C; Nims E; Cui Y; O’Connor SE An Alternative Route to Cyclic Terpenes by Reductive Cyclization in Iridoid Biosynthesis. Nature, 2012, 492, 138–142. [DOI] [PubMed] [Google Scholar]
- 41.Murata J; Roepke J; Gordon H; De Luca V. The Leaf Epidermome of Catharanthus Roseus Reveals its Biochemical Specialization. Plant Cell, 2008, 20, 524–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irmler S; Schröder G; St-Pierre B; Crouch NP; Hotze M; Schmidt J; Strack D; Matern U; Schröder J Indole Alkaloid Biosynthesis in Catharanthus Roseus: New Enzyme Activities and Identification of Cytochrome P450 CYP72A1 as Secologanin Synthase. The Plant Journal, 2008, 24, 797–804. [DOI] [PubMed] [Google Scholar]
- 43.De Waal A; Meijer AH; Verpoorte R Strictosidine synthase from Catharanthus roseus: Purification and Characterization of Multiple Forms. Biochem. J, 1995, 306, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKnight TD; Roessner CA; Devagupta R; Scott AI; Nessler CL Nucleotide Sequence of a cDNA Encoding the Vacuolar Protein Strictosidine Synthase from Catharanthus roseus. Nucleic Acids Res, 1990, 18, 4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemscheidt T; Zenk MH Glucosidases involved in indole alkaloid biosynthesis of Catharanthus roseus cell cultures. FEBS Lett. 1980, 110:187–191 [DOI] [PubMed] [Google Scholar]
- 46.Geerlings A; Martinez-Lozano Ibanez M; Memelink J; van der Heijden R; Verpoorte R Molecular Cloning and Analysis of Strictosidine β-D-Glucosidase, an Enzyme in Terpenoid Indole Alkaloid Biosynthesis in Catharanthus roseus. J Biol Chem. 2000, 275, 3051–3056. [DOI] [PubMed] [Google Scholar]
- 47.Qu Y; Thamm AMK; Czerwinski M; Masada S; Kim KH; Jones G; Liang P; Luca VD Geissoschizine Synthase Controls Flux in the Formation of Monoterpenoid Indole Alkaloids in a Catharanthus Roseus Mutant. Planta, 2017, 247, 625–634. [DOI] [PubMed] [Google Scholar]
- 48.Qu Y; Easson MEAM; Simionescu R; Hajicek J; Thamm AMK; Salim V; Luca VD Solution of the Multistep Pathway for Assembly of Corynanthean, Strychnos, Iboga, and Aspidosperma Monoterpenoid Indole Alkaloids from 19E-Geissoschizine. PNAS, 2018, 115, 3180–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caputi L; Franke J; Farrow SC; Chung K; Payne RME; Nguyen T-D; Dang T-TT; Carqueijeiro IST; Koudounas K; Bernonville TDD; Ameyaw B; Jones DM; Vieira IJC; Courdavault V; O’Connor SE Missing Enzymes in the Biosynthesis of the Anticancer Drug Vinblastine in Madagascar Periwinkle. Science, 2018, 360, 1235–1239. [DOI] [PubMed] [Google Scholar]
- 50.Farrow SC; Kamileen MO; Caputi L; Bussey K; Mundy JEA; Mcatee RC; Stephenson CRJ; O’Connor SE Biosynthesis of an Anti-Addiction Agent from the Iboga Plant. J. Am. Chem. Soc, 2019, 141, 12979–12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klas K; Tsukamoto S; Sherman DH; Williams RM Natural Diels–Alderases: Elusive and Irresistable. J. Org. Chem, 2015, 80, 11672–11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andriamialisoa RZ; Langlois N; Langlois Y Preparation of 15-oxo-16-methoxycarbonyl-15, 20-dihydrocleavamine and Coupling Reaction with Vindoline. Heterocycles., 1981,15, 245–250. [Google Scholar]
- 53.Caputi L; Franke J; Bussey K; Farrow SC; Vieira IJC; Stevenson CEM; Lawson DM; O’Connor SE Structural Basis of Cycloaddition in Biosynthesis of Iboga and Aspidosperma Alkaloids. Nat. Chem. Biol 2020, 16, 383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linder M; Brinck T Stepwise Diels-Alder: More Than Just An Oddity? A Computational Mechanistic Study. J. Org. Chem, 2012, 77, 6563–6573. [DOI] [PubMed] [Google Scholar]
- 55.Buonora P; Olsen J-C; Oh T Recent Developments in Imino Diels–Alder Reactions. Tetrahedron, 2001, 57, 6099–6138. [Google Scholar]
- 56.Farrow SC; Kamileen MO; Meades J; Ameyaw B; Xiao Y; O’Connor SE Cytochrome P450 and O-Methyltransferase Catalyze the Final Steps in the Biosynthesis of the Anti-addictive Alkaloid Ibogaine from Tabernanthe iboga. J Biol Chem, 2018, 293,13821–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dogru E; Warzecha H; Seibel F; Haebel S; Lottspeich F; Stöckigt J The Gene Encoding Polyneuridine Aldehyde Esterase of Monoterpenoid Indole Alkaloid Biosynthesis in Plants Is an Ortholog of Theα/β Hydrolase Super Family. Eur. J. Biochem. 2000, 267, 1397–1406. [DOI] [PubMed] [Google Scholar]
- 58.McCoy E; O’Connor SE Directed biosynthesis of alkaloid analogs in the medicinal plant Catharanthus roseus. J. Am. Chem. Soc 2006, 128,14276–14277. [DOI] [PubMed] [Google Scholar]
- 59.Runguphan W; Qu X; O’Connor SE Integrating Carbon-Halogen Bond Formation into Medicinal Plant Metabolism., Nature 2010, 468, 461–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glenn WS; Nims E; O’Connor SE Reengineering a Tryptophan Halogenase to Preferentially Chlorinate a Direct Alkaloid Precursor. J. Am. Chem. Soc, 2011, 133, 19346–19349. [DOI] [PubMed] [Google Scholar]
- 61.Jana GK; Paul S; Sinha S Progress in the Synthesis of Iboga-Alkaloids and Their Congeners. Organic Preparations and Procedures International, 2011, 43, 541–573. [Google Scholar]
- 62.Sundberg RJ; Smith SQ The Alkaloids, 2002, 59, 281–386. [DOI] [PubMed] [Google Scholar]
- 63.Büchi G; Coffen DL; Kocsis K; Sonnet PE; Ziegler FE The Total Synthesis of (±)-Ibogamine and of (±)-Epiibogamine. J. Am. Chem. Soc, 1965, 87, 2073–2075. [Google Scholar]
- 64.Jana GK; Sinha S Total Synthesis of Ibogaine, Epiibogaine and Their Analogues. Tetrahedron, 2012, 68, 7155–7165. [Google Scholar]
- 65.Kruegel AC; Rakshit S; Li X; Sames D Constructing Iboga Alkaloids via C–H Bond Functionalization: Examination of the Direct and Catalytic Union of Heteroarenes and Isoquinuclidine Alkenes. J. Org. Chem, 2015, 80, 2062–2071. [DOI] [PubMed] [Google Scholar]
- 66.Reding MT; Fukuyama T Stereocontrolled Total Synthesis of (±)-Catharanthine via Radical-Mediated Indole Formation. Org. Lett, 1999, 1, 973–976. [Google Scholar]
- 67.Kim SJ; Batey RA Enantioselective Isoquinuclidine Synthesis via Sequential Diels–Alder/Visible-Light Photoredox C–C Bond Cleavage: A Formal Synthesis of the Indole Alkaloid Catharanthine. Organic Chemistry Frontiers, 2018, 5, 2934–2939. [Google Scholar]
- 68.Höck S; Borschberg HJ Enantioselective Synthesis of Key Intermediates in a Novel Approach towards the Iboga-Alkaloid Family. Helvetica Chimica Acta, 2003, 86, 1397–1409. [Google Scholar]
- 69.Höck S; Borschberg HJ Enantioselective Synthesis of (−)-(19R)-Ibogamin-19-ol. Helvetica Chimica Acta, 2006, 89, 542–557. [Google Scholar]
- 70.Huffman JW; Rao CBS; Kamiya T The Synthesis of Desethylibogamine. J. Am. Chem. Soc, 1965, 87, 2288–2288. [Google Scholar]
- 71.Huffman JW; Rao CBS; Kamiya T The Synthesis of Desethylibogamine. J. Am. Chem. Soc, 1967, 32, 697–700. [DOI] [PubMed] [Google Scholar]
- 72.Huffman JW; Shanmugasundaram G; Sawdaye R; Raveendranath PC; Desai RC A Formal Synthesis of (±)-Ibogamine. J. Org. Chem, 1985, 50, 1460–1464. [Google Scholar]
- 73.Henry KJ; Grieco PA; Dubay WJ A Novel Approach to Iboga Alkaloids: Total Synthesis of (±)-Ibogamine and (±)-Epi-Ibogamine. Tetrahedron Letters, 1996, 37, 8289–8292. [Google Scholar]
- 74.Sallay SI Total Synthesis of DL-Ibogamine. J. Am. Chem. Soc,1967, 89, 6762–6763. [DOI] [PubMed] [Google Scholar]
- 75.White JD; Choi Y Catalyzed Asymmetric Diels−Alder Reaction of Benzoquinone. Total Synthesis of (−)-Ibogamine. Org. Lett, 2000, 2, 2373–2376. [DOI] [PubMed] [Google Scholar]
- 76.Nagata W; Hirai S; Okumura T; Kawata K A Stereochemical Controlled Total Synthesis of DL-Ibogamine and DL-Epiibogamine. J. Am. Chem. Soc, 1968, 90, 1650–1651. [DOI] [PubMed] [Google Scholar]
- 77.Hirai S; Kawata K; Nagata W Total Synthesis of (±)-Coronaridine and an Improved Synthesis of (±)–Ibogamine. Chem. Commun, 1968, 719, 1016–1017. [Google Scholar]
- 78.Nagata W; Hirai S; Kawata K; Aoki T One-Step Synthesis of Bridged Aziridines. J. Am. Chem. Soc, 1967, 89, 5045–5046. [Google Scholar]
- 79.Trost BM; Godleski SA; Genet JP A Total Synthesis of Racemic and Optically Active Ibogamine. Utilization and Mechanism of a New Silver Ion Assisted Palladium Catalyzed Cyclization. J. Am. Chem. Soc,1978, 100, 3930–3931. [Google Scholar]
- 80.Trost BM; Genet JP Palladium Catalyzed Cyclizations to Alkaloid Skeletons. Facile Synthesis of Desethylibogamine. J. Am. Chem. Soc,1976, 98, 8516–8517. [Google Scholar]
- 81.Hodgson DM; Galano JM Enantioselective Access to Isoquinuclidines by Tropenone Desymmetrization and Homoallylic Radical Rearrangement: Synthesis of (+)-Ibogamine. Org. Lett, 2005, 7, 2221–2224. [DOI] [PubMed] [Google Scholar]
- 82.Imanishi T; Yagi N; Hanaoka M 1,6-Dihydro-3(2H)-Pyridinones. X. 2-Azabicyclo(2.2.2)Octane Ring Formation via Intramolecular Michael Reaction: Total Synthesis of (±)-Ibogamine and (±)-Epiibogamine. Chemical & Pharmaceutical Bulletin, 1985, 33, 4202–4211. [Google Scholar]
- 83.Jana GK; Sinha S Reductive Heck Coupling: An Efficient Approach toward the Iboga Alkaloids. Synthesis of Ibogamine, Epiibogamine and Iboga Analogs. Tetrahedron, 2012, 53, 1671–1674. [Google Scholar]
- 84.Kutney JP; Fuller GB; Greenhouse R; Itoh I Selective Debenzylation of Quaternary Salts. The Benzyl Group as an Excellent Protecting Group for Basic Nitrogen Compounds. Synthetic Communications, 1974, 4, 183–187. [Google Scholar]
- 85.Marazano C; Goff M-TL; Fourrey JL; Das BC An Unequivocal Synthesis of 1-Benzyl-3-Ethyl-1,6-Dihydropyridine and Its Use for a Biogenetically Modelled Synthesis of (±)-Catharanthine. J. Chem. Soc. Chem. Commun, 1981, 148, 389–391. [Google Scholar]
- 86.Sundberg RJ; Hong J; Smith SQ; Sabat M; Tabakovic I Synthesis and Oxidative Fragmentation of Catharanthine Analogs. Comparison to the Fragmentation—Coupling of Catharanthine and Vindoline. Tetrahedron, 1998, 54, 6259–6292. [Google Scholar]
- 87.Alkayar ZTI; Coldham I Cascade Cyclization and Intramolecular Nitrone Dipolar Cycloaddition and Formal Synthesis of 19-Hydroxyibogamine. Organic & Biomolecular Chemistry, 2019, 17, 66–73. [DOI] [PubMed] [Google Scholar]
- 88.Kono M; Harada S; Nozaki T; Hashimoto Y; Murata S-I; Gröger H; Kuroda Y; Yamada K-I; Takasu K; Hamada Y; Nemoto T Asymmetric Formal Synthesis of (+)-Catharanthine via Desymmetrization of Isoquinuclidine. Org. Lett, 2019, 21, 3750–3754. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Y; Xue Y; Li G; Yuan H; Luo T Enantioselective Synthesis of Iboga Alkaloids and Vinblastine via Rearrangements of Quaternary Ammoniums. Chemical Science, 2016, 7, 5530–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coldham I; Burrell AJM; White LE; Adams H; Oram N Highly Efficient Synthesis of Tricyclic Amines by a Cyclization/Cycloaddition Cascade: Total Syntheses of Aspidospermine, Aspidospermidine, and Quebrachamine. Angewandte Chemie, 2007, 119, 6271–6274. [DOI] [PubMed] [Google Scholar]
- 91.Burrell AJM; Coldham I; Watson L; Oram N; Pilgram CD; Martin NG Stereoselective Formation of Fused Tricyclic Amines from Acyclic Aldehydes by a Cascade Process Involving Condensation, Cyclization, and Dipolar Cycloaddition. J. Org. Chem, 2009, 74, 2290–2300. [DOI] [PubMed] [Google Scholar]
- 92.Coldham I; Burrell AJM; Guerrand Hélène DS; Oram N Cascade Cyclization, Dipolar Cycloaddition to Bridged Tricyclic Amines Related to The Daphniphyllum Alkaloids. Org. Lett, 2011, 13, 1267–1269. [DOI] [PubMed] [Google Scholar]
- 93.Harada S; Kono M; Nozaki T; Menjo Y; Nemoto T; Hamada Y General Approach to Nitrogen-Bridged Bicyclic Frameworks by Rh-Catalyzed Formal Carbenoid Insertion into an Amide C–N Bond. The Journal of Organic Chemistry, 2015, 80, 10317–10333. [DOI] [PubMed] [Google Scholar]
- 94.Raheem IT; Thiara PS; Peterson EA; Jacobsen EN Enantioselective Pictet−Spengler-Type Cyclizations of Hydroxylactams: H-Bond Donor Catalysis by Anion Binding. J. Chem. Am. Soc, 2007, 129, 13404–13405. [DOI] [PubMed] [Google Scholar]
- 95.He W; Xie L; Xu Y; Xiang J; Zhang L Electrophilicity of α-Oxo Gold Carbene Intermediates: Halogen Abstractions from Halogenated Solvents Leading to the Formation of Chloro/Bromomethyl Ketones. Organic & Biomolecular Chemistry, 2012, 10, 3168–3171. [DOI] [PubMed] [Google Scholar]
- 96.Iwasaki K; Wan KK; Oppedisano A; Crossley SWM; Shenvi RA Simple, Chemoselective Hydrogenation with Thermodynamic Stereocontrol. J. Am. Chem. Soc, 2014, 136, 1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lo JC; Yabe Y; Baran PS A Practical and Catalytic Reductive Olefin Coupling. J. Am. Chem. Soc, 2014, 136, 1304–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lo JC; Gui J; Yabe Y; Pan C-M; Baran PS Functionalized Olefin Cross-Coupling to Construct Carbon–Carbon Bonds. Nature, 2014, 516, s343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harada M; Asaba K; Iwai M; Kogure N; Kitajima M; and Takayama H Asymmetric Total Synthesis of an Iboga-Type Indole Alkaloid, Voacangalactone, Newly Isolated from Voacanga Africana. Org. Lett, 2012, 14, 5800–5803. [DOI] [PubMed] [Google Scholar]
- 100.Jiang L; Job JE; Klapars A; Buchwald SL Copper-Catalyzed Coupling of Amides and Carbamates with Vinyl Halides. Org. Lett, 2003, 5, 3667–3669. [DOI] [PubMed] [Google Scholar]
- 101.Iritani K; Matsubara S; Utimoto K Palladium catalyzed reaction of 2-alkynylanilines with allyl chlorides. Formation of 3-allylindoles. Tetrahedron Lett, 1988, 29, 1799–1802. [Google Scholar]
- 102.Zhao G; Xie X; Sun H; Yuan Z; Zhong Z; Tang S; She X Bioinspired Collective Syntheses of Iboga-Type Indole Alkaloids. Org. Lett, 2016, 18, 2447–2450. [DOI] [PubMed] [Google Scholar]
- 103.Lim H; Seong S; Han S Syntheses of Post-Iboga Alkaloids. Synthesis, 2019, 51, 2737–2758. [Google Scholar]
- 104.Seong S; Lim H; Han S Biosynthetically Inspired Transformation of Iboga to Monomeric Post-iboga Alkaloids. Chem, 2019, 5, 353–363. [Google Scholar]
- 105.Mizoguchi H; Oikawa H; Oguri H Biogenetically Inspired Synthesis and Skeletal Diversification of Indole Alkaloids. Nat. Chem, 2013, 6, 57–64. [DOI] [PubMed] [Google Scholar]
- 106.Chen P; Cao L; Li C Protecting-Group-Free Total Synthesis of (±)-Subincanadine F. J. Org. Chem 2009, 74, 7533–7535. [DOI] [PubMed] [Google Scholar]
- 107.Sheppard SG A preliminary investigation of ibogaine: case reports and recommendations for further study. J. Subst. Abuse Treat, 1994, 11, 379–385. [DOI] [PubMed] [Google Scholar]
- 108.Alper KR; Lotsof HS; Frenken GM; Luciano DJ; Bastiaans J Treatment of acute opioid withdrawal with ibogaine. Am. J. Addict, 1999, 8, 234–242. [DOI] [PubMed] [Google Scholar]
- 109.Schenberg EE; Comis MADC; Chaves BR; Silveira DXD Treating Drug Dependence with the Aid of Ibogaine: A Retrospective Study. J. Psychopharmacol, 2014, 28, 993–1000. [DOI] [PubMed] [Google Scholar]
- 110.Mash DC; Duque L; Page B; Allen-Ferdinand K Ibogaine Detoxification Transitions Opioid and Cocaine Abusers Between Dependence and Abstinence: Clinical Observations and Treatment Outcomes. Front. Pharmacol, 2018, 9:529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mash DC; Kovera CA; Pablo J; Tyndale RF; Ervin FD; Williams IC; Singleton EG; Mayor M Ibogaine: Complex Pharmacokinetics, Concerns for Safety, and Preliminary Efficacy Measures. Ann N Y Acad. Sci, 2000, 914, 394–401. [DOI] [PubMed] [Google Scholar]
- 112.Cappendijk SL; Dzoljic MR Inhibitory Effects of Ibogaine on Cocaine Self-Administration in Rats. Eur. J. Pharmacol, 1993, 241(2–3), 261–265. [DOI] [PubMed] [Google Scholar]
- 113.Maisonneuve IM; Keller RW; Glick SD Interactions between Ibogaine, a Potential Anti-Addictive Agent, and Morphine: An in Vivo Microdialysis Study. Eur. J. Pharmacol, 1991, 199, 35–42. [DOI] [PubMed] [Google Scholar]
- 114.Parker LA; Siegel S Chapter 11 Modulation of the Effects of Rewarding Drugs by Ibogaine. The Alkaloids: Chemistry and Biology, 2001, 211–225. [DOI] [PubMed] [Google Scholar]
- 115.Brown TK Ibogaine in the treatment of substance dependence. Current Drug Abuse Reviews, 2013, 6, 3–16. [DOI] [PubMed] [Google Scholar]
- 116.Wasko MJ; Witt-Enderby PA; Surratt CK DARK Classics in Chemical Neuroscience: Ibogaine. ACS Chem. Neurosci, 2018, 9, 2475–2483. [DOI] [PubMed] [Google Scholar]
- 117.Maciulaitis R; Kontrimaviciute V; Bressolle FM; Briedis V Ibogaine, an anti-addictive drug: pharmacology and time to go further in development. A narrative review. Hum. Exp. Toxicol, 2008, 27, 181–94. [DOI] [PubMed] [Google Scholar]
- 118.Alper KR Ibogaine: a review. Alkaloids Chem Biol, 2001, 56, 1–38. [DOI] [PubMed] [Google Scholar]
- 119.Popik P; Layer RT; Skolnick P 100 Years of Ibogaine: Neurochemical and Pharmacological Actions of a Putative Anti-Addictive Drug. Pharmacol. Rev, 1995, 47, 235–253. [PubMed] [Google Scholar]
- 120.Ohearn E; Molliver M Degeneration of Purkinje Cells in Parasagittal Zones of the Cerebellar Vermis after Treatment with Ibogaine or Harmaline. Neuroscience, 1993, 55, 303–310. [DOI] [PubMed] [Google Scholar]
- 121.Koenig X; Kovar M; Boehm S; Sandtner W; Hilber K Anti-Addiction Drug Ibogaine Inhibits HERG Channels: A Cardiac Arrhythmia Risk. Addict. Biol, 2014, 19, 237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thurner P; Stary-Weinzinger A; Gafar H; Gawali VS; Kudlacek O; Zezula J; Hilber K; Boehm S; Sandtner W; Koenig X Mechanism of HERG Channel Block by the Psychoactive Indole Alkaloid Ibogaine. J. Pharmacol. Exp. Ther, 2014, 348, 346–358. [DOI] [PubMed] [Google Scholar]
- 123.Alper KR; Stajic M; Gill JR Fatalities Temporally Associated with the Ingestion of Ibogaine. J. Forensic Sci, 2012, 57, 398–412. [DOI] [PubMed] [Google Scholar]
- 124.Koenig X; Hilber K The Anti-Addiction Drug Ibogaine and the Heart: A Delicate Relation. Molecules, 2015, 20, 2208–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hough LB; Pearl SM; Glick SD Tissue Distribution of Ibogaine after Intraperitoneal and Subcutaneous Administration. Life Sci., 1996, 58, 119–122. [DOI] [PubMed] [Google Scholar]
- 126.Jamieson C; Moir EM; Rankovic Z; Wishart G Medicinal chemistry of hERG optimizations: Highlights and hang-ups. J. Med. Chem, 2006, 49, 5029–5046. [DOI] [PubMed] [Google Scholar]
- 127.Arias HR; Feuerbach D; Targowska-Duda KM; Jozwiak K Structure–Activity Relationship of Ibogaine Analogs Interacting with Nicotinic Acetylcholine Receptors in Different Conformational States. Int. J. Biochem. Cell Biol, 2011, 43, 1330–1339. [DOI] [PubMed] [Google Scholar]
- 128.Arias HR; Targowska-Duda KM; Feuerbach D; Jozwiak K Coronaridine Congeners Inhibit Human α3β4 Nicotinic Acetylcholine Receptors by Interacting with Luminal and Non-Luminal Sites. Int. J. Biochem. Cell Biol, 2015, 65, 81–90. [DOI] [PubMed] [Google Scholar]
- 129.Efange SMN; Mash DC; Khare AB; Ouyang Q Modified Ibogaine Fragments: Synthesis and Preliminary Pharmacological Characterization of 3-Ethyl-5-Phenyl-1,2,3,4,5,6-Hexahydroazepino[4,5-b] Benzothiophenes. J. Med. Chem, 1998, 41, 4486–4491. [DOI] [PubMed] [Google Scholar]
- 130.Pablo JP; Mash DC Noribogaine Stimulates Naloxone-Sensitive [35S]GTPγS Binding. NeuroReport, 1998, 9, 109–114. [DOI] [PubMed] [Google Scholar]
- 131.Pearl SM; Herrick-Davis K; Teitler M; Glick SD Radioligand-Binding Study of Noribogaine, a Likely Metabolite of Ibogaine. Brain Res., 1995, 675, 342–344. [DOI] [PubMed] [Google Scholar]
- 132.Mash DC; Staley JK; Baumann MH; Rothman RB; Hearn WL Identification of a Primary Metabolite of Ibogaine That Targets Serotonin Transporters and Elevates Serotonin. Life Sci., 1995, 57, 45–50. [DOI] [PubMed] [Google Scholar]
- 133.Bowen WD; Vilner BJ; Williams W; Bertha CM; Kuehne ME; Jacobson AE Ibogaine and Its Congeners Are σ2 Receptor-Selective Ligands with Moderate Affinity. Eur. J. Pharmacol, 1995, 279, 1–3. [DOI] [PubMed] [Google Scholar]
- 134.Rezvani AH; Overstreet D; Leef Y-W Attenuation of Alcohol Intake by Ibogaine in Three Strains of Alcohol-Preferring Rats. Pharmacol. Biochem. Behav, 1995, 52, 615–620. [DOI] [PubMed] [Google Scholar]
- 135.Glick SD; Maisonneuve IM; Visker KE; Fritz KA; Bandarage UK; Kuehne ME 18-Methoxycoronardine Attenuates Nicotine-Induced Dopamine Release and Nicotine Preferences in Rats. Psychopharmacology, 1998, 139, 274–280. [DOI] [PubMed] [Google Scholar]
- 136.Staley JK; Ouyang Q; Pablo J; Hearn WL; Flynn DD; Rothman RB; Rice KC; Mash DC Pharmacological Screen for Activities of 12-Hydroxyibogamine: a Primary Metabolite of the Indole Alkaloid Ibogaine. Psychopharmacology, 1996, 127, 10–18. [DOI] [PubMed] [Google Scholar]
- 137.Glick S; Pearl S; Cai J; Maisonneuve I Ibogaine-like Effects of Noribogaine in Rats. Brain Res., 1996, 713, 294–297. [DOI] [PubMed] [Google Scholar]
- 138.Rezvani AH; Mash DC; Hearn WL; Lee YW; Overstreet DH: Noribogaine, a primary ibogaine metabolite, Reduces alcohol intake in P and Fawn-Hooded rats. Alcohol Clin. Exp. Res, 1995, 19:15A; [Google Scholar]
- 139.Chang Q; Hanania T; Mash DC; Maillet EL Noribogaine Reduces Nicotine Self-Administration in Rats. J. Psychopharmacol, 2015, 29, 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Trouvin J-H; Jacqmin P; Rouch C; Lesne M; Jacquot C Benzodiazepine Receptors Are Involved in Tabernanthine-Induced Tremor: in Vitro and in Vivo Evidence. Eur. J. Pharmacol 1987, 140, 303–309. [DOI] [PubMed] [Google Scholar]
- 141.Arias HR; Jin X; Feuerbach D; Drenan RM Selectivity of Coronaridine Congeners at Nicotinic Acetylcholine Receptors and Inhibitory Activity on Mouse Medial Habenula. Int. J. Biochem. Cell Biol, 2017, 92, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Glick SD; Kuehne ME; Maisonneuve IM; Bandarage UK; Molinari HH 18-Methoxycoronaridine, a Non-Toxic Iboga Alkaloid Congener: Effects on Morphine and Cocaine Self-Administration and on Mesolimbic Dopamine Release in Rats. Brain Res., 1996, 719, 29–35. [DOI] [PubMed] [Google Scholar]
- 143.Rezvani AH; Overstreet DH; Yang Y; Maisonneuve IM; Bandarage UK; Kuehne ME; Glick SD Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacol. Biochem. Behav 1997, 58, 615–619. [DOI] [PubMed] [Google Scholar]
- 144.Glick SD; Maisonneuve IM; Dickinson HA 18-MC Reduces Methamphetamine and Nicotine Self-Administration in Rats. Neuroreport, 2000, 11, 2013–2015. [DOI] [PubMed] [Google Scholar]
- 145.Passarella D; Barilli A; Efange SMN; Elisabetsky E; Leal MB; Lesma G; Linck VM; Mash DC; Martinelli M; Peretto I; Silvani A; Danieli B Nature-Inspired Indolyl-2-Azabicyclo[2.2.2]Oct-7-Ene Derivatives as Promising Agents for the Attenuation of Withdrawal Symptoms: Synthesis of 20-Desethyl-20-Hydroxymethyl-11-Demethoxyibogaine. Nat. Prod. Res, 2006, 20, 758–765. [DOI] [PubMed] [Google Scholar]
- 146.Banerjee TS; Paul S; Sinha S; Das S Synthesis of Iboga-like Isoquinuclidines: Dual Opioid Receptors Agonists Having Antinociceptive Properties. Bioorg. Med. Chem, 2014, 22, 6062–6070. [DOI] [PubMed] [Google Scholar]
- 147.Gassaway MM; Jacques TL; Kruegel AC; Karpowicz RJ; Li X; Li S; Myer Y; Sames D Deconstructing the Iboga Alkaloid Skeleton: Potentiation of FGF2-Induced Glial Cell Line-Derived Neurotrophic Factor Release by a Novel Compound. ACS Chem. Biol, 2015, 11, 77–87. [DOI] [PubMed] [Google Scholar]
- 148.Sundberg RJ; Amat M; Fernando AM Analogs of the Iboga Alkaloids. Synthesis and Reactions of (±)-15-Oxo-20-deethylcoronaridine Derivatives. J. Org. Chem, 1987, 52, 3151–3159. [Google Scholar]
- 149.Layer RT; Skolnick P; Bertha CM; Bandarage UK; Kuehne ME; Popik P Structurally Modified Ibogaine Analogs Exhibit Differing Affinities for NMDA Receptors. Eur. J. Pharmacol, 1996, 309, 159–165. [DOI] [PubMed] [Google Scholar]
- 150.Maillet EL; Milon N; Heghinian MD; Fishback J; Schürer SC; Garamszegi N; Mash DC Noribogaine Is a G-Protein Biased κ-Opioid Receptor Agonist. Neuropharmacology, 2015, 99, 675–688. [DOI] [PubMed] [Google Scholar]
- 151.Obach RS, Pablo J, Mash DC Cytochrome P4502D6 catalyzes the Odemethylation of the psychoactive alkaloid ibogaine to 12-hydroxyibogamine. Drug Metab. Dispos, 1998, 26, 764–768. [PubMed] [Google Scholar]
- 152.Kivell BM; Ewald AW; Prisinzano TE Salvinorin A Analogs and Other Kappa-Opioid Receptor Compounds as Treatments for Cocaine Abuse. Emerging Targets & Therapeutics in the Treatment of Psychostimulant Abuse. Adv. in Pharmacol, 2014, 69, 481–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bandarage UK; Kuehne ME; Glick SD Total Syntheses of Racemic Albifloranine and Its Anti-Addictive Congeners, Including 18-Methoxycoronaridine. Tetrahedron, 1999, 55, 9405–9424. [Google Scholar]
- 154.Alper K; Bai R; Liu N; Fowler SJ; Huang X-P; Priori SG; Ruan Y HERG Blockade by Iboga Alkaloids. Cardiovasc. Toxicol, 2016, 16, 14–22. [DOI] [PubMed] [Google Scholar]
- 155.Kuehne ME; He L; Jokiel PA; Pace CJ; Fleck MW; Maisonneuve IM; Glick SD; Bidlack JM Synthesis and Biological Evaluation of 18-Methoxycoronaridine Congeners. Potential Antiaddiction Agents. J. Med. Chem, 2003, 46, 2716–2730. [DOI] [PubMed] [Google Scholar]
- 156.Pace CJ; Glick SD; Maisonneuve IM; He L-W; Jokiel PA; Kuehne ME; Fleck MW Novel Iboga Alkaloid Congeners Block Nicotinic Receptors and Reduce Drug Self-Administration. Eur. J. Pharmacol, 2004, 492, 159–167. [DOI] [PubMed] [Google Scholar]
- 157.Glick SD; Maisonneuve IM Development of Novel Medications for Drug Addiction. The Legacy of an African Shrub. Ann. N. Y. Acad. Sci, 2000, 909, 88–103. [DOI] [PubMed] [Google Scholar]
- 158.Glick SD; Maisonneuve IM; Szumlinski KK 18-Methoxycoronaridine (18-MC) and Ibogaine: Comparison of Antiaddictive Efficacy, Toxicity, and Mechanisms of Action. Ann. N. Y. Acad. Sci, 2000, 914, 369–386. [DOI] [PubMed] [Google Scholar]
- 159.Wender PA; Verma VA; Paxton TJ; Pillow TH Function Oriented Synthesis, Step Economy, and Drug Design. Acc. Chem. Res, 2008, 41, 40–49. [DOI] [PubMed] [Google Scholar]
- 160.Sundberg RJ; Amat M; Fernando AM Analogs of the Iboga Alkaloids. Synthesis and Reactions of (±)-15-Oxo-20-deethylcoronaridine Derivatives. J. Org. Chem, 1987, 52, 3151–3159. [Google Scholar]
- 161.Sundberg RJ; Cherney RJ Synthesis of Analogs of Iboga Alkaloids. Investigation of Electrophilic, Palladium-Catalyzed and Radical Cyclizations for Preparation of 5,6-Homoiboga Derivatives. J. Org. Chem, 1990, 55, 6028–6037. [Google Scholar]
- 162.Paul S; Pattanayak S; Sinha S Synthesis of New Series of Iboga Analogues. Tetrahedron, 2011, 52, 6166–6169. [Google Scholar]
- 163.Glick S; Kuehne M; Raucci J; Wilson T; Larson D; Keller R; Carlson J Effects of Iboga Alkaloids on Morphine and Cocaine Self-Administration in Rats: Relationship to Tremorigenic Effects and to Effects on Dopamine Release in Nucleus Accumbens and Striatum. Brain Res., 1994, 657, 14–22. [DOI] [PubMed] [Google Scholar]
- 164.Kuehne ME; Wilson TE; Bandarage UK; Dai W; Yu Q Enantioselective Syntheses of Coronaridine and 18-Methoxycoronaridine. Tetrahedron, 2001, 57, 2085–2094. [Google Scholar]
- 165.Deecher DC; Teitler M; Soderlund DM; Bornmann WG; Kuehne ME; Glick SD Mechanisms of Action of Ibogaine and Harmaline Congeners Based on Radioligand Binding Studies. Brain Res, 1992, 571, 242–247. [DOI] [PubMed] [Google Scholar]
- 166.Sershen H; Hashim A; Lajtha A Characterization of Multiple Sites of Action of Ibogaine. Alkaloids Chem. Biol, 2001, 115–133. [DOI] [PubMed] [Google Scholar]
- 167.Noller GE; Frampton CM; Yazar-Klosinski B Ibogaine Treatment Outcomes for Opioid Dependence from a Twelve-Month Follow-up Observational Study. Am. J. Drug Alcohol Abuse, 2018, 44, 37–46. [DOI] [PubMed] [Google Scholar]
- 168.Schenberg EE; De Castro Comis MA; Chaves BR; Da Silveira DX Treating Drug Dependence with the Aid of Ibogaine: A Retrospective Study. J. Psychopharmacol, 2014, 28, 993–1000. [DOI] [PubMed] [Google Scholar]
- 169.He DY; McGough NN; Ravindranathan A; Jeanblanc J; Logrip ML; Phamluong K; Janak PH; Ron D Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J. Neurosci, 2005, 25, 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Marton S; González B; Rodríguez-Bottero S; Miquel E; Martínez-Palma L; Pazos M; Prieto JP; Rodríguez P; Sames D; Seoane G; Scorza C; Cassina P; Carrera I Ibogaine Administration Modifies GDNF and BDNF Expression in Brain Regions Involved in Mesocorticolimbic and Nigral Dopaminergic Circuits. Front. Pharmacol, 2019, 10, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Olson DE Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. of Exp. Neurosci, 2018, 12, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baumann MH; Pablo JP; Ali SF; Rothman RB; Mash DC Noribogaine (12-Hydroxyibogamine): A Biologically Active Metabolite of the Antiaddictive Drug Ibogaine. Ann. N. Y. Acad. Sci, 2000, 914, 354–368. [DOI] [PubMed] [Google Scholar]
- 173.Ly C; Greb AC; Cameron LP; Wong JM; Barragan EV; Wilson PC; Burbach KF; Soltanzadeh Zarandi S; Sood A; Paddy MR; Duim WC; Dennis MY; McAllister AK; Ori-McKenney KM; Gray JA; Olson DE Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep., 2018, 23, 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bogenschutz MP; Johnson MW Classic hallucinogens in the treatment of addictions. Prog. Neuropsychopharmacol. Biol. Psychiatry, 2016, 64, 250–258. [DOI] [PubMed] [Google Scholar]
- 175.Carnicella S; He DY; Yowell QV; Glick SD; Ron D Noribogaine, but not 18-MC, exhibits similar actions as ibogaine on GDNF expression and ethanol self-administration. Addict. Biol, 2010, 15, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.