Abstract
While temporal lobe epilepsy (TLE) is a focal epilepsy, previous work demonstrates that TLE causes widespread brain-network disruptions. Impaired visuospatial attention and learning in TLE may be related to thalamic arousal nuclei connectivity. Our prior preliminary work in a smaller patient cohort suggests that patients with TLE demonstrate abnormal functional connectivity between central lateral (CL) thalamic nucleus and medial occipital lobe. Others have shown pulvinar connectivity disturbances in TLE, but it is incompletely understood how TLE affects pulvinar subnuclei. Also, the effects of epilepsy surgery on thalamic functional connectivity remains poorly understood. In this study, we examine the effects of TLE on functional connectivity of two key thalamic arousal-nuclei: lateral pulvinar (PuL) and CL. We evaluate resting-state functional connectivity of the PuL and CL in 40 patients with TLE and 40 controls using fMRI. In 25 patients, postoperative images (≥1 year) were also compared with preoperative images. Compared to controls, patients with TLE exhibit loss of normal positive connectivity between PuL and lateral occipital lobe (p<0.05), and a loss of normal negative connectivity between CL and medial occipital lobe (p<0.01, paired t-tests). FMRI amplitude of low frequency fluctuation (ALFF) in TLE trended higher in ipsilateral PuL (p=0.06) but was lower in the lateral occipital (p<0.01) and medial occipital lobe in patients versus controls (p<0.05, paired t-tests). More abnormal ALFF in the ipsilateral lateral occipital lobe is associated with worse preoperative performance on Rey Complex Figure Test Immediate (p<0.05, r=0.381) and Delayed scores (p<0.05, r=0.413, Pearson’s Correlations). After surgery, connectivity between PuL and lateral occipital lobe remains abnormal in patients (p<0.01), but connectivity between CL and medial occipital lobe improves and is no longer different from control values (p>0.05, ANOVA, post-hoc Fischer’s LSD). In conclusion, thalamic arousal nuclei exhibit abnormal connectivity with occipital lobe in TLE, and some connections may improve after surgery. Studying thalamic arousal centers may help explain distal network disturbances in TLE.
Keywords: Temporal lobe epilepsy, pulvinar, central lateral nucleus, functional connectivity, ALFF, visuospatial attention
1. Introduction
Approximately 1% of the global population suffers from epilepsy [1]. The most common type of focal epilepsy is temporal lobe epilepsy (TLE), and many patients with medically refractory seizures seek additional treatments, such as surgical removal of the seizure onset zone [2]. Although TLE is a focal epilepsy, it has been demonstrated to have widespread effects throughout the brain [3-7]. These abnormalities, such as reduced widespread hypometabolism [4], white matter structural connectivity [5], cortical thinning uncharacteristic of age [6], and deficits in neurocognitive functioning associated with extra-temporal structures [7], cannot be explained by temporal networks alone [8]. Thus, we have proposed that recurrent focal seizures may alter activity of structures in the subcortical arousal networks [9,10] such as the thalamus.
In patients with TLE, thalamic nuclei have been shown to have decreased volume [11], altered functional connectivity [12] interictally, and are involved in seizure propagation ictally [13]. Preliminary neuroimaging work by our group has suggested that the central lateral nucleus (CL), an intralaminar arousal nucleus, has altered functional connectivity with the medial occipital lobe in TLE [14]. Some other groups have evaluated functional connectivity within other thalamic nuclei important for arousal, such as the pulvinar, and have found alterations in TLE [15]. As the largest thalamic nucleus, the pulvinar is primarily involved in visual attention and is structurally connected with mesial temporal regions and the occipital lobe [16,17]. It has been demonstrated that visual attention is correlated with thalamo-occipital network activity in healthy individuals [18]. Some studies of TLE have shown that the pulvinar has altered connectivity with mesial temporal structures such as the hippocampus and parahippocampus [19-21]. One investigation demonstrated that the dorsolateral pulvinar has altered connectivity with large areas of the posterior quadrant [22], but prior studies have not evaluated in detail connectivity between pulvinar and occipital lobe in TLE.
As the pulvinar and CL are both related to awareness and visuospatial attention [16,23], which is affected in people with TLE [24], we seek to evaluate and contrast the relationship between these thalamic nuclei and the occipital lobe. In the present study, we use two fMRI measures, functional connectivity and amplitude of low frequency fluctuations (ALFF), to examine thalamo-occipital connectivity in TLE patients compared to controls. This investigation builds upon our preliminary work examining CL connectivity in a smaller patient cohort [14]. We also seek to understand potential relationships between these fMRI measures and neurocognitive test scores relevant to visual spatial attention and memory. Finally, we explore how thalamo-occipital fMRI connectivity patterns changes after epilepsy surgery.
2. Materials and Methods
2.1. Participants
Participants included 40 adult patients with mesial TLE who were evaluated for epilepsy surgery between 2012 to 2019 at Vanderbilt University Medical Center. Diagnosis of mesial TLE was established using standard clinical care at our institution which included epilepsy evaluation by neurologists, neurosurgeons, neuropsychologists, and other physicians. Evaluations included obtaining detailed patient history, MRI, neuropsychological testing, positron emission tomography, seizure semiology analysis, video electroencephalography, and localization of language and/or memory by fMRI and/or Wada test. Of these individuals, 39 patients proceeded with epilepsy surgery, and 25 of these patients underwent an additional research MRI 81.2±40.4 (mean±standard deviation) months after surgery (Table 1). The 40 healthy control participants were matched to patients (Table 1) individually using sex and age (typically ±3, maximum ±5 years). Written informed consent was obtained from all participants prior to participation in the study, and all procedures were approved by the Vanderbilt University Institutional Review Board.
Table 1.
Demographics of participants. Data for continuous variables are represented as mean ± standard deviation and paired t-tests were used for statistical testing. Data for categorical variables are shown as N (%) and chi-square tests were used for statistical testing. N=40 TLE patients and 40 controls.
| Patients | Controls | P Value | |
|---|---|---|---|
| Age, Years | 38.5 ± 11.9 | 38.5 ± 12.1 | 0.99 |
| Gender, female | 21 (52.5%) | 21 (52.5%) | 0.99 |
| Epilepsy duration, years | 21.1 ± 14.8 | ||
| Seizure frequency, monthly | |||
| Focal Aware Seizures | 9.1 ± 33.2 | ||
| Focal Impaired Awareness Seizures | 6.4 ± 9.4 | ||
| Focal to Bilateral Tonic-Clonic Seizures | 0.6 ± 1.6 | ||
| History of Tonic-Clonic Seizures, yes | 22 (55.0%) | ||
| Epileptogenic side, right | 30 (75.0%) | ||
| Mesial temporal sclerosis on MRI, yes | 27 (67.5%) | ||
| Type of Epilepsy Surgery | |||
| Selective Amygdalohippocampectomy | 29 | ||
| Anterior Temporal Lobectomy | 9 | ||
| Responsive Neurostimulation | 1 | ||
| Time between Surgery and Post-op MRI, months | 31.7 ± 15.8 |
2.2. Imaging
Data was obtained using Philips Achieva 3T MRI scanner (Philips Healthcare, Best, Netherlands) with 32-channel head coil. Imaging acquired consisted of (1) three-dimensional T1-weighted whole-brain images for normalization between participants and tissue segmentation (gradient echo, repetition time (TR)=9.1 ms, echo time (TE)=4.6 ms, 192 shots, flip-angle=8.0°, matrix=256×256, 1.0×1.0×1.0 mm3), (2) two-dimensional, T1-weighted axial images for functional to structural image coregistration (1.0×1.0×4.0 mm3), (3) two 10-minute, T2*-weighted blood oxygenation level dependent (BOLD) resting-state fMRI with eyes closed (field of view (FOV)=240.0 mm, TE=35.0 ms, TR=2.0 s, 34 axial slices, slice thickness=3.50 mm/0.50 mm gap, matrix=80×80, 3.0×3.0×4.0 mm3), with 300 volumes acquired per each 10 min acquisition [3]. Prior to acquisition of fMRI, all subjects were instructed to lay at rest with their eyes closed and not fall asleep. Acquisition of physiological signals, cardiac and respiratory rates, occurred at 500 Hz.
2.3. Regions of interest (ROIs)
Subject specific atlases [25] were created for the thalami of each subject using an active shape model [26] to segment the thalamus into 23 bilateral nuclei (46 total) based on the Morel stereotactic atlas [27] from T1 MRI (Fig. 1). These nuclei include the intralaminar thalamic nuclei, of which the largest is CL, and pulvinar subnuclei. Of note, in the Morel stereotactic atlas, the 4 pulvinar subregions are divided into three masks which includes a mask of the anterior pulvinar (PuA), lateral pulvinar (PuL) and a combined mask of the inferior and medial pulvinar (PuI/PuM) [27]. The active shape model used to segment the thalamic nuclei for each subject has been validated in a cohort of healthy controls [25]. Additionally, to confirm that the signal to noise ratio (SNR) was comparable between these small ROIs and a more commonly used larger ROI, the SNR was measured in each thalamic nuclei of interest and was compared to the whole mask of the thalamus from the Harvard-Oxford atlas. The correlation of fMRI signal of voxels within a region was also calculated within each thalamic nucleus and compared to the whole mask of the thalamus from the Harvard-Oxford atlas. This was measured by finding the Pearson’s correlation of each voxel pair within the region and then calculating the mean Pearson’s correlation for all voxels within an ROI.
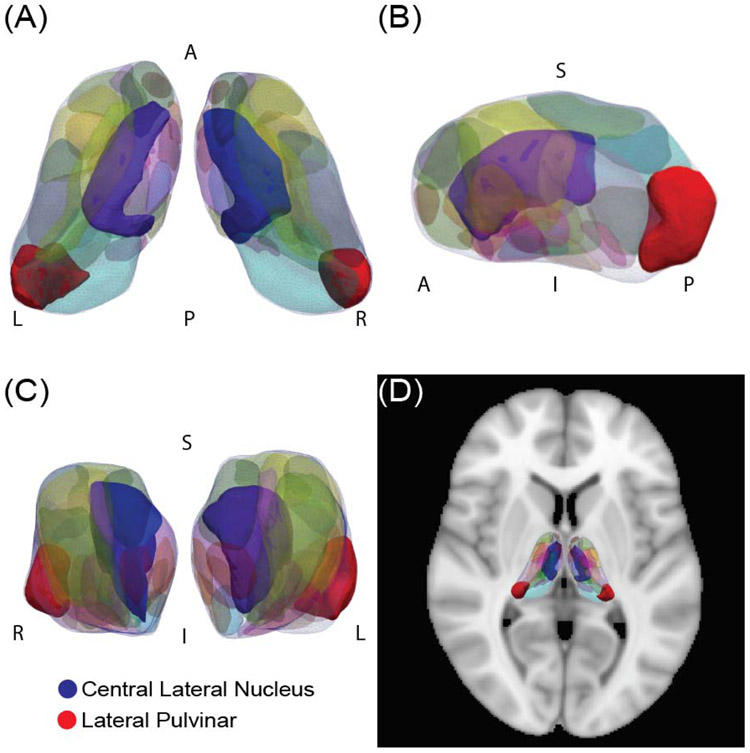
Fig. 1. Segmentation of the thalamus.
(A) A superior view, (B) lateral view, and (C) anterior view of the segmented thalamus are shown, as well as (D) the thalamus segmentation overlaid on an axial brain. Two nuclei of interest, the central lateral nucleus and the lateral pulvinar, are highlighted.
In addition, regions of the occipital lobe were defined using the Harvard-Oxford Atlas (http://www.fmrib.ox.ac.uk/fsl). Medial occipital lobe ROIs included cuneal cortex, intracalcarine cortex, lingual gyrus, occipital fusiform gyrus, occipital pole, and supracalcarine cortex. Lateral occipital lobe ROIs included the inferior lateral occipital cortex and the superior lateral occipital cortex. Analyses were performed on regions defined as ipsilateral or contralateral to the epileptogenic side.
2.4. Functional connectivity analysis
FMRI data was preprocessed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and MATLAB 2017a (The MathWorks, Natick, MA, USA). The preprocessing pipeline included slice-timing correction, motion correction, correction for physiological (cardiac and respiratory) noise using RETROspective Image CORrection [28], segmentation into cerebrospinal fluid and white and grey matters, and spatial normalization with the Montreal Neurological Institute template. Coregistration and normalization of fMRI with T1 MRI to the cortical/subcortical atlas was done using SPM. A band-pass filter of 0.0067 to 0.1 Hz was used. For region-wise analyses, functional connectivity was calculated between ROIs mentioned in section 2.3 by partial Pearson correlation between the time series of each region for both fMRI sessions for each participant. Confounds included six motion time series (three measures of translation: x, y, and z and three measures of rotation: roll, pitch, and yaw) and mean white matter BOLD signals. The Fisher z-transformation was used to transform Pearson correlations for each participant and the mean was calculated between both fMRI sessions. In addition, data visualization and voxel-wise analyses on fully preprocessed data were performed using the CONN toolbox 17 (https://www.nitrc.org/projects/conn/) [29]. For these analyses, images of patients’ functional connectivity were arranged according to side of epileptogenicity, and images of matched controls were arranged accordingly.
2.5. Amplitude of low-frequency fluctuations measurements
To improve understanding of the differences in fMRI signals in the thalamo-occipital regions, we calculated Amplitude of Low-Frequency Fluctuations (ALFF) in the thalamic nuclei and the occipital lobes and compared patients to controls. ALFF can be used to determine the power of the spontaneous low frequency fluctuations of the BOLD signal of the individual regions and may contribute to altered functional connectivity. As previous work has demonstrated that ALFF in slow frequency bands (<0.032 Hz) is altered in the pulvinar in patients with TLE [30], we chose to focus on the slow-5 frequency band (0.01-0.0267 Hz) for ALFF measurements [31].
Preprocessing of fMRI occurred as described in section 2.4. ALFF was measured by converting the time series BOLD signal to the frequency domain with the Fourier transform function in MATLAB. Next, the averaged square root of the absolute value of the transformed signal in the frequency band was measured and divided by the mean ALFF of the brain (equation 1).
| (1) |
2.6. Thalamus volume
To measure overall thalamus volume, the multiatlas approach was used, as described previously [32]. Upon visual inspection of each parcellation, no obvious defects were found. The thalamic volume was calculated.
2.7. Epilepsy disease measures and neuropsychological testing
Clinical assessments of epileptologists were used to determine subject demographics and disease measures in patients, such as seizure frequency, duration of epilepsy, history of focal to bilateral tonic-clonic (secondarily generalized) seizures, side of epileptogenicity, and imaging evidence of mesial temporal sclerosis (Table 1). Designation of seizure outcomes using Engel classification [33] occurred at the time of postoperative imaging, 31.7±15.8 (mean±Standard Deviation) months after surgery.
Preoperative neuropsychological testing for patients was administered by a licensed neuropsychologist. As the thalamic nuclei of interest are involved in visuospatial attention processes, we related altered functional connectivity between patients and controls with the Rey Complex Figure Test Immediate (RCFT-I) and the Rey Complex Figure Test Delayed (RCFT-D) in which performance is related to visuospatial attention and memory function. Two patients who did not receive these specific tests were excluded from this analysis.
2.8. Statistical analyses
Parametric tests were applied for normally distributed data, as defined using the Anderson-Darling test. Comparisons of the demographics between preoperative patients and controls were performed using paired t-tests for continuous variables and χ2 for categorical variables. Paired t-tests with post-hoc Bonferroni-Holm correction for multiple comparisons were used to compare functional connectivity and ALFF between preoperative patients and their matched controls, where stated. Comparison of functional connectivity and ALFF between preoperative patients, postoperative patients, and controls was done using analysis of variance (ANOVA), with post-hoc Fischer’s Least Significant Difference (LSD) procedure. Pearson’s correlations were employed to evaluate functional connectivity, ALFF, disease measures, neuropsychological testing, and thalamic volume. MATLAB 2019b and SPSS V.23 (Armonk, NY, USA) were used for statistical analyses. For all tests, significance was defined as p<0.05.
3. Results
3.1. Patients with TLE have altered functional connectivity in thalamo-occipital networks
Prior to evaluating functional connectivity, for quality assurance, we measured the SNR of fMRI signals and demonstrated that the SNR of individual thalamic nuclei of interest on each side of the brain were comparable or greater than the SNR of the commonly used thalamus mask from the Harvard-Oxford atlas (data not shown). In addition, we also measured the correlation of the fMRI signal within the voxels of each thalamic nuclei [0.39-0.59, range of mean Pearson correlation] and compared to the whole thalamus [0.49-0.59] and found these values to be in line with those of previous studies [34].
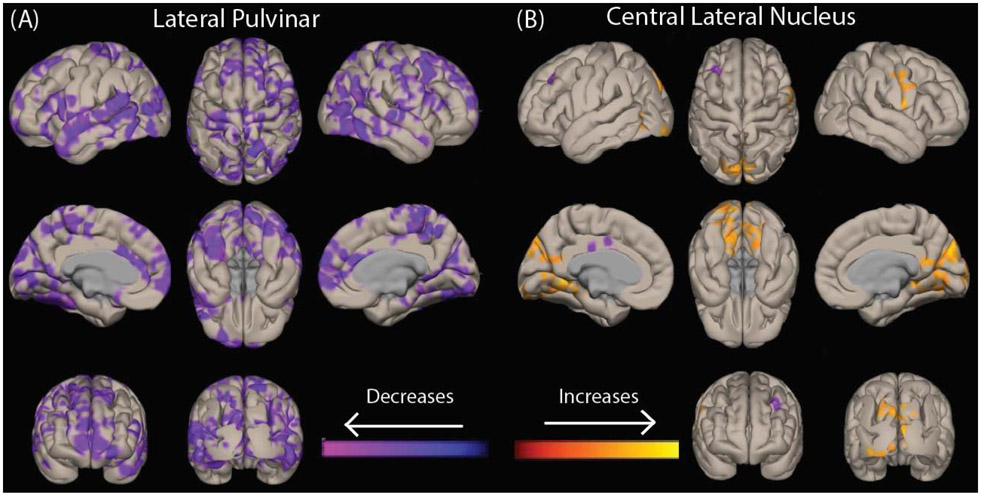
We then performed voxel-wise analyses to examine functional connectivity differences between preoperative patients and controls seeded from three pulvinar nuclei (PuL, PuA, and PuI/PuM) and CL. We observed decreases in connectivity between PuL and widespread bilateral neocortical areas in patients vs. controls, with differences most prominent in the lateral occipital lobe (Fig. 2a). We also saw increases in functional connectivity between CL and the bilateral medial occipital lobe in patients compared to controls (Fig. 2b). Of note, we also saw smaller decreases in connectivity between PuA and the bilateral superior temporal neocortex and between PuI/PuM and the bilateral medial frontal cortex (not shown), but no significant connectivity differences between PuA and PuI/PuM and the occipital lobe were observed. Given our specific interest in thalamo-occipital connectivity in the present study, we focused on PuL and CL for the remainder of the study.
Fig. 2. Patients with temporal lobe epilepsy have different patterns of abnormal functional connectivity in key thalamic arousal nuclei.
Voxel-wise t-tests of functional connectivity seeded from bilateral lateral pulvinar (A) and central lateral nucleus (B) are shown in 40 patients vs 40 controls. Data for patients with right-sided epilepsy and their corresponding matched controls are flipped, so that changes ipsilateral to the epileptogenic side are shown on the left while contralateral changes are seen on the right side of the brain. Images are corrected for false discovery rate (FDR), and a cluster correction (p<0.05) is applied.
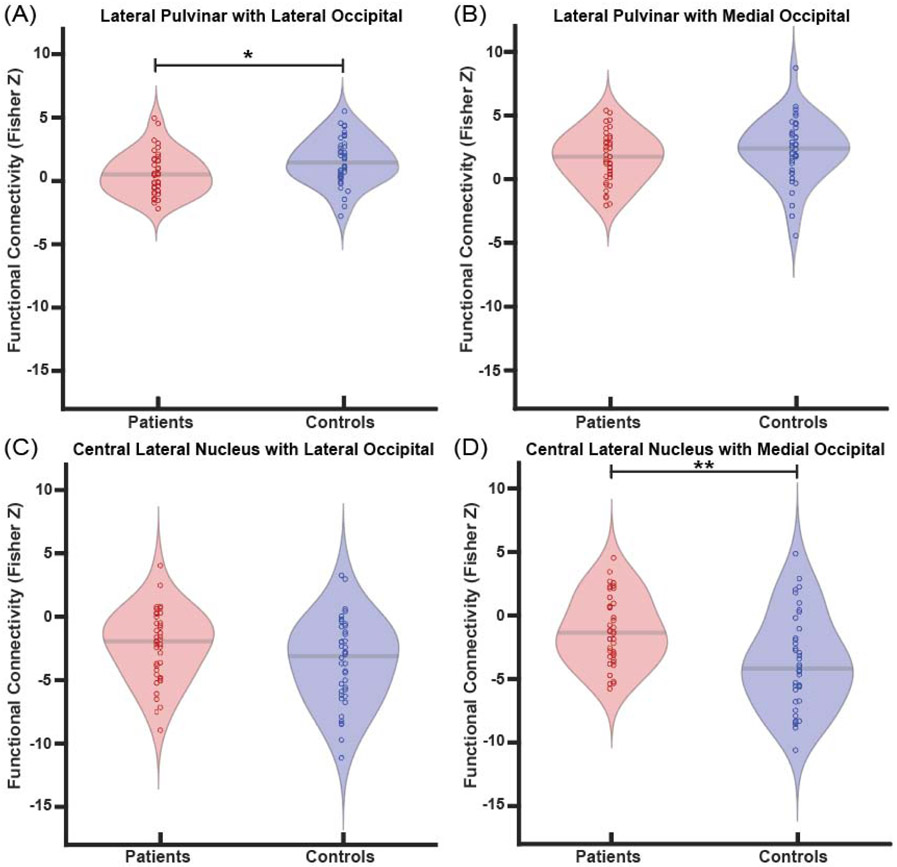
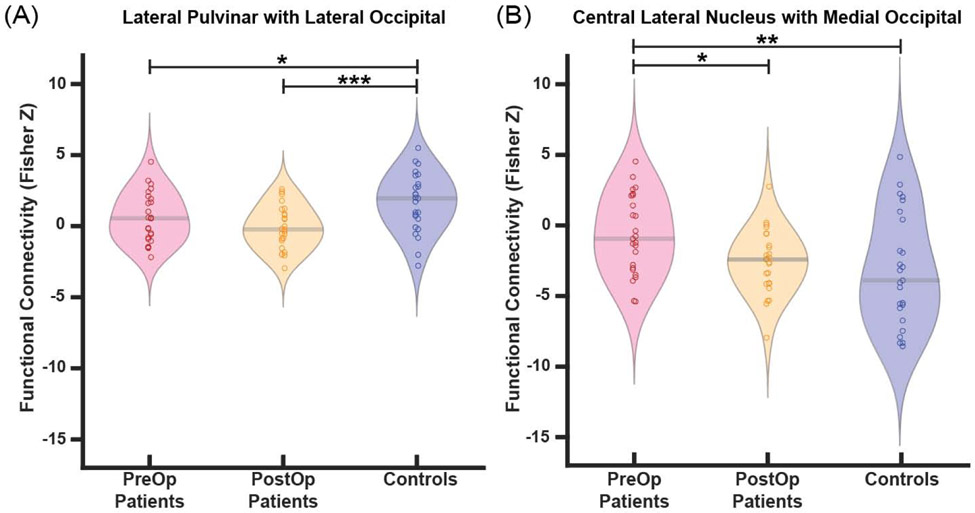
Next, we more closely examined abnormal thalamo-occipital functional connectivity of PuL and CL using region-wise analyses. In patients, we saw less positive functional connectivity between the PuL and the lateral occipital lobe compared to controls (p=0.02, paired t-tests with Bonferroni-Holm correction) (Fig. 3a), however, there were no differences between PuL and the medial occipital lobe (p=0.14) (Fig. 3b). Less negative functional connectivity was found between CL and the medial occipital lobe (p=0.01) in patients versus controls (Fig. 3d). No differences were seen between CL and the lateral occipital lobe (p=0.11) (Fig. 3c). Of note, no connectivity differences in patients vs. controls were seen between PuA or PuI/PuM and the medial or lateral occipital lobes (not shown). These results suggest differing patterns of thalamo-occipital connectivity alterations involving PuL and CL in patients vs. controls.
Fig. 3. Patients exhibit altered functional connectivity between thalamic arousal nuclei and regions of occipital lobe.
Patients demonstrate reduced connectivity between the lateral pulvinar and lateral occipital lobe compared to controls (A), but no differences in connectivity between the lateral pulvinar and medial occipital lobe (B). When examining central lateral nucleus connectivity, no connectivity differences to the lateral occipital lobe are seen (C), but connectivity to the medial occipital lobe is less negative in patients compared to controls (D). *p<0.5, **p<0.01, Bonferroni-Holm correction. N=40 patients with TLE vs 40 controls.
We also individually examined the functional connectivity between the PuL and CL with the lateral and medial occipital lobe, respectively, and in the ipsilateral and contralateral thalamic nuclei. These results demonstrated similar findings to the analysis with combined bilateral thalamic nuclei. Differences in thalamo-occipital connectivity were seen between patients (mean=0.69±1.69, mean±SD) versus controls (mean=1.6±2.7) between the ipsilateral PuL and lateral occipital lobe (p=0.05, uncorrected) and in patients (mean=0.43±2.6) versus controls (mean=1.48±2) between the contralateral PuL and lateral occipital lobe (p=0.04, uncorrected). Additionally, we found differences in connectivity in patients (mean=−1.64±2.8) versus controls (mean=−4.1±4) between the ipsilateral CL and medial occipital lobe (p<0.01, uncorrected) and in patients (mean=−0.86±3) versus controls (mean=−3.3±3.7) between the contralateral CL and medial occipital lobe (p<0.01, uncorrected).
3.2. Altered ALFF in patients with TLE
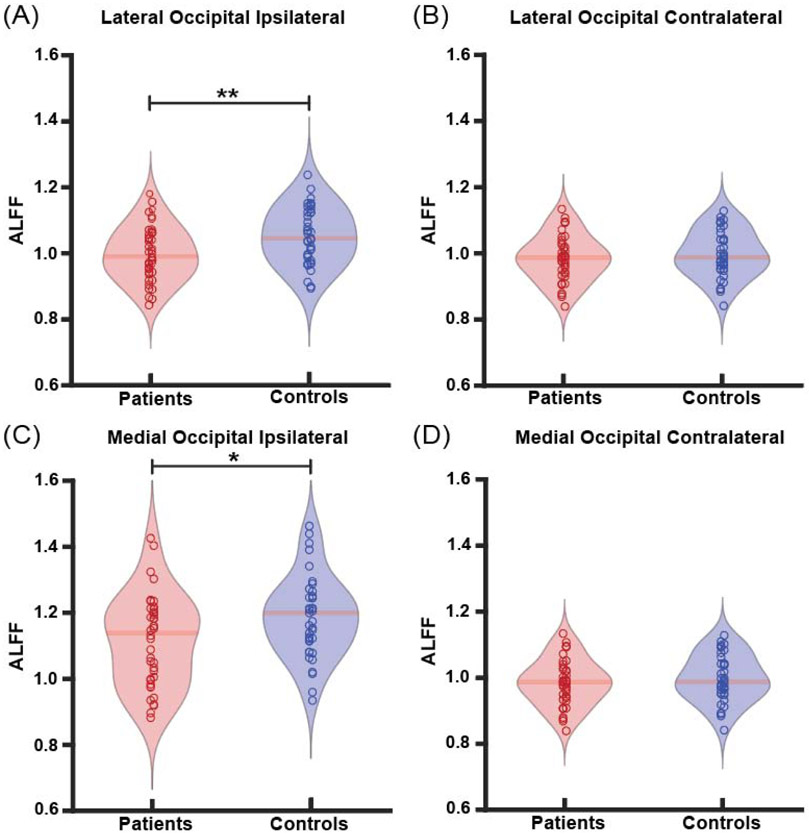
Whereas connectivity measurements may help probe aberrant functional connections between two structures, ALFF measurements can identify abnormalities in BOLD signal fluctuations in individual structures, which may provide insight about the driving forces of connectivity alterations. Given that we observed abnormal functional connections between two key thalamic arousal nuclei (PuL and CL) and the lateral and medial occipital lobe, respectively, we then measured ALFF in these four regions in patients and controls, both ipsilateral and contralateral to the epileptogenic side. We found that patients showed decreased ALFF in the ipsilateral lateral occipital lobe compared to controls (p<0.01, paired t-tests with Bonferroni-Holm correction) (Fig. 4a). Additionally, we found that patients showed decreased ALFF in the ipsilateral medial occipital lobe compared to controls (p=0.03) (Fig. 4c). No differences in ALFF in patients vs. controls were seen in the contralateral lateral occipital lobe (p=0.41) (Fig. 4b) or the contralateral medial occipital lobe (p=0.48) (Fig. 4d).
Fig. 4. Patients demonstrate altered ALFF in occipital lobe.
A) Patients demonstrate decreased ALFF compared to controls in the ipsilateral lateral occipital lobe, B) but no differences in ALFF in the contralateral lateral occipital lobe. Similarly, there is decreased ALFF between patients and controls in the C) ipsilateral medial occipital lobe, D) but no altered ALFF in the contralateral medial occipital lobe. *p<0.5, **p<0.01, Bonferroni-Holm correction. N=40 patients with TLE vs 40 controls.
ALFF was found to trend somewhat higher in patients (0.97±0.09, mean±SD) compared to controls (0.92±0.1) in the ipsilateral PuL (p=0.06), while no differences were measured in ALFF in the contralateral PuL between patients (0.93±0.12) and controls (0.92±0.1, p=0.73). No ALFF differences between patients or controls were seen in the ipsilateral (p=0.3) or contralateral CL (p=0.99).
Next, given our hypothesis that thalamo-occipital abnormalities may be related to visuospatial attention deficits in TLE, we examined whether ALFF abnormalities seen in ipsilateral occipital lobe or ipsilateral PuL were related to RCFT immediate (−I) or delayed (−D) scores (available in N=38 out of 40 patients). We observed a modest relationship trend between reduced ALFF values in the ipsilateral lateral occipital lobe and lower scores on RCFT-I (p=0.04, r=0.38) and RCFT-D (p=0.03, r=0.41), suggesting that ALFF values further away from those in controls may be associated with somewhat worse performance. We did not see relationships between ALFF in the ipsilateral medial occipital lobe (RCFT-I with ipsilateral medial occipital: p=0.28, r=0.18, RCFT-D with ipsilateral medial occipital: p=0.11, r=0.27) or in the ipsilateral PuL and RCFT scores (RCFT-I with ipsilateral PuL: p=0.06, r=0.73, RCFT-D with ipsilateral PuL: p=0.15, r=0.24). Also, to gain insight into whether abnormal ALFF observed in the lateral occipital lobe and the relationship to RCFT scores might be a progressive phenomenon in TLE, we measured its relationship to duration of disease and noted a modest relationship between longer duration of disease and lower (further from controls) ALFF values (p=0.03, r=−0.38). Overall, these ALFF results suggest possible inherent abnormalities of BOLD signal fluctuations in the occipital lobe and PuL in TLE, the former of which may have a modest relationship to impaired visuospatial attention.
3.3. Patients demonstrate decreased ipsilateral thalamic volume
To determine whether aberrant thalamic connectivity patterns in patients are also accompanied with overall structural abnormalities in the thalamus as a whole, we looked at thalamic volume. Thalamic volume ipsilateral to the epileptogenic side was reduced in patients (6829±791 mm3, mean±SD) compared to controls (7215±764 mm3, p=0.02, paired t-tests with Bonferroni-Holm correction), but contralateral thalamic volume in patients (7039±814 mm3) and controls (7300±827 mm3) did not differ (p=0.34, paired t-tests with Bonferroni-Holm correction). Next, given our prior observations of relationships between seizure frequency and structural abnormalities in other subcortical arousal structures [35], we measured whether ipsilateral thalamic volume in patients was related to frequency of focal impaired awareness seizures or focal aware seizures. While there was no relationship between ipsilateral thalamic volume and frequency of focal impaired awareness seizures (p=0.43, rho=−0.13), increased frequency of focal aware seizures was related to reduced ipsilateral thalamic volume (p<0.01, rho=−0.456, Spearman’s Rho with Bonferroni-Holm correction). This suggests overall abnormalities in ipsilateral thalamic structure that may be related to frequency of seizures without impaired awareness.
3.4. Improvements in functional connectivity after surgery may be seen in CL but not PuL
Next, we evaluated changes in thalamo-occipital functional connectivity and ALFF after surgical treatment of epilepsy. FMRI data were available for 25 patients 31.7±15.8 (mean±SD) months after treatment. Functional connectivity reductions between PuL and the lateral occipital lobe seen in preoperative patients versus controls (p=0.04) did not recover in postoperative patients compared to preoperative baseline (p=0.15), and connectivity after surgery remained different from controls (p<0.001, ANOVA with Fisher’s LSD) (Fig. 5a). However, abnormal connectivity between CL and the medial occipital lobe in patients versus controls (p<0.01) did recover after surgery, with connectivity in postoperative patients trending to be more negative than preoperative baseline connectivity (p=0.027) and no longer demonstrating differences from controls (p=0.52, ANOVA with Fisher’s LSD) (Fig. 5b).
Fig. 5. After epilepsy surgery, thalamo-occipital connectivity of the central lateral nucleus, but not the lateral pulvinar, may improve.
A) In post-operative patients, connectivity between the lateral pulvinar and the lateral occipital lobe is not different than pre-operative patients and remains different from controls. B) Connectivity between the central lateral nucleus and the medial occipital lobe changes in post-operative patients compared to pre-operative baseline, with connectivity in post-operative patients no longer different from controls. *p<0.05, **p<0.01, ANOVA & post-hoc Fischer’s LSD. N=25 patients before surgery, the same 25 patients after surgery, and 25 controls.
Finally, we looked at changes in ALFF values in the ipsilateral lateral occipital lobe and the ipsilateral medial occipital lobe after surgery, since ALFF in these structures were abnormal in preoperative patients. While ALFF in the ipsilateral lateral occipital lobe in postoperative patients (1.06±0.17, mean±SD) was different than in patients before surgery (0.97±0.07, p=0.01), ALFF after surgery recovered towards control values (1.06±0.17) and no longer differed from controls (p=0.87, ANOVA with Fisher’s LSD). Additionally, ALFF in the medial occipital lobe which was different in preoperative patients (1.06±0.12) and controls (1.17±0.14, p=0.03 using all 40 patients, p=0.07 in present subset of 25 patients) recovered towards control values in postoperative patients (1.15±0.26) and values were not different from controls (p=0.81, ANOVA with Fisher’s LSD). Overall, these results suggest that after epilepsy surgery for TLE, some thalamo-occipital network abnormalities may recover toward control network patterns, whereas others do not.
4. Discussion
In this study, we sought to investigate altered connectivity of the thalamic arousal nuclei, PuL and CL, with the occipital lobe, their relationship with visuospatial attention in TLE, and how these functional connections are affected by surgery. We show PuL has less positive functional connectivity with the lateral occipital lobe than controls. Similar to our previous work in a smaller patient cohort [14], we found that in patients with TLE, there is less negative functional connectivity between CL and the medial occipital lobe versus controls. As PuL and CL are both arousal thalamic nuclei, these results support our hypothesis of altered arousal network connectivity in patients with TLE [35,36]. This idea of interictal altered arousal networks is built upon the Network Inhibition Hypothesis, first proposed by Blumenfeld [9], which states that seizures in TLE result in ictal disturbances of activity in subcortical arousal structures [37].
Previously, studies have demonstrated negative correlations between the thalamus and the occipital lobe in resting-state healthy adults using fMRI [38,39]. In our study, we also found negative correlations between CL and the medial occipital lobe, however, we found a positive relationship between PuL and the lateral occipital lobe, indicating that not all thalamic arousal nuclei have a negative correlation with the occipital lobe. Interestingly, both positive and negative thalamo-occipital correlations were disturbed in patients with TLE, suggesting broadly altered thalamo-occipital networks. One method to provide complementary understanding of these altered relationships in the BOLD fMRI is ALFF [40] which has been shown to be correlated with spontaneous neuronal activity [41]. Though it has been demonstrated that people with TLE have increased ALFF of the ipsilateral thalamus [42], the specific intrathalamic nuclei contributing to this alteration have not been previously studied to the best of our knowledge. Our work suggests that ALFF may trend towards increased in some intrathalamic nuclei, such as PuL, but is not altered in others, such as CL. Previously, it has been suggested that altered ALFF in one region may drive the functional connectivity between two regions [36,43]. To deepen the understanding of which regions drive changes in functional connectivity, future work could focus on evaluating effective functional connectivity to determine the causal relationship between these structures [44].
Some of the cognitive deficits in people with TLE cannot be explained by altered activity of the mesial temporal regions alone, thus suggesting these regions may have a widespread effect on other cortical regions [8]. As visuospatial attention is affected in people with TLE [24], we evaluated the relationship between altered ALFF activity of the thalamic nuclei and occipital lobe, regions involved in visual tasks [16,23,45], with scores on neuropsychiatric tests that measure visuospatial attention and memory. We found that reduced power in the ALFF of the ipsilateral lateral occipital lobe was correlated with lower scores on both the RCFT-I and RCFT-D. To the best of our knowledge, this is the first time a relationship has been found between activity of the ipsilateral lateral occipital lobe and the RCFT in patients with TLE, supporting the hypothesis that epilepsy has widespread effects on regions beyond the epileptic zones. Additionally, decreased thalamic volume of the ipsilateral thalamus is commonly seen in patients with TLE [11,46,47] which we validated in this cohort of patients. We also found that reduced thalamic volume is correlated with increased frequency of consciousness-sparing seizures in a larger cohort, which contrasts with previous relationships we have reported between brainstem arousal networks and frequency of consciousness-impairing seizures in TLE [35].
Study of the effects of surgical treatment of epilepsy on functional connectivity in patients with TLE is a growing field. Previously, we saw connectivity improvements in certain brainstem arousal networks after TLE surgery [14]. Our work here suggests that while some functional relationships in thalamic arousal nuclei may improve after surgery (e.g. between CL and the medial occipital lobe), others may not (e.g. between PuL and the lateral occipital lobe). Unlike our functional connectivity results, we demonstrated that ALFF may recover towards control values in both the ipsilateral lateral occipital lobe and the ipsilateral medial occipital lobe, suggesting ALFF values of occipital regions may improve after epilepsy surgery. While to the best of our knowledge, no other groups have studied postoperative ALFF in the occipital lobe of patients with TLE, previous work by our group and others have demonstrated that ALFF does change in some brain regions after epilepsy surgery [36,48]. Together, these findings suggest that changes in postoperative ALFF vary between regions and that ALFF should be further studied to better interpret postoperative outcomes. Overall, network studies may help identify novel neuromodulation targets for epilepsy and other neurological disorders. Interestingly, CL has previously been explored as a neurostimulation target for traumatic brain injury [49], has been studied in rat models of epilepsy [50], and will soon be targeted in human epilepsy patients in an active clinical trial.
A limitation of this work is that postoperative neuropsychological testing data was not available in our patient cohort and, as such, postoperative visuospatial attention scores could not be correlated with postoperative fMRI. Additionally, our TLE group is not homogenous as our patients have had two types of epilepsy surgery, not all patients have evidence of mesial temporal sclerosis, and a history of focal to bilateral tonic-clonic seizures is not seen in all individuals. As such, our results may not be consistently seen in other TLE cohorts. Future work should include a larger cohort in which subgroup analysis can be performed. In addition, during the acquisition of resting-state fMRI, participants are asked to lie awake with their eyes closed, however, we cannot be certain that participants remain awake during the scans and this may impact functional connectivity measurements. Furthermore, visual identification of the thalamic nuclei using standard 3T MRI imaging is challenging, and these small regions may be more susceptible to motion artifact. Despite this, patient-specific masks of the thalamic nuclei were ascertained using active shape models that were fit to each patient. Despite these limitations, we found altered thalamo-occipital network behavior between PuL and CL with the occipital lobe.
5. Conclusions
In summary, this work evaluates functional connectivity of the thalamo-occipital network focusing on thalamic arousal nuclei PuL and CL. This is one of the first studies to demonstrate altered functional connectivity of PuL in TLE. Additionally, we demonstrated that there are different patterns of functional connectivity within thalamic nuclei and that they have altered ALFF on the ipsilateral side of seizures. Altered ALFF in the occipital lobe was demonstrated to be correlated with altered scores on visuospatial attention examinations. Finally, functional connectivity between thalamic nuclei and the occipital lobe may improve for some thalamic nuclei after successful epilepsy surgery, but not others. Further studies of the thalamic arousal nuclei in people with TLE may increase our understanding of how these regions are affected in this disorder, clarify the relationship of these regions with cognitive deficits, and assist in the identification of targets for neuromodulation for people with epilepsy.
Highlights:
In TLE, functional connectivity of PuL with lateral occipital lobe is less positive
Functional connectivity of CL with medial occipital lobe is less negative
TLE has altered ALFF in ipsilateral PuL, and the lateral and medial occipital lobe
Altered occipital lobe ALFF correlates with lower scores on Rey Complex Figure Test
After epilepsy surgery, functional connectivity of CL, but not PuL, improves
Acknowledgements
The authors would like to thank the subjects for volunteering to participate in this study. The authors also thank Srijata Chakravorti and Benoit Dawant for assistance generating participant specific thalamic masks. The authors thank Harvard Center for Morphometric Analysis (Harvard-Oxford Atlas) and McGovern Institute for Brain Research (CONN toolbox) for atlases and toolboxes.
Funding sources
This work was supported by National Institutes of Health grants F31 NS106735 (HFJG), R00 NS097618 (DJE), R01 NS108445 (VLM), R01 NS110130 (VLM), R01 NS112252 (DJE), T32 EB001628- 17 (SN), T32 EB021937 (HFJG), T32 GM07347 (HFJG, GWJ), and the Vanderbilt Institute for Surgery and Engineering (VISE).
Abbreviations
- TLE
temporal lobe epilepsy
- CL
central lateral nucleus
- ALFF
amplitude of low frequency fluctuations
- BOLD
blood oxygenation level dependent
- ROI
regions of interest
- PuA
anterior pulvinar
- PuL
lateral pulvinar
- PuI/PuM
inferior and medial pulvinar
- RCFT-I
Rey Complex Figure Test Immediate
- RCFT-D
Rey Complex Figure Test Delayed
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
None of the authors have competing interests to disclose.
References
- [1].Beghi E The Epidemiology of Epilepsy. Neuroepidemiology 2020;54:185–91. 10.1159/000503831. [DOI] [PubMed] [Google Scholar]
- [2].Asadi-Pooya AA, Stewart GR, Abrams DJ, Sharan A. Prevalence and Incidence of Drug-Resistant Mesial Temporal Lobe Epilepsy in the United States. World Neurosurg 2017;99:662–6. 10.1016/j.wneu.2016.12.074. [DOI] [PubMed] [Google Scholar]
- [3].Englot DJ, D’Haese PF, Konrad PE, Jacobs ML, Gore JC, Abou-Khalil BW, et al. Functional connectivity disturbances of the ascending reticular activating system in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2017;88:925–32. 10.1136/jnnp-2017-315732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chassoux F, Artiges E, Semah F, Desarnaud S, Laurent A, Landre E, et al. Determinants of brain metabolism changes in mesial temporal lobe epilepsy. Epilepsia 2016;57:907–19. 10.1111/epi.13377. [DOI] [PubMed] [Google Scholar]
- [5].Tsuda K, Tsuji T, Ishida T, Takahashi S, Yamada S, Ohoshi Y, et al. Widespread abnormalities in white matter integrity and their relationship with duration of illness in temporal lobe epilepsy. Epilepsia Open 2018;3:247–54. 10.1002/epi4.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Galovic M, Van Dooren VQH, Postma TS, Vos SB, Caciagli L, Borzì G, et al. Progressive Cortical Thinning in Patients with Focal Epilepsy. JAMA Neurol 2019;76:1230–9. 10.1001/jamaneurol.2019.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Englot DJ, Konrad PE, Morgan VL. Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia 2016;57:1546–57. 10.1111/epi.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aparicio J, Carreño M, Bargalló N, Setoain X, Rubí S, Rumià J, et al. Combined 18F-FDG-PET and diffusion tensor imaging in mesial temporal lobe epilepsy with hippocampal sclerosis. NeuroImage Clin 2015;12:976–89. 10.1016/j.nicl.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Englot DJ, Blumenfeld H. Consciousness and epilepsy: why are complex-partial seizures complex? Prog Brain Res 2009;177:147–70. 10.1016/S0079-6123(09)17711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Englot DJ, Morgan VL, Chang C. Impaired vigilance networks in temporal lobe epilepsy: Mechanisms and clinical implications. Epilepsia 2020;61:189–202. 10.1111/epi.16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bernhardt BC, Bernasconi N, Kim H, Bernasconi A. Mapping thalamocortical network pathology in temporal lobe epilepsy. Neurology 2012;78:129–36. 10.1212/WNL.0b013e31823efd0d. [DOI] [PubMed] [Google Scholar]
- [12].Doucet GE, He X, Sperling M, Sharan A, Tracy JI. Gray Matter Abnormalities in Temporal Lobe Epilepsy: Relationships with Resting-State Functional Connectivity and Episodic Memory Performance. PLoS One 2016;11:e0154660 10.1371/journal.pone.0154660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Guye M, Régis J, Tamura M, Wendling F, Gonigal AM, Chauvel P, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain 2006;129:1917–28. 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- [14].González HFJ, Chakravorti S, Goodale SE, Gupta K, Claassen DO, Dawant B, et al. Thalamic arousal network disturbances in temporal lobe epilepsy and improvement after surgery. J Neurol Neurosurg Psychiatry 2019. 10.1136/jnnp-2019-320748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jo HJ, Kenny-Jung DL, Balzekas I, Benarroch EE, Jones DT, Brinkmann BH, et al. Nuclei-specific thalamic connectivity predicts seizure frequency in drug-resistant medial temporal lobe epilepsy. Neuroimage Clin 2019;21:101671 10.1016/j.nicl.2019.101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Benarroch EE. CLINICAL IMPLICATIONS OF NEUROSCIENCE RESEARCH Section Editor Pulvinar Associative role in cortical function and clinical correlations. 2015. [DOI] [PubMed] [Google Scholar]
- [17].Saalmann YB, Kastner S. Cognitive and Perceptual Functions of the Visual Thalamus. Neuron 2011;71:209–23. 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Menegaux A, Bäuerlein FJB, Vania A, Napiorkowski N, Neitzel J, Ruiz-Rizzo AL, et al. Linking the impact of aging on visual short-term memory capacity with changes in the structural connectivity of posterior thalamus to occipital cortices. Neuroimage 2020; 208:116440 10.1016/j.neuroimage.2019.116440. [DOI] [PubMed] [Google Scholar]
- [19].Barron DS, Eickhoff SB, Clos M, Fox PT. Human pulvinar functional organization and connectivity. Hum Brain Mapp 2015;36:2417–31. 10.1002/hbm.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen C, Li H, Ding F, Yang L, Huang P, Wang S, et al. Alterations in the hippocampal-thalamic pathway underlying secondarily generalized tonic-clonic seizures in mesial temporal lobe epilepsy: A diffusion tensor imaging study. Epilepsia 2019;60:121–30. 10.1111/epi.14614. [DOI] [PubMed] [Google Scholar]
- [21].Evangelista E, Bénar C, Bonini F, Carron R, Colombet B, Régis J, et al. Does the thalamo-cortical synchrony play a role in seizure termination? Front Neurol 2015; 6 10.3389/fneur.2015.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].He X, Doucet GE, Sperling M, Sharan A, Tracy JI. Reduced thalamocortical functional connectivity in temporal lobe epilepsy. Epilepsia 2015;56:1571–9. 10.1111/epi.13085. [DOI] [PubMed] [Google Scholar]
- [23].Van Der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 2002;39:107–40. 10.1016/S0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- [24].Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol 1997;54:369–76. 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- [25].Chakravorti S, Morgan VL, Trujillo Diaz P, Wirz Gonzalez R, Dawant BM. A structural connectivity approach to validate a model-based technique for the segmentation of the pulvinar complex In: Gimi B, Krol A, editors. Med. Imaging 2018 Biomed. Appl. Mol. Struct. Funct. Imaging, vol. 10578, SPIE; 2018, p. 28 10.1117/12.2293685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu Y, D’Haese PF, Newton AT, Dawant BM. Generation of human thalamus atlases from 7 T data and application to intrathalamic nuclei segmentation in clinical 3 T T1-weighted images. Magn Reson Imaging 2020;65:114–28. 10.1016/j.mri.2019.09.004. [DOI] [PubMed] [Google Scholar]
- [27].Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol 1997;387:588–630. . [DOI] [PubMed] [Google Scholar]
- [28].Glover GH, Li T, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 2000;44:162–7. . [DOI] [PubMed] [Google Scholar]
- [29].Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect 2012;2:125–41. 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- [30].Morgan VL, Rogers BP, Abou-Khalil B. Segmentation of the thalamus based on BOLD frequencies affected in temporal lobe epilepsy. Epilepsia 2015;56:1819–27. 10.1111/epi.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: Complex and reliable. Neuroimage 2010;49:1432–45. 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Asman AJ, Landman BA. Non-local statistical label fusion for multi-atlas segmentation. Med Image Anal 2013;17:194–208. 10.1016/j.media.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Engel J, Pedley TA. Epilepsy : a comprehensive textbook. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- [34].Korhonen O, Saarimäki H, Glerean E, Sams M, Saramäki J. Consistency of Regions of Interest as nodes of fMRI functional brain networks. Netw Neurosci 2017;1:254–74. 10.1162/netn_a_00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Englot DJ, Gonzalez HFJ, Reynolds BB, Konrad PE, Jacobs ML, Gore JC, et al. Relating structural and functional brainstem connectivity to disease measures in epilepsy. Neurology 2018;91:E67–77. 10.1212/WNL.0000000000005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].González HFJ, Goodale SE, Jacobs ML, Haas KF, Landman BA, Morgan VL, et al. Brainstem Functional Connectivity Disturbances in Epilepsy may Recover After Successful Surgery. Neurosurgery 2020;86:417–28. 10.1093/neuros/nyz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci 2009;29:13006–18. 10.1523/JNEUROSCI.3846-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zou Q, Long X, Zuo X, Yan C, Zhu C, Yang Y, et al. Functional connectivity between the thalamus and visual cortex under eyes closed and eyes open conditions: A resting-state fMRI study. Hum Brain Mapp 2009;30:3066–78. 10.1002/hbm.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Goldman RI, Stern JM, Engel J Jr, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 2002;13:2487–92. 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, et al. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage 2007;36:144–52. 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- [41].Pelled G, Goelman G. Different physiological MRI noise between cortical layers. Magn Reson Med 2004;52:913–6. 10.1002/mrm.20229. [DOI] [PubMed] [Google Scholar]
- [42].Zhang Z, Lu G, Zhong Y, Tan Q, Chen H, Liao W, et al. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Hum Brain Mapp 2010;31:1851–61. 10.1002/hbm.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ji GJ, Zhang Z, Zhang H, Wang J, Liu DQ, Zang YF, et al. Disrupted Causal Connectivity in Mesial Temporal Lobe Epilepsy. PLoS One 2013;8 10.1371/journal.pone.0063183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stephan KE, Friston KJ. Analyzing effective connectivity with functional magnetic resonance imaging. Wiley Interdiscip Rev Cogn Sci 2010;1:446–59. 10.1002/wcs.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kelley TA, Serences JT, Giesbrecht B, Yantis S. Cortical mechanisms for shifting and holding visuospatial attention. Cerebral Cortex 2008;18:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pulsipher DT, Seidenberg M, Morton JJ, Geary E, Parrish J, Hermann B. MRI volume loss of subcortical structures in unilateral temporal lobe epilepsy. Epilepsy Behav 2007;11:442–9. 10.1016/j.yebeh.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lee HJ, Seo SA, Park KM. Quantification of thalamic nuclei in patients diagnosed with temporal lobe epilepsy and hippocampal sclerosis. Neuroradiology 2020;62:185–95. 10.1007/s00234-019-02299-6. [DOI] [PubMed] [Google Scholar]
- [48].Tang Y, Xia W, Yu X, Zhou B, Luo C, Huang X, et al. Short-term cerebral activity alterations after surgery in patients with unilateral mesial temporal lobe epilepsy associated with hippocampal sclerosis: A longitudinal resting-state fMRI study. Seizure 2017;46:43–9. 10.1016/j.seizure.2016.12.021. [DOI] [PubMed] [Google Scholar]
- [49].Kundu B, Brock A, Englot DJ, Butson CR, Rolston JD. Deep brain stimulation for the treatment of disorders of consciousness and cognition in traumatic brain injury patients: a review. J Neurosurg n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kundishora AJ, Gummadavelli A, Ma C, Liu M, McCafferty C, Schiff ND, et al. Restoring Conscious Arousal During Focal Limbic Seizures with Deep Brain Stimulation 2016. 10.1093/cercor/bhw035. [DOI] [PMC free article] [PubMed] [Google Scholar]